Abstract
Inhibition of return (IOR) represents a well-known mechanism of human perception that biases attentional orienting to novel locations in the environment. Behaviorally, IOR reflects slower reaction time (RT) to stimuli presented in previously-cued locations. In this study, we examined within patients with schizophrenia this inhibitory aftereffect using two different cue types --- eye gaze and standard peripheral cues. Results indicated that patients showed evidence of IOR, as reflected in a 3.2% slowing in RT to previously peripherally-cued locations. However for eye gaze, patients failed to show evidence of IOR, and instead had 1.7% faster RT to targets presented following delay in locations that had been previously cued. This inhibitory failure correlated strongly with reduced neuropsychological performance and global symptoms ratings of attention and bizarre behavior. Reduced inhibitory aftereffect in RT for eye-gaze cues may reflect disease-related abnormalities in social attention.
Keywords: social attention, schizophrenia, neuropsychology, symptoms
Introduction
Over a century ago, William James emphasized the ‘varieties of attention’ that allow for selection, whether of a train or thought, a particular location or a specific object (James, 1890; Parasuraman, 1998; Rees, Frackowiak, & Frith, 1997). Later, echoing James, Kraepelin (1919) described the many different aspects of attentional disturbances in schizophrenia. Today, contemporary neuroscience models often divide attention into two general domains --- facilitation and inhibition --- each of which entails distinct sets of processes and operations, supported by discrete neural circuitry (Parasuraman, 1998). From these models emerges an important question: are these general attentional domains and their constituent processes equally affected by schizophrenia (Nestor & O’Donnell, 1998)?
The current study investigated this question by focusing within the general domain of attentional inhibition on an experimentally well-established phenomenon, inhibition of return (IOR). IOR is a fundamental mechanism of human perception that biases attentional orienting to novel locations in the environment (Posner & Cohen, 1984). Behaviorally, IOR reflects slower reaction time (RT) to stimuli presented in previously-cued locations. Typically in a visually IOR task, a sudden onset spatial cue flashes at a peripheral location outside of central fixation, signaling an impending target. Subsequent stimuli occurring at or near the cued location are processed faster and more efficiently, but this advantage lasts for only about 300 ms following cue presentation or stimulus onset asynchrony (SOA), after which there is a slowing of processing for stimuli appearing in the originally-cued location (Posner & Cohen, 1984).
This inhibitory aftereffect reflects IOR, and is thought to play a major role in healthy cognition, in general, and efficient and adaptive visual search, in particular (Klein, 2000). It prevents attention from being locked into a particular location; it protects against redundant, distracting sensory information; and it presets perception to favor novel locations for foraging and exploration over already sampled, checked, and explored sources that are likely barren. For patients with schizophrenia, normal levels of IOR for standard (Carter et al., 1992; Maruff et al., 1998) as well as for relatively long SOA intervals have been reported (Fuentes & Santiago, 1999; Fuentes et al., 1999). However, other studies have reported delayed (e.g., Huey and Wexler, 1994; Sapir et al., 2001) as well as markedly disturbed (Gouzoulis-Mayfrank et al., 2004) IOR, which has been shown to be independent of medication (Gouzoloulis-Mayfrank, Arnold, & Heekeren, 2006).
One implication derived from this mixed pattern of findings is that visual IOR to peripheral cues is not uniformly and consistently affected by schizophrenia. If this is so then, would patients with schizophrenia show a similar level of IOR across different types of cues? Consider the novel IOR paradigm developed by Frischen and Tipper (2004) that uses eye-gaze cues embedded in a face stimulus to signal the location of an impending target. This paradigm with healthy participants showed an IOR effect for a target appearing at a previously gazed-at location following 2400 ms SOA. Their results pointed to a robust effect, evident in reaction times (RT) or saccades, across different cue and target faces, and they further noted that in comparison to standard peripheral cues, IOR evoked by gaze cues emerged more slowly.
For purposes of the current study, IOR to eye-gaze cues provides a means to examine a distinct aspect of attention important for social communication and interaction (see Friesen & Kingstone, 1998; Driver et al., 1999; Frischen & Tipper, 2004). Social attention, as is it often referred, has increasingly been seen as disrupted by schizophrenia (Sasson et al., 2007), and these disturbances are now often understood within a wider context of disease-related impairments in social motivation and functioning (e.g., Burns, 2004; Hoffman, 2007). This distinct form of attention is generally framed in reference to social cognition, which in turn is defined as a complex set of representations of internal bodily states, knowledge of self, perceptions of others, and interpersonal motivations that is supported by a widely-distributed network of diverse brain regions, including the medial prefrontal cortex, the temporoparietal junction, the temporal sulcus and the temporal poles (Adolphs 2003; Amodio & Frith, 2006). The burgeoning field of social neuroscience is now devoted to understanding the dynamic interplay of these informational, motivational, and neural processes from which our perceptions of self and others develop (e.g., Blakemore, 2007, Dunbar & Schultz, 2007; Hermann et al., 2007).
Two principal aims guided the current study. First is to examine IOR as a function of eye gaze and peripheral cues within the same group of patients with schizophrenia. Experimental studies with healthy participants have indicated these two types of cues serve as a simple but powerful independent variable to distinguish social and non-social forms of attention. The current study manipulates or varies cue type within patients with schizophrenia as a means to provide a direct experimental test of the hypothesis that failures in social attention may represent a core characteristic of the illness. Similarly, the second aim centers on the construct validity of social attention and how it may contribute to the ever-changing nomological net of schizophrenia. It is thought that eye-gaze IOR represents a key building block for the healthy development of social communication and learning (e.g., Frischen, Bayliss, & Tipper, 2007). Disruptions in both these areas are common in schizophrenia, and are typically measured, cognitively, by neuropsychological tests, and, socially, by objective ratings of positive and negative symptoms. Thus, using multiple regression techniques, the current study aims to quantify the relative contributions of eye-gaze and peripheral cues IOR to two core characteristics of schizophrenia: neuropsychological functioning and symptom ratings.
Method
Participants
All subjects were between the ages of 17 and 55 years, right-handed, native speakers of English, without histories of ECT, neurological illness, and without alcohol or drug abuse in the past 5 years, as assessed by the Addiction Severity Index (McClellan et al., 1992). Twenty-four persons with schizophrenia (22 males, 2 females) participated, with a mean age of 44.08 (S.D. = 10.29) and mean years of education 14.33 (S.D. = 3.88) All were part of an ongoing comprehensive, longitudinal study of schizophrenia, receiving neuroleptic medication, with a mean chlorpromazine (CPZ) equivalent daily dose of 317.98 mg (S.D. = 244.55). Mean duration of illness was 17.31 years (S.D. = 11.05). Diagnoses were ascertained by the Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-P), along with chart review (see e.g., Nestor et al., 2008). After the study was described to them, all subjects provided written informed consent. We also recruited 23 healthy participants from the University of Massachusetts Boston who participated for course credit as well as eight subjects recruited from the community (15 males, 16 females). Healthy participants had a mean age of 26.58 years (S.D. = 10.61) and mean years of education of 12.71 (S.D. = 1.47). We included these subjects as a validity check on the reproducibility of the relatively novel finding of Frischen and Tipper (2004) of eye-gaze IOR in their non-clinical, healthy samples. We did not directly compare healthy participant and patient groups given their differences in age, education, and other uncontrolled extraneous factors.
Stimuli and Task
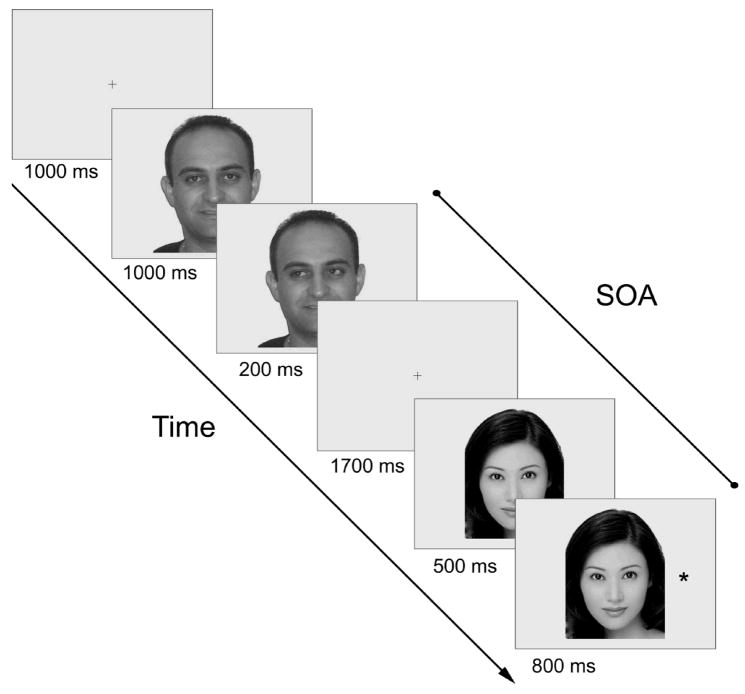
The stimuli and task followed from Frischen and Tipper (2004, Experiment 9). The experiment was run on a Windows PC using custom software written in the program language C. Stimuli were presented on a 19-in (48.3 cm) Dell P992 CRT monitor set to 32-bit color and 1024x 768 pixels screen resolution. Participants sat on a comfortable chair with their head position stabilized on a chin rest at a distance of 63 cm from the screen. For the eye-gaze IOR task, a central cross subtending 0.7 degrees served as a fixation point. Ten digitized photographs of faces (5 male and 5 female) with their eye gazing straight ahead were used to produce the cues for the eye-gaze IOR task. The faces subtended, on average, 13.1 degrees in height and 10.4 degrees in width. Following Frischen and Tipper (2004), we constructed from each face photograph left-gaze and right-gaze cues by cutting out the pupil and iris area of each eye and pasting it into the left and right corners, respectively, of each eye, using Adobe PhotoShop 7 software. This served to ensure that no other asymmetrical properties of the faces could confound orienting according to eye-gaze cues. The target was an asterisk. For eye-gaze IOR task, the asterisk (target) subtended 0.7 degrees and was presented 8 degrees to the left and right of the center of the screen, approximately on level with the eyes of the face in the vertical plane. On validly-cued trials, the target appeared at the side the eye gaze of the face was directed to, whereas on invalidly-cue trials, the target appeared at the opposite side (see Figure 1). Half the trials used valid cues and the other half used invalid cues.
Figure 1.
Graphic representation of the experimental procedures. The face that is presented in the cue display is different from that in the target display. SOA = stimulus onset asynchrony.
Procedure
In the standard IOR paradigm, subjects were instructed to fixate for 1000 ms on a central fixation cross that was flanked by two peripheral boxes at an eccentricity of 8 degrees horizontally from the central target. Subsequently, one of the boxes was brightened for 300 ms, and then the two peripheral boxes were shown uncued for 860 ms, followed by the target appearing in either the cued or uncued location. Participants were instructed to fixate on the central marker throughout the trial and were told to press one button on a hand-held device if the target appeared in the left square and another button if the target appeared in the right square. Before beginning the actual task, an experimental session consisted of ten practice trials followed by three blocks of 50 trials each, with 25 trials for each of the two peripheral target positions in randomized order. A fixed SOA of 1160 ms was used throughout the task based on the well-established time course of peripheral cueing, with IOR observed from about 200–300 ms after the onset of a peripheral cue until at least 3000 ms (Samuel and Kat, 2003). On valid trials, the target appeared at the cued location, and on invalid trials the target appeared in the opposite location. Half the trials were valid, and half were invalid.
As shown in Figure 1, in the gaze-evoked IOR paradigm, participants started a trial by pressing a button and were presented with a central fixation cross for 1000 ms. Subsequently, they were shown a photograph of a face looking straight ahead, and after another 1000 ms, the pupils of this face appeared to move to either the left or the right corners of the eye for 200 ms. The face then disappeared, and participants once again fixated on a central marker for 1,700 ms, followed by the photograph of a different face looking straight ahead. After another 500 ms, a target appeared to either the left or right of the face. Participants were instructed to fixate on the central marker throughout the trial and to press a button if the target appeared on the left side and another button if the target appeared on the right side of the screen. A fixed SOA of 2400 ms was used to replicate Frischen and Tipper’s (2004) findings (Experiment 9). Performance was measured by RT from the time when the target appeared to the time the button was pressed that corresponded to the target location. An experimental session consisted of ten practice trials for each of the two conditions, followed by six blocks of 50 trials each, with 25 trials for each of the two peripheral target positions in randomized order. There were three blocks of response trials in total. The order of tasks was counterbalanced across subjects.
Neuropsychological and Symptom Measures
The patient sample had available neuropsychological measures of intelligence Wechsler Adult Intelligence Scale-Third Edition (WAIS-III, Wechsler, 1997) including working memory as well as the Wechsler Memory Scale Third Edition (WMS-III, Wechsler, 1997), which also included a working memory index, and Trail Making Test, Trails B performance speed as an index of executive attention (Arbuthnott & Frank, 2000). The Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1983) and The Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984) were completed in the same session as the SCID-P. The SANS consists of 24 items that are summarized in five global ratings: affective flattening, alogia, avolition-apathy, anhedonia-asociality, and attention (Andreasen, 1983). The SAPS consists of 34 items that are summarized in four global ratings: hallucinations, delusions, bizarre behavior, and positive formal thought disorder (Andreasen, 1984). Individual items are rated on a scale of 0 to 5 for degree of severity as are the global ratings. The global ratings are therefore not simply an arithmetic sum of ratings on component items. The SANS/SAPS were done independently and blind to the IOR and neuropsychological results.
Statistical Analyses
Paired t-tests compared IOR performance for peripheral and eye-gaze cues within the healthy participants and schizophrenia groups. A series of hierarchical regression analyses then examined the contribution of IOR measures to neuropsychological functioning and symptom ratings for the schizophrenia group. To examine the unique contributions of the peripheral and eye-gaze IOR indices to each dependent measure (i.e., neuropsychological measures of intelligence, working memory, and executive attention) partial (rp) and semi-partial (rsp), correlations were computed in a series of hierarchical regression analyses, which permitted the evaluation of significant univariate relationships by partitioning total variance of the dependent variable (e.g., IQ) among independent variables (peripheral cue IOR, eye-gaze cue IOR). In this example, the partial correlation squared (rp2) is the proportion of variance of IQ shared by eye-gaze cue IOR, after the effects of peripheral cue IOR have been removed from both IQ and eye-gaze cue IOR measures (Cohen, 1988; Cohen & Cohen, 1975). This statistic answers the question, “What proportion of the remaining variance in IQ (i.e., that which is not estimated by the other IV in the equation, peripheral cue IOR) is uniquely estimated by eye-gaze IOR?” In contrast, the square of the semi-partial correlation (rsp2) estimates the amount of variance in IQ that is uniquely shared with eye-gaze IOR after the effects of peripheral cue IOR on eye-gaze cue IOR have been removed (Cohen, 1988; Cohen & Cohen, 1975). It is semi-partial because the effects of perceptual cue IOR have been removed from eye-gaze-cue IOR but not from the dependent variable of IQ. In conjunction with other linear regression statistics, partial and semi-partial correlations provide a comprehensive picture of how peripheral and eye-gaze cue IOR indices relate to symptom ratings and neuropsychological functioning when collinearity is controlled. For all regression analyses the F-to-enter probability was .05 and the F-to-exclude probability was 0.1. Significance levels are two-tailed.
Results
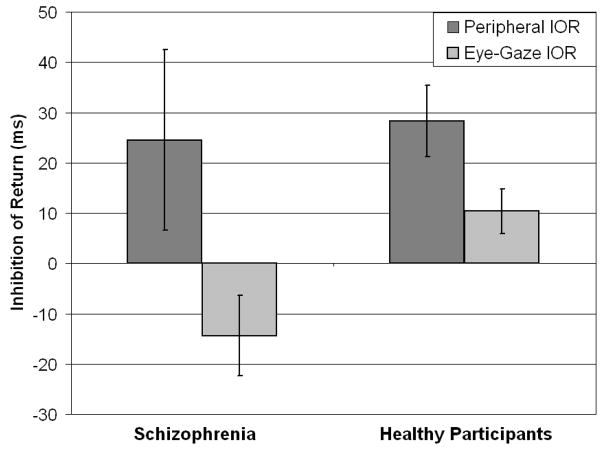
Figure 2 presents IOR performance to eye-gaze cues and peripheral box cues. The IOR effect is calculated by subtracting RT of invalidly-cued targets from that of validly-cued targets. This calculation is based on the premise that for long SOA time periods, IOR produces slower RT to a target presented in a previously validly-cued location than to a target presented in an uncued or invalidly-cued location.
Figure 2.
Bar graph with 2 IOR (peripheral and eye gaze) for each group (healthy participants and schizophrenia).
For IOR to peripheral box cues, the healthy participants showed the expected effect of slower RT for validly-cued than for invalidly-cued trials. The healthy participants had an IOR to peripheral box cues of 28.34 ms (S.D. = 38.85), which in comparison to their overall RT, reflected a 4.5% slowing in response time for validly- cued trials over invalidly-cued trials. A paired-samples t test with the valid and invalid trials as variable pairs showed that this 28-ms difference reached statistical significance, t (29) = 4.00, p<.001. For IOR to eye-gaze cues, the healthy participants also showed the expected effect of slower RT for validly-cued than for invalidly-cued trials. The healthy participants had an IOR to eye-gaze cues of 10.35 ms (S.D. = 22.91), which in comparison to their overall RT, reflected a 1.6% slowing in response time for validly- cued trials over invalidly-cued trials. A paired-samples t test with the valid and invalid conditions as variable pairs showed that this 10-ms difference reached statistical significance, t(26) = 2.35, p<.05. These data showed the IOR effect in healthy participants, replicating previous studies using peripheral box (e.g., Posner & Cohen, 1984) and eye-gaze (Frischen & Tipper, 2004) cues to manipulate attention.
For IOR to peripheral box cues, the patient group also showed the expected effect of slower RT for validly-cued than for invalidly-cued trials. The patients had an IOR to peripheral box cues of 24.51 ms (S.D. = 86.24), which in comparison to their overall RT, reflected a 3.2% slowing in response time for validly-cued trials over invalidly-cued trials. However, a paired-samples t test with the valid and invalid trials as variable pairs showed that this 24-ms difference did not reach statistical significance, t(22) = 1.36, p = .187, likely due to the marked variation of scores in the patient sample. For IOR to eye-gaze cues, the patient group did not show an IOR effect, but instead exhibited a 14.43 ms (S.D. = 37.43) advantage in RT for validly-cued than for invalidly-cued trials, which in relation to their overall RT reflected a 1.7% improvement in speed of response for validly-cued trials than for invalidly-cued trials. As shown in Figure 2, the patients showed IOR to peripheral box cues similar in absolute magnitude to that of the healthy participants. By contrast, the patient group did not show IOR to eye-gaze cues that the healthy participants did.
Table 1 presents neuropsychological test scores for the patient group. We used hierarchical regression to explore both the joint and unique contributions of IOR to neuropsychological functioning in patients with schizophrenia. The models entered the predictors of IOR to peripheral cues first, followed by IOR to eye-gaze cues. For overall intelligence, as measured by WAIS-III Full-Scale IQ, only IOR to eye-gaze cues produced a significant F change, R Square Change = .226, F Change (1, 18) = 6.123, p = .024. These values corresponded to partial and semi-partial correlation coefficients of .475 and .504 respectively, meaning that IOR to eye-gaze cues uniquely accounted for between 22.56% and 25.40% of the variance in WAIS-III Full-Scale IQ scores for patients with schizophrenia.
Table 1.
Neuropsychological scores and symptom ratings (SANS/SAPS) for patients with schizophrenia.
| Neuropsychological Measures | Score +/− S.D. |
|---|---|
| WAIS-III | |
| Full-Scale IQ | 93.92 +/− 12.93 |
| Verbal IQ | 96.46 +/− 11.84 |
| Performance IQ | 91.58 +/− 13.84 |
| Working Memory | 90.83 +/− 15.40 |
| Processing Speed | 83.42 +/− 16.43 |
| WMS-III | |
| Immediate Memory | 82.74 +/− 18.39 |
| Auditory | 85.83 +/− 22.06 |
| Visual | 83.00 +/− 19.34 |
| Delayed Memory | 88.41 +/− 16.29 |
| Auditory | 93.18 +/− 24.72 |
| Visual | 84.83 +/− 21.11 |
| Working Memory | 91.19 +/− 21.99 |
| Trail Making Test (sec) | |
| Trails A | 52.26 +/− 18.87 |
| Trails B | 126.51 +/− 69.95 |
| SANS/SAPS Global Ratings | |
| SANS | |
| Affective Flattening | 1.87 +/− 1.87 |
| Alogia | 1.57 +/− 1.44 |
| Apathy | 2.70 +/− 1.46 |
| Anhedonia | 2.78 +/− 1.62 |
| Attention | 1.89 +/− 1.57 |
| SAPS | |
| Delusions | 3.50 +/− 1.41 |
| Hallucinations | 2.89 +/− 2.00 |
| Bizarre Behavior | .91 +/− 1.31 |
| Thought Disorder | 1.86 +/− 1.64 |
Note. WAIS-III = Wechsler Intelligence Scale-Third Edition; WMS-III = Wechsler Memory Scale-Third Edition; SANS = The Scale for the Assessment of Negative Symptoms; SAPS = The Scale for the Assessment of Positive Symptoms (SAPS).
For working memory, a composite average of WAIS-III Working Memory Index and WMS-III Working Memory Index correlated significantly with only IOR to eye-gaze cues, r (19) = .530, p = .019. Higher working memory scores corresponded with increased IOR effect to eye-gaze cues. Only IOR to eye-gaze cues produced a significant F change, R Square Change = .227, F Change (1, 20) = 6.124, p = .022. These values corresponded to a partial correlation of .484 and a semi-partial correlation of .477, meaning that IOR to eye-gaze cues uniquely accounted for between 22.75% and 23.43% of the variance in composite working memory scores for patients with schizophrenia.
By contrast, for Trails B executive attention, IOR to peripheral cues accounted for a larger percentage of variance than did IOR to eye-gaze cues. Moreover, the two IOR indices influenced Trails B performance in opposite directions. That is, IOR to peripheral cues produced a highly significant F change, R Square Change = .457, F Change (1, 18) = 15.129, p = .001. These values corresponded to a partial correlation of .754 and a semi-partial correlation of .740, meaning that IOR to peripheral cues accounted for between 54.76% and 56.85% of the variance in Trails B performance for patients with schizophrenia. These positive correlation coefficients indicated that as IOR to peripheral cues increased, so did Trails B performance time. By comparison, IOR to eye-gaze cues produced a significant F change, R Square Change = .127, F Change (1, 17) = 5.21., p = .036. These values corresponded to a partial correlation of −.485 and a semi-partial correlation of −.357, meaning that IOR to peripheral cues accounted for between 12.75% and 23.52% of the variance in Trails B performance for patients with schizophrenia. These negative correlation coefficients indicated that as IOR to eye-gaze cues decreased, Trails B performance speed increased ---- a direction opposite to that of IOR to peripheral cues and Trails B.
Last, IOR to eye-gaze cues correlated significantly with SANS global rating of attention (r=−.511, p=.018) and SAPS global rating of bizarre behavior (r=−.451, p=.046). The negative direction of these significant correlations indicated that reduced IOR to eye-gaze cues corresponded to more severe ratings for bizarre behavior and attention. For the global rating of attention, as measured by the SANS, only IOR to eye-gaze cues produced a significant F change, R Square Change = .248, F Change (1, 17) = 5.637, p = .03. These values corresponded to partial and semi-partial correlation coefficients of −.499 and −.489, respectively, meaning that IOR to eye-gaze cues uniquely accounted for between 23.91% and 24.90% of the variance in SANS global ratings of attention for patients with schizophrenia. For the global rating of bizarre behavior, as measured by the SAPS, only IOR to eye-gaze cues produced a significant F change, R Square Change = .268, F Change (1, 16) = 5.93, p = .027. These values corresponded to partial and semi-partial correlations of −.520 and −.518, respectively, meaning that 26.8% to 27.04% of the variance in SAPS global ratings of bizarre behavior can be uniquely accounted by IOR to eye-gaze cues.
Discussion
The study used an IOR paradigm in which subjects viewed two kinds of spatial cues, eye-gaze shifts and brightening of peripheral boxes, each of which served to signal the impending location of a target. Eye gaze and peripheral cues represented social and nonsocial signals, respectively. In this sense, then, the paradigm allowed for within-subject comparison of social and non-social forms of attention. For patients with schizophrenia, their RT performance varied as a function of cue type. They responded more slowly to peripherally-cued targets presented following a delay in previously signaled locations. While failing to reach statistical significance, this IOR effect was in the expected direction. By contrast, for eye-gaze cues, the patient group showed no evidence of IOR, but rather responded faster to targets presented following a delay in previously signaled locations. The patients thus failed to show evidence of an inhibitory aftereffect only for eye-gaze cues.
To provide a context for these current results, we first aimed to establish the validity of the IOR paradigm in healthy subjects. And indeed, our results, replicating previous studies, indicated that the experimental paradigm elicited the hypothesized IOR effect in healthy subjects (Frischen & Tipper, 2004). Of particular interest is that these data showed that as did peripheral cues, social cues in the form of eye gaze evoked inhibition to previously attended locations. In so doing, these findings replicated Frischen and Tipper (2004), the first study to show that eye-gaze cues can produce IOR effects when the interval between cue and target is lengthened to 2400 ms. The current study thus extended the Frischen & Tipper study by showing IOR effects for both nonsocial and social cues within the same group of healthy participants. And while these effects have different time courses, and are likely mediated by distinct neural systems, they share a similar pivotal function, as Frischen and Tipper (2004) aptly stated: “to prevent reprocessing of information at locations previously found to lack any useful information.” (p. 530).
Patients with schizophrenia also showed evidence of an IOR effect for peripheral cues. Here, the patients demonstrated an absolute RT disadvantage of 24.51 ms (S.D. = 86.24), which in comparison to their overall RT, reflected a 3.2% slowing in response time for validly-cued trials over invalidly-cued trials. These values compared favorably to those of healthy participants who showed an absolute RT disadvantage of 28.34 ms (S.D. = 38.85), which in comparison to their overall RT, reflected a 4.5% slowing in response time for validly-cued trials over invalidly- cued trials. However, for the patients, their sizable IOR effect did not translate into the expected statistically significant effect of slower RT for validly-cued trials than invalidly-cued trials. These findings therefore suggested, but could not firmly establish, sparing of IOR to peripheral cues in this sample of patients with schizophrenia --- a pattern that is consistent with several studies that have indicated normal (e.g., Carter et al., 1992; Maruff et al., 1998) or delayed (e.g., Huey and Wexler, 1994; Sapir et al., 2001) IOR for patients with schizophrenia.
The current data showed no evidence for gaze-evoked IOR in the patient group. In fact, patients showed evidence of faster rather than slower RT for targets presented at previously gazed-at locations. That is, gaze cues failed to activate inhibitory processes Thus, a different pattern of IOR therefore emerged for the patient group in comparison to the healthy participants. For patients, whereas peripheral cues inhibited RT to targets appearing at previously-cued locations, eye-gaze cues that are used to guide the sharing of attention did not. By contrast, in the current study, healthy participants showed inhibitory effects of slower RT for targets appearing at previously-cued locations, regardless of the kind of cue used to signal the impending target.
Peripheral sudden onset cues trigger automatic and rapid orienting of attention. As these signals convey no social meaning, attention can be rapidly withdrawn from a location when no stimulus is presented (Frischen & Tipper, 2004). For healthy participants, peripheral cues can elicit an IOR effect for SOA intervals as short as 300 ms. But for patients with schizophrenia a much longer SOA is needed to elicit IOR effects to peripheral cues. In healthy participants, IOR effects to eye-gaze cues have a much slower time course than do peripheral cues (Frischen & Tipper, 2004). In fact, in healthy participants, SOA intervals as long as 1,005 ms have failed to elicit IOR to eye-gaze cues (Driver et al., 1999; Friesen & Kingstone, 1998). Only when the interval between eye cue and target was extended to 2400 ms did healthy participants for the first time show inhibition effects (Frischen & Tipper, 2004). Thus, while the current findings suggested an absence of IOR to eye-gaze cues in the patient group, the possibility of a delayed IOR response to eye-gaze cues cannot be ruled out. Just as the patients required longer SOA intervals for IOR to peripheral cues so too might they also require longer SOA intervals for eye-gaze cues
The current study focused on within-group analysis of patient performance. Often between-group comparisons are fraught with difficulties, as patients with schizophrenia typically differ from control samples on a host of variables apart from their diagnosis, whether related to education, SES, nutrition, IQ, medication, illness duration, or health. These extraneous factors, which are not easily controlled either statistically or by a subject matching scheme, confound between-group comparisons. Instead, the current study revealed distinct patterns of IOR as a function of cue within the patient group. And within the patient group, age, medication level or duration of illness did not correlate with IOR to either peripheral or eye-gaze cues. However, all patients had long histories of psychopharmacological treatment, and the effects of such treatment on these behavioral are unknown.
The findings nevertheless raise the possibility of a rather specific disease-related impairment in social attention. Both IOR tasks entailed encoding shits of attention in response to cues. But only did the eye-gaze IOR task tap a salient and fundamental social ability to encode the shifts of attention of another person. Only under these socially salient conditions in which encoding gaze direction of human faces regulated the distribution of visual attention did the patients fail. Other studies have also pointed to a similarly important role of social cues, such as those that are threatening or anxiety provoking, in the regulation and control of attention (e.g., Derryberry & Reed, 2008). In addition, IOR to eye-gaze cues correlated significantly with symptoms ratings of attention and bizarre behavior. In both instances, increased symptom severity corresponded with reduced eye-gaze IOR. These correlations emerged despite rather overall low levels of severity in positive and negative symptom ratings in this sample of patients with chronic schizophrenia. These data provide evidence linking abnormalities of encoding gaze direction of human faces with differential symptom expression in schizophrenia.
In a similar vein, our data showed IOR to eye-gaze cues as a strong contributor to neuropsychological impairment in the patient group. That is, hierarchical regression revealed attentional inhibition to social cues accounted for a significant portion of unique variance across neuropsychological measures of intelligence, working memory, and executive attention. Approximately 24% of variance in each of the neuropsychological summary measures, WAIS-II IQ, WAIS-III/WMSIII Working Memory, and Trails B, could be uniquely explained by IOR to eye-gaze cues. Higher levels of neuropsychological functioning corresponded with more effective use of gaze cues to guide visual attention. These findings are consistent with studies of healthy participants linking gaze perception to both basic information processes (Sasaki, Ishi, & Gyoba, 2004) and higher-order abilities of memory retrieval and visual attention (Frischen, et al., 2007, Frischen & Tipper, 2004), as well as to social cognition, particularly those processes related to disambiguating expressions during face-to-face conversation (Hanna & Brennan, 2007). In addition, for schizophrenia, recent findings have shown that psychometric measures of social and non-social cognition each account for a significant portion of unique variance in the disease-related neuropsychological impairment (Nestor et al., under review).
In summary, social attention represents a key human adaptation. As a theoretical construct, it presumably exists within a nomological net of interlocking empirical relationships with other relevant and meaningful behaviors and outcomes. The current findings indicated that, among its multiple empirical indicators, IOR to eye-gaze cues may capture a key property of social attention. Indeed, in the current study, persons with schizophrenia showed abnormalities to eye-gaze but not peripheral cues, with the former strongly associated with disease-related neuropsychological outcomes and symptoms. Future studies are needed to provide additional evidence of construct validity of social attention, which should include measuring eye movement as well as RT.
References
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; 1983. [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; 1984. [Google Scholar]
- Arbuthnott K, Frank J. Trail Making Test, Part B as a measure of executive control: Validation using a set-switching paradigm. Journal of Clinical and Experimental Neuropsychology. 2000;22:518–528. doi: 10.1076/1380-3395(200008)22:4;1-0;FT518. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. How does the brain deal with the social world? Science. 2006;314:60–61. [Google Scholar]
- Burns JK. An evolutionary theory of schizophrenia: Cortical connectivity, metarepresentation, and the social brain. Behavioral and Brain Sciences. 2004;27:831–855. doi: 10.1017/s0140525x04000196. [DOI] [PubMed] [Google Scholar]
- Carter CS, Robertson LC, Chaderjian MR, Celaya LJ, Nordahl TE. Attentional asymmetry in schizophrenia: controlled and automatic processes. Biological Psychiatry. 1992;31:909–918. doi: 10.1016/0006-3223(92)90117-i. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlational analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1975. [Google Scholar]
- Derryberry D, Reed M. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Driver J, Davis G, Ricciardelli P, Kidd P, Maxwell E, Baron-Cohen S. Gaze perception triggers reflexive visuospatial orienting. Visual Cognition. 1999;6:509–540. [Google Scholar]
- Dunbar RIM, Schultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- Friessen CK, Kingstone A. The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychonomic Bulletin & Review. 1998;5:490–495. [Google Scholar]
- Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychological Bulletin. 2007;133:694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischen A, Tipper SP. Orienting attention via observed gaze shifts evokes longer term inhibitory effects: Implications for social interactions, attention, and memory. Journal of Experimental Psychology: General. 2004;133:516–533. doi: 10.1037/0096-3445.133.4.516. [DOI] [PubMed] [Google Scholar]
- Fuentes LJ, Boucart M, Alvarez R, Zimmerman MA. Inhibitory processing in visuospatial attention in healthy adults and schizophrenic patients. Schizophrenia Research. 1999;40:75–80. doi: 10.1016/s0920-9964(99)00044-4. [DOI] [PubMed] [Google Scholar]
- Fuentes LJ, Boucart M, Vivas AB, Alvarez R, Zimmerman MA. Inhibitory tagging in inhibition of return is affected in schizophrenia: Evidence from the Stroop task. Neuropsychology. 2000;14:134–140. [PubMed] [Google Scholar]
- Fuentes LJ, Santiago E. Spatial and semantic inhibitory processing in schizophrenia. Neuropsychology. 1999;13:259–270. doi: 10.1037//0894-4105.13.2.259. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Arnold S, Heekeren K. Deficient inhibition of return in schizophrenia: further evidence from an independent sample. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2006;30:42–49. doi: 10.1016/j.pnpbp.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Heekeren K, Voss T, Moerth D, Thelen B, Meincke U. Blunted inhibition of return in schizophrenia- evidence from a longitudinal study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2004;28:389–396. doi: 10.1016/j.pnpbp.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Hanna JE, Brennan SE. Speakers’ eye gaze disambiguates referring expressions early during face-to-face conversation. Journal of Memory and Language. 2007;57:596–615. [Google Scholar]
- Hermann E, Call J, Hernandez-Lloreda, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science. 2007;317:1360–1366. doi: 10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]
- Hoffman RE. A social deafferentation hypothesis for induction of active schizophrenia. Schizophrenia Bulletin. 2007;33:1066–1070. doi: 10.1093/schbul/sbm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey ED, Wexler BE. Abnormalities in rapid, automatic aspects of attention in schizophrenia: blunted inhibition of return. Schizophrenia Research. 1994;14:57–63. doi: 10.1016/0920-9964(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Science. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Maruff P, Hay D, Malone V, Currie J. Asymmetries in the covert orienting of visual spatial attention in schizophrenia. Neuropsychologia. 1995;33:1205–1223. doi: 10.1016/0028-3932(95)00037-4. [DOI] [PubMed] [Google Scholar]
- McClellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Niznikiewicz M, McCarley RW. Distinct contributions of working memory and social cognition to neuropsychological disturbance in schizophrenia (under review) [Google Scholar]
- Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW, Shenton ME. Neuropsychological disturbance in schizophrenia: A Diffusion Tensor Imaging Study. Neuropsychology. 2008;22:246–254. doi: 10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen YA. Components of visual orienting. In: Bouma H, Bouwhuis DG, editors. Attention and performance. X. Hillsdale, NJ: Erlbaum; 1984. pp. 531–556. [Google Scholar]
- Samuel AG, Kat D. Inhibition of return: A graphical meta-analysis of its time course and an empirical test of its temporal and spatial properties. Psychonomic Bulletin & Review. 2003;10:897–906. doi: 10.3758/bf03196550. [DOI] [PubMed] [Google Scholar]
- Sapir A, Henik A, Dobrusin M, Hochman EY. Attentional asymmetry in schizophrenia: disengagement and inhibition of return deficits. Neuropsychology. 2001;15:361–370. doi: 10.1037//0894-4105.15.3.361. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Ishi H, Gyoba J. Effects of gaze perception on response to location or feature. Psychologia. 2004;47:104–112. [Google Scholar]
- Sasson N, Tsuchiya N, Hurley R, Couture SM, Penn DL, Adolphs R, Piven J. Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia. 2007;45:2580–2588. doi: 10.1016/j.neuropsychologia.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Weschler Adult Intelligence Scales. 3. San Antonio, TX: Harcourt Brace & Co; 1997. [Google Scholar]