Abstract
1,1-Bis(3-indolyl)-1-(p-substituted phenyl)methanes (C-DIMs) substituted in the phenyl ring with a para-, t-butyl, trifluoro or phenyl (DIM-C-pPhC6H5) group activates peroxisome proliferator-activated receptor γ (PPARγ) in several cancer cell lines. In this study, we have examined the effects of 5,5'-dihyroxy, 5,5'-dimethyl, 5,5'-dibromo, 5,5'-dinitro and 5,5'-dimethoxyindole ring substituted analogs of DIM-C-pPhC6H5 on their activity as PPARγ agonists. Introduction of the 5,5'-dihydroxy and 5,5'-dimethyl substituents enhanced activation of PPARγ in colon and pancreatic cancer cells. However, activation of p21 in Panc28 pancreatic cancer cells and induction of caveolin-1 and KLF4 in colon cancer cells by the cells by these C-DIMs was structure- and cell context-dependent. The results demonstrate that DIM-C-pPhC6H5 and indole ring-substituted analogs are selective PPARγ modulators.
Keywords: C-DIMs, PPARγ agonists, indole ring substituents
INTRODUCTION
1,1-Bis(3'-indolyl)methane (DIM) is a metabolite of the phytochemical indole-3-carbinol and DIM has been used as scaffold to synthesize a series of 1,1-bis(3'-indolyl)-1-(p-substituted phenyl)methanes (C-DIMs) [1–14]. These compounds are triarylmethane derivatives which differ from DIM and ring-substituted DIMs which are diarylmethanes. Initial studies showed that some C-DIMs inhibited carcinogen-induced rat mammary tumor growth and growth of various cancer cell lines [1–5; 8]. The activation of several orphan nuclear receptors by a series of C-DIMs containing various p-substituents has also been determined and the results showed that some analogs activated PPARγ in breast cancer cells [8]. Subsequent studies showed that one or more of the three most active compounds, namely the p-trifluoromethyl (DIM-C-pPhCF3), p-t-butyl (DIM-C-pPhtBu), and p-phenyl (DIM-C-pPhC6H5) analogs also activated PPARγ in colon, pancreatic, prostate, bladder, breast, endometrial and kidney cancer cell lines [1–6; 8–12]. The PPARγ-active C-DIMs exhibit highly tissue-specific receptor-dependent activation of responses and genes. For example, these compounds induced PPARγ-dependent p21 gene expression in Panc28 pancreatic cancer cells and caveolin-1 in colon and bladder cancer cells [1–3]. However, most other responses such as C-DIM-induced proapoptotic and growth inhibitory effects were PPARγ-independent.
PPARγ-active C-DIMs also induce other proapoptotic responses that are both receptor- and ER stress-independent. For example, in SW480 colon cancer cells, DIM-C-pPhCF3 and DIM-C-pPhC6H5 do not induce typical markers of ER stress and, in both SW480 and HCT116 colon cancer cells, C-DIM compounds induce expression of NAG-1 and activating transcription factor 3 (ATF3) which are proapoptotic genes and proteins [4; 15]. DIM also induces NAG-1 and ATF3 in HCT116 cells; however, the mechanism of this response has not been determined. Induction of NAG-1 in HCT116 cells by PPARγ-active C-DIMs is dependent on PI3-K-dependent activation of early growth response-1 (Egr-1) gene which in turn activates NAG-1 through interactions with a proximal Egr-1 element in the NAG-1 promoter [4]. In contrast, induction of NAG-1 by DIM-C-pPhCF3 in LNCaP cells is MAPK-dependent [6] and suggests that induction of some proapoptotic genes such as NAG-1 are dependent on activation of kinase pathways by C-DIMs. It is clear from studies on C-DIM compounds that DIM is an excellent scaffold from which new chemotherapeutic agents can be derived. In this study, we have synthesized a series of symmetrical 5'-indole ring substituted analogs of DIM-C-pPhC6H5 and have investigated their cytotoxicity and PPARγ-dependent activity in colon and pancreatic cancer cells.
METHODS AND MATERIALS
Cell lines and reagents
SW480, HT-29 and HCT-15 human colon cancer cells, Panc-1 and Panc-28 human pancreatic cancer cells were obtained from American Type Culture Collection (Manassas, VA). SW480, HT-29, Panc-1 and Panc-28 cells were maintained in Dulbecco's modified/Ham's F-12 (Sigma Aldrich, St Louis, MO) with phenol red supplemented with 0.22% sodium bicarbonate, 0.011% sodium pyruvate, 5% fetal bovine serum and 10 ml/l 100× antibiotic anti-mycotic solution (Sigma). HCT-15 cells were maintained in RPMI 1640 (Sigma) supplemented with 0.22% sodium bicarbonate, 0.011% sodium pyruvate, 0.45% glucose, 0.24% N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid, 10% fetal bovine serum and 10 ml/l of 100× antibiotic anti-mycotic solution (Sigma). Cells were maintained at 37°C in the presence of 5% CO2. Reporter lysis buffer and luciferase reagent for luciferase studies were supplied by Promega (Madison, WI). β-Galactosidase (β-Gal) reagent was obtained from Tropix (Bedford, MA), and LipofectAMINE reagent was purchased from Invitrogen (Carlsbad, CA). The PPARγ antagonist N-(4'-aminopyridyl)-2-chloro-5-nitrobenzamide (T007) was synthesized in our laboratory, and its identity and purity (>98%) was confirmed by gas chromatography mass spectrometry. The C-DIMs compounds were all prepared by condensation of indole or ring-substituted indoles with substituted benzaldehydes essentially as described (Qin et al., 2004).
Cell proliferation assay
This assay was performed in 12-well tissue culture plates using an initial concentration of 2 × 104 cells per well, and Dulbecco's modified Eagle's medium (DMEM)/Ham's F-12 media containing 2.5% charcoal-stripped fetal bovine serum (FBS). Cells were counted on the initial day using a Z1 cell counter (Beckman Coulter, Fullerton, CA) and then treated either with vehicle [dimethyl sulfoxide (DMSO)] or the indicated indole ring-substituted C-DIM compounds. Every 48 hr, fresh medium was added along with the indicated compounds. Cell counts were taken after 24, 48, 72 and 96 hr. The results are expressed as means ± standard errors for at least 3 samples for each treatment group.
Transfection Assays
The Gal4 reporter containing five Gal4 response elements (pGal4) was kindly provided by Dr. Marty Mayo (University of North Carolina, Chapel Hill, NC). Gal4DBD-PPARγ construct was a gift of Dr. Jennifer L. Oberfield (GlaxoSmithKline Research and Development). Cells were seeded in 12-well plates, and 0.4 µg of GAL4-Luc, 0.04 µg of β-GAL, 0.04 µg of GAL4DBD-PPARγ were transfected using LipofectAMINE reagent (Invitrogen) following the manufacturer's protocol. Cells were treated either with vehicle or respective compounds suspended in complete medium after 6 hr of transfection. Cell lysates were extracted after treatment for 20 – 22 hr by adding 100 µl of 1× reporter lysis buffer per well, and 30 µl of this extract was used to quantitate the luciferase activity using Lumicount (Perkin-Elmer Life and Analytical Sciences). Each experiment was conducted in triplicate and the results were normalized to the β-GAL activity.
Western blot analysis
SW480, HT-29 and HCT-15 colon cancer cells, Panc-28 (3 × 105) pancreatic cancer cells were seeded in 6-well tissue culture plates in DMEM/Ham's F-12 medium containing 2.5% charcoal-stripped FBS. Protein was extracted from the cells treated either with vehicle or the indicated compounds for 24 hr for p21 protein, or 72 hr for caveolin-1 protein. Samples were extracted in high salt buffer [50 mmol/l N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid, 500 mmol/l NaCl, 1.5 mmol/l MgCl2, 1 mmol/l ethyleneglycol-bis(aminoethylether)-tetraacetic acid, 10% glycerol and 1% Triton X-100 (pH 7.5) and 5 µl/ml protease inhibitor cocktail (Sigma-Aldrich)]. Extracts were incubated at 100°C for 2 min, separated on either 10 or 12% sodium dodecylsulfate-polyacrylamide gel electrophoresis gels and then transferred to polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). The polyvinylidene difluoride membrane was blocked in 5% TBST-Blotto (10 mM Tris-HCl, 150 mM NaCl, pH 8.0, 0.05% Triton X-100 and 5% non-fat dry milk) for 30 min and then incubated in fresh 5% TBST-Blotto with 1:1000 for caveolin-1 (Santa Cruz Biotechnology, Santa Cruz, CA), 1:1000 for p21 (BD Pharmingen, Franklin Lakes, NJ) and 1:2000 for β-tubulin (Santa Cruz Biotechnology, Santa Cruz, CA) primary antibody overnight with gentle shaking at 4°C. After washing with Tris-buffered saline containing Tween-20 (TBST) for 10 min, the membrane was incubated with respective secondary antibody (1:5000) (Santa Cruz Biotechnology) in 5% TBST-Blotto for 3 hr. The membrane is then washed with TBST for 10 min, incubated with chemiluminiscence reagent from Perkin-Elmer for 1 min and then exposed to Kodak X-OMAT AR autoradiography film (Eastman Kodak, Rochester, NY).
Semi-quantitative real-time polymerase chain reaction
SW480 and HT29 colon cancer cells were treated either with vehicle (DMSO) or indicated indole ring-substituted C-DIMs compounds and, after 24 hr total, RNA was extracted using RNeasy kit (Qiagen, Valencia, CA). RNA concentration was measured by UV 260:280 nm absorption ratio, and 2 µg RNA was used to synthesize cDNA using Reverse Transcription System (Promega). Polymerase chain reaction (PCR) conditions were as follows: initial denaturation at 94°C (1 min) followed by 28 cycles of denaturation for 30 sec at 94°C, annealing for 60 sec at 55°C, and extension at 72°C for 60 sec, and a final extension step at 72°C for 5 min. mRNA levels were normalized to GAPDH as an internal housekeeping gene. Primers were obtained from IDT (Coralville, IA) and used for amplification as follows: KLF4 (sense 5'-CTA TGG CAG GGA GTC CGC TCC-3'; anti-sense 5'-ATG ACC GAC GGG CTG CCG TAC-3') and GAPDH (sense 5'-ACG GAT TTG GTC GTA TTG GGC G-3'; anti-sense 5'-CTC CTG GAA GAT GGT GAT GG-3'). PCR products were electrophoresed on 1% agarose gels containing ethidium bromide and visualized under UV transillumination.
RESULTS
Growth Inhibition by Indole Ring-Substituted C-DIMs
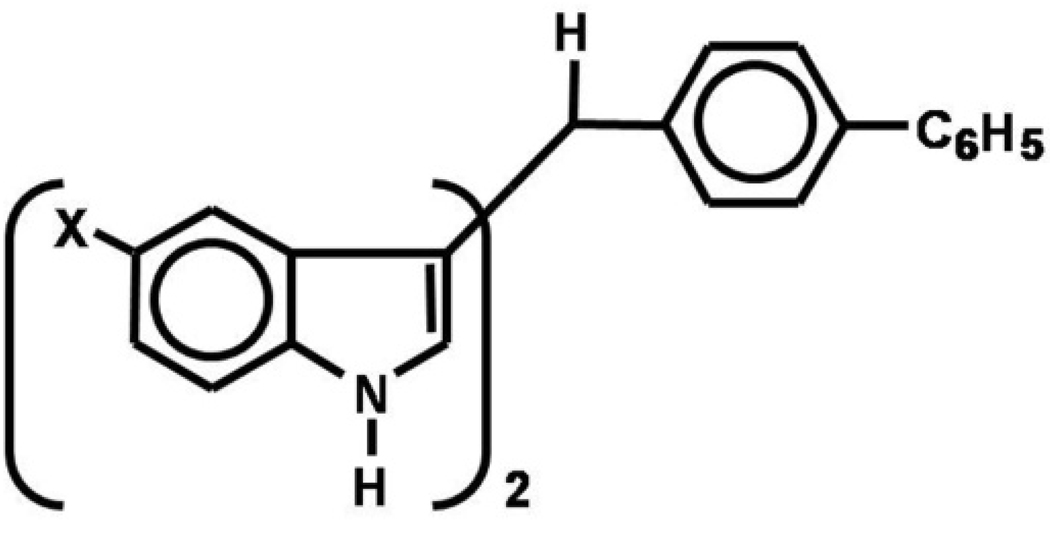
It has previously been shown that DIM-C-pPhC6H5 (Fig. 1) inhibited growth of several different cancer cell lines and also activated PPARγ [1; 3]. In this study, we have examined the effects of various symmetrical 5,5'-indole ring-substituted analogs of DIM-C-pPhC6H5 and determined the effects of substituent structure on the cytotoxicity and PPARγ agonist activity of these compounds in Panc28 pancreatic cancer cells and SW480 colon cancer cell lines.
Figure 1.
Structure of indole ring-substituted DIM-C-pPhC6H5.
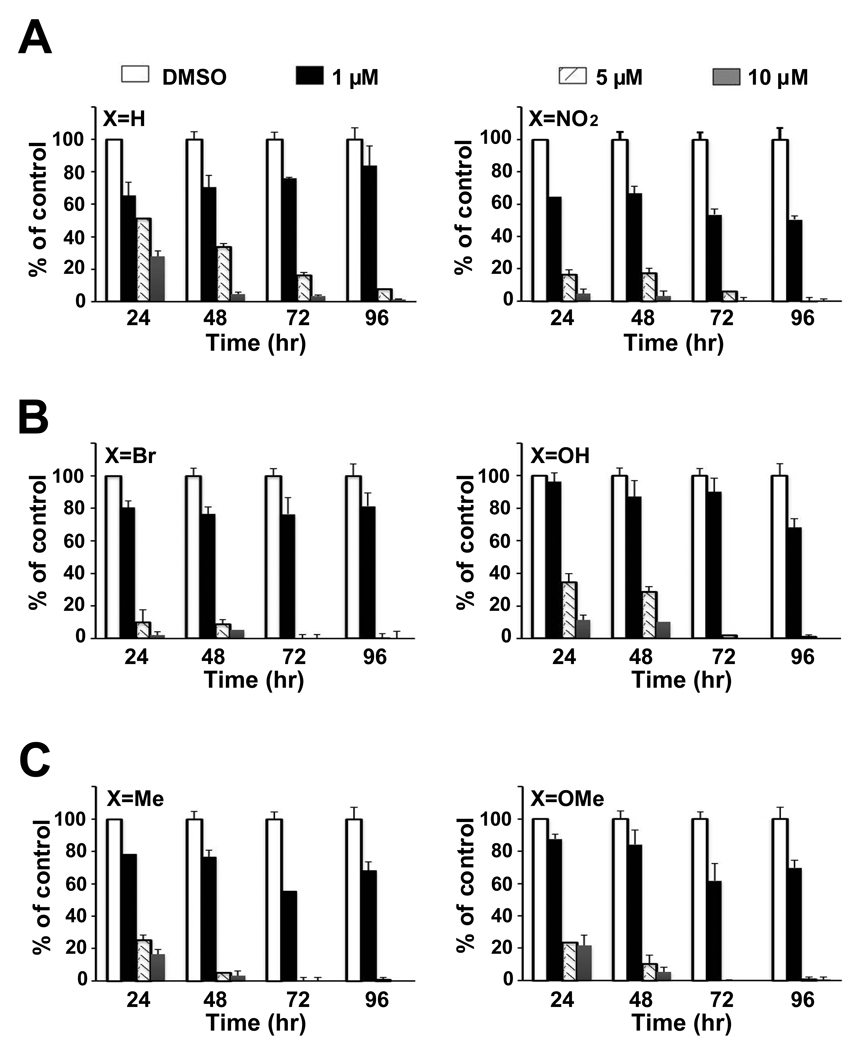
Figure 2 summarizes the cytotoxicity of DIM-C-pPhC6H5 and the 5,5'-substituted nitro (NO2), bromo (Br), hydroxyl (OH), methyl (Me) and methoxy (OMe) analogs in Panc28 cells. All compounds caused a concentration-dependent decrease in cell counts after treatment for 24, 48, 72 or 96 hr, and similar results were observed in colon cancer cells (data not shown). Table 1 summarizes the growth inhibitory IC50 values at all time points. There was less than a 2-fold difference in IC50 values among the six C-DIM compounds in both cell lines, suggesting that introduction of the 5,5'-substituents on the indole ring do not substantially enhance or decrease the cytotoxicity of DIM-C-pPhC6H5.
Figure 2.
Cell proliferation assays. Panc28 cells were treated with X-DIM-C-pPhC6H5 (X = H, X=NO2, X=Br, X=OH X=Me and X=OMe), and cell numbers were determined after treatment for 24, 48, 72 and 96 hr as described in Materials and Methods. Columns = mean of three replicate determinations for each treatment group; bars = SE.
Table 1.
Growth inhibitory IC50 values (µM) for X-IM-C-pPhC6H5 in Panc28 and SW480 cells treated for 24, 48, 72 or 96 hr.
| X=H | X=NO2 | X=Br | X=OH | X=Me | X=OMe | |
|---|---|---|---|---|---|---|
| Panc28 cells | ||||||
| Day 1 | 5.17 | 4.08 | 4.45 | 5.68 | 4.80 | 5.08 |
| Day 2 | 4.79 | 4.17 | 4.31 | 5.21 | 4.08 | 4.55 |
| Day 3 | 4.42 | 3.04 | 3.90 | 4.52 | 2.90 | 3.22 |
| Day 4 | 4.46 | 2.62 | 4.11 | 3.56 | 3.56 | 3.63 |
| SW480 cells | ||||||
| Day 1 | 4.77 | 2.25 | 3.31 | 2.14 | 3.62 | 3.06 |
| Day 2 | 4.51 | 2.20 | 2.31 | 2.04 | 2.28 | 2.65 |
| Day 3 | 3.99 | 2.03 | 1.94 | 2.15 | 2.28 | 2.82 |
| Day 4 | 3.95 | 1.93 | 1.88 | 1.20 | 1.25 | 2.29 |
Activation of PPARγ by Indole Ring-Substitued C-DIMs
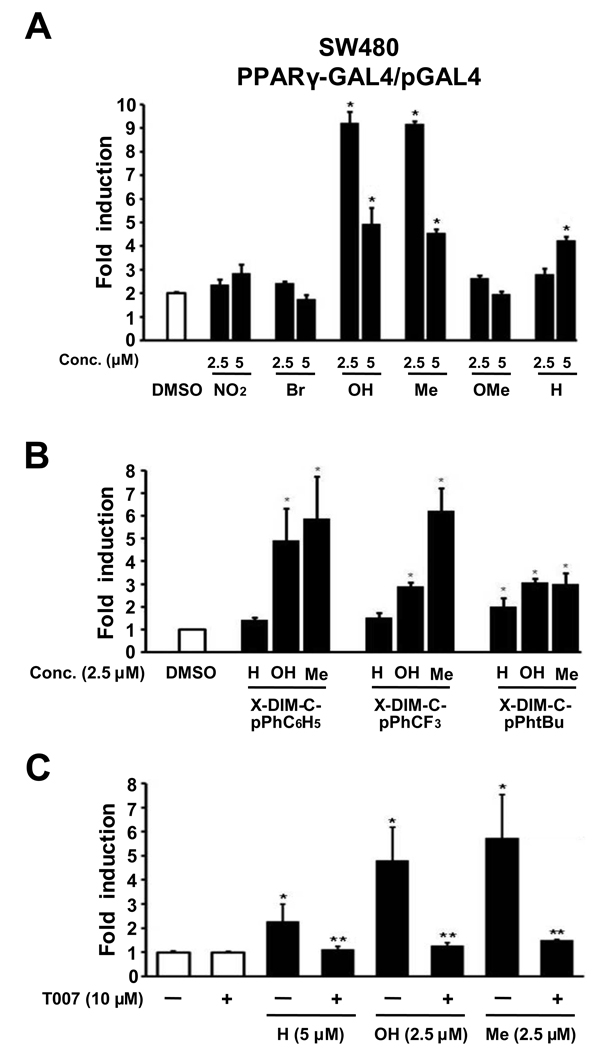
The effects of the indole ring substituents on the activation of PPARγ by DIM-C-pPhC6H5 and related compounds were investigated in SW480 colon cancer cells transfected with GAL4-PPARγ/GAL4-luc constructs. Treatment of SW480 colon cancer cells with 5.0 µM DIM-C-pPhC6H5 (X=H), showed that the 5,5'-dihydroxy and 5,5'-dimethyl (X=OH and Me, respectively) analogs significantly induced luciferase activity and, at a lower concentration of 2.5 µM, transactivation was induced only with the indole ring-substituted analogs and not DIM-C-pPhC6H5 (Fig. 3A). The results indicate that the 5,5'-dihydroxy and 5,5'-dimethyl compounds were more active than the unsubstituted compound DIM-C-pPhC6H5, and the 5,5'-dinitro, -dibromo and -dimethoxy analogs were inactive at this concentration and higher doses (> 5.0 µM) were cytotoxic. The effects of the 5,5'-dimethyl and dihydroxy substituents on activation of PPARγ-dependent activity by DIM-C-pPhCF3 and DIM-C-pPhtBu were compared to the results obtained for the corresponding X-DIM-C-pPhC6H5 compounds in SW480 cells transfected with PPARγ-GAL4/pGAL4 (Fig. 3B). The results show that introduction of 5,5'-dimethyl or -dihydroxy groups enhanced PPARγ-dependent activity for all three PPARγ-active C-DIMs. In SW480 cells transfected with PPARγ-GAL4/pGAL4, induction of luciferase activity by DIM-C-pPhC6H5 and the 5,5'-dihydroxy and -dimethyl analogs was inhibited after cotreatment with 10 µM T007, a PPARγ antagonist (Fig. 3C).
Figure 3.
X-DIM-C-pPhC6H5 activates PPARγ in SW480 cells. (A) Activation of X-DIM-C-pPhC6H5. SW480 cells were transfected with PPARγ-Gal4/pGal4 and treated with DMSO or different concentrations of X-DIM-C-pPhC6H5 (X=H, X=NO2, X=Br, X=OH, X=Me and X=OMe), and luciferase activity was determined as described in Materials and Methods. (B) Activation of PPARγ-active DIM-C-pPhCF3, DIM-C-pPhtBu and DIM-C-pPhC6H5 and their 5,5'-dimethyl and 5,5'-dihydroxy derivatives. SW480 cells were treated as in (A), and luciferase activity was determined as described in Materials and Methods. Columns = mean of three replicate determinations for each treatment group; bars = SE. * = P<0.05, significant induction. (C) Effects of PPARγ antagonist T007 on induced transactivation in SW480 cells transfected with PPARγ-GAL4/pGAL4. Induction of luciferase activity by DIM-C-pPhC6H5 and the 5,5’-dihydroxy and 5,5'-dimethyl analogs was inhibited in cells cotreated with 10 µM T007, a PPARγ antagonist. Columns = mean of three replicate determinations for each treatment group; bars = SE; * = P<0.05, significant induction; ** = P<0.05, significant inhibition.
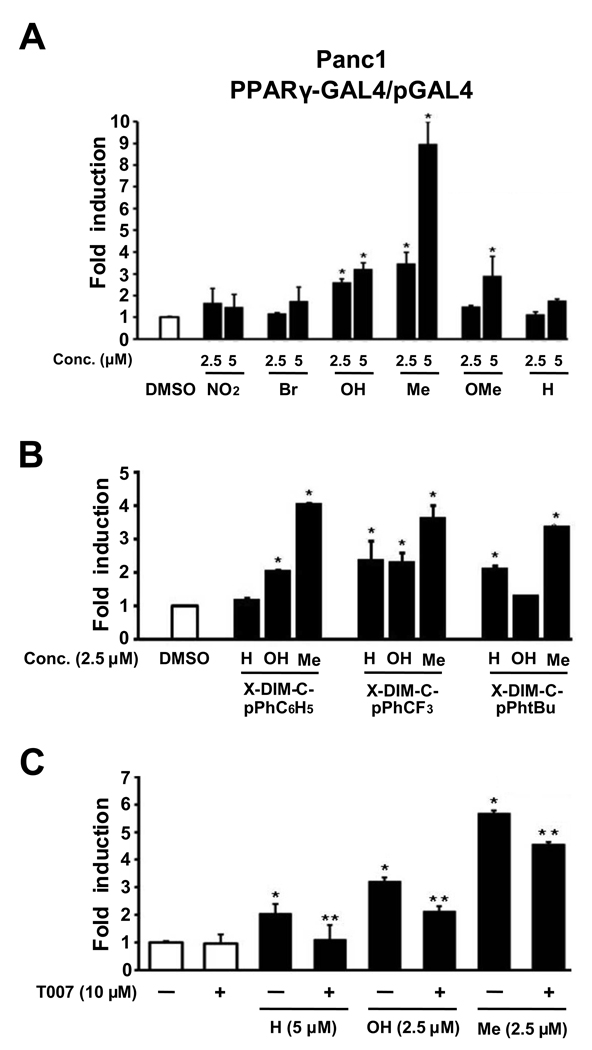
Figure 4 summarizes the structure-dependent activation of luciferase activity in Panc1 cells treated with DIM-C-pPhC6H5 and the 5,5'-ring substituted analogs of this C-DIM. In this cell line, 2.5 and 5 µM concentration of the 5,5'-dihydroxy, -dimethyl and -dimethoxy analogs were active, whereas 5 µM DIM-C-pPhC6H5 did not induce luciferase activity. Higher concentration of this compound only weakly induced luciferase activity (data not shown). Thus, the 5,5'-dihydroxy and 5,5'-dimethyl substituted compounds were PPARγ agonists in both cell lines, whereas the 5,5'-dimethoxy analog activated PPAR in Panc1 but not SW480 cells (Fig. 3A).
Figure 4.
X-DIM-C-pPhC6H5 activates PPARγ in Panc1 cells. (A) Activation of X-DIM-C-pPhC6H5. Panc1 cells were transfected with PPARγ-Gal4/pGal4 and treated with DMSO or different concentrations of X-DIM-C-pPhC6H5 (X=H, X=NO2, X=Br, X=OH, X=Me and X=OMe), and luciferase activity was determined as described in Materials and Methods. (B) Activation of PPARγ-active DIM-C-pPhCF3, DIM-C-pPhtBu and DIM-C-pPhC6H5 and their 5,5'-dimethyl and 5.5'-dihydroxy derivatives. Panc1 cells were treated as described in (A), and luciferase activity was determined as described in Materials and Methods. Columns = mean of three replicate determinations for each treatment group; bars = SE; * = P < 0.05, significant induction. (C) Effects of PPARγ antagonist T007 on induced transactivation in Panc1 cells. Cells were transfected with PPARγ-GAL4/pGAL4, induction of luciferase activity by DIM-C-pPhC6H5 and the 5,5’-dihydroxy and 5,5'-dimethyl analogs was inhibited after cotreatment with 10 µM T007, a PPARγ antagonist. Columns = mean of three replicate determinations for each treatment group; bars = SE; * = P < 0.05, significant induction; ** = P < 0.05, significant inhibition.
A comparison of the activation of PPARγ-active DIM-C-pPhCF3, DIM-C-pPhtBu and DIM-C-pPhC6H5 and their 5,5'-dimethyl and 5,5'-dihydroxy derivatives in Panc1 cells is shown in Figure 4B. Treatment with 2.5 µM concentration of the C-DIMs induced luciferase activity in Panc1 cells with 7 out of 9 compounds, only DIM-C-pPhC6H5 and the 5,5'-dihydroxy derivative of DIM-C-pPhtBu were inactive. These results were similar to those observed in SW480 cells (Fig. 3B), except that the 5,5'-dihydroxy derivative of DIM-C-pPhtBu induced transactivation in colon but not in pancreatic cancer cells.
The PPARγ agonist activity of DIM-C-pPhC6H5 and the 5,5'-dihydroxy and -dimethyl derivatives in Panc1 cells transfected PPARγ-GAL4/pGAL4 was inhibited in cells cotreated with the PPARγ antagonist T007 (10 µM) (Fig. 4C), and these results were similar to that observed in SW480 cells (Fig. 3).
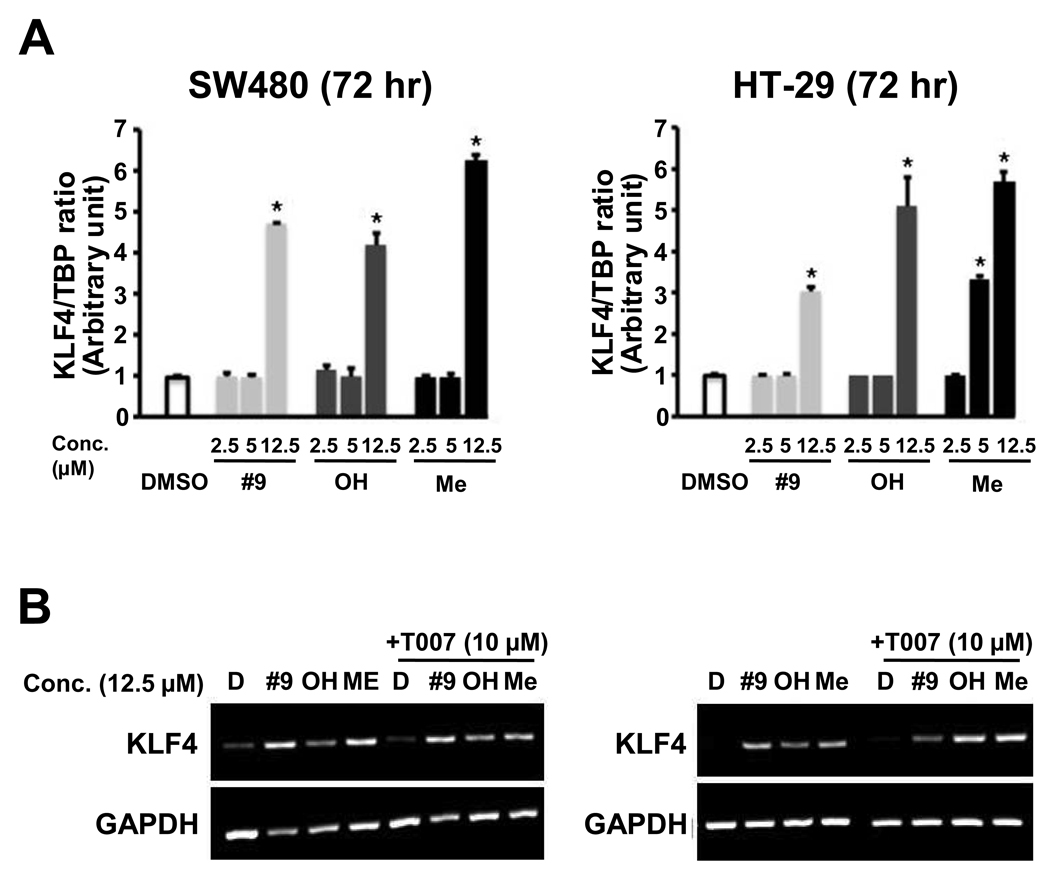
Activation of KLF4 and Caveolin-1 by Indole Ring-Substituted C-DIMs in Colon Cancer Cells
Previous studies show that KLF4 is activated by the PPARγ agonist methyl-2-cyano-3,11-dioxo-18β-olean-1,12-dien-30-oate (CDODA-Me) in colon cancer cells and these induction responses are inhibited by the PPARγ antagonist T007 [16]. Results in Figure 5A show that DIM-C-pPhC6H5 and the 5,5'-dihydroxy and -dimethyl analogs also induce KLF4 mRNA levels in SW480 colon cancer cells. However, the induction response is only observed after treatment of SW480 or HT-29 cells with a relatively high concentration (12.5 µM) of these compounds. Semi-quantitative RT-PCR analysis of KLF4 mRNA induction by the C-DIM compounds alone or in combination with the PPARγ antagonist T007 is illustrated in Figure 5B. The results confirm that the C-DIMs induce KLF4 expression in colon cancer cells; however, the lack of inhibition by T007 suggests that the induction response was PPARγ-independent. PPARγ-independent induction of KLF4 by the C-DIMs was similar to results of a previous report showing that induction of KLF4 mRNA in colon cancer cells by the PPARγ agonist 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2) was receptor-independent [17], whereas induction of KLF4 mRNA in colon cancer cells by CDODA-Me was receptor-dependent [16].
Figure 5.
Induction of KLF4 gene expression by DIM-C-pPhC6H5 and the 5,5’-dihydroxy and 5,5'-dimethyl analogs in SW480 and HT-29 cells. Induction of KLF-4 (A) and inhibition by T007 (B). Cells were treated with different concentrations of DIM-C-pPhC6H5 and the 5,5’-dihydroxy and 5,5'-dimethyl analogs or T007 alone or in combination for 24 hr, and KLF4 mRNA levels were determined by real-time polymerase chain reaction as described in the Materials and Methods. Each experiment was replicated ( > 3X). T007 did not inhibit KLF4 mRNA induction by DIM-C-pPhC6H5 and the 5,5’-dihydroxy and 5,5'-dimethyl analogs.
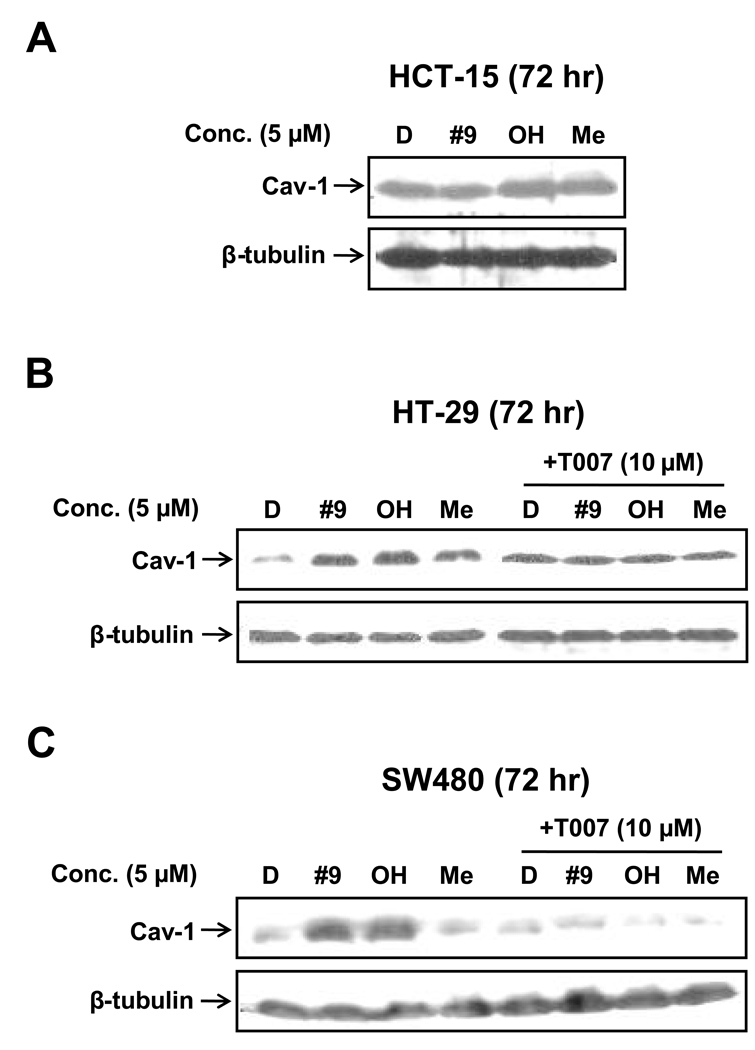
Previous studies showed that PPARγ-active C-DIMs also enhanced caveolin-1 protein expression in colon cancer cells (Chintharlapalli et al., 2004), and Figure 6 illustrates the effects of 5 µM DIM-C-pPhC6H5 and related indole ring-substituted compounds on expression of caveolin-1 in HT-19, HCT-15 and SW480 cells. Induction of caveolin-1 was not observed in HCT-15 cells (data not shown); however all three C-DIMs alone induced caveolin-1 in HT-29 cells, whereas only DIM-C-pPhC6H5 and the 5,5'-dihydroxy but not the 5,5'-dimethyl analogs induced caveolin-1 in SW480 cells (fig. 6A). The effects of T007 on induction of caveolin-1 by C-DIMs in HT-29 cells were difficult to decipher due to induction of caveolin-1 by T007 alone. However, T007 inhibited caveolin-1 induction by DIM-C-pPhC6H5 and the dihydroxy analog. These results show that induction of caveolin-1 by C-DIMs was dependent on cell context and also on the structure of the C-DIM (Fig. 6B), suggesting that these indole ring substituted analogs of DIM-C-pPhC6H5 are selective PPARγ modulators.
Figure 6.
Induction of caveolin-1 expression in colon cancer cells and effects of T007 on induction of caveolin-1. HCT-15 (A), HT-29 (B) or SW480 (C) cells were treated with DMSO or 5 µM DIM-C-pPhC6H5 and the 5,5’-dihydroxy and 5,5'-dimethyl analogs alone or in combination with 10 µM T007 and caveolin-1 expression was determined by western blot analysis as described in the Materials and Methods.
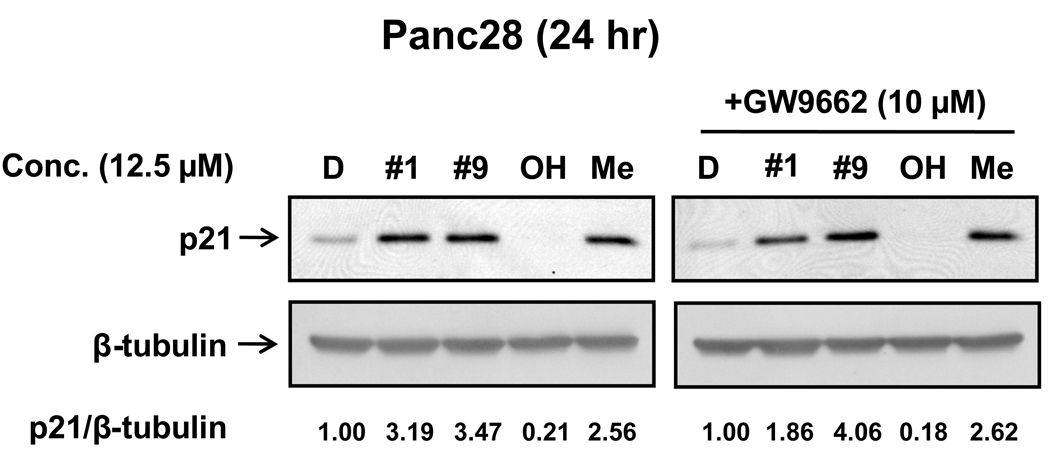
Activation of p21 by Indole Ring-Substituted C-DIMs in Pancreatic Cancer Cells
Figure 7A shows that both DIM-C-pPhC6H5 (#9) and DIM-C-pPhCF3 (#1) induce p21 protein expression in Panc28 cells and the induction response by the latter compound was previously reported [1]. The 5,5'-dimethyl analog of DIM-C-pPhC6H5 also induced p21 protein but the corresponding 5,5'-dihydroxy indole ring-substituted compound was inactive at a concentration of 12.5 µM. However, the effects of DIM-C-pPhC6H5 and the 5,5'-dihydroxy and 5,5'-dimethyl analogs on p21 expression were unaffected after cotreatment with the PPARγ antagonist T007. These results suggest that in Panc28 cells, DIM-C-pPhC6H5 and related compounds enhance PPARγ-independent expression of p21, whereas previous studies reported induction of p21 by DIM-C-pPhCF3 in Panc28 cells was PPARγ-dependent [1].
Figure 7.
Induction of p21 protein. Panc-28 cells were treated with the different compounds alone (A) or in combination with T007 (B) as indicated for 24 hr, and whole-cell lysates were obtained and analyzed by immunoblots as described in the Materials and Methods.
DISCUSSION
PPARγ is a member of the nuclear receptor family of ligand-activated receptors, and the transcriptionally-active complex is a heterodimer containing PPARγ and the retinoic acid × receptor (RXR). The thiazolidinedione class of PPARγ agonists has been used extensively for treatment of type II diabetes, and there is evidence that these compounds may have applications for treating other diseases including atherogenesis and cancer [18; 19]. PPARγ is overexpressed in multiple tumor types, and several studies shows the effectiveness of different structural classes of PPARγ agonists for inhibiting cancer cell growth and inducing apoptosis. Typically, these compounds induce differentiation in cancer cells and inhibit G0/G1 to S phase progression which is associated with decreased expression of cyclin D1 and induction of the cyclin-dependent kinase inhibitors p21 and/or p27. Mechanistic studies using PPARγ antagonists (T00s7 and GW9662) and PPARγ knockdown by RNA interference demonstrate that many of the growth inhibitory and proapoptotic responses induced by PPARγ agonists are receptor-independent [4–6; 14; 15].
Nuclear receptor agonists typically bind structurally-diverse compounds and this has also been observed for PPARγ. For example, PPARγ binds and is activated by endogenous biochemicals such as fatty acids and 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2), flavonoids, and various synthetic compounds including substituted indoles and chromane carboxylic acids, phosphono-phosphates, PPARγ-active C-DIMs, CDODA-Me and triterpenoids such as 2-cyano-3,12-dioxo-18β-oleana-1,9-dien-28-oic acid (CDDO) and related derivatives [20–26]. Not surprisingly, there is evidence that among this structurally-diverse group of compounds, PPARγ agonists exhibit tissue-/cell- and response-specific differences suggesting that these compounds are selective PPARγ modulators. For example, induction of non-steroidal anti-inflammatory drug-activated gene-1 (NAG-1) by PGJ2 in colon cancer cells was receptor-dependent, whereas induction of this gene by other PPARγ agonists such as thiazolidinediones and C-DIMs was receptor-independent [4; 27; 28]. Moreover, research in our laboratory on α-and β-CDODA-Me, which are triterpenoids that exhibit different stereochemistries at C18, and a structurally-related 2-cyanobetulinic acid derivative (CN-BA) also exhibited response- and cell context-dependent differences [16; 29]. Previous studies with PPARγ-active C-DIMs showed that DIM-C-pPhCF3 induced p21 in Panc28 cells and all three PPARγ-active C-DIMs induced caveolin-1 in colon cancer cells and these responses were inhibited after cotreatment with PPARγ antagonists T007 or GW9662 [1; 3]. Moreover, we have also shown that PPARγ-active triterpenoids induce KLF4 mRNA in a receptor-dependent manner in some colon cancer cell lines [16; 29]. Therefore, in this study we first examined the cytotoxicity of DIM-C-pPhC6H5 and symmetrical 5,5'-indole ring substituents and their activation of PPARγ (Fig. 1). The second objective was to determine whether these compounds were selective PPARγ modulators with respect to activation of caveolin-1, KLF-4 and p21.
Results illustrated in Figure 2 and Table 1 show that the growth-inhibitory IC50 values for DIM-C-pPhC6H5 and several indole ring-substituted analogs in SW480 colon and Panc28 pancreatic cancer cells were similar and varied between 1–10 µM at all time points in both cell lines. Thus, introduction of a 5-methyl, 5-nitro, 5-bromo, 5-hydroxy or 5-methoxy into both indole rings did not substantially enhance or inhibit, their cytotoxic effects compared to the unsubstituted DIM-C-pPhC6H5.
The comparative activation of PPARγ by DIM-C-pPhC6H5 and X-DIM-C-pPhC6H5 analogs was determined in Panc1 and SW480 cells transfected with PPARγ-GAL4/pGAL4 constructs (Fig. 3 – Fig 4). Panc1 cells were used instead of Panc28 cells because of the higher transfection efficiency in the former cell line. In SW480 cells, the 5,5'-dihydroxy and 5,5'-dimethyl analogs induced PPARγ-dependent transactivation and were clearly more potent than the DIM-C-pPhC6H5 (Fig. 3). Moreover, 5,5'-dihydroxy and 5,5'-dimethyl analogs of DIM-C-pPhC6H5 and DIM-C-pPhtBu also induced transactivation in SW480 cells transfected with PPARγ-GAL4/pGAL4 demonstrating that introduction of these substituents enhanced the PPARγ agonist activities of other PPARγ-active C-DIMs in SW480 (Fig. 2) and Panc1 cells (Fig. 4). With one exception, similar structure-activity relationships for activating PPARγ were observed in SW480 and Panc1 cells (Fig. 4) where the 5,5'-dihydroxy and 5,5'-dimethyl analogs were active and the 5,5'-dinitro and 5,5'-dibromo analogs were inactive. However, it was also observed that the 5,5'-dimethoxy-derivative also induced transactivation in Panc1 (but not SW480) cells at the 2.5 and 5.0 µM concentrations used in this study. Higher doses were not used due to cytotoxicity but it is possible that the cell-context-dependent differences observed for the 5,5'-dimethoxy derivative are due to potency differences for this compound as a PPARγ agonist in the two cell lines. The inhibitory effects of T007, a PPARγ antagonist confirms that DIM-C-pPhC6H5 and the 5,5'-dihydroxy and 5,5'-dimethyl analogs are PPARγ agonists (Fig. 3 and Fig 4) and these compounds and possibly the 5,5'-dimethoxy derivative further extend the number of C-DIMs that activate PPARγ. It is apparent that the PPARγ agonist activities of indole ring-substituted DIM-C-pPhC6H5 are structure-dependent; however, the cytotoxicities of these compounds are comparable, suggesting that this important aspect of their anticarcinogenic activity is PPARγ-independent. This observation is consistent with a recent study showing that the antiproliferative activities of PPARγ-active C-DIMs were not affected after cotreatment with PPARγ antagonists [1].
Previous reports show that some PPARγ agonists induce KLF4 in colon cancer cells; however, this response is structure and cell context-dependent. α- and β-CDODA-Me induced KLF4 expression in HT-29 and SW480 but not HCT-15 cells, whereas the 2-cyano derivative of betulinic induced KLF4 in HT-29 but not SW480 cells [16; 29]. All three compounds are PPARγ agonists but induction of KLF4 by α- and β-CDODA-Me was receptor-dependent and induction by the cyano betulinic acid derivative was PPARγ-independent. Similar results were observed in this study (Fig. 5) where induction of KLF4 by DIM-C-pPhC6H5 and the 5,5'-dihydroxy and 5,5'-dimethyl analogs in SW480 and HT-29 cells was not inhibited after cotreatment with T007.
Receptor-dependent induction of caveolin-1 by CDODA-Me, PPARγ-active C-DIMs and CN-BA has been reported in colon cancer cells; however, the induction responses were cell context-dependent [3; 16; 29]. For example, CN-BA induced caveolin-1 in HT-29 and HCT-15 cells but not SW480 cells, whereas β-CDODA-Me induces caveolin-1 is all three cell lines [16; 29]. In this study, 5 µM DIM-C-pPhC6H5 and the 5,5'-dihydroxy and 5,5'-dimethyl analogs induced caveolin-1 protein in HT-29 and SW480 cells (Fig. 6), and cotreatment with T007 inhibited the response in the latter cell line. In contrast, we did not observe induction of caveolin-1 in HCT-15 cells. The inhibitory response was not well-defined in HT-29 cells due to induction of caveolin-1 by T007 alone. Nevertheless, the pattern of caveolin-1 induction in the two cell lines by the indole ring-substituted compounds was similar to that previously described for PPARγ-active C-DIMs in these same cell lines (3). However, in this study the failure to observe induction in HCT-15 cells was inconsistent with previous studies with C-DIMs in this cell line, and the differences observed are currently being reinvestigated.
In Panc28 cells, DIM-C-pPhCF3 induced p21 expression that was PPARγ-dependent [1], and Figure 7A confirms that both DIM-C-pPhC6H5 (#9) and DIM-C-pPhCF3 (#1) induce p21 protein in this cell line. The 5,5'-dimethyl analog of DIM-C-pPhC6H5 also induced p21 protein but the corresponding 5,5'-dihydroxy analog was inactive at a concentration of 12.5 µM. Moreover, the effects of DIM-C-pPhC6H5 and the 5,5'-dihydroxy and 5,5'-dimethyl analogs on p21 expression were unaffected after cotreatment with the PPARγ antagonist T007. These results suggest that in Panc28 cells, DIM-C-pPhC6H5 and related compounds enhance PPARγ-independent expression of p21, whereas previous studies reported induction of p21 by DIM-C-pPhCF3 in Panc28 cells was PPARγ-dependent [1].
These results observed in colon and pancreatic cancer cells further demonstrate that indole ring-substituted analogs of DIM-C-pPhC6H5 activate PPARγ and some PPARγ-mediated responses in colon cancer cells. However the role of the receptor in mediating the responses is cell context- and structure-dependent. It was also apparent that the PPARγ-active and inactive C-DIM analogs exhibit comparable cytotoxicities suggesting that the antiproliferative activity of C-DIMs is primarily PPARγ-independent. Currently, we are investigating structural modification of C-DIMs at various positions in the indole and phenyl rings in order to maximize their PPARγ agonist activities and cytotoxic effects in cancer cell lines.
Acknowledgements
The financial assistance of the National Institutes of Health (ES09106, CA108718 and CA112337) and the Texas Agricultural Experiment Station is gratefully acknowledged.
REFERENCES
- 1.Hong J, Samudio I, Liu S, Abdelrahim M, Safe S. Peroxisome proliferator-activated receptor γ-dependent activation of p21 in Panc-28 pancreatic cancer cells involves Sp1 and Sp4 proteins. Endocrinology. 2004;vol. 145:5774–5785. doi: 10.1210/en.2004-0686. [DOI] [PubMed] [Google Scholar]
- 2.Vanderlaag K, Su Y, Frankel AE, Grage H, Smith R, III, Khan S, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes inhibit proliferation of estrogen receptor-negative breast cancer cells by activation of multiple pathways. Breast Cancer Res.Treat. 2007 doi: 10.1007/s10549-007-9648-y. [DOI] [PubMed] [Google Scholar]
- 3.Chintharlapalli S, Smith R, III, Samudio I, Zhang W, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substitutedphenyl)methanes induce peroxisome proliferator-activated receptor γ-mediated growth inhibition, transactivation and differentiation markers in colon cancer cells. Cancer Research. 2004;vol. 64:5994–6001. doi: 10.1158/0008-5472.CAN-04-0399. [DOI] [PubMed] [Google Scholar]
- 4.Chintharlapalli S, Papineni S, Baek SJ, Liu S, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substitutedphenyl)methanes are peroxisome proliferator-activated receptor gamma agonists but decrease HCT-116 colon cancer cell survival through receptor-independent activation of early growth response-1 and NAG-1. Mol.Pharmacol. 2005;vol. 68:1782–1792. doi: 10.1124/mol.105.017046. [DOI] [PubMed] [Google Scholar]
- 5.Chintharlapalli S, Papineni S, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through PPARγ-dependent and PPARγ-independent pathways. Mol.Cancer Ther. 2006;vol. 5(no. 5):1362–1370. doi: 10.1158/1535-7163.MCT-06-0002. [DOI] [PubMed] [Google Scholar]
- 6.Chintharlapalli S, Papineni S, Safe SH. 1,1-Bis(3'-indolyl)-1-(p-substitutedphenyl)methanes inhibit growth, induce apoptosis, and decrease the androgen receptor in LNCaP prostate cancer cells through PPARγ-independent pathways. Mol.Pharmacol. 2007;vol. 71(no. 2):558–569. doi: 10.1124/mol.106.028696. [DOI] [PubMed] [Google Scholar]
- 7.Contractor R, Samudio I, Estrov Z, Harris D, McCubrey JA, Safe S, Andreeff M, Konopleva M. A novel ring-substituted diindolylmethane 1,1-bis[3'-(5-methoxyindolyl)]-1-(p-t-butylphenyl)methane inhibits ERK activation and induces apoptosis in acute myeloid leukemia. Cancer Research. 2005;vol. 65:2890–2898. doi: 10.1158/0008-5472.CAN-04-3781. [DOI] [PubMed] [Google Scholar]
- 8.Qin C, Morrow D, Stewart J, Spencer K, Porter W, Smith R, III, Phillips T, Abdelrahim M, Samudio I, Safe S. A new class of peroxisome proliferator-activated receptor γ (PPARγ) agonists that inhibit growth of breast cancer cells: 1,1-bis(3'-indolyl)-1-(p-substitutedphenyl)methanes. Mol.Cancer Therap. 2004;vol. 3:247–259. [PubMed] [Google Scholar]
- 9.Vanderlaag K, Samudio I, Burghardt R, Barhoumi R, Safe S. Inhibition of breast cancer cell growth and induction of cell death by 1,1-bis(3'-indolyl)methane (DIM) and 5,5'-dibromoDIM. Cancer Lett. 2005;vol. 236(no. 2):198–212. doi: 10.1016/j.canlet.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 10.York M, Abdelrahim M, Chintharlapalli S, Lucero SD, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substitutedphenyl)methanes induce apoptosis and inhibit renal cell carcinoma growth. Clin.Cancer Res. 2007;vol. 13:6743–6752. doi: 10.1158/1078-0432.CCR-07-0967. [DOI] [PubMed] [Google Scholar]
- 11.Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. 3,3'-Diindolylmethane (DIM) and derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;vol. 27:717–728. doi: 10.1093/carcin/bgi270. [DOI] [PubMed] [Google Scholar]
- 12.Calabro P, Samudio I, Safe SH, Willerson JT, Yeh ET. Inhibition of tumor necrosis factor-α-induced endothelial cell activation by a new class of PPARγ agonists: an in vitro study. J.Vascular Res. 2005;vol. 42:509–516. doi: 10.1159/000088260. [DOI] [PubMed] [Google Scholar]
- 13.Kassouf W, Chintharlapalli S, Abdelrahim M, Nelkin G, Safe S, Kamat AM. Inhibition of bladder tumor growth by 1,1-bis(3'-indolyl)-1-(p-substitutedphenyl)methanes: a new class of peroxisome proliferator-activated receptor γ agonists. Cancer Research. 2006;vol. 66:412–418. doi: 10.1158/0008-5472.CAN-05-2755. [DOI] [PubMed] [Google Scholar]
- 14.Lei P, Abdelrahim M, Safe S. 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes inhibit ovarian cancer cell growth through peroxisome proliferator-activated receptor-dependent and independent pathways. Mol.Cancer Ther. 2006;vol. 5(no. 9):2324–2336. doi: 10.1158/1535-7163.MCT-06-0184. [DOI] [PubMed] [Google Scholar]
- 15.Cho SD, Yoon K, Chintharlapalli S, Abdelrahim M, Pei P, Hamilton S, Khan S, Ramaiah SK, Safe S. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and independent pathways. Cancer Research. 2007;vol. 67:674–683. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- 16.Chintharlapalli S, Papineni S, Jutooru I, McAlees A, Safe S. Structure-dependent activity of glycyrrhetinic acid derivatives as peroxisome proliferator-activated receptor γ (PPARγ) agonists in colon cancer cells. Mol.Cancer Therap. 2007;vol. 6:1588–1598. doi: 10.1158/1535-7163.MCT-07-0022. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZY, Tseng CC. 15-Deoxy-Δ12,14 prostaglandin J2 up-regulates Krüppel-like factor 4 expression independently of peroxisome proliferator-activated receptor γ by activating the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signal transduction pathway in HT-29 colon cancer cells. Mol.Pharmacol. 2005;vol. 68(no. 5):1203–1213. doi: 10.1124/mol.105.014944. [DOI] [PubMed] [Google Scholar]
- 18.Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat.Res. 2000;vol. 448(no. 2):121–138. doi: 10.1016/s0027-5107(99)00231-6. [DOI] [PubMed] [Google Scholar]
- 19.Fajas L, Debril MB, Auwerx J. Peroxisome proliferator-activated receptor-γ: from adipogenesis to carcinogenesis. Journal of Molecular Endocrinology. 2001;vol. 27(no. 1):1–9. doi: 10.1677/jme.0.0270001. [DOI] [PubMed] [Google Scholar]
- 20.Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, Maue RA, Place AE, Porter DM, Spinella MJ, Williams CR, Wu G, Dannenberg AJ, Flanders KC, Letterio JJ, Mangelsdorf DJ, Nathan CF, Nguyen L, Porter WW, Ren RF, Roberts AB, Roche NS, Subbaramaiah K, Sporn MB. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-inflammatory activity. Cancer Research. 1999;vol. 59(no. 2):336–341. [PubMed] [Google Scholar]
- 21.Rieusset J, Touri F, Michalik L, Escher P, Desvergne B, Niesor E, Wahli W. A new selective peroxisome proliferator-activated receptor γ antagonist with antiobesity and antidiabetic activity. Molecular Endocrinology. 2002;vol. 16(no. 11):2628–2644. doi: 10.1210/me.2002-0036. [DOI] [PubMed] [Google Scholar]
- 22.Berger JP, Petro AE, Macnaul KL, Kelly LJ, Zhang BB, Richards K, Elbrecht A, Johnson BA, Zhou G, Doebber TW, Biswas C, Parikh M, Sharma N, Tanen MR, Thompson GM, Ventre J, Adams AD, Mosley R, Surwit RS, Moller DE. Distinct properties and advantages of a novel peroxisome proliferator-activated protein γ selective modulator. Molecular Endocrinology. 2003;vol. 17(no. 4):662–676. doi: 10.1210/me.2002-0217. [DOI] [PubMed] [Google Scholar]
- 23.Koyama H, Miller DJ, Boueres JK, Desai RC, Jones AB, Berger JP, Macnaul KL, Kelly LJ, Doebber TW, Wu MS, Zhou G, Wang PR, Ippolito MC, Chao YS, Agrawal AK, Franklin R, Heck JV, Wright SD, Moller DE, Sahoo SP. (2R)-2-ethylchromane-2-carboxylic acids: discovery of novel PPARα/γ dual agonists as antihyperglycemic and hypolipidemic agents. J.Med.Chem. 2004;vol. 47(no. 12):3255–3263. doi: 10.1021/jm030621d. [DOI] [PubMed] [Google Scholar]
- 24.Acton JJ, III, Black RM, Jones AB, Moller DE, Colwell L, Doebber TW, Macnaul KL, Berger J, Wood HB. Benzoyl 2-methyl indoles as selective PPARγ modulators. Bioorg.Med.Chem.Lett. 2005;vol. 15(no. 2):357–362. doi: 10.1016/j.bmcl.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 25.Liu K, Black RM, Acton JJ, III, Mosley R, Debenham S, Abola R, Yang M, Tschirret-Guth R, Colwell L, Liu C, Wu M, Wang CF, Macnaul KL, McCann ME, Moller DE, Berger JP, Meinke PT, Jones AB, Wood HB. Selective PPARγ modulators with improved pharmacological profiles. Bioorg.Med.Chem.Lett. 2005;vol. 15(no. 10):2437–2440. doi: 10.1016/j.bmcl.2005.03.092. [DOI] [PubMed] [Google Scholar]
- 26.Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor γ ligand. Proc.Natl.Acad.Sci.U.S.A. 2005;vol. 102(no. 7):2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baek SJ, Wilson LC, Hsi LC, Eling TE. Troglitazone, a peroxisome proliferator-activated receptor γ (PPARγ) ligand, selectively induces the early growth response-1 gene independently of PPARγ. A novel mechanism for its anti-tumorigenic activity. Journal of Biological Chemistry. 2003;vol. 278(no. 8):5845–5853. doi: 10.1074/jbc.M208394200. [DOI] [PubMed] [Google Scholar]
- 28.Baek SJ, Kim JS, Nixon JB, DiAugustine RP, Eling TE. Expression of NAG-1, a transforming growth factor-β superfamily member, by troglitazone requires the early growth response gene EGR-1. Journal of Biological Chemistry. 2004;vol. 279(no. 8):6883–6892. doi: 10.1074/jbc.M305295200. [DOI] [PubMed] [Google Scholar]
- 29.Chintharlapalli S, Papineni S, Liu S, Jutooru I, Chadalapaka G, Cho SD, Murthy R, You YJ, Safe S. 2-Cyano-lup-1-en-3-oxo-20-oic acid, a cyano derivative of betulinic acid, activates peroxisome proliferator-activated receptor γ in colon and pancreatic cancer cells. Carcinogenesis. 2007;vol. 28:2337–2346. doi: 10.1093/carcin/bgm189. [DOI] [PubMed] [Google Scholar]