Abstract
Background.
Age-related alterations of neuromuscular activation may contribute to deficits in muscle power and mobility function. This study assesses whether impaired activation of the agonist quadriceps and antagonist hamstrings, including amplitude- and velocity-dependent characteristics of activation, may explain differences in leg extension torque and power between healthy middle-aged, healthy older, and mobility-limited older adults.
Methods.
Torque, power, and electromyography were recorded during maximal voluntary leg extension trials across a range of velocities on an isokinetic dynamometer.
Results.
Neuromuscular activation was similar between middle-aged and older healthy groups, with differences in torque and power explained predominantly by muscle size. However, the older mobility–limited group demonstrated marked impairment of torque, power, and agonist muscle activation, with the greatest deficits occurring at the fastest movement velocities. Agonist muscle activation was found to be strongly associated with torque output.
Conclusions.
Similar neuromuscular activation between the middle-aged and older healthy groups indicates that impaired voluntary activation is not an obligatory consequence of aging. However, the finding that the mobility-limited group exhibited impaired activation of the agonist quadriceps and concomitant deficits in torque and power output suggests that neuromuscular activation deficits may contribute to compromised mobility function in older adults.
Keywords: Aging, Electromyography, Strength
MUSCLE power, the product of torque and movement velocity, declines more rapidly than static muscle strength with advancing age (1–4). Furthermore, power is more closely associated with physical functioning compared with traditional measures of strength that do not emphasize movement speed (eg, one-repetition maximum and static maximal voluntary force) (5–8). Power may therefore be superior to strength as an indicator of physiological impairment underlying functional deficits.
Impaired strength and muscle power may result from a variety of factors (for review see (9)), one of which is impaired neuromuscular activation (10). Neuromuscular activation is the process by which excitation of motor neurons leads to force production in a population of muscle fibers. Each motor neuron and its associated muscle fibers constitute a motor unit, and the number and firing rate of recruited motor units are the major intrinsic determinant of muscular force (extrinsic factors such as muscle length and contraction velocity also influence force output). Weakness may directly result from impaired capacity of the nervous system to maximize motor unit recruitment and/or rate coding in agonist muscles (prime movers) or may be indirectly caused by poor intermuscular coordination or by excessive activation of antagonist muscles (which oppose the agonist). Neuromuscular activation is commonly evaluated using surface electromyography (EMG), which is a noninvasive technique where small recording electrodes are placed on the skin over the muscle(s) of interest to record the bioelectrical activity associated with muscle contraction.
Given the emerging literature regarding muscle power in older adults, it is notable that most investigations of neuromuscular activation in this population have assessed only static muscle contractions (10). Some investigations have revealed reduced maximal motor unit firing rates (11–14) and inability to voluntarily elicit peak muscle force (15–17) with aging. However, other studies have concluded that activation is not impaired (18–22). There are also conflicting findings as to whether antagonist coactivation may be increased (1,23,24) or decreased (4,17,25,26) with aging. A few studies have assessed agonist activation capability during maximal dynamic contractions (ie, during movement) but have also yielded inconclusive findings (26,27).
The first objective of this study was to determine the effect of movement velocity on torque and power production in older healthy adults (OH) and older mobility–limited (OML) adults relative to middle-aged healthy adults (MH). We chose to compare older adults with middle-aged rather than with young adults because we believe that identifying age-related differences across a narrower age range may better capture the key differences contributing to mobility limitations. The second objective was to assess whether potential age- and velocity-dependent deficits in torque and power were associated with differences in neuromuscular activation of the agonist and antagonist muscle groups. We hypothesized that both older groups, but particularly the older mobility limited, would exhibit deficits in torque and power that are most severe at fast movement velocity. We further hypothesized that impaired agonist activation, and not excessive antagonist coactivation, would be associated with these deficits.
METHODS
Participants
Volunteers were recruited by local newspaper advertisements, direct mailing to volunteers from earlier studies at our center, and posting of flyers around the Tufts University Health Sciences Campus. Three specific groups were recruited: MH (aged 40–55 years), OH (aged 70–85 years), and OML (aged 70–85 years). A preliminary screening was conducted by telephone using the following exclusion criteria: presence of unstable chronic disease, acute or terminal illness, myocardial infarction within 6 months (or other symptomatic coronary artery disease), uncontrolled hypertension (>150/90 mmHg), fracture in the previous 6 months, diseases or medications affecting neuromuscular function, anticoagulation therapy (due to a muscle biopsy procedure; data not presented here), hormone replacement therapy, body mass index less than 19 or more than 33, weight loss or gain within 6 months, and participation in an exercise program within 6 months. Volunteers being considered for MH and OH were also required to not be taking any prescription medications. Individuals who passed the telephone screening were further screened by a licensed physician or nurse practitioner, including assessment of the presence of lower extremity joint pain and administration of the Mini-Mental State Examination (MMSE) and Short Physical Performance Battery (SPPB). Persons with MMSE score more than 23 or with joint pain were excluded. The SPPB, which probes the domains of strength, ambulation, and balance and is predictive of future disability (28), was used to classify the older adults into the OH and OML groups. Older adults with SPPB less than or equal to 9 (out of a possible 12 points) were classified as OML, whereas older adults with SPPB more than 9 who were not taking prescription medication were classified as OH.
Protocol
Testing was conducted using a Cybex-II dynamometer (Cybex, Ronkonkoma, NY). Participants were seated upright with the hip and knee of the dominant leg flexed to approximately 85° and 90°, respectively. The lateral epicondyle of the knee was aligned with the dynamometer axis of rotation, and the limb secured to the dynamometer lever arm by a padded attachment placed 1 inch above the ankle. Straps were secured over the shoulders, lap, and thigh, and hands were kept on the lap or folded across the chest.
Prior to testing, limb weight was measured throughout the full range of motion and was later used to correct for the resistive effects of gravity. Five consecutive maximal isokinetic (constant velocity) knee extensions were performed at 60, 90, 180, and 240 degrees second, with the participants instructed to kick out as fast and hard as possible. Testing proceeded sequentially from the slowest to the fastest velocity to minimize confusion (29). Three static maximal voluntary contractions were then performed for 3–5 seconds for both knee flexion and extension, with the leg fixed at 60° of knee flexion. To avoid fatigue, each testing condition was separated by at least 1 minute of rest. The total duration of muscle contractions for the entire protocol was less than 1 minute.
Data Acquisition
Torque, position, and velocity signals were acquired directly from the dynamometer. Muscle activation was assessed by surface EMG using a commercially available system and single-differential surface electrodes with 1-cm intersensor distance (Delsys, Boston, MA). EMG was recorded from quadriceps muscles (knee extensors) rectus femoris (rf), vastus medialis (vm), and vastus lateralis (vl) and hamstrings muscles (knee flexors) biceps femoris (bf) and semimembranosus (sm). Data were acquired at a sampling rate of 1 kHz using a Powerlab/16SP system and Chart software (ADInstruments, Colorado Springs, CO) and saved to computer disk.
Computed tomography (CT) scans of the nondominant thigh were obtained at the midpoint of the femur using a Siemens Somatom Scanner (Erlangen, Germany) operating at 120 kV and 100 mA, a slice width of 10 mm and a scanning time of 1 second. All scans were analyzed by a single blinded assessor using SliceOmatic v4.2 software (Tomovision, Montreal, Canada). Images were reconstructed on a 512 × 512 matrix with a 25-cm field of view, and anterior compartment muscle cross-sectional area (CSA) was measured by manual tracing using, when applicable, intermuscular adipose tissue as a guide. Muscle CSA was measured in the range of 0–100 Hounsfield units and calculated as the sum of low- and normal-density area.
Data Analysis
Torque, power, and EMG data were analyzed using Matlab 7.0 (The Mathworks, Natick, MA) and JMP statistical software (v7.0; SAS Institute Inc., Cary, NC). For each movement repetition, torque and velocity were calculated as the mean value between 55° and 70° of knee flexion. Power was then calculated as the product of the mean torque and mean velocity within this same 15° region. Static torque was quantified by locating the absolute peak torque of the trial and calculating the average over a 250-millisecond window centered at this peak. Raw EMG signals were corrected for baseline offset, filtered using a zero phase lag second-order Butterworth band-pass filter (10–200 Hz), and root-mean-square amplitude was calculated over the same range of motion used for calculating torque and power. To allow familiarization with the procedures, the data presented here are from the second of two identical testing sessions, performed approximately 1 week apart.
To assess differences between groups, we expressed torque and power in absolute terms (Newton meter and Watts, respectively) and normalized to (ie, divided by) the anterior compartment muscle CSA. The resultant specific torque (Newton meter per square centimeter) and specific power (Watts per square centimeter) provide insight regarding neuromuscular determinants of motor output independent of muscle size. To investigate influences of velocity on torque production, we normalized torque from all the movement conditions to static peak torque. Similarly, to assess velocity-dependent effects, muscle activation was expressed in absolute units (volts) and also normalized to activation during the maximal static contractions.
EMG data were corrected as follows to account for the influence of subcutaneous adipose tissue thickness on activation amplitude. Activation data from all study participants were pooled for each agonist muscle at each testing velocity and plotted against subcutaneous adipose CSA. For each velocity, this relationship was fit with a third-order polynomial equation and the residuals were calculated. The original mean activation magnitude calculated across all participants was then added back to the residuals. These residuals were then separated by group for comparison.
Statistical Analyses
Data from each trial were averaged within-subject and criterion velocity, and the Box–Cox power transformation was applied to non-normally distributed variables (torque, specific torque, power, specific power, and non-normalized EMG from each muscle). To determine whether the velocity dependence of torque and activation differed for men and women, we examined the Group × Sex interaction for normalized torque and muscle activation. Torque and power were analyzed using a two-factor repeated measures analysis of variance (ANOVA; Group × Velocity) and Tukey's honestly significant difference post hoc analysis with significance set at p < .05. The amplitude of absolute (non-normalized) muscle activation was examined using a single-factor ANOVA (group). The association between activation amplitude and movement velocity in each group was examined using Pearson's correlation analysis for both absolute and normalized muscle activation. Correlation analysis was also used to examine the association between normalized torque and normalized activation at each velocity. Figures show mean ± SE.
RESULTS
Participants
A total of 786 individuals responded to advertisements, of which 743 were screened by telephone. Of those, 122 met our inclusion and exclusion criteria and were invited to come to our facility for additional screening. Ninety-three individuals passed the second screening, and 89 ultimately performed the study procedures, including 29 MH, 28 OH, and 32 OML. Full descriptive characteristics are presented in Table 1. A small but significant difference in age was present between OH and OML (p < .001). Consistent with our recruitment criteria, the score for the SPPB was significantly lower in OML compared with MH and OH (p < .0001). Anterior compartment muscle CSA differed significantly between all groups, with MH having the greatest CSA followed by OH and OML (p < .001). The Group × Sex interaction was not significant (p > .05) for normalized torque or normalized muscle activation; thus, data from both sexes were pooled within each group for further analysis.
Table 1.
Participant Characteristics
| MH | OH | OML | |
| Age (yr) | 47.2 ± 4.7 | 74.0 ± 3.6* | 78.1 ± 4.5† |
| Weight (kg) | 75 ± 14.1 | 71.4 ± 20.3 | 70.4 ± 11.1† |
| Height (cm) | 170 ± 10.7 | 170.1 ± 10.8 | 163.3 ± 8.3† |
| Body mass index (kg/m2) | 25.8 ± 3.1 | 25.3 ± 3.7 | 26.4 ± 3.1 |
| SPPB score (out of 12) | 11.7 ± 0.5 | 11.0 ± 0.9 | 7.9 ± 1.3† |
| Anterior compartment muscle CSA (cm2) | 64.2 ± 15.0 | 52 ± 13.7* | 44.2 ± 11.3† |
| Subcutaneous adipose tissue CSA (cm2) | 63.9 ± 30.1 | 55.8 ± 30.0 | 66.8 ± 37.6 |
| Sex (male/female) | 14/15 | 16/12 | 15/17 |
| Presence of knee osteoarthritis (self-report), % | 0 | 7 | 16 |
Notes: All values are mean ± SD. CSA = cross-sectional area; MH = middle-aged healthy adults; OH = older healthy adults; OML = older adults with mobility limitation.
OH different from MH (p < .001).
OML different from MH and OH (p < .001).
Torque
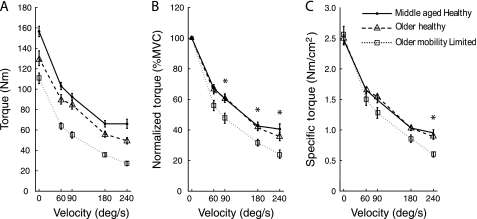
The main effects of group and velocity were significant for absolute torque, specific torque, and normalized torque (all p < .0001; Figure 1). Post hoc analysis of the group effect for each torque variable revealed that MH produced more absolute torque than OH but that the two groups did not differ for specific torque or normalized torque. MH and OH produced significantly more absolute, specific, and normalized torque than OML. There was a significant Group × Velocity interaction effect for normalized torque (p = .03) and specific torque (p = .0009) but not for absolute torque (p = .40). Of note, normalized torque in OML was significantly lower than MH and OH at 90, 180, and 240 degrees per second. For specific torque, the static condition did not differ between groups (p > .05), and indeed, specific torque in OML was 103% of that observed in both MH and OH. Across the slower velocities, there was a gradual differentiation between groups, which reached statistical significance at 240 degrees per second, with OML producing just 63% and 67% of MH and OH, respectively (p < .05).
Figure 1.
Absolute torque (Newton meter, A), normalized torque (percent maximal voluntary, B), and specific torque (Newton meter per square centimeter, C) plotted against movement velocity. Significant post hoc results where older adults with mobility limitation was less than middle-aged healthy adults and older healthy adults are indicated by *.
Power
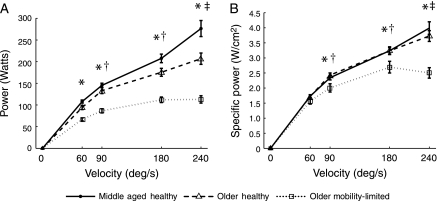
Significant main effects of group and velocity as well as a Group × Velocity interaction effect were found for absolute and specific power (all p < .0001; Figure 2). Post hoc analysis of the group effect for both power variables revealed that all groups differed significantly, with MH producing the greatest power followed by OH and OML. The interaction effect revealed that OML produced significantly less absolute power than MH and OH at each velocity and significantly less specific power at each velocity except 60 degrees per second. Furthermore, we found that MH had a significant increase in absolute and specific power with each velocity increment and that OH also significantly increased absolute and specific power with each increment except between 180 and 240 degrees per second. In marked contrast, OML showed no difference in specific or absolute power between 60 and 90 degrees per second or between 90, 180, and 240 degrees per second, indicating a plateau in the ability to produce power at faster speeds.
Figure 2.
Absolute power (Watts, A) and specific power (Watts per square centimeter, B) plotted against movement velocity. The following significant post hoc results are indicated: *older adults with mobility limitation (OML) was less than middle-aged healthy adults (MH) and older healthy adults (OH); †power has increased from the previous (slower) velocity in MH and OH but not in OML; and ‡power has increased from the previous (slower) velocity in MH but not in OH or OML.
Amplitude of Muscle Activation
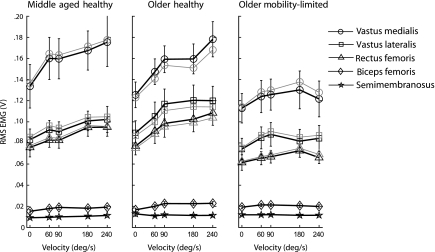
When pooled across all groups, agonist quadriceps muscle activation was significantly associated with subcutaneous adipose CSA for each muscle at each velocity (all p < .0001, R2 = .31–.52 based on a third-order polynomial fit). Muscle activation corrected for subcutaneous adipose (see the Methods section) did not differ from the original activation values (p = .41–.92; Figure 3); thus, the original values were used for further analysis.
Figure 3.
Absolute activation (thick black lines) and activation corrected for subcutaneous adipose (thin gray lines provided for agonist muscles only) plotted against movement velocity. Agonist activation amplitude for each muscle was higher in middle-aged healthy adults (MH) and older healthy adults (OH) compared with older adults with mobility limitation (OML). Furthermore, both MH and OH demonstrated significant positive associations between activation amplitude and velocity, whereas OML did not.
There was a significant effect of group (p < .0001) in all three agonist muscles when all velocities were included in the model, with post hoc analysis revealing that vm, vl, and rf were significantly higher in MH and OH compared with OML (Figure 3). However, when only the static condition was analyzed, we found that agonist activation amplitude did not differ across groups for vm or vl (p = .72 and .24, respectively), but there was a significant difference in rf (p = .04) with MH producing greater activation than with OML.
For antagonist hamstrings muscle activation amplitude, there was a significant effect of group for both sm (p < .01) and bf (p = .01). Post hoc analysis revealed that sm activation in MH was significantly less than that in OH and OML, and bf activation in MH was significantly less than that in OH but the same as that in OML. Although these significant differences were identified, the mean hamstrings activation was generally quite low in all groups at all velocities, ranging from 12% to 18% of mean quadriceps activation (Figure 3).
Modulation of Agonist Activation With Faster Contraction Velocity
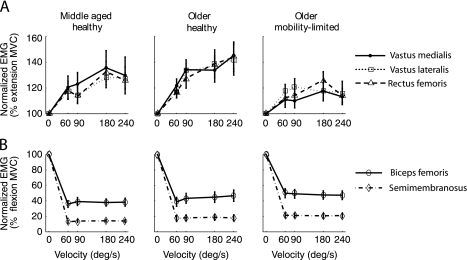
In MH, agonist activation amplitude (Figure 3) was positively associated with velocity for vm (r = .92, p = .03), vl (r = .96, p = .01), and rf (r = .91, p = .03). Similar significant correlations were observed in OH for vm (r = .93, p = .02), vl (r = .83, p = .04), and rf (r = .93, p = .02). In contrast, OML demonstrated no significant associations for vm (r = .64, p = .34), vl (r = .55, p = .48), or rf (r = .42, p = .24), indicating that the change (ie, the slope) across velocities did not significantly differ from zero. To more closely examine how agonist activation changes across velocities, activation at each velocity was normalized to activation from the maximal voluntary isometric knee extension condition (Figure 4A). This helps to account for intersubject differences in activation amplitude and allows meaningful comparisons of the slope of the activation–velocity relationship for each muscle (which are expressed in units of percent of maximal voluntary activation per degree per second increment of velocity). A significant positive relationship between normalized activation and velocity was revealed in vm, vl, and rf for OH (all p < .0001) with slopes ranging from .16 to .18 and MH (p = .0003, .02 and .001, respectively) with slopes ranging from .11 to .12. OML demonstrated a significant positive relationship in vm and rf (p = .01 and .04, respectively) with slopes of .08 and .06 but not in vl (p = .11), which had a slope of .05. Within each group, the slopes of each muscle did not significantly differ so the data from all three muscles were pooled to form a composite measure of normalized quadriceps activation. The slope of this normalized quadriceps activation was significantly higher in OH compared with OML (p = .03) and higher with borderline significance in MH compared with OML (p = .05). MH and OH did not differ (p = .82). For normalized antagonist activation (expressed relative to maximal isometric knee flexion), there was no associated with velocity for any group (Figure 4B) so no further comparisons were made.
Figure 4.
Normalized activation in the agonist quadriceps and the antagonist hamstrings muscles (% of activation during maximal effort knee extension and flexion, in A and B, respectively) plotted against movement velocity. The agonist activation–velocity slopes were higher in middle-aged healthy adults and older healthy adults compared with older adults with mobility limitation.
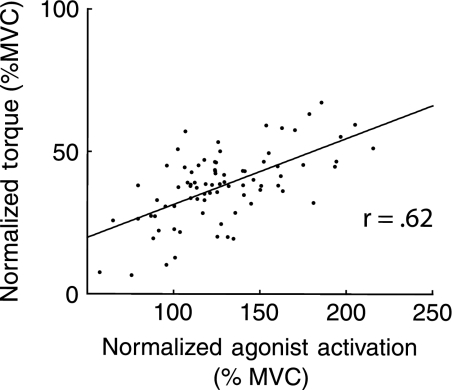
The implications of velocity-dependent agonist activation modulation on torque production were examined by the association between normalized agonist activation (average of vm, vl, and rf) and normalized torque at each criterion velocity. For this analysis, data from all three groups were pooled and revealed strong correlations (r = .56–.62, p < .001; see, eg, Figure 5). This result indicates that velocity-dependent loss of torque (ie, the torque–velocity relationship) is attenuated by a concurrent velocity-dependent increase in agonist activation.
Figure 5.
An example of the significant association between normalized torque and normalized agonist muscle activation. This figure contains data for all study participants at 180 degrees per second and indicates that persons with greater modulation of activation (relative to isometric) also have higher torque production (relative to isometric).
DISCUSSION
Our findings indicate that impaired voluntary neuromuscular activation is not an obligatory consequence of aging but may instead reveal emerging pathology in the nervous system that contributes to the onset of mobility disability in some older adults. Lower torque and muscle power in OH compared with MH were explained by reduced muscle size as specific torque and power did not differ between the two groups. In contrast, specific torque and power were lower in OML compared with both healthy groups, indicating that muscle size alone does not fully account for weakness. Rather, weakness in OML is likely also due to impaired agonist activation, as evidenced by reduced EMG amplitude (see Figure 3) and a reduction in the positive amplitude modulation that typically occurs with increased movement velocity (see Figures 3 and 4). A number of age-related changes to the nervous system may contribute to impaired neuromuscular activation in our OML group. For instance, loss of cortical projections to spinal motoneurons (30), decreased inhibition between cerebral hemispheres (31), and reduced excitability of the corticospinal pathway (32) may lead to compromised ability to fully drive the motor pool.
The differences observed between OML and OH suggest that discrepant findings in the literature with regard to age-related activation impairment may be due in part to heterogeneity of the participants included. All participants in the present study were medically stable, functionally independent, and did not present with overt motor dysfunction. Although many of our OML participants would likely meet the inclusion criteria for many studies of normal aging, we instead took the unique approach of distinguishing between elders who do or do not exhibit subtle mobility deficits that indicate risk for future mobility disability. Using this classification, we revealed markedly different neuromotor performance between these older groups. Another factor that may contribute to discrepancies among previous research is the muscle group being tested, as the functional roles and habitual usage (eg, weight bearing vs nonweight bearing) may affect the susceptibility of a muscle to impaired activation. The methodological approach might also be a contributing factor to earlier conflicting reports. For instance, studies using the twitch interpolation technique have concluded that both younger and older adults can produce full or near full activation, whereas EMG studies (including surface and motor unit recordings) have often detected differences.
The phenomenon of increased activation amplitude with faster concentric contraction velocity has been established previously (26,33,34), although the contribution of this phenomenon to torque production had not been systematically assessed. Although decreased torque with increased velocity is a fundamental mechanical property of muscle (35), evidence indicates that in vivo torque loss can be attenuated by positive modulation of activation (33,36). Consistent with this evidence, our data revealed strong correlations between normalized torque and normalized agonist activation at each criterion velocity (eg, see Figure 5). Notably, we did not observe an association between normalized antagonist activation and velocity, indicating that changes in coactivation cannot be responsible for deficits in dynamic force production. Cumulatively, these findings indicate that the lower modulation of agonist activation amplitude observed in OML likely contributes to deficits in dynamic torque and power.
Some methodological considerations should be considered when interpreting the results of this investigation. OML was an average of 4 years older than OH, although the differences we found between groups exceed the expected age-related differences according to a recent longitudinal study from our laboratory (37). Differences in habitual activity may affect neuromuscular performance, although potential differences were minimized by excluding volunteers who had exercised regularly during the previous 6 months. CT scans and neuromuscular testing were performed on different legs, but bilateral thigh measurements have been shown to be quite consistent in persons of varying age and body composition (38). Many sources of variability affect the EMG signal (39). Of greatest concern are factors with systematic influence, and signal attenuation caused by subcutaneous adipose tissue is well recognized. We compared the absolute activation amplitude with a corrected activation value that accounted for between-subject differences in adipose CSA and found that the two measures did not significantly differ for our study sample (see Figure 3). Random sources of variability in the EMG signal challenge the capacity to detect statistically significant differences between groups and/or testing conditions. Were it not for this random variability, it is probable that additional differences in neuromuscular activation could be identified among the groups and that the differences that we did observe would be even more prominent.
In conclusion, our data reveal impaired power and neuromuscular activation, specifically in OML, and suggest emerging pathology of the neuromuscular system that may contribute to declines in mobility function. Future research should investigate the potential neural mechanisms contributing to activation impairment and mobility dysfunction in older adults.
FUNDING
This research was supported by the National Institute on Aging (AG-18844 to RA Fielding) and the Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679). This material is based upon work supported by the U.S. Department of Agriculture, under agreement No. 58-1950-7-707. D.J.C. was supported by U.S. Department of Veterans Affairs Career Development Award B4888M.
Acknowledgments
Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture or U.S. Department of Veterans Affairs.
References
- 1.Izquierdo M, Ibanez J, Gorostiaga E, et al. Maximal strength and power characteristics in isometric and dynamic actions of the upper and lower extremities in middle-aged and older men. Acta Physiol Scand. 1999;167:57–68. doi: 10.1046/j.1365-201x.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 2.Lanza IR, Towse TF, Caldwell GE, Wigmore DM, Kent-Braun JA. Effects of age on human muscle torque, velocity, and power in two muscle groups. J Appl Physiol. 2003;95:2361–2369. doi: 10.1152/japplphysiol.00724.2002. [DOI] [PubMed] [Google Scholar]
- 3.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65-89 years. Age Ageing. 1994;23:371–377. doi: 10.1093/ageing/23.5.371. [DOI] [PubMed] [Google Scholar]
- 4.Valour D, Ochala J, Ballay Y, Pousson M. The influence of ageing on the force-velocity-power characteristics of human elbow flexor muscles. Exp Gerontol. 2003;38:387–395. doi: 10.1016/s0531-5565(02)00265-6. [DOI] [PubMed] [Google Scholar]
- 5.Bean JF, Kiely DK, Herman S, et al. The relationship between leg power and physical performance in mobility-limited older people. J Am Geriatr Soc. 2002;50:461–467. doi: 10.1046/j.1532-5415.2002.50111.x. [DOI] [PubMed] [Google Scholar]
- 6.Foldvari M, Clark M, Laviolette LC, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55:M192–M199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 7.Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci. 2004;59:1200–1206. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 8.Hazell T, Kenno K, Jakobi J. Functional benefit of power training for older adults. J Aging Phys Act. 2007;15:349–359. doi: 10.1123/japa.15.3.349. [DOI] [PubMed] [Google Scholar]
- 9.Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- 10.Klass M, Baudry S, Duchateau J. Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol. 2007;100:543–551. doi: 10.1007/s00421-006-0205-x. [DOI] [PubMed] [Google Scholar]
- 11.Kamen G, Sison SV, Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol. 1995;79:1908–1913. doi: 10.1152/jappl.1995.79.6.1908. [DOI] [PubMed] [Google Scholar]
- 12.Patten C, Kamen G, Rowland DM. Adaptations in maximal motor unit discharge rate to strength training in young and older adults. Muscle Nerve. 2001;24:542–550. doi: 10.1002/mus.1038. [DOI] [PubMed] [Google Scholar]
- 13.Christie A, Kamen G. Doublet discharges in motoneurons of young and older adults. J Neurophysiol. 2006;95:2787–2795. doi: 10.1152/jn.00685.2005. [DOI] [PubMed] [Google Scholar]
- 14.Patten C, Kamen G. Adaptations in motor unit discharge activity with force control training in young and older human adults. Eur J Appl Physiol. 2000;83:128–143. doi: 10.1007/s004210000271. [DOI] [PubMed] [Google Scholar]
- 15.Harridge SD, Kryger A, Stensgaard A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve. 1999;22:831–839. doi: 10.1002/(sici)1097-4598(199907)22:7<831::aid-mus4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Stevens JE, Stackhouse SK, Binder-Macleod SA, Snyder-Mackler L. Are voluntary muscle activation deficits in older adults meaningful? Muscle Nerve. 2003;27:99–101. doi: 10.1002/mus.10279. [DOI] [PubMed] [Google Scholar]
- 17.Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV. Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. Eur J Appl Physiol. 2004;92:219–226. doi: 10.1007/s00421-004-1056-y. [DOI] [PubMed] [Google Scholar]
- 18.De Serres SJ, Enoka RM. Older adults can maximally activate the biceps brachii muscle by voluntary command. J Appl Physiol. 1998;84:284–291. doi: 10.1152/jappl.1998.84.1.284. [DOI] [PubMed] [Google Scholar]
- 19.Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol. 1999;87:843–852. doi: 10.1152/jappl.1999.87.2.843. [DOI] [PubMed] [Google Scholar]
- 20.Kent-Braun JA, Ng AV. Specific strength and voluntary muscle activation in young and elderly women and men. J Appl Physiol. 1999;87:22–29. doi: 10.1152/jappl.1999.87.1.22. [DOI] [PubMed] [Google Scholar]
- 21.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve. 1999;22:1094–1103. doi: 10.1002/(sici)1097-4598(199908)22:8<1094::aid-mus14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of the human ankle joint with aging. J Appl Physiol. 1986;61:361–367. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]
- 23.Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G. Contractile muscle volume and agonist-antagonist coactivation account for differences in torque between young and older women. Muscle Nerve. 2002;25:858–863. doi: 10.1002/mus.10113. [DOI] [PubMed] [Google Scholar]
- 24.Klein CS, Rice CL, Marsh GD. Normalized force, activation, and coactivation in the arm muscles of young and old men. J Appl Physiol. 2001;91:1341–1349. doi: 10.1152/jappl.2001.91.3.1341. [DOI] [PubMed] [Google Scholar]
- 25.Simoneau EM, Billot M, Martin A, Van Hoecke J. Antagonist mechanical contribution to resultant maximal torque at the ankle joint in young and older men. J Electromyogr Kinesiol. 2009;19:e123–e131. doi: 10.1016/j.jelekin.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Pousson M, Lepers R, Van Hoecke J. Changes in isokinetic torque and muscular activity of elbow flexors muscles with age. Exp Gerontol. 2001;36:1687–1698. doi: 10.1016/s0531-5565(01)00143-7. [DOI] [PubMed] [Google Scholar]
- 27.Klass M, Baudry S, Duchateau J. Aging does not affect voluntary activation of the ankle dorsiflexors during isometric, concentric, and eccentric contractions. J Appl Physiol. 2005;99:31–38. doi: 10.1152/japplphysiol.01426.2004. [DOI] [PubMed] [Google Scholar]
- 28.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohannon RW. Relative decreases in knee extension torque with increased knee extension velocities in stroke patients with hemiparesis. Phys Ther. 1987;67:1218–1220. doi: 10.1093/ptj/67.8.1218. [DOI] [PubMed] [Google Scholar]
- 30.Eisen A, Entezari-Taher M, Stewart H. Cortical projections to spinal motoneurons: changes with aging and amyotrophic lateral sclerosis. Neurology. 1996;46:1396–1404. doi: 10.1212/wnl.46.5.1396. [DOI] [PubMed] [Google Scholar]
- 31.Peinemann A, Lehner C, Conrad B, Siebner HR. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett. 2001;313:33–36. doi: 10.1016/s0304-3940(01)02239-x. [DOI] [PubMed] [Google Scholar]
- 32.Oliviero A, Profice P, Tonali PA, et al. Effects of aging on motor cortex excitability. Neurosci Res. 2006;55:74–77. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Clark DJ, Condliffe EG, Patten C. Activation impairment alters muscle torque-velocity in the knee extensors of persons with post-stroke hemiparesis. Clin Neurophysiol. 2006;117:2328–2337. doi: 10.1016/j.clinph.2006.07.131. [DOI] [PubMed] [Google Scholar]
- 34.Aagaard P, Simonsen EB, Andersen JL, Magnusson SP, Halkjaer-Kristensen J, Dyhre-Poulsen P. Neural inhibition during maximal eccentric and concentric quadriceps contraction: effects of resistance training. J Appl Physiol. 2000;89:2249–2257. doi: 10.1152/jappl.2000.89.6.2249. [DOI] [PubMed] [Google Scholar]
- 35.Hill AV. The heat of shortening and the dynamic constants of muscle. 1938;126:135–195. [Google Scholar]
- 36.Dudley GA, Harris RT, Duvoisin MR, Hather BM, Buchanan P. Effect of voluntary vs. artificial activation on the relationship of muscle torque to speed. J Appl Physiol. 1990;69:2215–2221. doi: 10.1152/jappl.1990.69.6.2215. [DOI] [PubMed] [Google Scholar]
- 37.Frontera WR, Reid KF, Phillips EM, et al. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol. 2008;105:637–642. doi: 10.1152/japplphysiol.90332.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irving BA, Weltman JY, Brock DW, Davis CK, Gaesser GA, Weltman A. NIH ImageJ and Slice-O-Matic computed tomography imaging software to quantify soft tissue. Obesity (Silver Spring) 2007;15:370–376. doi: 10.1038/oby.2007.573. [DOI] [PubMed] [Google Scholar]
- 39.Farina D. Interpretation of the surface electromyogram in dynamic contractions. Exerc Sport Sci Rev. 2006;34:121–127. doi: 10.1249/00003677-200607000-00006. [DOI] [PubMed] [Google Scholar]