Abstract
The results of the GUARDIAN/EXPEDITION trials demonstrate the need for more precisely controlled studies to inhibit Na/H exchange (NHE1) during ischemia/reperfusion. This is because overwhelming evidence is consistent with the hypothesis that myocardial ischemic injury results in part from increases in intracellular Na (Nai) mediated by Na/H exchange (NHE1) that in turn promote Na/Ca exchanger (NCX) mediated increases in intracellular Ca ([Ca]i) and Ca-dependent cell damage. We used a more potent and specific NHE1 inhibitor HOE 694 (HOE) to test whether inhibition of NHE1 during ischemia limits increases in Nai and [Ca]i in newborns. NMR was used to measure pHi, Nai, [Ca]i, and ATP in isolated newborn rabbit hearts. Perfusion pressure (PP) and left ventricular developed pressure (LVDP) and CK were measured. HOE was added before global ischemia. Results are reported as mean±SE. Nai (mEq/kg dry wt) rose from 11.6±0.9 prior to ischemia to 114.0±16.1 at the end of ischemia and recovered to 55.2±11.8 in the control group. During ischemia and reperfusion the corresponding values for Nai in the HOE group (63.1±8.4 and 15.9±2.5, respectively, p<0.05) were lower than control. In the control group [Ca]i (nM/L) rose from 331±41 to 1069±71 and recovered to 814±51 whereas in the HOE group [Ca]i rose less (p<0.05): 359±50, 607±85 and 413±40, respectively. Total CK release was significantly reduced in the HOE group. PP and LVDP also recovered significantly better in the HOE group than control. In conclusion, NHE1 inhibition diminishes ischemia-induced increases in Nai and therefore [Ca]i and thus diminishes myocardial injury in neonatal hearts.
Keywords: Developmental biology, ischemia/reperfusion, Sodium, Calcium, Na/H exchanger, Na/Ca exchanger, NMR
Introduction
Current methods of myocardial protection during adult open-heart surgery have been shown to improve myocardial preservation. In contrast, myocardial protection during pediatric open-heart surgery is relatively less successful and associated with greater morbidity and mortality.1,2 Of particular interest in this context are findings that Na/H exchanger (NHE1) inhibition diminishes intracellular Na (Nai) and Ca (Cai) accumulation and thereby diminishes myocardial injury during I/R under a wide variety of conditions.3, 4-7
However, the less than optimal design and outcome of the Guardian/Expedition trials aimed at inhibiting NHE1 under clinical conditions have made it necessary to revisit the issue of NHE1 inhibition in limiting myocardial ischemic injury. 8,9 This will require studies to precisely determine optimal treatments, perhaps including promising NHE1 inhibitors not yet tested clinically.
Successful recovery from open-heart surgery is directly related to limitation of myocardial injury associated with ischemia/reperfusion (I/R) and many pathophysiological processes in cardiac I/R are associated with derangement of cellular ion homeostasis.1,3,11,12 Decreases in intracellular pH (pHi), increases in intracellular Na (Nai), and Ca overload play key roles in the impairment of I/R tissue13. The Na dependence of myocyte Ca uptake suggested Na/Ca exchange (NCX) plays a major role in increasing cytosolic Ca concentration ([Ca]i) and therefore Ca-dependent myocardium injury.14-17 Although it has been suggested that Na/H exchange (NHE1) is inhibited by excess extracellular protons during ischemia,18 most studies are consistent with the hypothesis that myocardial hypoxic/ischemic injury is the result of low pHi stimulation of NHE1 leading to increased Na uptake that in turn promotes NCX mediated increases in [Ca]i and a cascade of Ca-dependent responses that cause injury, necrosis, and/or apoptosis. Furthermore, given numerous well-documented differences between the adult and newborn hearts’ responses to hypoxia, ischemia, and reperfusion and lack of consensus on age-related susceptibility to associated injury and treatment it should not be concluded that inhibition of NHE1 will have the same effects in both age groups. 10,11,19-27 Thus it is more important than ever to assess the response of the newborn heart to NHE1 inhibition under precisely controlled conditions. Only by doing so can we rationally develop interventions to protect the newborn heart from I/R injury. Finally, although many studies have demonstrated that NHE1 inhibition is effective in protection against ischemic damage in adult hearts,3,7,18,28 the results in this study are the first to assess the effect of HOE694 on pHi, Nai and [Ca]i in newborn hearts.
Methods
The study protocol was approved by the Animal Care Committee of the University of California, Davis (Davis, California, USA) and all experiments were conducted in accordance with “The Guide for Care and Use of Laboratory Animals” (NIH publication vol.25 no.28, revised 1996) and policies of the University of California, Davis.
New Zealand white rabbits (4-7 days old, weight 70 to 150 g) were anesthetized with sodium pentobarbital (65 mg/kg) and heparinized (1000 USP units/kg). Hearts were removed and perfused at a constant rate (9 ml/min) at 36±1°C (normothermic) for 60 minutes of baseline perfusion (which included 30 to 40 minute FBAPTA loading as necessary to measure [Ca]i). Perfusion pressure (PP) and left ventricular develop pressure (LVDP) were also measured. LVDP was calculated as end systolic minus end diastolic pressure as measured by isovolumic balloon after end diastolic pressure was set to 8±1 mmHg under baseline conditions. Normothermic ischemia started at time 0 (t = 0 min), lasted for 40 minutes, and 40 minutes normothermic reperfusion followed ischemia. Control perfusate contained (mmole/liter): 133 NaCl, 4.75 KCl, 1.25 MgCl2, 1.82 CaCl2, 25 NaHCO3, 11.1 dextrose. The NHE1 inhibitor 3-methylsulfonyl-4-piperidinobenzoyl guanidine methanesulfonate (HOE 694 - 5 μM, hereafter HOE), (Hoechst Marion Roussel, Germany), was added into the perfusate 10 minutes before ischemia in the treated group. NHE1 inhibitor was not added to the reperfusion solution. 23Na, 19F, and 31P NMR spectroscopy were used to measure Nai, [Ca]i, and pHi and ATP, respectively.22,29 For Nai measurement 7.5mM dysprosium triethylenetetraminehexaacetic acid (DyTTHA) was substituted iso-osmotically for NaCl in the perfusate and Ca was added to reach a perfusate concentration of 2 mM as measured by Ca electrode. For [Ca]i measurement, hearts were perfused during the baseline interval (30-40 min) with perfusate containing the acetoxymethyl ester of 5F-1,2-bis(2-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (FBAPTA) at 2.5 μM.24 FBAPTA was then washed out of the extracellular space with control solution before measurement of [Ca]i. Perfusates were equilibrated with 95% O2/5% CO2 which provided a pH of 7.35-7.45. At the end of perfusion, hearts were weighed wet and dried to constant weight (at least 48 hr) at 65°C to determine dry weight.
NMR spectroscopy
A Bruker AMX400 spectrometer (Bruker, Rheinstetten, Germany) was used for all experiments. Spectra were acquired and analyzed as previously described.29 Briefly, 23Na, 19F, and 31P spectra were generated from the summed free induction decays of 1000, 1500, and 148 excitation pulses (90°, 45° and 60°) using 2K, 2K, and 4K word data files and ±4000, ±5000, and ±4000 Hz sweep widths, respectively. For all nuclei data files were collected over 5 minute intervals. Nai was calculated from the calibrated area under the unshifted peak of the 23Na spectra after subtracting out the extracellular peak.22 [Ca]i in nmoles/liter cell water was calculated as the product of the ratio of the areas of the Ca-bound and Ca-free peaks in the FBAPTA spectrum and the 500 nM Ca-FBAPTA dissociation constant.30 pHi was determined from the chemical shift of the inorganic phosphate (Pi) resonance with reference to control phosphocreatine (PCr) calibrated at 37°C. ATP is reported as percent of control peak intensity where the control for each heart is the mean value for data acquired during 30 minutes of baseline perfusion prior to ischemia. Nai, [Ca]i, and pHi were measured in different hearts in order to maximize signal to noise for the nucleus being measured with a single tuned probe.
Creatine Kinase (CK) and left ventricular function measurements
Perfusate was collected during the baseline and reperfusion intervals and a CK kit from Sigma Diagnostics (Sigma Diagnostics, St. Louis, MO, USA) was used along with a Shimadzu UV-VIS recording photospectrometer (Shimadzu, Columbia, MD, USA) to measure the time integrated total CK release from the myocardium29. Strain gauge transducers connected to a Powerlab (AD Instruments, Colorado Springs, CO, USA) analogue-to-digital converter in conjunction with a computer were used to measure PP (mmHg) and LVDP where the latter is reported as % of control where the control for each heart is the mean of three measurements acquired during 30 minutes of baseline perfusion prior to ischemia.
Results are reported as mean±SE. Analysis of variance for repeated measures was used to test for the differences between the treatments. If differences were found, the unpaired t test was used to determine the times at which differences between treatments occurred. For all comparisons differences were considered significant when p < 0.05.
Results
Nai increases during ischemia and NHE1 inhibition diminishes ischemia-induced Nai accumulation
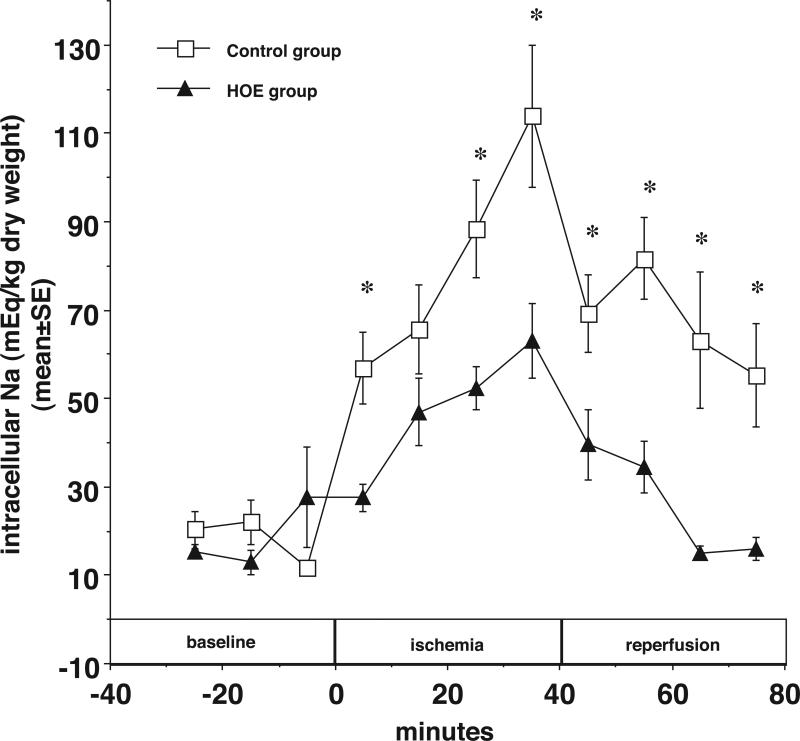
The hypothesis predicts that during ischemia, anaerobic metabolism increases H production and decreases pHi and thus stimulates NHE1 to increase Nai. 31,32 The data shown in Figure 1 demonstrate that HOE diminished the increase in Nai during ischemia and improved Nai recovery during reperfusion. Nai (mEq/kg dry wt) rose from 11.6±0.9 before ischemia to 114.0±16.1 at the end of ischemia and recovered to 55.2±11.8 in the control group. When the NHE1 inhibitor HOE (5 μM) was added to the perfusate 10 min prior to ischemia, the corresponding values for Nai in HOE treated hearts were 27.7±11.4, 63.1±8.4 and 15.9±2.5, respectively (*p < 0.05 during I/R).
Figure 1.
In newborn rabbit hearts, the NHE inhibitor HOE694 (5 μM) decreases accumulated intracellular Na during ischemia and reperfusion. Intracellular Na content (mEq/kg dry weight) is plotted vs. time before, during, and after ischemia with HOE694 (closed triangles), and without any treatment (open squares). * p<0.05.
[Ca]i increases during ischemia and NHE1 inhibition diminishes this increase
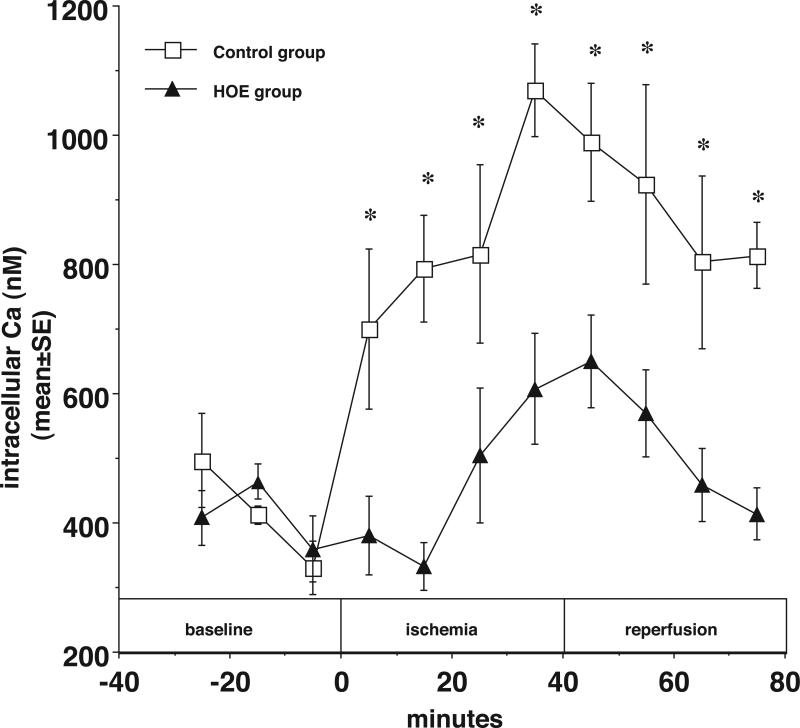
NHE1 inhibition also diminished the increase in [Ca]i during ischemia and was associated with relatively lower [Ca]i during reperfusion (*p < 0.05). As shown in Figure 2, in control hearts [Ca]i (nM/L) rose from 331±41 to 1069±71 and recovered to 814±51 whereas in HOE treated hearts the values were 359±50, 607±85 and 413±40, respectively. Again, this is consistent with the general hypothesis.
Figure 2.
The cytosolic Ca accumulation was significantly decreased in the HOE694 (5 μM) treated hearts during ischemia and reperfusion. Cytosolic Ca concentration (nM) is plotted vs. time before, during, and after ischemia with HOE694 (closed triangles) and without HOE694 (open squares). * p<0.05.
NHE1 inhibition has no measurable effect on pHi during ischemia but increases pHi during reperfusion
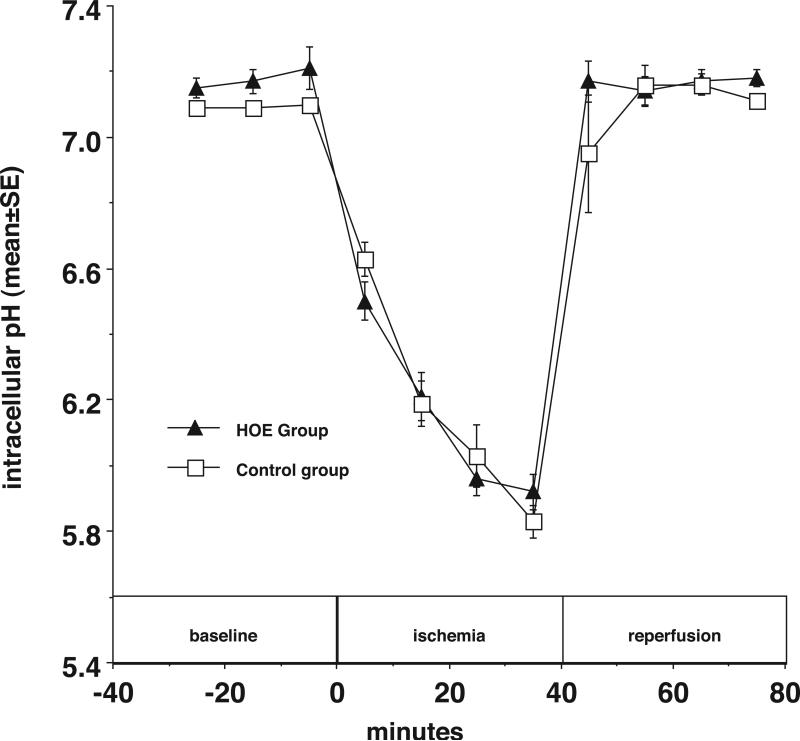
In contrast to what one might predict from the hypothesis, the data shown in Figure 3 demonstrate that after 40 minute ischemia pHi in HOE treated hearts (5.92 ± 0.05) was nominally greater, but not significantly different from that of control hearts (5.83± 0.05). The recovery of pHi in the HOE treated group was also similar to the control group during 40 min of reperfusion. There were no significant differences in pHi between the groups with and without HOE prior to ischemia.
Figure 3.
The NHE inhibitor HOE694 (5 μM) decreases intracellular proton concentration at the end of reperfusion. Intracellular pH is plotted vs. time before, during, and after ischemia with HOE694 (closed triangles) and without HOE694 (open squares).
NHE1 inhibition preserves myocardial high-energy phosphates during I/R
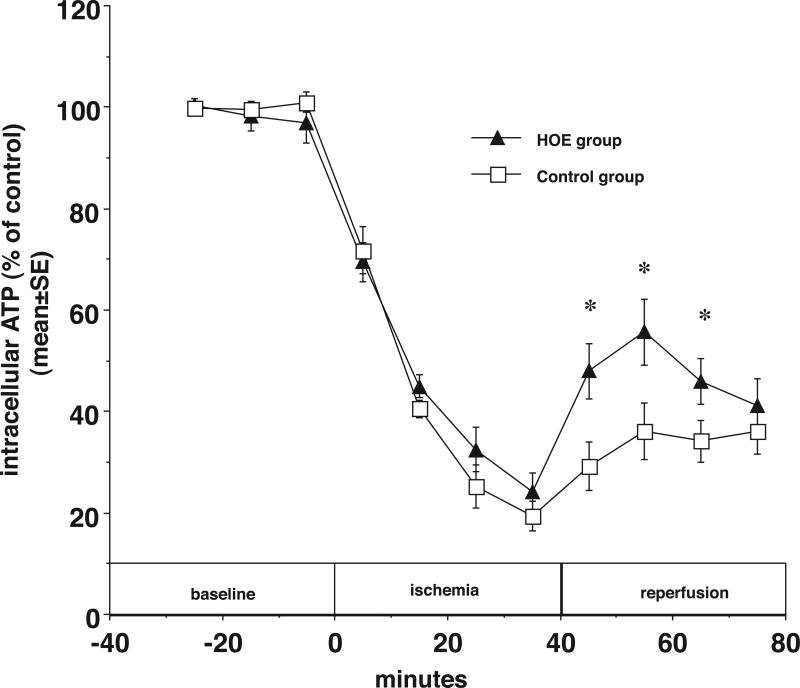
Figures 4 summarize the results of experiments aimed at determining the effects of NHE1 inhibition on high-energy phosphate metabolism in newborn ischemic myocardium. During 40 minutes of ischemia myocardial ATP declined to 24±4% of pre-ischemic baseline in HOE treated hearts. This was not significantly different from control group (19±3%, p>0.05). After 20 min of reperfusion ATP had recovered to 36±6% of baseline in control hearts and 56±7% in the HOE hearts (*p<0.05).
Figure 4.
In newborn rabbit hearts, the NHE inhibitor HOE694 (5 μM) improves ATP recovery during reperfusion. Myocardial ATP (% of control) is plotted vs. time before, during, and after ischemia with HOE694 (closed triangles) and without HOE694 (open squares). * p<0.05.
NHE1 inhibition decreases myocardial CK release and PP and improves LVDP recovery during reperfusion
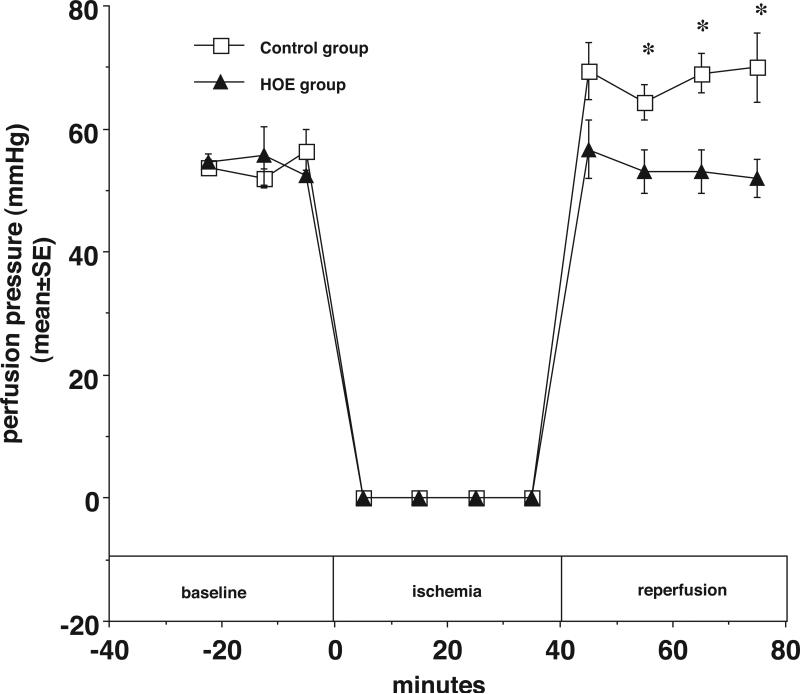
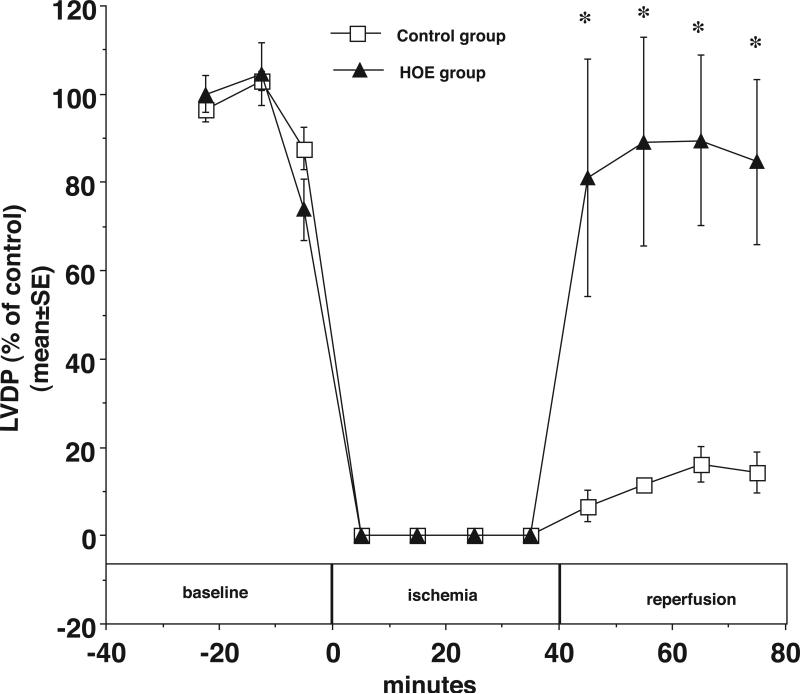
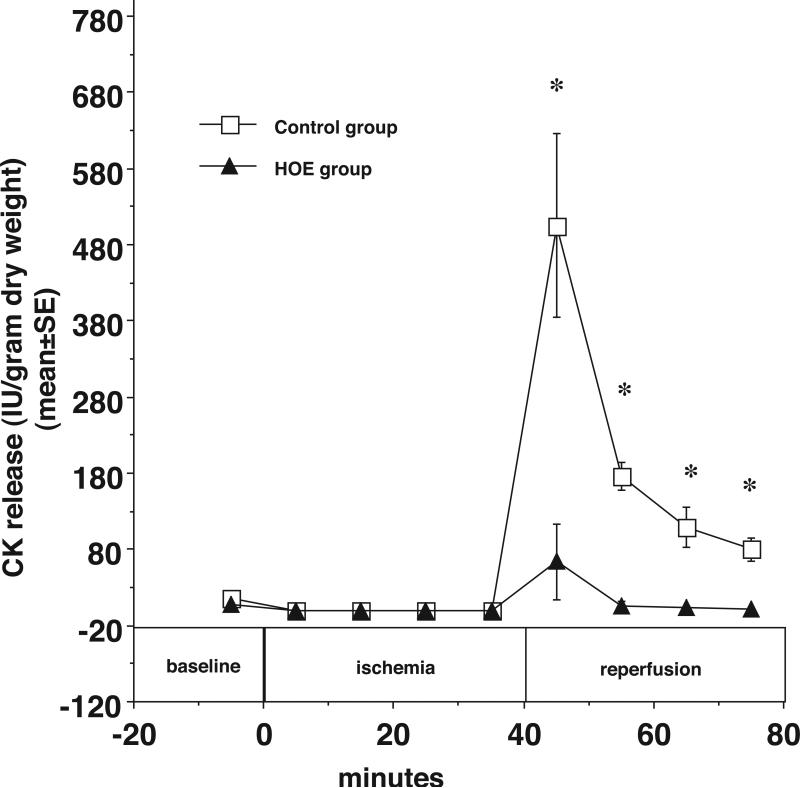
The results also demonstrate that NHE1 inhibition diminishes the increase in coronary resistance during reperfusion compared with the control group. In Figure 5, at the end of 40 minutes of reperfusion, the PP was 70±6 mmHg in control hearts and 52±3 in HOE treated hearts (*p<0.05). As shown in Figure 6, LVDP also recovered better during reperfusion in HOE treated hearts. At the end of reperfusion, LVDP was 14±4% of baseline in control hearts and 85±18% of baseline in HOE treated hearts (*p<0.05). Finally, as shown in Figure 7, HOE decreased total myocardial CK release during reperfusion from 870.6±118.8 (IU/g dry weight) in the control group to 74.6±56.4 (IU/g dry weight) in the HOE treated group (*p<0.05)
Figure 5.
In newborn rabbit hearts, the NHE inhibitor HOE694 (5 μM) limits the increase in perfusion pressure (a linear function of coronary resistance in this model) during reperfusion. Perfusion pressure (% of control) is plotted vs. time before, during, and after ischemia with HOE694 (closed triangles) and without HOE694 (open squares). * p<0.05.
Figure 6.
During reperfusion LVDP was significantly greater in the HOE treated hearts than the control hearts. LVDP (% of control) is plotted vs. time before, during, and after ischemia with HOE694 (closed triangles) and without HOE694 (open squares). * p<0.05.
Figure 7.
Myocardial CK release was significantly reduced during reperfusion in HOE treated hearts compared to untreated hearts. CK (IU/g dry weight) is plotted vs. time before, during, and after ischemia with HOE694 (closed triangles) and without HOE694 (open squares). *p<0.05
Discussion
It has been suggested that NHE1 is inhibited by low extracellular pH during ischemia.18,33 Nevertheless, we and others have demonstrated that hypoxia and ischemia cause increases in Hi, Nai, and [Ca]i in adult and newborn hearts and that such increases are limited by NHE1 inhibition. 22,29,31,34-36 That is, even if NHE1 is inhibited by low extracellular pH during ischemia, it remains capable of mediating a large net Na uptake, which is decreased by NHE1 inhibitors. Furthermore, these results provide further support for our hypothesis that also predicts any intervention that inhibits any step in this process will diminish cell damage. Although numerous studies have demonstrated that NHE1 inhibition is effective in protection against ischemic damage in adult and immature hearts,34-36 the results in this study are the first to assess the effect of HOE 694 on pHi, Nai and [Ca]i in newborn hearts of this age group.
By using a putatively more specific and potent NHE1 inhibitor, HOE 694, 37 this study provides further evidence to confirm NHE1 makes a significant contribution to Nai accumulation in the ischemic newborn heart. It also supports our previous work by demonstrating that, on a qualitative basis, newborn and adult hearts respond to ischemia similarly in terms of these ions and NHE1 inhibition. 22,29 This result is consistent with a previous report using isolated rabbit cardiac myocytes that concluded there was no developmental change in the NHE1 activity. 38
Effect of NHE1 inhibition on pHi during ischemia
It has been reported that after inhibiting the NHE1, pHi is lower than control in adult hearts during ischemia as well as during simulated “ischemia” in myocytes isolated from neonatal rat hearts. 39-41 However, in both adult and newborn hearts it has also been reported that inhibition of NHE1 has no significant effect on pHi during ischemia.2,4,22 The present study demonstrates that HOE had no significant effect on pHi during ischemia, but consistent with our previous study 22, pHi was significantly higher in the HOE group at the end of reperfusion. Our explanation of this result is as follows. During ischemia, anaerobic metabolism increases H production and Hi, which stimulates NHE1. H efflux is coupled to Na uptake via NHE1. Increased Na uptake stimulates Na-K ATPase to increase the consumption of ATP which increases H production as the result of an increased rate of ATP hydrolysis during ischemia. 13, 42 HOE inhibition of Na accumulation may thus decrease H production such that Hi accumulation is not increased even though proton efflux via NHE1 is inhibited.
HOE694, a more specific NHE1 inhibitor, decreases Nai and [Ca]i during I/R
Our results demonstrate that in newborn rabbit hearts the NHE1 inhibitor HOE (5 μM) diminishes the Nai accumulation at the end of ischemia by 44.7%. This may be compared to 75% inhibition of Nai accumulation in newborn hearts pretreated with 10 μM EIPA or in adult rat hearts, about 83% inhibition achieved by treatment with 2.7 μM EIPA, and essentially 100% inhibition achieved by treatment with 1mM amiloride.4, 22,43 HOE 694 has been identified as one of the more specific NHE1 inhibitors.37 Compared with the results of the effect of EIPA on Nai during ischemia, newborn hearts pretreated with EIPA show greater inhibition of Nai accumulation than those pretreated with HOE694 (p<0.01). This difference between HOE694 and EIPA could be the result of an added effect of EIPA on non-inactivating Na channels.44 This result is also consistent with the preliminary results which show that in adult rabbit hearts EIPA inhibits veratridine stimulated Na uptake whereas HOE does not (unpublished observation from this laboratory). Given that increases in [Ca]i appear to be dependent upon the magnitude of increase in Nai, regardless of whether accumulation occurs during ischemia or reperfusion, we speculate that non-specific inhibitors of Na uptake, e.g. those that inhibit both NHE1 and non-inactivating Na channels, may provide more protection from I/R injury that any specific inhibitor of Na uptake administered alone.
Our result is also consistent with that of previous studies which conclude the accumulation of Nai during myocardial ischemia plays a critical role in irreversible injury because of its effect on [Ca]i as mediated by NCX.10,11,34,45 Assuming the stoichiometry of NCX is 3/1 and the membrane potential remains at its normal resting level, if Nai increases by a factor of 2 to 3, the driving force for NCX will reach equilibrium and therefore NCX will no longer extrude Ca. If Nai increases further and/or if the membrane potential becomes more positive, flux through the NCX will reverse direction and it will mediate net Ca uptake. Thus when NHE1 was inhibited by HOE694, the decrease in Na accumulation was accompanied by more than a 66% decrease in [Ca]i accumulation during ischemia. This may be compared with an 84% decrease in ischemia-induced [Ca]i accumulation in adult rat hearts after Na uptake was decreased using the non-specific inhibitor amiloride (1mM). 4
NHE1 inhibition preserves high-energy phosphates and contractile function and decreases myocardial injury during reperfusion
While the above-described alterations in ion homeostasis are vitally important, changes in energy metabolism may also play an important role in the development of irreversible myocyte injury during I/R. NHE1 inhibition has been reported to reduce depletion of myocardial ATP during I/R in newborn and adult hearts. 22,36 In this study, after exposure to HOE, ATP recovers significantly better (*p<0.05) and Pi accumulation is significantly decreased (*p<0.05) early during reperfusion (Figs. 4 & 6). We postulate that NHE1 inhibition could diminish changes in ATP and Pi during I/R as a result of the relative decrease it causes in Nai and [Ca]i. In particular, decreases in Nai and [Ca]i accumulation during ischemia, would decrease Na- and Ca-dependent energy consuming processes (e.g. Na/K ATPase and Ca-ATPase).5 The Na accumulation during ischemia might also induce mitochondrial damage and decrease ATP production.46 NHE1 inhibition prevents the Nai accumulation and may thus preserve the mitochondria function to limit decreases in high-energy phosphates. Consistent with a previous report,28 after HOE treatment, LVDP recovered better (14.3% vs. 84.7% of baseline at the end of reperfusion). Also at the end of reperfusion, the coronary resistance, proportional to PP at constant flow, was decreased nearly 26% by HOE. Similarly, the total CK release is more than 11.6 times higher in the control group compared to the HOE treated group.
Our study has several limitations. First of all, it is well known that numerous pathways are involved in myocardial I/R injury. We only focused on the pathways outlined in the hypothesis. Second, it was designed to study the newborn, not the adult hearts, so we only used the newborn rabbit and did not include adult for comparison. Third, the Nai, [Ca]i, and pHi were measured in separated hearts. Fourth, in order to further improve signal to noise for the 19F data, data from 2 acquisitions was added to provide an average over 10 min. Thus the values of [Ca]i reported in this study are “average” calcium, rather than diastolic or systolic calcium and since these hearts are not beating during ischemia, we cannot ascribe associated injury to changes in systolic or diastolic [Ca]i per se.
Conclusion
We conclude that the data in this study are consistent with the hypothesis that newborn hearts, like adult hearts, respond to ischemia with an increase in NHE1 activity that leads to increased Nai, collapse of the transmembrane Na gradient and, consequently, increased accumulation of Ca (due in part to alterations in NCX) which in turn leads to myocardial cell damage and dysfunction. Also as predicted by the hypothesis, in newborn rabbit hearts NHE1 inhibition limits increases in intracellular Nai and [Ca]i during 40 minutes of warm ischemia. The results are further consistent with the postulate that HOE 694 is a more specific NHE1 inhibitor than EIPA.47 Finally, the observed decreases in Nai and [Ca]i accumulation are associated with significant preservation of high-energy phosphates, decreased CK release, decreased coronary resistance, and improved recovery of left ventricular function during reperfusion. Thus the results provide strong evidence suggesting further studies must be conducted to learn how NHE1 inhibition can be exploited clinically to limit morbidity and mortality associated with myocardial ischemia/reperfusion in the newborn.
Sources
This work was supported by NIH grants: HL07682, HL56681, HL21179 and was supported by a UCDHS Research Award. Spectrometers were further supported by NIH RR08206. HOE 694 was a gift from Hoechst Marion Roussel, The Pharmaceutical Company of Hoechst, Germany.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Imura H, Caputo M, Parry A, Pawade A, Angelini G, Suleiman M. Age-dependent and hypoxia-related differences in myocardial protection during pediatric open heart surgery. Circulation. 2001;103:1551–6. doi: 10.1161/01.cir.103.11.1551. [DOI] [PubMed] [Google Scholar]
- 2.Karimi M, Wang LX, Hammel JM, Mascio CE, Abdulhamid M, Barner EW, Scholz TD, Segar JL, Li WG, Niles SD, Caldarone CA. Neonatal vulnerability to ischemia and reperfusion: Cardioplegic arrest causes greater myocardial apoptosis in neonatal lambs than in mature lambs. J Thorac Cardiovasc Surg. 2004 Feb;127(2):490–7. doi: 10.1016/j.jtcvs.2003.07.052. [DOI] [PubMed] [Google Scholar]
- 3.Myers M, Mathur S, Li G, Karmazyn M. Sodium-hydrogen exchange inhibitors improve postischaemic recovery of function in the perfused rabbit heart. Cardiovasc Res. 1995;29:209–214. [PubMed] [Google Scholar]
- 4.Murphy E, Perlman M, London RE, Steenbergen C. Amiloride delays the ischemia-induced rise in cytosolic free calcium. Circ. Res. 1991;68:1250–1258. doi: 10.1161/01.res.68.5.1250. [DOI] [PubMed] [Google Scholar]
- 5.Scholz W, Albus U. Potential of selective sodium-hydrogen exchange inhibitors in cardiovascular therapy. Cardiovasc Res. 1995;29:184–188. [PubMed] [Google Scholar]
- 6.Levitsky J, Gurell D, Frishman W. Sodium ion/hydrogen ion exchange inhibition: a new pharmacologic approach to myocardial ischemia and reperfusion injury. J Mol Cell Cardiol. 1998;31:1985–95. doi: 10.1002/j.1552-4604.1998.tb04383.x. [DOI] [PubMed] [Google Scholar]
- 7.Stromer H, de Groot MC, Horn M, Faul C, Leupold A, Morgan JP, Scholz W, Neubauer S. Na(+)/H(+) exchange inhibition with HOE642 improves postischemic recovery due to attenuation of Ca(2+) overload and prolonged acidosis on reperfusion. Circulation. 2000;13:2738–45. doi: 10.1161/01.cir.101.23.2749. [DOI] [PubMed] [Google Scholar]
- 8.Boyce SW, GUARDIAN Study Investigators Impact of sodium-hydrogen exchange inhibition by cariporide on death or myocardial infarction in high-risk CABG surgery patients: results of the CABG surgery cohort of the GUARDIAN study. J Thorac Cardiovasc Surg. 2003 Aug;126(2):420–7. doi: 10.1016/s0022-5223(03)00209-5. [DOI] [PubMed] [Google Scholar]
- 9.Mentzer RM, EXPEDITION Study Investigators Sodium-Hydrogen Exchange Inhibition by Cariporide to Reduce the Risk of Ischemic Cardiac Events in Patients Undergoing Coronary Artery Bypass Grafting: Results of the EXPEDITION Study. Ann Thorac Surg. 2008 Apr;85(4):1261–70. doi: 10.1016/j.athoracsur.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara T, Heinle J, Britton L, Mayer JJ. Myocardial preservation in neonatal lambs. Comparison of hypothermia with crystalloid and blood cardioplegia. J Thorac. Cardiovasc. Surg. 1991;101:703–712. [PubMed] [Google Scholar]
- 11.Scholz W, Albus U. Na/H exchange and its inhibition in cardiac ischemia and reperfusion. Basic Res. Cardiol. 1993;88:443–455. doi: 10.1007/BF00795411. [DOI] [PubMed] [Google Scholar]
- 12.Fliegel L, Dyck J. Molecular biology of cardiac sodium/hydrogen exchanger. Cardiovasc Res. 1995;29:155–159. [PubMed] [Google Scholar]
- 13.Dennis SC, Gevers W, Opie LH. Protons in ischemia: where do they come from; where do they go to? J. Mol. Cell. Cardiol. 1991;23:1077–1086. doi: 10.1016/0022-2828(91)91642-5. [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Okada A, Smith TW. Control of cytosolic calcium activity during low sodium exposure in cultured chick heart cells. Circ. Res. 1987;61:29–41. doi: 10.1161/01.res.61.1.29. [DOI] [PubMed] [Google Scholar]
- 15.Philipson KC, Bersohn MM, Nishimoto AY. Effects of pH on Na-Ca exchange in canine cardiac sarcolemmal vesicles. Circ Res. 1982;50:287–293. doi: 10.1161/01.res.50.2.287. [DOI] [PubMed] [Google Scholar]
- 16.Grinwald PM, Brosnahan C. Sodium imbalance as a cause of calcium overload in post-hypoxic reoxygenation injury. J. Mol. Cell. Cardiol. 1987;19:487–495. doi: 10.1016/s0022-2828(87)80400-5. [DOI] [PubMed] [Google Scholar]
- 17.Hearse DJ, Tosaki A. Free radicals and calcium: simultaneous interacting triggers as determinants of vulnerability to reperfusion-induced arrhythmias in the rat heart. J. Mol. Cell. Cardiol. 1988;20:213–223. doi: 10.1016/s0022-2828(88)80054-3. [DOI] [PubMed] [Google Scholar]
- 18.Park C, Xiao X, Allen D. Changes in intracellular Na+ and pH in rat heart during ischemia: role of Na+/H+ exchanger. Am J Physiol. 1999;276:H1581–90. doi: 10.1152/ajpheart.1999.276.5.H1581. [DOI] [PubMed] [Google Scholar]
- 19.Kempsford RD, Hearse DJ. Protection of the immature heart. J. Thorac. Cardiovasc. Surg. 1990;99:269–279. [PubMed] [Google Scholar]
- 20.Yano Y, Braimbridge MV, Hearse DJ. Differential susceptibility to ischemic injury of the neonatal rat heart. J. Thorac. Cardiovasc. Surg. 1987;94:887–896. [PubMed] [Google Scholar]
- 21.Wittnich C, Peniston C, Ianuzzo D, Abel JG, Salerno TA. Relative vulnerability of neonatal and adult hearts to ischemic injury. Circulation. 1987;76:V156–V160. [PubMed] [Google Scholar]
- 22.Liu H, Cala P, Anderson S. Ethyl-isopropylamiloride diminishes changes in intracellular Na, Ca, and pH in ischemic newborn myocardium. J Mol Cell Cardiol. 1997;29:2077–86. doi: 10.1006/jmcc.1997.0442. [DOI] [PubMed] [Google Scholar]
- 23.Anderson SE, Carr LJ, Schierling TD, Kost GJ. Are age-related differences in response to myocardial ischemia and cardioplegia pH dependent? Biology of the Neonate. 1994;65:25–35. doi: 10.1159/000244023. [DOI] [PubMed] [Google Scholar]
- 24.Anderson SE, Liu H, Ho HS, Lewis EJ, Cala PM. Age-related differences in Na+ - dependent Ca2+ accumulation in rabbit hearts exposed to hypoxia and acidification. Am J Physiol Cell Physiol. 2003;284:C1123–C1132. doi: 10.1152/ajpcell.00148.2002. [DOI] [PubMed] [Google Scholar]
- 25.Artman M. Sarcolemmal Na+-Ca2+ exchange activity and exchanger immunoreactivity in developing rabbit hearts. Am. J. Physiol. 1992;263:H1506–H1513. doi: 10.1152/ajpheart.1992.263.5.H1506. [DOI] [PubMed] [Google Scholar]
- 26.Carr LJ, VanderWerf QM, Anderson SE, Kost GJ. Age-related response of rabbit heart to normothermic ischemia: a 31P-MRS study. Am J Physiol. 1992;262:H391–H398. doi: 10.1152/ajpheart.1992.262.2.H391. [DOI] [PubMed] [Google Scholar]
- 27.Pridjian AK, Levitsky S, Krukenkamp I, Silverman NA, Feinberg H. Developmental changes in reperfusion injury. J. Thorac. Cardiovasc. Surg. 1987;93:428–433. [PubMed] [Google Scholar]
- 28.Kusumoto K, Haist J, Karmazyn M. Na(+)/H(+) exchange inhibition reduces hypertrophy and heart failure after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2001;280:H738–45. doi: 10.1152/ajpheart.2001.280.2.H738. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Cala P, Anderson S. Ischemic preconditioning: effects on pH, Na and Ca in newborn rabbit hearts during ischemia/reperfusion. J Mol Cell Cardiol. 1998;30:686–97. doi: 10.1006/jmcc.1997.0636. [DOI] [PubMed] [Google Scholar]
- 30.Kirschenlohr HL, Metcalfe JC, Morris PG, Rodrigo GC, Smith GA. Ca2+ transient, Mg2+, and pH measurements in the cardiac cycle by 19F NMR. Proc. Natl. Acad. Sci. USA. 1988;85:9017–9021. doi: 10.1073/pnas.85.23.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson SE, Murphy E, Steenbergen C, London RE, Cala PM. Na/H exchange in myocardium: effects of hypoxia and acidification on Na and Ca. Am. J. Physiol. 1990;259:C940–C948. doi: 10.1152/ajpcell.1990.259.6.C940. [DOI] [PubMed] [Google Scholar]
- 32.Cala PM. Volume-sensitive ion fluxes in Amphiuma red blood cells: General principles governing Na/H and K/H exchange transport and Cl/HCO3 exchange coupling. In: Benos DJ, Mandel LJ, editors. Current Topics in Membranes and Transport. Vol. 27. Academic Press; New York: 1986. pp. 193–218. [Google Scholar]
- 33.Lazdunski M, Frelin C, Vigne P. The sodium/hydrogen exchange system in cardiac cells: Its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. J. Mol. Cell. Cardiol. 1985;17:1029–1042. doi: 10.1016/s0022-2828(85)80119-x. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann M, Decking U. Blocking Na(+)-H+ exchange by cariporide reduces Na(+)-overload in ischemia and is cardioprotective. J Mol Cell Cardiol. 1999;31:1985–95. doi: 10.1006/jmcc.1999.1029. [DOI] [PubMed] [Google Scholar]
- 35.Zhou RH, Long C, Liu J, Liu B. Inhibition of the Na+/H+ exchanger protects the immature rabbit myocardium from ischemia and reperfusion injury. Pediatr Cardiol. 2008 Jan;29(1):113–20. doi: 10.1007/s00246-007-9072-4. [DOI] [PubMed] [Google Scholar]
- 36.Sett SS, Galanopoulos PT, Kashihara H, Talling DN, LeBlanc JG, Tibbits GF. Na+/H+ exchange inhibition with HOE 642 improves recovery of the injured neonatal rabbit heart. Can J Cardiol. 2003 Dec;19(13):1515–9. [PubMed] [Google Scholar]
- 37.Orlowski J, Kandasamy RA. Delineation of transmembrane domains of the Na+/H+ exchanger that confer sensitivity to pharmacological antagonists. Journal of Biological Chemistry. 1996;271:19922–7. doi: 10.1074/jbc.271.33.19922. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi T, Gu H, Seguchi M, Cragoe EJ, Momma K. HCO3(-)-dependent intracellular pH regulation in the premature myocardium. Circ Res. 1992;71:1314–23. doi: 10.1161/01.res.71.6.1314. [DOI] [PubMed] [Google Scholar]
- 39.Hurtado C, Pierce G. Inhibition of Na(+)/H(+) exchange at the beginning of reperfusion is cardioprotective in isolated, beating adult cardiomyocytes. J Mol Cell Cardiol. 2000;32:1897–907. doi: 10.1006/jmcc.2000.1222. [DOI] [PubMed] [Google Scholar]
- 40.Harper IS, Bond JM, Chacon E, Reece JM, Herman B, Lemasters JJ. Inhibition of Na/H exchange preserves viability, restores mecheincal function, and prevents the pH paradox in reperfusion injury to rat neonatal myocytes. Basic Res. in cardiol. 1993;88:430–442. doi: 10.1007/BF00795410. [DOI] [PubMed] [Google Scholar]
- 41.Dulce RA, Hurtado C, Ennis IL, Garciarena CD, Alvarez MC, Caldiz C, Pierce GN, Portiansky EL, Chiappe de Cingolani GE, Camilión de Hurtado MC. Endothelin-1 induced hypertrophic effect in neonatal rat cardiomyocytes: involvement of Na+/H+ and Na+/Ca2+ exchangers. J Mol Cell Cardiol. 2006 Nov;41(5):807–15. doi: 10.1016/j.yjmcc.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Anderson S, Dickinson C, Liu H, Cala P. Effects of Na-K-2Cl cotransport inhibition on myocardial Na and Ca during ischemia and reperfusion. Am J Physiol. 1996 Feb;270(2 Pt 1):C608–18. doi: 10.1152/ajpcell.1996.270.2.C608. [DOI] [PubMed] [Google Scholar]
- 43.Pike MM, Luo CS, Clark MD, Kirk KA, Kitakaze M, Madden MC, Cragoe EJ, Jr, Pohost GM. NMR measurements of Na+ and cellular energy in ischemic rat heart: role of Na+-H+ exchange. Am. J. Physiol. 1993;265:H2017–H2026. doi: 10.1152/ajpheart.1993.265.6.H2017. [DOI] [PubMed] [Google Scholar]
- 44.Haigney M, Lakatta E, Stern M, Silverman H. Sodium channel blockade reduces hypoxic sodium loading and sodium-dependent calcium loading. Circulation. 1994;90:391–9. doi: 10.1161/01.cir.90.1.391. [DOI] [PubMed] [Google Scholar]
- 45.Haigney M, Lakatta E, Stern M, Silverman H. Sodium channel blockade reduces hypoxic sodium loading and sodium-dependent calcium loading. Circulation. 1994;90:391–9. doi: 10.1161/01.cir.90.1.391. [DOI] [PubMed] [Google Scholar]
- 46.Iwai T, Tanonaka K, Inoue R, Kasahara S, Kamo N, Takeo S. Mitochondrial damage during ischemia determines post-ischemic contractile dysfunction in perfused rat heart. J Mol Cell Cardiol. 2002;34(7):725–38. doi: 10.1006/jmcc.2002.2002. [DOI] [PubMed] [Google Scholar]
- 47.Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na(+)/H(+) exchanger. Eur J Med Chem. 2003 Jun;38(6):547–54. doi: 10.1016/s0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]