Abstract
Background.
Chronic stress may lead to health decline through metabolic syndrome. Thus, persons in stressful caregiving situations who also have more indicators of metabolic syndrome may experience more decline than other caregivers or noncaregivers.
Methods.
The sample included 921 women (338 caregivers and 583 noncaregivers) from the Caregiver-Study of Osteoporotic Fractures study. Participants had home-based baseline and 1-year follow-up interviews between 1999 and 2003. At baseline, caregivers were categorized as long term (³4 years) versus short term (<4 years), and caring for someone with Alzheimer's disease/dementia or not. A metabolic risk composite score was the sum of four indicators: body mass index ³30, and diagnosis or using medications for hypertension, diabetes, or high cholesterol. Walking speed (m/second) was measured at both interviews.
Results.
Walking speed declined for the total sample (adjusted mean = −0.005 m/second, ±0.16) over an average of 1.04 years (±0.16). Overall, caregiving was not associated with decline. Increasing metabolic risk score was associated with greater decline for the total sample and long-term and dementia caregivers, but not other caregivers or noncaregivers. Metabolic risk score modified the adjusted associations between years of caregiving and dementia caregiving with walking speed decline (p values for interaction terms were 0.039 and 0.057, respectively). The biggest declines were in long-term caregivers and dementia caregivers who also had 3–4 metabolic indicators (−0.10 m/second and −0.155 m/second, respectively).
Conclusions.
Walking speed declined the most among older women who had both stressful caregiving situations and more metabolic syndrome indicators, suggesting these caregiver subgroups may have increased risk of health decline.
Keywords: Caregiving, Dementia caregivers, Metabolic indicators, Gait speed
CHRONIC stress, such as that experienced by informal caregivers, can adversely affect physical health. Recent theories suggest that stress-induced metabolic syndrome may contribute to this association (1–3). Evidence supporting these theories comes from findings that workers in high-stress occupations have a higher prevalence of metabolic syndrome (4,5) and that neuroendocrine and inflammatory markers that reflect chronic stress are associated with metabolic syndrome (1,5–7). Metabolic syndrome is a risk factor for functional decline (8–11) and mortality (12), and thus could be an intermediary between chronic caregiving-based stress and health decline. However, few studies have evaluated the role of metabolic syndrome on health decline in older caregivers (13–15).
Studies of caregiving and health decline have mixed results (16,17), but caregivers consistently have higher levels of self-reported stress and stress-related biomarkers than noncaregivers (18–20). Moreover, perceived stress, biomarkers, and physical symptoms are worse in persons engaged in more stressful caregiving situations, such as caregiving for more years (21), or for a relative with Alzheimer's disease (AD) or dementia (17,18,22). In the single study that evaluated relationships among caregiving, metabolic syndrome, and health decline, both older AD caregivers and noncaregivers who also had metabolic syndrome had a higher incidence of coronary heart disease (14), but there was no consistent association between caregiving and prevalence of metabolic syndrome (14,15). Thus, the prevalence of metabolic syndrome may be similar in caregivers and noncaregivers because of differences in lifestyle and other risk factors for metabolic syndrome (23), whereas the combination of stressful caregiving circumstances and metabolic syndrome (whether resulting from chronic stress or from other factors) may increase health decline.
The current study evaluated whether having more indicators of metabolic syndrome modified the association between caregiving and 1-year decline in walking speed among older community-dwelling women. Decline in walking speed was selected as the outcome because it is influenced by metabolic syndrome (8–10) and increases the risk of disability and mortality (24,25), and hence is likely related to the ability to continue performing caregiving activities. Because stress and physical health parameters vary according to caregiving responsibilities, we examined these associations in subgroups based on years of caregiving, and whether the care recipient had AD or dementia. Furthermore, the study lacked measures to replicate the National Cholesterol Education Program Adult Treatment Panel III (ATP III) definition of metabolic syndrome (23), so we constructed a composite score from existing study variables. We hypothesized that caregivers who had more stressful situations (ie, long-term caregivers and AD or dementia caregivers) combined with more indicators of metabolic syndrome would decline more in walking speed than caregivers with fewer indicators or noncaregivers.
METHODS
Sample
Study participants came from the Study of Osteoporotic Fractures (SOF) (26). The SOF sample included 9,704 women who were at least 65 years old and were recruited between 1986 and 1988 from population-based listings in four areas of the United States: Baltimore County, Maryland; Minneapolis, Minnesota; Portland, Oregon; and the Monongahela Valley, Pennsylvania. Women were excluded if they could not walk without help or had a history of bilateral hip replacement. Although African American women were initially excluded, 662 African American women with similar characteristics were enrolled in 1996–1997. Participants in caregiver-SOF included members of the original and African American cohorts who participated in the sixth biennial SOF examination that was conducted between 1997 and 1999.
Caregiver-SOF subsample.—
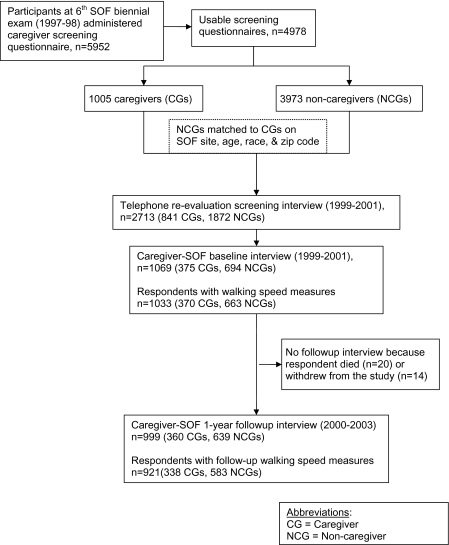
The study sample was identified in two phases, described elsewhere (27). The first phase consisted of administering a caregiver screening questionnaire to 5,952 SOF participants who had their sixth biennial examination at their home or SOF clinic and were not cognitively impaired, or living in long-term care facilities (Figure 1). The questionnaire asked SOF participants if they currently helped a relative or friend with each of seven instrumental activities of daily living (ADL) and seven basic ADL tasks (28) because that person was physically, cognitively, or mentally unable to perform that task independently. Caregivers were SOF participants who helped someone with at least one instrumental activity of daily living (IADL) or ADL task; noncaregivers did not help anyone with these tasks.
Figure 1.
Flow chart of creation of caregiver-SOF sample, and sample for current analyses. Notes: CG = caregiver; NCG = noncaregiver.
The second phase occurred from 1999 to 2001. We readministered this questionnaire by telephone to 841 caregivers and 1,872 noncaregivers identified in the first phase. Respondents who had stopped caregiving (n = 493) were excluded. For each participating caregiver, we matched one or two noncaregivers on SOF site, age, race, and zip code. The resulting sample included 375 caregivers and 694 noncaregivers.
Data Collection
Face-to-face interviews were conducted in respondents’ homes within 2 weeks of the telephone reevaluation (ie, caregiver-SOF baseline interview) and 1 year later. This study was approved by the institutional review boards at each SOF site and at the Boston University Medical Center. All participants provided written informed consent.
Independent Variables
Caregiving status.—
Respondents were classified as caregivers or noncaregivers based on whether they assisted someone with any IADL/ADL tasks, as described above, at the caregiver-SOF baseline interview. Caregivers were asked questions about the main person whom they helped with these tasks.
Long-term caregivers were respondents who had been caregiving for 4 years or more; short-term caregivers had been caregiving from several months to less than 4 years.
Dementia caregivers were caregivers whose main care recipient had been diagnosed with AD or dementia; other caregivers were classified as nondementia caregivers.
Metabolic risk composite score was based on four existing study variables. Hypertension, diabetes, and high cholesterol were considered present if the respondent reported being told by a physician or health care provider that she had the condition or used a medication for that condition in the past month. Interviewers recorded the names of prescription medications taken in the past month. These names were coded according to indication using the Physicians’ Desk Reference and online prescription databases, and cross-checked for reliability. Body mass index (kg/m2) was based on the respondent's height at her baseline SOF visit and her weight, measured at the baseline caregiver-SOF interview. Respondents with body mass index (BMI) of 30 or more were classified as “high BMI”, consistent with the cutpoint used in the World Health Organization definition of metabolic syndrome (23). We calculated the total number of these four indicators. Because few respondents had all indicators present, we combined respondents with three or four indicators (possible values ranged from 0 to 3).
Outcome Variable
Walking speed.—
At the caregiver-SOF baseline and follow-up interviews, respondents were timed on the number of seconds it took to walk a 2-m or 3-m course at their usual pace (29). After a trial walk, respondents were timed on two walks at usual pace. We calculated the average of these two walks (m/second). Respondents who used canes or walkers performed timed walks if the interviewer and respondent felt it was safe. Change in walking speed was calculated as the difference between average walking speeds at follow-up and baseline.
Covariables
Sociodemographic variables included the respondent’s self-report of age, race (white or black), highest level of education (dichotomized at college or more), and current marital status.
Leisure time exercise was determined by a positive response to one of two questions: “Do you usually walk for exercise (walk one block or more without stopping)?” (30), and “Do you usually engage in any regular exercise other than walking at least once a week, such as stretching or strengthening exercises, swimming or any other exercise”?
Limitations in IADLs and ADLs were based on the respondent’s self-report of her ability to perform each of seven IADLs and seven ADLs independently. The IADLs were use the telephone, get to places out of walking distance, shop, prepare meals, manage medications, manage finances, and do heavy housework (31). The ADLs included walk across a room, groom, transfer from bed to chair, eat, dress, bathe, and use the toilet. Separate variables were constructed for the total number of ADL (0–7) and IADL (0–7) limitations.
Perceived stress was measured by the Perceived Stress scale (32). This 14-item scale measures general stress experienced in the past month, with higher scores indicating more stress, and possible values ranging from 0–56.
Caregiving characteristics.—
Caregivers reported if they were the care recipient's spouse versus another relative or other friend, if they lived with the care recipient, and the number of ADL tasks (0–7) and IADL tasks (0–7) with which they helped the care recipient.
Statistical Analyses
We compared the baseline characteristics of respondents across the caregiver status categories using ANOVAs for continuous variables and chi-square tests for categorical variables. We conducted separate multiple linear regression analyses for the two stressful caregiving situations (ie, caregiving) on change in walking speed from baseline to the first follow-up interview. Individual covariables that were associated with both caregiving and change in walking speed were considered as potential confounders. These variables were included in the baseline regression model and were eliminated one at a time if they were not statistically significant (p ≤ .10) and their exclusion did not markedly change the association between caregiving and change in walking speed. To determine whether number of indicators of metabolic syndrome modified this association, we evaluated the statistical significance of the interaction term, caregiving × metabolic risk composite score, in regression models containing independent variables for these terms, baseline walking speed and confounders. These models produced adjusted mean values for change in walking speed. We also evaluated whether increasing number of metabolic syndrome indicators was associated with greater decline in walking speed by conducting tests for linear trend of the metabolic risk composite score within the total sample and within each caregiving subgroup.
RESULTS
Sample Characteristics
The sample included 921 respondents who had walking speed measures at the baseline and follow-up interviews. Of these respondents, 583 were noncaregivers, 156 (16.9%) were long-term caregivers, and 90 (27.5% of the caregivers) cared for someone with AD or dementia. Compared with respondents included in these analyses, the 148 subjects who were excluded due to lacking follow-up interviews (n = 34) or walking speed measures (n = 114) were significantly older, had more ADL and IADL limitations, slower baseline walking speeds, more metabolic indicators, and were more likely to care for a spouse.
The mean age of respondents was 81.1 (±3.6) years and 88% were white (Table 1). Caregivers were significantly more likely than noncaregivers to be married and stressed, but had fewer IADL limitations. Long-term caregivers had the fastest baseline walking speeds, were younger and better educated than other respondents, and less likely to be caring for a spouse. Noncaregivers were more likely than caregivers to have 3–4 metabolic syndrome indicators.
Table 1.
Sample Characteristics for the Total Sample and by Length of Time Caregiving, Among 921 Caregiver-SOF Participants
| Characteristic | Total sample (n = 921) | NCGs (n = 583) | Short-term CGs (n = 182) | Long-term CGs (n =156) | p Value comparing NCGs, short- and long-term CGs |
| Caregiver demographics and health characteristics | |||||
| Age in years, mean (SD) | 81.1 (3.6) | 81.2 (3.7) | 81.3 (3.6) | 80.3 (3.5) | .009 |
| Race: % African American | 11.7 | 11.7 | 13.2 | 10.3 | .70 |
| Highest education level: % attended college or more education | 53.6 | 51.1 | 54.4 | 62.2 | .047 |
| Marital status: % married | 37.1 | 27.1 | 59.9 | 48.1 | <.0001 |
| Number ADL limitations: mean (SD) | 0.4 (0.6) | 0.4 (0.7) | 0.3 (0.6) | 0.3 (0.5) | .28 |
| Number IADL limitations, mean (SD) | 0.6 (1.0) | 0.7 (1.2) | 0.3 (0.6) | 0.3 (0.6) | <.0001 |
| Leisure time exercise (%) | 58.6 | 58.5 | 60.8 | 56.8 | .75 |
| Perceived stress scale, mean (SD) | 16.0 (7.1) | 15.2 (6.7) | 17.4 (7.1) | 17.4 (8.0) | <.0001 |
| Metabolic risk composite score (%) | |||||
| 0 components | 29.1 | 29.1 | 26.4 | 31.4 | .05 |
| 1 component | 39.6 | 41.3 | 37.4 | 35.9 | |
| 2 components | 22.0 | 18.9 | 29.7 | 25.0 | |
| 3–4 components | 9.3 | 10.5 | 6.6 | 7.7 | |
| Walking speed (m/s), mean (SD) | 0.71 (0.19) | 0.70 (0.19) | 0.72 (0.20) | 0.75 (0.17) | .03 |
| 1-year change in walking speed (m/s), mean (SD) | −0.005 (0.16) | −0.003 (0.16) | 0.001 (0.17) | −0.018 (0.18) | .51 |
| Caregiving characteristics | |||||
| Caregiver to spouse (%) | 45.9 | 51.7 | 39.1 | .02 | |
| Caregiver lives with care recipient (%) | 48.8 | 51.7 | 45.5 | .26 | |
| Care recipient has AD or dementia (%) | 27.5 | 29.1 | 25.7 | .48 | |
| Number of ADLs caregiver helps with, mean (SD) | 1.5 (1.8) | 1.5 (1.7) | 1.6 (1.8) | .59 | |
| Number of IADLs caregiver helps with, mean (SD) | 3.8 (2.0) | 3.9 (2.1) | 3.7 (1.9) | .35 |
Notes: CGs = caregivers; NCGs = noncaregivers.
Change in walking speed.—
Walking speed declined for the total sample over the follow-up period (mean = 1.04 years, ±0.16). Mean decline, adjusted for baseline speed, was −0.005 m/second (±0.16). Neither long-term caregiving (β = −.004, p = .57) nor dementia caregiving (β =.0005, p = .95) was associated with change in walking speed, adjusted for confounders.
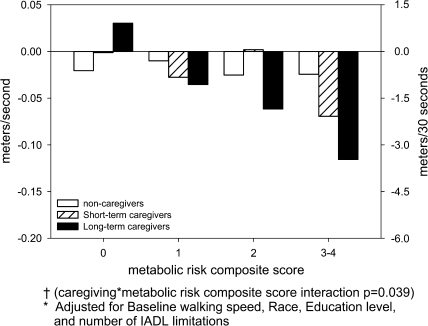
However, stressful caregiving situations combined with multiple metabolic syndrome indicators resulted in greater walking speed decline. In multiple regression analyses, long-term caregivers with 3–4 metabolic indicators declined more than other respondents: mean =−0.10 m/second; interaction term p = .039 (Table 2 and Figure 2). There was a significant linear trend between increasing number of metabolic syndrome indicators and walking speed decline among long-term caregivers (p = .002), but not short-term caregivers (p = .24) or noncaregivers (p = .70).
Table 2.
Adjusted* Mean Change in Walking Speed Over 1-year by Length of Time Caregiving and Metabolic Risk Composite Score Combined
| Length of time caregiving, mean (SE) |
||||
| Metabolic risk composite score | Total sample | Noncaregivers | Short-term caregivers | Long-term caregivers |
| 0 | 0.004 (0.013) | −0.007 (0.012) | 0.012 (0.022) | 0.044 (0.022) |
| 1 | −0.023 (0.012) | 0.004 (0.010) | −0.014 (0.018) | −0.022 (0.020) |
| 2 | −0.027 (0.013) | −0.012 (0.014) | 0.016 (0.021) | −0.048 (0.024) |
| 3–4 | −0.069 (0.023) | −0.011 (0.020) | −0.056 (0.044) | −0.102 (0.044) |
| Linear trend for increasing metabolic risk composite score | p = .003 | p = .70 | p = .24 | p = .002 |
Adjusted for baseline walking speed (β = −.355, SE = 0.029, p < .001), black race (β =−.034, SE = 0.016, p = .04), high school graduate+ (β = .018, SE = 0.010, p = .08), and IADL limitations (β = −.024, SE = 0.005, p < .001). p Value for caregiving × metabolic risk composite score interaction term = .039.
Figure 2.
Adjusted* mean change in walking speed over 1 year by length of time caregiving and metabolic risk composite score combined†. Notes: *Adjusted for baseline walking speed, race, education level, and number of IADL limitations. †Caregiving × metabolic risk composite score interaction, p = .039).
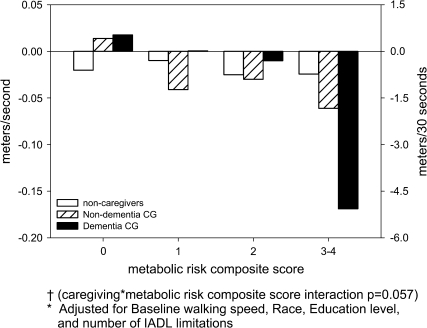
Similarly, caregivers for persons with dementia who also had 3–4 metabolic risk indicators experienced more decline in walking speed (−0.155 m/second) than other caregivers and noncaregivers. The interaction between dementia caregiving and metabolic risk composite score was borderline statistically significant (p = .057; Table 3 and Figure 3). There was also more of a linear trend between increasing number of metabolic syndrome indicators and walking speed decline among dementia caregivers (p = .004) than nondementia caregivers (p = .09).
Table 3.
Adjusted* Mean Change in Walking Speed Over 1-year by Dementia Status and Metabolic Risk Composite Score Combined
| Dementia caregiving status, mean (SE) |
||||
| Metabolic risk composite score | Total sample | Noncaregivers | Nondementia caregivers | Dementia caregivers |
| 0 | 0.005 (0.014) | −0.007 (0.012) | 0.027 (0.019) | 0.031 (0.029) |
| 1 | −0.016 (0.013) | 0.004 (0.010) | −0.028 (0.016) | 0.014 (0.028) |
| 2 | −0.021 (0.014) | −0.012 (0.014) | −0.016 (0.018) | 0.003 (0.031) |
| 3–4 | −0.084 (0.025) | −0.011 (0.020) | −0.048 (0.037) | −0.155 (0.057) |
| Linear trend for increasing metabolic risk composite score | p ≤ .001 | p = .70 | p = .09 | p = .004 |
Adjusted for baseline walking speed (β = −.359, SE = 0.029, p < .001), black race (β =−.033, SE = 0.016, p=−.04), high school graduate+ (β = .018, SE = 0.010, p = 0.077), and IADL limitations (β = −.024, SE = 0.005, p < .001). p Value for the caregiving × metabolic risk composite score interaction term = .057.
Figure 3.
Adjusted* mean change in walking speed over 1 year by dementia versus nondementia caregiving and metabolic risk composite score combined†. Notes: *Adjusted for Baseline walking speed, race, education level, and number of IADL limitations. †Caregiving × metabolic risk composite score interaction p = .057.
In both models, walking speed declined more in respondents who were African American, had less education, and more IADL limitations.
DISCUSSION
This study found that older women who were caregivers for 4 years or more and who also had 3–4 metabolic syndrome indicators declined more in walking speed than other respondents. Older women who cared for a person with dementia and who had more metabolic syndrome indicators showed a borderline significant trend toward more decline in walking speed. These results support our hypothesis and corroborate previous observations of poorer health outcomes in AD caregivers than other caregivers (17). They are consistent with studies that found that older adults (8,9,11) and adults with peripheral arterial disease (10) who also had metabolic syndrome had a higher risk of incident mobility limitations (11), decline in ADLs (8,9), mobility-related ADLs (9), and walking speed (10). They are also consistent with studies that found linear trends between increasing number of metabolic syndrome components and poorer outcomes in mobility (11) and cognitive functioning (33).
The decline in walking speed may appear minimal. Yet, even small declines increase the risk of mortality (25). Moreover, if translated into distance walked over 30 seconds (the time often allotted to cross a street), then long-term and dementia caregivers with 3–4 metabolic syndrome indicators would walk 3.0 and 4.7 m less in 30 seconds at 1-year follow-up than at baseline (Figures 2 and 3). This change may affect caregivers’ quality of life and ability to provide optimal care for the care recipient.
There are several pathways by which chronic caregiving stress combined with multiple metabolic syndrome indicators may lead to a decline in walking speed. First, the presence of both conditions may exacerbate their individual effects, such as reduced walking speed in obese persons (34) or in depressed adults resulting from chronic stress (35). Second, there may be indirect effects through disruptions in cytokine activity, notably interleukin-6 (IL-6). Both AD caregivers (20,36) and adults with metabolic syndrome (5) have higher levels of IL-6. Older adults with elevated IL-6 levels have greater risk of mobility impairment (37). Third, chronic stress may create a cascade of disruptions in multiple physiological systems (1,3). Caregivers with more metabolic syndrome indicators may experience more decline because they are further along this pathway toward health decline than other caregivers.
This study had several limitations. The metabolic risk composite score differed from ATP-III–defined metabolic syndrome, which is based on waist circumference and a combination of clinical assessments and medications for identifying hypertension, dyslipidemia, and elevated glucose (23), whereas our measure used BMI instead of waist circumference and a combination of self-report and medications for the other components. However, various measures of metabolic syndrome exist for clinical (23) and research purposes (33,38). Previous studies of caregivers used a “metabolic risk composite” that did not include high-density lipoprotein or triglyceride levels (15) and other modifications of metabolic variables (14), and other studies also substituted BMI for waist circumference (33). Additionally, our measure did not include prevalent cardiovascular disease, but when terms for heart disease and stroke were added to multivariable models, they were not statistically significant.
Other potential limitations were that long-term caregivers did not have higher perceived stress than short-term caregivers. However, caregiving-related stressors and psychological distress fluctuate over time. Caregivers in this sample reported being in this role from less than 1 year to 53 years. Thus, long-term caregivers had the longest exposure to caregiving-related stressors. The fact that we found a significant, dose–response association between higher metabolic risk composite score and walking speed decline in long-term caregivers supports our hypothesis. However, interpretation of these results should consider the small number of respondents in subgroups of respondents with 3–4 metabolic indicators who were also in stressful caregiving subgroups (12 long-term caregivers and 7 dementia caregivers) as well as the possibility of regression to the mean because this group had the fastest baseline walking speed. We also lacked measures of physical activity and diet, risk factors for metabolic syndrome (23), and mobility limitations. (39) Although residual confounding may be a concern, our results were consistent with studies that adjusted for physical activity, suggesting that this was not a major limitation. Additionally, the sample was comprised of older women who were mainly white and high functioning, raising the question of generalizability of these results. However, as most caregivers in the United States are older women (40), these results apply to the majority of caregivers.
Nonetheless, this study's strengths included its large, multisite community-based sample of older women. Caregivers and noncaregivers came from the same source population, thus reducing possible biases that may result from recruiting caregivers from patient registries and noncaregivers from other sources. The inclusion criteria required that caregivers were helping the care recipient with at least one IADL/ADL at baseline, so misclassification of caregiver status was unlikely.
In summary, these results add to studies of the combined effects of caregiving and metabolic risk factors (13–15), and are the first, to our knowledge, to compare different caregiving situations. Additional studies are needed to replicate these findings, including studies with longer follow-up periods, larger samples, and alternative health and functioning outcomes. Given the increasing population of older caregivers, such studies will provide important information for identifying subgroups of older caregivers that may be at risk of health decline.
FUNDING
AG18037, AG05407, AR35582, AG05394, AR35584, and AR35583. An earlier version of this manuscript was presented at the 59th Annual meeting of the Gerontological Society of America, Dallas, Texas, 2006.
References
- 1.Bjorntorp P. Do stress reactions cause abdominal obesity and comorbidities? Obes Rev. 2001;2:73–86. doi: 10.1046/j.1467-789x.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 2.Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24(suppl 2):S50–S55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- 3.Rosmond R. Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology. 2005;30:1–10. doi: 10.1016/j.psyneuen.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Keltikangas-Jarvinen L, Ravaja N, Raikkonen K, et al. Relationships between the pituitary-adrenal hormones, insulin, and glucose in middle-aged men: moderating influence of psychosocial stress. Metab. Clin. Exp. 1998;47:1440–1449. doi: 10.1016/s0026-0495(98)90067-1. [DOI] [PubMed] [Google Scholar]
- 5.Brunner EJ, Hemingway H, Walker BR, et al. Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation. 2002;106:2659–2665. doi: 10.1161/01.cir.0000038364.26310.bd. [DOI] [PubMed] [Google Scholar]
- 6.Black PH. The inflammatory response is an integral part of the stress response: implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain Behav Immun. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 7.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 8.Blazer DG, Hybels CF, Fillenbaum GG. Metabolic syndrome predicts mobility decline in a community-based sample of older adults. J Am Geriatr Soc. 2006;54:502–506. doi: 10.1111/j.1532-5415.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 9.Blaum CS, West NA, Haan MN. Is the metabolic syndrome, with or without diabetes, associated with progressive disability in older Mexican Americans? J Gerontol A Biol Sci Med Sci. 2007;62:766–773. doi: 10.1093/gerona/62.7.766. [DOI] [PubMed] [Google Scholar]
- 10.Gardner AW, Montgomery PS, Parker DE. Metabolic syndrome impairs physical function, health-related quality of life, and peripheral circulation in patients with intermittent claudication. J Vasc Surg. 2006;43:1191–1196. doi: 10.1016/j.jvs.2006.02.042. discussion 1197. [DOI] [PubMed] [Google Scholar]
- 11.Penninx BW, Nicklas BJ, Newman AB, et al. Metabolic syndrome and physical decline in older persons: results from the Health, Aging And Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009;64:96–102. doi: 10.1093/gerona/gln005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford ES. The metabolic syndrome and mortality from cardiovascular disease and all-causes: findings from the National Health and Nutrition Examination Survey II Mortality Study. Atherosclerosis. 2004;173:309–314. doi: 10.1016/j.atherosclerosis.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Vitaliano PP, Scanlan JM, Siegler IC, et al. Coronary heart disease moderates the relationship of chronic stress with the metabolic syndrome. Health Psychol. 1998;17:520–529. doi: 10.1037//0278-6133.17.6.520. [DOI] [PubMed] [Google Scholar]
- 14.Vitaliano PP, Scanlan JM, Zhang J, et al. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med. 2002;64:418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Vitaliano PP, Echeverria D, Yi J, et al. Psychophysiological mediators of caregiver stress and differential cognitive decline. Psychol Aging. 2005;20:402–411. doi: 10.1037/0882-7974.20.3.402. [DOI] [PubMed] [Google Scholar]
- 16.Fredman L, Cauley JA, Satterfield S, et al. Caregiving, mortality, and mobility decline: the Health, Aging, and Body Composition (Health ABC) study. Arch Intern Med. 2008;168:2154–2162. doi: 10.1001/archinte.168.19.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinquart M, Sorensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. 2003;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- 18.Ory MG, Hoffman RR, 3rd, Yee JL, et al. Prevalence and impact of caregiving: a detailed comparison between dementia and nondementia caregivers. Gerontologist. 1999;39:177–185. doi: 10.1093/geront/39.2.177. [DOI] [PubMed] [Google Scholar]
- 19.Bauer ME, Vedhara K, Perks P, et al. Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. J Neuroimmunol. 2000;103:84–92. doi: 10.1016/s0165-5728(99)00228-3. [DOI] [PubMed] [Google Scholar]
- 20.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth DL, Haley WE, Owen JE, et al. Latent growth models of the longitudinal effects of dementia caregiving: a comparison of African American and White family caregivers. Psychol Aging. 2001;16:427–436. [PubMed] [Google Scholar]
- 22.Bertrand RM, Fredman L, Saczynski J. Are all caregivers created equal? Stress in caregivers of adults with and without dementia. J Aging Health. 2006;18:534–551. doi: 10.1177/0898264306289620. [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13:322–327. [PubMed] [Google Scholar]
- 24.Hardy SE, Perera S, Roumani YF, et al. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55:1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 25.Perera S, Studenski S, Chandler JM, et al. Magnitude and patterns of decline in health and function in 1 year affect subsequent 5-year survival. J Gerontol A Biol Sci Med Sci. 2005;60:894–900. doi: 10.1093/gerona/60.7.894. [DOI] [PubMed] [Google Scholar]
- 26.Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263:665–668. [PubMed] [Google Scholar]
- 27.Fredman L, Tennstedt S, Smyth KA, et al. Pragmatic and internal validity issues in sampling in caregiver studies: a comparison of population-based, registry-based, and ancillary studies. J Aging Health. 2004;16:175–203. doi: 10.1177/0898264303262639. [DOI] [PubMed] [Google Scholar]
- 28.Katz S, Ford A, Moskowitz R, et al. Studies on illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:94–99. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 29.Ensrud KE, Nevitt MC, Yunis C, et al. Correlates of impaired function in older women. J Am Geriatr Soc. 1994;42:481–489. doi: 10.1111/j.1532-5415.1994.tb04968.x. [DOI] [PubMed] [Google Scholar]
- 30.Guralnik JM, Fried LP, Simonsick EM, et al. The Women's Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. NIH Pub. No. 95-4009. [Google Scholar]
- 31.Multidimensional Functional Assessment: the OARS Methodology. Durham, NC: Duke University Center for the Study of Aging and Human Development; 1978. [Google Scholar]
- 32.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 33.Yaffe K, Weston AL, Blackwell T, et al. The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol. 2009;66:324–328. doi: 10.1001/archneurol.2008.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visser M, Langlois J, Guralnik JM, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr. 1998;68:584–590. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- 35.Ruo B, Liu K, Tian L, et al. Persistent depressive symptoms and functional decline among patients with peripheral arterial disease. Psychosom Med. 2007;69:415–424. doi: 10.1097/PSY.0b013e318063ef5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.von Kanel R, Dimsdale JE, Ancoli-Israel S, et al. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer's disease. J Am Geriatr Soc. 2006;54:431–437. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 37.Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52:1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- 38.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arterioscler Thromb Vasc Biol. 2000;20:2255–2260. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 39.Visser M, Simonsick EM, Colbert LH, et al. Type and intensity of activity and risk of mobility limitation: the mediating role of muscle parameters. J Am Geriatr Soc. 2005;53:762–770. doi: 10.1111/j.1532-5415.2005.53257.x. [DOI] [PubMed] [Google Scholar]
- 40.National Alliance for Caregiving. Caregiving in the U.S. Bethesda, MD: National Alliance for Caregiving (NAC) and the American Association of Retired Persons (AARP); 2004. [Google Scholar]