Abstract
Extraordinary strides have been made toward understanding the complexities and regulatory mechanisms of sleep over the past two decades, thanks to the help of rapidly evolving technologies. At its most basic level, mammalian sleep is a restorative process of the brain and body. Beyond its primary restorative purpose, sleep is essential for a number of vital functions. Our primary research interest is to understand the cellular and molecular mechanisms underlying the regulation of sleep and its cognitive functions. Here I will reflect on our own research contributions to fifty years of extraordinary advances in the neurobiology of slow-wave sleep (SWS) and rapid eye movement (REM) sleep regulation. I conclude this review by suggesting some potential future directions to further our understanding of the neurobiology of sleep.
Keywords: Wakefulness, Slow-wave sleep, Rapid eye movement sleep, Wake-promoting structures of the brain, Metabolite homeostasis, Cellular-Molecular-Network model, Neurotransmitters, Intracellular signal transduction
1. Introduction
The rest-activity cycle is one of the basic signs of all living organisms. Rest is a passive process during which living organisms “slow down” and become rejuvenated for subsequent activities. In homeothermic vertebrates, like mammals and birds, the rest part of this rest-activity cycle evolved as sleep. In homeothermic vertebrates, sleep, in addition to providing rest, provides a biological advantage in the formation of complex memory and in physiological fight against infections [1-3]. Therefore, sleep is a highly evolved global behavioral state in homeothermic vertebrates. Sleep in mammals could be defined by: (1) characteristic changes in posture; (2) raised sensory threshold; and (3) distinctive electrographic signs. Sleep is usually associated with a marked diminution of motor activity and with the assumption of recumbent postures. Typically the eyes close and the somatic musculature becomes hypotonic. The threshold to external stimulation increases and animals become progressively less responsive to external stimuli as sleep deepens. In some mammalian species which regulate temperature at lower levels during winter, sleep constitutes the state of entry to and exit from hibernation.
Beneath the calm exterior of sleeping mammals, dynamic forces are at play. There are mechanisms that regulate the two alternating sleep states that occur while mammals are “asleep” [4]. Normally, when we first enter the sleep state, it is via quiet (non rapid eye movement; NREM) sleep, which is a state that, behaviorally, is not very dramatic. We simply lie still, breathing slowly. Our eyes drift slowly back and forth and every once in a while we shift our sleep position. On first falling asleep, individuals may progressively lose awareness of the outside world and experience microhallucinations and illusions of movements of the body in space. During NREM sleep there are decreases in body temperature, blood pressure, heart rate, and respiratory rate. However, the pulsatile release of growth and sex hormones from the pituitary gland and production of antibodies increases during NREM sleep. The concomitance of these events gives further credence to the notion that NREM sleep may be functionally associated with anabolic processes benefiting the somatic tissues [5].
NREM sleep is characterized by a change in the electroencephalogram (EEG) from a low-amplitude, high-frequency to a high-amplitude, low-frequency pattern. The degree to which the EEG is progressively synchronized (that is, of high-amplitude and low-frequency) can be subdivided into four stages in humans: Stage one (NREM-I) sleep is characterized by relatively low-amplitude (<50 μV) theta frequency activity (4-7 cycles per second (Hz)) and vertex sharp waves in the EEG. Stage two (NREM-II) sleep is characterized by the appearance of distinctive sleep spindles (lasting between 0.5-1.0 sec and peak amplitudes of 100 μV) composed of augmenting and decrementing waves at a frequency of 12-14 Hz and K-complex (a negative sharp wave followed immediately by a slower positive component) waveforms in the EEG. Stage three (NREM-III) sleep is characterized by the addition of high-amplitude (>100 μV) slow waves (1-4 Hz), with no more than 50% of the EEG record occupied by the slow waves. In stage four (NREM-IV), the EEG record is dominated by high-amplitude (150-250 μV) slow waves (1-4 Hz). Throughout this process, as the EEG frequency is decreasing and the amplitude is increasing, muscle tone progressively declines and may be lost altogether in most of the somatic musculature. Slow rolling eye movements first replace rapid saccadic eye movements of waking and then subside, with the eyes finally assuming a divergent upward gaze.
After varying amounts of time (depending upon the size of the animal and its brain), this progressive set of changes in the EEG reverses itself and the EEG eventually resumes the low-amplitude, fast character previously seen in waking. Instead of waking, however, behavioral sleep persists. Muscle tone, at first passively decreased during NREM sleep, is now actively inhibited. Stereotyped bursts of saccadic eye movements, called rapid eye movements (REMs), appear in the electrooculogram (EOG) and give this state the name REM sleep. This phase of sleep has also been called activated sleep (due to increased EEG activation) and paradoxical sleep (to signal the maintenance of increased behavioral arousal threshold in the presence of the activated brain). Supplemental to these polysomnographic signs, other REM sleep-specific physiological signs are: myoclonic twitches in the facial, digital, and even major proximal skeletal muscles, pronounced fluctuations in cardio-respiratory rhythms and core body temperature and penile erection in males and clitoral engorgement in females.
In the fifty years since brain transection experiments began to identify specific centers for well-characterized states of NREM and REM sleep [6,7], our understanding of the neurobiology of sleep in terms of brain structures, cell types, neurotransmitters and their receptors, a neuronal network, and synaptic interactions has progressed dramatically. Similarly, considerable progress has also been made in identifying some of the positive functions of sleep. Although it is remarkable how far we have come in understanding the different aspects of sleep neurobiology, its molecular aspect is not one of them. This is unfortunate, as molecular aspects of sleep mechanisms will likely yield significant new insights into both the causes and solutions of sleep and other disorders that are affected by the sleep disorders.
This review summarizes work over the past fifty years in our and in other laboratories investigating the neurobiology of sleep. In this review, I first describe the similarities and differences between human sleep stages and those of animals. I then highlight the differences in neurochemical status of the brain in wakefulness and different stages of sleep. Finally, based on what we know at this time, I discuss neural mechanisms of SWS and then REM sleep without detailing the results of individual studies. Although much remains to be learned, this review is intended to provide the most simple but most complete description of the neurobiological mechanisms of sleep. It is expected that this simplified knowledge would be valuable for designing future experiments to understand the molecular mechanisms of sleep. While, in this review, I do not discuss the pathophysiological aspects of sleep disorders and pharmacological treatments for any specific sleep disorders, this simplified knowledge of the neurobiological mechanisms of sleep will aid in the development of the rational schematics of behavioral and pharmacological therapies both for sleep disorders and their comorbidities.
2. Sleep Stages in human and other mammals
Sleep in mammals is not a homogenous behavioral state, rather it is a continuum of mixed states that differ in their physiology, chemistry, and phenomenological experiences [4]. Because some human sleep stages are named differently from those in animals, and since the present description on the neurobiological mechanisms of sleep is based on animal research, the following text describes the similarities and differences between human sleep stages and those of animals.
Human NREM and REM sleep alternate throughout each of the four to six sleep cycles that occur every night. The most common and preferred animal models used in sleep research include the mouse, rat, and cat; in these animals, NREM-REM sleep cycles are much shorter than in human and non-human primates. These cyclic NREM-REM sleep epochs in rodents and cats continue throughout the day and night, except when the animal is engaged in activities that require wakefulness. In humans, NREM sleep is further subdivided into four stages (I, II, III and IV), each corresponding to an increasing depth of sleep. The deepest stages of NREM sleep, III and IV, are collectively called SWS (also known as delta sleep). Distinctions between stages of NREM sleep in animal models differ slightly from those in humans. In the aforementioned animals, NREM sleep is normally subdivided into two stages (SWS-I and SWS-II). SWS-I is identified by the presence of sleep spindles in the cortical EEG. SWS-II is considered deep sleep and is identified by the presence of high-amplitude, low frequency waves in the cortical EEG (delta sleep). Human NREM stages I and II sleep are comparable to mouse, rat, and cat SWS-I, whereas stages III and IV NREM sleep in humans are comparable to the animal SWS-II.
We spend about 80% of our total sleep time in NREM sleep compared to only 20% time in REM sleep; and some recent studies have indicated that the specific stages of NREM sleep are beneficial for specific functions of our brain and body. Since the total period of NREM sleep is much longer than the total period of REM sleep and the physiological significance of NREM sleep stages are equally important, the use of its old name began to disappear in some recent publications. The proposed new names for “NREM I” and “NREM II” sleep stages are “stage I sleep” and “stage II sleep”, respectively. Also, NREM III and NREM IV sleep stages are no longer considered as two separate states. These two sleep stages are now considered as a single sleep stage under the name of “SWS”.
Physiological signs of REM sleep in humans are, for the most part, comparable to physiological signs of REM sleep in the rat and cat. However, another sleep stage, one between SWS-II and REM sleep, has been identified mainly in rats and cats and is called the transitional sleep stage (tS-R). During this transitional stage, the appearance of cortical EEG signs is similar to the cortical EEG of SWS-I and hippocampal and pontine EEG signs are similar in appearance to those during REM sleep, but with less intensity. Since most animal sleep studies have not recorded hippocampal and/or pontine EEG, such studies have often considered this stage to be SWS-I. Similarly, in sleep studies of healthy humans, hippocampal and/or pontine EEG cannot be recorded to help identify sleep stages. Therefore, based on EEG signs in humans, this transitional sleep stage has always been identified as stage II NREM sleep.
3. Behavioral state-dependent neurochemical status of the brain
Studies that measure state-dependent neurotransmitter levels of the brain have suggested that the levels of different neurotransmitters in the brain are tightly regulated by the behavioral states of wake and sleep [8,9]. Conversely, neuropharmacological studies have shown that the changes in the brain levels of specific neurotransmitters could trigger or suppress specific stages of wake and sleep [4]. Interestingly, one of the principles of psychiatric medicine is that abnormal mental states can be normalized by changing neurotransmitter activity levels of the brain. It is also known that if the levels of neurotransmitters in the brain remain high and/or low for an extended period of time, a number of neurological and neurodegenerative disorders may occur. Therefore, understanding the behavioral state-specific normal levels of neurotransmitter activity could help us to understand not only the mechanisms of sleep regulation, but also could help us to understand the patho-physiological mechanisms of a number of psychiatric, neurological, and neurodegenerative disorders.
Collective evidence from many years of research on animals using single cell recordings, immunohistochemistry, and neurotransmitter measurement techniques suggests that during the awake state the brain levels of acetylcholine (Ach), norepinephrine (NE), serotonin (5-HT), dopamine (DA), histamine (HA), and glutamate (Glut) are at their highest levels [4,10-25]. During SWS-I the levels of Ach and Glut drop and remain at their lowest level until the end of SWS-II. During tS-R the levels of Ach and Glut begin to rise. During REM sleep, Ach reaches to about 65% of its highest waking levels, and Glut matches its highest level during wakefulness. The NE, 5-HT, DA, and HA levels in all parts of the brain slightly decline during SWS-I and then slowly drop down to their lowest levels during SWS-II. During REM sleep, NE, 5-HT, and HA remain at their lowest levels. But the level of DA begins to increase during tS-R, and during REM sleep it reaches a level close to its highest during wakefulness. During wakefulness, the levels of GABA are at their lowest [26-30]. The levels of GABA in the cortical and subcortical areas of the forebrain slowly increase during SWS-I and then reach their highest level during SWS-II. During REM sleep, the level of GABA in almost all parts of the brain, except some specific areas of the brainstem, drops almost to its lowest level.
4. Wake-promoting systems of the brain
Utilizing only a macroscopic view of our external behavior, sleep is defined by the absence of wakefulness. Therefore, to understand the basic neurobiological mechanisms for the initiation and maintenance of sleep, it is critical to understand wake-promoting systems of our brain. Activation of these systems results in arousal, thus preventing an organism from falling asleep. The state of wakefulness is a complex, coordinated expression of behaviors that are constantly changing in response to variations in the internal and external milieu. The waking state is characterized by low-amplitude synchronization of fast oscillations in the cortical EEG (also called activated EEG) in the range of 20 to 60 Hz and the presence of muscle tone in the electromyogram (EMG). While we are awake, our voluntary movements are present and thresholds for sensory responses are lowest, while thought processes are logical and progressive.
In mammals, there exist multiple systems that promote wakefulness [4,10]. Individual activation of these wake-promoting systems contributes in specific ways to maintain our general state of wakefulness. Identification of these wake-promoting systems began almost 60 years ago using transection and electrical stimulation experiments in the cat. In these early experiments, Moruzzi and Magoun suggested that the waking state required a critical level of brain activity maintained by a steady flow of ascending impulses arising in the brainstem reticular formation [31-33]. At that time, the excitable area of this ascending reticular activating system (ARAS) was broadly localized within the core of pons and midbrain; this area includes the central region of the brainstem extending forward from the bulbar reticular formation, through the pontine and mesencephalic tegmentum, and into the caudal diencephalons [31]. Over many years, the advancement of anatomical and physiological techniques has enabled us to identify neurochemically-specific wake-promoting cell groups within the ARAS [4]. These wake-promoting cell groups are: 1) NE-synthesizing (noradrenergic) cells in the locus coeruleus (LC), 2) 5-HT-synthesizing (serotonergic) cells in the raphe nuclei (RN), 3) Ach-synthesizing (cholinergic) cells in the pedunculopontine tegmentum (PPT) and laterodorsal tegmentum (LDT), 4) Glut-synthesizing (glutamatergic) cells in the midbrain, and 5) DA-synthesizing (dopaminergic) cells in the substantia nigra compacta (SNc) and ventral tegmental area (VTA). Projections from these pontine and midbrain wake-promoting cells travel dorsally to activate the thalamo-cortical system, as well as ventrally to activate the hypothalamo-cortical and basalo-cortical systems [10, 34-36].
In addition to brainstem ARAS wake-promoting cell groups, there are at least five other groups of cells in the forebrain that could promote wakefulness independently and/or in coordination with wake-promoting cells of the brainstem [37-51]. These forebrain cell groups are: 1) HA-containing (histaminergic) cells in the tuberomammillary nuclei (TMN) of the posterior hypothalamus (PH), 2) hypocretin (Hcrt, also known as orexins)-containing (hypocretinergic) cells in the lateral hypothalamus (LH), 3) Cholinergic cells in the basal forebrain (BF), 4) neuropeptide Y (NPY)-containing cells in the suprachiasmatic nucleus (SCN), and 5) glutamatergic cells in the ventro-medial prefrontal cortex (vmPFC). Spontaneous or experimental activation of these systems results in the cortical activation patterns necessary to maintain wakefulness [4]. Each of these wake-promoting cell groups contribute in a unique way to different aspects of wakefulness; however, none of these cell groups appears to be absolutely necessary for the generation and maintenance of wakefulness [49]. This may be the reason that a lesion of any specific cell groups has failed to reveal any significant changes in the total amount of time spent in wakefulness.
5. Neural mechanisms of SWS
Historically, sleep, especially SWS, has been considered a passive process during which cerebral sensory gates close, leading to functional deafferentation of the cerebral cortex. This passive deafferentation of the cerebral cortex provides our brain and body a chance to slow down and be refreshed. Over the last four decades, this view has been challenged and replaced. Evidence has shown that neurons in the preoptic/anterior hypothalamic (POA/AH) area act with inhibitory influences on wake-promoting neurons in the ARAS and other wake-promoting areas of the brain to suppress their excitatory influences on the brain. Like passive deafferentation, this active process of SWS generation could also lead to functional deafferentation of the cerebral cortex; there has been an on-going debate about which ostensibly opposing mechanism (passive or active) accurately explains the initiation of SWS The following discussion describes an integrative explanation suggesting that the passive and active mechanisms of SWS generation are actually successive steps within a chain of events and are complementary phenomena of a common goal of generating and maintaining SWS.
5.1. Initiation of sleep
The transition from wakefulness to SWS-I in animals is equivalent to stage I sleep in humans. This initial part of sleep is a passive process. The notion that the initiation of SWS-I is a passive process was the principle mantra of the reticular deactivation theory [33]. The reticular deactivation theory was based on two assumptions. First, “the state of wakefulness requires a critical level of brain activity, which is maintained by a steady flow of ascending impulses arising in the brainstem reticular formation.” The second is “a reduction of tonic activity of the ascending reticular system is responsible for physiological sleep.” Over the past three decades, sleep researchers have gathered more knowledge about widespread physiological changes during the transition from wakefulness to SWS-I, including recorded electrical activity of the brain, sensory, motor, and metabolic processes. This new information suggests that the passive mechanism of SWS-I initiation could only be explained as a homeostatic regulation of neuronal activity-dependent metabolites [4]. Neuronal activity-dependent metabolites are endogenous metabolites produced during wakefulness that increase proportionately with increased duration and intensity of wakefulness. At present, among hundreds of known metabolites, adenosine, neuroinhibitory amino acids GABA and glycine, prostaglandin D2 (PGD2), and cytokines (interleukin-I beta (IL-1β) and tumor necrosis factor alpha (TNFα)) have been identified as SWS initiating metabolic factors. The slow accumulation of these metabolic factors increases sleep inertia and, with sufficient accumulation, facilitates the transition from wakefulness to SWS-I by suppressing wakefulness.
According to the “activity-dependent metabolites homeostatic theory”, during wakefulness, our use-dependent metabolic rate in the brain and body is much higher than it is during sleep and/or resting periods [4]. The increased metabolic rate during wakefulness is accompanied by an increased rate of metabolite synthesis that is higher than the rate of clearance; as a result, the levels of specific metabolites tend to accumulate in the brain and body. When these metabolites reach a critical level, our metabolic process responds by slowing down wake-promoting neuronal activities, thus lowering the rate of production until the metabolites return to basal levels. During this period, the rate of metabolite clearance remains unchanged. The diminished activity of wake-promoting neuronal systems results in reduced synthesis of metabolites. This reduction of metabolites from critical to basal levels is considered a process of metabolite homeostasis. Homeostatic demand for a lower metabolic state begins at the cellular level, ultimately affects behavior at the systemic level, and is the primary factor necessary to initiate SWS-I. Since this passive process operates on a larger timescale compared to the active process that ultimately deepens and maintains SWS-I, the time it takes to fall asleep is therefore quite longer than the time it takes to transition from SWS-I to SWS-II. Interestingly, the homeostatic demand for a lower metabolic state that ultimately initiates the process of sleep generation also triggers complex motor behaviors directed to find a safe area for sleep in a number of mammalian species.
5.2. Mechanisms of SWS-I
Electrophysiological and behavioral signs of SWS-I in animals are comparable to electrophysiological and behavioral signs of stage II sleep in humans. The defining EEG signs of stage II sleep are the sleep spindle and the K-complex. Sleep spindles are waxing-and-waning waves, grouped in sequences that occur every 2-5 sec. The pacemaker of sleep spindles is the thalamic reticular nucleus, which consists exclusively of GABAergic neurons [52]. The thalamus is also a gateway for most sensory and internal signals (generated in the brainstem) enroute to the cerebral cortex [53]. The thalamus contains two functionally different types of neurons: 1) thalamo-cortical relay neurons, which relay incoming sensory information to the cerebral cortex, and 2) thalamic reticular neurons, the activation of which prevents thalamo-cortical relay cells from transferring sensory information to the cerebral cortex. During wakefulness, thalamo-cortical relay cells remains in a ‘ready state’ for relaying incoming sensory information to the specific parts of the cerebral cortex. The levels of readiness of those thalamo-cortical relay cells depend positively on the levels of activity of wake-promoting noradrenergic, serotonergic, and cholinergic cells in the brainstem [10]. In contrast, the neuronal activity of thalamic reticular cells is dampened by the activity of those wake-promoting noradrenergic, serotonergic, and cholinergic cells of the brainstem. Therefore, during the passive step of sleep generation (initiation of sleep), when activity levels of wake-promoting noradrenergic, serotonergic, and cholinergic cells of the brainstem are reduced by the increased levels of metabolites, the excitability of thalamo-cortical relay cells decreases, and excitability of thalamic reticular cells increases. Increased excitation of thalamic reticular cells then actively inhibits thalamo-cortical relay cells by the activation of postsynaptic GABA-B receptors. The consequence is that the incoming sensory signals are blocked in the thalamus and the cerebral cortex is deprived of information from the outside world (functional deafferentation of the cerebral cortex). At this stage, thalamic reticular neurons fire as spike bursts that impose inhibitory postsynaptic potentials (IPSPs) on the thalamo-cortical relay neurons. In turn, these IPSPs hyperpolarize those thalamo-cortical relay neurons. When the level of hyperpolarization reaches to -65 mV it de-inactivates the low-threshold calcium channels of those thalamo-cortical relay cells. Activation of low-threshold calcium channels then generates postinhibitory rebound spike bursts in the thalamo-cortical relay cells [52]. These signals from the thalamo-cortical relay cells are transmitted to the cortex, where they rhythmically excite cortical neurons that ultimately generate cortical sleep spindles [54]. Therefore, this sleep spindle generating mechanism combines both passive and active physiological processes.
The sleep spindle generating mechanism is also the mechanism for functional deafferentation of cerebral cortex. Functional deafferentation of the cerebral cortex facilitates progression of sleep from lighter to deeper stages. It is worth mentioning here that this sleep spindle generating mechanism that involves rhythmic excitation of cortical neurons makes those cortical neurons physiologically unsuitable for receiving other subcortical inputs. They could, however, easily send cortical information to subcortical areas. This mechanism provides a physiological explanation as to why the behavioral state of stage II sleep (in human)/ SWS-I (in non-human primates and other animals) are not conducive behavioral states for the transfer of long-term memories from the hippocampus and/or amygdala to the long-term memory storage in the cortex. Yet, these behavioral states remain conducive to transfer short-term memory information from the cortex to the hippocampus and/or amygdala for the sorting and deleting steps of long-term memory formation processes [2,55].
5.3. Mechanisms of SWS-II
Human SWS is comparable to the animal SWS-II. The SWS/SWS-II generating mechanisms are active processes. The active steps of the SWS generating mechanisms, compared to the passive steps of the initiation of sleep, operate on shorter timescales. An active hypnogenic role for the POA was first suggested by von Economo [56] about eighty years ago. In post-mortem brain tissue analysis of patients exhibiting insomnia in association with viral encephalitis, von Economo documented inflammatory lesions within the region recognized as the POA. Nauta [57] supported this hypothesis by experimentally replicating behavioral insomnia in rats using bilateral knife cut lesions in the POA. Finally, polygraphic recordings of sleep-wake stages in cats with localized electrolytic lesions of the anterior hypothalamus objectively demonstrated the role of the POA in SWS generation [58].
The advancement of experimental techniques has confirmed, as well as expanded, the findings of these early lesion studies. For example, specific lesioning of cell bodies in the POA of the anterior hypothalamus has been shown to effectively suppress SWS in mammals [59-62]. Utilizing single cell recording techniques, various researchers have identified a large population of cells within the POA that are more active during the electrophysiological and behavioral signs of SWS than during wakefulness or REM sleep [63-73]. Consistent with these single cell recording studies, a study using functional magnetic resonance imaging (fMRI) in the behaving rat has demonstrated that the POA is more active than other parts of the hypothalamus and basal forebrain during SWS [74]. A number of local microinjection studies have supported the critical role of the POA in the generation of SWS [75-79].
Immunohistochemical analysis of sleep-active neurons in the POA has revealed that a majority contain the inhibitory neurotransmitters GABA and galanin [80-82]. These sleep-active neurons innervate many wake-promoting areas of the brain, including the TMN, LH, LC, DRN, and PPT/LDT [83-85]. Thus, it is possible that the increased activity of SWS-active GABAergic cells in the POA could release GABA to targets within the wake-promoting areas of the brain. Released GABA may then suppress activity in these areas in two different ways: 1) GABA receptor-activation mediated inhibition of wake-promoting cells or 2) Inhibition of presynaptic neurotransmitter release that is necessary for the activation of wake-promoting cells [86,87]. Neuropharmacological studies have shown that SWS is also induced by a number of sedative and hypnotic drugs that involve potentiation of POA GABAergic neurotransmission [88-91]. Heightened activity of GABAergic neurons throughout the entire POA during SWS substantiates the theory that the primary factor responsible for the induction of SWS is the activation of GABA-containing POA neurons.
Recent research has indicated that the presence of growth hormone-releasing hormone (GHRH) may be critical for the generation of GABAergic-POA-cell-activation-mediated-SWS. There are two distinct clusters of GHRHergic (GHRH-synthesizing) neurons in the hypothalamus: one cluster, containing the majority of GHRHergic cells is located in the arcuate nucleus and another cluster, with fewer cells, is located around the ventromedial nucleus and the periventricular nucleus [92-94]. In the rat, hypothalamic GHRH mRNA levels peak around light onset, decrease towards the end of the light period and remain at very low levels throughout the night [95,96]. The light onset is followed by a short rise in GHRH suggesting that the transcribed mRNA is translated into protein very rapidly [97]. The GHRH mRNA levels and GHRH content could indicate that the time of day where maximum GHRH synthesis and release occur corresponds to the period of SWS. It has also been shown that sleep deprivation increases hypothalamic GHRH mRNA levels and depletes GHRH peptide [96-98]. This anatomical and temporal expression of GHRH mRNA and GHRH synthesis data suggest the possibility that GHRH is involved in the induction of SWS.
The GHRHergic neurons in the arcuate nucleus are the major source of GHRH released at the median eminence; thus, the control of pituitary GH secretion is the major function of this group of neurons [99] The anterior pituitary somatotroph cells produce growth hormone (GH); its secretion occurs in pulses throughout the day but, after sleep onset, SWS is associated with large bursts of GH secretion. The GH secretion during SWS can amount to two-thirds of the total GH secreted in young males. The majority of the extra-arcuate GHRHergic neurons as well as part of the arcuate GHRHergic neurons project predominantly to the POA. This GHRHergic neuronal projection to the POA is significant because the activation of POA GABAergic cells is shown to be involved in the generation and maintenance of SWS. Indeed, systemic injection of GHRH increases deep SWS in humans [100-103] and rats [104]. Intracerebroventricular administration of GHRH results in an increase in SWS in the rat [105-107]. Inhibition of endogenous GHRH, using either a peptide antagonist [108] or anti-GHRH antibodies [109] suppresses SWS. Inhibition of endogenous GHRH by feedback inhibition after application of GH also suppresses SWS [110]. Studies have also shown that SWS is reduced in transgenic animal models with mutation in the GHRH gene as dw/dw rat [111] and lit/lit mice [112] compared to their wild counterpart rats and mice. Based on the above evidence, there is no doubt that GHRH is an important hypothalamic peptide for the induction of SWS. Another study has demonstrated that the application of GHRH directly into the medial POA increases SWS [98]. The same study also demonstrated that spontaneous and rebound SWS after 3 hr total sleep deprivation is suppressed when a GHRH antagonist is microinjected into the medial POA. This study indicated that induced SWS generating mechanisms by application of GHRH most likely involve the medial POA. More recently, it has been demonstrated that the application of GHRH in the hypothalamic cell culture increases intracellular calcium levels in the GABAergic cells [113]. Collectively, the studies discussed above could be interpreted to show that, for the induction of SWS, GHRH is released in the POA and binds to the GHRH receptors to activate POA GABAergic cells. Indeed, a recent study has demonstrated that the localized administration of GHRH into the POA induces NREM sleep by activating GABAergic sleep-active cells [114].
In addition to the POA, GABAergic cells in the cerebral cortex are also actively involved in the generation of SWS [4]. These GABAergic interneurons within the cortex become active during the initial stages of sleep and these neurons remain active for the entire period of SWS. This process, initially observed in the rat, was later supported by another study using three different species of rodents [115]. The presence of large-amplitude, slow waves (delta-frequency waves) in the cortical EEG is the hallmark of deep SWS. The origin of these delta-waves is the cortex itself and they require activation of hyperpolariztion-activated cation current (Ih) and low-threshold calcium channels in the cortico-cortical and cortico-thalamic pyramidal cells [52,116]. The activation of Ih and low-threshold calcium channels in these pyramidal cells requires GABAergic input. Therefore, it is likely that this GABAergic input to these cortico-cortical and cortico-thalamic cells are originating in those SWS-active GABAergic neurons within the cortex.
In summary, during wakefulness, neuronal activity-dependent metabolic processes yield adenosine, GABA, glycine, PGD2, IL-1β and TNF and many other unknown substances in the brain as metabolic end products. These excess levels of metabolites then passively act on the wake-promoting neuronal systems of our brain to dampen their activities. Coinciding with reduced neuronal activity, the transmission of incoming sensory signals to the cortex via thalamic relay neurons is suspended. Sensory gating at the level of the thalamus is achieved when thalamic relay neurons are hyperpolarized by bursting activities of GABAergic neurons in the thalamic reticularis. While the sensory gating process is occurring, GABAergic neurons in the POA of the hypothalamus become active, and activation of these cells then further hyperpolarizes those wake-promoting cells. Almost concurrently, cortical GABAergic cells become active and impose their inhibitory influence on cortico-cortical and cortico-thalamic neurons. While GABAergic neurons in the POA are active, the release of GHRH intensifies activity of these GABAergic POA neurons to increase the depth and duration of ongoing SWS.
6. Neural mechanisms of REM sleep
REM sleep is characterized by a constellation of events including the following: (1) an activated cortical EEG activity pattern; (2) marked atonia of the postural muscles; (3) rapid eye movements; (4) a theta rhythm within the hippocampus; (5) field potentials in the pons (P-wave), lateral geniculate nucleus and occipital cortex (ponto-geniculo-occipital [PGO]) spikes; (6) myoclonic twitches, most apparent in the facial and distal limb musculature; (7) pronounced fluctuations in cardio-respiratory rhythms and core body temperature; and (8) penile erection and clitoral tumescence [4]. In research over the last four decades considerable progress has been made in identifying the brain structures, neurotransmitters, receptors, and neuronal networks critical to the regulation of REM sleep [4]. Following is a summary of this research on the neuronal mechanisms of REM sleep regulation.
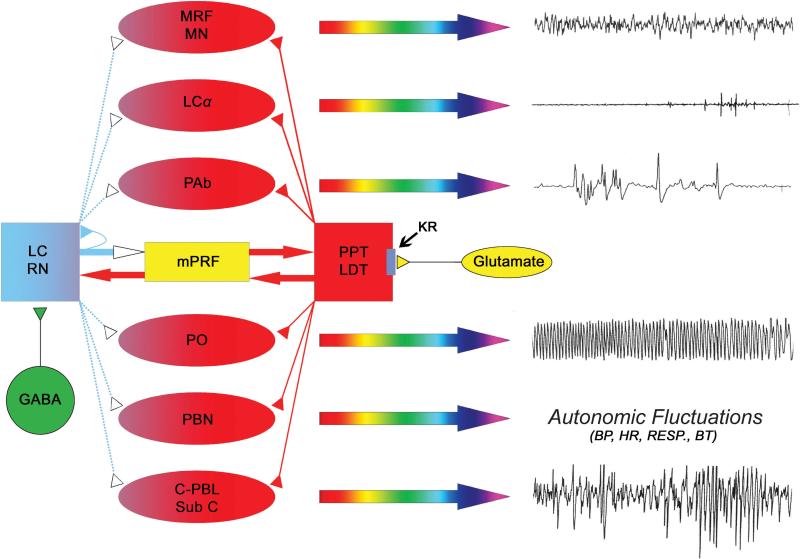
During the past decade, evidence from both rat and cat studies has suggested that each of the events of REM sleep is executed by distinct cell groups located in the brainstem [4,10,117]. These cell groups are discrete components of a widely distributed network, rather than a single REM sleep “center” (Fig. 1). For example, muscle atonia is executed by the activation of neurons in the locus coeruleus alpha (LCα); rapid eye movements result from the activation of neurons in the peri-abducens reticular formation (PAb); PGO waves emerge from activation of neurons in the caudo-lateral peribrachial area (C-PBL) of predator mammals and in the dorsal part of the nucleus subcoeruleus (SubCD) of prey mammals; hippocampal theta rhythm is produced via the activation of neurons in the pontis oralis (PO), muscle twitches appear with the activation of neurons in the nucleus gigantocellularis (especially the caudal part), and increased brain temperature and cardio-respiratory fluctuations result from the activation of neurons in the parabrachial nucleus (PBN). The cortical EEG activation sign of REM sleep, however, is collaboratively executed by activation of neurons in the mesencephalic reticular formation (MRF) and rostrally-projecting bulbar reticular formation (also called medullary magnocellular nucleus, MN). It should be noted here that the mentioned cell groups simply represent the executive neurons for the individual signs. Final expression of each is executed by the relevant neuronal circuit unique to that REM sleep sign. In essence, each of these REM sleep signs has a separate, specialized network. Thus, each of these REM sleep signs could be modulated by multiple neurotransmitters at multiple sites of their circuit.
Figure 1.
Cellular-Molecular-Network model of physiological mechanisms for the generation of REM sleep. Each of the individual signs of REM sleep (right column, polygraphic signs) is executed by the increased activation of distinct cell groups in the brainstem (red-colored oval shapes). For example, cortical EEG activation sign of REM sleep is executed jointly by the activation of neurons in the mesencephalic reticular formation (MRF) and the rostrally-projecting bulbar reticular formation (medullary magnocellular nucleus, MN); muscle atonia is executed by the neurons in the locus coeruleus alpha (LCα); rapid eye movements are executed by the neurons in the peri-abducens reticular formation (PAb); PGO/P-waves are executed by the neurons in the caudo-lateral peribrachial area (C-PBL) of predator mammals and in the dorsal part of the nucleus subcoeruleus (Sub C) of prey mammals; hippocampal theta rhythm is executed by the neurons in the pontis oralis (PO), and increased brain temperature and cardio-respiratory fluctuations are executed by the neurons in the parabrachial nucleus (PBN). These REM sleep sign generating executive neurons are excited by the increased release of cholinergic neurotransmitter while the releases of aminergic neurotransmitter are reduced and/or absent. The sources of the cholinergic neurotransmitter (red) are the cholinergic neurons in the pedunculopontine tegmentum (PPT) and lateral dorsal tegmentum (LDT). The sources of the aminergic neurotransmitters (blue) are the noradrenergic neurons in the locus coeruleus (LC) and serotonergic neurons in the raphe nucleus (RN). For the initiation of REM sleep, kainate receptors on the cholinergic cells are activated by the increased release of glutamate (yellow) that ultimately activates cholinergic cells and increase release of acetylcholine in each of the REM sleep sign-generators and in the cholinoceptive REM sleep inducing site in the medial pontine reticular formation (mPRF). While PPT/LDT cholinergic cells are activated, local GABAergic cells (green) in the LC and RN are also activated. Activation of these local GABAergic cells actively inhibits aminergic cells in the LC and RN. Active inhibition of those aminergic cells reduces and/or stops releasing aminergic neurotransmitters to those REM sleep sign-generators. For the maintenance of REM sleep episodes, increased acetylcholine release in the mPRF activates glutamatergic cells that continue to release glutamate in the PPT/LDT to maintain activity of cholinergic cells. Thus, PPT/LDT cholinergic cells and mPRF glutamatergic cells create a positive feedback loop to maintain REM sleep. Activation of mPRF glutamatergic cells also releases glutamate in the LC and RN. This glutamate could also activate both aminergic cells and local GABAergic cells in the LC and RN. Activation of GABAergic cells intensifies their inhibitory response to those aminergic cells. But, the possibility of mPRF glutamate activating these aminergic cells is eliminated by the increased release of GABA in the LC and RN and also auto-inhibition. (Reproduced with permission from [4]).
Turn-on and turn-off conditions of REM sleep generating executive neurons are regulated by the ratio of available aminergic and cholinergic neurotransmitters within those cell groups. Aminergic neurotransmitters are released from the LC and RN, while cholinergic neurotransmitters originate from the PPT. The activity of both aminergic and cholinergic cells is approximately equal during wakefulness, and the onset of SWS results in an equal reduction in activity. Therefore, the ratio of aminergic to cholinergic neurotransmitters in REM sleep generators is proportionate during wakefulness and through SWS. During REM sleep, however, aminergic cell activities are markedly reduced or absent and cholinergic cell activities are comparatively high [118-124]. The level of cholinergic cell activity during REM sleep is roughly 35% less than that of wakefulness. Thus, when a hypothetical ratio of aminergic and cholinergic neurotransmitters is 1:1, the REM sleep sign-generator remains in turned-off condition; however, when this ratio is 0:0.65, the generator is turned-on to express REM sleep signs [4,123].
The PPT is situated in the dorsolateral tegmentum and contains a prominent group of cholinergic neurons that project widely throughout the brainstem and forebrain [10,125-127]. To date, almost all studies have consistently demonstrated that the activation of cholinergic cells in the PPT is one of the most critical steps for the generation and maintenance of REM sleep [4,124]. Interestingly, the studies have also shown that the activation of cholinergic cells in the PPT is involved not only in the generation and maintenance of REM sleep but also involved in the termination of REM sleep episodes by inducing wakefulness [4]. Single cell recordings from the PPT in behaving cats and rats have identified several different classes of cells whose firing rates correlate with both wakefulness and REM sleep [22,123,128,129]. The results of single cell recordings and neuropharmacological studies have provided some direct evidence to suggest that when the activity level in the cholinergic cell compartment of the PPT reaches to about a 65% level by tonic release of glutamate, REM sleep is induced [123,130,131]. Throughout SWS, activation of GABA-B receptors inhibits cells in the cholinergic cell compartment of the PPT maintaining a population activity level of approximately 7.4% [123,132]. Activation of low-threshold, kainate-type glutamate receptors on the PPT cholinergic cells results in a population activity level increase in the cholinergic cell compartment of the PPT to about 65%, resulting in REM sleep generation [133,134]. When high-threshold NMDA-type receptors on those PPT cholinergic cells are also activated, the population activity in the cholinergic cell compartment reaches approximately 100%, resulting in wakefulness [123,134,135]. In summary, PPT cholinergic cell activity and REM sleep are regulated by interactions of the neurotransmitters glutamate and GABA, as well as through activation of kainate, NMDA, and GABA-B receptors.
It is well known that activation of kainate receptors increases cytoplasmic free calcium concentration [136-140]. In neurons, calcium ions can stimulate the production of cyclic adenosine monophosphate (cAMP) and the activation of protein kinase A (PKA) via the activation of adenylyl cyclase (AC) [141-144]. Thus, it is possible that the activation of kainate receptors in the PPT could also activate the cAMP-PKA signal transduction pathway to induce REM sleep. Indeed, one recent study has demonstrated that the inhibition of AC in the cholinergic cell compartment of the PPT suppresses REM sleep in the freely moving rat [145]. Yet another recent study demonstrated that REM sleep increases with increased catalytic subunit of PKA and PKA enzymatic activity in the cholinergic cell compartment of the PPT [146]. This study also demonstrated that the pharmacological inhibition of PPT intracellular cAMP-PKA activity suppresses REM sleep. Accordingly, it appears that the cAMP-PKA intracellular signaling pathway is critically involved in the cholinergic cell compartment of the PPT for the regulation of cholinergic tone in the REM sleep sign-generators. Rapid desensitization (in seconds) is one of the important characteristics of the kainate receptors (GluR6) but, increased cytosolic PKA activity can phosphorylate GluR6 subunits and modulate channel function for about 3-25 minutes [147]. In turn, this cytosolic PKA activity mediated phosphorylation increases the number of active receptors and effectively enhances the gating properties of the channels [147]. So, the increased PKA activity may also be responsible for the sustained activity of the PPT cells and maintenance of normal REM sleep episodes.
It is also known that Gi/Go G proteins inhibit AC and that inhibition of AC prevents activation of the cAMP-PKA signal transduction pathway [148,149]. Recently we have demonstrated that the PPT GABA-B receptor-activation-induced suppression of REM sleep involves inhibition of AC that ultimately prevents activation of the cAMP-PKA signaling pathway [150,151]. Therefore, suppression of neuronal activity during NREM sleep may be due to activation of GABA-B receptors in the PPT that ultimately inhibit the cAMP-PKA signal transduction pathway.
Given that activated NMDA receptors conduct calcium ions (Ca2+), it is reasonable to suggest that the PPT NMDA receptor activation-induced wakefulness may involve the Ca2+/calmodulin-dependent protein kinase II (CaMKII), a serine/threonine kinase constituting roughly 1-2% of total brain protein [151,152]. Importantly, activation of NMDA receptors potentiates CaMKII activity [153,154]. When active, CaMKII transduces membrane-mediated Ca2+ currents to affect transcriptional targets, such as the cAMP response element binding protein (CREB) [155]. Notably, CaMKII is capable of autophosphorylation, which unhinges the enzyme from direct Ca2+ -mediated activity [156]. Recently we have demonstrated that the levels of CaMKII and phosphorylated CaMKII expression in the PPT decreases with low wakefulness/high REM sleep and increases with high wakefulness/low REM sleep periods [157]. This study also demonstrated that with increased PPT CaMKII activity observed during high wakefulness/low REM sleep, there were marked shifts in the expression of genes that are involved in components of various signal transduction pathways. Collectively, the results of this study suggest that the increased CaMKII activity within the PPT neurons is associated with increased wakefulness at the expense of REM sleep, and this process is accomplished through the activation of a specific gene expression profile [157]. Since within the normal sleep-wake cycle, the end of REM sleep episodes corresponds with the beginning of wakefulness and this transition from REM sleep to wakefulness requires increased PPT neuronal activity, and based on the results discussed above, it is therefore reasonable to suggest that increased CaMKII activity in the PPT neurons is the intracellular signaling mechanism for the behavioral transition from REM sleep to wakefulness.
In summary, the generation and maintenance of REM sleep involves a complex system of neuronal connections. Distinct cell groups within the brainstem are responsible for the expression of individual events that characterize REM sleep (e.g. cortical activation, muscle atonia, rapid eye movement, P-waves). The turn-on/turn-off condition for the executive neurons unique to each cell group is regulated by the ratios of available aminergic and cholinergic neurotransmitters. Accordingly, the expression of REM sleep signs is a result of a significant reduction in aminergic tone and a comparatively high level of cholinergic tone within each REM sleep sign-generator. For these individual REM sleep sign generators, the source of cholinergic neurotransmitter is the PPT and sources of aminergic neurotransmitters are the LC and DRN. There are two types of cholinergic cells in the cholinergic cell compartment of the PPT: REM-on and W-REM-on types. REM-on cells increase firing rate during the transition periods from SWS to REM sleep. The second group of neurons, W-REM-on cells, increases firing activity during both wakefulness and REM sleep. Activation of low-threshold, kainate-type glutamate receptors on the PPT cholinergic cells generates REM sleep episodes by increasing their activity. Recent research has indicated that the activation of kainate receptors in the PPT induce REM sleep via activation of the cAMP-PKA signaling pathway. Endogenously released acetylcholine from the PPT also activates glutamatergic cells in the medial pontine reticular formation (mPRF). These activated mPRF cells subsequently release glutamate in the cholinergic cell compartment of the PPT, causing the continued release of acetylcholine into the mPRF and REM sleep sign generators. Therefore, once REM sleep is initiated by the kainate receptor activation-mediated excitation of PPT cholinergic cells, REM sleep episodes are maintained via a mutually excitatory positive feedback loop between the PPT cholinergic and the mPRF glutamatergic cells.
7. Conclusion and Future directions
As with all biomedical research, the ultimate goal of sleep research is to understand the basic mechanisms of sleep regulation and thereby find causes and cures for sleep disorders. The wealth of information presented here shows just how much progress neurobiological research has made in identifying the brain areas, cell types, neurotransmitters and their receptors, neuronal networks, and synaptic interactions integral to the regulation of mammalian sleep.
Based on our present understanding of the mechanisms of SWS and REM sleep regulation, a number of remedies for insomnia and other sleep disorders have been developed. At this time, the principal drugs used in sleep medicine are GABA-mimetic drugs, which are effective in restoring SWS. Unfortunately, nearly all of these SWS-inducing drugs suppress REM sleep. Therefore, the immediate challenge to sleep research is to identify novel pharmacological agents that restore SWS as well as REM sleep.
Ongoing molecular studies suggest that progressive research in intracellular signaling pathways holds perhaps the greatest potential to fully elucidate the mechanisms by which normal sleep architecture is generated and maintained. In the future, it is expected that more and more studies will seek to expand our knowledge of sleep on the molecular level. The results of those molecular studies will provide insight in designing a new generation of drugs that will be more effective in the treatment of sleep disorders and other ailments that are affected by the sleep disorders.
Acknowledgements
This work was supported by grants from the US National Institutes of Health (MH59839 and NS34004). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. I thank Mr. Brian W. Macone for his assistance in the production of this manuscript. I thank Dr. E. Patterson and Dr. Donald Siwek for their helpful suggestions on this manuscript. I would like to gratefully acknowledge my mentor, Professor J. Allan Hobson, for providing his wisdom and unconditional support to the research program of the Datta Laboratory at Boston University School of Medicine. This publication is dedicated to Dr. J. Allan Hobson for his critical and novel contributions to our understanding of the basic mechanisms of sleep regulation and its functions over the past four decades.
Footnotes
Disclosure/conflict of interest
The author declares there is no conflict of interest.
References
- 1.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3:679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 2.Datta S, Patterson EH. Activation of phasic pontine wave (P-wave): A mechanism of learning and memory processing. In: Maquet P, Smith C, Stickgold R, editors. Sleep and Brain Plasticity. Oxford University Press; New York: 2003. p. 379. [Google Scholar]
- 3.Hobson JA. REM sleep and dreaming: towards a theory of protoconsciousness. Nat Rev Neurosci. 2009;10:803–813. doi: 10.1038/nrn2716. [DOI] [PubMed] [Google Scholar]
- 4.Datta S, Maclean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pace-Schott EF, Hobson JA. The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci. 2002;3:591–605. doi: 10.1038/nrn895. [DOI] [PubMed] [Google Scholar]
- 6.Jouvet M, Michel F. [Electromyographic correlations of sleep in the chronic decorticate & mesencephalic cat.]. C R Seances Soc Biol Fil. 1959;153:422–425. [PubMed] [Google Scholar]
- 7.Hobson JA. The Effects of Chronic Brain-Stem Lesions on Cortical and Muscular Activity During Sleep and Waking in the Cat. Electroencephalogr Clin Neurophysiol. 1965;19:41–62. doi: 10.1016/0013-4694(65)90006-4. [DOI] [PubMed] [Google Scholar]
- 8.Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–1295. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 9.Lydic R, Baghdoyan HA. Acetylcholine modulates sleep and wakefulness: a synaptic perspective. In: Monti JM, Pandi-Perumal SR, Sinton CM, editors. Neurochemistry of Sleep and Wakefulness. Cambridge University Press; Cambridge: 2008. pp. 109–143. [Google Scholar]
- 10.Datta S. Neuronal activity in the peribrachial area: relationship to behavioral state control. Neurosci Biobehav Rev. 1995;19:67–84. doi: 10.1016/0149-7634(94)00043-z. [DOI] [PubMed] [Google Scholar]
- 11.Kodama T, Takahashi Y, Honda Y. Enhancement of acetylcholine release during paradoxical sleep in the dorsal tegmental field of the cat brain stem. Neurosci Lett. 1990;114:277–282. doi: 10.1016/0304-3940(90)90576-u. [DOI] [PubMed] [Google Scholar]
- 12.Day J, Damsma G, Fibiger HC. Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo microdialysis study. Pharmacol Biochem Behav. 1991;38:723–729. doi: 10.1016/0091-3057(91)90233-r. [DOI] [PubMed] [Google Scholar]
- 13.Lydic R, Baghdoyan HA, Lorinc Z. Microdialysis of cat pons reveals enhanced acetylcholine release during state-dependent respiratory depression. Am J Physiol. 1991;261:R766–770. doi: 10.1152/ajpregu.1991.261.3.R766. [DOI] [PubMed] [Google Scholar]
- 14.Mochizuki T, Yamatodani A, Okakura K, Horii A, Inagaki N, Wada H. Circadian rhythm of histamine release from the hypothalamus of freely moving rats. Physiol Behav. 1992;51:391–394. doi: 10.1016/0031-9384(92)90157-w. [DOI] [PubMed] [Google Scholar]
- 15.Smith AD, Olson RJ, Justice JB. Quantitative microdialysis of dopamine in the striatum: effect of circadian variation. J Neurosci Methods. 1992;44:33–41. doi: 10.1016/0165-0270(92)90111-p. [DOI] [PubMed] [Google Scholar]
- 15.Kurosawa M, Okada K, Sato A, Uchida S. Extracellular release of acetylcholine, noradrenaline and serotonin increases in the cerebral cortex during walking in conscious rats. Neurosci Lett. 1993;161:73–76. doi: 10.1016/0304-3940(93)90143-9. [DOI] [PubMed] [Google Scholar]
- 17.Williams JA, Comisarow J, Day J, Fibiger HC, Reiner PB. State-dependent release of acetylcholine in rat thalamus measured by in vivo microdialysis. J Neurosci. 1994;14:5236–5242. doi: 10.1523/JNEUROSCI.14-09-05236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marrosu F, Portas C, Mascia MS. Microdialysis measurement of cortical and hippocampal acetylcholine release during sleep-wake cycle in freely moving cats. Brain Res. 1995;671:329–332. doi: 10.1016/0006-8993(94)01399-3. [DOI] [PubMed] [Google Scholar]
- 19.Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 1996;742:89–97. doi: 10.1016/s0006-8993(96)00967-5. [DOI] [PubMed] [Google Scholar]
- 20.Portas CM, Thakkar M, Rainnie D, McCarley RW. Microdialysis perfusion of 8-hydroxy-2-(din-propylamino)tetralin (8-OH-DPAT) in the dorsal raphe nucleus decreases serotonin release and increases rapid eye movement sleep in the freely moving cat. J Neurosci. 1996;16:2820–2828. doi: 10.1523/JNEUROSCI.16-08-02820.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodama T, Lai YY, Siegel JM. Enhanced glutamate release during REM sleep in the rostromedial medulla as measured by in vivo microdialysis. Brain Res. 1998;780:176–179. [PubMed] [Google Scholar]
- 22.Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J Neurosci. 1998;18:5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berridge CW, Abercrombie ED. Relationship between locus coeruleus discharge rates and rates of norepinephrine release within neocortex as assessed by in vivo microdialysis. Neurosci. 1999;93:1263–1270. doi: 10.1016/s0306-4522(99)00276-6. [DOI] [PubMed] [Google Scholar]
- 24.Kawahara Y, Kawahara H, Westerink BH. Tonic regulation of the activity of noradrenergic neurons in the locus coeruleus of the conscious rat studied by dual-probe microdialysis. Brain Res. 1999;823:42–48. doi: 10.1016/s0006-8993(99)01062-8. [DOI] [PubMed] [Google Scholar]
- 25.Feenstra MG, Botterblom MH, Mastenbroek S. Dopamine and noradrenaline efflux in the prefrontal cortex in the light and dark period: effects of novelty and handling and comparison to the nucleus accumbens. Neurosci. 2000;100:741–748. doi: 10.1016/s0306-4522(00)00319-5. [DOI] [PubMed] [Google Scholar]
- 26.Nitz D, Siegel JM. GABA release in posterior hypothalamus across sleep-wake cycle. Am J Physiol. 1996;271:R1707–R1712. doi: 10.1152/ajpregu.1996.271.6.R1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleepwake state. Neurosci. 1997;78:795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kodama T, Lai YY, Siegel JM. Changes in inhibitory amino acid release linked to pontine-induced atonia: an in vivo microdialysis study. J Neurosci. 2003;23:1548–1554. doi: 10.1523/JNEUROSCI.23-04-01548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson CJ, Lydic R, Baghdoyan HA. Sleep and GABA levels in the oral part of rat pontine reticular formation are decreased by local and systemic administration ofmorphine. Neurosci. 2007;144:375–386. doi: 10.1016/j.neuroscience.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vanini G, Watson CJ, Lydic R, Baghdoyan HA. Gamma-aminobutyric acid-mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology. 2008;109:978–988. doi: 10.1097/ALN.0b013e31818e3b1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 32.Moruzzi G. Reticular Influences on the Eeg. Electroencephalogr Clin Neurophysiol. 1964;16:2–17. doi: 10.1016/0013-4694(64)90021-5. [DOI] [PubMed] [Google Scholar]
- 33.Moruzzi G. The sleep-waking cycle. Ergeb Physiol. 1972;64:1–165. doi: 10.1007/3-540-05462-6_1. [DOI] [PubMed] [Google Scholar]
- 34.Morgane PJ, Stern WC. Chemical anatomy of brain: circuits in relation to sleep and wakefulness. In: Weitzman ED, editor. Advances in Sleep Research. Spectrum Publications; Flushing, New York: 1974. pp. 1–131. [Google Scholar]
- 35.Garcia-Rill E. Mechaninsm of Sleep and Wakefulness. In: Lee-Chiong TL Jr., Sateia MJ, Carskadon MA, editors. Sleep Medicine. Hanley & Belfus, Inc.; Phildelphia, PA: 2002. pp. 31–39. [Google Scholar]
- 36.Sakai K, Crochet S. A neural mechanism of sleep and wakefulness. Sleep and Biol Rhythms. 2003;1:29–42. [Google Scholar]
- 37.Swett CP, Hobson JA. The effects of posterior hypothalamic lesions on behavioral and electrographic manifestations of sleep and waking in cats. Arch Ital Biol. 1968;106:283–293. [PubMed] [Google Scholar]
- 38.Szymusiak R, McGinty D. Sleep-waking discharge of basal forebrain projection neurons in cats. Brain Res Bull. 1989;22:423–430. doi: 10.1016/0361-9230(89)90069-5. [DOI] [PubMed] [Google Scholar]
- 39.Maquet P, Dive D, Salmon E, Sadzot B, Franco G, Poirrier R, von Frenckell R, Franck G. Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and [18F]2-fluoro-2-deoxy-D-glucose method. Brain Res. 1990;513:136–143. doi: 10.1016/0006-8993(90)91099-3. [DOI] [PubMed] [Google Scholar]
- 40.Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol. 1999;521:679–690. doi: 10.1111/j.1469-7793.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koyama Y, Kodama T, Takahashi K, Okai K, Kayama Y. Firing properties of neurones in the laterodorsal hypothalamic area during sleep and wakefulness. Psychiatry Clin Neurosci. 2002;56:339–340. doi: 10.1046/j.1440-1819.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- 43.Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological, and pharmacological characteristics of histidine decarboxylase knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deboer T, Vansteensel MJ, Detari L, Meijer JH. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat Neurosci. 2003;6:1086–1090. doi: 10.1038/nn1122. [DOI] [PubMed] [Google Scholar]
- 45.Gerashchenko D, Blanco-Centurion C, Greco MA, Shiromani PJ. Effects of lateral hypothalamic lesion with the neurotoxin hypocretin-2-saporin on sleep in Long-Evans rats. Neurosci. 2003;116:223–235. doi: 10.1016/s0306-4522(02)00575-4. [DOI] [PubMed] [Google Scholar]
- 46.Mendelson WB, Bergmann BM, Tung A. Baseline and post-deprivation recovery sleep in SCN-lesioned rats. Brain Res. 2003;980:185–190. doi: 10.1016/s0006-8993(03)02896-8. [DOI] [PubMed] [Google Scholar]
- 47.Easton A, Meerlo P, Bergmann B, Turek FW. The suprachiasmatic nucleus regulates sleep timing and amount in mice. Sleep. 2004;27:1307–1318. doi: 10.1093/sleep/27.7.1307. [DOI] [PubMed] [Google Scholar]
- 48.Gerashchenko D, Shiromani PJ. Effects of inflammation produced by chronic lipopolysaccharide administration on the survival of hypocretin neurons and sleep. Brain Res. 2004;1019:162–169. doi: 10.1016/j.brainres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 49.Gerashchenko D, Shiromani PJ. Different neuronal phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol Neurobiol. 2004;29:41–59. doi: 10.1385/MN:29:1:41. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi K, Lin JS, Sakai K. Neuronal activity of histaminergic tuberomammillary neurons during wake-sleep states in the mouse. J Neurosci. 2006;26:10292–10298. doi: 10.1523/JNEUROSCI.2341-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neurosci. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 52.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 53.Jones EG. The Thalamus. Plenum; New York: 1985. [Google Scholar]
- 54.Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- 55.Datta S. Activation of phasic pontine-wave generator: a mechanism for sleep-dependent memory processing. Sleep and Biological Rhythms. 2006;4:16–26. [Google Scholar]
- 56.von Economo C. Sleep as a problem of localization. J Nerv and Ment Dis. 1930;71:249–259. [Google Scholar]
- 57.Nauta W. Hypothalamic regulation of sleep in rats. J Neurophysiol. 1946;9:285–316. doi: 10.1152/jn.1946.9.4.285. [DOI] [PubMed] [Google Scholar]
- 58.McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–1255. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- 59.John J, Kumar VM, Gopinath G, Ramesh V, Mallick H. Changes in sleep-wakefulness after kainic acid lesion of the preoptic area in rats. Jpn J Physiol. 1994;44:231–242. doi: 10.2170/jjphysiol.44.231. [DOI] [PubMed] [Google Scholar]
- 60.John J, Kumar VM. Effect of NMDA lesion of the medial preoptic neurons on sleep and other functions. Sleep. 1998;21:587–598. doi: 10.1093/sleep/21.6.587. [DOI] [PubMed] [Google Scholar]
- 61.Kumar VM, Khan NA, John J. Male sexual behaviour not abolished after medial preoptic lesion in adult rats. Neuroreport. 1996;7:1481–1484. doi: 10.1097/00001756-199606170-00007. [DOI] [PubMed] [Google Scholar]
- 62.Srividya R, Mallick HN, Kumar VM. Differences in the effects of medial and lateral preoptic lesions on thermoregulation and sleep in rats. Neuroscience. 2006;139:853–864. doi: 10.1016/j.neuroscience.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Alam MN, McGinty D, Szymusiak R. Preoptic/anterior hypothalamic neurons: thermosensitivity in rapid eye movement sleep. Am J Physiol. 1995;269:R1250–R1257. doi: 10.1152/ajpregu.1995.269.5.R1250. [DOI] [PubMed] [Google Scholar]
- 64.Findlay AL, Hayward JN. Spontaneous activity of single neurones in the hypothalamus of rabbits during sleep and waking. J Physiol. 1969;201:237–258. doi: 10.1113/jphysiol.1969.sp008753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glotzbach SF, Heller HC. Changes in the thermal characteristics of hypothalamic neurons during sleep and wakefulness. Brain Res. 1984;309:17–26. doi: 10.1016/0006-8993(84)91006-0. [DOI] [PubMed] [Google Scholar]
- 66.Kaitin KI. Preoptic area unit activity during sleep and wakefulness in the cat. Exp Neurol. 1984;83:347–357. doi: 10.1016/S0014-4886(84)90103-1. [DOI] [PubMed] [Google Scholar]
- 67.Koyama Y, Hayaishi O. Firing of neurons in the preoptic/anterior hypothalamic areas in rat: its possible involvement in slow wave sleep and paradoxical sleep. Neurosci Res. 1994;19:31–38. doi: 10.1016/0168-0102(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 68.Kumar VM, Datta S, Singh B. The role of reticular activating system in altering medial preoptic neuronal activity in anesthetized rats. Brain Res Bull. 1989;22:1031–1037. doi: 10.1016/0361-9230(89)90016-6. [DOI] [PubMed] [Google Scholar]
- 69.Lincoln DW. Correlation of unit activity in the hypothalamus with EEG patterns associated with the sleep cycle. Exp Neurol. 1969;24:1–18. doi: 10.1016/0014-4886(69)90002-8. [DOI] [PubMed] [Google Scholar]
- 70.McGinty D, Szymusiak R. Keeping cool: a hypothesis about the mechanisms and functions of slow-wave sleep. Trends Neurosci. 1990;13:480–487. doi: 10.1016/0166-2236(90)90081-k. [DOI] [PubMed] [Google Scholar]
- 71.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–188. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 72.McGinty D, Szymusiak R. The sleep-wake switch: A neuronal alarm clock. Nat Med. 2000;6:510–511. doi: 10.1038/74988. [DOI] [PubMed] [Google Scholar]
- 73.Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D. Sleep-waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol. 2002;543:665–677. doi: 10.1113/jphysiol.2002.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khubchandani M, Jagannathan NR, Mallick HN, Mohan Kumar V. Functional MRI shows activation of the medial preoptic area during sleep. Neuroimage. 2005;26:29–35. doi: 10.1016/j.neuroimage.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Datta S, Mohan Kumar V, Chhina GS, Singh B. Tonic activity of medial preoptic norepinephrine mechanism for body temperature maintenance in sleeping and awake rats. Brain Res Bull. 1985;15:447–451. doi: 10.1016/0361-9230(85)90034-6. [DOI] [PubMed] [Google Scholar]
- 76.Datta S, Kumar VM, Chhina GS, Singh B. Interrelationship of thermal and sleep-wakefulness changes elicited from the medial preoptic area in rats. Exp Neurol. 1988;100:40–50. doi: 10.1016/0014-4886(88)90199-9. [DOI] [PubMed] [Google Scholar]
- 77.Kumar VM, Datta S, Chhina GS, Singh B. Alpha-adrenergic system in medial preoptic area involved in sleep-wakefulness in rats. Brain Res Bull. 1986;16:463–468. doi: 10.1016/0361-9230(86)90174-7. [DOI] [PubMed] [Google Scholar]
- 78.Mendelson WB, Martin JV. Characterization of the hypnotic effects of triazolam microinjections into the medial preoptic area. Life Sci. 1992;50:1117–1128. doi: 10.1016/0024-3205(92)90349-t. [DOI] [PubMed] [Google Scholar]
- 79.Ticho SR, Radulovacki M. Role of adenosine in sleep and temperature regulation in the preoptic area of rats. Pharmacol Biochem Behav. 1991;40:33–40. doi: 10.1016/0091-3057(91)90317-u. [DOI] [PubMed] [Google Scholar]
- 80.Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–294. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 81.Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gvilia I, Turner A, McGinty D, Szymusiak R. Preoptic area neurons and the homeostatic regulation of rapid eye movement sleep. J Neurosci. 2006;26:3037–3044. doi: 10.1523/JNEUROSCI.4827-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gritti I, Mainville L, Jones BE. Projections of GABAergic and cholinergic basal forebrain and GABAergic preoptic-anterior hypothalamic neurons to the posterior lateral hypothalamus of the rat. J Comp Neurol. 1994;339:251–268. doi: 10.1002/cne.903390206. [DOI] [PubMed] [Google Scholar]
- 84.Zardetto-Smith AM, Johnson AK. Chemical topography of efferent projections from the median preoptic nucleus to pontine monoaminergic cell groups in the rat. Neurosci Lett. 1995;199:215–219. doi: 10.1016/0304-3940(95)12003-m. [DOI] [PubMed] [Google Scholar]
- 85.Steininger TL, Gong H, McGinty D, Szymusiak R. Subregional organization of preoptic area/anterior hypothalamic projections to arousal-related monoaminergic cell groups. J Comp Neurol. 2001;429:638–653. [PubMed] [Google Scholar]
- 86.Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111:231–239. doi: 10.1016/s0306-4522(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 87.Ulloor J, Mavanji V, Saha S, Siwek DF, Datta S. Spontaneous REM sleep is modulated by the activation of the pedunculopontine tegmental GABAB receptors in the freely moving rat. J Neurophysiol. 2004;91:1822–1831. doi: 10.1152/jn.01104.2003. [DOI] [PubMed] [Google Scholar]
- 88.Sallanon M, Denoyer M, Kitahama K, Aubert C, Gay N, Jouvet M. Long-lasting insomnia induced by preoptic neuron lesions and its transient reversal by muscimol injection into the posterior hypothalamus in the cat. Neuroscience. 1989;32:669–683. doi: 10.1016/0306-4522(89)90289-3. [DOI] [PubMed] [Google Scholar]
- 89.Mendelson WB. The sleep-inducing effect of ethanol microinjection into the medial preoptic area is blocked by flumazenil. Brain Res. 2001;892:118–121. doi: 10.1016/s0006-8993(00)03243-1. [DOI] [PubMed] [Google Scholar]
- 90.Tung A, Bluhm B, Mendelson WB. The hypnotic effect of propofol in the medial preoptic area of rat. Life Sci. 2001;69:855–862. doi: 10.1016/s0024-3205(01)01179-1. [DOI] [PubMed] [Google Scholar]
- 91.Tung A, Mendelson WB. Anesthesia and sleep. Sleep Med Rev. 2004;8:213–225. doi: 10.1016/j.smrv.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 92.Merchenthaler I, Vigh S, Schally AV, Petrusz P. Immunocytochemical localization of growth hormone-releasing factor in the rat hypothalamus. Endocrinology. 1984;114:1082–1085. doi: 10.1210/endo-114-4-1082. [DOI] [PubMed] [Google Scholar]
- 93.Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985;241:138–153. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- 94.Daikoku S, Kawano H, Noguchi M, et al. GRF neurons in the rat hypothalamus. Brain Res. 1986;399:250–261. doi: 10.1016/0006-8993(86)91515-5. [DOI] [PubMed] [Google Scholar]
- 95.Bredow S, Taishi P, Obel F, Jr., Guha-Thakurta N, Krueger JM. Hypothalamic growth hormone-releasing hormone mRNA varies across the day in rats. Neuroreport. 1996;7:2501–2505. doi: 10.1097/00001756-199611040-00020. [DOI] [PubMed] [Google Scholar]
- 96.Toppila J, Alanko L, Asikainen M, Tobler I, Stenberg D, Porkka-Heiskanen T. Sleep deprivation increases somatostatin and growth hormone-releasing hormone messenger RNA in the rat hypothalamus. J Sleep Res. 1997;6:171–178. doi: 10.1046/j.1365-2869.1997.00049.x. [DOI] [PubMed] [Google Scholar]
- 97.Gardi J, Obal F, Jr., Fang J, Zhang J, Krueger JM. Diurnal variations and sleep deprivation-induced changes in rat hypothalamic GHRH and somatostatin contents. Am J Physiol. 1999;277:R1339–R1344. doi: 10.1152/ajpregu.1999.277.5.R1339. [DOI] [PubMed] [Google Scholar]
- 98.Zhang J, Chen Z, Taishi P, Obal F, Fang J, Krueger J. Sleep deprivation increases rat hypothalamic growth hormone-releasing hormone messenger RNA in the rat hypothalamus. American Journal of Physiology. 1999;275:R1755–R1761. doi: 10.1152/ajpregu.1998.275.6.R1755. [DOI] [PubMed] [Google Scholar]
- 99.Obal F, Jr., Krueger JM. GHRH and sleep. Sleep Med Rev. 2004;8:367–377. doi: 10.1016/j.smrv.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 100.Steiger A, Guldner J, Hemmeter U, Rothe B, Wiedemann K, Holsboer F. Effects of growth hormone-releasing hormone and somatostatin on sleep EEG and nocturnal hormone secretion in male controls. Neuroendocrinology. 1992;56:566–573. doi: 10.1159/000126275. [DOI] [PubMed] [Google Scholar]
- 101.Kerkhofs M, Van Cauter E, Van Onderbergen A, Caufriez A, Thorner MO, Copinschi G. Sleep-promoting effects of growth hormone-releasing hormone in normal men. Am J Physiol. 1993;264:E594–E598. doi: 10.1152/ajpendo.1993.264.4.E594. [DOI] [PubMed] [Google Scholar]
- 102.Marshall L, Derad I, Strasburger CJ, Fehm HL, Born J. A determinant factor in the efficacy of GHRH administration in promoting sleep: high peak concentration versus recurrent increasing slopes. Psychoneuroendocrinology. 1999;24:363–370. doi: 10.1016/s0306-4530(98)00085-7. [DOI] [PubMed] [Google Scholar]
- 103.Schussler P, Yassouridis A, Uhr M, et al. Growth hormone-releasing hormone and corticotropin-releasing hormone enhance non-rapid-eye-movement sleep after sleep deprivation. Am J Physiol Endocrinol Metab. 2006;291:E549–E556. doi: 10.1152/ajpendo.00641.2005. [DOI] [PubMed] [Google Scholar]
- 104.Obal F, Jr., Floyd R, Kapas L, Bodosi B, Krueger JM. Effects of systemic GHRH on sleep in intact and hypophysectomized rats. Am J Physiol. 1996;270:E230–E237. doi: 10.1152/ajpendo.1996.270.2.E230. [DOI] [PubMed] [Google Scholar]
- 105.Ehlers CL, Reed TK, Henriksen SJ. Effects of corticotropin-releasing factor and growth hormone-releasing factor on sleep and activity in rats. Neuroendocrinology. 1986;42:467–474. doi: 10.1159/000124489. [DOI] [PubMed] [Google Scholar]
- 106.Nistico G, De Sarro GB, Langer SZ. Behavioural and electrocortical power spectrum effects of 5-methoxytryptoline and other analogs after intraventricular administration in rats. Eur J Pharmacol. 1987;142:121–128. doi: 10.1016/0014-2999(87)90660-1. [DOI] [PubMed] [Google Scholar]
- 107.Obal F, Jr., Alfoldi P, Cady AB, Johannsen L, Sary G, Krueger JM. Growth hormone-releasing factor enhances sleep in rats and rabbits. Am J Physiol. 1988;255:R310–R316. doi: 10.1152/ajpregu.1988.255.2.R310. [DOI] [PubMed] [Google Scholar]
- 108.Obal F, Jr., Payne L, Kapas L, Opp M, Krueger JM. Inhibition of growth hormone-releasing factor suppresses both sleep and growth hormone secretion in the rat. Brain Res. 1991;557:149–153. doi: 10.1016/0006-8993(91)90128-i. [DOI] [PubMed] [Google Scholar]
- 109.Obal F, Jr., Payne L, Opp M, Alfoldi P, Kapas L, Krueger JM. Growth hormone-releasing hormone antibodies suppress sleep and prevent enhancement of sleep after sleep deprivation. Am J Physiol. 1992;263:R1078–R1085. doi: 10.1152/ajpregu.1992.263.5.R1078. [DOI] [PubMed] [Google Scholar]
- 110.Stern WC, Jalowiec JE, Shabshelowitz H, Morgane PJ. Effects of growth hormone on sleep-waking patterns in cats. Horm Behav. 1975;6:189–196. doi: 10.1016/0018-506x(75)90035-5. [DOI] [PubMed] [Google Scholar]
- 111.Obal F, Jr., Fang J, Taishi P, Kacsoh B, Gardi J, Krueger JM. Deficiency of growth hormone-releasing hormone signaling is associated with sleep alterations in the dwarf rat. J Neurosci. 2001;21:2912–2918. doi: 10.1523/JNEUROSCI.21-08-02912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Obal F, Jr., Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:d520–50. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- 113.De A, Churchill L, Obal F, Jr., Simasko SM, Krueger JM. GHRH and IL1beta increase cytoplasmic Ca(2+) levels in cultured hypothalamic GABAergic neurons. Brain Res. 2002;949:209–212. doi: 10.1016/s0006-8993(02)03157-8. [DOI] [PubMed] [Google Scholar]
- 114.Peterfi Z, McGinty D, Sarai E, Szymusiak R. Growth hormone-releasing hormone activates sleep regulatory neurons of the rat preoptic hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2010;298:R147–R156. doi: 10.1152/ajpregu.00494.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gerashchenko D, Wisor JP, Burns D, et al. Identification of a population of sleep-active cerebral cortex neurons. Proc Natl Acad Sci U S A. 2008;105:10227–10232. doi: 10.1073/pnas.0803125105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vertes RP. Brainstem control of the events of REM sleep. Prog Neurobiol. 1984;22:241–288. doi: 10.1016/0301-0082(84)90020-0. [DOI] [PubMed] [Google Scholar]
- 118.McGinty DJ, Harper RM. Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res. 1976;101:569–575. doi: 10.1016/0006-8993(76)90480-7. [DOI] [PubMed] [Google Scholar]
- 119.Hobson JA. Arrest of firing of aminergic neurones during REM sleep: implications for dream theory. Brain Res Bull. 1999;50:333–334. doi: 10.1016/s0361-9230(99)00146-x. [DOI] [PubMed] [Google Scholar]
- 120.Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Foote SL, Bloom FE, Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 122.Lydic R, McCarley RW, Hobson JA. The time-course of dorsal raphe discharge, PGO waves, and muscle tone averaged across multiple sleep cycles. Brain Res. 1983;274:365–370. doi: 10.1016/0006-8993(83)90720-5. [DOI] [PubMed] [Google Scholar]
- 123.Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. Journal of Neuroscience Research. 2002;70:611–621. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 124.Datta S, Siwek DF, Stack EC. Identification of cholinergic and non-cholinergic neurons in the pons expressing phosphorylated cyclic AMP response element-binding protein as a function of rapid eye movement sleep. Neurosci. 2009;163:397–414. doi: 10.1016/j.neuroscience.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- 126.Lai YY, Clements JR, Siegel JM. Glutamatergic and cholinergic projections to the pontine inhibitory area identified with horseradish peroxidase retrograde transport and immunohistochemistry. J Comp Neurol. 1993;336:321–330. doi: 10.1002/cne.903360302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jones BE. Paradoxical REM sleep promoting and permitting neuronal networks. Arch Ital Biol. 2004;142:379–396. [PubMed] [Google Scholar]
- 128.El-Mansari M, Sakai K, Jouvet M. Responses of presumed cholinergic mesopontine tegmental neurons to carbachol microinjections in freely moving cats. Exp Brain Res. 1990;83:115–123. doi: 10.1007/BF00232199. [DOI] [PubMed] [Google Scholar]
- 129.Steriade M, Pare D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Datta S, Patterson EH, Siwek DF. Endogenous and exogenous nitric oxide in the pedunculopontine tegmentum induces sleep. Synapse. 1997;27:69–78. doi: 10.1002/(SICI)1098-2396(199709)27:1<69::AID-SYN7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 131.Datta S, Spoley EE, Patterson EH. Microinjection of glutamate into the pedunculopontine tegmentum induces REM sleep and wakefulness in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;280:R752–R759. doi: 10.1152/ajpregu.2001.280.3.R752. [DOI] [PubMed] [Google Scholar]
- 132.Ulloor J, Mavanji V, Saha S, Siwek DF, Datta S. Spontaneous REM sleep is modulated by the activation of the pedunculopontine tegmental GABAB receptors in the freely moving rat. J Neurophysiol. 2004;91:1822–1831. doi: 10.1152/jn.01104.2003. [DOI] [PubMed] [Google Scholar]
- 133.Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- 134.Datta S, Spoley EE, Mavanji VK, Patterson EH. A novel role of pedunculopontine tegmental kainate receptors: a mechanism of rapid eye movement sleep generation in the rat. Neuroscience. 2002;114:157–164. doi: 10.1016/s0306-4522(02)00250-6. [DOI] [PubMed] [Google Scholar]
- 135.Datta S, Patterson EH, Spoley EE. Excitation of the pedunculopontine tegmental NMDA receptors induces wakefulness and cortical activation in the rat. J Neurosci Res. 2001;66:109–116. doi: 10.1002/jnr.1202. [DOI] [PubMed] [Google Scholar]
- 136.Haak LL, Heller HC, van den Pol AN. Metabotropic glutamate receptor activation modulates kainate and serotonin calcium response in astrocytes. J Neurosci. 1997;17:1825–1837. doi: 10.1523/JNEUROSCI.17-05-01825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bleakman D, Lodge D. Neuropharmacology of AMPA and kainate receptors. Neuropharmacology. 1998;37:1187–1204. doi: 10.1016/s0028-3908(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 138.Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54:369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 139.Bernard A, Ferhat L, Dessi F, et al. Q/R editing of the rat GluR5 and GluR6 kainate receptors in vivo and in vitro: evidence for independent developmental, pathological and cellular regulation. Eur J Neurosci. 1999;11:604–616. doi: 10.1046/j.1460-9568.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- 140.Kovacs AD, Cebers G, Liljequist S. Kainate receptor-mediated activation of the AP-1 transcription factor complex in cultured rat cerebellar granule cells. Brain Res Bull. 2000;52:127–133. doi: 10.1016/s0361-9230(00)00247-1. [DOI] [PubMed] [Google Scholar]
- 141.Ginty DD, Glowacka D, Bader DS, Hidaka H, Wagner JA. Induction of immediate early genes by Ca2+ influx requires cAMP-dependent protein kinase in PC12 cells. J Biol Chem. 1991;266:17454–17458. [PubMed] [Google Scholar]
- 142.Cali JJ, Zwaagstra JC, Mons N, Cooper DM, Krupinski J. Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem. 1994;269:12190–12195. [PubMed] [Google Scholar]
- 143.Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Waltereit R, Dammermann B, Wulff P, et al. Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J Neurosci. 2001;21:5484–5493. doi: 10.1523/JNEUROSCI.21-15-05484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Datta S, Prutzman SL. Novel role of brain stem pedunculopontine tegmental adenylyl cyclase in the regulation of spontaneous REM sleep in the freely moving rat. J Neurophysiol. 2005;94:1928–1937. doi: 10.1152/jn.00272.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bandyopadhya RS, Datta S, Saha S. Activation of pedunculopontine tegmental protein kinase A: a mechanism for rapid eye movement sleep generation in the freely moving rat. J Neurosci. 2006;26:8931–8942. doi: 10.1523/JNEUROSCI.2173-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang LY, Taverna FA, Huang XP, et al. Phosphorylation and modulation of kainate receptor (GluR6) by cAMP-dependent protein kinase. Science. 1993;259:1173–1175. doi: 10.1126/science.8382377. [DOI] [PubMed] [Google Scholar]
- 148.Gilman AG. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- 149.Marinissen MJ, Gutkind JS. G-protein-coupled receptors and signaling networks: emerging paradigms. Trends Pharmacol Sci. 2001;22:368–376. doi: 10.1016/s0165-6147(00)01678-3. [DOI] [PubMed] [Google Scholar]
- 150.Datta S. Activation of pedunculopontine tegmental PKA prevents GABAB receptor activation-mediated rapid eye movement sleep suppression in the freely moving rat. J Neurophysiol. 2007;97:3841–50. doi: 10.1152/jn.00263.2007. [DOI] [PubMed] [Google Scholar]
- 151.Colbran RJ. Regulation and role of brain calcium/calmodulin-dependent protein kinase II. Neurochem Int. 1992;21:469–497. doi: 10.1016/0197-0186(92)90080-b. [DOI] [PubMed] [Google Scholar]
- 152.Soderling TR. CaM-kinases: modulators of synaptic plasticity. Curr Opin Neurobiol. 2000;10:375–380. doi: 10.1016/s0959-4388(00)00090-8. [DOI] [PubMed] [Google Scholar]
- 153.Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/ calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl- D-aspartate receptor. J Biol Chem. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- 154.Leonard AS, Lim IA, Hemsworth DE, Horne MC, Hell JW. Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 1999;96:3239–3244. doi: 10.1073/pnas.96.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, McKnight GS. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol. 1994;14:6107–6116. doi: 10.1128/mcb.14.9.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hudmon A, Schulman H. Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- 157.Stack EC, Desarnaud F, Siwek DF, Datta S. A novel role for calcium/calmodulin kinase II within the brainstem pedunculopontine tegmentum for the regulation of wakefulness and rapid eye movement sleep. J Neurochem. 2010;112:271–281. doi: 10.1111/j.1471-4159.2009.06452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]