Abstract
The requirements for engineering clinically sized cardiac constructs include medium perfusion (to maintain cell viability throughout the construct volume) and the protection of cardiac myocytes from hydrodynamic shear. To reconcile these conflicting requirements, we proposed the use of porous elastomeric scaffolds with an array of channels providing conduits for medium perfusion, and sized to provide efficient transport of oxygen to the cells, by a combination of convective flow and molecular diffusion over short distances between the channels. In this study, we investigate the conditions for perfusion seeding of channeled constructs with myocytes and endothelial cells without the gel carrier we previously used to lock the cells within the scaffold pores. We first established the flow parameters for perfusion seeding of porous elastomer scaffolds using the C2C12 myoblast line, and determined that a linear perfusion velocity of 1.0 mm/s resulted in seeding efficiency of 87 ± 26% within 2 hours. When applied to seeding of channeled scaffolds with neonatal rat cardiac myocytes, these conditions also resulted in high efficiency (77.2 ± 23.7%) of cell seeding. Uniform spatial cell distributions were obtained when scaffolds were stacked on top of one another in perfusion cartridges, effectively closing off the channels during perfusion seeding. Perfusion seeding of single scaffolds resulted in preferential cell attachment at the channel surfaces, and was employed for seeding scaffolds with rat aortic endothelial cells. We thus propose that these techniques can be utilized to engineer thick and compact cardiac constructs with parallel channels lined with endothelial cells.
Keywords: Bioreactor, scaffold, perfusion, cell seeding, cardiac tissue engineering
Introduction
Following myocardial infarction, cardiac myocytes have an extremely limited ability to regenerate, and cardiovascular diseases lead to the death of more than 850,000 patients in the United States each year 1. Cardiac tissue engineering offers the promise of recreating thick, compact, and functional cardiac constructs for in vitro screening of treatment options and for the in vivo implantation.
Optimization of the cell seeding technique is an essential step for the successful in vitro generation of cardiac constructs of clinically relevant size (millimeter-scale thicknesses). An optimal seeding process should yield a high seeding efficiency (to maximize the utilization of cells) and a spatially uniform cell distribution (to provide a basis for uniform tissue development and regeneration) 2. In addition, the initial cell density should approach the cell density found in rat myocardium (~108 cells/cm3) because cardiomyocytes have practically no ability to proliferate.
Initial attempts at engineering cardiac tissue have been limited by the use of static culture environments. A viable cell layer in statically grown constructs is only <100 μm thick, a value corresponding to the diffusional penetration depth of oxygen 3. Beyond the 100 μm thick outer region, the engineered constructs are mostly hypoxic and acellular, whether constructs are cultured statically in petri dishes (molecular diffusion throughout the culture system) 4, 5 or dynamically in stirred flasks (external mass transport enhanced by stirring; internal transport by molecular diffusion) 6, 7 or rotating vessels (external mass transport enhanced by construct motion; internal transport by molecular diffusion) 8. Perfusion systems have been used in an attempt to enhance oxygen mass transport throughout the construct volume, and thereby create thicker and fully viable constructs. However, the exposure of myocytes to hydrodynamic shear (a non-physiologic stimulus) leads to a decrease in their functionality 9, 10.
To mimic the function of the in vivo cardiac capillary network and shield cardiac cells from hydrodynamic shear, our group has developed channeled scaffolds made from the porous elastomer poly(glycerol sebacate) (PGS). A parallel array of channels with diameters of 250 μm can be formed by laser piercing. By mathematical modeling it was shown that perfusion of culture medium through a channel array can support the requirements for oxygen transport in constructs that are several millimeters thick, and that the addition of synthetic oxygen carriers further enhanced this effect 11.
A main limitation to overcome prior to study of channeled cardiac constructs is the selection and optimization of an appropriate cell seeding method. In previous studies, cardiac cells were uniformly distributed throughout the construct by the use of Matrigel for cell innoculation. Cells were suspended in liquid Matrigel, the suspension was loaded onto the porous scaffolds, Matrigel was allowed to gel, and the perfusion flow through the seeded scaffold was then initiated 9, 10, 12. However, if used with channeled scaffolds, Matrigel would fill the small-diameter channels, blocking the perfusion flow path. Additionally, Matrigel is inappropriate for use in engineered tissues intended for clinical applications, because it is currently and will likely remain unapproved by the FDA for use in humans 13.
We therefore hypothesized that perfusion of a cardiac cell suspension in culture medium through the scaffold pores can be utilized to efficiently seed a cell-dense channeled scaffold. Following seeding the channels should remain open, allowing us to perfuse culture medium without exposing the cardiac cells to direct hydrodynamic shear. To test this hypothesis, we utilized a cartridge perfusion system that was designed to hold the scaffolds in place and allow the cell suspension to be perfused through the scaffold pores10, 14. An alternative to this approach, which may be more appropriate for seeding a larger number of constructs simultaneously, is to confine scaffolds and move them in a controlled manor through a cell suspension15. In our study, the parameters of flow rate and duration of seeding were first established by perfusing C2C12 cell (mouse skeletal myoblast) suspensions through the scaffolds. The optimized parameters were then used for seeding of neonatal rat cardiac cells into the pores of channeled scaffolds that were placed into perfusion cartridges either alone or stacked one on top of another.
Ultimately the channels of engineered cardiac tissue should be lined with endothelial cells (ECs), to further shield the myocytes from hydrodynamic shear, and provide paracrine signaling. Endothelialized scaffold channels could also serve as precursors for blood vessel formation. The proposed protocol for sequential perfusion seeding of cardiac cells (in the scaffold pores) followed by EC cell seeding (at the channel walls) yields an millimeters thick engineered constructs with cardiac myocytes and endothelial cells seeded in a spatially defined manner.
Methods
C2C12 cell culture
Mouse skeletal myoblasts (C2C12) were subcultured in T-75 flasks in Dulbecco’s Modified Eagle Medium (DMEM) containing 4.5 g/L glucose, supplemented with 10% fetal bovine serum (FBS), 10mM N-2-hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES), 2mM L-glutamine, and 100 units/mL penicillin. Cells were dissociated with trypsin and counted using a hemocytometer prior to seeding.
Cardiac cells
Cardiac cells were obtained from 1- to 2-day-old neonatal Sprague-Dawley (Harlan) rats according to procedures approved by the Institutional Animal Care and Use Committee, as previously described10. Briefly, ventricles were quartered, incubated at 4°C in a 0.06% (w/v) solution of trypsin in Hank’s balanced salt solution (HBSS, Gibco), and subjected to a series of digestions (3–4 min, 37°C, 150 rpm) in a 0.1% (w/v) solution of collagenase type II in HBSS. The cell suspensions from the 4–5 digestions were collected and labeled unseparated. The cells were preplated in T75 flasks for one 75-min period to enrich for cardiomyocytes. The non-adherent cells were then collected and counted using a hemocytometer prior to seeding.
Endothelial cells
Rat aortic endothelial cells (RAECs) were purchased from VEC Technologies (Rensselaer, NY). Cells were cultured in 0.2% gelatin-coated (Sigma, St. Louis, MO) polystyrene tissue culture flasks in MCDB-131 “complete medium” (VEC Technologies) supplemented with 10% fetal bovine serum (FBS), antibiotics and growth factors. Fresh culture medium was exchanged every 3–4 days. RAECs were passaged when they reached 80% confluency using 0.25% trypsin-EDTA (Gibco BRL). For retroviral transduction of RAECs, EGFP DNA was inserted in the LZRSpBMN retroviral vector as previously described16. Phoenix packaging cell lines were transfected with the retrovirus DNA vectors using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) to generate virus-producing cell lines and supernatants from these cells were collected in DMEM containing 10% FBS with 2mM L-glutamine, and 100 units/mL penicillin. Supernatants were then filtered using a 0.45 μm syringe filter (BD Biosciences) and supplemented with polybrene (8 μg/mL, Sigma-Aldrich, St. Louis, MO). EGFP+ RAECs were sorted using FACSaria (Becton Dickinson) and passage 15 cells were used in this study.
Scaffolds
Porous poly(glycerol sebacate) (PGS) scaffolds were fabricated by a salt leaching technique as previously described10. The PGS scaffolds used for this study had an elastic modulus in tension of 282 ± 25 kPa, porosity of 89%, and pore sizes in the range of 75 – 150 μm, as measured previously 17, 18. For channeled scaffolds, an array of cubically packed parallel channels (250 μm in diameter, with 500 μm wall-to-wall spacing) were created using a computerized 40W carbon dioxide laser (Epilog, Pololu Robotics & Electronics). Scaffolds were cored into disks (8 mm diameter, 1 mm thickness), sterilized by autoclave and ethanol gradient (75, 50, 25, and 0% EtOH), and soaked overnight in culture medium containing 10% FBS.
Perfusion loop setup, cell seeding and culture
Perfusion loops were assembled as previously described (Figure 1) 10. Briefly, scaffolds were placed into custom-designed perfusion cartridges and held in place by gaskets made from silicone tubing. In this configuration, perfusion of culture medium is forced through the inner 5 mm core of the scaffold. The tubing forming the chamber was secured by a triple barb on each side to ensure a tight-fitting connection that minimized leaks and ensured sterility of the construct during the entire seeding process. The cartridges were then connected to PVC tubing in a two-cartridge loop, as shown (Figure 1).
Figure 1. Perfusion seeding of channeled elastomer scaffolds.
Schematic diagram showing the perfusion seeding loop (A) and perfusion cartridge setup in the single (B) and stacked (C) scaffold configuration. Alternating the direction of perfusion of cell suspension through the scaffold (D) results in cell lodging in the scaffold pores (E).
An appropriate number of cells was then suspended in 7 mL of culture medium and added into the tubing above and below each cartridge. The loops were connected via tubing to a peristaltic pump. A custom program (Labview) controlled the flow rate and alternated the flow direction after 3 mL of suspension had been perfused in either direction. The cells were forced into the scaffold pores by the flow, where they became entrapped.
The seeding flow parameters (flow rate, duration of seeding) were determined using a single non-channeled scaffold in each cartridge. To optimize the seeding of channeled scaffolds, two channeled scaffolds were stacked on top of one another in each cartridge; single channeled scaffolds served as controls. When stacked, scaffolds were positioned such that the channels were not aligned, but instead the channels of each scaffold were effectively blocked by the bulk phase of the adjacent scaffold.
The cartridges were then connected to stopcocks, tubing, and a peristaltic pump. Perfusion loops were loaded by a cell suspension containing 3.3 million cells per scaffold (C2C12, cardiac, or ECs), a volume density (~150.106 cells/cm3 tissue) that corresponds to the physiologic range of cell density for rat myocardium. Throughout the duration of seeding, a custom program (Labview) was used to change the flow direction, with reversals after flowing each 3 mL of fluid through the scaffold.
For EC culture, scaffolds were first soaked in a laminin solution (10 μg/mL in distilled water, Sigma) to enhance cell interaction with PGS surfaces, as previously demonstrated19. After seeding channeled scaffolds with ECs under optimized conditions (2 hour duration, 0.1 mm/sec flow velocity, single scaffold in each cartridge, reversal of flow direction after flowing 3 mL of fluid through the scaffold), the resulting constructs were cultured for an additional 4 days, with the flow velocity of 0.1 mm/s and the reversal of flow direction after flowing 3 mL of fluid through the scaffold. The resulting constructs were harvested for analytical assays.
Cell seeding efficiency
The seeding efficiency, defined as the percentage of initially seeded cells that attached to the scaffolds, was determined as follows. Seeded scaffold were cut in half, each half was weighed and digested in 1 mL proteinase K solution for 18 h at 56°C. Proteinase K was prepared at a concentration of 1 mg/mL in 10 mM Tris with 1 mM EDTA. Aliquots of the solution were analyzed using the Picogreen DNA assay kit (Invitrogen) according the manufacturer’s instructions. DNA concentrations were compared to reference lambda DNA standards supplied in the kit.
At the time of seeding, 2–3 aliquots of the seeding cell suspension were collected, identical in volume to the aliquot seeded onto one scaffold. Cells were centrifuged to obtain pellets, digested with proteinase K solution, and analyzed using the Picogreen assay. Since these samples contained the same number of cells initially seeded per scaffold, they served as a reference (100% value) for seeding efficiency. The linear relationship of the Picogreen fluorescence with the number of cells allowed us to calculate the seeding efficiency as a percentage of the reference after adjusting by the weight percent of the scaffold digested.
Histology and fluorescence imaging
For histological analysis, one half of each scaffold was fixed overnight in 4% neutral buffered formalin, embedded in paraffin, sectioned either perpendicular or parallel to the scaffold diameter to 5 μm, and stained with hematoxylin and eosin (H&E). Color images were acquired by a Q-imaging camera mounted to a light microscope (Olympus). For fluorescence imaging of GFP-labeled ECs, samples were transferred into PBS and imaged under a fluorescence microscope (Olympus) at an excitation wavelength of 395 nm and an emission wavelength of 510 nm. For comparison purposes, an exposure time of 200 ms was used to image all samples.
Cell viability
Scaffolds were removed from perfusion cartridges and stained using the Live/Dead viability assay kit (Invitrogen) according to the manufacturer’s instructions immediately after the two hour seeding period. The kit contains two viability probes, calcein AM and ethidium homodimer-1, that stain live cells green and dead cells red, respectively. Scaffolds were imaged an hour after staining using a scanning confocal microscope (Zeiss LSM 510 META) at excitation/emission wavelengths of 494/517 nm for calcein and 528/617 nm for Ethidium homodimer-1. With this system, we were able to obtain optical cross sections of about 100 μm thickness that were reconstructed as projections using software (Zeiss LSM Image Browser).
Scanning electron microscopy
Samples were fixed in 2.5% gluteraldehyde-paraformaldehyde in 100 mM sodium cacodylate solution (Electron microscopy sciences) at pH 7.4 for 2 hr 20. After fixation, samples were rinsed in 100 mM sodium cacodylate at pH 7.4. Samples were dehydrated with 10 minute exchanges in each of 50, 70, 80, 90% ethanol solution and in absolute ethanol for three times, and were then immersed in Hexamethyldisilazane (HMDS) for 15 minutes and air dried at room temperature overnight. The dried samples were mounted on stainless steel SEM stubs and coated with gold by a sputter coater for 30 seconds. Samples were examined and imaged using scanning electron microscopy (XL30 ESEM, FEI company, Hillsboro, OR).
Results
Perfusion seeding of channeled scaffolds
We developed a perfusion loop system to seed cells in the pores of (PGS) scaffolds (Figure 1). The cells were forced into the scaffold pores by the flow, where they became entrapped. Selection of optimal flow parameters (flow rate and duration) for seeding was performed using C2C12 mouse skeletal myoblasts and non-channeled PGS scaffolds. A single scaffold was placed in each cartridge and a cell suspension containing 3.3 million cells per scaffold was perfused through the PGS pores. After 2 hours of perfusion seeding, a superficial flow rate of 1.0 mm/s yielded a higher seeding efficiency (87 ± 26%) and lower variability between seeded scaffolds than lower flow rates (69 ± 51 and 66 ± 26 for the flow velocity of 0.1mm/s and 0.4mm/s respectively, Figure 2A). In addition, the higher flow rate (0.4 mm/s) during seeding resulted in deposited cells more uniformly distributed across the thickness of the scaffold as compared to the lower flow rate (0.1 mm/s) where most of the cells attached within the upper half of the scaffold volume (Figure 3A-C). The flow rate was not increased further to avoid exposing cells to additional shear stress and based on the relatively high efficiency and uniform distribution at the 1.0 mm/s flow rate.
Figure 2. Efficiency of C2C12 cell seeding for non-channeled scaffolds.
Data are shown for C2C12 cells, as a function of flow rate for seeding time of 2 hr (A), and as a function of seeding time at a superficial velocity of perfusion of 1.0 mm/s (B).
Figure 3. Spatial uniformity of C2C12 cell seeding of non-channeled scaffolds.
Representative H&E stained cross sections showing uniformity of C2C12 cell seeding as a function of flow rate (A, B, C) or seeding time (D, E, F).
The effects of duration of seeding on efficiency and cell distribution were then investigated at the flow rate of 1.0 mm/s. A seeding period of 1 hr had a slightly lower average efficiency than a period of 2 hr (Figure 2B). Seeded scaffolds showed similar uniform cross-sectional cell distributions at all time points, due to the dispersion provided at the 1.0 mm/s flow rate (Figure 3D-F). Therefore the flow rate of 1.0mm/s and duration of 2 hr were selected for further investigation with cardiac cells.
Seeding of channeled scaffolds with cardiac cells
Two cartridge setups were used to develop a method for seeding the pores of channeled PGS scaffolds with cardiac myocytes. At first, a single scaffold was placed in between the cartridge gaskets and perfused with a suspension of 3.3 million freshly isolated cardiac cells in 7 mL volume of culture medium. The technique resulted in a relatively low seeding efficiency (59.7 ± 27.1%) compared to the non-channeled seeding of C2C12 cells (87 ± 26%). Additionally, the majority of cells were found closer to the channel surfaces instead of being uniformly distributed throughout the scaffold pores (Figure 4A).
Figure 4. Spatial uniformity of cardiac cell seeding of channeled scaffolds.
Representative H&E stained cross (A, B) or face (C) sections for scaffolds seeded with cardiac cells in the single (A) or stacked (B,C) configuration. Dotted lines indicate the approximate position of channels in each section
In the second configuration, two channeled scaffolds were stacked one on top of another, placed in the perfusion cartridges between gaskets and perfused with 3.3 million cardiac cells per scaffold (am total 0f 6.6 million cells in 7 mL volume for each cartridge containing two scaffolds). With this setup the channels of the upper scaffold were effectively blocked by the lower scaffold and vice versa. Seeding yielded a higher efficiency (77.2 ± 23.7%) than the single scaffold setup, and much closer to the efficiency obtained for C2C12 cells on non-channeled scaffolds (87 ± 26%). The cells were distributed uniformly throughout the scaffold pores with not much preference for the channel edges seen in either the thickness or diameter cross sections (Figure 4B-C).
Cardiac cell viability
To assess any loss of cell viability associated with medium perfusion, we stained the stacked channeled scaffolds seeded with cardiac cells with Live/Dead assay and performed confocal imaging. Constructs showed a dense distribution of viable cells (green) throughout the construct face and cross section (Figure 5). The PGS scaffold was auto-fluorescent (red), however we were able to distinguish the single dead round cells (red), which from our observation were present in rather small numbers as compared to the numbers of viable cells (green).
Figure 5. Cardiac cell viability in seeded scaffolds.
Projection of images taken via confocal microscope of channeled scaffolds seeded with cardiac cells in stacked configuration and stained with live-dead assay Images show the top-down view of the channels (A) and the cross sectional view of the channel walls (B).
Seeding of channel lumens with endothelial cells
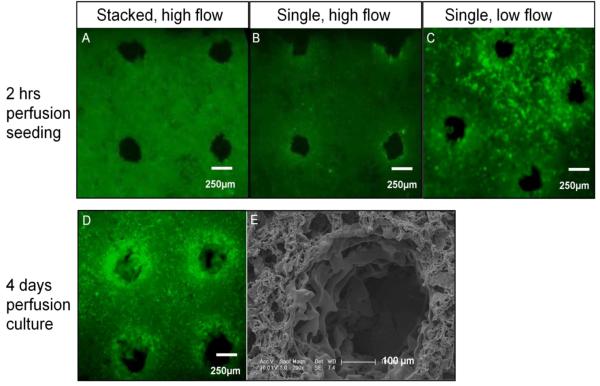
Seeding of channeled PGS scaffolds with ECs in the stacked and single configurations resulted in seeding efficiencies of 76.1 ± 13.2% and 39.1 ± 13.6% respectively. As expected, and documented by both the fluorescence imaging and histology, ECs were localized near the channel surfaces in the single scaffolds setup, whereas cells were uniformly distributed throughout the scaffold volume in the stacked setup (Figure 6). Since our intention is to specifically seed the channel surfaces with ECs, the single scaffold configuration was selected. Seeding ECs at a lower flow rate (0.1 mm/sec) increased the number of cells at the channel walls. The continuation of culture with flow perfusion for an additional 4 days resulted in spreading of ECs around the channels as shown by SEM imaging (Figure 6).
Figure 6. Seeding of channel walls with endothelial cells.
Fluorescence and SEM images following perfusion seeding (A-C) or culture (D-E) of endothelial cells (ECs). Scaffolds were seeded at either the high (1.0 mm/s) or low (0.1 mm/s) perfusion velocity (top row). After 4 days of cultivation, cell-seeded scaffolds from the low-flow group show high viability (D), and attachment to the channel walls with characteristic flat morphology seen in scanning electron microscopy (E). All fluorescence images of live-dead assayed samples were taken using the same exposure time (200 ms) for comparison purposes.
Discussion
In native vascularized myocardium, the very high cell density (~108 cells/cm3) is supported by the flow of oxygen-rich blood through a dense capillary network that shields cardiomyocytes from the shear stress of blood perfusion. Our intent was to use a “biomimetic” in vitro culture in which myocytes are shielded from shear stress by an array of channels cut in a porous elastomer scaffold that permit fluid flow. Furthermore, perfusion through the channels will enable development of a thicker construct (as compared to static culture) with enhanced functionality (as compared to perfusion through non-channeled scaffolds).
In previous studies of cardiac cells on porous scaffolds, seeding was accomplished by inoculation of cells suspended in Matrigel, with or without medium perfusion used to establish spatially uniform cell distribution. The Matrigel delivery - perfusion technique allows for a uniform distribution of metabolically active cardiac cells in relatively thick constructs, but is not suitable for seeding of channeled scaffolds because the gel would block the small diameter (~250μm) channels. Since Matrigel remains unapproved for human use, its elimination from our in vitro cardiac culture system also represents an important step in developing methods for generating constructs to be used in clinical applications.
Perfusion of a cell suspension through the pores of elastomeric scaffolds proved to be effective in seeding cells at high densities, an important factor in engineering tissues that mimic the physiologic cell density. We selected a range of flow velocities for seeding based upon a previous study that showed perfusion of a cell suspension through highly porous foams without the use of gel carriers could be achieved with linear flow rates ranging from 0.1 – 1.0 mm/s (using chondrocytes seeded onto porous Hyaff-11 meshes) 14. For cardiomyocyte cultures, perfusion at 0.14 – 0.71 mm/s 21, 22, 0.425 – 1.275 mm/s 2, 9, or 0.523 mm/s 23 induced efficient assembly of compacted cardiac tissue, suggesting that our selected flow rates for cardiac cell perfusion seeding fall within acceptable limits.
Another key requirement is to maximize the utilization of available cells for scaffold seeding, and to maintain cell viability. The relatively high flow rate applied during initial seeding was therefore limited to a relatively short 2 hr period. The cells maintained their viability, indicating that the flow was not damaging. Since the culture medium flow will be mainly through the channels during the remainder of culture, we expect the cells to be able to survive, regenerate contractile proteins, and develop functionality without suffering due to shear stress exposure. Indeed, cardiac cells seeded and cultured in perfusion for a period of one week in porous PGS scaffolds were able to contract synchronously in response to electrical pacing 24.
Direct perfusion of cells through scaffold pores also proved to be effective for seeding cardiac cells at a high density, and for achieving homogeneous spatial distribution throughout the construct volume. When channeled scaffolds were stacked on top of one another for perfusion with cell suspension, there was no preferential seeding at either surface of the construct or near the channel walls. In contrast, perfusion seeding at a low flow rate did not provide enough force to distribute the cells throughout the scaffold, leaving most cells in the upper half of the scaffold nearest to where the suspension was injected. This is similar to the case when cells are loaded statically onto PGS, with or without Matrigel, because the driving force to distribute cells (i.e. gravity and capillary action) is also insufficient to seed a thick cardiac construct 10.
The perfusion flow can also be exploited to seed ECs on the surfaces of channel walls. Perfusion through a single scaffold placed in each cartridge, in which the majority of flow is through the channels, resulted in seeding of the regions near the channel surfaces, and few ECs entering the bulk of the construct. We established a method of sequqntial seeding that can be utilized to engineer cardiac constructs with endothelialized channels. Cardiac cells were seeded uniformly by perfusing through stacked scaffolds and maintained in perfusion culture following separation and placement of the two scaffolds into individual cartridges. The scaffolds were then perfused with a suspension of ECs through the scaffold channels, to endothelialize the channel walls. EC seeding was best perfored in single scaffold configuration, and at relatively low perfusion rates, to avoid washout of already seeded cardiomycytes from the scaffold pores. As an alternative, ECs can be seeded first, at a low flow rate, and the and keep the resulting constructs in perfusion culture for several days to allow cells to spread around the channel surfaces. These constructs can then be stacked and loaded into perfusion cartridges to seed cardiac myocytes into the scaffold pores. In either case, the result is an engineered cardiac construct with channels lined with ECs.
An important feature of the perfusion seeding process is that the channels remain open. With the stacked scaffold configuration, the channels of the upper scaffold are effectively blocked by the lower scaffold and vice versa. Uniform cell distribution is achieved with no observable preference for cell attachment near the scaffold surfaces or channel edges. If a single scaffold configuration is used, a lower percentage of cells are seeded and most attach near the channel surfaces. This probably occurs because the majority of the flow is through the scaffold channels, not the PGS pores. A potential disadvantage of the method is the risk of contamination during transfer of seeded scaffolds. However, recent trials in our laboratory have shown that the cartridge design allows for easy, and quick sterile transfer of constructs following perfusion seeding.
Conclusions
Our ultimate goal is to engineer cardiac tissue patches with a primitive vasculature that could enable the engineering of thicker cardiac constructs in vitro and enhance the ability of the construct to survive upon in vivo implantation. A perfusion bioreactor system was used for seeding cardiac cells homogeneously throughout the pores of an elastomeric scaffold containing an array of parallel channels, 250 μm in diameter. By perfusing cell suspension back and forth through the scaffold, cells became distributed in the scaffold pores. The flow parameters were optimized to show that one can achieve a seeding that is both spatially uniform and at a high cell density, by using a flow rate of 1.0 mm/s and a seeding duration of 2 hr. Placing one scaffold on top of another in a perfusion cartridge effectively blocks the channels of both scaffolds, allowing cells to be homogeneously seeded throughout both scaffolds. Cardiac cells remain viable throughout seeding and subsequent cultivation. Since the channels remain open, perfusion of medium during culture is mainly through the channels, thus shielding cardiomyocytes in the scaffold pores from hydrodynamic shear. Furthermore, perfusion of an endothelial cell suspension through individual scaffolds resulted in the attachment of ECs to channel walls, a method that could be exploited to establish a confluent lining of the channel surfaces.
Acknowledgment
The authors gratefully acknowledge funding by NIH (R01 HL076485 and R21 HL089913 to GVN). Elastomer scaffolds were kindly provided by Professor Yadong Wang.
Contributor Information
Robert Maidhof, Columbia University, Department of Biomedical Engineering, 622 west 168th street, VC12-234, New York, NY 10032, 212-305-9239, rtm2001@columbia.edu
Anna Marsano, Columbia University, Department of Biomedical Engineering, 622 west 168th street, VC12-234, New York, NY 10032, 212-305-9239, am2805@columbia.edu
Eun Jung Lee, Yale University, Department of Anesthesiology, 10 Amistad Street 314, P.O.Box. 208089, New Haven, CT 06520, 203-737-1428 203-737-1484(fax), el368@email.med.yale.edu
Gordana Vunjak-Novakovic, Columbia University, Department of Biomedical Engineering, 622 west 168th street, VC12-234, New York, NY 10032, 212-305-2304 212-305-4692 (fax), gv2131@columbia.edu.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009 Jan 27;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003 May 20;82(4):403–414. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 3.Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006 Feb 5;93(2):332–343. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- 4.Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM. Survival and function of bioengineered cardiac grafts. Circulation. 1999 Nov 9;100(19 Suppl):II63–69. doi: 10.1161/01.cir.100.suppl_2.ii-63. [DOI] [PubMed] [Google Scholar]
- 5.Radisic M, Park H, Shing H, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. 2004 Dec 28;101(52):18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrier RL, Papadaki M, Rupnick M, et al. Cardiac tissue engineering: cell seeding, cultivation parameters, and tissue construct characterization. Biotechnol Bioeng. 1999 Sep 5;64(5):580–589. doi: 10.1002/(sici)1097-0290(19990905)64:5<580::aid-bit8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Bursac N, Papadaki M, Cohen RJ, et al. Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies. Am J Physiol. 1999 Aug;277(2 Pt 2):H433–444. doi: 10.1152/ajpheart.1999.277.2.H433. [DOI] [PubMed] [Google Scholar]
- 8.Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. Am J Physiol Heart Circ Physiol. 2001 Jan;280(1):H168–178. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 9.Radisic M, Yang L, Boublik J, et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. 2004 Feb;286(2):H507–516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 10.Radisic M, Marsano A, Maidhof R, Wang Y, Vunjak-Novakovic G. Cardiac tissue engineering using perfusion bioreactor systems. Nat Protoc. 2008;3(4):719–738. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol. 2005 Mar;288(3):H1278–1289. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- 12.Radisic M, Park H, Chen F, et al. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006 Aug;12(8):2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 13.Polykandriotis E, Arkudas A, Horch RE, Kneser U. To matrigel or not to matrigel. Am J Pathol. 2008 May;172(5):1441. doi: 10.2353/ajpath.2008.071215. author reply 1441-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wendt D, Marsano A, Jakob M, Heberer M, Martin I. Oscillating perfusion of cell suspensions through three-dimensional scaffolds enhances cell seeding efficiency and uniformity. Biotechnol Bioeng. 2003 Oct 20;84(2):205–214. doi: 10.1002/bit.10759. [DOI] [PubMed] [Google Scholar]
- 15.Timmins NE, Scherberich A, Fruh JA, Heberer M, Martin I, Jakob M. Three-dimensional cell culture and tissue engineering in a T-CUP (tissue culture under perfusion) Tissue Eng. 2007 Aug;13(8):2021–2028. doi: 10.1089/ten.2006.0158. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd BR, Jay SM, Saltzman WM, Tellides G, Pober JS. Human aortic smooth muscle cells promote arteriole formation by coengrafted endothelial cells. Tissue Eng Part A. 2009 Jan;15(1):165–173. doi: 10.1089/ten.tea.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002 Jun;20(6):602–606. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 18.Gao J, Crapo PM, Wang Y. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 2006 Apr;12(4):917–925. doi: 10.1089/ten.2006.12.917. [DOI] [PubMed] [Google Scholar]
- 19.Eun Jung Lee YW, Maidhof Robert, Marsano Anna, Vunjak-Novakovic Gordana, Niklason Laura. Biocompatibility of rat aortic endothelial cell with a novel biodegradable elastomeric scaffold. in press. [Google Scholar]
- 20.Niklason LE, Abbott W, Gao J, et al. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001 Mar;33(3):628–638. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 21.Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Effects of oxygen on engineered cardiac muscle. Biotechnol Bioeng. 2002 Jun 20;78(6):617–625. doi: 10.1002/bit.10245. [DOI] [PubMed] [Google Scholar]
- 22.Carrier RL, Rupnick M, Langer R, Schoen FJ, Freed LE, Vunjak-Novakovic G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002 Apr;8(2):175–188. doi: 10.1089/107632702753724950. [DOI] [PubMed] [Google Scholar]
- 23.Dvir T, Benishti N, Shachar M, Cohen S. A novel perfusion bioreactor providing a homogenous milieu for tissue regeneration. Tissue Eng. 2006 Oct;12(10):2843–2852. doi: 10.1089/ten.2006.12.2843. [DOI] [PubMed] [Google Scholar]
- 24.Marsano A, Maidhof R, Tandon N, Gao J, Wang Y, Vunjak-Novakovic G. Engineering of functional contractile cardiac tissues cultured in a perfusion system. Conf Proc IEEE Eng Med Biol Soc. 2008;1:3590–3593. doi: 10.1109/IEMBS.2008.4649982. [DOI] [PubMed] [Google Scholar]