Abstract
The purpose of this research project was to develop nanoparticles with improved targeting, adhesion, and cellular uptake to activated or inflamed endothelial cells (ECs) under physiological flow conditions. Our hypothesis is that by mimicking platelet binding to activated ECs through the interaction between platelet glycoprotein Ibα (GP Ibα) and P-selectin on activated endothelial cells, GP Ibα-conjugated nanoparticles could exhibit increased targeting and higher cellular uptake in injured or activated endothelial cells under physiological flow conditions. To test this hypothesis, fluorescent carboxylated polystyrene nanoparticles were selected for the study as a model particle due to its narrow size distribution as a “proof-of-concept”. Using confocol microscopy, fluorescent measurement, and protein assays, cellular uptake properties were characterized for these polystyrene nanoparticles. The study also found that conjugation of 100 nm polystyrene nanoparticles with glycocalicin (the extracellular segment of GP Ibα) significantly increased the particle adhesion on P-selectin-coated surfaces and cellular uptake of nanoparticles by activated endothelial cells under physiological flow conditions. The results demonstrate that these novel endothelial-targeting nanoparticles could be the first step towards developing a targeted and sustained drug delivery system that can improve shear-regulated particle adhesion and cellular uptake.
INTRODUCTION
Recent studies have focused on targeting endothelium with nanoparticles in order to deliver drug and/or gene therapy for a variety of pathological conditions in the vascular system because of its large population and proximity to blood flow.1–6 Under diseased conditions such as thrombosis, inflammation, and restenosis, endothelial cells (ECs) become activated and express endothelial cell adhesion molecules (ECAMs) such as P-selectin and E-selectin.3–5,7,8 Using ECAM ligands has been considered for effective targeting of diseased ECs. For instance, immunoliposomes and microparticles coated with ligands (sLex, PSGL-1), and humanized monoclonal antibodies (mAbs) bound to P-selectin highly expressed on activated ECs, have demonstrated varying degrees of success.4,7–12
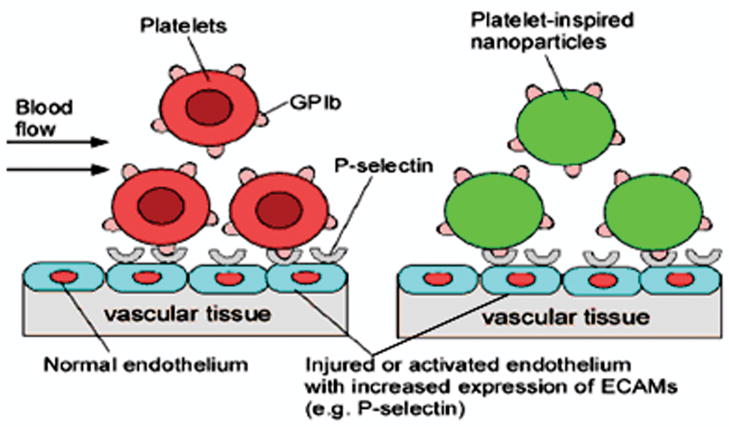
The long-term goal of our research is to develop biodegradable nanoparticles that can target and deliver therapeutic agents to treat injured and inflamed ECs after angioplasty and/or stenting treatments. Using carboxylated polystyrene (non-biodegradable) nanoparticles as model particles, an effective targeting strategy will be determined in this study. These nanoparticles have a uniform composition, narrow size distribution, and are stable. These properties of the carboxylated polystyrene particles will allow us to investigate the adhesion and uptake properties without concern for the possibility that the particle size and degradation influence on the targeting strategy would affect the outcome. GP Ibα was selected as an ideal ligand for conjugating onto the surface of nanoparticles in order to increase their adhesiveness under high shear conditions. GP Ibα is well-known for its role on the platelet adhesion onto the vascular wall in the high shear stress regions.13,14 It can also serve as a targeting ligand that binds specifically to P-selectin expressed on activated ECs.13,15 These “platelet-mimicking nanoparticles” should specifically adhere onto damaged or activated ECs under conditions of high shear stress, inducing cellular retention and uptake of nanoparticles (Figure 1).
Figure 1.
Platelet-mimicking Nanoparticles for Targeting Dysfunctional Endothelium
Understanding the cellular uptake process of polystyrene nanoparticles and characterizing interaction of platelet-mimicking polystyrene nanoparticles with P-selectin and activated ECs is needed in order to reach a long-term goal to develop drug-loaded endothelial targeting nanoparticles. Although previous studies successfully characterized cellular uptake of polystyrene nanoparticles in the human colon adenocarcinoma cell line (Caco-2) and umbilical vein endothelial cells (HUVECs),1,16,17 these cell types are different from the human aortic endothelial cells (HAECs) used in this study. Since we want to target restenosis after vascular intervention, HAECs are a better choice for in vitro cell culture characterization. In this study, the cellular uptake properties of polystyrene nanoparticles of various sizes, doses, and incubation times were investigated using HAECs. The effects of shear stress on the cellular uptake of 100-nm size nanoparticles by HAECs were also studied using the parallel plate flow chamber system. The cellular uptake of these particles in HAECs was then determined using both fluorescent intensity measurements and confocal microscopy imaging of cells after the incubation time. In addition, purified glycocalicin (the extracellular segment of platelet GP Ibα) was conjugated to the polystyrene nanoparticles to produce GP Ib-nanoparticles. The experiments were then performed to determine the effects of GP Ib conjugation on particle adhesion on the P-selectin coated surfaces and cellular uptake of these nanoparticles in activated HAECs under physiological flow conditions.
MATERIALS AND METHODS
Materials
Fluoresbrite® YG Carboxylate Polystyrene particles were purchased from Polysciences, Inc. (Warrington, PA). Chemicals, if not specified, were purchased from Sigma-Aldrich (St. Louis, MO). HAECs and low serum growth supplement (LSGS, 2 % fetal bovine serum, hydrocortisone (1 μg/ml), human epidermal growth factor (10 ng/ml), basic fibroblast growth factor (3 ng/ml), and heparin (10 μg/ml)) were purchased from Cascade Biologics (Portland, OR). Cell culture media, supplements, and buffers, including Medium 199 (M199), trypsin, fetal bovine serum (FBS), penicillin-streptomycin, and phosphate buffered saline (PBS) were purchased from Invitrogen Corp. (Carlsbad, CA).
Cell Culture
HAECs were grown in M199 supplemented with 1% penicillin-streptomycin and LSGS (complete M199). Upon reaching confluence, cells were passaged or used for experiments. Cells up to passage 9 were used for the study.
Cellular Uptake of Polystyrene Nanoparticles by Human Aortic Endothelial Cells
To determine cellular uptake of nanoparticles, HAECs were seeded onto 24-well plates at a density of 30,000 cells/well and allowed to grow for 2 days. Since high serum media would contribute to the variation in the assessment of particle uptake in HAECs,18 we chose to use low serum media in all particle uptake studies. The first study was conducted to determine the effects of particle size on cellular particle uptake in HAECs. Particles ranging in size from 100–1000 nm were suspended in low serum growth medium at the concentration of 100 μg/ml. Medium from the 24-well plate was replaced with the particle suspensions, and cells were allowed to incubate with the nanoparticles for one hour. The second study was to optimize nanoparticle concentration. Nanoparticle (100 nm in size) solutions (100–800 μg/ml) were prepared in low serum growth medium and added to HAECs for one hour. The third study was to evaluate the effect of incubation time on cellular uptake of nanoparticles (100-nm in size). Cells were incubated with 100 μg/ml nanoparticle solution for various time periods up to 6 hrs. Cells without nanoparticle solutions served as controls.
An additional study was performed to investigate the effects of shear stress on cellular uptake of nanoparticles by HAECs. Cells were seeded onto pre-etched glass slides at a density of 105 cells/cm2. Upon reaching confluence, cells on glass slides were exposed to either 1 or 5 dyne/cm2 of media containing 100-nm nanoparticles at concentration of 100 μg/ml for 30 minutes using the parallel plate flow chamber system as described previously.19 The parallel flow chamber was chosen over other flow systems because of its ability to produce constant levels of shear stress. Cells in static condition served as the control.
Quantification of nanoparticle uptake by HAECs using a fluorometer
After experiments, cells were washed carefully at least three times with cold PBS to remove any remaining nanoparticles. After washing, 1ml of 1% Triton® X-100 was added to each cell sample and incubated for one hour in order to lyse the cells. Twenty-five microliters of cell lysate from each well was used to determine total cell protein content using the BCA protein assay (Pierce) following the manufacturer’s instructions. Total protein concentration in each sample was used for normalizing cellular uptake of nanoparticles. To quantify nanoparticle uptake by HAECs, the fluorescent intensity in cell lysates was measured at EM 480 nm/EX 510 nm (VersaFluor™, Bio-Rad Laboratories, Hercules, CA). A standard curve was obtained by serial dilution of same stock nanoparticle solutions in 1% Triton® X-100, and the fluorescent intensities were measured using the same filters. The nanoparticle uptake by HAECs was calculated by normalizing the nanoparticle concentration in each cell lysate sample with the total cellular protein, which correlates to the number of cells in the sample. For size uptake, we seeded HAEC’s at different seeding densities and generated a standard plot of varying number of known cells to a standard of known BSA. We then calculated the amount of protein per 1000 cells and converted the cellular uptake per total cellular protein to the cellular uptake per 1000 cells.
Evaluation of nanoparticle uptake using confocal microscopy
After experiments, cover slips were washed with cold PBS, followed by the addition of cold FM® 4–64 FX (5 μg/ml of Texas-Red® dye, Invitrogen) in PBS for 10 min to stain cell plasma membranes. HAECs were then imaged using a confocal laser scanning microscope (Carl Zeiss LSM Meta 510, Goettingen, Germany) equipped with FITC and RITC filters (Ex(λ) 488 nm, Em(λ) 543 nm). Images of HAECs were taken using a fast scan option to section the cells. Slice thickness was set at 1 μm, with an average of 18 slices taken per image. The images were then analyzed using Carl Zeiss LSM Image Browser (version 3.5).
Nanoparticle conjugation with glycocalicin
Carboxylated nanoparticles, 100 nm in size, were conjugated with glycocalicin using carbodiimide chemistry and avidin-biotin affinity. First, glycocalicin were biotinylated using the Biotin-X-NHS Kit (EMD Biosciences, Inc., San Diego, CA) following the manufacturer’s instructions. Second, polystyrene nanoparticles were added to a 15 mg/ml EDC solution in 0.1 M MES buffer, pH 4.75 and incubated at room temperature for four hours to ensure carboxyl group activation. After four hours, 500 μg of avidin (EMD Biosciences, Inc.) was added to the nanoparticle solution and allowed to interact overnight in 0.1 M sodium bicarbonate solution (pH 8.5). Biotinylated glycocalicin (120 μl of 50 μg/ml) described in the first step was added to the avidin-conjugated polystyrene nanoparticle solution and reacted at room temperature under gentle agitation, for one hour. The nanoparticle solution was then dialyzed against 0.1 M PBS for three hours to remove any unreacted materials. Great care was taken to avoid exposure of the nanoparticles to light throughout the entire procedure. To confirm the conjugation of ligands onto nanoparticles, 100 μl of 30 μg/ml primary mouse antibody monoclonal against glycocalicin (HIP1, BioLegend), was added to glycocalicin-conjugated nanoparticles in PBS. A fluorescent (red) secondary antibody (anti-mouse IgG1, BioLegend) was added to the nanoparticle solution and incubated for one hour. After washing, nanoparticles were analyzed using confocal microscopy method.
Preparation of P-selectin Coated Slides and activated ECs
To prepare P-selectin coated surfaces, glass slides were incubated with 500 μl of 20 μg/ml P-selectin (R&D Systems) for four hours at 37 °C, followed by one hour of incubation with a 1% BSA solution in PBS to block any nonspecific binding sites. Half the slides were then further incubated with P-selectin antibodies for one hour at room temperature in order to serve as a negative control. The slides were then washed gently with a 0.9% NaCl solution to remove any unbound P-selectin or antibody. To prepare activated HAECs, HAECs seeded on glass slides as described earlier were treated with 25 mM histamine for 12 minutes at room temperature to induce P-selectin expression on HAECs. Stimulated (activated) cells were used immediately in flow chamber experiments.
Effects of GPIb conjugation on particle adhesion and cellular uptake under physiological flow conditions
Slides (coated with either P-selectin or P-selectin/anti-P-selectin for surface studies and containing HAEC monolayer for cell studies) were assembled into the parallel plate flow systems.19 For non-cellular flow studies, that flow chamber was set to produce 5 dyne/cm2 of shear stress, and three types of samples were used. These samples were PBS solution consisting of control nanoparticles, GPIbα- conjugated nanoparticles, and GPIbα-conjugated nanoparticles preincubated with antibodies against GPIbα (or GPIbα mAbs). After the flow experiments, the amount of nanoparticles bound to the glass cover slides were measured using a fluorometer. The glass slides were also observed using confocal microscopy. For cell studies, the shear stress was varied between 0 dyne/cm2 to 15 dyne/cm2. The nanoparticle solutions were diluted to 100 μg/ml concentration in low serum media for cell studies. Activated HAECs seeded on the glass slides were exposed to low serum media consisting of either non-conjugated (control) or GPIb-conjugated nanoparticles under various levels of shear stress. Samples in the static condition served as controls.
Statistical Analysis
Analysis of the results was performed using ANOVA and t-tests with p < 0.05 (StatView 5.0 software, SAS Institute). Post-hoc comparisons were made using the Fisher’s least significant differences (LSD). All the results are given as mean ± SD.
RESULTS
Characteristics of cellular uptake of nanoparticles by HAECs
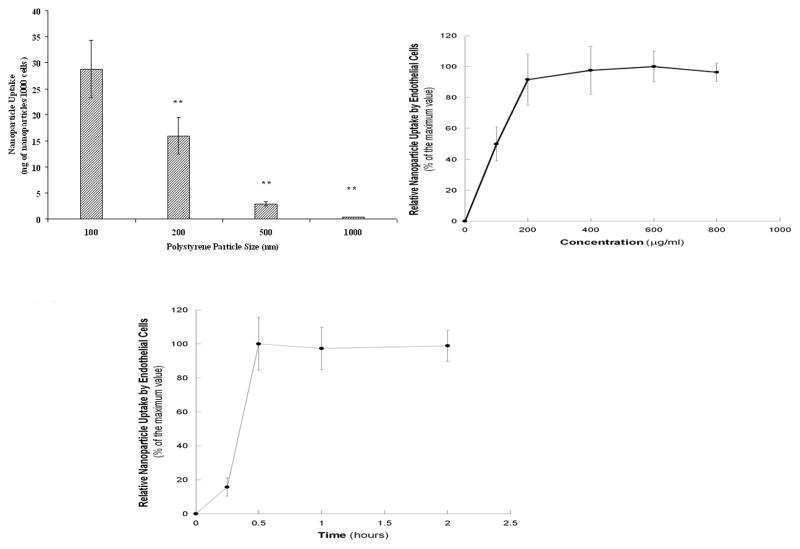
To determine the effects of nanoparticle size on cellular uptake by HAECs, experiments were conducted using polystyrene nanoparticles of varying sizes (100–1000 nm) due to their narrow size distribution. The results displayed a clear trend of decreased cellular uptake with increase in nanoparticle size. Particles of 1000 nm displayed least uptake by HAECs, while the smallest nanoparticles (100 nm) were uptaken the most (Figure 2A).
Figure 2.
Figure 2A. Effects of the particle size on the cellular uptake of polystyrene nanoparticles in ECs. Values were obtained after one hour of incubation with nanoparticle solutions and represent mean ± SD (n=6). ** indicates significant differences compared to the 100-nm nanoparticle samples (p<0.001).
Figure 2B. Effect of concentration on the cellular uptake of 100-nm polystyrene nanoparticles in ECs. Values were obtained after one hour of incubation with nanoparticle solutions and represent mean ± SD (n=6).
Figure 2C. Effect of the incubation time on the cellular uptake of 100-nm polystyrene nanoparticles in ECs. Samples were incubated with 100 μ/ml of nanoparticle solution. Values represent mean ± SD (n=6).
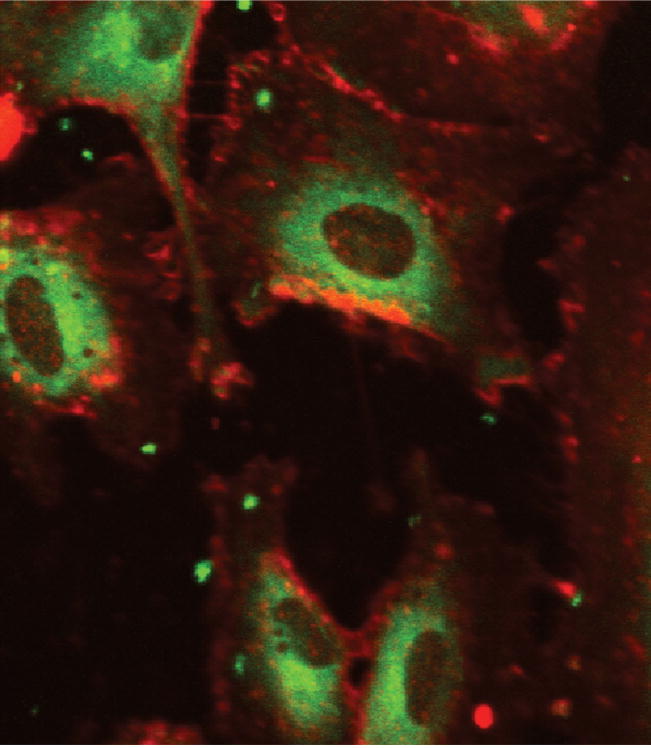
Nanoparticle uptake for 100-nm polystyrene nanoparticles displayed trends towards to the concentration and incubation time dependency. For the former, cellular uptake by HAECs reached saturation at the nanoparticle concentration of 200 μg/ml (Figure 2B). For the latter, the saturation was reached in 30 minutes for the polystyrene nanoparticles (Figures 2C). Confocal microscopy was used to confirm the uptake of particles inside the cells (Figure 3). Images at the middle point of the cell confirmed that the fluorescent nanoparticles were localized inside cells.
Figure 3.
Confocal Images of Nanoparticle Uptake in ECs. Represents cells incubated with polystyrene nanoparticles. Plasma members were dyed using Texas Red® and imaged using a RITC filter. Fluorescent nanoparticles were imaged using a FITC filter. Images represent an overlay of RITC and FITC filters and were taken at Ex(λ) 488 nm, Em(λ) 543 nm.
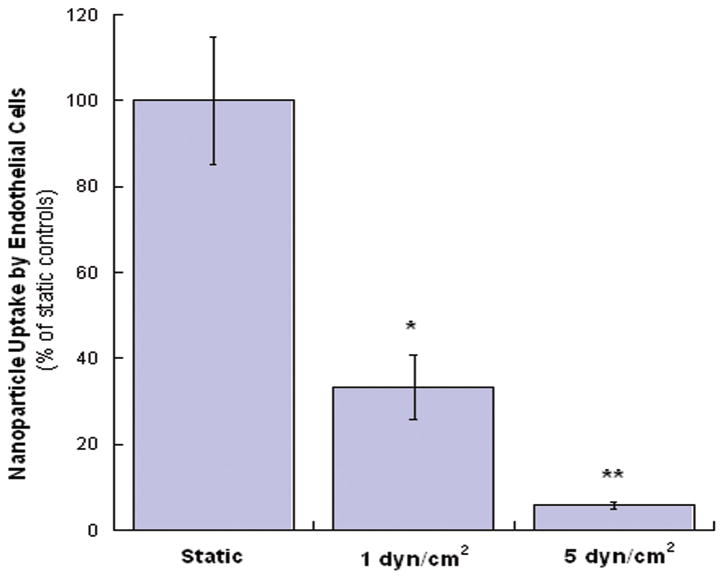
To investigate the effects of shear stress on cellular uptake of nanoparticles by HAECs, the parallel plate flow chamber system was used to generate different levels of shear stress. We found that the cellular uptake of nanoparticles was decreased with the increase of shear stress magnitude (Figure 4), indicating an inverse correlation between nanoparticle uptake and the levels of shear stress.
Figure 4.
Effects of shear stress on the cellular uptake of 100-nm nanoparticles by HAECs. Values were obtained after 30 minutes of flow with nanoparticle solutions and represent mean ± SD (n=4). *and ** indicate the significant differences compared to the static samples (p< 0.05 and p< 0.001, respectively).
Conjugation of GPIb enhanced adhesion of particles on P-selectin surfaces and cellular uptake of particles by activated HAECs
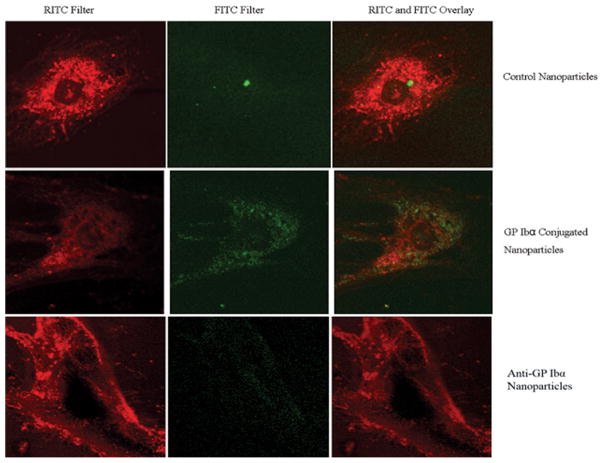
Because of the size of nanoparticles is 100 nm, below the detection limit of the available flow cytometry, the conjugation of GP Ib onto nanoparticles was determined using the Zeiss Cytoviva Microscope Imaging System (at a magnification of 10X). As shown in Figure 5, fluorescent tagged secondary antibody against GP Ib antibody was detected around nanoparticles, suggesting that glycocalicin had indeed been conjugated onto the nanoparticles.
Figure 5.
Cytoviva Images of GPIb conjugation onto 100nm polystyrene nanoparticles. the Flurocent nanoparticles were imaged using aFITC(green) filter (A) and Image (B) represents the image obtained using both the FITC and Texas Red (Red) filters. Magnification is at 10X.
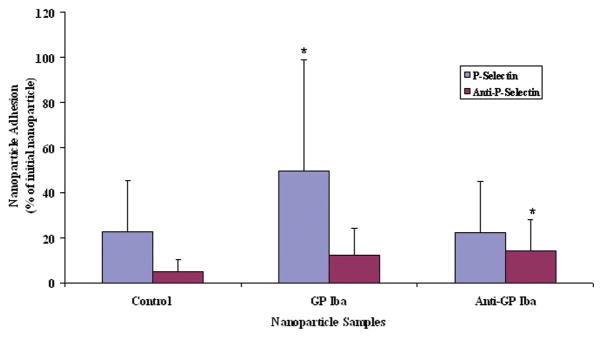
Specific interaction between P-selectin coated surfaces and GP Ibα-conjugated nanoparticles under flow conditions was further analyzed using fluorescent intensity measurements (Figure 6). Approximately 50% GP Ibα nanoparticles adhered to P-selectin coated surfaces, while about 22% of control nanoparticles and GP Ib nanoparticles pre-incubated with anti-GP Ibα antibodies adhered, respectively. The control nanoparticles adhered minimally to P-selectin and P-selectin/anti-P-selectin surfaces, indicating that non-selective binding under shear stress was minimal. Using antibodies against GPIb with GPIb-conjugated nanoparticles also showed insignificant levels of nanoparticle adhesion onto P-selectin coated surfaces, indicating the importance of GPIb binding onto P-selectin. In addition, surfaces coated with anti-P-selectin displayed no specific interactions with any of the nanoparticles. Confocal microscopy analysis also confirmed the observation using a fluorometer: the highest fluorescence detected on P-selectin coated slides with adherent GP Ibα-conjugated nanoparticles (data not shown). These results indicate that GP Ibα-conjugated nanoparticles display an enhanced adhesion onto P-selectin coated surfaces under physiological flow conditions.
Figure 6.
Nanoparticle Adhesion onto P-selection and Anti-P-selection Coated Surfaces Using Different Nanoparticle Samples. Values represent mean ± SD (n=6). *indicates the significant differences compared to the control nanoparticle samples (p< 0.05)
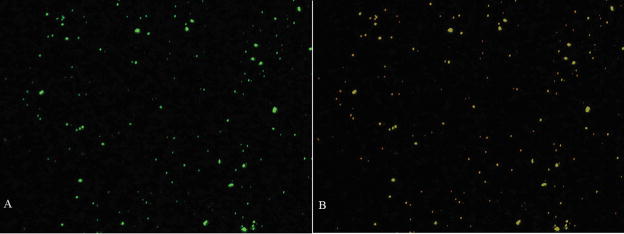
Glass slides seeded with activated HAECs were run on the flow chamber to investigate the effects of shear stress on the cellular uptake of non-conjugated and GPIb-conjugated nanoparticles by activated endothelial cells. Results showed significant cellular uptake when GP Ibα nanoparticles were perfused, while control nanoparticles displayed insignificant cellular uptake. Nanoparticles conjugated with GP Ibα displayed much higher uptake in HAECs activated with histamine as detected within cells, with a very low concentration of nanoparticles present in extracellular spaces (Figure 8). Samples were observed at the middle point of each cell using the stack imaging option, with slice thickness set at 1 μm. In contrast, unconjugated nanoparticles were mostly detected in extracellular spaces, with minimal within the cells (Figure 8). Control nanoparticles had the lowest cellular uptake under exposure to shear stress with almost no nanoparticles seen within the cells, and most nanoparticles were localized on the surface of the glass slides. Negative control samples by blocking the GP Ibα ligand with an antibody resulted in minimal cellular uptake (results not shown), indicating the specific interaction between GP Ibα and P-selectin. These observations confirm that GP Ibα adhesion with P-selectin expressed in activated HAECs is necessary for cellular uptake under conditions of fluid shear stress.
Figure 8.
Confocal Images of Cellular Uptake of Polystyrene Nanoparticles and (C) anti-GP Ibα Conjugated Nanoparticles. Plasma membranes were dyed using Texas Red® and imaged using a RITC filter. Fluorescent nanoparticles were imaged using a FITC filter. The third image (far right) represents an overlay of RITC and FITC filters. All images were taken at Ex(λ) 488 nm, Em(λ) 543 nm.
DISCUSSION
The purpose of this study was to characterize the cellular uptake of nanoparticles by endothelial cells and to investigate a new strategy to improve cellular uptake and targeting of nanoparticles in activated or inflamed HAECs. Particle uptake studies showed that decrease in particle size increased cellular uptake. In addition, under static conditions, optimal nanoparticle dosage was found to be 200 μg/ml while optimal incubation time was found to be 30 minutes for 100-nm polystyrene nanoparticles. Our results have shown that nanoparticles conjugated with the GP Ibα significantly increased adhesion of nanoparticles to P-selectin coated surfaces. Cellular uptake studies conducted under fluid shear stress also displayed increased nanoparticle uptake and targeting ability when HAECs were activated with histamine to express P-selectin. Results from our endothelial cell uptake and nanoparticle targeting characterization studies suggest that the GP Ibα can increase nanoparticle targeting abilities and endothelial cell uptake under conditions of fluid shear stress.
The cellular uptake of polystyrene nanoparticles in HAECs was investigated in order to determine the effects of particle size, concentration, incubation time, and levels of shear stress. Carboxylated polystyrene particles were chosen as they are frequently used as model particles (for “a proof-of-concept”) to investigate drug targeting strategies because of their narrow size distribution, uniform composition, and non-degradation property.20,21 Cellular uptake of polystyrene nanoparticles by HAECs decreased as the size of nanoparticles increased (Figure 3). Similar to HAECs, cellular uptake of polystyrene particles (ranging from 1 μm to 6 μm) by both leukocytes and macrophages reduced with an increase in the particle size.22 In contrast to this study, Tabata and Ikada found that the maximal cellular uptake of polystyrene particles (ranging from 0.5 to 5 μm in size) in mouse peritoneal macrophages was reached when the particle size was 2 μm.23 Yet these studies use particles range at a larger size and different cell types compared to our studies. For cellular uptake of nanoparticles as the same range as our studies, previous studies have shown that nanoparticles below 200 nm are internalized in Caco-216,17 and HUVEC cells.16,24–26 These studies also found that cells uptake or internalize nanoparticles through receptor-mediated endocytosis process.16,24–26 Understanding the mechanism of cellular nanoparticle uptake plays a large role in intracellular trafficking, thus, greatly modulating the effectiveness of any internalized drug.18,27–30 Results from our studies and previous studies again confirm the advantage of nanoparticles compared to microparticles for intracellular drug delivery; by showing that HAECs prefer to uptake only small size particles. Other advantages of nanoparticles include little or no local inflammation and less risk of arterial occlusion.31,32
Beside size-dependence, the uptake of nanoparticles in HAECs was also dose- and incubation time-dependent. HAEC uptake of polystyrene nanoparticles was saturated at low concentrations (200 μg/ml). Other studies using similarly sized polystyrene nanoparticles (100 nm) in Caco-2 cells also displayed cellular uptake proportional to dosage, but reached saturation at higher particle concentrations (500 μg/ml).16,17 The difference in results between this study and previous studies may be due to different cell types. Nevertheless, our result, combined with previous data, suggest that a drug can be delivered using nanoparticles with particle concentration-based dosages. In fact, increasing nanoparticle concentration in the infusate has been shown to significantly increase arterial uptake of drug (anti-proliferative reagents)-loaded nanoparticles, leading to the increased levels of anti-proliferative reagents at the arterial wall using acute dog models.33–35
In addition to dose-dependence, the uptake of nanoparticles by cells is also dependent on the incubation or exposure time. Previous studies using Caco-2 cells displayed a similar trend in cellular uptake, but reached saturation after two hours of incubation.16,17 In contrast, our study found that the uptake in endothelial cells was saturated after 30 minutes of incubation. These results might imply that particle uptake in endothelial cells is more active, requiring lower incubation times, and reaching the saturation limit more rapidly than Caco-2 cells. Interestingly, in vivo studies using dogs have found that the nanoparticle arterial uptake was two-fold for repeated short infusions of nanoparticle suspension (15 s × 4) than a single prolonged infusion (60 s).34 These results indicate that optimized concentration and time could be used to enhance the particle delivery and reduce the drug side effects.
Besides the particle composition, particle size, and cell type, cellular uptake of nanoparticles can also be affected by other factors such as surface properties of nanoparticles, high concentration of serum, and levels of shear stress. For instance, studies have shown that cells do not uptake polystyrene nanoparticles as efficiently as PLGA nanoparticles, possibly due to increased surface hydrophobicity or charges.17,24 In addition to particle surface properties, media with high serum content may also result in excessive cell exocytosis of nanoparticles, due to the energy dependent nature of the process.18 The level of shear stress is another factor that can affect particle adhesion to and uptaken by endothelial cells. Similar to our observation, others have found that adhesion of nanoparticles on surfaces and/or endothelial cells is inversely correlated to the levels of shear stress.36,37
To enhance recruitment of nanoparticles to endothelial cells under physiological flow conditions, previous studies use “endothelial targeting particles” coated with humanized antibodies against E- and P-selectins36,37 and especially “leukocyte-inspired” particles.4,7–12 Micro- and nano-particles conjugated with antibodies against P-selectin,36,37 the selectin ligand, sialyl Lewisx (sLex)4,8,10–12,38 and LFA-139 were found to adhere to surfaces coated with P-selectin, E-selectin and ICAM-1 and activated endothelial cells. Similar to leukocyte-inspired nanoparticles, our “novel endothelial-targeting nanoparticles” that mimic the adhesion of platelets on activated endothelial cells were able to adhere to P-selectin coated surfaces under physiological flow conditions. The nanoparticles may also adhere better with von Willebrand factor, the ligand of GP Ibα, deposited on injured vessel wall and expressed P-selectin on activated HAECs compared to “leukocyte-inspired nanoparticles” due to the higher binding strength of the platelet ligands under high shear stress conditions.15,40–42 Of interests in the leukocyte-mimicking approaches, Eniola and Hammer developed novel endothelial targeting nanoparticles that mimic leukocyte adhesion onto endothelial cells using multiple-receptor targeting: the selectin ligand sLex and an antibody against intercellular cell adhesion molecule-1 (ICAM-1).4 These couple receptors have been involved in the initial dynamic interaction (sLex) and the firm arrest of leukocytes on the endothelium (ICAM-1). Therefore, nanoparticles with these double ligand bindings would have a great selective adhesion onto the inflamed endothelium.
One final note for this study is its limitations. There is a concern regarding the GP Ibα and its role in mediating adhesion onto vWF surfaces. Previous discussions have defined the role of GP Ibα in adhesion of platelets towards vWF in vitro and in vivo.13,14,43–45 Our current study did not include vWF; however, its binding action with GP Ibα can occur under much higher fluid shear stress than found in P-selectin mediated adhesion. Other studies have successfully characterized this vWF mediated adhesion under shear stress,13,14,43–45 while less work has been done on the role of P-selectin. Another limitation of this study is that nanoparticles made of polystyrene are non-degradable and can not be used as a real drug delivery carrier. Finally, the relationship between the bond forces upon the GP Ibα-vWF and/or GP Ibα P-selectin interaction and shear stress with the size of the particles has not been investigated in this study. Although our studies have these shortcomings, using polystyrene nanoparticles as model nanoparticles to confirm “a-proof-of-concept” is necessary to help us further in our next step, to design drug carriers that can selectively target endothelium under physiological flow conditions. Future work on this project will include studies involving vWF coated surfaces and our GP Ibα-conjugated and drug-loaded biodegradable nanoparticles.
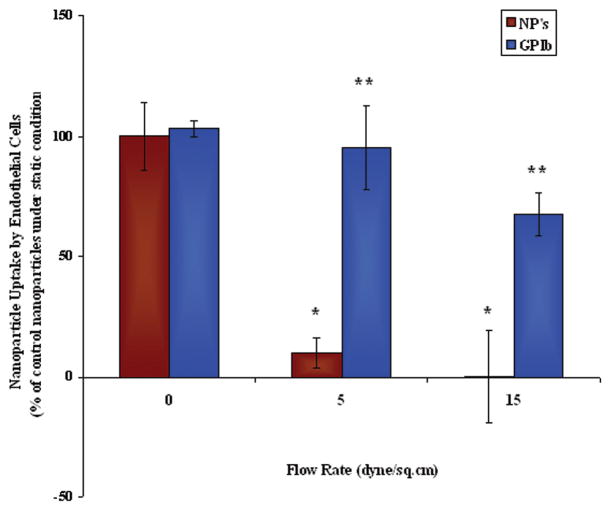
Figure 7.
Cellular Adhesion of GP Ib conjugated nanoparticles and control nanoparticles by HAECs under varying shear stress. values were obtained after 30 minutes of flow with nanoparticle solutions represent mean ± SD(n=3). * indicates the significant differences compared to the control nanoparticle static samples (p< 0.001). ** indicates the significant differences compared to the control nanoparticles (p< 0.001) at each flow rate.
Acknowledgments
We acknowledge Chad Larson and Dr. Sophia Passy from the University of Texas at Arlington Department of Biology for their assistance with the confocal microscope supported in part by the National Science Foundation Grant 0215852. The authors are grateful for the assistance provided by Hao Xu and Maham Rahimi in the Nanomedicine and Tissue Engineering Laboratory at UTA. We also acknowledge the financial support from the American Heart Association Scientist Development Award 0735270N and the NIH grant HL091232 (K.N.).
References
- 1.Davda J, Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. International journal of pharmaceutics. 2002;233(1–2):51–9. doi: 10.1016/s0378-5173(01)00923-1. [DOI] [PubMed] [Google Scholar]
- 2.Kuldo JM, Ogawara KI, Werner N, Asgeirsdaottir SA, Kamps JA, Kok RJ, Molema G. Molecular pathways of endothelial cell activation for (targeted) pharmacological intervention of chronic inflammatory diseases. Current vascular pharmacology. 2005;3(1):11–39. doi: 10.2174/1570161052773898. [DOI] [PubMed] [Google Scholar]
- 3.Lutters BC, Leeuwenburgh MA, Appeldoorn CC, Molenaar TJ, Van Berkel TJ, Biessen EA. Blocking endothelial adhesion molecules: a potential therapeutic strategy to combat atherogenesis. Current opinion in lipidology. 2004;15(5):545–52. doi: 10.1097/00041433-200410000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Omolola Eniola A, Hammer DA. In vitro characterization of leukocyte mimetic for targeting therapeutics to the endothelium using two receptors. Biomaterials. 2005;26(34):7136–44. doi: 10.1016/j.biomaterials.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Sakhalkar HS, Hanes J, Fu J, Benavides U, Malgor R, Borruso CL, Kohn LD, Kurjiaka DT, Goetz DJ. Enhanced adhesion of ligand-conjugated biodegradable particles to colitic venules. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19(7):792–4. doi: 10.1096/fj.04-2668fje. [DOI] [PubMed] [Google Scholar]
- 6.Yao SN, Wilson JM, Nabel EG, Kurachi S, Hachiya HL, Kurachi K. Expression of human factor IX in rat capillary endothelial cells: toward somatic gene therapy for hemophilia B. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(18):8101–5. doi: 10.1073/pnas.88.18.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakhalkar HS, Dalal MK, Salem AK, Ansari R, Fu J, Kiani MF, Kurjiaka DT, Hanes J, Shakesheff KM, Goetz DJ. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15895–900. doi: 10.1073/pnas.2631433100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou X, Shinde Patil VR, Dagia NM, Smith LA, Wargo MJ, Interliggi KA, Lloyd CM, Tees DF, Walcheck B, Lawrence MB, et al. PSGL-1 derived from human neutrophils is a high-efficiency ligand for endothelium-expressed E-selectin under flow. American journal of physiology. Cell physiology. 2005;289(2):C415–24. doi: 10.1152/ajpcell.00289.2004. [DOI] [PubMed] [Google Scholar]
- 9.Burch EE, Shinde Patil VR, Camphausen RT, Kiani MF, Goetz DJ. The N-terminal peptide of PSGL-1 can mediate adhesion to trauma-activated endothelium via P-selectin in vivo. Blood. 2002;100(2):531–8. doi: 10.1182/blood.v100.2.531. [DOI] [PubMed] [Google Scholar]
- 10.Eniola AO, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes II: effect of degradation on targeting activity. Biomaterials. 2005;26(6):661–70. doi: 10.1016/j.biomaterials.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Eniola AO, Rodgers SD, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes. Biomaterials. 2002;23(10):2167–77. doi: 10.1016/s0142-9612(01)00349-0. [DOI] [PubMed] [Google Scholar]
- 12.Eniola AO, Hammer DA. Artificial polymeric cells for targeted drug delivery. Journal of controlled release : official journal of the Controlled Release Society. 2003;87(1–3):15–22. doi: 10.1016/s0168-3659(02)00346-2. [DOI] [PubMed] [Google Scholar]
- 13.Kumar RA, Dong JF, Thaggard JA, Cruz MA, Lopez JA, McIntire LV. Kinetics of GPIbalpha-vWF-A1 tether bond under flow: effect of GPIbalpha mutations on the association and dissociation rates. Biophys J. 2003;85(6):4099–109. doi: 10.1016/S0006-3495(03)74822-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong J, Schade AJ, Romo GM, Andrews RK, Gao S, McIntire LV, Lopez JA. Novel gain-of-function mutations of platelet glycoprotein IBalpha by valine mutagenesis in the Cys209-Cys248 disulfide loop. Functional analysis under statis and dynamic conditions. J Biol Chem. 2000;275(36):27663–70. doi: 10.1074/jbc.M909952199. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Lopez JA. Interactions of platelets with subendothelium and endothelium. Microcirculation. 2005;12(3):235–46. doi: 10.1080/10739680590925484. [DOI] [PubMed] [Google Scholar]
- 16.Desai MP, Labhasetwar V, Walter E, Levy RJ, Amidon GL. The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharmaceutical research. 1997;14(11):1568–73. doi: 10.1023/a:1012126301290. [DOI] [PubMed] [Google Scholar]
- 17.Win KY, Feng SS. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(15):2713–22. doi: 10.1016/j.biomaterials.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 18.Panyam J, Labhasetwar V. Dynamics of endocytosis and exocytosis of poly(D,L-lactide-co-glycolide) nanoparticles in vascular smooth muscle cells. Pharmaceutical research. 2003;20(2):212–20. doi: 10.1023/a:1022219003551. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen KT, Eskin SG, Patterson C, Runge MS, McIntire LV. Shear stress reduces protease activated receptor-1 expression in human endothelial cells. Ann Biomed Eng. 2001;29(2):145–52. doi: 10.1114/1.1349700. [DOI] [PubMed] [Google Scholar]
- 20.Florence AT, Hillery AM, Hussain N, Jani PU. Factors affecting the oral uptake and translocation of polystyrene nanoparticles: histological and analytical evidence. Journal of drug targeting. 1995;3(1):65–70. doi: 10.3109/10611869509015936. [DOI] [PubMed] [Google Scholar]
- 21.Hussain N. Fluorometric method for the simultaneous quantitation of differently-sized nanoparticles in rodent tissue. International journal of pharmaceutics. 2001;214(1–2):55–61. doi: 10.1016/s0378-5173(00)00631-1. [DOI] [PubMed] [Google Scholar]
- 22.Ayhan H, Tuncel A, Bor N, Piskin E. Phagocytosis of monosize polystyrene-based microspheres having different size and surface properties. J Biomater Sci Polym Ed. 1995;7(4):329–42. doi: 10.1163/156856295x00355. [DOI] [PubMed] [Google Scholar]
- 23.Tabata Y, Ikada Y. Effect of the size and surface charge of polymer microspheres on their phagocytosis by macrophage. Biomaterials. 1988;9(4):356–62. doi: 10.1016/0142-9612(88)90033-6. [DOI] [PubMed] [Google Scholar]
- 24.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. International journal of pharmaceutics. 2005;298(2):315–22. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 25.Qaddoumi MG, Ueda H, Yang J, Davda J, Labhasetwar V, Lee VH. The characteristics and mechanisms of uptake of PLGA nanoparticles in rabbit conjunctival epithelial cell layers. Pharmaceutical research. 2004;21(4):641–8. doi: 10.1023/b:pham.0000022411.47059.76. [DOI] [PubMed] [Google Scholar]
- 26.Wiewrodt R, Thomas AP, Cipelletti L, Christofidou-Solomidou M, Weitz DA, Feinstein SI, Schaffer D, Albelda SM, Koval M, Muzykantov VR. Size-dependent intracellular immunotargeting of therapeutic cargoes into endothelial cells. Blood. 2002;99(3):912–22. doi: 10.1182/blood.v99.3.912. [DOI] [PubMed] [Google Scholar]
- 27.Qaddoumi MG, Gukasyan HJ, Davda J, Labhasetwar V, Kim KJ, Lee VH. Clathrin and caveolin-1 expression in primary pigmented rabbit conjunctival epithelial cells: role in PLGA nanoparticle endocytosis. Molecular vision [electronic resource] 2003;9:559–68. [PubMed] [Google Scholar]
- 28.Muro S, Wiewrodt R, Thomas A, Koniaris L, Albelda SM, Muzykantov VR, Koval M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. Journal of cell science. 2003;116(Pt):1599–609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 29.Muro S, Cui X, Gajewski C, Murciano JC, Muzykantov VR, Koval M. Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress. American journal of physiology. Cell physiology. 2003;285(5):C1339–47. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- 30.Muro S, Koval M, Muzykantov V. Endothelial endocytic pathways: gates for vascular drug delivery. Current vascular pharmacology. 2004;2(3):281–99. doi: 10.2174/1570161043385736. [DOI] [PubMed] [Google Scholar]
- 31.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Advanced drug delivery reviews. 2003;55(3):329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 32.Vasir JK, Labhasetwar V. Polymeric nanoparticles for gene delivery. Expert opinion on drug delivery. 2006;3(3):325–44. doi: 10.1517/17425247.3.3.325. [DOI] [PubMed] [Google Scholar]
- 33.Labhasetwar V, Song C, Humphrey W, Shebuski R, Levy RJ. Arterial uptake of biodegradable nanoparticles: effect of surface modifications. Journal of pharmaceutical sciences. 1998;87(10):1229–34. doi: 10.1021/js980021f. [DOI] [PubMed] [Google Scholar]
- 34.Song C, Labhasetwar V, Cui X, Underwood T, Levy RJ. Arterial uptake of biodegradable nanoparticles for intravascular local drug delivery: results with an acute dog model. Journal of controlled release : official journal of the Controlled Release Society. 1998;54(2):201–11. doi: 10.1016/s0168-3659(98)00016-9. [DOI] [PubMed] [Google Scholar]
- 35.Guzman LA, Labhasetwar V, Song C, Jang Y, Lincoff AM, Levy R, Topol EJ. Local intraluminal infusion of biodegradable polymeric nanoparticles. A novel approach for prolonged drug delivery after balloon angioplasty. Circulation. 1996;94(6):1441–8. doi: 10.1161/01.cir.94.6.1441. [DOI] [PubMed] [Google Scholar]
- 36.Blackwell JE, Dagia NM, Dickerson JB, Berg EL, Goetz DJ. Ligand coated nanosphere adhesion to E- and P-selectin under static and flow conditions. Annals of biomedical engineering. 2001;29(6):523–33. doi: 10.1114/1.1376697. [DOI] [PubMed] [Google Scholar]
- 37.Dickerson JB, Blackwell JE, Ou JJ, Shinde Patil VR, Goetz DJ. Limited adhesion of biodegradable microspheres to E- and P-selectin under flow. Biotechnol Bioeng. 2001;73(6):500–9. doi: 10.1002/bit.1085. [DOI] [PubMed] [Google Scholar]
- 38.Eniola AO, Willcox PJ, Hammer DA. Interplay between rolling and firm adhesion elucidated with a cell-free system engineered with two distinct receptor-ligand pairs. Biophysical journal. 2003;85(4):2720–31. doi: 10.1016/s0006-3495(03)74695-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eniola AO, Krasik EF, Smith LA, Song G, Hammer DA. I-domain of lymphocyte function-associated antigen-1 mediates rolling of polystyrene particles on ICAM-1 under flow. Biophysical journal. 2005;89(5):3577–88. doi: 10.1529/biophysj.104.057729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nesbitt WS, Mangin P, Salem HH, Jackson SP. The impact of blood rheology on the molecular and cellular events underlying arterial thrombosis. J Mol Med. 2006;84(12):989–95. doi: 10.1007/s00109-006-0101-1. [DOI] [PubMed] [Google Scholar]
- 41.Andrews RK, Shen Y, Gardiner EE, Berndt MC. Platelet adhesion receptors and (patho)physiological thrombus formation. Histol Histopathol. 2001;16(3):969–80. doi: 10.14670/HH-16.969. [DOI] [PubMed] [Google Scholar]
- 42.Grabowski EF. Thrombolysis, flow, and vessel wall interactions. J Vasc Interv Radiol. 1995;6(6 Pt 2 Su):25S–29S. doi: 10.1016/s1051-0443(95)71245-3. [DOI] [PubMed] [Google Scholar]
- 43.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100(12):4033–9. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 44.Kumar RA, Moake JL, Nolasco L, Bergeron AL, Sun C, Dong JF, McIntire LV. Enhanced platelet adhesion and aggregation by endothelial cell-derived unusually large multimers of von Willebrand factor. Biorheology. 2006;43(5):681–91. [PubMed] [Google Scholar]
- 45.Simon DI, Chen Z, Xu H, Li CQ, Dong J, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Exp Med. 2000;192(2):193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]