Abstract
Calorie restriction (CR) has been known for more than 70 years to extend life span and delay disease in rodent models. Metformin administration in rodent disease models has been shown to delay cancer incidence and progression, reduce cardiovascular disease and extend life span. To more directly test the potential of metformin supplementation (300 mg/kg/day) as a CR mimetic, life-span studies were performed in Fischer-344 rats and compared with ad libitum feeding and CR (30%). The CR group had significantly reduced food intake and body weight throughout the study. Body weight was significantly reduced in the metformin group compared with control during the middle of the study, despite similar weekly food intake. Although CR significantly extended early life span (25th quantile), metformin supplementation did not significantly increase life span at any quantile (25th, 50th, 75th, or 90th), overall or maximum life span (p > .05) compared with control.
Keywords: Calorie restriction, Metformin, Mimetic, Life span, Aging
FROM the nutrition life-span studies of rodents in the 1930s (1,2) to the microarray and high-throughput techniques of the 2000s (3,4), calorie restriction (CR) continues to be the most robust, nongenetic intervention for extending life span and delaying the development of disease in model organisms (5,6). Research from the last 30 years has begun to enumerate many metabolic and physiological changes that accompany CR in laboratory organisms, including mice and rats. As more CR-responsive pathways are identified, the goal of fully understanding the molecular underpinnings of these benefits seems closer now than ever. Several prominent theories offer explanations for at least part of the CR data, but a unified theory is still incomplete.
Preliminary results from ongoing studies in humans are revealing many similar metabolic benefits as are observed in laboratory organisms (7,8). Even so, a long-term randomized control CR life-span study in humans is highly unlikely. If CR were shown to be beneficial for health and longevity in humans, the prevailing environment of energy excess with increased obesity suggests that humans are unwilling or unable to voluntarily undergo the amount and duration of restriction normally required to observe benefits in laboratory studies (9,10). As such, the feasibility of CR in humans, particularly on a lifelong scale, is impractical and thus far unproven. With these difficulties in mind, a search for calorie restriction mimetics (CRM), compounds, or conditions that result in the beneficial effects of CR without the imposed restriction of food/calorie intake is under way (4,11–17).
Metabolic profiling of specific pathways that are altered with CR has been used to compare dietary supplementation with compounds that may mimic the CR response, despite normal calorie intake. The use of microarray expression data offers a more global insight into the potential mechanisms of CR and has been utilized during the last decade (3,4,18–22). By assessing transcriptional changes that occur in organisms subjected to CR, compared with control ad libitum (AL) feeding, a metabolic signature (transcriptional profile) can be identified and used for comparison with other types of dietary interventions (4,17). Previous research has demonstrated that short-term CR, 8 weeks in duration, initiated at 19–20 months of age in rodents shares a similar, although incomplete, transcriptional response to long-term CR (4) and similarly extends health and life span in older mice (22). Using the long-term CR transcriptional profile, metformin, glipizide, glipizide plus metformin, rosiglitazone, and soy isoflavone supplementation (for 8 weeks) were found to share a partial response with CR, with the biguanide metformin demonstrating the most similar transcriptional profile to both short-term and long-term CR (4).
Supporting the potential for metformin and related biguanides (phenformin and buformin) to mimic CR, previous reports have shown delayed incidence and development of cancers and other disease conditions, as well as life-span extension, in a variety of rodent models that are prone to cancer or other diseases (23–34). Metformin is known to improve insulin sensitivity and reduce liver production of glucose (35), phenotypes similar to CR. The role of insulin/insulin-like growth factor 1 in aging has received much support from both model organisms and studies with CR in mammals, including nonhuman primates (29,36,37). Thus, supplementation of a normal AL-fed condition with metformin may mimic the metabolic and physiological benefits of CR and increase life span. To test the potential of metformin supplementation as a CRM and eliminate the confounding of early death caused by a specific disease condition present in previous rodent studies, male Fischer-344 (F344) rats were studied under AL, 30% CR, and metformin supplementation to assess metabolic and life-span effects.
METHODS
Animals
Male F344 rats (Charles River Laboratories, Raleigh, NC) were individually housed in stainless steel cages with AL access to a standard chow diet (Teklad NTP-2000; Harlan Teklad, Madison, WI) (38) provided weekly in clean feeders and AL tap water until 6 months of age. Food and water were routinely analyzed for composition, contamination, and specific microbes. The room was maintained at 21 ± 3°C with 30%–70% relative humidity and a 12:12 hour light:dark cycle. At 6 months of age, the animals were randomized to one of four diet groups: (a) control (CON; n = 45), (b) calorie restricted (CR; n = 45), (c) metformin (300 mg/kg/day; MET; n = 45), and (d) pair fed to metformin (PF–MET; n = 45). Metformin supplementation was accomplished by incorporation into the diet, and all diet formulations were prepared weekly. CON and MET groups were provided AL food throughout the study. CR rats were provided 70% of the CON group's AL intake daily at 16:00 hours. PF–MET rats were provided with food equivalent to the MET groups mean intake to better separate any effect of metformin supplementation on life span from potential reductions in calorie intake. Body weight and food intake were measured weekly during weeks 1–14 and every 3–4 weeks thereafter.
Glucose and Insulin
Approximately 1 mL of blood was collected by orbital sinus puncture under CO2/O2 anesthesia for all surviving rats in each group at age 27, 39, 52, and 65 weeks (1, 13, 26, and 39 weeks following group randomization) at 06:00 hours (±1:00 hour). Blood glucose levels were determined at all four time points for CON: n = 44, 45, 45, and 44; CR: n = 44, 43, 43, and 43; MET: n = 44, 45, 43, and 43; and PF–MET: n = 45, 44, 44, and 43. Insulin was assessed in selected samples (n = 12) of rats from each group at each time point (with the exception of 11 rats in the CR group at age 39 weeks).
Core Body Temperature
Core body temperature (CBT) was measured by thermistor probe (at 27 and 39 weeks of age) or subcutaneous implant (52 and 65 weeks of age; BioMedic Data System, Inc., Monitoring System, Seaford, DE) at 06:00 hours (±1:00 hour) to the nearest 0.1°C. The total numbers of rats measured at each point were CON: n = 44, 45, 45, and 44; CR: n = 45, 43, 43, and 43; MET: n = 44, 45, 43, and 43; and PF–MET: n = 45, 44, 44, and 43.
Survival
Animals were inspected daily for health status and survival status. Moribund animals were euthanized and day of death was recorded. Animals removed at interim points during the study for other experimental end points were not included in survival analysis (CON: n = 14, CR: n = 5, MET: n = 5, and PF–MET: n = 5). The total number of deaths for survival analysis, both euthanized (moribund animals) and natural deaths, for the groups was CON: n = 31; CR: n = 40; MET: n = 40; and PF–MET: n = 40. Gross necroscopic examinations were performed following death.
Statistical Analysis
Data were analyzed with SAS 9.1 statistical software (SAS Institute, Cary, NC). Food intake, body weight, glucose, insulin, and CBT were analyzed with a group by time repeated measures analysis of variance (ANOVA), with a post hoc Bonferroni correction for repeated comparisons between groups. Results were considered significant when p < .05 (two tailed). To assess life span, Cox proportional hazards regression, quantile regression, and a test for maximum life span were performed (39–41). Overall mean life span and the mean life span of the last 10% of survivors of each group were analyzed by ANOVA. Parameters of the Gompertz model (α and mortality rate doubling time) were calculated as described (42) and analyzed by analysis of covariance (ANCOVA).
The Cox proportional hazards regression is used to model survival time by group. There were no covariates used in the model because all the F344 rats are male and of the same age. However, a model with a time-dependent covariate is used to test the proportional hazards assumption. A possible issue regarding censoring arose as there were approximately 80 moribund kills during the study period, and thus the time to the event of natural death could not be determined with specificity. Despite this, the results obtained from a regression model including the moribund kills as censored data did not differ to any significant degree from the regression model not including censored data. The results of the regression model using noncensored data are reported.
Quantile regression analysis is performed to determine whether there are differences in the group's survival rates at the 25th, 50th, 75th, and 90th quantiles. The maximum life-span test is used to detect differences in group life span with the groups consisting of animals who survived at or beyond a specified upper quantile. In this analysis, the approximate 90th quantile of the control group is used as the specified upper quantile. In this study, the approximate 90th quantile of the control equates to study Day 821 (1,001 days old).
Also, Kaplan–Meier survival curves are used to graphically illustrate differences and similarities in various group survival patterns. The analyses were conducted using the following SAS procedures: PROC PHREG, PROC REG, PROC ANOVA, PROC ANCOVA, PROC GLM, PROC QUANTREG, PROC NPAR1WAY, and PROC LIFETEST.
RESULTS
Food Intake and Body Weight
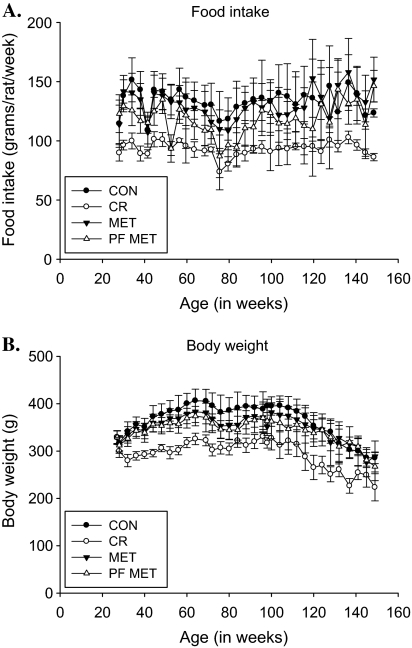
As designed, the CR group had a significantly lower food intake compared with CON for the duration of the study (Figure 1A). Additionally, the MET group consumed similar amounts of food as the CON (after Bonferroni correction) but significantly more than the CR group (Figure 1A). Mean body weight increased for all groups following randomization and subsequently declined with advanced age (Figure 1B). Mean body weight of the CR group was significantly reduced compared with CON from the second week postrandomization until near the end of the study (p < .05; Figure 1B), whereas both MET and PF–MET groups were significantly heavier than the CR group and significantly lighter than CON from approximately 48–74 weeks of age (Figure 1B).
Figure 1.
Food intake and body weight. (A) Food intake (mean ± SD in grams/rat/week) for CON, CR, MET, and PF–MET groups recorded every 3–4 weeks following group randomization. (B) Body weight (mean ± SD in grams/rat) for CON, CR, MET, and PF–MET groups every 4 weeks following group randomization. Note: CON = control; CR = calorie restriction; MET = metformin; PF–MET = pair fed to metformin.
Glucose, Insulin, and CBT
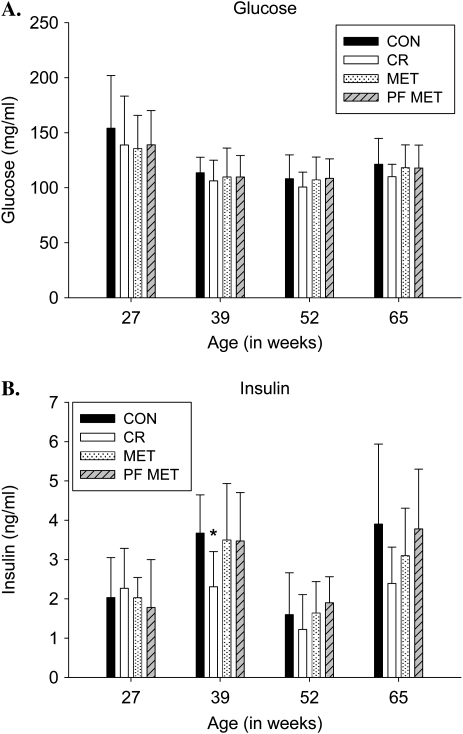
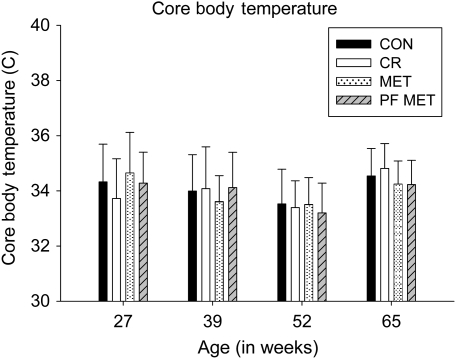
A significant time and group effect was observed for measured glucose levels (p < .01); however, glucose levels were statistically similar between groups at 27, 39, 52, and 65 weeks of age (Figure 2A). Additionally, there was a significant time and group effect for insulin measures (p < .01) with a reduction in insulin levels in the CR group during Week 39 (p < .01; Figure 2B). Insulin levels were similar between CON, MET, and PF–MET for the duration of the study (Figure 2B). Although there was a significant time effect (p < .01) and group by time interaction (p < .01) on CBTs across the four measures, there was no significant main effect for group in measured mean CBT or in pairwise comparisons with CON at any given week (Figure 3).
Figure 2.
Glucose and insulin response. (A) Glucose levels (milligrams per deciliter) obtained at 06:00 hours during the indicated week (age 27, 39, 52, and 65 weeks) for CON, CR, MET, and PF–MET (mean ± SD for all surviving rats). (B) Insulin levels (nanograms per milliliter) obtained at 06:00 hours during the indicated week (age 27, 39, 52, and 65 weeks; mean ± SD for 12 rats per study group). *p < .05. Note: CON = control; CR = calorie restriction; MET = metformin; PF–MET = pair fed to metformin.
Figure 3.
Core body temperature (mean ± SD) at age 27, 39, 52, and 65 weeks (at 06:00 hours).
Survival
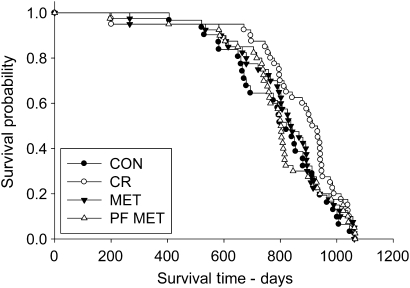
To assess differences in life span across the survival curve, multiple analyses were performed. Cox regression analysis of life span showed no significant differences in the CR, MET, or PF–MET group compared with CON (Figure 4; Table 1). There were no significant differences in overall mean life span or the mean of the last surviving 10% of each group (Table 2). However, the aging rate estimate (α—slope, rate of increase of mortality) of the Gompertz model of the CON group alone was significantly different from the CR, MET, and PF–MET groups (Table 2), reflecting the early deaths in the CR, MET, and PF–MET groups. Additionally, CR significantly increased life span in the 25th quantile but not the 50th, 75th, or 90th quantile (Table 3). MET and PF–MET groups were not significantly different from CON at any quantile (Figure 4; Table 3). Maximum life span was not significantly different from CON for any group (CR, MET, or PF–MET; Figure 4; Table 4).
Figure 4.
Kaplan–Meier survival plots for CON, CR, MET, and PF–MET (CON: n = 31; CR: n = 40; MET; n = 40; and PF–MET: n = 40). Note: CON = control; CR = calorie restriction; MET = metformin; PF–MET = pair fed to metformin.
Table 1.
Cox Proportional Hazard Regression of Life Span
| Group | Parameter Estimate | Hazard Ratio | Lower 95% Confidence Limit | Upper 95% Confidence Limit |
| CR vs CON | −0.399 (0.241) | 0.671 | 0.418 | 1.076 |
| MET vs CON | −0.017 (0.040) | 0.983 | 0.908 | 1.065 |
| PF–MET vs CON | −0.010 (0.035) | 0.990 | 0.926 | 1.060 |
Note: CON = control; CR = calorie restriction; MET = metformin; PF–MET = pair fed to metformin.
Table 2.
Effect of CR and Metformin on Life Span of Fischer-344 Male Rats
| Group | Mean Life Span*,† | Range (Minimum–Maximum)* | Mean Life Span of Last 10%*,†,‡ | Gompertz Aging Rate (α)§ | MRDT |
| CON | 796.4 ± 169.7 | 406–1,065 | 1,039.3 ± 29.6 | 2.19 (2.04; 2.34) | 0.32 |
| CR | 866.5 ± 189.8 | 200–1,065 | 1,057.0 ± 8.4 | 1.92 (1.70; 2.13) | 0.36 |
| MET | 814.5 ± 185.8 | 266–1,062 | 1,060.8 ± 2.5 | 2.04 (1.91; 2.16) | 0.34 |
| PF–MET | 803.3 ± 178.0 | 196–1,065 | 1,058.3 ± 11.6 | 2.07 (1.88; 2.27) | 0.33 |
Notes: CON = control; CR = calorie restriction; MET = metformin; MRDT = mortality rate doubling time (years); PF–MET = pair fed to metformin.
Days.
Mean ± SD.
Last 10% survivors in each group.
Constant α in years; 95% confidence limits are given in parentheses.
Table 3.
Quantile Regression Analysis of Life Span (p value)
| Group | 25th Quantile | 50th Quantile | 75th Quantile | 90th Quantile |
| CR vs CON | 0.020 | 0.070 | 0.329 | 0.228 |
| MET vs CON | 0.219 | 0.675 | 0.849 | 0.268 |
| PF–MET vs CON | 0.169 | 0.615 | 0.990 | 0.369 |
Note: CON = control; CR = calorie restriction; MET = metformin; PF–MET = pair fed to metformin.
Table 4.
Maximum Life-Span Test Results
| Group | Sum of Scores | Expected Score | p Value (one-sided Pr < Z) |
| CR vs CON | 1,488 vs 1,068 | 1,440 vs 1,116 (56.31) | 0.1995 |
| MET vs CON | 1,457 vs 1,099 | 1,440 vs 1,116 (52.17) | 0.3759 |
| PF–MET vs CON | 1,473 vs 1,084 | 1,440 vs 1,116 (54.31) | 0.2779 |
Note: CON = control; CR = calorie restriction; MET = metformin; PF–MET = pair fed to metformin.
DISCUSSION
The search for compounds that mimic the health and longevity benefits of CR without requiring actual calorie reduction is an attractive proposition in current nutrition research. Although previous studies have suggested that metformin supplementation provides a transcriptional response highly consistent with actual CR (4), the results from this study do not support the proposition that metformin is a CRM, at least at the one dose investigated. Previous studies published over the past three decades have shown that metformin and other related biguanides can extend life span in rodent models of disease, including cancer, hypertension, and Huntington’s (23–34). However, the use of a normal, highly researched rodent model for basic life-span assessment was indicated. The previous reports of life-span extension with biguanide supplementation may be related to a reduction of early mortality associated with the disease condition in strains heavily prone to disease, especially cancer, a situation resulting in relative life-span extension compared with untreated controls. A recent study suggests that this may not be the sole explanation as life span was extended in female outbred Swiss-derived SHR mice, even without a reduction in tumor onset or incidence in the treated mice, hinting at the possibility of metformin altering aging pathways independent of disease states (24). Even so, the use of this particular rodent strain (male F344 rats) may have contributed to the lack of an observed benefit. However, no other reports are available that conclusively demonstrate life-span extension in a “normal” rodent model with metformin supplementation. Importantly, the dose of metformin used in the present study was efficacious for disease prevention and life-span extension in previous rodent studies, although it should be noted that this dose is ∼10-fold higher than the maximum daily dose used in human treatment (milligrams per kilogram body weight) (33). Recently, metformin supplementation (50 mM dose) was shown to increase the median life span (but not maximum) of Caenorhabditis elegans, although 10 or 100 mM doses showed no significant life-span benefits (43).
One limitation of the current study is the lack of a robust CR response for extension of maximum life span (Figure 4; Tables 2 and 3). CR initiated at 6 months of age in male F344 rats was previously reported to increase life span (44). Therefore, the possibility that the strain of rodent used does not possess the ability to respond to CR seems unlikely. Rather, the reduced CR response could arise from a sampling artifact resulting in a reduced significance of the life-span effect for this particular set of rats. As such, the reduced efficacy of CR in the current study might provide a partial explanation for the lack of a significant increase in life span with MET treatment. Additionally, the type of diet used in the current study (NTP-2000) was optimized for health and longevity benefits when used in AL feeding but has not yet been reported for life-span extension during CR. Comparing the observed life-span measures with previous studies, the mean and maximum life span of the AL CON group is longer, whereas the mean and maximum life span of the CR group is shorter than was previously reported (Table 2) (45,46). Although these were male F344 rats, a different supplier and diet were used in the previous report compared with the current study. Thus, there is some question as to whether the reduced protein concentration of the diet would be adequate for long-term studies in a restricted state (38).
There were two early deaths in the CR group within 3 weeks of group randomization and CR initiation (Days 20 and 21). The life-span results included these two early deaths (Tables 1–4). Censoring these two animals had no effect on the significance of the life-span comparison with the exceptions of a significant increase in the 50th quantile of life span in the CR group compared with CON and a significant increase in the mean life span of the CR group (901.6 ± 112.9 days after censoring; Table 2). When including the two early deaths in the CR group, CR still delayed early mortality compared with CON (Figure 4; Table 3—25th quantile), whereas MET did not. Therefore, any MET life span response is not as robust as CR in the current investigation. Importantly, although life span was not extended with chronic metformin supplementation, the MET group life span was also not reduced compared with CON (Figure 4; Table 3). Regarding the Gompertz α parameter, it is important to note that the small sample size makes the model susceptible to early deaths observed in the CR, MET, and PF–MET groups (Table 2). This causes the “aging rate” to appear elevated in the CON group, despite there being no significant difference in mean, maximal, or overall life span among groups (Table 2).
In addition to the dampened CR response, metformin supplementation did not significantly affect glucose/insulin levels in the current study. As such, the tested concentration may have been insufficient to trigger a full CR-like response, resulting in altered life span. Despite this, previous research using similar or lower concentrations of biguanides has reported beneficial effects (24,26,33), although the method of administration differed between studies. Additionally, the metformin concentration utilized in the diet is approximately 10 times that of the highest dose used in human treatments (33), implying that any increase necessary to observe life-span benefits is questionable for a human application.
Metformin supplementation resulted in reduced body weight, despite similar food intake, during the middle of the study (Figure 1A and B). A reduction of adipose tissue is proposed to contribute to longevity-associated benefits of CR (47). Body composition measures were not acquired in the present study, making it impossible to determine if reduced body fat percentage accompanied the reduction in weight. Future studies may benefit from the inclusion of lean and fat mass measures to more fully explore the relationship between metformin supplementation on body composition in relationship to longevity.
The four aspects of a CRM were previously outlined (13) as (i) metabolic, hormonal, and physiological effects similar to CR, (ii) no significant reduction of long-term food intake, (iii) activated stress response pathways similar to CR, and (iv) beneficial effects on longevity and reduction of age-related disease. Using these criteria to assess the effect of metformin supplementation in this study, there were no significant reductions in insulin, glucose, or CBT with metformin (Figures 2 and 3). However, the CR group was also not consistently reduced in these same measures (Figures 2 and 3). Long-term food intake was not significantly reduced with metformin supplementation, although body weight was slightly reduced (Figure 1). There were no specific measures of stress response or protection performed in the present study, and necroscopic findings were similar among groups (coded “mass”: CON-8, CR-9, and MET-11). Finally, with multiple life-span analyses, no significant life-span extension was observed with metformin supplementation, a critical component for the validation of a CRM. In contrast with previous reports using disease models in rodents and transcriptional profiling, these results challenge the proposition of metformin supplementation at the specified dosage acting as a bona fide CRM.
FUNDING
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. This work was performed under a contract from the National Institute on Aging to TherImmune Research Corporation (now GeneLogic, Gaithersburg, MD). D.L.S. was supported by T32DK062710.
DISCLOSURES
Dr. Allison has received grants, honoraria, donations, and consulting fees from numerous food, beverage, pharmaceutical companies, and other commercial and nonprofit entities with interests in obesity and receives royalties from obesity-related books. The opinions expressed herein are those of the authors and not necessarily those of the National Institutes of Health or any other organization with which the authors are affiliated.
Acknowledgments
We would like to thank Drs. Bin Zhang and Nick Pajewski for assistance with the Gompertz analysis.
References
- 1.McCay CM, Crowell MF. Prolonging the life span. Sci Mon. 1934;39:405–414. [Google Scholar]
- 2.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- 3.Weindruch R, Kayo T, Lee CK, Prolla TA. Microarray profiling of gene expression in aging and its alteration by caloric restriction in mice. J Nutr. 2001;131:918S–923S. doi: 10.1093/jn/131.3.918S. [DOI] [PubMed] [Google Scholar]
- 4.Dhahbi JM, Mote PL, Fahy GM, Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiol Genomics. 2005;23:343–350. doi: 10.1152/physiolgenomics.00069.2005. [DOI] [PubMed] [Google Scholar]
- 5.Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 6.Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C. Thomas Publisher; 1988. [Google Scholar]
- 7.Redman LM, Martin CK, Williamson DA, Ravussin E. Effect of caloric restriction in non-obese humans on physiological, psychological and behavioral outcomes. Physiol Behav. 2008;94:643–648. doi: 10.1016/j.physbeh.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke LE, Dunbar-Jacob JM, Hill MN. Compliance with cardiovascular disease prevention strategies: a review of the research. Ann Behav Med. 1997;19:239–263. doi: 10.1007/BF02892289. [DOI] [PubMed] [Google Scholar]
- 10.Knauper B, Cheema S, Rabiau M, Borten O. Self-set dieting rules: adherence and prediction of weight loss success. Appetite. 2005;44:283–288. doi: 10.1016/j.appet.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Lane MA, Ingram DK, Roth GS. 2-Deoxy-D-glucose feeding in rats mimics physiological effects of calorie restriction. J Anti Aging Med. 1998;1:327–337. [Google Scholar]
- 12.Ingram DK, Anson RM, de Cabo R, et al. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–423. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 13.Ingram DK, Zhu M, Mamczarz J, et al. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 14.Lane MA, Roth GS, Ingram DK. Caloric restriction mimetics: a novel approach for biogerontology. Methods Mol Biol. 2007;371:143–149. doi: 10.1007/978-1-59745-361-5_11. [DOI] [PubMed] [Google Scholar]
- 15.Lane MA, Ingram DK, Roth GS. The serious search for an anti-aging pill. Sci Am. 2002;287:36–41. doi: 10.1038/scientificamerican0802-36. [DOI] [PubMed] [Google Scholar]
- 16.Roth GS, Lane MA, Ingram DK. Caloric restriction mimetics: the next phase. Ann N Y Acad Sci. 2005;1057:365–371. doi: 10.1196/annals.1356.027. [DOI] [PubMed] [Google Scholar]
- 17.Spindler SR. Use of microarray biomarkers to identify longevity therapeutics. Aging Cell. 2006;5:39–50. doi: 10.1111/j.1474-9726.2006.00194.x. [DOI] [PubMed] [Google Scholar]
- 18.Higami Y, Pugh TD, Page GP, Allison DB, Prolla TA, Weindruch R. Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long-term caloric restriction. FASEB J. 2004;18:415–417. doi: 10.1096/fj.03-0678fje. [DOI] [PubMed] [Google Scholar]
- 19.Kayo T, Allison DB, Weindruch R, Prolla TA. Influences of aging and caloric restriction on the transcriptional profile of skeletal muscle from rhesus monkeys. Proc Natl Acad Sci USA. 2001;98:5093–5098. doi: 10.1073/pnas.081061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 21.Dhahbi JM, Tsuchiya T, Kim HJ, Mote PL, Spindler SR. Gene expression and physiologic responses of the heart to the initiation and withdrawal of caloric restriction. J Gerontol A Biol Sci Med Sci. 2006;61:218–231. doi: 10.1093/gerona/61.3.218. [DOI] [PubMed] [Google Scholar]
- 22.Dhahbi JM, Kim HJ, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci USA. 2004;101:5524–5529. doi: 10.1073/pnas.0305300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dilman VM, Anisimov VN. Effect of treatment with phenformin, diphenylhydantoin or L-dopa on life span and tumour incidence in C3H/Sn mice. Gerontology. 1980;26:241–246. doi: 10.1159/000212423. [DOI] [PubMed] [Google Scholar]
- 24.Anisimov VN, Berstein LM, Egormin PA, et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7:2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 25.Anisimov VN, Berstein LM, Popovich IG, et al. Central and peripheral effects of insulin/IGF-1 signaling in aging and cancer: antidiabetic drugs as geroprotectors and anticarcinogens. Ann N Y Acad Sci. 2005;1057:220–234. doi: 10.1196/annals.1356.017. [DOI] [PubMed] [Google Scholar]
- 26.Anisimov VN, Egormin PA, Bershtein LM, et al. Metformin decelerates aging and development of mammary tumors in HER-2/neu transgenic mice. Bull Exp Biol Med. 2005;139:721–723. doi: 10.1007/s10517-005-0389-9. [DOI] [PubMed] [Google Scholar]
- 27.Anisimov VN, Berstein LM, Egormin PA, et al. Effect of metformin on life span and on the development of spontaneous mammary tumors in HER-2/neu transgenic mice. Exp Gerontol. 2005;40:685–693. doi: 10.1016/j.exger.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Anisimov VN, Ukraintseva SV, Anikin IV, et al. Effects of phentermine and phenformin on biomarkers of aging in rats. Gerontology. 2005;51:19–28. doi: 10.1159/000081430. [DOI] [PubMed] [Google Scholar]
- 29.Anisimov VN, Semenchenko AV, Yashin AI. Insulin and longevity: antidiabetic biguanides as geroprotectors. Biogerontology. 2003;4:297–307. doi: 10.1023/a:1026299318315. [DOI] [PubMed] [Google Scholar]
- 30.Anisimov VN. Insulin/IGF-1 signaling pathway driving aging and cancer as a target for pharmacological intervention. Exp Gerontol. 2003;38:1041–1049. doi: 10.1016/s0531-5565(03)00169-4. [DOI] [PubMed] [Google Scholar]
- 31.Anisimov VN. [Effect of buformin and diphenylhydantoin on the life span, estrous function and spontaneous tumor incidence in rats] Vopr Onkol. 1980;26:42–48. [PubMed] [Google Scholar]
- 32.Dil'man VM, Anisimov VN. [Increase in longevity and a decrease in the frequency of tumors in C3H/Sn mice under the influence of fenformin and diphenin] Dokl Akad Nauk SSSR. 1979;245:753–757. [PubMed] [Google Scholar]
- 33.Ma TC, Buescher JL, Oatis B, et al. Metformin therapy in a transgenic mouse model of Huntington’s disease. Neurosci Lett. 2007;411:98–103. doi: 10.1016/j.neulet.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 34.Anisimov VN, Egormin PA, Piskunova TS, et al. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle. 2010;9:188–197. doi: 10.4161/cc.9.1.10407. [DOI] [PubMed] [Google Scholar]
- 35.Radziuk J, Bailey CJ, Wiernsperger NF, Yudkin JS. Metformin and its liver targets in the treatment of type 2 diabetes. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:151–169. doi: 10.2174/1568008033340298. [DOI] [PubMed] [Google Scholar]
- 36.Roth GS, Ingram DK, Lane MA. Caloric restriction in primates and relevance to humans. Ann N Y Acad Sci. 2001;928:305–315. doi: 10.1111/j.1749-6632.2001.tb05660.x. [DOI] [PubMed] [Google Scholar]
- 37.Longo VD, Fabrizio P. Regulation of longevity and stress resistance: a molecular strategy conserved from yeast to humans? Cell Mol Life Sci. 2002;59:903–908. doi: 10.1007/s00018-002-8477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao GN. New diet (NTP-2000) for rats in the National Toxicology Program toxicity and carcinogenicity studies. Fundam Appl Toxicol. 1996;32:102–108. doi: 10.1006/faat.1996.0112. [DOI] [PubMed] [Google Scholar]
- 39.Gao G, Wan W, Zhang S, Redden DT, Allison DB. Testing for differences in distribution tails to test for differences in “maximum” lifespan. BMC Med Res Methodol. 2008;8:49. doi: 10.1186/1471-2288-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redden DT, Fernandez JR, Allison DB. A simple significance test for quantile regression. Stat Med. 2004;23:2587–2597. doi: 10.1002/sim.1839. [DOI] [PubMed] [Google Scholar]
- 41.Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on “maximum lifespan. Mech Ageing Dev. 2004;125:629–632. doi: 10.1016/j.mad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 42.de Magalhaes JP, Cabral JA, Magalhaes D. The influence of genes on the aging process of mice: a statistical assessment of the genetics of aging. Genetics. 2005;169:265–274. doi: 10.1534/genetics.104.032292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Onken B, Driscoll M. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5:e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40:657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 45.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 46.Holmes DJ. Experimental rodent strains. Sci Aging Knowledge Environ. 2003;36:as2. [Google Scholar]
- 47.Muzumdar R, Allison DB, Huffman DM, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]