Abstract
Recognizing the specific speech act (Searle, 1969) that a speaker performs with an utterance is a fundamental feature of pragmatic competence. However, little is known about neurocognitive mediation of speech act comprehension. The present research examined the extent to which people with Parkinson's Disease (PD) comprehend specific speech acts. In the first experiment, participants read conversational utterances and then performed a lexical decision task (decide whether a target string of letters is a word). Consistent with past research, non-impaired participants performed this task more quickly when the target string was the speech act associated with the preceding utterance. In contrast, people with Parkinson's disease did not demonstrate this effect, suggesting that speech act activation is slowed or is not an automatic component of comprehension for people with PD. In a second study, participants were given unlimited time to indicate their recognition of the speech act performed with an utterance. PD participants were significantly poorer at this task than were control participants. We conclude that a previously undocumented language disorder exists in PD and that this disorder involves a selective deficit in speech act comprehension. Frontostriatal systems (the systems impaired in PD) likely contribute to normal speech act comprehension.
Pragmatic Comprehension Deficit in Parkinson's Disease
Although Parkinson's Disease (PD) is primarily associated with debilitating extrapyramidal motor dysfunction, PD also affects thinking, reasoning, planning and language functions. The nature of the language-related deficits of PD have been hotly debated, but, with the partial exception of the attentional and short-term memory related sentence processing deficit, (Lieberman et al., 1990; Grossman et al, 1992; Grossman, 1999) they are largely understudied. In particular, impairment in the domain of pragmatics has not yet been studied adequately.
Pragmatics refers to the use of language in social contexts (e.g., knowing what to say and when to say it, correctly interpreting another person's meaning, etc.). Recent evidence suggests that people with PD are impaired in certain pragmatic abilities such as conversational fluency/appropriateness and topic-coherence (McNamara & Durso, 2003), inferencing and humor appreciation (Berg, Bjornram, Hartelius, Laakso, & Johnels, 2003; Bhat, Iyengar & Chengappa, 2001) and the interpretation of figurative language (Lewis, Lapointe, Murdoch & Chenery (1998). However, the extent and the nature of pragmatic impairment are unclear. Importantly, examining the nature of the pragmatic impairment in PD may yield clues as to the underlying neurobiology and cognitive architecture of pragmatic competence itself. And clinically it is important to study pragmatic competence in PD because pragmatic dysfunction may be a key component of both the communication disorders associated with PD and the social-cognitive and behavioral disorders of PD. In this research we investigated the ability of people with PD to comprehend speech acts - a capacity theorized by some to constitute the core of pragmatic competence and pragmatic processing functions (e.g., Kasher, 1991; Soroker et al., 2005). We first provide a brief overview of speech act theory and research demonstrating the importance of speech act recognition in comprehension.
Speech Act Theory
According to speech act theory (Austin, 1962; Searle, 1969), utterances involve the simultaneous performance of multiple acts: a locutionary act (i.e., propositional meaning), an illocutionary act (the force associated with the use of the utterance in a specific context), and a perlocutionary act (i.e., the effects on the recipient of the performed speech act). It is the illocutionary act (or speech act) that represents the speaker's intention or goal in producing a particular conversation turn. For example, when Andy says to Bob ‘I definitely will do it tomorrow’, in many contexts this utterance will have the illocutionary force of a promise.
Recognizing the speech act performed with an utterance is critical for successful communication. This is complicated, however, by the fact that there are many ways in which the same speech act can be performed. One important distinction in this regard is between speech acts that are explicit and those that are implicit. Explicit speech acts are relatively clear and direct and include the relevant performative verb, the verb that names (in the appropriate contexts) the speech act that it performs. One can promise to shut the door, for example, by simply saying ‘I promise to shut the door’. However, the use of performative verbs is relatively rare. Instead, people frequently perform implicit speech acts, or speech acts that do not contain the performative verb. For example, explicit speech acts such as ‘I promise to do it’ and ‘I forbid you to do it’ can also be performed implicitly with ‘I guarantee that I'll have it finished tomorrow’, and ‘You are not allowed to do that again’, neither of which contain the performative verbs ‘promise’ and ‘forbid’.1
In a series of studies, Holtgraves and colleagues (Holtgraves, 2008; Holtgraves & Ashley, 2001) demonstrated that people automatically recognize the speech acts performed with implicit performatives. In these experiments, participants read descriptions of situations that were followed by remarks said by one interactant to another interactant. On some trials the final utterance (e.g., ‘Don't forget to go to your dentist appointment today’) performed a specific speech act (e.g., remind). In the control version the wording was almost identical but it did not perform that speech act (e.g., ‘I'll bet you forgot to go to your dentist appointment today’). After indicating comprehension of the final utterance, participants performed a secondary task. In some experiments they performed a recognition probe task and were required to indicate if a probe word had appeared in the last utterance that they read. On critical trials the probe word named the speech act performed with the preceding utterance (e.g., warn, beg, thank, etc.). If illocutionary force is activated when people comprehend implicit speech acts, then participants should be poorer at verifying that a probe had not literally been present in the utterance when the utterance performed the speech act named with the probe, relative to the control version. For example, participants should be slower at verifying that “remind” had not literally been present in the remark “Don't forget to go to your dentist appointment today” than in the (control) remark “I'll bet you forgot to go to your dentist appointment today.” This is exactly what happened, suggesting that comprehension of the former involved the on-line activation of the speech act “remind.”
In other experiments, participants performed a lexical decision task (i.e., decide whether or not the probe is a word), a task for which performance should be the opposite of that obtained with the recognition probe task (Holtgraves, 2008; Holtgraves & Ashley, 2001). That is, participants should be faster at verifying that a probe is a word when it follows an utterance performing the speech act named with the probe, relative to a control. Hence, participants should be significantly faster at verifying that “remind” is a word when it follows “Don't forget to go to your dentist appointment today” than when it follows the (control) remark “I'll bet you forgot to go to your dentist appointment today.” Again, this is exactly what happened.
Overall, then, evidence suggests that the comprehension of implicit speech acts entails activation of illocutionary force (i.e., speech act recognition). We conducted two experiments to determine whether speech act activation is automatic during comprehension for people with PD. In the first experiment we examined on line speech act activation. In a second experiment we used an off-line task to examine speech act recognition without a time constraint.
Experiment 1
In this experiment we used a lexical decision task similar to that used in Holtgraves (2008). Participants read scenarios and subsequent utterances and then performed a lexical decision task. On some trials the lexical decision probes represented the speech act (illocutionary force) performed with the immediately prior utterance. For nonimpaired participants, we expected lexical decisions to be faster for the speech act probes than for the control probes because of the activation of illocutionary force (as demonstrated previously, Holtgraves, 2008). In contrast, if PD patients exhibit core pragmatic processing deficits, then automatic speech act activation, as measured by this priming effect, should be reduced or altogether absent.
Method
Participants
PD participants were 28 (2 female; 2 non-white) individuals diagnosed with idiopathic Parkinson's disease (mean age = 66.5). The majority (> 90%) were either Stage two or three on the Hoehn and Yahr scale (M = 2.74). Most (93%) of the PD participants had completed high school with half (50%) having completed some college.
The patient's diagnosis was agreed upon by a specialist in PD and at least one other neurologist. None of the patients were demented according to clinical examinations and DSM-IV criteria. All were on some form of dopaminergic medication and were tested while on medications with optimal effects (i.e., motor signs were well controlled). All patients were required to have had at least one CT- or MRI scan during their illness to rule out history of brain injury. Patients with Parkinsonism from known causes (e.g., encephalitis, trauma, carbon monoxide exposure, manganese poisoning, hypoparathyroidism, a multi-infarct state or medications (such as neuroleptics) interfering with dopaminergic functions) were excluded. Similarly, other degenerative diseases mimicking PD (e.g., striatonigral degeneration, progressive supranuclear palsy or olivopontocerebellar degeneration) were excluded. PD patients with concurrent Alzheimer-like dementia were excluded. Other exclusion criteria included: 1) An abnormal CT or MRI scan showing basal ganglia atrophy or calcification and or stroke. 2) For patients who had received levodopa (LD) for more than 1 year, a history of no response to LD even in the initial stages of the disease, as this would be consistent with striatonigral degeneration disorders other than PD. 3) The presence of pyramidal, downward gaze or cerebellar dysfunction on examination, as these would be consistent with other diagnoses such as multi-infarct state, progressive supranuclear palsy or olivopontocerebellar degeneration respectively. 4) Other: (a) inability to obtain informed consent from patient due to an incapacity on the part of patient to understand purpose and risks of study, as these individuals are likely demented; (b) history of ongoing alcohol or drug abuse; (c) patients with a history of psychiatric or psychotic disorder and patients currently on anti-depressant or anti-psychotic medications as these medications may influence communication functions.
Medication information was obtained from each patient's records and levodopa equivalent dosages (LDE) were calculated with 100 mg levodopa=83 mg levodopa with a COMT inhibitor=1 mg Pramipexole=1 mg Pergolide. LDEs were later examined to assess the impact of medications on performance outcomes.
Nonimpaired participants were 32 (12 female; 7 non-white) individuals (mean age = 56.3) recruited through the Movement Disorders Clinics of the V.A New England Health Care System. All of these participants had completed high school with the majority (80%) having completed some college. Hence, relative to the PD participants, the control group had more females (37.5 % vs. 7.1%, χ2 = 7.69, p < .05), was younger (56.3 vs. 66.5, t(58) = −3.74, p < .05), more highly educated (15.27 years vs. 13.93 years, t(58) = 1.8, p < .1), more ethnically diverse (22% nonwhite vs. 7% nonwhite, χ2 = 5.28, p < .05), and scored higher on the MMMSE (28.27 vs. 26.57, t(58) = 4.17, p < .01).
Language Materials
The stimulus materials for this experiment were adapted from Holtgraves (2008) and consisted of a set of 48 scenarios (24 target scenarios and 24 filler scenarios). Each scenario (2-6 sentences) described a situation between two people and was followed by a remark or remarks. The last remark was always the target utterance that either performed a specific speech act (speech act version) or did not perform that speech act (control version). Following the target utterance was a probe word naming the speech act performed with the target utterance (e.g., beg, brag, etc.). An example is presented in Table 1 and all speech act utterances are presented in Table 2. All materials are available from the first author upon request.
Table 1.
Sample Scenario and Speech Act Manipulation
| Cheryl and Dan have been married for 20 years. |
| Dan tends to be somewhat forgetful. |
| Today, Cheryl is sure Dan didn=t remember (had forgotten) his dentist appointment. |
| They are eating breadfast (dinner) together when Cheryl says to Dan: |
| Cheryl: Don=t forget (I=ll bet you forgot) to go to your dentist appointment today. |
| Probe: Remind |
Note: The speech act version contained the italicized material; the control version was created by replacing the italicized material with the material in parentheses.
Table 2.
Target Utterances used in Experiment 1
| Assertives | |
| Speech Act | Utterance |
|---|---|
| Agree: | You=re right. It=s wrong to experiment on animals. |
| Blame: | It=s all Mary=s fault. |
| Remind: | Don=t forget to go to your dentist appointment today. |
| Guess: | I don=t really know, but would estimate around $100. |
| Deny: | I did not take your chainsaw. |
| Correct: | The proper way is to ask by saying Awhy aren=t we going to the park today.@ |
| Introduce: | Brad, this is my friend Charles |
| Excuse: | I have not been feeling well. |
| Expressives | |
| Thank: | I appreciate your help so much. I couldn=t have done it without you. |
| Apologize: | I=m so sorry that I ruined your shirt. |
| Complain: | I can=t believe they raised their rates again. Cable just keeps on getting more and more expensive. |
| Brag: | Fantastic. I caught a bigger fish than anyone else. I was super happy. |
| Congratulate: | That=s awesome. I=m so happy for you. |
| Compliment: | I like your coat. |
| Directives | |
| Warn: | Watch out, there=s a lot of cops around. I almost got caught speeding. |
| Encourage: | Don=t stop now. You can do it. |
| Beg: | Please, please, please let me play. I will do anything you want me to do. |
| Demand: | The bank must pay for the fee. |
| Ask: | What time is it? |
| Invite: | Would you like to come over for dinner tomorrow night? |
| Commissives | |
| Threaten: | If you don=t stop I=ll tell your parents and you=ll be grounded. |
| Promise: | I swear I will be neater after the weekend. |
| Offer: | If you need some help, just give me a call. |
| Reassure: | I want to make sure you know that we're definitely not moving. We're stayingm right here. |
The development of these materials is described in detail elsewhere (Holtgraves, 2005; 2008). In general, an attempt was made to include a large and varied set of speech acts. There were 24 speech act scenarios (8 assertives, 6 directives, 6 expressives, and 4 commissives). Control versions were created for each of the 24 scenarios. The goal was to create versions of the scenarios that would share as many words as possible with the speech act scenarios but for which the final utterance did not perform the relevant speech act. For example, the utterance performing the speech act Aapologize@ was AI=m so sorry that I ruined your shirt @ and the control version was AEd is so sorry that he ruined your shirt. @ This was done in order to keep the semantic associates roughly equal for the speech act and control versions. In this way, any processing differences between the speech act and control scenarios would not be due to the semantic associates of the individual words but to the action performed with the speech act utterance (and not performed with the control utterance).
Control versions were created in four different ways as follows: (1) by switching the tense of the utterance (e.g. Promise: I swear I will be neater after the weekend vs. I swear I was neater after the weekend ), (2) by switching the sentence subject (e.g., Apologize: I=m so sorry that I ruined your shirt vs. Ed is so sorry that he ruined your shirt ), (3) by negating the speech act (e.g., Offer: If you need some help just give me a call vs. If you need some help don't give me a call) and (4) by performing a different speech act (e.g., Agree: You're right. It's wrong to experiment on animals vs. That's right. It's wrong to experiment on animals). A pretest demonstrated that the speech act versions were significantly more likely to be perceived as performing the intended speech act relative to their matched controls (Holtgraves, 2008). For the present research, slight modifications were made to the materials used in Holtgraves (2008) in order to make them more relevant for elderly participants (rather than college undergraduates). For example, the complain speech act in Holtgraves (2008) was changed from a complaint about rising tuition to a complaint about rising cable rates.
Two sets of the stimulus materials were created that were mirror images of each other; if a scenario appeared in the speech act version in one stimulus set it appeared in the control version in the other set. Each stimulus set contained 12 speech act and 12 control scenarios. In this way, each participant saw an equal number of speech act and control versions of the scenarios, and across the experiment, an equal number of participants saw the speech act and control versions of each scenario. The probe word for each of the 24 scenarios was always the verb naming the speech act performed in the speech act version. Hence, the probe for these 24 scenarios was the speech act performed with the final utterance in the speech act version but not in the control version. Finally, each participant saw an equal number of the speech act and control versions of each of the four illocutionary points (directive, assertive, expressive, commissive).
Additional Materials
Participants provided basic demographic data (age, education level, ethnicity, gender). They also completed a version of the Stroop task which served as a measure of executive cognitive function. Executive cognitive function includes a fairly wide range of skills and there is no agreed-upon “gold standard” for its assessment. However, we used a version of the Stroop task (Delis, Kaplan, & Kramer, 2001) that involves planning as well as inhibitory power (Delis, Kramer, Kaplan, & Holdnack, 2004) and hence represents a reasonable (though far from perfect) measure of executive cognitive function. Specifically, in this version of the test participants were required to name the color of the inks presented on card one, and then to name the words printed in black ink on card two. This was followed by two interference cards. One interference test card (card three) consisted of rows of color words printed in ink colors incongruent with the word represented, with the task being to name the ink colors as quickly as possible. A second interference card (card four) also consisted of rows of color words printed in ink colors incongruent with the word represented, but 28 of the words were placed in a box. Participants were to name the ink color if the word was not in a box; otherwise they were to name the word. We computed susceptibility to cognitive interference as the total time taken to name the colors or read the words (when presented in a box) on the block four interference card, minus the time taken to read the colors on the color only card (card one).
Procedure
The language comprehension portion of the study was conducted on a personal computer using the Eprime software. Participants read detailed instructions and then performed eight practice trials. They received feedback as they performed the practice trials. To begin a trial, participants pushed a button and the first sentence of the scenario then appeared on the screen. Participants read at their own pace and pushed a button to proceed through the material. After indicating comprehension of the last remark in a scenario, a 500-Hz tone sounded and a cross (+) appeared in the center of the screen. Two hundred fifty ms. later the cross was then replaced with the probe. Participants were instructed to indicate, as quickly as possible, whether or not the probe was a word. They were instructed to push the button if the probe was a word and to do nothing if it was not a word. If no response was made within 8000 ms the next trial was presented. Pretesting indicated that this single-button procedure was easier for PD participants than a two-button (word – nonword) procedure, and that PD participants were able to respond within the 8000 ms time frame.
For 24 trials the probe was always the relevant speech act verb and hence the correct answer was yes. Lexical decision speed and judgment (correct/incorrect), as well as reading time for the final utterance, were automatically recorded. To ensure that participants did not develop the expectation that the target string was always a word, there were 24 filler trials in which the target string was not a word. The format of the filler trials was identical to that of the 24 critical trials, but the filler trials did not duplicate the content of any of the critical trials. The non-word letter strings presented on the filler trials were created by reversing two letters of real words (e.g., amdit, adivse, rejcet). Immediately after making a judgment, feedback (correct/incorrect and response time) was provided on the screen for 1500 ms. Feedback was provided in order to increase participant task motivation. Presentation order was randomized for each participant.
Results
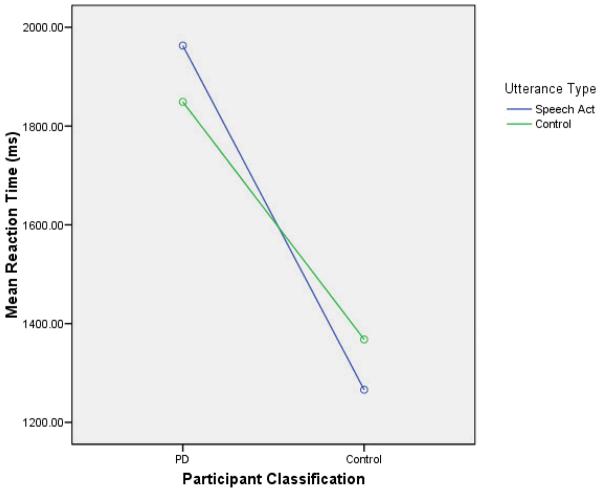
The data from five participants (two PD and three control) were incomplete (due either to an equipment malfunction or the participant's inability to complete the task) and excluded from all analyses. Lexical decision accuracy and speed for the probe word were analyzed with a 2 × 2 (Participant Classification: PD vs. Control × Speech Act Activation: Speech Act vs. Control) Analysis of Variance (ANOVA), with repeated measures on the second factor. For decision speed, only error-free trials were included. Response times greater than three standard deviations above the mean for a participant were treated as outliers and not included in the analyses (less than 1% of the trials). All analyses were conducted twice, once with participants as a random variable (F1), and once with stimuli as a random variable (F2). Although there is no prior research suggesting that any of the demographic variables we assessed would be related to speech act performance, preliminary analyses were conducted that included gender and ethnicity (white vs. nonwhite) as factors. Neither of these variables were significant (neither main effects nor interactions) and were dropped from subsequent analyses. In addition, preliminary analyses were conducted that included age and education as covariates. Neither of these variables interacted with speech act priming (although they both had significant effects on overall response times) and were dropped from subsequent analyses. The results are summarized in Table 3 and Figure 1.
Table 3.
Lexical Decision Times and Error Rates as a Function of Utterance Type and Participant Classification: Experiment 1
| Utterance Type |
|||
|---|---|---|---|
| Speech Act | Control | Mean | |
| Control Participants | |||
| Reaction Time | 1266 (155) |
1368 (131) |
1317 (140) |
| Error Rates | 6.6% (1.5) |
4.0% (1.3) |
5.3% (1.2) |
| PD Participants | |||
| Reaction Time | 1963 (164) |
1849 (139) |
1906 (148) |
| Error Rates | 5.8% (1.6) |
6.4% (1.4) |
6.1% (1.3) |
Note: Standard errors are presented in parentheses.
Figure 1.
Mean Reaction Time as a Function of Participant Classification and Utterance Type
Error rates were low and similar for PD (6.1%) and control participants (5.3%), F1(1,53) < 1, MSe = .008, F2(1,46) < 1, MSe = .015. Hence, PD participants were quite capable of performing this task. In addition, there was no difference in error rates for the speech act (6.2%) and control (5.2%) trials, F1(1,53) < 1, MSe = .004, F2(1,46) < 1, MSe = .005.
For reaction times, there was a significant main effect for participant classification, F1(1,53) = 8.35, MSe = 1139932, p < .01, F2 (1,46) = 40.7, MSe = 190889, p < .01; PD participants were significantly slower (1906 ms) than control participants (1317 ms). Most importantly, there was a reliable Speech Act Activation × Participant Classification interaction, F1(1,53) = 5.36, p < .05, MSe = 59341; F2(1,46) = 5.73, MSe = 40832; p < .05. Follow-up tests indicated that control participants were significantly faster at verifying the probe word when it followed the speech act utterances (1266 ms) than when it followed the control utterances (1368 ms), F1(1,28) = 4.56, MSe = 32710, p < .05, F2(1,23) = 10.61, MSe = 11991, p < .01, an effect that did not occur for PD participants (1963 vs. 1849), F1(1,25) = 1.89, p > .15, MSe = 89168; F2(1,23) = 1.54, MSe = 69673, p > .2.
Subsidiary Analyses
Analyses were conducted to examine the effects of disease severity, executive cognitive function (Stroop), and medication level on speech act priming. Disease stage (Stages two and three only) was included as a variable in an analysis of speech act priming for PD participants. There was a significant Speech Act × Disease Stage interaction, F(1,21) = 8.72, MSe = 1518017, p < .01. Simple effects tests indicated that speech act priming did occur for stage two participants (speech act probes: 1281; control probes: 1440), F(1,8) = 14.08, MSe =113685, p < . 01. For Stage three participants, in contrast, speech act priming did not occur and was, in fact, reversed (speech act probes: 2490; control probes: 2169), F(1,13) = 6.46, MSe =1453446, p < .05.
Overall, PD participants displayed significantly more interference on the Stroop task (M = 80.42) than control participants (M = 47.13), F(1,56) = 5.86, MSe = 2677, p < .001. We also computed the relationship between Stroop interference scores and priming (reaction times for speech act probe minus reaction time for control probe). For PD participants there was a large and significant inverse (r = −.81, p < .001) relationship between priming and Stroop performance. Hence, greater interference was associated with a reduced priming effect. For PD participants, there was also a negative correlation between priming and card one color naming scores. However, this correlation was much smaller than the priming-Stroop interference correlation and only marginally significant (r = − .37, p < .07), suggesting that the absent priming effect for PD participants was not simply the result of slower responding. In addition, for control participants there was no relationship between Stroop interference scores and priming (r = .05, ns).
Finally, the relationship between levodopa dose equivalent (LDE) and priming was computed. There was a nonsignificant (r = .16) relationship between priming and LDE.
Discussion
Consistent with past research (Holtgraves, 2008; Holtgraves & Ashley, 2001), nonimpaired participants in this study displayed on-line speech act activation. That is, their ability to make a lexical decision was facilitated when the preceding utterance performed the speech act named with the to-be-judged word. In contrast, PD participants did not display on-line speech act activation in this study; their performance on the lexical decision task was independent of whether the prior utterance did or did not perform a speech act. PD performance furthermore was significantly related to their performance on the Stroop task, poorer performance on the Stroop was related to poorer performance on the speech act activation task. These results do not mean necessarily that PD participants cannot recognize the speech acts performed with these utterances, only that it did not occur on-line. The purpose of Experiment 2 was to examine whether PD participants display a speech act recognition deficit if given adequate time.
Experiment 2
Participants in this study read materials similar to those used in Experiment 1. However, rather than performing a lexical decision task following utterance comprehension, participants instead were asked to provide a single word that they believed described what action the speaker was performing with the prior remark. Participants were not under any time constraints; hence, this represents an off-line analogue of the Experiment 1 task.
Method
Participants
The participants were the same as in Experiment 1. All participants completed this study. The order with which participants completed Experiments 1 and 2 was counterbalanced.
Materials
Twelve scenarios, similar to those used in Experiment 1, were written specifically for this experiment. Unlike Experiment 1, there was only one version (the speech act version) of each scenario. There were an equal number (four) of assertive, directive, expressive, and commissive speech acts. The structure of the scenarios and remarks was similar to the materials used in Experiment 1, but the content was different. All target utterances used in this experiment are presented in Table 4.
Table 4.
Target Utterances used in Experiment 2
| Assertives | |
| Speech Act | Utterance |
|---|---|
| Agree: | You=re right. We do need to discount some of our items. |
| Blame: | It=s all Mary=s fault. |
| Remind: | Don=t forget to stop at the store on your way home from work. |
| Deny: | I did not delete any files. |
| Expressives | |
| Thank: | I appreciate your help so much. We couldn=t have done it without you. |
| Complain: | I can=t believe they're asking us to work more overtime. They just keep piling and piling it on. |
| Brag: | I played in a tournament last week and did great – placed 2nd out of 180. I was super happy. |
| Compliment: | Hi. I really like your new car. It's very nice |
| Directives | |
| Warn: | Watch out. There's a lot of ice on the roads. I almost slid off the road a couple of times. |
| Encourage: | Don=t stop now. You can do it. |
| Demand: | You must take this steak back and bring me another. |
| Invite: | Hi. Would you like to come to our picnic this weekend? |
| Commissives | |
| Threaten: | If you don=t stop your dog from coming over here, I'm going to call animal control and they'll take him away. |
| Promise: | I swear I will be on time from now on. |
| Offer: | If you need some help with it, just let me know. |
| Reassure: | I want to make sure you know that I'm definitely not planning on leaving. I plan on staying with this company. |
Procedure
Participants were asked to read a scenario and corresponding remarks, and to then write down a single word that they believed described the action that the speaker was performing with the final remark. Participants then indicated their degree of confidence in their judgment on a seven-point (1 = Extremely Unconfident to 7 = Extremely Confident) scale. Participants first performed one practice trial. Scenarios were presented two to a page, with pages randomized for each participant.
Participants' responses were coded for the presence of the correct speech act term. To be counted as correct, participants had to provide the specific speech act word or a derivative for that utterance. For example, thank, thanks, thankful, thanking were the only acceptable responses for the thanking scenario.
Results
Number of correct responses and confidence ratings were analyzed with a 2 × 4 (Participant Classification × Speech Act type) ANOVA with repeated measures on the last factor. All analyses were conducted twice, once with participants as a random variable, and once with stimuli as a random variable. Preliminary analyses were conducted that included gender and ethnicity (white vs. nonwhite) as factors. Neither of these variables were significant (neither main effects nor interactions) and were dropped subsequent analyses. In addition, preliminary analyses were conducted that included age and education as covariates. Neither of these variables qualified the effects we report below (although they both were related to speech act recognition) and were dropped from subsequent analyses. The results are summarized in Table 5.
Table 5.
Interpretation Accuracy and Confidence as a Function of Speech Act and Participant Classification: Experiment 2
| Percent Correct | Confidence | |
|---|---|---|
| Control Participants | 47.9 (3.3) |
6.28 (.166) |
| PD Participants | 33.7 (3.5) |
5.95 (.178) |
Note: Standard errors are in parentheses.
For correct identification, there was a significant main effect for participant classification, F1(1,58) = 8.84, MSe = .135, p < .01, F2 (1,24) = 6.24, MSe = .026, p < .01; PD participants correctly identified significantly fewer speech acts (33.7%) than did the control participants (47.9%). There was also a significant effect for speech act type, F1(3,174) = 10.4, MSe =.057, p < .01, F2(3,24) = 3.02, MSe = .026 p = .05, and (participants analysis only) a Speech Act type × Participant Classification interaction F1(3, 174) = 3.06, MSe = .057, p < .05, F2 (3,24) < 1, MSe = .026. Follow-up tests in the participants analysis indicated that the difference between PD and control participants was significant for all speech act types except expressives.
In contrast to accuracy, the difference between PD (M = 5.95) and control (M = 6.28) participants was not significant for confidence in the participants analysis, F1(1,58) = 8.42, MSe = 3.533, p > .15, although it was in the item analysis, F2(1,24) = 8.39, MSe =.105, p < .01. In addition, there was a significant effect for speech act in the participants analysis, F1 (3,174) = 5.69, MSe = .299, p < .01, but not in the item analysis, F2(3,24) = 2.18, MSe = .105, p > 10. Follow-up tests indicated that in the participants analysis, participants were significantly more confident in their interpretation of directives (M = 6.37) than the other three speech act types, with no difference between the latter three speech act types (Ms = 6.0 to 6.05).
Subsidiary Analyses
Exploratory analyses were conducted in which confidence was computed separately for correct and incorrect identification. Separate analyses for control and PD participants indicated that the former were significantly more confident when they were correct (M = 6.48) than when they were incorrect (M = 6.02), F(1,30) = 4.46, MSe = .712, p < .05. In contrast, PD participants were no more confident when they were correct (M = 6.21) than when they were incorrect (M = 6.12), F(1,25) < 1, MSe = .888.
Analyses were conducted to examine the effects of disease severity and medication level on speech act recognition. Disease stage (Stages two and three only) was included as a variable in an analysis of speech act identification for PD participants. Consistent with Experiment 1, there was a trend for more severe disease to be associated with poorer performance. Stage two participants correctly identified more utterances (M = .43) than Stage three participants (M = .30), F(1,23)=2.56, MSe = .038, p < .13. The relationship between levodopa dose equivalent (LDE) and speech act interpretation was computed and was negative (r = −.18, n.s.).
In summary, the results of this experiment demonstrate a deficit in speech act recognition in PD that is independent of temporal constraints. That is, even without the time constraint, PD participants demonstrated a deficit in recognizing the speech acts performed with an utterance. As in Experiment 1, the size of this deficit was associated with disease severity.
General Discussion
Recognizing the specific speech act (Searle, 1969) that a speaker performs with an utterance is a fundamental feature of pragmatic competence and this feature of language use appears to be selectively impaired in patients with Parkinson's Disease (PD). In the present studies, people with Parkinson's Disease did not demonstrate normal, automatic activation of speech act verbs and this deficit could not be ascribed to a general slowing of processing language materials. Instead it was related to Stroop performance which in turn implicates executive cognitive functions in support of this particular system of pragmatic competence. We conclude that a previously undocumented language disorder exists in PD and that this disorder involves a selective deficit in speech act comprehension.
Speech act comprehension is considered core to pragmatic competence because it supports the ability of a listener to recognize a speaker's intention. In fact, many theorists have argued that intention recognition is the basis of successful communication. Originally articulated by Grice (1957), this assumption is fundamental to many psychological theories of pragmatic comprehension (Gibbs, 1999), as well as relevance theory (Sperber & Wilson, 1995), speech act theory (Austin, 1962; Searle, 1969), and certain computational models of discourse comprehension (Cohen & Perrault, 1979; Stone, 2005). In this view language is for doing - it is used for performing various actions. Hence, recognizing the intentions that others implement with their utterances is a critical component of successful language use. On-line speech act comprehension clearly has its advantages because it allows interlocutors to perform numerous speech acts in a very short period of time. Not being able to quickly and automatically recognize the actions of one's interlocutors can seriously disrupt and hinder one's communicative performance.
There is some descriptive evidence that PD patients do in fact exhibit clinically severe communication difficulties. Using the Prutting and Kirchener (1987) inventory of pragmatic language skills, McNamara and Durso (2003) found that patients with PD were significantly impaired on selected measures of pragmatic communication abilities, including the areas of conversational fluency/appropriateness, speech act production and comprehension, topic-coherence, prosodics and proxemics. They also found that PD patients were relatively unaware of their pragmatic deficits. Bhat, Iyengar and Chengappa (2001) reported similar results in a series of case studies of PD patients who also evidenced deficits in contextual inferencing and in humor appreciation. Berg, Bjornram, Hartelius, Laakso, and Johnels (2003) reported ‘high-level’ language dysfunction in PD including a significant inferencing deficit (i.e., drawing appropriate inferences from short narratives about social interactions) in mid-stage patients. Similarly, Lewis, Lapointe, Murdoch & Chenery (1998) found that PD patients are at a disadvantage interpreting figurative language. Our demonstration in this set of studies of selective deficits in speech act activation and comprehension may go some way in accounting for the clinical communication disorders of PD.
Importantly, it appears that the ability to recognize speech acts (Experiment 2) and to do so automatically (Experiment 1) decreases as the disease progresses. This link between performance and disease severity raises the issue of potential neurobiologic systems that support speech act comprehension. In PD, the Braak six-stage descriptive system of pathologic Lewy Body (LB) progression suggests that early stage disease begins in the brainstem and then ascends up the neuroaxis over several years until the cortex is affected. The first two Braak stages are presymptomatic while stages three and four involve pathology in the basal ganglia and neostriatum with onset and progression of motor symptoms, and the last two (cortical) stages are associated with cognitive impairment (see Jellinger, 2008, for review and critique). Our patients were Hoehn-Yahr stages II and III which corresponds roughly to Braak stages four and five, presumably indicating pathology in dopaminergic frontostriatal circuitry. Although several other neurotransmitter systems are implicated in PD, the primary pathology involves loss of dopaminergic cells in the substantia nigra (SN) and in the ventral tegmental area (VTA; Agid, Javoy-Agid and Ruberg, 1987). These two subcortical dopaminergic sites give rise to two projection systems important for functioning. The nigrostriatal system originates in the pars compacta of the SN and terminates in the striatum. The mesocortical system originates in the VTA and terminates in the frontal lobes. Dopamine levels in the ventral striatum and frontal lobes of PD patients are approximately 40% of normal (Javoy-Agid & Agid 1980; Scatton et al. 1983; Agid et al. 1987; Shinotoh & Calne 1995) and is correlated with the degree of intellectual impairment (Torack & Morris 1988; German et al. 1989; Rinne et al. 1989) in affected individuals.
Role of executive functions in speech act comprehension
We found that speech act priming was correlated with performance on the Stroop task, a measure of executive cognitive function (ECF). This relationship held for PD participants but not for control participants. In addition, the relationship was not due to a simple slowing because the correlation between priming and color naming (card one performance) was much smaller than the Stroop interference – priming correlation. This pattern of results suggests that part of the reason for the automatic speech act recognition deficit in PD is a reduction in executive function. Research demonstrates that PD patients perform abnormally on a variety of ECF components such as planning (such as the Tower of London or its automated analog ‘Stockings of Cambridge’), and verbal and semantic fluency (generative word fluency) (Bayles et al., 1996; Dubois, Boller, Pillon, & Agid, 1991; McNamara & Durso, 2000; Piccirilli, D'Alessandro, Finali, Piccinin, & Agostini, 1989; Troster & Woods, 2003; Wolters & Scheltens, 1995). Both the profile of neurobiologic impairment in PD (neostrial and prefrontal pathology) and our finding of a correlation of performance with a measure of executive function suggests that speech act comprehension depends crucially on frontostriatal circuitry.
Consistent with McNamara and Durso's (2003) findings, there was also some evidence that PD participants did not have awareness of their diminished communicative capacities. In Experiment 2, nonimpaired participants were more confident of their interpretations when they were correct than when they were incorrect; in other words, confidence was positively related to accuracy. In contrast, PD participants did not show this effect; they were just as confident when they were wrong as when they were right.
We could not, in this study, measure differential contributions of right vs left sided prefrontal function to speech act comprehension. Soroker et. al (2005) examined processing of basic speech acts after right and left sided lesions and found greater impairment after left-sided lesions, particularly lesions close to the classical language areas in the perisylvian cortex of the temporal and parietal lobes. On the other hand patients with right sided lesions also demonstrated abnormalities in producing basic speech acts. Thus, the relative contribution of each hemisphere, particularly each prefrontal network needs clarification in future studies.
Taken together, the current findings, along with previous studies (McNamara and Durso, 2003), point to a previously unrecognized language disorder in PD involving pragmatic comprehension. This deficit may contribute to difficulties that PD patients display during social interaction; no doubt the ability to comprehend the actions others are performing with their talk is important for successfully interacting with others. In addition, this deficit may increase caregiver burden (Edwards & Scheetz, 2002), reduce quality of life (Global PD Steering Committee, 2002) and may compromise complex decision-making capacities around long term care. Importantly, changes in the quality of social interactions may predate the onset of overt extrapyramidal motor signs of PD by several years (Hubble & Koller, 1995; Mendelsohn et al., 1995). Thus, selected social cognitive deficits (including pragmatic comprehension) may predict risk for disease severity.
Acknowledgments
This research was supported by a grant from the NIDCD: ‘Pragmatic Language Skills in Patients with Parkinson's Disease’, 1R01DC007956-01A2.
Footnotes
Note that we use the implicit/explicit terminology here rather than a distinction between direct and indirect speech acts in order to avoid some of the controversy surrounding the latter distinction (e.g., Dascal, 1987; Gibbs, 1984). For the present research, we defined explicit speech acts as those containing the performative verb and all implicit speech acts as those that do not contain the performative verb.
Contributor Information
Thomas Holtgraves, Ball State University.
Patrick McNamara, Boston University.
References
- Agid, Javoy-Agid, Ruberg M. Biochemistry of neurotransmitters in Parkinson's disease. In: Marsden CD, Fahn S, editors. Movement disorders. Vol. 2. Butterworths and Co. Publishers; 1987. pp. 166–230. [Google Scholar]
- Austin JL. How to do things with words. Harvard University Press; Cambridge, MA: 1962. [Google Scholar]
- Bench CJ, Frith CD, Grasby PM, et al. Investigations of the functional anatomy of attention using the Stroop test. Neuropsychologia. 1993;31(9):907–922. doi: 10.1016/0028-3932(93)90147-r. [DOI] [PubMed] [Google Scholar]
- Berg E, Bjornram C, Hartelius L, Laakso K, Johnels B. High-level language difficulties in Parkinson's disease. Clinical Linguistics & Phonetics. 2003;17(1):63–80. doi: 10.1080/0269920021000055540. [DOI] [PubMed] [Google Scholar]
- Bhat S, Iyengar KR, Chengappa S. Pragmatic deficits in Parkinson's disease: Description of two case studies. Journal of the Indian Speech & Hearing Association. 2001;15:79–84. [Google Scholar]
- Cohen PR, Perrault CR. Elements of a plan based theory of speech acts. Cognitive Science. 1979;3:177–212. [Google Scholar]
- Dascal M. Defending literal meaning. Cognitive Science. 1987;11:259–281. [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. The Delis/Kaplan Executive Function System (D-KEFS) The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: An update. Journal of the International Neuropsychological Society. 2004;10:301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Dubois B, Boller F, Pillon B, Agid Y. Cognitive deficits in Parkinson's disease. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Vol. 5. Elsevier; Amsterdam: 1991. pp. 195–240. [Google Scholar]
- Edwards NE, Scheetz PS. Predictors of burden for caregivers of patients with Parkinson's disease. Journal of Neuroscience Nursing. 2002;34(4):184–190. doi: 10.1097/01376517-200208000-00003. [DOI] [PubMed] [Google Scholar]
- German D, Manaye K, Smith W, Woodward D, Saper C. Mid-brain dopaminergic cell loss in Parkinson's disease: Computer visualization. Annals of Neurology. 1989;26:507–514. doi: 10.1002/ana.410260403. [DOI] [PubMed] [Google Scholar]
- Gibbs RW., Jr. Literal meaning and psychological theory. Cognitive Science. 1984;8:225–304. [Google Scholar]
- Gibbs RW., Jr. Intentions in the experience of meaning. Cambridge University Press; Cambridge: 1999. [Google Scholar]
- Global Parkinson's Disease Survey Steering Committee Factors impacting on quality of life in Parkinson's disease: results from an international survey. Movement Disorders. 2002;17(1):60–67. doi: 10.1002/mds.10010. [DOI] [PubMed] [Google Scholar]
- Grice HP. Meaning. Philosophical Review. 1957;66:377–388. [Google Scholar]
- Grice HP. Logic and conversation. In: Cole P, Morgan J, editors. Syntax and semantics 3: Speech acts. Academic Press; New York: 1975. pp. 41–58. [Google Scholar]
- Grossman M. Sentence processing in Parkinson's disease. Brain and Cognition. 1999;40:387–413. doi: 10.1006/brcg.1999.1087. [DOI] [PubMed] [Google Scholar]
- Grossman M, Grossman M, Crino P, Reivich M, Stern MB, Hurtig HI. Attention and sentence processing deficits in Parkinson's disease: The role of anterior cingulate cortex. Cerebral Cortex. 1992;2:513–525. doi: 10.1093/cercor/2.6.513. [DOI] [PubMed] [Google Scholar]
- Holtgraves TM. Comprehending indirect replies: When and how are their conveyed meanings activated? Journal of Memory and Language. 1999;41:519–540. [Google Scholar]
- Holtgraves T. The production and perception of implicit performatives. Journal of Pragmatics. 2005;37:2024–2043. [Google Scholar]
- Holtgraves T. Automatic intention recognition in conversation processing. Journal of Memory and Language. 2008;58:627–645. [Google Scholar]
- Holtgraves TM, Ashley A. Comprehending illocutionary force. Memory & Cognition. 2001;29:83–90. doi: 10.3758/bf03195743. [DOI] [PubMed] [Google Scholar]
- Hubble JP, Koller WC. The Parkinsonian personality. In: Weiner WJ, Lang AE, editors. Behavioral neurology of movement disorders. Raven Press; New York: 1995. pp. 43–48. [PubMed] [Google Scholar]
- Javoy-Agid F, Agid Y. Is the mesocortical dopaminergic system involved in Parkinson's disease? Neurology. 1980;30:1326–1330. doi: 10.1212/wnl.30.12.1326. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 2008;116(1):1–16. doi: 10.1007/s00401-008-0406-y. Epub 2008 Jul 1. Review. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Attention and effort. Prentice Hall; Englewood Cliffs, NJ: 1973. [Google Scholar]
- Kasher On the pragmatic modules: A lecture. Journal of Pragmatics. 1991;16:381–397. [Google Scholar]
- Lewis FM, Lapointe LL, Murdoch BE, Chenery HJ. Language impairment in Parkinson's disease. Aphasiology. 1998;12(3):193–206. [Google Scholar]
- Lieberman P, Friedman J, Feldman LS. Syntax comprehension deficits in Parkinson's disease. Journal of Nervous & Mental Disease. 1990;178:360–365. doi: 10.1097/00005053-199006000-00003. [DOI] [PubMed] [Google Scholar]
- McNamara P, Durso R. Language functions in Parkinson's Disease: Evidence for a neurochemistry of language. In: Obler L, Connor LT, editors. Neurobehavior of Language and Cognition: Studies of Normal Aging and Brain Damage. Kluwer Academic Publishers; New York: 2000. pp. 201–212. [Google Scholar]
- McNamara P, Durso R. Pragmatic communication skills in Parkinson's disease. Brain & Language. 2003;84:414–423. doi: 10.1016/s0093-934x(02)00558-8. [DOI] [PubMed] [Google Scholar]
- Neely JH. Semantic priming and retrieval from lexical memory: Roles of inhibitionless spreading activation and limited-capacity attention. Journal of Experimental Psychology - General. 1977;106:226–254. [Google Scholar]
- Orfei MD, Robinson RG, Bria P, Caltagirone C, Spalletta G. Unawareness of illness in neuropsychiatric disorders: phenomenological certainty versus etiopathogenic vagueness. Neuroscientist. 2008;14(2):203–22. doi: 10.1177/1073858407309995. Epub 2007 Dec 5. Review. [DOI] [PubMed] [Google Scholar]
- Piccirilli M, D'Alessandro P, Finali G, Piccinin GL, Agostini L. Frontal lobe dysfunction in Parkinson's disease: Prognostic value for dementia? European Neurology. 1989;29(2):71–76. doi: 10.1159/000116381. [DOI] [PubMed] [Google Scholar]
- Prutting CA, Kirchner DM. A clinical appraisal of the pragmatic aspects of language. Journal of Speech & Hearing Disorders. 1987;52(2):105–119. doi: 10.1044/jshd.5202.105. Rinne et al. 1989. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, neuroadrenaline, seratonin, and their metabolites in Parkinson's disease. Brain Research. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: 1. Detection, search, and attention. Psychological Review. 1977;84:1–66. [Google Scholar]
- Searle J. Speech acts. Cambridge University Press; Cambridge: 1969. Servan-Schreiber and Cohen, 1998. [Google Scholar]
- Shinotoh H, Calne D. The use of PET in Parkinson's disease. Brain & Cognition. 1995;28:297–310. doi: 10.1006/brcg.1995.1259. [DOI] [PubMed] [Google Scholar]
- Soroker N, Kasher A, Giora R, Batori G, Corn C, Gil M, Zaidel E. Processing of basic speech acts following localized brain damage: a new light on the neuroanatomy of language. Brain & Cognition. 2005;57(2):214–217. doi: 10.1016/j.bandc.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Sperber D, Wilson D. Relevance. 2nd edition Harvard University Press; Cambridge, MA: 1995. [Google Scholar]
- Stone M. Communicative intentions and conversational processes in human-human and human-computer dialogue. In: Trueswell JC, Tanenhaus MK, editors. Approaches to studying world-situated language use. MIT Press; Cambridge, MA: 2005. pp. 39–69. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Troster AI, Woods SP. Neuropsychological aspects of Parkinson's disease and parkinsonian syndromes. In: Pahwa R, Lyons KE, Koller WC, editors. Handbook of Parkinson's Disease. Dekker; New York: 2003. pp. 127–157. [Google Scholar]
- Torack RM, Morris JC. The association of ventral tegmental area histopathology with adult dementia. Archives of Neurology. 1988;45(5):497–501. doi: 10.1001/archneur.1988.00520290025008. [DOI] [PubMed] [Google Scholar]
- Wolters E, Scheltens P, editors. Mental dysfunction in Parkinson's disease. ICG; The Netherlands: 1995. [Google Scholar]