Abstract
Acute alcohol challenge has been associated with a selective impairment of right hemisphere function. A hallmark of visuospatial neglect syndrome is that patients with right hemisphere lesions misbisect horizontal lines far rightward of veridical center. Neurologically intact subjects misbisect lines with a systematic leftward bias (pseudoneglect). Neuroimaging studies in neurologically intact subjects reveal predominant right hemisphere activation during performance of line bisection tasks. The current study assessed whether acute alcohol challenge alters global visuospatial attention. Subjects (N=18; 10 male; strongly right-handed; mean age=23 years) engaged in a forced-choice tachistoscopic line bisection task in both ethanol challenge (mean BAC=.077) and no ethanol control conditions. Mean leftward bisection error in the control challenge condition was −0.238 degrees visual angle (1.05% line length), and leftward bisection error significantly increased (p=.001) under ethanol challenge (−0.333 degrees visual angle, 1.47% line length). Mean bisection precision in the control condition was 0.358 degrees visual angle (1.58% line length); bisection precision significantly deteriorated (p=.008) under ethanol challenge (0.489 degrees, 2.17% line length). Decreased bisection precision indicates that ethanol disrupts the fidelity of visuospatial performance. The exaggerated leftward bisection error implies that ethanol may exert a differential effect on left versus right hemispheric function with respect to the control of global visuospatial attention.
Keywords: Line Bisection, Pseudoneglect, Visuospatial Attention, Ethanol
INTRODUCTION
Ethanol influences many aspects of visual performance: it decreases visual acuity (Mortimer, 1963; Wilson & Mitchell, 1983), spatial contrast sensitivity (Pearson & Timney, 1998; 1999; Roquelaure et al., 1995), particularly for moving targets (Andre et al., 1994; Nicholson et al., 1995), and critical flicker fusion frequency (Pearson & Timney, 1998; Virsu et al., 1973). It also modestly degrades color vision (Wallgren & Barry, 1970; Watten & Lie, 1996, Hill & Toffolon, 1990), as well as accommodation (Watten & Lie, 1996), stereoscopic depth perception (Hill & Toffolon, 1990; Neill et al., 1990; Watten & Lie, 1996; Wegner & Fahle, 1999; Nawrot et al., 2004), and motion perception per se (MacArthur & Sekuler, 1982; McNamee et al., 1980; Bates, 1989; Nawrot et al., 2004). Ethanol delays the initiation and decreases the velocity of saccadic eye movements, and reduces the speed and accuracy of slow eye movements (Guedry et al., 1975; Baloh et al., 1979; Moser et al., 1998; Holdstock & de Wit, 1999), hence impairing perception of depth from motion parallax (Nawrot et al., 2004).
Ethanol also affects attention. Its effect on divided attention is robust (Moskowitz & Depry, 1968; Hamilton & Copeman, 1970; Moskowitz & Sharma, 1974; Leigh et al., 1977; Vuchinich & Sobell, 1978; Landauer & Howat, 1983; Moskowitz et al., 1985; Post et al., 1996; 2000; Schulte et al., 2001). Even modest doses interfere with the ability to efficiently multitask, and there is a marked increase in task-switching costs (Newman et al., 1997). Ethanol impairs performance on vigilance tasks, which require the maintenance of attention continuously over time (Rohrbaugh et al., 1987; 1988; Schulte et al., 2001). Ethanol reduces the amplitude of components of the event-related potential (ERP) associated with reflexive (exogenously recruited) attention and decreases the potency of exogenous cues to capture attention (Jaaskelainen et al., 1996; Jaaskelainen, 1999). Covert (voluntary) attention is also affected. Ethanol decreases cue-validity effects in Posner-type detection tasks for targets presented in left hemispace (i.e., to the right hemisphere), and increases cue-validity effects for targets presented in right hemispace (to the left hemisphere) (Fillmore et al., 2000; Schulte et al., 2001). Ethanol impairs the ability to tightly focus attention at fixation, and compromises the ability to covertly attend to peripheral regions (Canto-Pereira et al., 2007). Thus, while there is much evidence suggesting that it exerts a powerful influence on numerous facets of attention, its effect on the deployment of global visuospatial attention has yet to be assessed.
Global spatial attention refers to that type of environmental monitoring that is continuously deployed across a wide expanse of space, including perhaps regions that lie outside the visual field (i.e., overhead or behind). Disruptions of visuospatial attention (i.e., global spatial attention confined to the visual field) are a hallmark of the clinical neurological syndrome known as hemispatial neglect.
Line bisection is commonly used in the clinical assessment of hemispatial neglect, a syndrome typically (but not exclusively) associated with vascular lesions of the right hemisphere (Vallar & Perani, 1987; Cappa et al., 1991; Mesulam, 2000; Na et al., 2000; Kerkhoff, 2001). In line bisection tasks subjects manually mark, or otherwise indicate, the perceived midpoint of a horizontal line. Patients with hemineglect bisect lines far rightward of true center (Robertson & Halligan, 1999). There is consensus that while both left and right parietal cortices participate in the deployment of spatial attention to right hemispace, the right hemisphere is uniquely responsible for deploying attention into left hemispace (Heilman & Van Den Abell, 1980; Weintraub & Mesulam, 1987). Lesions to the right hemisphere therefore often render patients pathologically inattentive to left hemispace.
This hemispheric specialization hypothesis of neglect is further supported by the phenomenon known as “pseudoneglect” (Bowers & Heilman, 1980; Jewell & McCourt, 2000), which refers to the systematic misbisection of lines to the left of veridical midpoint made by neurologically intact observers. This leftward error in normal observers is a corollary of the profound parietal asymmetry in attentional control that underlies hemineglect syndrome. Thus, in normal observers, leftward bisection error is theorized to be a byproduct of the right hemisphere’s prepotent vector of attention into left hemispace (McCourt, 2001; McCourt & Jewell, 1999; McCourt et al., 2000). Functional imaging (fMRI) studies have established the pivotal role played by the right hemisphere in the performance of line bisection tasks (Weiss et al., 2000; Fink et al., 2000; 2001; 2002). High-density electrical mapping techniques have also revealed that spatial judgments in line bisection tasks are associated with enhanced activity in right cerebral cortex; the time-course of these scalp-recorded electrical potentials, and the locations of the putative intracranial generators, have been described in detail (Foxe et al., 2003).
A variety of studies has suggested that ethanol exerts a differential influence on the two cerebral hemispheres, with most studies disclosing a selective deleterious effect on right hemisphere function. For example, during ethanol challenge the relative amplitude of visual evoked potentials is shifted in favor of the left hemisphere (Lewis et al., 1969; Rhodes et al., 1975; Sternberg et al., 1994); ethanol decreases the right hemisphere fMRI BOLD response to visual stimuli (Levin et al., 1998), and decreases right hemisphere regional cerebral blood flow (Wendt et al., 1994; but see Volkow et al., 1988). Ethanol selectively impairs perception of the “global” level of hierarchical patterns (Lamb & Robertson, 1987), increases choice reaction time (Chandler & Parsons, 1977; Poppel & Steinbach, 1986), and increases detection thresholds and inspection times for stimuli presented in the left visual field (Damkot & Frysinger, 1978; Kostandov et al., 1982).
Present Study
The present study used forced-choice tachistoscopic line bisection to assay the effect of acute ethanol intoxication on global visuospatial attention. We have two predictions. First, because ethanol has a putative differential effect on right versus left hemisphere function we hypothesized that intoxicated subjects would, compared to their performance in a nonintoxicated control condition, exhibit decreased pseudoneglect as indexed by smaller leftward error (or a frank rightward bias) in the line-bisection task. Second, because ethanol exerts a general deleterious effect on a variety of behavioral capacities, such as increasing sensory thresholds (Zulauf et al., 1988; Watten et al., 1998; Pearson & Timney, 1998; 1999; Andre et al., 1994; Nicholson et al., 1995) and reaction times (Young, 1970; Chandler & Parsons, 1977; Poppel & Steinbach, 1986; Canto-Pereira et al., 2007), we hypothesized that ethanol-challenged subjects would, compared to their performance in a no-alcohol control condition, exhibit decreased precision in their bisection judgments.
METHODS
Subjects
A total of 18 right-handed subjects (10 male, mean age = 23.0 years; 8 female, mean age = 23.0 years) participated in the experiment. All subjects were at least 21 years of age. Subject laterality was assessed using a standard instrument (Oldfield, 1971) on which a composite score of −100 denotes exclusive left-handedness, and +100 denotes exclusive right-handedness. The mean laterality score for male subjects was +78.5 (S.E. = 7.1); that for female subjects was +75.0 (S.E. = 6.6). There was no significant difference in mean age or laterality across male and female subjects, t16 = 0.0, p = 1.00 and t16 = 0.36, p = 0.72, respectively; all subsequent inferential statistical tests were therefore conducted on data collapsed across subject sex. No subjects reported any neurological abnormalities, and all possessed normal or corrected-to-normal visual acuity. Subjects were instructed to refrain from eating for one hour prior to the experiment. All subjects provided informed consent forms and responded to a list of 11 exclusionary criteria: e.g., prior drinking problems, familial history of alcoholism, characterizing oneself as a “non-drinker” (less than 2 drinks per year), etc. Four subjects endorsed one or more list items and were excluded from the study. After the alcohol challenge subjects were allowed to leave the laboratory when their blood alcohol content (BAC) fell below 0.05%. Taxi service was provided. Subjects were monetarily compensated for their participation. The experiment was conducted in accordance with the ethical standards described in the 1964 Declaration of Helsinki, and all procedures were approved by the Institutional Review Board of North Dakota State University.
Instrumentation and Calibration
Subject responses were collected by computer and stimuli were presented using CRT monitors driven by graphics adaptors with 640 × 480 pixel resolution. Monitor frame refresh rate was 60 Hz. Luminance and contrast calibrations were made using a spot photometer (Konica Minolta model LS-110).
Ethanol Administration and Measurement
Subjects were requested to abstain from alcohol use for 24 hours prior to both sessions. Prior to each session blood alcohol concentration (BAC) was estimated using an Intoxilyzer 5000 (CMI Inc., Owensboro, KY) which is an evidentiary breath analyzer using infra-red absorption. Prior to each session subjects had a BAC of zero.
In the ethanol challenge condition subjects were administered a dose of 0.8 g/kg body weight, delivered as a mixture of 100 proof Vodka and orange juice designed to achieve a BAC target level of 0.1%. The total dose was delivered in four equal installments given at 10 minute intervals. BAC was assessed by breath analysis. BAC as measured using the Intoxilyzer has excellent agreement with venous blood analysis (Jones et al., 1992). Factory calibration was checked with a wet bath alcohol breath simulator (Toxitest Model ABS 120, CMI Inc., Owensboro, KY). The first four BAC measurements were taken immediately prior to the administration of each dose. Twenty minutes following the fourth and final dose, BAC was measured to determine whether subjects’ readings had reached the target BAC. In the event that BAC was not in range, 10 additional minutes were allowed to elapse at which point BAC was remeasured and the line bisection task was initiated. Subjects commenced the line bisection task at the plateau stage of intoxication (BAC levels from 0.06–0.11%). The line bisection task took between 5–7 minutes to complete. Following completion of the line bisection task BAC was measured every 25 minutes until the reading fell below .05%.
Stimuli
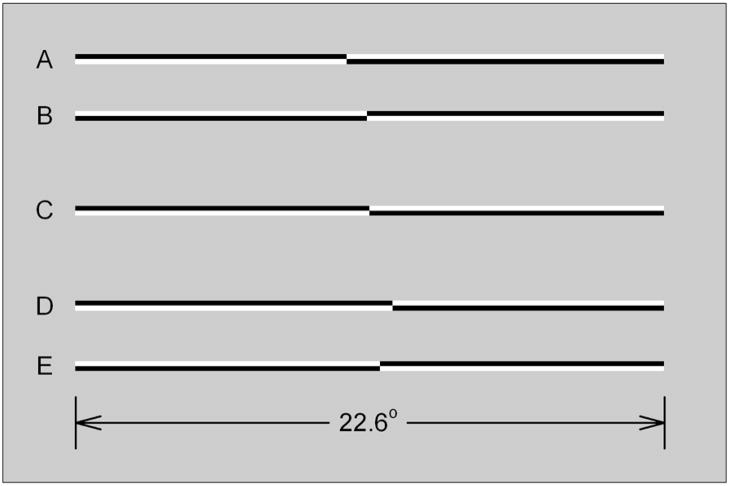
Stimuli were horizontally oriented lines of 100% Michelson contrast presented on a gray background (mean luminance = 50 cd/m2). At a viewing distance of 45 cm lines subtended 22.6° in width by 0.39° in height. All lines were pre-transected prior to presentation. Transectors were located at 25 positions ranging from +/−0.89° visual angle relative to veridical line center. This range of transector locations was sufficient to produce near-asymptotic “left” or “right” judgments in control subjects.
Figure 1 illustrates examples of line stimuli used in the experiments. Both members of the upper pair of lines (A, B) are transected to the left (by −0.89° and −0.10°, respectively). Both members of the lower pair of lines (D, E) are transected to the right of veridical center (by +0.89° and +0.39°, respectively). Line C is veridically transected. The members of line pairs (A, B) and (D, E) differ in contrast polarity. Lines of opposite contrast polarity appeared with equal frequency and the order of appearance of lines with different transector locations and elevations was randomized within blocks of trials.
Figure 1.
Examples of line stimuli used in the experiments. The members of the upper pair, A and B, are transected to the left (by −0.89° and −0.10°, respectively). The members of the lower pair, D and E, are transected to the right of veridical center (by +0.89° and +0.39°, respectively). Line C is veridically transected. The members of line pairs AB and DE differ in contrast polarity.
Procedure
Subjects were seated upright in straight-backed chairs in a dimly lit room. Their midsagittal planes were aligned with the display monitor, and viewing distance and head orientation were controlled using a chinrest. On each trial subjects made single-interval forced-choice decisions regarding transector location relative to perceived line midpoint by depressing either the left or right mouse button as appropriate. Button orientation corresponded to the axis of perceptual discrimination (i.e., the “left” response button was to the left of the “right” response button). Subjects responded using both right and left hands in separate blocks of trials. Order of hand use was counterbalanced within and across subjects. Response time was unlimited. All statistical tests are conducted on mean bisection performance averaged across the two hands.
Tachistoscopic presentation (McCourt & Olafson, 1997) was used to control for scanning eye movements. Pre-transected lines were presented for 150 ms; inter-trial intervals were variable since subsequent trials began 750 ms following previous responses.
In each experimental session subjects made eight “left-right” judgments in conjunction with each line transector location. Each determination of subjective line midpoint, described below, was therefore computed based on 200 (25 transector locations × 8 judgements per location) forced- choice bisection trials. Line transector position was randomly interleaved within blocks of trials.
Design and Analysis
Each subject completed two experimental sessions (ethanol challenge, no ethanol) separated by a minimum of 24 hours (and up to 1 week). The order of completion of the sessions was randomized with the constraint that order was counterbalanced across participants.
The dependent measure was the proportion of trials on which subjects indicated that the transector was located to the “left” of perceived line midpoint. The method of constant stimuli was used to derive psychometric functions; nonlinear regression was performed to fit a cumulative Gaussian distribution to these psychometric functions by method of least-squares. The cumulative Gaussian function is described by the equation:
| (1) |
where x is transector location, β is the x-axis location corresponding to the mean of the underlying Gaussian density function (i.e., the transector location at which left-right responses occur with equal frequency = p.s.e.), and σ is its standard deviation (SD). The parameters x, β and σ are expressed in degrees visual angle. The expression (erf) refers to the error function, an approximation to the cumulative Gaussian distribution.
Based on these least-squares fits, the transector location corresponding to a 50% “left” response rate (β) was extracted, as well as the distribution’s standard deviation (σ). The transector location for which “left” and “right” responses occur with equal frequency is known as the “point of subjective equality” (p.s.e.) and is an objective measure of perceived line midpoint. Subsequent inferential statistical tests including one-sample and paired sample t-tests were conducted on these regression-determined values of p.s.e. and standard deviation. The t-statistics were used to calculate estimates of effect size using the formula d = 2t/√df (Cohen, 1988). By convention, an effect size of +/−0.2 is considered to be a small effect, a value of +/−0.4 is a moderate effect and a value of +/−0.6 or greater is considered a relatively large effect (Cooper & Hedges, 1994).
RESULTS
Bisection Accuracy (Bias)
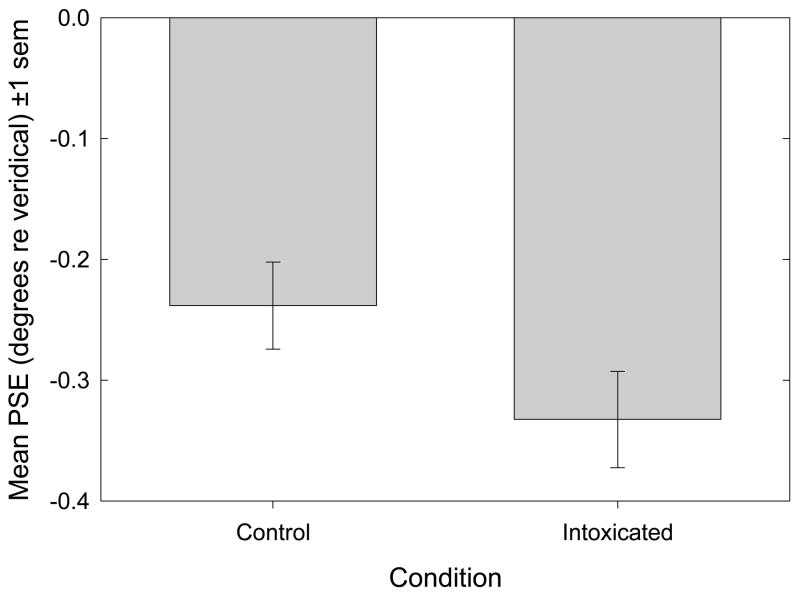
Figure 2 plots mean p.s.e (+/− 1 s.e.m.) for the two conditions. With regard to bisection accuracy, the mean leftward bisection error recorded in the control condition was −0.238 degrees visual angle (1.05% line length). A one-sample t-test indicates that this leftward error differed significantly from zero [t17 = −4.14, p<.001, d = −2.01]. The mean leftward bisection error observed in the ethanol challenge condition was −0.333 degrees visual angle (1.47% line length). A one-sample t-test indicates that this leftward deviation also differed significantly from zero [t17 = −5.77, p<.001, d = −2.80]. The surprising principal finding is that, contrary to our hypothesis, leftward bisection error significantly increased during ethanol challenge [t17 = −3.88, p=.001, d = −1.88; paired-samples t-test].
Figure 2.
Bisection Accuracy (Bias). Mean p.s.e (±1 s.e.m.) is plotted as a function of experimental condition. Mean leftward bisection errors in both control and intoxicated conditions were significantly leftward of zero: t17 =−4.14, p<.001, d =−2.01 and t17 = −5.77, p<.001, d =−2.80, respectively. A paired-samples t-test discloses that ethanol causes a significant increase in bisection error: t17 = −3.88, p=.001, d = −1.88.
Bisection Precision
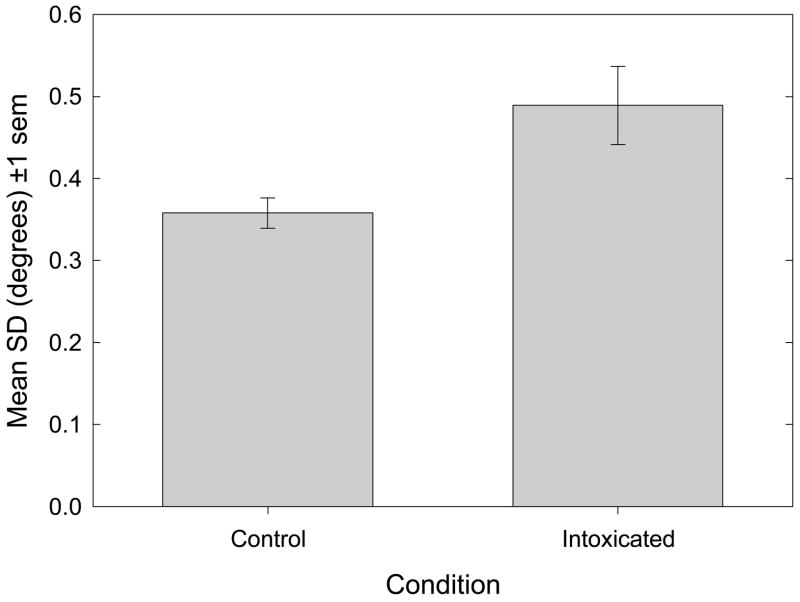
Figure 3 plots the mean standard deviation (σ, +/− 1 s.e.m.) of the cumulative normal distributions used to fit the psychometric data, as a function of experimental condition. Individual values of σ were obtained by fitting cumulative normal distributions (equation 1) to each subject’s psychometric data. The value of σ is inversely related to the slope of the psychometric function as it passes through the point of subjective equality (p.s.e.) Thus, the steeper the slope of the psychometric function, the smaller is the value of σ, and the narrower is the range of transector locations over which “left” judgments transit to “right” judgments. Small values of σ indicate that observers are highly sensitive (i.e., can respond differentially) to changes in transector location around the p.s.e. and that perceived line midpoint is judged with a high degree of precision. Note that bisection accuracy (bias) and precision are independent anddissociable measures of bisection performance.
Figure 3.
Bisection Precision. The mean standard deviation parameter (+/− 1 s.e.m.) is plotted as a function of experimental condition. A paired-samples t-test discloses that ethanol causes a significant increase in the standard deviation of the cumulative normal function used to fit the psychometrics, indicating decreased bisection precision: t17 = 3.03, p=.008, d = 1.47.
Figure 3 illustrates that with regard to bisection precision, the mean value of σ in the control condition was 0.358 degrees visual angle (1.58% line length). During ethanol challenge the mean value of this regression parameter rose to 0.489 degrees (2.17% line length), which is consistent with our prediction, and represents a significant increase over values recorded in the no ethanol control condition [t17 = 3.03, p=.008, d = 1.47; paired-samples t-test].
DISCUSSION
Bisection Accuracy (Bias)
Surprisingly, contrary to our expectation of reduced leftward bias (pseudoneglect), the effect of ethanol on visuospatial attention was to exaggerate the tonic leftward error typically found in line bisection tasks.
The significant leftward bisection error of subjects in the control condition (−0.238 degrees visual angle; 1.05% line length) is comparable (in terms of effect size) to that reported in numerous previous studies using the forced-choice tachistoscopic line bisection procedure (McCourt & Jewell, 1999; McCourt & Garlinghouse, 2000; 2001; McCourt et al., 2000; 2001a; b; 2005 b; 2008; McCourt, 2001; Foxe et al., 2003), and replicates the nearly universal finding that neurologically normal observers exhibit a significant leftward error on line bisection tasks, i.e., display pseudoneglect (Jewell & McCourt, 2000). It should be noted that there was no significant differential effect of the ethanol and no-ethanol treatments which depended upon the order of their administration.
The profound rightward shifts in perceived line midpoint exhibited by hemineglect patients and the systematic leftward shifts evinced by neurologically intact observers are theorized to be twin manifestations of the right hemispheric dominance for the allocation of spatial attention. According to the hemispheric specialization theory of spatial attention (Heilman & Van Den Abell, 1980; Weintraub & Mesulam, 1987) the exaggerated (40% greater) leftward shift in perceived line midpoint of neurologically intact subjects under acute ethanol challenge could imply that alcohol potentiates right hemisphere dominance for the allocation of spatial attention.
Ethanol exerts effects on both excitatory (glutamatergic) and inhibitory (GABAergic) neurotransmission (Nie et al., 2000; Ticku & Mehta, 1995). One functional outcome of such influence is a significant reduction of lateral inhibition in visual processing at mesopic adaptation levels following ethanol intake (Johnston & Timney, 2008). Based on such neurochemical influences an alternative interpretation of our results follows from the activation-orientation theory of attention (Kinsbourne, 1973; 1993), according to which the two cerebral hemispheres compete for control of various functions, including spatial attention, in an opponent fashion. According to this explanation the exaggerated leftward bisection error induced by ethanol might not reflect an absolute increase in right hemisphere dominance, but could instead result from reduced left hemisphere competition. A direct test of these alternative explanations could be made by recording event-related potentials associated with line bisection performance, using a high-density electrode montage (Foxe et al., 2003). A within-subject comparison of the (predominantly right-hemispheric) bisection-related activations in ethanol challenge versus control conditions would be informative. If ethanol induces an increase in the “gain” of right hemisphere networks which allocate spatial attention, then the amplitude of the difference wave termed the “line-bisection effect” (Foxe et al., 2003) should be larger for subjects in the ethanol challenge condition. On the other hand, if the increased leftward bisection error results from a selective impairment of left hemispheric function, then a decrease in the amplitude of the difference wave recorded over the left hemisphere is predicted.
One possible consequence of exaggerated leftward directed attention is a relative inattention to right hemispace. In locomotor tasks normal subjects collide more frequently with obstacles on the right than on the left (Turnbull & McGeorge, 1998; Nicholls et al., 2007; 2008). One implication of our findings is that ethanol might exaggerate this propensity to collide with right-hand obstacles, perhaps even translating into a preponderance of right-hand-related collisions in motor vehicle accidents involving alcohol intoxication. This hypothesis might be tested by assaying the deployment of attention of intoxicated subjects in a driving simulation, or by analyzing motor vehicle accident records (across both left- and right-hand driving countries).
Bisection Precision
Similar to results for bisection accuracy (p.s.e.), bisection precision in the control condition is similar to that observed in previous studies using the forced-choice tachistoscopic line bisection procedure (McCourt & Jewell, 1999; McCourt & Garlinghouse, 2000; McCourt et al., 2000; 2001a; b; 2005 b; 2008; McCourt, 2001; Foxe et al., 2003). The decrease in bisection precision caused by ethanol challenge, indexed by the significant increase in the value of the regression parameter sigma (σ), which implies elevated thresholds for the discrimination of transector position, indicates that ethanol impairs the fidelity of spatial localization. This result parallels prior observations that ethanol causes reductions in other visuoperceptual capabilities such as spatial contrast sensitivity (Zulauf et al., 1988; Andre et al., 1994; Nicholson et al., 1995; Pearson & Timney, 1998), spatial frequency discrimination (Watten et al., 1998), and visual reaction time (Young, 1970; Chandler & Parsons, 1977; Poppel & Steinbach, 1986; Canto-Pereira et al., 2007).
Acknowledgments
The experiment described in this publication was made possible by grants to MEM: NIH P20 RR020151; NIH R15 EY12267; and NSF EPS-0132289. The National Center for Research Resources (NCRR) and the National Eye Institute (NEI) are components of the National Institutesof Health (NIH). EPSCoR (EPS) is a division of the National Science Foundation (NSF). The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NSF, NIH, NCRR, or NEI. The authors thank Dr. Mark Nawrot for informative discussion, and Wren Pasieka and Caitlin Schultz for help with conducting the experiment. There are no commercial conflicts of interest.
References
- Andre JT, Tyrrell RA, Leibowitz HW, Nicholson ME, Wang M. Measuring and predicting the effects of alcohol consumption on contrast sensitivity for stationary and moving gratings. Perception & Psychophysics. 1994;56:261–267. doi: 10.3758/bf03209760. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Sharma S, Moskowitz H, Griffith R. Effect of alcohol and marijuana on eye movements. Aviation, Space, and Environmental Medicine. 1979;50:18–23. [PubMed] [Google Scholar]
- Bates ME. The effect of repeated occasions of alcohol intoxication on two processes involved in the visual discrimination of movement. Journal of Studies on Alcohol. 1989;50:143–154. doi: 10.15288/jsa.1989.50.143. [DOI] [PubMed] [Google Scholar]
- Bowers D, Heilman KM. Pseudoneglect: Effects of hemispace on a tactile line bisection task. Neuropsychologia. 1980;18:491–498. doi: 10.1016/0028-3932(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Canto-Pereira LHM, David IPA, Machado-Pinheiro W, Ranvaud RD. Effects of acute alcohol intoxication on visuospatial attention. Human &Experimental Toxicology. 2007;26:311–319. doi: 10.1177/0960327106070490. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Guariglia C, Messa C, Pizzamiglio L, Zoccolotti P. Computed tomography correlates of chronic unilateral neglect. Neuropsychology. 1991;5:195–204. [Google Scholar]
- Chandler BC, Parsons OA. Altered hemispheric functioning under alcohol. Journal of Studies on Alcohol. 1977;38:381–391. doi: 10.15288/jsa.1977.38.381. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- Cooper H, Hedges LV. The handbook of research synthesis. New York: Russell Sage Foundation; 1994. [Google Scholar]
- Damkot DK, Frysinger RC. Alcohol influence on hemisphere differences and signal detection thresholds. Psychopharmacology. 1978;56:173–177. doi: 10.1007/BF00431845. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Dixon MJ, Schweizer TA. Alcohol affects processing of ignored stimuli in a negative priming paradigm. Journal of Studies on Alcohol. 2000;61:571–578. doi: 10.15288/jsa.2000.61.571. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Shah NJ, Weiss PH, Halligan PW, Grosse-Ruyken M, Ziemons K, Zilles K, Freund HJ. Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology. 2000;28:1324–1331. doi: 10.1212/wnl.54.6.1324. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Zilles K. The neural basis of vertical and horizontal line bisection judgments: An fMRI study of normal volunteers. NeuroImage. 2001;14:S59–67. doi: 10.1006/nimg.2001.0819. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Toni I, Zilles K. Task instructions influence the cognitive strategies involved in line bisection judgments: Evidence from modulated neural mechanisms revealed by fMRI. Neuropsychologia. 2002;40:119–130. doi: 10.1016/s0028-3932(01)00087-2. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, McCourt ME, Javitt DC. Parietal control of visuospatial attention: Line bisection judgments evaluated with high-density electrical mapping and source analysis. NeuroImage. 2003;19:710–726. doi: 10.1016/s1053-8119(03)00057-0. [DOI] [PubMed] [Google Scholar]
- Guedry FE, Jr, Gilson RD, Schroeder DJ, Collins WE. Some effects of alcohol on various aspects of oculomotor control. Aviation, Space, and Environmental Medicine. 1975;46:1008–1013. [PubMed] [Google Scholar]
- Hamilton P, Copeman A. The effect of alcohol and noise on components of a tracking and monitoring task. British Journal of Psychology. 1970;61:149–156. doi: 10.1111/j.2044-8295.1970.tb01232.x. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Van Den Abell T. Right hemisphere dominance for attention: The mechanism underlying hemispheric asymmetries of inattention (neglect) Neurology. 1980;30:327–330. doi: 10.1212/wnl.30.3.327. [DOI] [PubMed] [Google Scholar]
- Hill JC, Toffolon G. Effect of alcohol on sensory and sensorimotor visual functions. Journal of Studies on Alcohol. 1990;51:108–113. doi: 10.15288/jsa.1990.51.108. [DOI] [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Ethanol impairs saccadic and smooth pursuit eye movements without producing self-reports of sedation. Alcoholism: Clinical and Experimental Research. 1999;23:664–672. [PubMed] [Google Scholar]
- Jaaskelainen I. Alcohol, attention and event-related brain potentials. Psykologia. 1996;31:191–193. [Google Scholar]
- Jaaskelainen IP, Schroger E, Naatanen R. Electrophysiological indices of acute effects of ethanol on involuntary attention shifting. Psychopharmacology. 1999;141:16–21. doi: 10.1007/s002130050801. [DOI] [PubMed] [Google Scholar]
- Jewell G, McCourt ME. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38:93–110. doi: 10.1016/s0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Johnston KD, Timney B. Effects of acute ethyl alcohol consumption on a psychophysical measure of lateral inhibition in human vision. Vision Research. 2008;48:1539–1544. doi: 10.1016/j.visres.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Jones AW, Beylich KM, Bjorneboe A, Ingum J, Morland J. Measuring ethanol in blood and breath for legal purposes: Variability between laboratories and between breath-test instruments. Clinical Chemistry. 1992;38:743–747. [PubMed] [Google Scholar]
- Kerkhoff G. Spatial hemineglect in humans. Progress in Neurobiology. 2001;63:1–27. doi: 10.1016/s0301-0082(00)00028-9. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M. The control of attention by interaction between the cerebral hemispheres. In: Kornblum S, editor. Attention and performance, IV. New York: Academic Press; 1973. pp. 239–256. [Google Scholar]
- Kinsbourne M. Orientational bias model of unilateral neglect: Evidence from Attentional gradients within hemispace. In: Robertson IH, Marshall JC, editors. Unilateral neglect: Clinical and experimental studies. Hove, U.K.: Lawrence Erlbaum Associates; 1993. pp. 63–86. [Google Scholar]
- Kostandov EA, Arsumanov YL, Genkina OA, Restchikova TN, Shostakovich GS. The effects of alcohol on hemispheric functional asymmetry. Journal of Studies on Alcohol. 1982;43:411–426. doi: 10.15288/jsa.1982.43.411. [DOI] [PubMed] [Google Scholar]
- Lamb MR, Robertson LC. Effect of acute alcohol on attention and the processing of hierarchical patterns. Alcoholism: Clinical and Experimental Research. 1987;11:243–248. doi: 10.1111/j.1530-0277.1987.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Landauer AA, Howat P. Low and moderate alcohol doses, psychomotor performance and perceived drowsiness. Ergonomics. 1983;26:647–657. doi: 10.1080/00140138308963386. [DOI] [PubMed] [Google Scholar]
- Leigh G, Tong JE, Campbell JA. Effects of ethanol and tobacco on divided attention. Journal of Studies on Alcohol. 1977;38:1233–1239. doi: 10.15288/jsa.1977.38.1233. [DOI] [PubMed] [Google Scholar]
- Levin JM, Ross MH, Mendelson JH, Kaufman MJ, Lange N, Maas LC, Mello NK, Cohen BM, Renshaw PF. Reduction in BOLD fMRI response to primary visual stimulation following alcohol ingestion. Psychiatry Research: Neuroimaging. 1998;82:135–146. doi: 10.1016/s0925-4927(98)00022-5. [DOI] [PubMed] [Google Scholar]
- Lewis EG, Dustman RE, Beck EC. The effects of alcohol on sensory phenomena and cognitive and motor tasks. Quarterly Journal of Studies on Alcohol. 1969;30:618–633. [PubMed] [Google Scholar]
- MacArthur RD, Sekuler R. Alcohol and motion perception. Perception & Psychophysics. 1982;31:502–505. doi: 10.3758/bf03204860. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Freeman P, Tahmahkera-Stevens C, Chaussee M. The influence of unimanual response on pseudoneglect magnitude. Brain &Cognition. 2001b;45:52–63. doi: 10.1006/brcg.2000.1255. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M. Stimulus modulation of pseudoneglect: Influence of line geometry. Neuropsychologia. 2000;38:520–524. doi: 10.1016/s0028-3932(99)00085-8. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M. Asymmetries of visuospatial attention are modulated by viewing distance and visual field elevation: Pseudoneglect in peripersonal and extrapersonal space. Cortex. 2001;36:715–732. doi: 10.1016/s0010-9452(08)70548-3. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Jewell G. Visuospatial attention in line bisection: Stimulus modulation of pseudoneglect. Neuropsychologia. 1999;37:843–855. doi: 10.1016/s0028-3932(98)00140-7. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Olafson C. Cognitive and perceptual influences on visual line bisection: Psychometric and chronometric analyses of pseudoneglect. Neuropsychologia. 1997;35:369–380. doi: 10.1016/s0028-3932(96)00143-1. [DOI] [PubMed] [Google Scholar]
- McCourt ME. Performance consistency of normal observers in forced-choice tachistoscopic visual line bisection. Neuropsychologia. 2001;39:1065–1076. doi: 10.1016/s0028-3932(01)00044-6. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Butler J. The Influence of viewing eye on pseudoneglect magnitude. Journal of the International Neuropsychological Society. 2001a;7:391–395. doi: 10.1017/s1355617701003137. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Reuter-Lorenz PA. A common origin for the effects of unilateral cueing and line geometry in the modulation of pseudoneglect. Cortex. 2005;41:499–511. doi: 10.1016/s0010-9452(08)70190-4. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Slater J. Centripetal versus centrifugal bias in visual line bisection: Focusing attention on two hypotheses. Frontiers in Bioscience. 2000;5:d58–71. doi: 10.2741/a496. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Shpaner M, Javitt DC, Foxe JJ. Hemispheric asymmetry and callosal integration of visuospatial attention in schizophrenia: A tachistoscopic line bisection study. Schizophrenia Research. 2008;102(1–3):189–196. doi: 10.1016/j.schres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamee JE, Tong JE, Piggins DJ. Effect of alcohol on velocity perceptions: I. Stimulus velocity and change in performance over time. Perceptual &Motor Skills. 1980;51:779–785. doi: 10.2466/pms.1980.51.3.779. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Attentional networks, confusional states, and neglect syndromes. In: Mesulam M-M, editor. Principles of behavioral and cognitive neurology. 2. Oxford: Oxford UniversityPress; 2000. pp. 174–256. [Google Scholar]
- Mortimer RG. Effect of low blood alcohol concentrations in simulated day and night driving. Perceptual & Motor Skills. 1963;17:399–408. doi: 10.2466/pms.1963.17.2.399. [DOI] [PubMed] [Google Scholar]
- Moser A, Heide W, Kompf D. The effect of oral ethanol consumption on eye movements in healthy volunteers. Journal of Neurology. 1998;245:542–550. doi: 10.1007/s004150050240. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, Depry D. Differential effect of alcohol on auditory vigilance and divided attention tasks. Quarterly Journal of Studies on Alcohol. 1968;29:54–63. [Google Scholar]
- Moskowitz H, Sharma S. Effects of alcohol on peripheral vision as a function of attention. Human Factors. 1974;16:174–180. doi: 10.1177/001872087401600209. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, Burns MM, Williams AF. Skills performance at low blood alcohol levels. Journal of Studies on Alcohol. 1985;46:482–485. doi: 10.15288/jsa.1985.46.482. [DOI] [PubMed] [Google Scholar]
- Na DL, Adair JC, Hye Choi S, Won Seo D, Kang Y, Heilman KM. Ipsilesional versus contralesional neglect depends on attentional demands. Cortex. 2000;36:455–467. doi: 10.1016/s0010-9452(08)70532-x. [DOI] [PubMed] [Google Scholar]
- Nawrot M, Nordenstrom B, Olson A. Disruption of eye movements by ethanol intoxication affects perception of depth from motion parallax. Psychological Science. 2004;15:858–865. doi: 10.1111/j.0956-7976.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- Neill RA, Delahunty AM, Fenelon B. Discrimination of motion in depth trajectory following acute alcohol ingestion. Biological Psychology. 1990;31:1–22. doi: 10.1016/0301-0511(90)90075-8. [DOI] [PubMed] [Google Scholar]
- Newman D, Speake DJ, Armstrong PJ, Tiplady B. Effects of ethanol on control of attention. Human Psychopharmacology. 1997;12:235–241. [Google Scholar]
- Nicholls MER, Loftus A, Mayer K, Mattingley JB. Things that go bump in the right: The effect of unimanual activity on rightward collisions. Neuropsychologia. 2007;45:1122–1126. doi: 10.1016/j.neuropsychologia.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Nicholls MER, Loftus AM, Orr CA, Barre N. Rightward collisions and their association with pseudoneglect. Brain & Cognition. 2008 doi: 10.1016/j.bandc.2008.04.003. (in press) [DOI] [PubMed] [Google Scholar]
- Nicholson ME, Andre JT, Tyrrell RA, Wang M, Leibowitz HW. Effects of moderate dose alcohol on visual contrast sensitivity for stationary and moving targets. Journal of Studies on Alcohol. 1995;56:261–266. doi: 10.15288/jsa.1995.56.261. [DOI] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. A metabotropic hipotesis for etanol sensitivity of GABAergic and glutamatergic central synapses. In: Liu Y, Hunt WA, editors. The drunken synapse: Studies of alcohol related disorders. New York: Plenum Publishers; 2000. pp. 135–144. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pearson P, Timney B. Effects of moderate blood alcohol concentrations on spatial and temporal contrast sensitivity. Journal of Studies on Alcohol. 1998;59:163–175. doi: 10.15288/jsa.1998.59.163. [DOI] [PubMed] [Google Scholar]
- Pearson P, Timney B. Differential effects of alcohol on rod and cone temporal processing. Journal of Studies on Alcohol. 1999;60:879–883. doi: 10.15288/jsa.1999.60.879. [DOI] [PubMed] [Google Scholar]
- Poppel E, Steinbach T. Selective vulnerability of the two cerebral hemispheres under alcohol. Naturwissenschaften. 1986;73:327–329. doi: 10.1007/BF00451482. [DOI] [PubMed] [Google Scholar]
- Post RB, Chaderjian MR, Maddock RJ. Effects of alcohol on exogenous precueing of attention. Journal of Studies on Alcohol. 2000;61:232–238. doi: 10.15288/jsa.2000.61.232. [DOI] [PubMed] [Google Scholar]
- Post RB, Lott LA, Maddock RJ, Beede JI. An effect of alcohol on the distribution of spatial attention. Journal of Studies on Alcohol. 1996;57:260–266. doi: 10.15288/jsa.1996.57.260. [DOI] [PubMed] [Google Scholar]
- Rhodes LE, Obitz FW, Creel D. Effect of alcohol and task on hemispheric asymmetry of visually evoked potentials in man. Electroencephalography and Clinical Neurophysiology. 1975;38:561–568. doi: 10.1016/0013-4694(75)90156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IH, Halligan PW. Spatial neglect: A clinical handbook for diagnosis and treatment. Philadelphia: Psychology Press; 1999. [Google Scholar]
- Rohrbaugh JW, Stapleton JM, Parasuraman R, Zubovic EA, Frowein HW, Varner JL, Adinoff B, Lane EA, Eckardt MJ, Linnoila M. Dose-related effects of ethanol on visual sustained attention and event-related potentials. Alcohol. 1987;4:293–300. doi: 10.1016/0741-8329(87)90026-7. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh JW, Stapleton JM, Parasuraman R, Frowein HW, Adinoff B, Varner JL, Zubovic EA, Lane EA, Eckardt MJ, Linnoila M. Alcohol intoxication reduces visual sustained attention. Psychopharmacology. 1988;96:442–446. doi: 10.1007/BF02180021. [DOI] [PubMed] [Google Scholar]
- Roquelaure Y, Gargasson LE, Kupper S, Girre C, Hispard E, Dally S. Alcohol consumption and visual contrast sensitivity. Alcohol and Alcoholism. 1995;30:681–685. [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Strasburger H, Warzel H, Sabel BA. Acute effects of alcohol on divided and covert attention in men. Psychopharmacology. 2001;154:61–69. doi: 10.1007/s002130000603. [DOI] [PubMed] [Google Scholar]
- Stenberg G, Sano M, Rosen I, Ingvar DH. EEG topography of acute ethanol effects in resting and activated normals. Journal of Studies on Alcohol. 1994;55:645–656. doi: 10.15288/jsa.1994.55.645. [DOI] [PubMed] [Google Scholar]
- Ticku MK, Mehta AK. Effects of alcohol on GABA mediated neurotransmission. In: Kranzler HR, editor. Pharmacology of alcohol abuse. New York: Springer- Verlag; 1995. pp. 103–119. [Google Scholar]
- Turnbull OH, McGeorge P. Lateral bumping: A normal-subject analog to the behaviour of patients with hemispatial neglect? Brain &Cognition. 1998;37:31–33. [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Research. 1988;24:201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Vallar G, Perani D. The anatomy of spatial neglect in humans. In: Jeannerod M, editor. Neurophysiological and neuropsychological aspects of spatial neglect. Amsterdam: North Holland; 1987. pp. 235–258. [Google Scholar]
- Virsu V, Kykka T, Vahvelainen M. Reports from the Institute of Psychology. The University of Helsinki; 1973. Effects of alcohol on inhibition in the human visual system: 1. Flicker and apparent spatial frequency. [Google Scholar]
- Vuchinich RE, Sobell MB. Empirical separation of physiologic and expected effects of alcohol on complex perceptual motor performance. Psychopharmacology. 1978;60:81–85. doi: 10.1007/BF00429183. [DOI] [PubMed] [Google Scholar]
- Wallgren H, Barry H. Actions of alcohol. New York: Elsevier; 1970. [Google Scholar]
- Watten RG, Lie I. Visual functions and acute ingestion of alcohol. Ophthalmic and Physiological Optics. 1996;16:460–466. [PubMed] [Google Scholar]
- Watten RG, Magnussen S, Greenlee MW. Spatial-frequency discrimination, brain lateralization, and acute intake of alcohol. Perception. 1998;27:729–736. doi: 10.1068/p270729. [DOI] [PubMed] [Google Scholar]
- Wegner AJ, Fahle M. Alcohol and visual performance. Program in Neuro- Psychopharmacology & Biological Psychiatry. 1999;23:465–482. doi: 10.1016/s0278-5846(99)00009-3. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Mesulam MM. Right cerebral dominance in spatial attention. Further evidence based on ipsilateral neglect. Archives of Neurology. 1987;44:621–625. doi: 10.1001/archneur.1987.00520180043014. [DOI] [PubMed] [Google Scholar]
- Weiss PH, Marshall JC, Wunderlich G, Tellmann L, Halligan PW, Freund HJ, Zilles K, Fink GR. Neural consequences of acting in near versus far space: a physiological basis for clinical dissociations. Brain. 2000;123:2531–2541. doi: 10.1093/brain/123.12.2531. [DOI] [PubMed] [Google Scholar]
- Wendt PE, Risberg J, Stenberg G, Rosen I, Ingvar DH. Ethanol reduces asymmetry of visual rCBF responses. Journal of Cerebral Blood Flow and Metabolism. 1994;14:963–973. doi: 10.1038/jcbfm.1994.129. [DOI] [PubMed] [Google Scholar]
- Wilson G, Mitchell R. The effect of alcohol on the visual and ocular motor systems. Australian Journal of Ophthalmology. 1983;11:315–319. doi: 10.1111/j.1442-9071.1983.tb01099.x. [DOI] [PubMed] [Google Scholar]
- Young JR. Blood alcohol concentration and reaction time. Quarterly Journal of Studies on Alcohol. 1970;31:823–831. [PubMed] [Google Scholar]
- Zulauf M, Flammer J, Signer C. Short-term influence of alcohol on spatial brightness contrast sensitivity. Ophthalmologica. 1988;197:159–165. doi: 10.1159/000309937. [DOI] [PubMed] [Google Scholar]