Abstract
Multi-layered composites composed of mammalian cells arranged in a hydrogel have been prepared using an acoustic focusing technique. Acoustic focusing is a simple, non-chemical technique that allows for the fast arrangement of cells in matrices where the control of cell geometry is beneficial. Breast cancer cells (MDA-MB231), were dispersed in a 30 wt % solution of poly(ethylene glycol) diacrylate (PEGDA) of molecular weight 400 at a density of 5 × 106 cells per ml of PEGDA solution. An ultrasonic field was used to organize the cells into multiple layers prior to polymerization of PEGDA. Disk-shaped hydrogel composites, typically 1 cm in diameter and 2 mm thick were prepared based on a PEGDA solution volume of 130 uL. At an acoustic frequency of 2.32 MHz, composites were fabricated, where concentric rings/layers of cells were interspersed with cell-free hydrogel. The cells were located in annuli approximately 80 μm thick and about 300 μm apart. The structure and viability of the cells within these constructs were studied using a fluorescent LIVE/DEAD assay. The viability of the cells was on the order of 50%. Cell death was primarily attributed to exposure of cells to the PEGDA solution prior to polymerization, rather than adverse effects of polymerization or the sound field itself.
Introduction
Acoustic fields have been used to manipulate particles of different size or density for the purpose of separating the dispersed phase from the continuous phase, driving coalescence in emulsions, or organizing particles within liquids to create periodic structures (1-3). More specifically, acoustic focusing has been used extensively in the area of biotechnology for such applications as cell deposition on surfaces, cell organization and immobilization in a gel matrix, and the examination of cell-cell contact in an ultrasonic trap (4-6). Further work has involved the immobilization and patterning of cells in gels in multiple geometries using ultrasonic standing waves (7). Here, we utilize a similar approach using ultrasonic fields to arrange human breast cancer cells (serving as analogs for future work involving mesenchymal stem cells for tissue engineering applications) in a concentric, cylindrical geometry, within UV-polymerized PEGDA hydrogels.
Starting with an unstructured polymer-precursor and cell suspension, cells can be organized into bands or layers provided that there is a difference in the acoustic properties of the cells relative to their suspending fluid. The cells are pushed to fixed pressure equilibrium positions within the fluid after application of a standing ultrasonic wave. The ultrasound can be produced by a pair of piezoelectric transducers or a transducer and a material acting as a reflector (8). Linear bands can be created that traverse a gel in a direction parallel or perpendicular to the surface by creating a standing wave in the same parallel or perpendicular direction. Using similar methods, it is also possible to produce 2-D banding when two perpendicular standing waves are utilized (9). The use of a cylindrical sound geometry, created by cylindrical transducers, can produce concentric ring patterns in a composite (7). The thickness of the bands is dependent on a number of factors including the overall cell concentration, the frequency of operation (as more node positions would disperse a given concentration of cells over a greater number of sites), and the application time of the acoustic field.
The objective of the study was to examine cell layers formed by the application of a cylindrical acoustic field in UV-crosslinked hydrogels and the effect of acoustic processing on cell viability within the resulting composites. The information presented here is important for optimizing the design and operation of future acoustic processing strategies for cell-hydrogel composites with tailored microstructures, formed in from cell encapsulation in UV-crosslinked matrices. By spatially concentrating the cells, cell-to-cell contact is increased and intercellular communication promoting tissue growth is expected (10-11). Ultimately, it may be possible to grow tissues from reduced initial cell loadings by use of acoustic focusing.
Materials and Methods
Human breast cancer cells (MDA-MB231) were cultured in 10 vol % fetal bovine serum comprised of HyQ® DMEM/High Glucose powder (Logan, UT), penicillin/streptomycin, and sodium bicarbonate media in BD Biosciences® T-75 (Sparks, Maryland) flasks at 37 °C in a 5% CO2 environment. When the cells reach about 80% confluence, a portion of them were passaged and others were used for experimentation. The cells were dispersed in 130 μL of a 30 wt % PEGDA400 (molecular weight is 400, Polysciences Inc., Warrington, PA) solution, that contained 0.1 wt % of the water-soluble photoinitiator Irgacure 2959® (CIBA, Basel, Switzerland) to give a final cell concentration of 1× 106 and 5 × 106 cells/ml of fluid, depending on the particular experiment performed. The cell suspension was then transferred to an acoustic chamber composed of an annular EC-64 (EDO Ceramic, Salt Lake City, UT) piezoelectric lead-zirconate-titanate transducer that was fixed at one end to a glass plate. The transducer had an inner diameter of 9 mm, a height of 13.5 mm, and a wall thickness of 1 mm. The transducer was connected to a Fluke 6011A synthesized signal generator (Everett, WA) via electrical leads that controlled the frequency and energy input into the transducer. The cell suspension was polymerized with UV-light provided from a Blak-Ray® long-wave (365 nm) ultraviolet lamp (Cole Parmer, Vernon Hills, IL) at an intensity of 15 mW/cm2 as measured by a Cole-Parmer Series 9811 Radiometer (Vernon Hills, IL). All experiments were preformed under sterile conditions in a biosafety hood. A schematic of the acoustic chamber is shown in Fig. 1. Similar acoustic chamber designs have been used in the literature (7).
Figure 1.
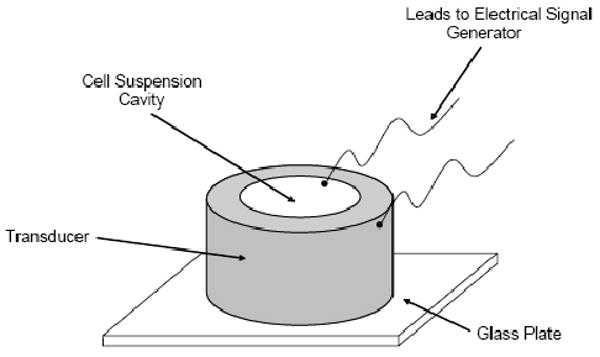
Schematic of cylindrical acoustic chamber.
The transducer was operated at a fundamental frequency of 2.32 MHz. During initial studies, digital images of the banded cells were captured while varying the voltage, prior to any polymerization. It was found that the transducer could be driven at 4 VRMS, without significantly distorting the cellular ring structure that might arise from field non-uniformities in the acoustic field or intense fluid perturbations. Additionally, this voltage produced little heating beyond the ambient, for the duration of the applied acoustic field. Standing waves with radial symmetry were produced within the cell suspension cavity formed by the transducer and its base, and the resulting acoustic forces on suspended cells moved them in the radial direction. The sound field emanated from the inner wall of the annular transducer and traveling towards the center point of the fluid layer, at which points it reflects with another converging wave, to produce a standing wave. The distance between nodal planes (which corresponds to the distance between cell layers) is approximately equal to one-half the wavelength of the sound field (12). As the cells traveled to these close to these regions, positions in the fluid at which the pressure was equal to zero, concentric layers of cells were created. This geometry is shown in Fig. 2.
Figure 2.
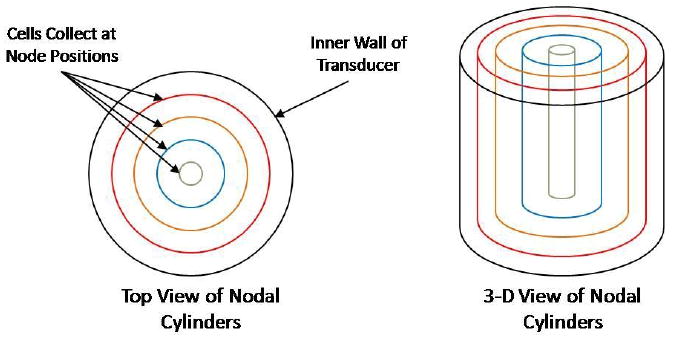
Arrangement of cells at radial positions emanating from a cylindrical sound source
Based on visual observation, application of the sound field for 20 min was sufficient to achieve a sharp organization of cells into the annular pattern shown in Fig. 2. Once the organization of the cells was complete, the PEGDA was polymerized by 3 min of UV light exposure. The resulting layered composite was ∼ 1 cm in diameter and 2 mm thick based on an initial solution volume of 130 μL.
Initial experiments done with cells were completed at a cell concentration of 1 × 106 per ml of fluid to study the cell arrangement after banding. After sound field treatment and encapsulation, the discs were fixed with a para-formaldehyde solution and sent to the histology department of the Case Western Reserve University Pathology center for methacrylate embedding and staining using hematoxylin and eosin. Once embedded in the methacrylate, the discs were cut in 5 μm slices every 100-150 μm cut perpendicular to the vertical axis of the disc. The samples were then analyzed using light microscopy.
Additional experiments were performed to study the effect of UV light, the acoustic field, and exposure to the polymer solution on cell viability. In these experiments, the cells (either encapsulated or not) were analyzed using 0.5 μL each of calcein and ethidium homodimer (LIVE/DEAD, Molecular Probes®, Carlsbad, CA) was added to the well. After an incubation period of 30 min at 37 °C, the discs were analyzed using an Olympus Model IX71S1F fluorescent microscope.
Results
Fig. 3 shows the center and inner most rings of the discs that were studied using the histology staining and methacrylate embedding process. While producing useful images, the embedding of the PEGDA discs and the microtome process tended to tear and ripple the PEGDA, which distorted the ring structure on most of the images. Additionally, settling during the focusing process meant that majority of the microtome slices had little or no cells present.
Figure 3.
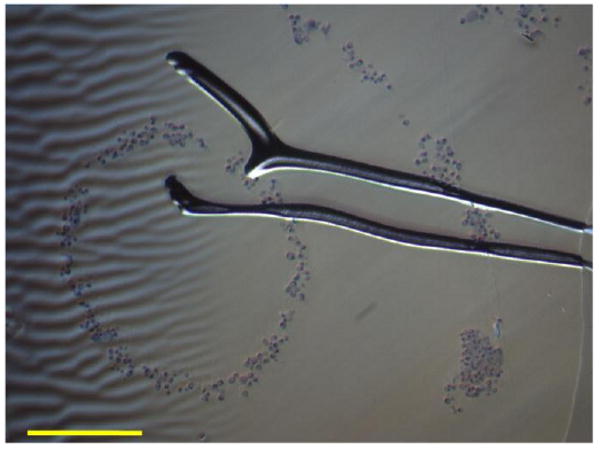
Bright-field image of microtome slice of inner rings composed of cancer cells arranged in 30 wt % PEGDA disc at a concentration of 1 × 106 cells per ml of fluid. Scale bar is equal to 300 μm. Distortions and the tear in the image was the result of the methacrylate embedding process.
In an effort to produce a better ring structure, the cell concentration and was increased to 5 × 106cells per ml of fluid and a fluorescent LIVE/DEAD assay was used to image the banded cells and to estimate viability. This analysis procedure significantly decreased the time required for analyzing the banded composites. Fig. 4 shows top-down views of the arrangement of cells near the axis of the cylindrical sample. For these images, the LIVE/DEAD assay was performed 40 hr after the initial polymerization to determine if cells could survive the initial acoustic focusing process. Cell viability was estimated to be about 50%.
Figure 4.
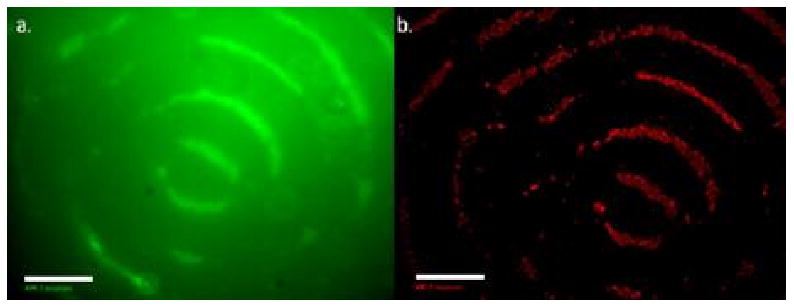
Fluorescent images of live (a) and dead cells (b) after acoustic focusing in a PEGDA solution, following by UV light photopolymerization. The frequency of the sound field was 2.32 MHz and the cell initial concentration was 5 × 106 cells/ml. The LIVE/DEAD assay was applied 40 hr after encapsulation and incubation. Scale bar is equal to 500 μm.
Within the entire sample, eleven annular layers were produced, where the average distance between layers was 302 ±33 μm with an average thickness of 80 ±21 μm (n=3). These results are consistent with the applied acoustic frequency of 2.32 MHz and a speed of sound through the cell suspension of ∼1400 m/s based on the distance between the bands, which is near the speed of sound in water which is ∼1500 m/s (13).
The effects of various process conditions on cell viability were investigated. Fig. 5 a and b shows LIVE and DEAD images of untreated cells (i.e. no sound field, UV light) suspended in cell media. As expected there should be very little cell death (as shown in Fig. 5-b). In a separate experiment, the cells were suspended in cell growth media and subjected to 20 minutes of sound field at 4 VRMS, only. The cells banded and agglomerated during application of the sound field, and then sank to the bottom of the acoustic chamber (losing the concentric banding geometry) after the sound field was discontinued. LIVE/DEAD viability studies were done on these agglomerated, sedimented cells as shown in Fig 5-c, and 5-d, respectively. The results indicated that the sound field at these conditions was not significantly damaging the cells. Therefore, cell death seen in Fig. 4-b must have resulted from other parameters such as UV-light exposure, or a combination of the polymer and photoinitiator.
Figure 5.
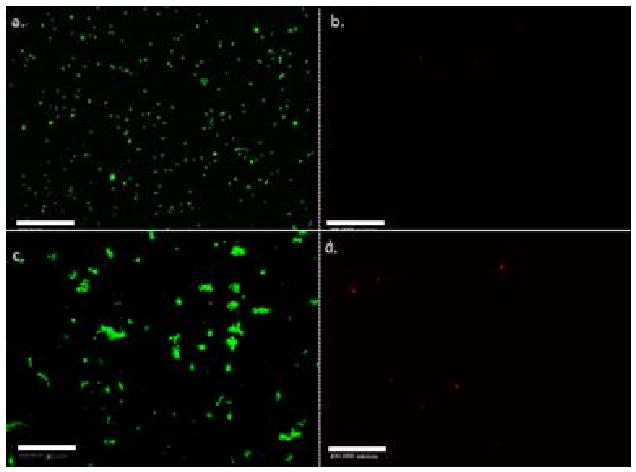
Fluorescent images of living (a) and dead (b) cells exposed to no sound field or UV-light in cell growth media; images of living (c) and dead (d) cells exposed to 20 min of 2.32 MHz ultrasound at and 4 VRMS with no UV-light. Images indicated that there was no significant cell death from application of the sound field. The cell concentration was equal to 5 × 106 per ml of fluid. The scale bar is equal to 500 μm.
To see determine if UV exposure affected the cell viability, the cells were suspended in cell growth media at a concentration of 5 × 106 per ml of fluid, and exposed to UV light for times of up to 15 minutes. Viability was assessed via the fluorescent LIVE/DEAD assay. The results of these UV experiments showed that after 15 minutes of UV exposure, the cell viability dropped from an initial value of 96 ±1.5% to 93% ±5.0% (n=3). In the acoustic focusing experiments show in Fig. 4, the UV exposure was only 3 minutes suggesting that UV irradiation alone was an unlikely cause of cell death.
As reported in the literature, when cells are exposed to polymer solutions, it can have a negative impact on cell viability (14). To simulate the acoustic focusing experiments, cells were added to a 30 wt % PEGDA solution at a concentration of 5 × 106 and treated with 20 minutes ultrasound (2.32 MHz, 4 VRMS) and 3 minutes of UV light. No photoinitiator was present in the PEGDA-cell suspension. After analyzing the images of the LIVE/DEAD assay it was found that the viability of the cells was equal to 52.8 ±16% (n=3), which is estimated to be the approximate cell viability of the acoustic focusing experiments shown in Fig. 4. The data suggest that the main cause of cell death is prolonged exposure of the cells to the polymer solution itself, noting that the photoinitiator can also adversely affect the cells as well.
Discussion
The specifics of particle positioning under the influence of an acoustic field have been discussed in the literature (15). In our particular case, the cells responded to the standing wave field by traveling to nodal regions, which, for the case of the cylindrically symmetric geometry used in our experiments, are a series of concentric cylindrical shells located as positions wherein the acoustic pressure is zero. Additional motion of the cells resulting from azimuthal primary or secondary acoustic forces, or by hydrodynamic stresses induced by acoustic streaming, may induce agglomeration (16). Examination of Fig. 4 shows small breaks in the ring structure which could be attributed to acoustic streaming, tangential variations in the field intensity which can lead to fluid motion that may interfere with the congruity of the bands, and lateral radiation forces acting within the nodal plane. This was reported by Gheradini (7). It also possible that distortions in the rings structure could result from imperfect matching of the chamber geometry, with the operation frequency causing secondary wave fronts that might interfere with the primary standing wave producing the banded geometry.
Uniformity of the ring structure could be preserved better by reducing the intensity of the sound field; however a weaker field would require a longer time for cell organization, which could introduce other complications, e.g., agglomeration of the cell and the expose cells to the polymer solution for extended time periods. There was some sedimentation of the cells during acoustic focusing, since gravitational forces were unopposed in the cell suspension cavity of the transducer (see Fig. 1.) This problem is compounded by the fact that the acoustic field created large agglomerates that are more prone to gravitational forces. To counter this effect in the future, the density of the fluid matrix should be matched closely to that of the cells so they are neutrally buoyant or, alternatively, a more intense sound field could be used to reduce the time for cell band formation, if is not detrimental to the cells.
The properties of the polymer matrix that most directly affected cell viability were the total polymer content and the presence of the photoinitiator. In Fig. 5, it was clear that the sound field was not significantly damaging the cells. After 20 minutes of sound field treatment, the amount of red cells was small relative to the amount of green cells as depicted in Fig. 5-c and 5-d. Also UV-light in and of itself seemed to have little effect, as cell viability dropped very little after exposure to UV light, while suspended in cell media (in the absence of the photoinitiator). Evidence of this has been seen in the literature by Bryant, where cells exposed to UV light for periods of 10 minutes with a low photoinitiator concentration (Igracure 2959 at 0.05 wt %), showed no significant cell death (17). In the similar study, the effect of the photoinitiator was also studied at 0.1 wt %, the level used here (18). It reported that cell death fell to ∼ 80% from an initial value of ∼ 95%, so it is probable in our case that the photoinitiator is contributing to cell death, but it is likely less significant than exposure to the polymer solution during the banding process. Other researchers have attributed significant cell death to short UV exposure times; however these were in concert with high photoinitiator concentrations that were roughly 10 times the concentration used in this study (19), which induced large amount of free-radical molecules.
The extent of cell death was estimated at 50% in Fig. 4 after 20 minutes of focusing in 30 wt% PEGDA, followed by 3 min of UV encapsulation. A similar viability was measured when the cells were exposed to the sound field and ultraviolet light while suspended in the 30 wt % PEGDA solution that contained no photoinitiator. This suggested that the polymer solution rather than the photoinitiator was more significant in reducing cell viability. Similar reductions in cell viabilities from PEGDA exposure have been reported in the literature at increased polymer loadings. For example, Burdick found that viability fell to roughly 60% when the concentration of PEGDA4600 was increased from 10% to 30% (20). Panda reported a cell drop in cell viability from 90% to 70% when the PEGDA concentration was increased from 10 to 30 wt % (21). Panda reported however, that they could not polymerize the system adequately at concentrations of 10 and 20 wt %, a reason why a fairly high polymer concentration of 30 wt % was used in this study. Presumably, our cell viability is lower at 50%, because 20 min was required to arrange the cells.
So, while not optimal in a biocompatibility sense, the 30 wt % PEGDA400 matrix and a photoinitiator concentration of 0.1 wt % was chosen in the present study because of its fast polymerization rate, so the banded cell constructs could be easily preserved and examined. A cell viability of 50% after 40 hours, while not ideal, is significant because it shows that the cells are surviving the acoustic focusing process, even if the system is not optimized. Additionally, a low molecular weight polymer molecule like PEGDA400 is potentially more damaging then a larger polymer chain since the smaller molecule can interact with the cells more directly (22). And while it is the combination of UV irradiation, photoinitiator concentration, and polymer loading, that affects viability, in our case, total polymer concentration is most detrimental to the cells, and will be addressed in future experimentation, while reducing the photoinitiator concentration to a level that is proven not to harm the cells.
Although the LIVE/DEAD fluorescent assay is widely used for estimating overall cell viability, the identification and subsequent counting of individual cells in a concentrated, layered state is difficult due to the staining characteristics of the “LIVE” calcien AM dye (the entire body of the cell fluoresces) as opposed to the “DEAD” ethidium homodimer, which only stains of the nucleus cell. The calcein AM dye does show that banding did occur (Fig-4a) and that a significant number of cells were viable due to increased, intense fluorescence (relative to the background) of the banded geometry, however the absorbance of the dye by the PEGDA, limits the resolution of the images and makes counting of individual cells very difficult. Thus, the viability results in the banded samples may be better than what was observed after assaying our PEGDA matrix. Alternative colorimetric-based assays, such as the aqueous and non-aqueous versions of the MTT test were tried, but were unsuccessful in providing useful viability data because the purple formazan product from the living cells could not be removed from the polymerized PEGDA matrix. This problem may be avoided if longer chained PEGDA molecules are for the matrix or if lower polymer concentrations are utilized, since a less dense matrix would be formed.
Conclusions
Human cells were successfully concentrated into annular layers and then encapsulated in a UV crosslinked (polymerized) PEGDA hydrogel matrix using an acoustic focusing technique. The layered cells exhibited a viability of 50 %, 40 hours after encapsulation. Through additional experimentation, it was concluded that the acoustic field and UV exposure had little detrimental effect on cell viability. Cell exposure to the PEGDA solution of relatively high concentration during the application of the sound field was the main cause of cell death. In the future reduced polymer loadings and photoinitiator concentration should improve cell viability. This preliminary study demonstrated that acoustic focusing is a viable technique for creating multi-layered cellular biocomposites for tissue engineering applications. Future work will involve the transition to more functional cells, such as human mesenchymal stem cells, for cartilage and tendon tissue engineering applications. Optimization of the sound field characteristics will increase cell-cell contact by reducing the thickness of the cell bands and decreasing the distance between bands, which will lead to better cell-cell communication during in vitro tissue development.
Acknowledgments
This research was supported in part by grants from the National Institutes of Health (EB006203, AR053622).
References
- 1.Gupta S, Feke DL, Manas-Zloczower I. Fractionation of mixed particulate solids according to compressibility using ultrasonic standing wave fields. Chemical Engineering Science. 1995;50(20):3275–3284. [Google Scholar]
- 2.Pangu G, Feke DL. Droplet transport and coalescence kinetics in emulsions subjected to acoustic fields. Ultrasonics. 2007;46(4):289–302. doi: 10.1016/j.ultras.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Saito M, et al. Fabrication of polymer composite with periodic structure by the use of ultrasonic waves. Journal of Applied Physics. 1998;83(7):3490–3494. [Google Scholar]
- 4.Hawkes JJ, et al. Ultrasonic deposition of cells on a surface. Biosensors and Bioelectronics. 2004;19(9):1021–1028. doi: 10.1016/j.bios.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Gherardini L, et al. A study of the spatial organization of microbial cells in a gel matrix subjected to treatment with ultrasound standing waves. Bioseparation. 2001;10(4):153–162. doi: 10.1023/a:1016311410219. [DOI] [PubMed] [Google Scholar]
- 6.Coakley WT, et al. Cell–cell contact and membrane spreading in an ultrasound trap. Colloids and Surfaces B: Biointerfaces. 2004;34(4):221–230. doi: 10.1016/j.colsurfb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Gherardini L, et al. A new immobilization method to arrange particles in a gel matrix by ultrasound standing waves. Ultrasound in Medicine and Biology. 2005;31(2):261–272. doi: 10.1016/j.ultrasmedbio.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Saito, et al. Host-guest composites containing ultrasonically arranged particles. Journal of Materials Science. 2000;35:2373–2377. [Google Scholar]
- 9.Oberti, et al. Manipulation of micron-sized within a micromachined fluidic device to form two-dimensional pattern using ultrasound. J Acoust Soc Am. 2006;121(2):778–785. doi: 10.1121/1.2404920. [DOI] [PubMed] [Google Scholar]
- 10.Kavalkovich KW, Boynton RE, Murphy JM, Barry F. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cellular & Developmental Biology - Animal. 2002;38(8):457–466. doi: 10.1290/1071-2690(2002)038<0457:cdohms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Wieser RJ, et al. Growth control in mammalian cells by cell-cell contacts. Environmental Health Perspectives. 1990;88:251–253. doi: 10.1289/ehp.9088251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skudrzyk E. Fundamentals of Acoustics. Springer-Verlag; New York: 1971. pp. 425–426. [Google Scholar]
- 13.Marrin DL. Sound in Water. In: Lehr J, Keeley J, Lehar Janet, editors. Water Encyclopedia. 1-5. John Wiley and Sons; New York: 2005. p. 569. [Google Scholar]
- 14.Burdick JA, Anseth KS. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- 15.Mandralis ZI, et al. Transient response of fine particle suspensions to mild planar ultrasonic fields. Fluid/Particle Separation Journal. 1990;3(3):115–121. [Google Scholar]
- 16.Nyborg WL. Acoustic Streaming. In: Hamilton MF, Blackstock DT, editors. Nonlinear Acoustics. Academic Press; San Diego: 1998. pp. 207–228. [Google Scholar]
- 17.Nuttleman CR, et al. In vitro osteogenic differentiation of human mesenchymal stem cells photoencapsulated in PEG hydrogels. J Biomed Mat Res A. 2004;68:773–782. doi: 10.1002/jbm.a.20112. [DOI] [PubMed] [Google Scholar]
- 18.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatiblity of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. Journal of Biomaterials Science Polymer Edition. 2000;11(5):439–457. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 19.Yeh J, et al. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27:5391–5398. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Burdick JA, et al. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;1(6):386–391. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panda P, et al. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip. 2008;8:1056–1061. doi: 10.1039/b804234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds CR, Tedeschi H. Permeability properties of mammalian cell nuclei in living cells and in vitro. Journal of Cell Science. 1984;70(1):197–207. doi: 10.1242/jcs.70.1.197. [DOI] [PubMed] [Google Scholar]


