Abstract
Background.
In the call for papers (Alley DE, Ferrucci L, Barbagallo M, Studenski SA, Harris TB. A research agenda: the changing relationship between body weight and health in aging. J Gerontol A Biol Sci Med Sci. 2008;63(11):1257–1259.), it is assumed that the association between body mass index (BMI [kilogram per square meter]) and mortality becomes increasingly U-shaped with advancing age. The aim of this study is to examine the association between BMI and mortality and to test whether the association is changing with advancing age for persons aged 70–95 years in Denmark.
Methods.
The study populations comprised two surveys: the Longitudinal Study of Aging of Danish Twins (LSADT) and the Danish 1905 Cohort Survey. From 1995 to 1999, 4253 individuals aged 70–95 years from the LSADT were interviewed at home. In 1998, 2,262 individuals aged 92–93 years from the 1905 Cohort were interviewed at home. The information in both surveys included self-reported weight and height. With virtually no loss to follow-up, survival was assessed through a 10-year follow-up period, during which 4,664 (72%) of the persons died.
Results.
The association between BMI and mortality is changing with advancing age for people aged 70–95 years. There was a significant decrease in the association between mortality and low BMI with advancing age for both genders (p ≤ .03). There was also a tendency for the association between mortality and high BMI to decrease with advancing age for males (p = .06).
Conclusion.
In a large contemporary Danish population–based sample, the association of BMI and mortality became decreasingly U-shaped with advancing age for the age range 70–95 years.
Keywords: Body mass index, Mortality, Age factors, Aged 75 years and older, Nonagenarians
INTRODUCTION
In the call for papers for the research agenda: “the changing relationship between body weight and health in aging,” it was stated that: “… the relationship between BMI and mortality is increasingly U-shaped with advancing age, with higher mortality among both underweight and obese older person. …” (1). A previous American study of 83,744 persons, followed for an average of 14.7 years, provides support for this statement based on two very broad age categories, one below the age of 55 years and one equal or above the age of 55 years (2). Based on this, it is not possible to assess whether the U-shape increases with age at the very old ages.
The relationship between body mass index (weight adjusted for height [kilogram per square meter]) and mortality is observed as being U-shaped in many cohorts (2–5). Other studies support an increasing linear association between body mass index (BMI) and mortality in younger persons, when adjusting for health at intake, while other studies found no association in older persons (4–7).
A few studies suggest that the association between BMI and mortality is declining with advancing age (5–7). In the study by Waaler (5) of 1.8 million Norwegians, this was shown as cumulative incidence, which has little discriminative power as recognized by the author (5). In the study of 6,139 German persons, who were followed for an average of 14.8 years, the focus was on obese persons, and the effect of age was only analyzed between age 18 and 74 years (6). In the study of 324,135 American persons, who were followed for 12 years, the number of persons above the age of 85 years was only 553 (7).
Here, we investigate the relationship between BMI and mortality for persons aged 70–95 years in a large population–based contemporary sample of Danes over a 10-year follow-up period.
METHODS
Study Population
The study population consists of two surveys. The Longitudinal Study of Aging of Danish Twins (LSADT) began in 1995 and includes all Danish same-sex twins aged 70+ years, who were assessed every 2 years up to 2005 (8). From 1995 to 1999, 5,882 twins were asked to make their first entry into the survey and 4,271 twins (73%) volunteered. These twins consist of 2,247 broken pairs (ie, only one twin in a pair participated) and 1,012 complete pairs of which 392 were monozygotic pairs. Eighteen were discarded because they were older than 95 years. The Danish 1905 Cohort comprises all individuals born in 1905 living in Denmark on April 1, 1968, and was identified through the Danish Civil Registration System. The 1905 Cohort Survey consists of four waves and was started in 1998, at which point 3,600 persons were still alive, and of these 2,262 (63%) participated (9).
In both surveys, they were asked about their present weight and height, and the two surveys resulted in a study population of 6,515 persons (2,275 males and 4,240 females) of which 742 were proxy interviews. Each person was followed from the baseline interview date.
Body Mass Index
The BMI was calculated from the self-reported height and weight at baseline. Thirteen persons had a BMI which was less than 15 or greater than 45, and it was considered that the recorded values might have been flawed so that the BMI was recorded as missing (10). One hundred forty-seven had no height or weight recorded. Hence, a total of 160 persons (2.5%) had a missing BMI value.
Survival
Each participant was followed from the baseline interview date through to December 31, 2008, in the Danish Civil Registration system, which registers date of death or emigration of all Danish persons (11). Two persons emigrated in the follow-up period; thus, they were lost in the follow-up process. The interview dates from LSADT conducted in 1995 and 1997 were only approximate. The actual month and day within these years were not recorded, but the time interval of the interviews was known (3–4 months), and the middle of these dates was used.
Analyses
Survival analyses using Cox's proportional hazards models were performed to study the association between BMI and mortality separately within each age group 70–74, 75–79, 80–89, and 90–94. In these models, the participants’ age was the time axis adjusting for birth cohort (12). Risk was assessed from the age of the interview to age of death or age after the 10-year follow-up period for those surviving more than 10 years. The survival was truncated at 10 years of follow-up because very few participants had more than 10 years of follow-up, making interpretation difficult. Survival analysis was also performed using the entire data but allowing for separate associations of BMI and mortality in the different age groups. This analysis has the advantage of more power when modeling the age effect but assumes BMI to be constant over the follow-up period.
For each of the above-mentioned age groups, we fitted a linear spline Cox regression model with one knot. This model assumes piecewise linear association between BMI and log hazard with different slopes below and above the knot (change point). The optimal knot was found using the log likelihood function under the working independence assumption. Models with two knots were also applied, but they did not provide an appreciably better fit, and we thus used the one-knot spline model only. This class of models gave an adequate fit to the data compared to the models with BMI divided into quantiles, and they also gave a significantly better fit to the data than linear associations (without a knot). These results were based on the log likelihood ratio test under the working independence assumption. In the spline models, the analyses were also performed, restricted from the 1st to the 99th percentile to test for potential high leverage of outliers. The proportional hazards assumption, underlying the Cox models, was tested using the Schoenfeld residual test (13). To examine whether the association between BMI and mortality changed with age, a linear trend analysis was performed, using variance-weighted least squares regression. A goodness of fit was calculated to test for the linear association.
Statistical analysis was done using Stata 9.2 (STATA Corp, College Station, TX). As the data partly pertain to twin pairs, and because observation within twin pairs might be correlated, the analyses were performed using the robust estimator of variance, assuming independence between pairs (14).
RESULTS
BMI generally decreased with age, and males had a higher BMI than females (Table 1). The variation of the BMI values was generally higher for females than males, and for males the variation was constant over age, but for females it decreased. The same pattern emerged if the analyses were restricted from the 1st to 99th percentile showing that the results were not influenced by outliers. During the 10-year follow-up period, 4,664 (72%) persons died. The mortality rates were higher for males than females, and the rates increased with increasing age (Table 2). Furthermore, higher mortality among both underweight and overweight persons was present in all age groups except for males aged 90–94 years, where it seemed that higher BMI led to better survival.
Table 1.
Summary statistics for each gender and age groups
| Male |
Female |
|||||||
| 70–74 y | 75–79 y | 80–89 y | 90–94 y | 70–74 y | 75–79 y | 80–89 y | 90–94 y | |
| N* | 590 | 610 | 459 | 616 | 706 | 938 | 819 | 1,777 |
| N BMI† | 586 | 605 | 450 | 605 | 698 | 918 | 797 | 1,696 |
| Median BMI | 25.35 | 25.03 | 24.51 | 23.88 | 23.88 | 23.71 | 23.12 | 22.31 |
| Mean BMI | 25.66 | 25.34 | 24.83 | 24.05 | 24.30 | 24.09 | 23.46 | 22.75 |
| SD BMI | 3.36 | 3.50 | 3.60 | 3.46 | 4.22 | 4.08 | 3.97 | 3.76 |
Notes: BMI, body mass index.
*Total number.
Number with a BMI measurement.
Table 2.
Mortality rates* according to median BMI†, by gender and age groups
| 70–74 y |
75–79 y |
80–89 y |
90–94 y |
|||||||||
| Quantiles BMI | N | Median BMI | Mortality Rate | N | Median BMI | Mortality Rate | N | Median BMI | Mortality Rate | N | Median BMI | Mortality Rate |
| Male | ||||||||||||
| 1 | 98 | 21.30 | 8.8 | 101 | 20.90 | 17.3 | 75 | 20.32 | 18.3 | 101 | 19.49 | 47.3 |
| 2 | 107 | 23.66 | 4.3 | 102 | 23.12 | 8.0 | 78 | 22.66 | 16.2 | 101 | 21.97 | 37.5 |
| 3 | 90 | 24.80 | 4.2 | 100 | 24.39 | 7.9 | 75 | 24.09 | 20.9 | 101 | 23.39 | 32.8 |
| 4 | 96 | 26.08 | 5.1 | 103 | 25.71 | 8.3 | 72 | 25.39 | 16.5 | 104 | 24.49 | 32.9 |
| 5 | 98 | 27.47 | 4.3 | 100 | 27.43 | 10.3 | 75 | 26.84 | 15.6 | 98 | 25.95 | 32.1 |
| 6 | 97 | 30.74 | 5.9 | 99 | 30.46 | 9.3 | 75 | 29.73 | 18.4 | 100 | 29.38 | 30.8 |
| Female | ||||||||||||
| 1 | 117 | 18.87 | 5.5 | 153 | 18.96 | 7.4 | 134 | 18.37 | 17.5 | 283 | 17.94 | 34.6 |
| 2 | 119 | 21.45 | 3.0 | 154 | 21.32 | 6.5 | 132 | 20.56 | 13.4 | 284 | 20.03 | 29.8 |
| 3 | 114 | 23.19 | 2.5 | 154 | 23.03 | 5.1 | 133 | 22.35 | 13.9 | 283 | 21.51 | 26.9 |
| 4 | 117 | 24.77 | 3.0 | 151 | 24.34 | 5.2 | 140 | 23.88 | 11.8 | 281 | 23.19 | 25.4 |
| 5 | 115 | 26.57 | 3.0 | 153 | 26.44 | 5.3 | 126 | 25.90 | 10.1 | 284 | 24.97 | 24.7 |
| 6 | 116 | 30.43 | 3.8 | 153 | 30.04 | 6.3 | 132 | 29.30 | 12.3 | 281 | 28.13 | 26.5 |
Notes: BMI, body mass index.
*Mortality rates are per 100 years.
BMI was divided into 6 quantiles.
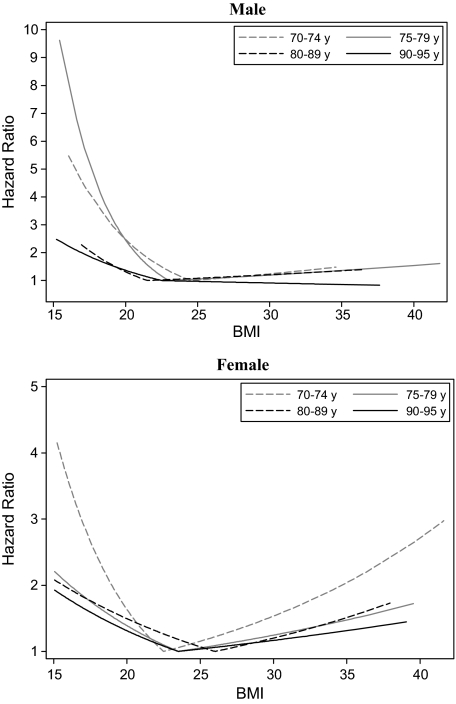
The association between BMI and mortality decreased with advancing age for both males and females (Figure 1). There was a significant decrease in the association between low BMI and mortality with advancing age (p ≤ .03) as well as a tendency for the association between high BMI and mortality to decrease with advancing age for males (p = .06) (Figure 2). This suggests a decrease in the “U-shape” with advancing age for both genders. The figure also illustrates that being underweight was associated with a higher mortality rate for both genders, contrary to being overweight for all age groups.
Figure 1.
Graphical representation of the linear spline Cox regression for each gender and age groups.
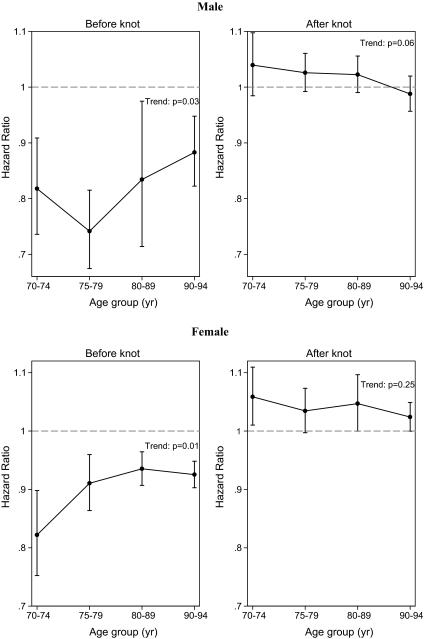
Figure 2.
Linear trend analysis for the hazard ratio with an increment of 1.0 in body mass index (BMI) according to age groups having a low (below knot) or high (above knot) BMI for each gender. The hazard ratios are from the separate linear spline models of the four age groups. The bars represent 95% confidence intervals. The p value refers to a linear trend test. The graph on the left shows that the association between mortality and low BMI decreases with advancing age, and the graph on the right also shows that the association between mortality and high BMI decreases with advancing age.
The linear assumption in the trend analysis was accepted for having a high BMI, but for having a low BMI, there was some evidence of the trend not being linear for females (p = .08) when analyzing all the age groups. Hence, the trend was evaluated excluding the age group 90–94, and the linear assumption of this model was accepted. This and Figure 2 suggest that the association between low BMI and mortality decreases significantly with advancing age for females, but the association might reach a plateau after the age of approximately 85 years.
The test for proportional hazard assumption was not compromised in the applied models, except for males aged 80–89 years. Further analysis showed that it was only four persons, who made the proportional hazard assumption fail; hence, we chose to accept the model. Restricting the data from the 1st to the 99th percentile gave virtually identical results in the linear spline model, indicating that the results were robust. When analyzing the entire data, allowing for separate associations of BMI and mortality in the different age groups, we achieved similar results compared to analyzing the data separately for each age group, which again substantiate the robustness of the conclusions.
DISCUSSION
This study found that the U-shaped association between BMI and mortality is decreasing from age 70 to 95 years in a large contemporary Danish population–based sample. The mortality rates in the lowest and highest BMI quantile, within each age group, increased with age but not as much as the rate in the other quantiles, suggesting that the relationship became less U-shaped with age. The association between low BMI (values below the knot) and mortality decreased significantly with advancing age, and there was a tendency for the association between high BMI (values above the knot) and mortality to decrease with advancing age for males. The test for the linear assumption was not fulfilled for low BMI for females, and further analyses showed that the association between mortality and low BMI decreased significantly with advancing age and that the association may reach a plateau after the age of approximately 85 years.
Having a low BMI was associated with a higher mortality at all age groups for both genders. For females, having a high BMI was also associated with a higher mortality for age group 70–74, and for age groups 75–79, 80–89, and 90–94, there was a tendency for an association. For males, having a high BMI was not associated with a higher mortality for any of the age groups. But having a low BMI was strongly associated with mortality compared to having a high BMI in all age groups and for both genders.
These results are different from the assumption in the present call for papers (1) but more in line with the articles by Waaler (5), Bender et al. (6), and Stevens et al. (7). The study by Waaler (5) on 1.8 million Norwegians had the problem in the relation to the present study that it was done using cumulative incidence, which reduces the power to detect differences in survival among the elderly. The study by Bender et al. (6) focused on the effect of obesity on mortality in different age groups, and the study by Stevens et al. (7) had few very old persons. None of the three studies had an analysis of nonagenarians, which is a large group in the present study.
None of the mentioned studies (2–7) did report whether the proportional hazard assumption was fulfilled, which is the main assumption in the Cox model. Violation of the proportional hazard assumption can indeed result in biased estimates of the effect of BMI on survival. A false nonsignificant result, regarding the effect of a given variable, can even be obtained if the effect is initially positive and later on the reverse.
Our study has the strength that it is population based. Some of the participants were twins, and it could be argued that twins are not good representatives of the large population on health, aging, and mortality. Twins might be a selective group because twins have birth-weight that on average is about 1 kg less than that of singletons. Hence, growth restriction in the third trimester is severe, which is hypothesized to “program” increased risk of cardiovascular diseases and diabetes (15–17). However, large-scale Swedish and Danish studies of mortality have been unable to detect any differences in all-cause mortality or cardiovascular mortality in the age range 6–90 between twins and the general population (18–20).
The weakness of our study is primarily that the BMI is calculated from self-reported height and weight. Older persons might be more likely to report their maximal attained height rather than current height, but this would not have an effect on the change in the association with age. Older persons might also be more likely to report with error than younger persons, which could have an effect on the change in the association with age. Using the longitudinal aspect of LSADT did, however, show a high reliability between the reported heights at the next follow-up 2 years later for the age groups 70–74 (0.96), 75–79 (0.95), and 80–89 (0.92) but still highest for the youngest group. For the age group 90–94, there were too few to assess this. Using the longitudinal aspect of LSADT and the 1905 Cohort Survey also showed that the average change in weight (kilograms) at the next follow-up 2 years later was fairly similar within the four age groups 70–74 (−0.4), 75–79 (−0.7), 80–89 (−1.0), and 90–94 (−0.9). We also performed the analyses excluding the proxies and the twins without mini-mental state examination recordings at baseline, and we found the same overall results. This suggests that the reporting is reliable, which is in agreement with the literature of self-reported height and weight (21).
We did not control for initial health, and it could be argued that the U-shaped association reflects the direct effect of diseases already present at measurement. However, this would particularly apply in the time just after measurement, and because the proportional hazard assumption was fulfilled in a 10-year follow-up period, this would not account for the overall U-shaped association.
The participation rate from the two surveys was high (69%), but nonresponse from the most frail persons could create a biased association. Using data from the Danish Civil Registration system, we tested the participants against the nonparticipants and found a higher death rate for the nonparticipants, compared with the survey participants. Because the effect was similar within each age group, this is unlikely to have an effect on the change in the association between BMI and mortality with age (data available on request).
The reason for the decrease in the association between high BMI and mortality with advancing age may be a selection effect. The proportion with a relative high BMI among the 90- to 94-year olds is much smaller than among the 70- to 74-year olds. The reason for the decrease in the association between low BMI and mortality may be due to different causes of low BMI among the 70- to 74-year olds and the 90- to 94-year olds. In the youngest group, a low BMI could be a sign of serious diseases, for example, cancer. In the oldest group, a low BMI is most likely a sign of the terminal phase. The decreasing association between BMI and mortality could also reflect the increasing impact of other risk factors.
All these factors could explain why the variation in BMI is larger among the 70- to 74-year-old females compared with 90- to 94-year-old females. This is, however, not the case for males, who also generally have a smaller variation than females, which may well be an effect of males having a higher mortality rate compared with females throughout life. Hence, there has already been a strong selection in males compared with females in these age groups.
In conclusion, this large population–based study found that the association between BMI and mortality became decreasingly U-shaped with advancing age in the age group 70–95 years. This suggests that BMI is less predictable of mortality with age, and for all persons older than 70 years a very low BMI is associated with the highest mortality.
FUNDING
The Longitudinal Study of Aging of Danish Twins and the Danish 1905 Cohort Survey were based on grants from National Institute on Aging (NIA-P01-AG08761). The Danish Aging Research Center is supported by a grant from the VELUX foundation.
Acknowledgments
The work was carried out at the Department of Epidemiology, Institute of Public Health, University of Southern Denmark, J.B. Winsløws Vej 9B, 5000 Odense C, Denmark.
References
- 1.Alley DE, Ferrucci L, Barbagallo M, Studenski SA, Harris TB. A research agenda: the changing relationship between body weight and health in aging. J Gerontol A Biol Sci Med Sci. 2008;63(11):1257–1259. doi: 10.1093/gerona/63.11.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman DM, Ron E, Ballard-Barbash R, Doody MM, Linet MS. Body mass index and all-cause mortality in a nationwide US cohort. Int J Obes (Lond) 2006;30(5):822–829. doi: 10.1038/sj.ijo.0803193. [DOI] [PubMed] [Google Scholar]
- 3.Al SS, Ottenbacher KJ, Markides KS, Kuo YF, Eschbach K, Goodwin JS. The effect of obesity on disability vs mortality in older Americans. Arch Intern Med. 2007;167(8):774–780. doi: 10.1001/archinte.167.8.774. [DOI] [PubMed] [Google Scholar]
- 4.Gelber RP, Kurth T, Manson JE, Buring JE, Gaziano JM. Body mass index and mortality in men: evaluating the shape of the association. Int J Obes (Lond) 2007;31(8):1240–1247. doi: 10.1038/sj.ijo.0803564. [DOI] [PubMed] [Google Scholar]
- 5.Waaler HT. Height, weight and mortality. The Norwegian experience. Acta Med Scand Suppl. 1984;679:1–56. doi: 10.1111/j.0954-6820.1984.tb12901.x. [DOI] [PubMed] [Google Scholar]
- 6.Bender R, Jockel KH, Trautner C, Spraul M, Berger M. Effect of age on excess mortality in obesity. JAMA. 1999;281(16):1498–1504. doi: 10.1001/jama.281.16.1498. [DOI] [PubMed] [Google Scholar]
- 7.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338(1):1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 8.McGue M, Christensen K. Social activity and healthy aging: a study of aging Danish twins. Twin Res Hum Genet. 2007;10(2):255–265. doi: 10.1375/twin.10.2.255. [DOI] [PubMed] [Google Scholar]
- 9.Nybo H, Gaist D, Jeune B, et al. The Danish 1905 cohort: a genetic-epidemiological nationwide survey. J Aging Health. 2001;13(1):32–46. doi: 10.1177/089826430101300102. [DOI] [PubMed] [Google Scholar]
- 10.Herskind AM, McGue M, Sorensen TI, Harvald B. Sex and age specific assessment of genetic and environmental influences on body mass index in twins. Int J Obes Relat Metab Disord. 1996;20(2):106–113. [PubMed] [Google Scholar]
- 11.Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449. [PubMed] [Google Scholar]
- 12.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145(1):72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 13.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 14.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:408, 1074–1078. [Google Scholar]
- 15.Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301(6761):1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 17.Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993;307(6918):1519–1524. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vagero D, Leon D. Ischaemic heart disease and low birth weight: a test of the fetal-origins hypothesis from the Swedish Twin Registry. Lancet. 1994;343(8892):260–263. doi: 10.1016/s0140-6736(94)91112-6. [DOI] [PubMed] [Google Scholar]
- 19.Christensen K, Vaupel JW, Holm NV, Yashin AI. Mortality among twins after age 6: fetal origins hypothesis versus twin method. BMJ. 1995;310(6977):432–436. doi: 10.1136/bmj.310.6977.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christensen K, Wienke A, Skytthe A, Holm NV, Vaupel JW, Yashin AI. Cardiovascular mortality in twins and the fetal origins hypothesis. Twin Res. 2001;4:344–349. doi: 10.1375/1369052012506. [DOI] [PubMed] [Google Scholar]
- 21.Schousboe K, Willemsen G, Kyvik KO, et al. Sex differences in heritability of BMI: a comparative study of results from twin studies in eight countries. Twin Res. 2003;6(5):409–421. doi: 10.1375/136905203770326411. [DOI] [PubMed] [Google Scholar]