Abstract
Background
Cell-free DNA (CFDNA) reflects both normal and tumor-derived DNA released into the circulation through cellular necrosis and apoptosis. We sought to determine the role of pre-operative total plasma CFDNA levels in predicting clinical outcome in patients with ovarian cancer.
Methods
Following IRB consent, DNA was extracted from plasma of 164 women with invasive epithelial ovarian carcinoma (EOC), 49 with benign ovarian neoplasms and 75 age-matched controls. The samples were randomly divided into training (n=144) and validation (n=144) sets. Quantification of CFDNA was performed using real-time PCR for beta-globin and the number of genome equivalents (GE) per ml of plasma was determined. CFDNA was correlated with clinicopathologic parameters.
Results
The training and validation sets were similar in terms of demographic features. In the training set, EOC patients had a median pre-operative CFDNA level of 10,113 GE/ml, compared to patients with benign ovarian neoplasms (median 2,365 GE/ml; p<0.0001) and controls (median 1,912 GE/ml, p<0.0001). CFDNA >22,000GE/ml was significantly associated with decreased patient survival (p<0.001). After adjusting for other clinical variables, pre-operative CFDNA > 22,000 GE/ml was an independent predictor (p=0.02) for disease-specific survival. Analysis of the validation set confirmed significantly higher CFDNA levels in EOC (median 13,672 GE/ml) and that CFDNA >22,000 GE/ml was associated with a 2.83-fold increased risk of death from disease (p<0.001).
Conclusions
Pre-operative plasma total CFDNA levels are significantly elevated in patients with EOC. Elevated plasma CFDNA is an independent predictor for death from disease in ovarian cancer.
Keywords: Cell-free DNA, Real time PCR, prognosis, ovarian cancer, biomarker
Introduction
Ovarian cancer remains the leading cause of death from a gynecologic malignancy in the United States (1). Most patients present with widely metastatic disease at diagnosis, which in turn contributes to the high mortality associated with this disease (2). In stark contrast, survival rates for women with tumor confined to the ovary are nearly 90% (3). CA125 levels are used to distinguish benign from malignant ovarian masses (4). Although CA125 levels have a high positive predictive value in post-menopausal patients, they have low specificity in premenopausal women (4). Additionally, since 50% of early-stage patients have normal CA125 levels, its prognostic value is limited (5). Thus, there is a critical need to develop novel diagnostic and prognostic biomarkers.
The presence of cell-free nucleic acids within the plasma was first reported in 1948 (6). Several decades later, elevated cell-free DNA (CFDNA) levels were discovered in the circulation of cancer patients (7). It is now known that majority of the plasma CFDNA in cancer patients is tumor-derived (8, 9). Recent studies have demonstrated a consistent correlation between tumor load and quantity of CFDNA detected in a wide range of malignancies including ovarian cancer (8-11). Using an orthotopic mouse model for ovarian cancer, we have demonstrated that tumor-specific CFDNA levels correlate with increasing tumor burden and decline in response to therapy (12). Thus, strategies for analysis of CFDNA in plasma using quantification of total (normal and tumor) plasma DNA or tumor-specific (genetic and epigenetic) sequence alterations (11-15) have been proposed. The availability of highly sensitive real-time PCR assays and optimal methods of plasma DNA isolation make the possibility of a plasma-based molecular test very attractive. Due to the paucity of data, we evaluated the utility of preoperative plasma CFDNA levels for detecting ovarian cancer. Additionally, based on the assumption that CFDNA reflects tumor volume, we also evaluated CFDNA as a prognostic factor. There are limited data regarding the clinical relevance of preoperative plasma CFDNA levels in predicting clinical outcomes in patients with ovarian carcinoma, which is the focus of our study.
Methods and Methods
Patient Plasma Samples
Following IRB approval, archived plasma samples were obtained from tumor banks at M.D. Anderson Cancer Center, Cedars-Sinai Medical Center and Fox Chase Cancer Center. Our cohort included 164 patients with invasive epithelial ovarian carcinoma (EOC), 49 patients with benign ovarian neoplasms and 75 unaffected age-matched controls. All patients were surgically staged based on International Federation of Gynecology and Obstetrics (FIGO) staging system. Blood was collected prior to surgery, plasma was separated by 2 rounds of centrifugation at 1000×g to remove cellular contamination; supernatant was used for DNA extraction. To control for differences in sample processing and storage, samples were pooled and assigned to two groups: training set (n=144) and validation set (n=144) using a random number generator.
Extraction and Quantification of Total Plasma Cell-free DNA
DNA extraction was performed using Qiagen DNA extraction Mini kit (QIAGEN Biosciences, MD). Quantification of total plasma DNA was performed using real-time PCR with TaqMan Assay (Applied Biosystems, Foster City, CA) with primers directed to beta-globin (15). Primer sequences used were: forward 5′- GTG CAC CTG ACT CCT GAG GAG A -3′; reverse 5′- CCT TGA TAC CAA CCT GCC CAG -3′; probe 5′-AAG GTG AAC GTG GAT GAA GTT GGT GG -3′. For the pilot study, primers for GAPDH and beta-actin were used (15, 16, Applied Biosystems, Foster City, CA). A standard curve was created and DNA concentration, expressed as genome equivalents/ml (GE/ml), was calculated using the following equation (16, 17):
C is target concentration in plasma (GE/ml), Q is target quantity (copies), VDNA is total volume of DNA extraction (50 μL), VPCR is volume of DNA used per PCR reaction (5 μL), and Vext is volume of plasma used to extract DNA (800 μL). All samples were run in triplicate.
Clinicopathologic variable analysis
All patients initially underwent surgical cytoreduction followed by adjuvant therapy as determined by the treating gynecologic oncologist. Platinum based chemotherapy was given to all patients who received chemotherapy. Based on FIGO stage, patients were divided into two groups, low stage (FIGO stage I and II) and high-stage (FIGO stage III and IV). A clinical remission was defined as no evidence of disease based on physical examination, imaging studies and CA 125 levels. Optimal cytoreduction was defined as less than 1 cm of residual disease at the end of surgery. Disease specific survival (DSS) was defined as the time from entry into the study until the date of death from disease.
Statistical Analysis
All tests were carried out separately for the training and validation sets. Fisher's exact test and Wilcoxon rank sums tests were used to compare demographics and clinical characteristics. A receiver operating characteristic (ROC) curve was generated to assess CFDNA level as a diagnostic biomarker. A cutoff point was chosen, and sensitivity, false-positive rate and their 95% score confidence intervals were calculated. Among ovarian cancer patients, an optimal cutoff point to predict mortality was created that yielded the lowest p-value while also maintaining the highest hazards ratio. Univariate and multivariate Cox regression analysis were performed using this cutoff point. We also combined CFDNA and CA125, along with a multiplicative interaction term, η, to determine a cutoff point for mortality using both variables (η = 0.000019 · CFDNA + 0.000126 · CA125 + -5.8988E-9 · CFDNA · CA125). Patients alive at last follow-up or dead from other causes were censored; overall survival time was estimated using the Kaplan-Meier product limit method. A p value < 0.05 was considered statistically significant.
Results
Optimizing quantification of CFDNA using real-time PCR
We performed a pilot study to optimize quantification of CFDNA sequences by real-time PCR using plasma samples from 20 patients with high-grade, advanced-stage serous, invasive EOC and 12 unaffected, age-matched controls. We tested primers to three genes: beta-globin, beta-actin and GAPDH, to correct for efficiency of amplification. Although CFDNA levels were significantly higher in the cancer group for all 3 loci (controls: 3,205 – 5,266 GE/ml vs. ovarian cancer: 2,581-20,018 GE/ml, p≤ 0.02), amplification efficiency was greatest using beta-globin (data not shown). Based on this, we used the beta-globin gene for subsequent analyses.
Demographic characteristics
We evaluated plasma CFDNA levels using archived plasma samples from the M. D. Anderson Cancer Center, Fox Chase Cancer Center and Cedars-Sinai Medical Center. Samples from all three institutions were pooled and randomly assigned to a training set (n=144; 38 controls, 24 benign masses, 82 ovarian cancers) and validation set (n=144; 37 controls, 25 benign masses; 82 ovarian cancers), to control for differences in sample processing and storage. In the training set, median age was 53 years (range 33-80) for controls, 46 years (range, 29-81) for women with benign ovarian masses; and 62 years (range, 28-84) for those with EOC. The median ages were similar for each group in both sets. Among benign ovarian masses, most common histopathological diagnoses were serous or mucinous cystadenoma (n=27), endometriotic or corpus luteum cyst (n=12), epithelial inclusion cysts (n=3), mature teratoma (n=5), and ovarian fibroma (n=2). There were no significant differences between the groups in terms of demographic or clinical characteristics (Table 1). Majority of patients with EOC were white and had high-stage, high-grade disease of serous histology. Median follow-up for all patients was 2.78 years (range, 0.07 – 6.2 years).
Table 1. Demographic characteristics of the training and validation sets.
| Variable | Training Set n=144 (%) |
Validation Set n=144 (%) |
p |
|---|---|---|---|
| Site | |||
| MDACC/Fox Chase | 50 (61) | 54 (66) | 0.6 |
| Cedars Sinai | 32 (39) | 28 (34) | |
| Median Age in yrs (range) | 61.5 (28-84) | 60 (25-88) | 0.11 |
| Race | |||
| White | 66 (80) | 67 (82) | |
| Other (Black/Hispanic) | 16 (20) | 15 (18) | 1.0 |
| Stage | |||
| Low (I/II) | 20 (24) | 18 (22) | |
| High (III/IV) | 62 (76) | 64 (78) | 0.9 |
| Histology | |||
| Serous | 66 (80) | 67 (82) | |
| Non-serous | 16 (20) | 15 (18) | 1.0 |
| Grade | |||
| Low grade | 9 (11) | 4 (5) | |
| High grade | 73 (89) | 78 (95) | 0.2 |
| Median CA-125 IU/mL (range) | 147 (12-5,893) | 120.5 (14 – 12,450) | 0.98 |
| Median CFDNA GE/mL (range) | 10,113 (1,460 – 104,544) | 13,672 (2,747 – 162,708) | 0.07 |
Preoperative plasma CFDNA: predictive marker of survival and diagnosis
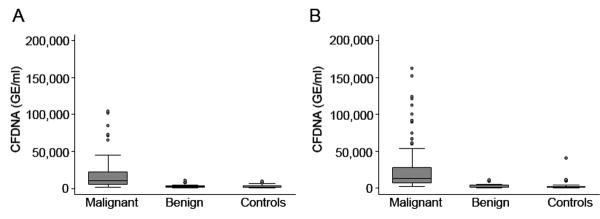
Preoperative CFDNA concentrations were measured for controls, women with benign masses and ovarian cancers in the training set. The CFDNA levels were significantly higher in patients with invasive cancer (median, 10,113 GE/mL) compared to women with benign ovarian tumors (median, 2,365 GE/mL; p<0.001) and controls (median, 1,912 GE/mL; p<0.001, Figure 1). CFDNA levels were significantly higher in EOC compared to both groups combined (p<0.001), but the difference in CFDNA levels between the benign and control groups was not significant. Interestingly, although the median CFDNA levels were lower among patients with early-stage (I/II) disease (median 4,807 GE/ml), these levels were significantly higher compared to those with benign disease and controls (p<0.001).
Figure 1.
Box plot of preoperative plasma CFDNA (GE/ml) for controls, patients with benign ovarian masses and ovarian carcinoma in the (A) Training Set (n=144) and (B) Validation Set (n=144). CFDNA level is significantly higher in the invasive carcinoma group when compared to women with benign ovarian tumors and controls with normal ovaries in both datasets.
Using exploratory statistical analysis, patients with ovarian cancer were dichotomized into two groups, high CFDNA and low CFDNA, based on a cutoff value of 22,000 GE/mL, which yielded the greatest hazard for mortality. Using this cutoff, we examined the association of CFDNA with age, stage, grade, histology, ascites, level of cytoreduction, nodal status and CA125 levels among those patients where detailed clinical information was available (n=104). CFDNA ≥ 22,000 GE/mL was significantly associated with high-stage (p<0.0001), high-grade (p=0.02), suboptimal cytoreduction (p=0.02), positive nodal status (p=0.02), presence of ascites (p=0.04) and CA-125 (p<0.001). The association of elevated CFDNA with age, histology and race was not significant. Among these patients, on multivariate analysis, only high-stage (p=0.003), suboptimal cytoreduction (p<0.001) and high CFDNA ≥22,000 GE/mL (p=0.01) retained their statistical significance.
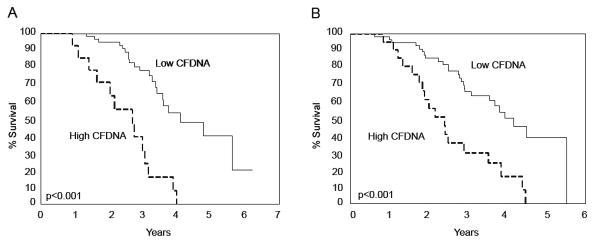
Next, in order to study the potential impact of high CFDNA on patient survival, we examined the effects of traditional prognostic factors and preoperative CFDNA on DSS using univariate analysis in the training set (Table 2). Serous histology, high-stage and CFDNA ≥ 22,000 GE/mL were each associated with decreased survival. The median survival for patients with high CFDNA was 3 years compared to 4.1 years for those with low CFDNA. CA125 levels > 35 IU/mL were also associated with decreased survival in the training set (Table 2). We studied the combination of CFDNA and CA125 (η) and determined an optimal cutoff for η=0.45, which yielded a hazard ratio of 1.46. Interestingly, the association of η ≥ 0.45 with DSS was highly significant. The Kaplan-Meier survival curves, based on CFDNA dichotomization (low < 22,000 GE/ml vs. high ≥ 22,000 GE/ml) and η ≥ 0.45 for the training set are depicted in Figure 2.
Table 2. Univariate analysis of survival for ovarian cancer patients*.
| Training Set (n = 82) | Validation Set (n = 82) | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p | HR | 95% CI | p |
| Age | 1.01 | 0.98 -1.04 | 0.48 | 1.01 | 0.99 – 1.04 | 0.32 |
| Race | 0.66 | 0.26 – 1.72 | 0.4 | 0.86 | 0.38 -1.94 | 0.72 |
| High-stage | 32.47 | 4.34 – 243 | <0.001 | 25.63 | 3.49 – 188.5 | 0.001 |
| Serous histology | 2.93 | 1.03 -8.37 | 0.04 | 1.65 | 0.73 -3.72 | 0.23 |
| High CA125 (≥ 35 IU/mL) |
4.26 | 1.01 – 17.88 | 0.05 | 3.68 | 0.89 – 15.31 | 0.07 |
| High CFDNA (≥ 22,000 IU/mL) |
3.67 | 1.87 – 7.31 | <0.001 | 2.83 | 1.53 – 5.24 | <0.001 |
| η ≥ 0.45 | 4.31 | 2.08 – 8.89 | <0.001 | 3.76 | 2.01 – 7.03 | <0.001 |
Grade could not be evaluated since there were no deaths among patients with low-grade disease.
Figure 2.
Kaplan-Meier estimates of the probability of survival in patients with high CFDNA levels (≥ 22,000 GE/ml, dotted line) vs. those with low CFDNA levels (< 22,000 GE/ml, full line) in Training Set (A). This cutoff was validated in a separate cohort of patients, Validation Set (B). Two-sided log rank test provided a significant p-value < 0.001.
To assess whether there was an independent association between any of the clinicopathologic variables and DSS, we performed multivariate Cox proportional hazards analyses. After adjusting for effects of age, race, histology, stage and CA125, high CFDNA and high-stage remained significantly associated with poor survival (Table 3). Next, we studied the relative contribution of CFDNA alone vs. η for predicting prognosis using the -2 log likelihood statistic in the training set. The likelihood for predicting death from disease for CFDNA and η was 198.8 and 199.8 respectively, indicating that CFDNA alone was as good a predictor of clinical outcome as CA125 combined with CFDNA. Since CA125 can be unreliable as a prognostic marker in patients with early-stage disease, we questioned whether CFDNA alone or in combination with CA125 could be used for predicting outcome in this subset of patients. In this study, there were only 38 patients with stage I or II disease with a total of 4 deaths due to disease among these early-stage patients. Although high CFDNA or elevated CA125 individually did not predict poor outcome in these patients, a combination of both these tests resulted in a significant association with shorter DSS (HR=15.91, p=0.03).
Table 3. Multivariate Analysis of Survival in Ovarian Cancer.
| Training Set (n = 82) | Validation Set (n = 82) | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p | HR | 95% CI | p |
| High-stage | 24.23 | 3.12 – 188.3 | 0.002 | 24.13 | 3.12 – 186.7 | 0.003 |
| Serous histology | 1.42 | 0.47 – 4.31 | 0.5 | 0.67 | 0.29 -1.57 | 0.36 |
| High CA125 (≥ 35 IU/mL)* |
0.99 | 0.98 – 1.005 | 0.4 | 0.99 | 0.99 – 1.0 | 0.32 |
| High CFDNA (≥ 22,000 IU/mL) |
2.52 | 1.16 – 5.48 | 0.02 | 2.22 | 1.16 – 4.21 | 0.01 |
Results based on 10-unit increase in CA125 values
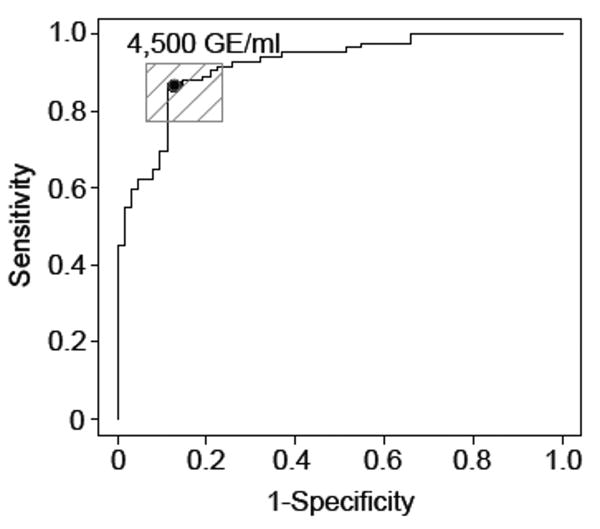
Next, to determine the optimal cut-off for CFDNA as a potential diagnostic biomarker for ovarian malignancy, we used an ROC curve to determine the sensitivity and specificity of CFDNA to detect malignancy. For diagnosis of invasive ovarian cancer, a cut-off value of 4,500 GE/ml yielded a sensitivity of 87% and specificity of 87% (Figure 3). The sensitivity and specificity of predicting malignancy in our cohort using CA125 cutoff of ≥ 35 IU/mL was 89% and 77%, respectively. Although the sensitivity of CFDNA was similar to that of CA125, the false positive rate of CFDNA (12.9%) was lower than that for CA125 (23.4%). Among patients with early-stage disease, CFDNA ≥ 4,500 GE/ml had a sensitivity of 55% and specificity of 87.1% to distinguish between benign and malignant disease.
Figure 3.
Receiver operating characteristic (ROC) curve of plasma cell-free DNA provides a sensitivity of 87% and a specificity of 87% to detect cancer, using a cut-off point of 4,500 GE/ml in the Training Set.
Validation Analysis of CFDNA
We applied the cut-offs generated from the training set to an independent validation set. The CFDNA levels among EOC patients (median 13,672 GE/ml) were significantly higher compared to the benign group (median 1,978 GE/ml) and controls (median 2,010 GE/ml; p<0.001). Levels of CFDNA were also statistically higher among low-stage patients (median, 6,060 GE/ml, p<0.0001). In women with EOC and plasma CFDNA levels ≥ 22,000 GE/ml, the risk of death from disease was 2.83 times higher than their counterparts with CFDNA < 22,000 GE/ml (p<0.001, Table 2). Importantly, high CFDNA remained an independent indicator for shorter DSS in this independent validation set (Table 3). We also tested the diagnostic ability of CFDNA for detecting malignant ovarian masses. Based on the cut-off identified from the training set, the sensitivity of CFDNA for detecting ovarian malignancy was 91.5% with a specificity of 85.5%.
Discussion
The key findings of this study are that preoperative CFDNA levels are significantly elevated in patients with ovarian carcinoma when compared to individuals with benign ovarian disease and controls. CFDNA levels were elevated even among patients with early-stage ovarian cancer. Plasma CFDNA >4,500 GE/ml provided 87% sensitivity for detecting cancer, similar to that of CA125 (89%), but with an improved false-positive rate of 12.9% vs. 23.4% for CA125. Interestingly, high CFDNA levels correlated with aggressive phenotypic features such as high-stage and high-grade. Elevated plasma CFDNA ≥22,000 GE/ml was an independent predictor for DSS in patients with ovarian carcinoma, and was superior to CA125 in predicting mortality. We validated our results using samples from three different institutions in order to take into account differences in CFDNA yields based on sample processing and storage. These results indicate that plasma CFDNA may be a useful biomarker for ovarian cancer and merits further evaluation.
Although the origin of CFDNA is unclear, the physiochemical characteristics of plasma DNA suggest that it may originate from internucleosomal cleavage of chromatin, a hallmark of the apoptotic process (20, 21). Similar DNA patterns were identified in plasma of several cancer patients (22). Studies quantifying the amount of plasma DNA have used a variety of approaches including quantitative real-time PCR (13, 23). In our experience, amplification efficiency with quantitative real-time PCR was greatest for beta-globin primers, which may be due to varying primer sequence or presence of differential representation of annealing sites in the cell-free DNA fragments. Although most studies have reported elevated levels of plasma DNA in cancer patients, absolute amounts vary between different studies and whether the DNA is derived from plasma or serum samples, with lower levels detected in the plasma (24, 25). Other factors affecting detection are storage (EDTA or citrate) and centrifugation speed (25-27). Therefore, to control for some of these variations, we used plasma samples from three separate institutions.
Cell-free DNA as a prognostic marker has recently been investigated in several solid tumors (24, 28-30). Gautschi and colleagues found that tumor progression was significantly correlated with increasing plasma DNA concentrations in patients with non-small cell lung cancer (28). Wei and colleagues performed quantitative analysis of Epstein-Barr virus (EBV) DNA in the plasma of nasopharyngeal carcinoma (NPC) patients and found that surgical resection of the tumor was associated with a significant decrease in the EBV DNA copy numbers (29). Another group has reported that significantly elevated pre-therapy plasma EBV DNA levels is a powerful predictor of clinical outcome in patients with early-stage NPC (30). CFDNA levels correlate with clinical stage, lymph node metastasis and tumor size in breast cancer (24). A recent study performed a quantitative comparison of matched serum and plasma DNA in patients with colorectal liver metastasis and found that only plasma DNA was predictive of recurrence. These authors concluded that plasma DNA better reflects the in vivo levels of circulating DNA (31). Using an orthotopic mouse model of ovarian cancer to detect tumor-derived CFDNA, we showed that CFDNA closely correlate with tumor load and levels decline appreciably with chemotherapy (12). Zachariah and colleagues have reported elevated levels of both cell-free nuclear and mitochondrial DNA among ovarian cancer patients compared to controls, but levels of cell-free DNA did not correlate with prognosis in their cohort (14). In the present study, we have shown that CFDNA ≥22,000 GE/ml is a powerful independent predictor of poor outcome in patients with ovarian carcinoma. In addition, on applying this cutoff to a separate validation set, CFDNA levels maintain their statistical significance. Interestingly, the combination of CA125 and CFDNA levels did not improve the likelihood of predicting mortality over CFDNA levels alone.
We also attempted to characterize the utility of preoperative CFDNA levels for detecting malignancy. The sensitivity and specificity for detecting ovarian cancer using CFDNA cut-off at 4,500 GE/ml were 87-91.5% and 85-87%, respectively, with a lower false-positive rate than CA125 levels in this cohort. Chang and colleagues provided some of the early evidence for the use of allelic imbalance (AI) to detect patients with ovarian cancer (10). They reported that the area under the ROC curve using AI in plasma DNA was 0.95. In their cohort, area under the curve for CA125 alone was 0.78; addition of total plasma DNA concentration increased this to 0.84. Recently, hypermethylation of the normally unmethylated BRCA1 and RAS association domain family protein 1a tumor suppressor genes was detected in the serum of patients with ovarian cancer with 82% sensitivity (32). In contrast, these authors report no hypermethylation in non-neoplastic tissue, peritoneal fluid, or serum from 40 control women (100% specificity) (32). In the present study, although levels of CFDNA were significantly higher among patients with early-stage disease, the sensitivity was low (55%). This may be a reflection of low numbers of early-stage patients included in this study. Thus, detection of total or tumor-specific CFDNA holds promise as a diagnostic test for women with ovarian cancer, alone or in combination with available modalities such as CA125 levels and transvaginal ultrasound.
In summary, results from this study add to the mounting evidence that levels of plasma CFDNA are significantly elevated in patients with ovarian cancer compared to those with benign ovarian disease and controls. However, to the best of our knowledge, this is the first study to demonstrate that plasma CFDNA levels are an independent predictor of DSS for women with ovarian cancer. Future prospective investigations will be instructive to determine whether CFDNA presents a novel biomarker for diagnostic and prognostic applications in patients with ovarian carcinoma.
Acknowledgments
AAK was supported by the NIH/NICHD Baylor WRHR scholarship grant (HD050128). Portions of this work were supported by U.T.M.D. Anderson Cancer Center SPORE in Ovarian Cancer (P50 CA083639), the Silicon Valley Foundation, the Marcus Foundation, a Program Project Development Grant from the Ovarian Cancer Research Fund, Inc., the EIF Foundation, and the Betty Ann Asche Murray Distinguished Professorship to A.K.S.
Footnotes
None of the authors have any financial disclosures.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Berek J, Hacker N. Practical Gynecologic Oncology. 3rd. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. [Google Scholar]
- 3.Ahmed FY, Wiltshaw E, et al. Natural history and prognosis of untreated stage I epithelial ovarian carcinoma. J Clin Oncol. 1996;14:2968–2975. doi: 10.1200/JCO.1996.14.11.2968. [DOI] [PubMed] [Google Scholar]
- 4.Malkasian GD, Jr, knapp RC, Lavin PT, et al. Preoperative evaluation of serum CA125 levels in premenopausal and postmenopausal patients with pelvic masses: discrimination of benign from malignant disease. Am J Obstet Gynecol. 1988;159:341–6. doi: 10.1016/s0002-9378(88)80081-4. [DOI] [PubMed] [Google Scholar]
- 5.Tuxen MK, Soletormos G, Dombernowsky P. Tumor markers in the management of patients with ovarian cancer. Cancer Treat Rev. 1995;21:215–245. doi: 10.1016/0305-7372(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 6.Mandel P, Metais P. Les acides nucleiques du plasma sanguine chez l'homme (in French) C R Acad Sci Paris. 1948;142:241–3. [PubMed] [Google Scholar]
- 7.Leon SA, Green A, Yaros MJ, Shapiro B. Radioimmunoassay for nanogram quantities of DNA. J Immunol Methods. 1975;9:157–64. doi: 10.1016/0022-1759(75)90106-4. [DOI] [PubMed] [Google Scholar]
- 8.Stroun M, Maurice P, et al. The origin and mechanism of circulating DNA. Ann NY Acad Sci. 2000;906:161–168. doi: 10.1111/j.1749-6632.2000.tb06608.x. [DOI] [PubMed] [Google Scholar]
- 9.Anker P, Mulcahy H, et al. Detection of circulating tumor DNA in the blood (plasma/serum) of cancer patients. Cancer Metastasis Rev. 1999;18:65–73. doi: 10.1023/a:1006260319913. [DOI] [PubMed] [Google Scholar]
- 10.Nawroz H, Koch W, et al. Microsatellite alterations in serum DNA of head and neck cancer patients. Nat Med. 1996;2:1035–1037. doi: 10.1038/nm0996-1035. [DOI] [PubMed] [Google Scholar]
- 11.Wu TL, Zhang D, et al. Cell-free DNA: measurement in various carcinomas and establishment of normal reference range. Clin Chim Acta. 2002;321:77–87. doi: 10.1016/s0009-8981(02)00091-8. [DOI] [PubMed] [Google Scholar]
- 12.Kamat AA, Bischoff FZ, Dang D, et al. Circulating cell-free DNA: A novel biomarker for response to therapy in ovarian carcinoma. Cancer Biol Therapy. 2006;5:1369–74. doi: 10.4161/cbt.5.10.3240. [DOI] [PubMed] [Google Scholar]
- 13.Chang HW, Lee SM, et al. Assessment of plasma DNA levels, allelic imbalance, and CA 125 as diagnostic tests for cancer. J Natl Cancer Inst. 2002;94:1697–1703. doi: 10.1093/jnci/94.22.1697. [DOI] [PubMed] [Google Scholar]
- 14.Zachariah RR, Schmid S, Buerki N, Radpor R, Holzgreve W, Zhong X. Levels of circulating cell-free nuclear and mitochondrial DNA in benign and malignant ovarian tumors. Obset Gynecol. 2008;112:843–50. doi: 10.1097/AOG.0b013e3181867bc0. [DOI] [PubMed] [Google Scholar]
- 15.Sozzi G, Conte D, Leon M, et al. Quantification of free circulating DNA as a diagnostic marker in lung cancer. J Clin Oncol. 2003;21:3902–8. doi: 10.1200/JCO.2003.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Fiegl H, Millinger S, Mueller-Holzner E, et al. Circulating tumor-specific DNA: a marker for monitoring efficacy of adjuvant therapy in cancer patients. Cancer Res. 2005;65:1141–5. doi: 10.1158/0008-5472.CAN-04-2438. [DOI] [PubMed] [Google Scholar]
- 17.Otsuka J, Okuda T, Amemiya A, et al. Detection of p53 mutations in the plasma DNA of patients with ovarian cancer. Int J Gynecol Cancer. 2004;14:459–64. doi: 10.1111/j.1048-891x.2004.014305.x. [DOI] [PubMed] [Google Scholar]
- 18.Bischoff FZ, Dang DX, et al. Detecting fetal DNA from dried maternal blood spots: another step towards broad scale non-invasive prenatal genetic screening and feasibility testing. Reprod Biomed Online. 2003;6:349–351. doi: 10.1016/s1472-6483(10)61856-1. [DOI] [PubMed] [Google Scholar]
- 19.Lo YM, Tein MS, et al. Quantitative analysis of fetal DNA in maternal plasma and serum: implications for noninvasive prenatal diagnosis. Am J Hum Genet. 1998;62:768–775. doi: 10.1086/301800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boddy JL, Gal S, Malone PR, Harris AL, Wainscoat JS. Prospective study of quantification of plasma DNA levels in the diagnosis of malignant versus benign prostate disease. Clin Cancer Res. 2005;11:1394–9. doi: 10.1158/1078-0432.CCR-04-1237. [DOI] [PubMed] [Google Scholar]
- 21.Stroun M, Anker P, Lyautey J, et al. Isolation and characterization of DNA from the plasma of cancer patients. Eur J Cancer Clin Oncol. 1987;23:707–12. doi: 10.1016/0277-5379(87)90266-5. [DOI] [PubMed] [Google Scholar]
- 22.Jahr S, Hentze H, English S, et al. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for theit origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–65. [PubMed] [Google Scholar]
- 23.Ziegler A, angemeister-Wittke U, Stahel RA. Circulating DNA: a new diagnostic gold mine? Cancer Treatment Reviews. 2002;28:255–71. doi: 10.1016/s0305-7372(02)00077-4. [DOI] [PubMed] [Google Scholar]
- 24.Wei WI, Yuen AP, Ng RW, Kwong DL, Sham JS. Quantitative analysis of plasma cell-free Epstein-Barr virus DNA in nasopharyngeal carcinoma after salvage nasopharyngectomy: a prospective study. Head Neck. 2004;26:878–83. doi: 10.1002/hed.20066. [DOI] [PubMed] [Google Scholar]
- 25.Shao ZM, Wu J, Shen ZZ, et al. p53 mutation in plasma DNA and its prognostic value in breast cancer patients. Clin Cancer Res. 2001;7:2222–7. [PubMed] [Google Scholar]
- 26.Lee TH, Montalvo L, Chrebtow V, et al. Quantitation of genomic DNA in plasma and serum samples: higher concentrations found in serum than in plasma. Transfusion. 2001;41:276–82. doi: 10.1046/j.1537-2995.2001.41020276.x. [DOI] [PubMed] [Google Scholar]
- 27.Chiu RWK, Poon LLM. Effects of blood-processing protocols on fetal and total DNA quantification in maternal plasma. Clin Chem. 2001;47:1607–1613. [PubMed] [Google Scholar]
- 28.Gautschi O, Bigosch C, Huegli B, et al. Circulating deoxyribonucleic acid as a prognostic marker in non-small cell lung cancer patients undergoing chemotherapy. J Clin Oncol. 2004;22:4157–64. doi: 10.1200/JCO.2004.11.123. [DOI] [PubMed] [Google Scholar]
- 29.Wei WI, Yuen AP, Ng RW, Kwong DL, Sham JS. Quantitative analysis of plasma cell-free Epstein-Barr virus DNA in nasopharyngeal carcinoma after salvage nasopharyngectomy: a prospective study. Head Neck. 2004;26:878–83. doi: 10.1002/hed.20066. [DOI] [PubMed] [Google Scholar]
- 30.Leung SF, Chan AT, Zee B, et al. Pretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer. 2003;98:288–91. doi: 10.1002/cncr.11496. [DOI] [PubMed] [Google Scholar]
- 31.Thijssen MA, Swinkles DW, Ruers TJ, et al. Difference between free circulating plasma and serum DNA in patients with colorectal cancer metastasis. Anticancer Res. 2002;22:421–5. [PubMed] [Google Scholar]
- 32.Ibanez de Caceres I, Battagli C, Esteller M, et al. Tumor specific BRCA1 and RASSF1A hypermethylation in serum, plasma and peritoneal fluid from ovarian cancer patients. Cancer Res. 2004;64:6476–81. doi: 10.1158/0008-5472.CAN-04-1529. [DOI] [PubMed] [Google Scholar]