Abstract
A high serum uric acid is common in subjects with pulmonary hypertension. The increase in serum uric acid may be a consequence of the local tissue ischemia and/or hypoxia, and it may also result from other factors independent of ischemia or hypoxia that occur in various forms of pulmonary hypertension. While classically viewed as a secondary phenomenon, recent studies suggest that hyperuricemia may also have a role in mediating the local vasoconstriction and vascular remodeling in the pulmonary vasculature. If uric acid does have a contributory role in pulmonary hypertension, we may see an increasing prevalence of pulmonary hypertension as hyperuricemia is common in subjects with obesity and metabolic syndrome. We propose studies to investigate the role of uric acid in pulmonary hypertension and to determine if lowering serum uric acid may have clinical benefit in this condition.
Keywords: Uric acid, Hyperuricemia, Pulmonary Hypertension, Nitric Oxide
Pulmonary hypertension (PH) is defined as elevated mean pulmonary arterial pressure (PAP, typically > 25 mm Hg at rest, or >30 mm Hg with exercise, or > 40 mm Hg for systolic) and is associated with the development of right heart failure and increased mortality [1]. The pathogenesis and etiologies of PH are complex, but in many cases (lung disease, primary hypoventilation syndromes, and chronic high altitude residence) it appears to be due to hypoxia-dependent pulmonary vasoconstriction with secondary vascular remodeling [2]. In this paper we propose that uric acid may contribute to its pathogenesis. We further suggest that the epidemic of obesity, in which subjects are commonly hyperuricemic, may lead to increasing numbers of patients with clinically significant PH.
An elevated serum uric acid is common in pulmonary hypertension
Up to 80 % of adult patients [3, 4] and 60% of pediatric patients with PH [5] have serum uric acid concentrations more than 5.5 mg/dl. In these subjects the level of uric acid correlates with right atrial pressure (RAP) [6]. The relationship of uric acid with PH is observed for multiple etiologies, including with both primary PH and PH associated with a variety of conditions such as congenital heart disease, collagen vascular disease, and recurrent venous thromboembolism. Hemolytic disorders associated with PH, such as sickle cell disease, are also commonly associated with elevated serum uric acid [7, 8].
Subjects with metabolic syndrome (central obesity, dyslipidemia, elevated systemic blood pressure) also commonly have an elevated serum uric acid, and many show evidence for PH [9]. In some subjects with metabolic syndrome the PH may be due to obstructive sleep apnea (OSA) [10–12]. In other subjects it may be a consequence of pulmonary venous hypertension secondary to left ventricular diastolic dysfunction from systemic hypertension or longstanding insulin resistance or diabetes [13].
Potential mechanisms for an elevated serum uric acid in pulmonary hypertension
A variety of mechanisms could account for an elevated serum uric acid in PH. Some of these factors are listed below.
Tissue hypoxia
Uric acid is an end product of purine metabolism that is generated from xanthine by xanthine oxidoreductase (XOR) during the metabolism of ATP, DNA and RNA. One of the more common mechanisms for an elevated uric acid is via tissue ischemia [14, 15]. Specifically, ischemia both activates XOR and results in the local consumption of ATP with the release of adenine nucleotides that provide substrate for uric acid generation [16, 17]. Lactate also interferes with the organic anion transporters in the proximal tubule resulting in enhanced urate reabsorption [18]. As a consequence, an elevated serum uric acid is common in ischemic states, such as in congestive heart failure [19], in congenital heart disease associated with hypoxia [20], and with humans chronically living at high altitude [21].
Other mechanisms
Other mechanisms may also account for the presence of hyperuricemia in subjects with PH. For example, the PH associated with hemolytic disorders, such as thalassemia [22], spherocytosis [23], paroxysmal nocturnal hemoglobinuria [24] and sickle cell disease (SCD) [25] may be due to the release of adenosine deaminase (ADA) from injured erythrocytes which may shunt adenosine towards uric acid production [26]. The hyperuricemia associated with OSA and metabolic syndrome could be due to the effects of hyperinsulinemia, since insulin is known to increase urate reabsorption in the proximal tubule [27]. In subjects with OSA the increase in uric acid may also reflect both the intermittent arterial hypoxemia and sympatho-adrenal activation. Hyperuricemia is also common in subjects with end stage renal disease since renal excretion is prevented. These subjects may account for up to 10–15% of all patients with PH [10], in which the mechanism is not fully attributable to left ventricular diastolic dysfunction, high cardiac output secondary to hypervolemia, anemia and arterio-venous fistulae, or hyperparathyroidism [11]. Thus, there are numerous potential mechanisms to account for an elevated uric acid in PH. These studies suggest that an elevated uric acid is simply a marker for severity of PH, and may not have any modifying role.
Uric acid: a predictor of outcome in pulmonary hypertension
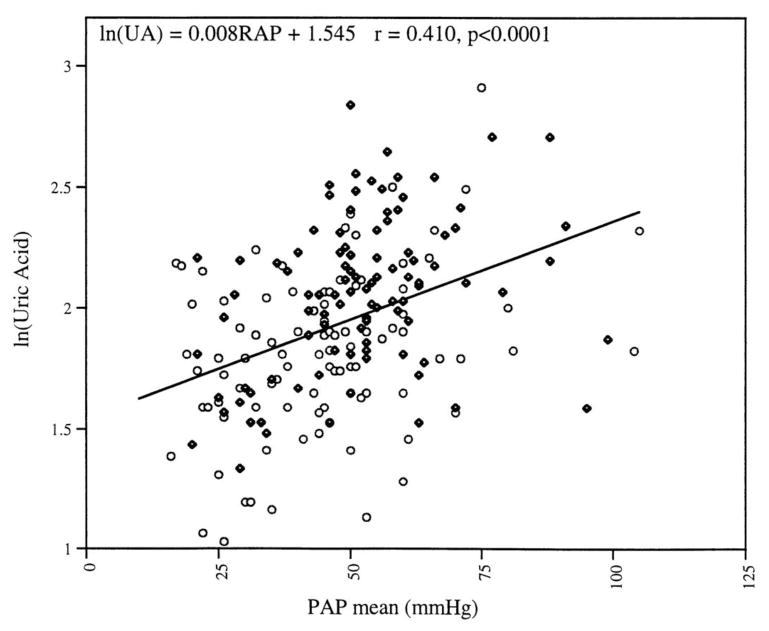
Serum uric acid correlates with mean pulmonary artery pressure (Figure 1)[6] is also a significant predictor of outcomes in PH [3]. PH patients with hyperuricemia live dramatically less long than patients without hyperuricemia [3, 28, 29]. Similarly, an elevated serum uric acid has also been associated with increased mortality in congestive heart failure patients as well as in the general and hypertensive population (reviewed in [30]).
Figure 1. Serum uric acid correlates with pulmonary arterial pressure.
The relationship of the log of serum uric acid with mean pulmonary artery pressure in 191 patients (r √ 0.41; p ≤ 0.0001). Solid circles reflect subjects with primary pulmonary hypertension, and open circles show subjects with secondary pulmonary hypertension. Reproduced with permission from Voelkel et al.[6]
Several mechanisms could account for the increased mortality associated with hyperuricemia. For example, serum uric acid could simply reflect the degree of local or systemic hypoxia, the presence of coexistent renal dysfunction, or the presence of local oxidative stress [16]. However, it also raises the intriguing possibility that an elevated uric acid may have a contributory role in PH. If so, what would be the mechanism?
Uric acid: a potential mediator of pulmonary hypertension?
Uric Acid as a Vasoconstrictor: Role in Endothelial Dysfunction
Uric acid is an unusual molecule in that it has both oxidative and antioxidant properties [31]. The antioxidant properties are well known, and include the ability of uric acid to neutralize various oxidants in cell free systems, including superoxide anion, hydroxyl radical, and peroxynitrite [32]. Uric acid can also protect endothelial cells from exogenously applied oxidants [33]. The infusion of uric acid acutely in humans has also been associated with an improvement in endothelial function, as determined by measuring brachial artery reactivity [34, 35].
Although these studies suggest a beneficial effect of uric acid, nonetheless it is a consistent finding that chronic hyperuricemia is associated with endothelial dysfunction, including in subjects with congestive heart failure, diabetes, smoking, OSA, and asymptomatic hyperuricemia [36–40]. Furthermore, lowering uric acid in these subjects with allopurinol has been routinely shown to improve endothelial function [36–40]. In these studies the investigators attributed the benefit of allopurinol to the reduction of XOR-induced oxidants. Nevertheless, recent studies suggest uric acid itself may be able to induce endothelial dysfunction.
When vascular endothelial cells are incubated with uric acid in the range of physiological concentrations, one can demonstrate a dose-dependent decrease in nitric oxide (NO) production [41–43]. This has been demonstrated in human, porcine, and bovine endothelial cells and has been shown by a variety of methods, including the Griess reaction and by various intracellular fluorescent probes [41–43]. Hyperuricemic rats also show a reduction in plasma nitrate and nitrite consistent with endothelial dysfunction and decreased NO production [42].
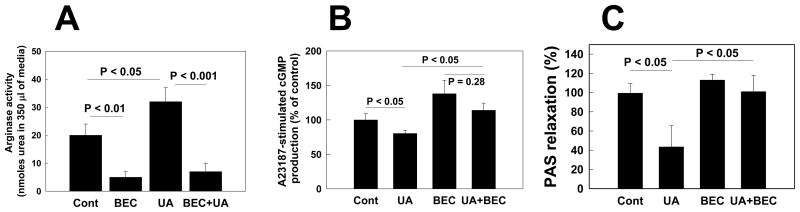
Several cellular mechanisms account for the reduction in NO bioavailability. For example, we have found that uric acid can stimulate arginase in pulmonary endothelial cells, which reduces local levels of L-arginine which are required for NO generation [44]. Inhibition of arginase prevented the inhibitory effect of uric acid on cGMP (the NO second messenger) production in pulmonary endothelial cells and abolished uric acid-induced inhibition of acetylcholine-stimulated vasodilation of pulmonary artery segments (Figure 2). Uric acid, while an antioxidant in the extracellular setting, has also been shown to stimulate oxidant production in endothelial cells, vascular smooth muscle cells and adipocytes [45–48]. The mechanism may relate to stimulation of NADPH oxidase, which produces the oxidants that then secondarily reduce local NO resulting in endothelial cell dysfunction. Additionally, uric acid can generate free radicals on combining with various oxidants such as peroxynitrite [49], and may also directly react with NO to form the product 6-aminouracil [50].
Figure 2. Arginase inhibition prevents the UA effect on cGMP production in pulmonary artery endothelial (PAEC) cells and on vasorelaxation of U-46619-contracted porcine pulmonary artery segments (PAS).
A: An arginase inhibitor (S)-(2-Boronoethyl)-L-cysteine (BEC) blocks UA-induced stimulation of urea production. PAEC were grown on 100 mm dishes in 7 ml of RPMI without (Control) or with an arginase inhibitor BEC (100 μM), UA (7.5 mg/dl), or UA + BEC for 24 h. At the end of the incubation period, aliquots of culture media were taken for analysis of urea contents. Data are from 3 experiments made in duplicate.
B: The arginase inhibitor BEC prevents the UA-induced reduction of cGMP production by PAEC. PAEC grown on 60 mm dishes were treated without (Control) or with an arginase inhibitor BEC (100 μM), UA (7.5 mg/dl), or UA + BEC for 1 h. At the end of the treatment period, the phosphodiesterase inhibitor IBMX (0.3 mM) was added to cells and 15 min later PAEC were stimulated with A23187 (10 μM) for 15 min. At the end of the stimulation, PAEC were washed with PBS and scraped in 0.3 ml of 65%, and the ethanol extract was collected. Contents of cGMP in 50 μl of the ethanol extract were determined using a cGMP detection kit. Bars are the means +/− SEM of 4 experiments made in duplicate.
C: The arginase inhibitor BEC prevents the UA effects on vasorelaxation of U-46619-contracted porcine PAS. PAS were washed and incubated without (Control) or with BEC (100 μM), UA (7.5 mg/dl), or UA + BEC for 1 h in Earl’s buffer at 37°C for 1h. PAS were pre-contracted with a thromboxane mimetic U-46619 (0.5 μM), and then the vasorelaxation responses to acethylcholine were assessed in PAS incubated without (control) or with corresponding drugs. To standardize the data, the vascular tone of U-46619-contracted PAS was set to 0% relaxation. For each treatment, 4 separate PAS were used.
[Reproduced with permission from [44]]
Uric Acid: Stimulation of Other Vasoconstrictor Systems
Other vasoconstrictive mediator systems can be activated by soluble uric acid. For example, uric acid increases cyclo-oxygenase 2 (COX2) expression both in the kidney and vascular smooth muscle cells, and in the latter this was shown to result in thromboxane production [51, 52]. Uric acid can also increase renin expression in vivo [53], and stimulate the production of angiotensin II and increase AT1 receptor expression in cultured vascular smooth muscle cells [43, 47]. Raising uric aid has also been reported to increase endothelin-1 expression in cardiac fibroblasts and vascular smooth muscle cells and to increase serum aldosterone levels [54–56]. Theoretically these effects could enhance hypoxia-mediated pulmonary artery vasoconstriction.
Recently new findings have demonstrated that hypoxia-induced PH depends on iron status: infusions of iron can blunt the vasoconstrictor responses to acute hypoxia and reducing iron availability enhances the constrictor response to hypoxia [57, 58]. It is known that uric acid can form stable complexes with ferric ions, thereby protecting against iron-induced oxidative damage [59, 60]. The chelation of iron by uric acid might present another possible mechanism underlying the pulmonary hypertensive effects of hyperuricemia.
Uric Acid-Mediated Vascular Remodeling
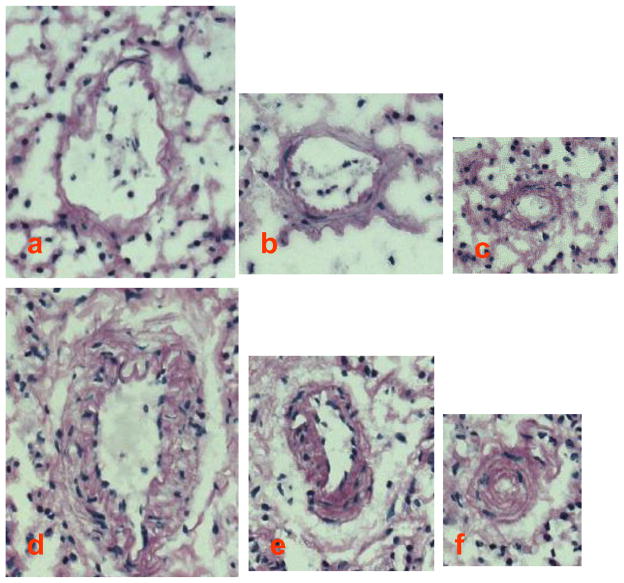
In experimental hyperuricemic animals elevated serum uric acid levels are associated with arteriolosclerotic vascular disease, characterized by vascular medial wall thickening, vascular smooth muscle cell proliferation, and luminal narrowing [51, 61]. Reducing uric acid with a XOR inhibitor can block the development of these vascular changes [51, 61]. Studies in vascular smooth muscle cells in culture have shown that uric acid enters cells via specific transporters such as URAT1 [45] where it engages mitogen activated protein kinases (p38 and ERK), nuclear transcription factors (NF-κB and AP-1) and stimulates growth factors (PDGFA- and C), vasoconstrictive agents (COX-2 induced thromboxane), oxidants, and components of the renin angiotensin system (including angiotensin II and the AT1 receptor) [43, 47, 51, 52, 62–64]. In preliminary data we have found that acutely raising uric acid in rats with a uricase inhibitor allantoxanamide also causes an increase in medial thickness of the pulmonary arterioles (Figure 3). These studies suggest uric acid could be a potentially modifiable risk factor in PH.
Figure 3. Morphological changes in the lung blood vessels in rats treated with allantoxanamide (AX).
Sprague-Dawley rats were injected i.p. daily with AX (120 mg/kg body weight) or vehicle (control rats) for 15 days. At the end of the treatment, rats were sacrificed and the lungs were fixed in 15% buffered formalin solution. Paraffin-embedded samples were cut into 3 μm sections and stained with the periodic acid-Schiff reagent (PAS). Randomly chosen fields on sections from each animal were photographed. Typical images of the blood vessels are presented; a through c are control rats; d through f are rats treated with AX. Magnification × 200.
Proinflammatory Mechanisms of Hyperuricema
Uric acid has also been found to activate both the innate and adaptive immune system [65, 66]. Uric acid stimulates the release of several proinflammatory molecules including MCP-1 and C- reactive protein in vascular cells [43, 62]. In experimental lung injury, local uric acid generation has been reported to activate the NALP3 inflammasome with IL-1β generation [67] which in turn may activate processes mediated by hypoxia-inducible factor-1 (HIF-1α) [68]. Uric acid-induced production of pro-inflammatory cytokines could have a key role in the vascular remodeling that occurs in PH [69].
Conclusions
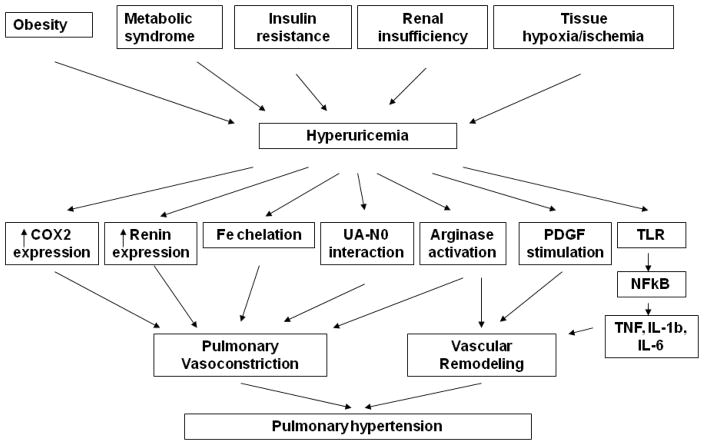
In conclusion, an elevated serum uric acid is common in subjects with PH and may simply reflect the presence of tissue ischemia or oxidative stress common in these subjects. Even if it is strictly a biomarker, the levels of serum uric acid may carry prognostic value since high levels predict poor outcomes. Most interestingly, recent studies suggest that the elevation in uric acid could have a secondary contributory role in the pathogenesis or progression of PH, since experimental studies suggest uric acid may have proinflammatory and vasoconstrictive effects as well as direct effects on vascular remodeling. Because hyperuricemia is associated with obesity and diabetes, we can predict that PH in patients with these diseases may have a more severe manifestation and have a pore prediction of outcome (Figure 4). We propose clinical studies to determine if lowering uric acid, especially with XOR inhibitors, may be of benefit in the management of PH, as we have shown in pediatric systemic hypertension [70].
Figure 4. Possible mechanisms of contribution of hyperuricemia to the severity of pulmonary hypertension.
COX-2 = cyclo-oxygenase-2; UA = uric acid; NO = nitric oxide; PDGF = platelet derived growth factor; TLR = Toll-like receptor; TNF = tumor necrosis factor; IL = interleukin
Acknowledgments
This work was supported by the National Institute of Health [Grants DK-52121, HL-68607, HL-85133]; and VA Merit Review
Abbreviations
- AP-1
activator protein-1
- AT
angiotensin
- COX-2
cyclo-oxygenase-2
- IL
interleukin
- MCP-1
monocyte chemotactic protein-1
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NOS
nitric oxide synthase
- OSA
obstructive sleep apnea
- PAP
pulmonary arterial pressure
- PDGF
platelet derived growth factor
- PH
pulmonary hypertension
- TNF
tumor necrosis factor
- UA
uric acid
- XOR
xanthine oxidoreductase
Footnotes
Dr. Zharikov has no conflicts of interest to disclose. Dr. Swenson has no conflicts of interest to disclose. Dr. Block has no conflicts of interest to disclose. Dr. Lanaspa has no conflicts of interest to disclose. Dr. Patel has no conflicts of interest to disclose. Dr. Johnson has no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barst RJ, McGoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. Journal of the American College of Cardiology. 2004;43:40S–47S. doi: 10.1016/j.jacc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Presberg KW, Dincer HE. Pathophysiology of pulmonary hypertension due to lung disease. Curr Opin Pulm Med. 2003;9:131–138. doi: 10.1097/00063198-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Bendayan D, Shitrit D, Ygla M, Huerta M, Fink G, Kramer MR. Hyperuricemia as a prognostic factor in pulmonary arterial hypertension. Respir Med. 2003;97:130–133. doi: 10.1053/rmed.2003.1440. [DOI] [PubMed] [Google Scholar]
- 4.Hoeper MM, Hohlfeld JM, Fabel H. Hyperuricaemia in patients with right or left heart failure. Eur Respir J. 1999;13:682–685. doi: 10.1183/09031936.99.13368299. [DOI] [PubMed] [Google Scholar]
- 5.Van Albada ME, Loot FG, Fokkema R, Roofthooft MT, Berger RM. Biological serum markers in the management of pediatric pulmonary arterial hypertension. Pediatr Res. 2008;63:321–327. doi: 10.1203/PDR.0b013e318163a2e7. [DOI] [PubMed] [Google Scholar]
- 6.Voelkel MA, Wynne KM, Badesch DB, Groves BM, Voelkel NF. Hyperuricemia in severe pulmonary hypertension. Chest. 2000;117:19–24. doi: 10.1378/chest.117.1.19. [DOI] [PubMed] [Google Scholar]
- 7.Bayazit AK, Noyan A, Aldudak B, Ozel A, Anarat A, Kilinc Y, et al. Renal function in children with sickle cell anemia. Clin Nephrol. 2002;57:127–130. doi: 10.5414/cnp57127. [DOI] [PubMed] [Google Scholar]
- 8.al-Naama LM, al-Sadoon EA, al-Sadoon TA. Levels of uric acid, urea and creatinine in Iraqi children with sickle cell disease. J Pak Med Assoc. 2000;50:98–102. [PubMed] [Google Scholar]
- 9.Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, et al. Insulin resistance in pulmonary arterial hypertension. Eur Respir J. 2009;33:318–324. doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peled N, Kassirer M, Shitrit D, Kogan Y, Shlomi D, Berliner AS, et al. The association of OSA with insulin resistance, inflammation and metabolic syndrome. Respir Med. 2007;101:1696–1701. doi: 10.1016/j.rmed.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. 2007;3:467–472. [PMC free article] [PubMed] [Google Scholar]
- 12.Golbin JM, Somers VK, Caples SM. Obstructive sleep apnea, cardiovascular disease, and pulmonary hypertension. Proc Am Thorac Soc. 2008;5:200–206. doi: 10.1513/pats.200708-143MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robbins IM, Newman JH, Johnson RF, Hemnes AR, Fremont RD, Piana RN, et al. Association of the Metabolic Syndrome With Pulmonary Venous Hypertension. Chest. 2009;136:31–36. doi: 10.1378/chest.08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedl HP, Till GO, Trentz O, Ward PA. Role of oxygen radicals in tourniquet-related ischemia-reperfusion injury of human patients. Klin Wochenschr. 1991;69:1109–1112. doi: 10.1007/BF01645168. [DOI] [PubMed] [Google Scholar]
- 15.De Scheerder IK, van de Kraay AM, Lamers JM, Koster JF, de Jong JW, Serruys PW. Myocardial malondialdehyde and uric acid release after short-lasting coronary occlusions during coronary angioplasty: potential mechanisms for free radical generation. The American journal of cardiology. 1991;68:392–395. doi: 10.1016/0002-9149(91)90838-c. [DOI] [PubMed] [Google Scholar]
- 16.Roy JS, McCord JM. Superoxide and ischemia: Conversion of xanthine dehydrogenase to xanthine oxidase. In: Greenwald RA, Cohen G, editors. Free Radicals and their Scavenger System. Elsevier; New York: 1983. pp. 145–153. [Google Scholar]
- 17.Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol. 1996;174:288–291. doi: 10.1016/s0002-9378(96)70410-6. [DOI] [PubMed] [Google Scholar]
- 18.Roch-Ramel F, Guisan B, Diezi J. Effects of uricosuric and antiuricosuric agents on urate transport in human brush-border membrane vesicles. The Journal of pharmacology and experimental therapeutics. 1997;280:839–845. [PubMed] [Google Scholar]
- 19.Leyva F, Anker SD, Godsland IF, Teixeira M, Hellewell PG, Kox WJ, et al. Uric acid in chronic heart failure: a marker of chronic inflammation. European heart journal. 1998;19:1814–1822. doi: 10.1053/euhj.1998.1188. [DOI] [PubMed] [Google Scholar]
- 20.Hayabuchi Y, Matsuoka S, Akita H, Kuroda Y. Hyperuricaemia in cyanotic congenital heart disease. Eur J Pediatr. 1993;152:873–876. doi: 10.1007/BF01957519. [DOI] [PubMed] [Google Scholar]
- 21.Jefferson JA, Escudero E, Hurtado ME, Kelly JP, Swenson ER, Wener MH, et al. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis. 2002;39:1135–1142. doi: 10.1053/ajkd.2002.33380. [DOI] [PubMed] [Google Scholar]
- 22.Morris CR, Kuypers FA, Kato GJ, Lavrisha L, Larkin S, Singer T, et al. Hemolysis-associated pulmonary hypertension in thalassemia. Ann N Y Acad Sci. 2005;1054:481–485. doi: 10.1196/annals.1345.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verresen D, De Backer W, Van Meerbeeck J, Neetens I, Van Marck E, Vermeire P. Spherocytosis and pulmonary hypertension coincidental occurrence or causal relationship? Eur Respir J. 1991;4:629–631. [PubMed] [Google Scholar]
- 24.Heller PG, Grinberg AR, Lencioni M, Molina MM, Roncoroni AJ. Pulmonary hypertension in paroxysmal nocturnal hemoglobinuria. Chest. 1992;102:642–643. doi: 10.1378/chest.102.2.642. [DOI] [PubMed] [Google Scholar]
- 25.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- 26.Tofovic SP, Jackson EK, Rafikova O. Adenosine deaminase-adenosine pathway in hemolysis-associated pulmonary hypertension. Med Hypotheses. 2009;72:713–719. doi: 10.1016/j.mehy.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 27.Quinones GA, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, et al. Effect of insulin on uric acid excretion in humans. The American journal of physiology. 1995;268:E1–E5. doi: 10.1152/ajpendo.1995.268.1.E1. [DOI] [PubMed] [Google Scholar]
- 28.Nagaya N, Uematsu M, Satoh T, Kyotani S, Sakamaki F, Nakanishi N, et al. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am J Respir Crit Care Med. 1999;160:487–492. doi: 10.1164/ajrccm.160.2.9812078. [DOI] [PubMed] [Google Scholar]
- 29.Wensel R, Opitz CF, Anker SD, Winkler J, Hoffken G, Kleber FX, et al. Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation. 2002;106:319–324. doi: 10.1161/01.cir.0000022687.18568.2a. [DOI] [PubMed] [Google Scholar]
- 30.Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 31.Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides, nucleotides & nucleic acids. 2008;27:608–619. doi: 10.1080/15257770802138558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite with uric acid in the presence of ascorbate and thiols: implications for uncoupling endothelial nitric oxide synthase. Biochem Pharmacol. 2005;70:343–354. doi: 10.1016/j.bcp.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Waring WS, McKnight JA, Webb DJ, Maxwell SR. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabete. 2006;55:3127–3132. doi: 10.2337/db06-0283. [DOI] [PubMed] [Google Scholar]
- 35.Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. Journal of cardiovascular pharmacology. 2001;38:365–371. doi: 10.1097/00005344-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Butler R, Morris AD, Belch JJ, Hill A, Struthers AD. Allopurinol normalizes endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension. 2000;35:746–751. doi: 10.1161/01.hyp.35.3.746. [DOI] [PubMed] [Google Scholar]
- 37.Mercuro G, Vitale C, Cerquetani E, Zoncu S, Deidda M, Fini M, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. The American journal of cardiology. 2004;94:932–935. doi: 10.1016/j.amjcard.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 38.Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation. 2002;106:221–226. doi: 10.1161/01.cir.0000022140.61460.1d. [DOI] [PubMed] [Google Scholar]
- 39.Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107:416–421. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- 40.Doehner W, Anker SD. Xanthine oxidase inhibition for chronic heart failure: is allopurinol the next therapeutic advance in heart failure? Heart (British Cardiac Society) 2005;91:707–709. doi: 10.1136/hrt.2004.057190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feig DI, Nakagawa T, Karumanchi SA, Oliver WJ, Kang DH, Finch J, et al. Hypothesis: Uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney International. 2004;66:281–287. doi: 10.1111/j.1523-1755.2004.00729.x. [DOI] [PubMed] [Google Scholar]
- 42.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney International. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 43.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 44.Zharikov S, Krotova K, Hu H, Baylis C, Johnson RJ, Block ER, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2008;295:C1183–C1190. doi: 10.1152/ajpcell.00075.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sautin YY, Nakagawa T, Zharikov S, Johnson RJ. Adverse effects of the classical antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am J Physiol Cell Physiol. 2007;293:C584–C596. doi: 10.1152/ajpcell.00600.2006. [DOI] [PubMed] [Google Scholar]
- 47.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. Journal of hypertension. 2008;26:269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 48.Yu MA, Sanchez-Lozada LG, Johnson RJ, Kang DH. Uric acid: An inducer of oxidative stress and renin angiotensin system in human vascular endothelial cells. American journal of physiology. 2009 submitted. [PubMed] [Google Scholar]
- 49.Santos CX, Anjos EI, Augusto O. Uric acid oxidation by peroxynitrite: multiple reactions, free radical formation, and amplification of lipid oxidation. Arch Biochem Biophy. 1999;372:285–294. doi: 10.1006/abbi.1999.1491. [DOI] [PubMed] [Google Scholar]
- 50.Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides, nucleotides & nucleic acids. 2008;27:967–978. doi: 10.1080/15257770802257952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. 2002;40:355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 53.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 54.Chao HH, Liu JC, Lin JW, Chen CH, Wu CH, Cheng TH. Uric acid stimulates endothelin-1 gene expression associated with NADPH oxidase in human aortic smooth muscle cells. Acta Pharmacol Sin. 2008;29:1301–1312. doi: 10.1111/j.1745-7254.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 55.Cheng TH, Lin JW, Chao HH, Chen YL, Chen CH, Chan P, et al. Uric acid activates extracellular signal-regulated kinases and thereafter endothelin-1 expression in rat cardiac fibroblasts. Int J Cardiol. 2008 doi: 10.1016/j.ijcard.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 56.Eraranta A, Kurra V, Tahvanainen AM, Vehmas TI, Koobi P, Lakkisto P, et al. Oxonic acid-induced hyperuricemia elevates plasma aldosterone in experimental renal insufficiency. Journal of hypertension. 2008;26:1661–1668. doi: 10.1097/HJH.0b013e328303205d. [DOI] [PubMed] [Google Scholar]
- 57.Smith TG, Balanos GM, Croft QP, Talbot NP, Dorrington KL, Ratcliffe PJ, et al. The increase in pulmonary arterial pressure caused by hypoxia depends on iron status. J Physiol. 2008;586:5999–6005. doi: 10.1113/jphysiol.2008.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith TG, Talbot NP, Privat C, Rivera-Ch M, Nickol AH, Ratcliffe PJ, et al. Effects of iron supplementation and depletion on hypoxic pulmonary hypertension: two randomized controlled trials. JAMA. 2009;302:1444–1450. doi: 10.1001/jama.2009.1404. [DOI] [PubMed] [Google Scholar]
- 59.Ghio AJ, Kennedy TP, Stonehuerner J, Carter JD, Skinner KA, Parks DA, et al. Iron regulates xanthine oxidase activity in the lung. Am J Physiol Lung Cell Mol Physiol. 2002;283:L563–L572. doi: 10.1152/ajplung.00413.2000. [DOI] [PubMed] [Google Scholar]
- 60.Miura T, Muraoka S, Ogiso T. Inhibitory effect of urate on oxidative damage induced by adriamycin-Fe3+ in the presence of H2O2. Res Commun Chem Pathol Pharmacol. 1993;79:75–85. [PubMed] [Google Scholar]
- 61.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. American journal of physiology. 2002;282:F991–997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 62.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension. 2003;41:1287–1293. doi: 10.1161/01.HYP.0000072820.07472.3B. [DOI] [PubMed] [Google Scholar]
- 63.Kang DH, Han L, Ouyang X, Kahn AM, Kanellis J, Li P, et al. Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. American journal of nephrology. 2005;25:425–433. doi: 10.1159/000087713. [DOI] [PubMed] [Google Scholar]
- 64.Price KL, Sautin YY, Long DA, Zhang L, Miyazaki H, Mu W, et al. Human vascular smooth muscle cells express a urate transporter. J Am Soc Nephrol. 2006;17:1791–1795. doi: 10.1681/ASN.2006030264. [DOI] [PubMed] [Google Scholar]
- 65.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 66.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 67.Gasse P, Riteau N, Charron S, Girre S, Fick L, Petrilli V, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 68.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 69.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 70.Feig DI, Soletsky B, Johnson RJ. Effect of Allopurinol on the Blood Pressure of Adolescents with Newly Diagnosed Essential Hypertension. JAMA. 2008;300:922–930. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]