Abstract
Background.
Low levels of sex hormone–binding globulin (SHBG) and total testosterone (T) in men have been associated with increased risk of type 2 diabetes mellitus (T2DM). As total T and SHBG levels are highly correlated, we determined whether SHBG influences the risk of T2DM through T or whether SHBG is an independent predictor of T2DM.
Methods.
Longitudinal analyses were conducted on men participating in the Massachusetts Male Aging Study, a population-based study of men aged 40–70 years. Of 1,709 men enrolled in 1987–1989, 1,156 were evaluated 7–10 years later and 853 after 15–17 years. Analyses were restricted to 1,128 men without T2DM at baseline.
Results.
Ninety new cases of T2DM were identified. After adjustment for age, body mass index, hypertension, smoking, alcohol intake, and physical activity, the hazard ratio (HR) for incident T2DM was 2.0 for each 1 SD decrease in SHBG (95% confidence interval [CI], 1.42–2.82, p < .001) and 1.29 for each 1 SD decrease in total T (95% CI, 1.01–1.66, p = .04). Free T was not associated with T2DM (HR = 1.03, 95% CI, 0.81–1.31, p = .79). The strong association of T2DM risk with SHBG persisted even after additional adjustment for free T (HR = 2.04, 95% CI, 1.44–2.87, p < .0001) or total T (HR = 1.95, 95% CI, 1.34–2.82, p = .0004).
Conclusions.
SHBG is an independent predictor of incident T2DM even after adjusting for free T or total T. Free T is not significantly associated with T2DM. SHBG may contribute to the risk of T2DM through nonandrogenic mechanisms, which should be investigated as they may provide novel targets for diabetes prevention.
Keywords: SHBG, Type-2 diabetes, Testosterone
THERE is strong epidemiological evidence that low levels of total testosterone (T) in men are associated with type 2 diabetes mellitus (T2DM) both cross sectionally and longitudinally (1–8). As a substantial fraction of circulating T is bound to sex hormone–binding globulin (SHBG), it is not clear if the observed association between total T and T2DM reflects an independent androgenic influence on diabetes risk or whether it operates via SHBG. The association of free T and T2DM has been inconsistent; some studies have reported a weak relationship (1,2,6), whereas others have failed to find any relationship (3,5). The lack of a strong correlation between free T and T2DM suggests that SHBG may be the primary determinant of the apparent relationship between total T levels and T2DM. However, none of the previous studies that have examined the association of SHBG and diabetes risk has adjusted for total or free T levels, rendering these studies unable to determine whether the reported association of T and diabetes risk simply reflects the effects of SHBG. SHBG and its polymorphisms have been associated with insulin resistance (9). Ding and colleagues (10), in their recent publication, showed prospectively that low SHBG levels predicted T2DM and that carriers of the rs6257 variant allele of the SHBG single nucleotide polymorphism had 10% lower plasma SHBG levels; however, neither multivariate model was controlled for T levels. Again, this leaves an important question unanswered—does SHBG mediate T2DM risk by modulating androgen action or does it confer this risk independently?
This issue has important clinical implications. If low T levels confer the increased risk of T2DM, then T therapy in men with low T levels would be expected to reduce the risk of T2DM. On the other hand, if SHBG is the primary determinant of T2DM risk, then totally different clinical and public health strategies that target factors that regulate circulating SHBG levels would be desirable. Indeed, the observed association of low T levels with T2DM in epidemiological studies has been used as an argument to rationalize clinical trials of T therapy to prevent T2DM in middle-aged and older men with low T levels. Although some small short-term trials of T therapy have been conducted in men with T2DM or in men at risk for T2DM, the results of these trials have been conflicting. For instance, Basu and colleagues (11) showed that 2 years of T treatment in elderly men with low or low normal T levels did not improve carbohydrate tolerance, insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, or the pattern of postprandial glucose metabolism. Other studies also have failed to confirm a beneficial effect of androgen therapy on insulin sensitivity or T2DM risk (12–16). In fact, neither the long-term benefit nor the potential harm of T therapy has been established. Therefore, it is important to ascertain whether T or SHBG is the primary determinant of the risk of incident T2DM.
Accordingly, we used the longitudinal data from a large sample of community-dwelling men participating in the Massachusetts Male Aging Study (MMAS) to evaluate prospectively whether SHBG is a significant predictor of incident T2DM, independent of T. In our previous analysis of the MMAS cohort in which sex hormones were used to predict T2DM at the first follow-up visit (2), multivariate models containing SHBG were adjusted for free T but not for total T. In that report, SHBG and free T were shown to both jointly and independently predict T2DM. In the current analysis, we included data from the second follow-up and examined the predictive ability of SHBG by adjusting for free T as well as total T in multivariate models in which we also adjusted for other covariates that might affect the risk of T2DM. We hypothesized that SHBG would independently predict T2DM after adjusting for free T or total T. If so, this would imply that the risk of T2DM associated with low levels of sex steroids is mediated exclusively by SHBG.
METHODS
Participants
The MMAS is a population-based observational cohort study of aging men, who were observed at three time points: baseline, 1987–1989; first follow-up, 1995–1997; and second follow-up, 2002–2004. The sampling design and field protocol have been described in detail previously (17). Briefly, men aged 40–70 years were randomly selected from 11 cities and towns in the Boston, Massachusetts area. At baseline, a total of 1,709 men were enrolled in the study. MMAS participants were typically employed (78%), married (75%), had completed high school (71%), and nearly half were Catholic (48%). Many had earned at least a bachelor's degree (42%). The racial distribution at baseline was as follows: 1,629 (95.5%) white, 52 (3.0%) black, 14 (0.8%) Asian, 1 (0.1%) American Indian, and 10 (0.6%) reported their race as other; three men were missing information on race. The low representation of racial minorities (4%) was consistent with the demographic composition of the Greater Boston, Massachusetts, population at the time of survey (18). At the first follow-up phase, 1,156 completed an interview. At the second follow-up phase, 853 were interviewed. The analysis of the relationship between sex hormones and T2DM at the first follow-up has been previously reported by Stellato and colleagues (2). In that analysis, data from 1,030 men were pooled to identify the predictors of incident T2DM (n = 54) at the first follow-up.
The sample used for this analysis was obtained as follows. Data were available on 1,707 men at baseline. Men not followed at both follow-ups were excluded (423 men). An additional 142 men with T2DM at baseline were excluded. Finally, 14 men were excluded because of missing data on hormone levels and diabetes status on follow-up. Thus, the final analysis for this study was conducted on 1,128 men.
Data Collection and Measures Used
A trained field technician and phlebotomist visited each participant in his home and administered a health questionnaire and performed a psychological assessment. Height, weight, and waist and hip circumferences were measured using standardized procedures developed for large-scale epidemiological field studies. Blood pressure measurements while the participant was seated were obtained at two points, 25 minute apart, and were averaged. The interview included questions concerning smoking, physical activity (19), and alcohol (20). The outcome variable for this study was a new diagnosis of T2DM postbaseline. T2DM was defined by either use of insulin or oral hypoglycemic agents or a positive response to the question, “Have you ever been told by a health professional that you have diabetes?” Self-report of diabetes has been shown to be a valid and accurate marker of T2DM (21).
Hormone Measurements
Nonfasting blood samples were drawn within 4 hours of the participant's awakening to control for diurnal variation in hormone levels. Two samples were drawn 30 minutes apart and pooled for analysis to control for episodic secretion. Blood was kept in an ice-cooled container for transport and was centrifuged within 6 hours. Serum was stored in 5-mL scintillation vials at −20°C, shipped to the laboratory within 1 week by same-day courier, and stored at –70°C until assay. All hormone measurements were performed in the laboratory of Dr. Christopher Longcope at the University of Massachusetts Medical Center (Worcester, MA). Total T and SHBG were measured by previously described immunoassays (Diagnostic Products Corp., Los Angeles, CA, for total T and Farmos kit, Oulunsalo, Finland, for SHBG). Intra-assay coefficients of variation for these assays were 5.4% and 5.0%, respectively. Interassay coefficients of variation were 8.0% and 6.0%, respectively. Free T was calculated from total T and SHBG measurements using Vermeulen's equation (22).
Statistical Analysis
Descriptive statistics, proportions for categorical variables, and means and standard deviations for continuous variables were used to describe the baseline analytic sample (n = 1,128). The purpose of the analysis was to determine whether sex hormone levels were predictors of incident T2DM during follow-up. Person-years were accumulated from baseline to the year of event or date of last contact. Proportional hazards regression models with time-dependent covariates were used to assess the association of SHBG and T levels with risk of developing T2DM during follow-up. Hazard ratios (HRs) were reported for each 1 SD decrease in hormone concentration and for quartiles of the hormone distributions. We chose men in the highest sex hormone quartile as the reference category for computing HRs. Multiple regression models, estimated using Cox regression models, were used to adjust for potential confounders. Significance was determined as p < .05.
RESULTS
Table 1 displays baseline characteristics of the analytic sample that contained 1,128 men, who did not have T2DM at baseline. Their total and free T levels were in the mid-normal range and so was their SHBG level. The mean age of the cohort was 53.7 ± 8.3 years, and the mean body mass index (BMI) was 27 ± 4.1 kg/m2. In comparison with men not included in the analytic sample, men in the analytic sample were younger, had a slightly lower BMI, and had smaller proportions of those with heart disease and hypertension. This was expected as men who had T2DM at baseline were excluded and those who were deceased or seriously ill were not followed. Importantly, hormone concentrations did not differ in the analytic sample from those excluded.
Table 1.
Baseline Participants Characteristics
|
M ± SD or N (%) |
||
| Variable | Participants Not in Analytic Sample (N = 579) | Participants in Analytic Sample (N = 1,128)* |
| Total T (ng/dL)† | 502.0 ± 183.0 | 524.6 ± 172.1 |
| Free T (ng/dL)† | 11.1 ± 4.3 | 11.8 ± 4.4 |
| SHBG (nmol/L) | 32.5 ± 17.1 | 32.0 ± 15.7 |
| Age (y) | 58.0 ± 8.6 | 53.7 ± 8.3 |
| BMI (kg/m2) | 28.1 ± 4.9 | 27.0 ± 4.1 |
| Physical activity (kcal/d) | 3,215 ± 972 | 3,264 ± 1,013 |
| Hypertension | 233 (40.2) | 285 (25.3) |
| Heart disease | 111 (19.2) | 104 (9.2) |
| Smoking | 170 (29.4) | 247 (21.9) |
| Alcohol intake | ||
| 0 drinks/d | 337 (58.9) | 573 (51.1) |
| 1–3 drinks/d | 134 (23.4) | 332 (29.6) |
| >3 drinks/d | 101 (17.7) | 216 (19.3) |
Notes: BMI = body mass index; SHBG = sex hormone–binding globulin; T = testosterone.
Men in the analytic sample did not have type 2 diabetes mellitus (T2DM) at baseline, were interviewed at the first follow-up, were not missing information on baseline T and SHBG, and were not missing information on T2DM status at all time points. Two participants missing age information were excluded from the comparison.
To convert total and free T values from nanograms per deciliter to nanomoles per liter, multiply the values in nanograms per deciliter by 0.0347.
Men were followed for an average of 13 years for the development of T2DM. There were 90 men in the analytic sample who had a new diagnosis of T2DM during 14,638 person-years of follow-up, yielding an overall incidence rate of 6.2 per 1,000 person-years. Correlations between the hormones of interest were as follows: total T with SHBG = 0.29, total T with free T = 0.80, and SHBG with free T = −0.30.
Table 2 displays the age-adjusted mean SHBG and total and free T levels at baseline according to incident T2DM status. Serum SHBG and total T levels were significantly lower in men who developed T2DM compared with men who did not (SHBG: 26.0 ± 1.6 vs 32.5 ± 0.5 nmol/L, p < .001 and total T: 468.3 ± 18.0 vs 529.3 ± 5.3 ng/dL, p = .001). On the other hand, baseline free T levels were not significantly different among men who developed T2DM and those who did not develop T2DM (11.3 ± 0.4 vs 11.8 ± 0.1 ng/dL, p = .34). Free T levels did not differ in men with and without T2DM even prior to age adjustment.
Table 2.
Age-Adjusted Mean Hormone Levels at Baseline According to T2DM Status
|
M ± SE |
|||
| Variable | No T2DM (N = 1,038) | Incident T2DM (N = 90) | p Value* |
| Total T (ng/dL)† | 529.3 ± 5.3 | 468.3 ± 18.0 | .001 |
| Free T (ng/dL)† | 11.8 ± 0.1 | 11.3 ± 0.4 | .34 |
| SHBG (nmol/L) | 32.5 ± 0.5 | 26.0 ± 1.6 | <.001 |
Notes: SHBG = sex hormone–binding globulin; T = testosterone; T2DM = type 2 diabetes mellitus.
p Value associated with F test from Type III sum of squares.
To convert total and free T values from nanograms per deciliter to nanomoles per liter, multiply the values in nanomoles per liter by 0.0347.
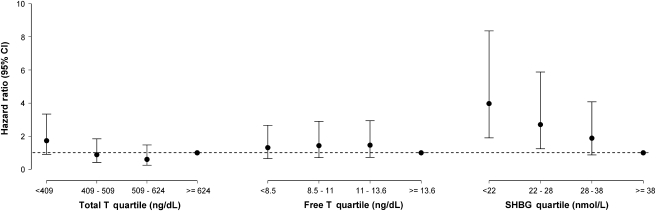
Because age and BMI are important contributors to T2DM risk, we adjusted for these covariates in the analyses. Age- and BMI-adjusted HRs with 95% confidence interval (CI) for developing incident T2DM are displayed in Table 3. The risk of developing T2DM per standard deviation decrease in hormone concentration was greatest for SHBG (HR = 1.93, 95% CI, 1.38−2.70, p = .0001). Total T and free T were not significantly associated with T2DM. Figure 1 shows the risks of T2DM associated with hormone quartiles. SHBG displayed a dose–response relation with incident T2DM. The highest risk of developing T2DM was associated with the lowest quartile of SHBG (HR = 3.97, 95% CI, 1.9–8.36, p = .0019), followed by the lowest quartile of total T (HR = 1.73, 95% CI, 0.9–3.33, p = .01); the lowest free T quartile was not predictive of future T2DM (HR = 1.31, 95% CI, 0.65–2.66, p = .74).
Table 3.
Age- and Body Mass Index–Adjusted Hazard Ratios (HRs) for Incident Type 2 Diabetes Mellitus
| Hormone | SD | HR | 95% Confidence Interval | p Value* |
| Total T | 172.1 ng/dL† | 1.23 | 0.96–1.59 | .1 |
| Free T | 4.4 ng/dL† | 0.99 | 0.78–1.25 | .92 |
| SHBG | 15.7 nmol/L | 1.93 | 1.38–2.70 | .0001 |
Notes: SHBG = sex hormone–binding globulin; T = testosterone.
p Value associated with Wald-type test.
To convert total and free T values from nanograms per deciliter to nanomoles per liter, multiply the values in nanomoles per liter by 0.0347.
Figure 1.
Age- and body mass index–adjusted hazard ratios (HRs) for incident type 2 diabetes mellitus associated with hormone quartile. Participants with hormone values in the 4th quartile serve as the reference category (HR = 1.00). Dashed line represents the reference line, where HR = 1.00.
Table 4 shows the results of Cox regression models for predicting T2DM after multivariate adjustment for age, BMI, smoking, high blood pressure, alcohol intake, and physical activity. In models that tested the role of the sex hormones in separate models, SHBG was most strongly associated with T2DM, with HR = 2.0 (95% CI, 1.42–2.82, p < .0001) associated with a 1 SD decrease in SHBG. Total T was relatively weakly associated with T2DM (HR = 1.29, 95% CI, 1.01–1.66, p = .04), and free T was not statistically significantly associated with T2DM. The strong association of SHBG with T2DM persisted even after additional adjustment for total T (HR = 1.95, 95% CI, 1.34–2.82, p = .0004) or free T (HR = 2.04, 95% CI, 1.44–2.87, p < .0001).
Table 4.
Multivariate Models of Hormones Predicting Incident Type 2 Diabetes Mellitus
| Hazard Ratio (95% Confidence Interval) | ||||
| Hormone | SD | Model 1* | Model 2† | Model 3‡ |
| Total T | 172.1 ng/dL§ | 1.29 (1.01–1.66) | 1.05 (0.80–1.38) | — |
| Free T | 4.4 ng/dL§ | 1.03 (0.81–1.31) | — | 1.13 (0.88–1.45) |
| SHBG | 15.7 nmol/L | 2.00 (1.42–2.82) | 1.95 (1.34–2.82) | 2.04 (1.44–2.87) |
Notes: SHBG = sex hormone–binding globulin; T = testosterone.
Model including SHBG, total T, or free T alone plus age, body mass index (BMI), high blood pressure, smoking, alcohol intake, and physical activity.
Model including total T and SHBG plus age, BMI, high blood pressure, smoking, alcohol intake, and physical activity.
Model including free T and SHBG plus age, BMI, high blood pressure, smoking, alcohol intake, and physical activity.
To convert total and free T values from nanograms per deciliter to nanomoles per liter, multiply the values in nanomoles per liter by 0.0347.
DISCUSSION
In a prospective study of 40- to 70-year-old men, among the sex hormones examined, SHBG is the most powerful predictor of T2DM; total T weakly predicts T2DM, whereas free T does not. The predictive ability of SHBG is independent of total and free T, whereas neither total nor free T predicts T2DM independent of SHBG. This confirms our hypothesis that the observed risk of T2DM in men with low T levels is mediated primarily through SHBG. We posit that previously reported associations between total T and T2DM are a reflection of the underlying relationship between SHBG and T2DM. The magnitude of the associations of lower SHBG and lower total T in men with incident T2DM is comparable with those of previous studies (1–3,5,6), although this study has a different conceptual framework—one that posits SHBG as the primary determinant for the risk of incident T2DM. These results not only confirm the findings of our previous analysis (2) in terms of a central role for SHBG but also differ from it in that free T did not predict T2DM in the current analysis. There are several possible explanations for this difference—the current analysis represents a longer follow-up, a larger sample size, and a higher number of incident cases of T2DM, thereby adding greater power to the analysis. Furthermore, the final models in this analysis were adjusted for additional covariates such as age, smoking, and physical activity. The difference in method of free T estimation is another possible factor; the previous analysis measured free T using a centrifugal ultrafiltration technique, whereas in the current analysis, free T levels were calculated by using mass action equations. Our findings support the recent work of Ding and colleagues (10) who demonstrated the role of low SHBG levels in T2DM risk prospectively as well as the association SHBG genotypes with this risk. The unique aspect of our manuscript is that unlike any previous study, we adjusted the analyses for total and free T levels and demonstrated a pivotal role of SHBG in diabetes risk.
Recent elucidations of SHBG structure, gene expression, and specific membrane receptor (SHBG-R) (23–27) have added further complexity to our understanding of SHBG function. Earlier studies had suggested that SHBG receptor is either a G protein–coupled receptor or functionally linked to one and that the receptor-mediated action of sex steroid–bound SHBG uses cyclic adenosine monophosphate as a second messenger, which in turn modulates androgen receptor transcriptional activity (28,29). The SHBG/SHBG-R system was believed to work as an additional control mechanism to modulate the effects of dihydrotestosterone and estradiol in cells (23). Recently, Hammes and colleagues (30) described the presence of megalin, an endocytic receptor for SHBG, which transports SHBG-bound sex steroids into the cell. This provocative finding challenges the free hormone hypothesis—the idea that only free sex steroids are biologically active—and implies that SHBG has a role beyond that of a circulating transport protein; our data support this viewpoint. Additionally, there is growing evidence that SHBG levels are genetically determined (31,32). Polymorphisms of SHBG promoter have been linked not only to SHBG levels and diabetes risk (10) but also to levels of androgens and glucuronidated androgen metabolites (33), underscoring the potential of SHBG as an independent marker for several pathophysiological processes.
Our findings suggest a need for a revised interpretation of the previously reported relationship between T levels and the risk of T2DM. We assert that these data do not support the use of T therapy to prevent T2DM. Rather, prevention strategies should focus on factors that regulate circulating SHBG, such as adiposity, inflammation, and insulin resistance. It is possible that the observed inverse correlation between T and inflammatory cytokines (34,35) may be mediated primarily by SHBG levels. Conflicting results of the response of inflammatory markers to T therapy (36–42) strengthens this proposition further. Although SHBG has received little attention as an inflammatory marker, its role as a marker of insulin resistance has been well recognized. Insulin negatively regulates SHBG production in the liver (43–46). Population studies have consistently reported a negative correlation between SHBG and insulin resistance (47–51). Hence, SHBG appears to be a marker of insulin resistance that can be used to identify men at risk for developing T2DM. Further studies would be needed to test if SHBG is an earlier and a stronger predictor of inflammation and insulin resistance than the existing components of metabolic syndrome and whether SHBG should be considered as an additional component of the metabolic syndrome.
Although MMAS allowed us to follow a large cohort of community-dwelling men over a significant period of time, this study has some limitations. Glucose and insulin concentrations were not available as the blood samples drawn were not in the fasting state. Additional adjustment for these in the final model would have given additional validation to our findings and helped determine whether low SHBG levels are a cause rather than a consequence of elevated insulin levels. However, this question has been addressed in longitudinal studies (1,5) by adjusting for insulin levels in multivariate models predicting metabolic syndrome and T2DM even after which, SHBG has remained a significant risk predictor. We relied on self-report to record incident cases of T2DM. Although this leaves open the possibility that not all cases of T2DM were detected and that some asymptomatic individuals with mild T2DM might have been missed, underreporting would not compromise the positive associations of T2DM with total T and SHBG and makes these strong associations all the more remarkable. These limitations should be considered in light of the strengths of the study, which include a random population-based sample of generally healthy well-characterized men from a defined geographic area, the ability to statistically adjust for a number of factors that could confound the association of SHBG and T with T2DM, the length of follow-up, and the relatively sizable number of events.
In conclusion, although a predictive relationship between SHBG and T2DM has been suggested in previous studies, using prospective data from a large sample of normally aging men, we demonstrated that SHBG is a robust predictor of T2DM, whose predictive ability is independent of total and free T, suggesting that SHBG mediates its effects through nonandrogenic pathways. The implication of our findings is that the strategies for prevention of T2DM should be directed at factors that determine SHBG rather than at raising T levels. Further studies are needed to determine if SHBG can be used as a reliable marker to assess the efficacy of currently prescribed interventions, such as therapeutic lifestyle changes and/or pharmacological methods, aimed at preventing the development of T2DM.
FUNDING
MMAS was funded by the following grants: R01AG04673 from the National Institute on Aging and R01DK44995 and R01DK51345 from the National Institute of Diabetes and Digestive and Kidney Disorders; additional support was provided by the Boston Claude D. Pepper Older Americans Independence Center grant 5P30AG031679 and 1R01AG22356.
Acknowledgments
The authors appreciate the statistical programming assistance of Gretchen R. Chiu. The authors have no conflicts of interest to disclose.
References
- 1.Haffner SM, Shaten J, Stern MP, Smith GD, Kuller L. Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. MRFIT Research Group. Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;143(9):889–897. doi: 10.1093/oxfordjournals.aje.a008832. [DOI] [PubMed] [Google Scholar]
- 2.Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23(4):490–494. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- 3.Oh J-Y, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo Study. Diabetes Care. 2002;25(1):55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 4.Svartberg J, Jenssen T, Sundsfjord J, Jorde R. The associations of endogenous testosterone and sex hormone-binding globulin with glycosylated hemoglobin levels, in community dwelling men. The Tromso Study. Diabetes Metab. 2004;30(1):29–34. doi: 10.1016/s1262-3636(07)70086-1. [DOI] [PubMed] [Google Scholar]
- 5.Laaksonen DE, Niskanen L, Punnonen K, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27(5):1036–1041. doi: 10.2337/diacare.27.5.1036. [DOI] [PubMed] [Google Scholar]
- 6.Selvin E, Feinleib M, Zhang L, et al. Androgens and diabetes in men: results from the third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30(2):234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 7.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor D, Aldred H, Clark S, Channer KS, Jones TH. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30(4):911–917. doi: 10.2337/dc06-1426. [DOI] [PubMed] [Google Scholar]
- 9.Zhao JL, Chen ZJ, Zhao YR, et al. Study on the (TAAAA)n repeat polymorphism in sex hormone-binding globulin gene and the SHBG serum levels in putative association with the glucose metabolic status of Chinese patients suffering from polycystic ovarian syndrome in Shandong province. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2005;22(6):644–647. [PubMed] [Google Scholar]
- 10.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361(12):1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basu R, Dalla Man C, Campioni M, et al. Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diabetes Care. 2007;30(8):1972–1978. doi: 10.2337/dc07-0359. [DOI] [PubMed] [Google Scholar]
- 12.Jedrzejuk D, Medras M, Milewicz A, Demissie M. Dehydroepiandrosterone replacement in healthy men with age-related decline of DHEA-S: effects on fat distribution, insulin sensitivity and lipid metabolism. Aging Male. 2003;6(3):151–156. [PubMed] [Google Scholar]
- 13.Basu R, Dalla Man C, Campioni M, et al. Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes. 2007;56(3):753–766. doi: 10.2337/db06-1504. [DOI] [PubMed] [Google Scholar]
- 14.Haddad RM, Kennedy CC, Caples SM, et al. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82(1):29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- 15.Corrales JJ, Burgo RM, Garca-Berrocal B, et al. Partial androgen deficiency in aging type 2 diabetic men and its relationship to glycemic control. Metabolism. 2004;53(5):666–672. doi: 10.1016/j.metabol.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Nair KS, Rizza RA, O'Brien P, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- 17.O'Donnell AB, Araujo AB, McKinlay JB. The health of normally aging men: the Massachusetts Male Aging Study (1987–2004) Exp Gerontol. 2004;39(7):975–984. doi: 10.1016/j.exger.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 18.US Census Bureau. Census of Population and Housing, 1990: Summary Tape File 3 on CD-ROM / prepared by the Bureau of the Census. Washington, DC: The Bureau [producer and distributor]; 1992; [Google Scholar]
- 19.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the five-city project. Am J Epidemiol. 1985;121(1):91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 20.Khavari KA, Farber PD. A profile instrument for the quantification and assessment of alcohol consumption. The Khavari Alcohol Test. J Stud Alcohol. 1978;39(9):1525–1539. doi: 10.15288/jsa.1978.39.1525. [DOI] [PubMed] [Google Scholar]
- 21.Harlow SD, Linet MS. Agreement between questionnaire data and medical records. The evidence for accuracy of recall. Am J Epidemiol. 1989;129(2):233–248. doi: 10.1093/oxfordjournals.aje.a115129. [DOI] [PubMed] [Google Scholar]
- 22.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 23.Fortunati N. Sex hormone-binding globulin: not only a transport protein. What news is around the corner? J Endocrinol Invest. 1999;22(3):223–234. doi: 10.1007/BF03343547. [DOI] [PubMed] [Google Scholar]
- 24.Hryb DJ, Khan MS, Rosner W. Testosterone-estradiol-binding globulin binds to human prostatic cell membranes. Biochem Biophys Res Commun. 1985;128(1):432–440. doi: 10.1016/0006-291x(85)91697-3. [DOI] [PubMed] [Google Scholar]
- 25.Krupenko SA, Krupenko NI, Danzo BJ. Interaction of sex hormone-binding globulin with plasma membranes from the rat epididymis and other tissues. J Steroid Biochem Mol Biol. 1994;51(1–2):115–124. doi: 10.1016/0960-0760(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 26.Kahn SM, Hryb DJ, Nakhla AM, Romas NA, Rosner W. Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. 2002;175(1):113–120. doi: 10.1677/joe.0.1750113. [DOI] [PubMed] [Google Scholar]
- 27.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16(10):2181–2187. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 28.Rosner W, Hryb DJ, Khan MS, Nakhla AM, Romas NA. Androgen and estrogen signaling at the cell membrane via G-proteins and cyclic adenosine monophosphate. Steroids. 1999;64(1–2):100–106. doi: 10.1016/s0039-128x(98)00108-1. [DOI] [PubMed] [Google Scholar]
- 29.Nakhla AM, Leonard J, Hryb DJ, Rosner W. Sex hormone-binding globulin receptor signal transduction proceeds via a G protein. Steroids. 1999;64(3):213–216. doi: 10.1016/s0039-128x(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 30.Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122(5):751–762. doi: 10.1016/j.cell.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 31.Ring HZ, Lessov CN, Reed T, et al. Heritability of plasma sex hormones and hormone binding globulin in adult male twins. J Clin Endocrinol Metab. 2005;90(6):3653–3658. doi: 10.1210/jc.2004-1025. [DOI] [PubMed] [Google Scholar]
- 32.Meikle AW, Stephenson RA, Lewis CM, et al. Age, genetic, and nongenetic factors influencing variation in serum sex steroids and zonal volumes of the prostate and benign prostatic hyperplasia in twins. Prostate. 1997;33(2):105–111. doi: 10.1002/(sici)1097-0045(19971001)33:2<105::aid-pros4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson AL, Lorentzon M, Mellstrom D, et al. SHBG gene promoter polymorphisms in men are associated with serum sex hormone-binding globulin, androgen and androgen metabolite levels, and hip bone mineral density. J Clin Endocrinol Metab. 2006;91(12):5029–5037. doi: 10.1210/jc.2006-0679. [DOI] [PubMed] [Google Scholar]
- 34.Khosla S, Atkinson EJ, Dunstan CR, O'Fallon WM. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J Clin Endocrinol Metab. 2002;87(4):1550–1554. doi: 10.1210/jcem.87.4.8397. [DOI] [PubMed] [Google Scholar]
- 35.Bellido T, Jilka RL, Boyce BF, et al. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J Clin Invest. 1995;95(6):2886–2895. doi: 10.1172/JCI117995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156(5):595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- 37.Lambert CP, Sullivan DH, Evans WJ. Effects of testosterone replacement and/or resistance training on interleukin-6, tumor necrosis factor alpha, and leptin in elderly men ingesting megestrol acetate: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2003;58(2):165–170. doi: 10.1093/gerona/58.2.m165. [DOI] [PubMed] [Google Scholar]
- 38.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 39.Ng MK, Liu PY, Williams AJ, et al. Prospective study of effect of androgens on serum inflammatory markers in men. Arterioscler Thromb Vasc Biol. 2002;22(7):1136–1141. doi: 10.1161/01.atv.0000022167.80130.a6. [DOI] [PubMed] [Google Scholar]
- 40.Singh AB, Hsia S, Alaupovic P, et al. The effects of varying doses of T on insulin sensitivity, plasma lipids, apolipoproteins, and C-reactive protein in healthy young men. J Clin Endocrinol Metab. 2002;87(1):136–143. doi: 10.1210/jcem.87.1.8172. [DOI] [PubMed] [Google Scholar]
- 41.Vincent A, Riggs BL, Atkinson EJ, Oberg AL, Khosla S. Effect of estrogen replacement therapy on parathyroid hormone secretion in elderly postmenopausal women. Menopause. 2003;10(2):165–171. doi: 10.1097/00042192-200310020-00009. [DOI] [PubMed] [Google Scholar]
- 42.Maggio M, Basaria S, Ble A, et al. correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91(1):345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 43.Crave JC, Lejeune H, Brebant C, Baret C, Pugeat M. Differential effects of insulin and insulin-like growth factor I on the production of plasma steroid-binding globulins by human hepatoblastoma-derived (Hep G2) cells. J Clin Endocrinol Metab. 1995;80(4):1283–1289. doi: 10.1210/jcem.80.4.7536204. [DOI] [PubMed] [Google Scholar]
- 44.Katsuki A, Sumida Y, Murashima S, et al. Acute and chronic regulation of serum sex hormone-binding globulin levels by plasma insulin concentrations in male noninsulin-dependent diabetes mellitus patients. J Clin Endocrinol Metab. 1996;81(7):2515–2519. doi: 10.1210/jcem.81.7.8675570. [DOI] [PubMed] [Google Scholar]
- 45.Pasquali R, Casimirri F, De Iasio R, et al. Insulin regulates testosterone and sex hormone-binding globulin concentrations in adult normal weight and obese men. J Clin Endocrinol Metab. 1995;80(2):654–658. doi: 10.1210/jcem.80.2.7852532. [DOI] [PubMed] [Google Scholar]
- 46.Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67(3):460–464. doi: 10.1210/jcem-67-3-460. [DOI] [PubMed] [Google Scholar]
- 47.Yki-Jarvinen H, Makimattila S, Utriainen T, Rutanen EM. Portal insulin concentrations rather than insulin sensitivity regulate serum sex hormone-binding globulin and insulin-like growth factor binding protein 1 in vivo. J Clin Endocrinol Metab. 1995;80(11):3227–3232. doi: 10.1210/jcem.80.11.7593430. [DOI] [PubMed] [Google Scholar]
- 48.Birkeland KI, Hanssen KF, Torjesen PA, Vaaler S. Level of sex hormone-binding globulin is positively correlated with insulin sensitivity in men with type 2 diabetes. J Clin Endocrinol Metab. 1993;76(2):275–278. doi: 10.1210/jcem.76.2.8432768. [DOI] [PubMed] [Google Scholar]
- 49.Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005;90(5):2618–2623. doi: 10.1210/jc.2004-1158. [DOI] [PubMed] [Google Scholar]
- 50.Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27(4):861–868. doi: 10.2337/diacare.27.4.861. [DOI] [PubMed] [Google Scholar]
- 51.Osuna JA, Gomez-Perez R, Arata-Bellabarba G, Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch Androl. 2006;52(5):355–361. doi: 10.1080/01485010600692017. [DOI] [PubMed] [Google Scholar]