Abstract
Background: Conjugated linoleic acid (CLA) is a supplemental dietary fatty acid that decreases fat mass accretion in young animals.
Objective: The aim of this study was to determine CLA's efficacy with regard to change in fat and body mass index (BMI; in kg/m2) in children.
Design: We conducted a 7 ± 0.5-mo randomized, double-blind, placebo-controlled trial of CLA in 62 prepubertal children aged 6–10 y who were overweight or obese but otherwise healthy. The subjects were randomly assigned to receive 3 g/d of 80% CLA (50:50 cis-9,trans-11 and trans-10,cis-12 isomers) or placebo in chocolate milk.
Results: Fifty-three subjects completed the trial (n = 28 in the CLA group, n = 25 in the placebo group). CLA attenuated the increase in BMI (0.5 ± 0.8) compared with placebo (1.1 ± 1.1) (P = 0.05). The percentage change in body fat measured by dual-energy X-ray absorptiometry was smaller (P = 0.001) in the CLA group (−0.5 ± 2.1%) than in the placebo group (1.3 ± 1.8%). The change in abdominal body fat as a percentage of total body weight was smaller (P = 0.02) in the CLA group (−0.09 ± 0.9%) than in the placebo group (0.43 ± 0.6%). There were no significant changes in plasma glucose, insulin, or LDL cholesterol between groups. Plasma HDL cholesterol decreased significantly more (P = 0.05) in the CLA group (−5.1 ± 7.3 mg/dL) than in the placebo group (−0.7 ± 8 mg/dL). Bone mineral accretion was lower (P = 0.04) in the CLA group (0.05 ± 0.03 kg) than in the placebo group (0.07 ± 0.03 kg). Reported gastrointestinal symptoms did not differ significantly between groups.
Conclusions: CLA supplementation for 7 ± 0.5 mo decreased body fatness in 6–10-y-old children who were overweight or obese but did not improve plasma lipids or glucose and decreased HDL more than in the placebo group. Long-term investigation of the safety and efficacy of CLA supplementation in children is recommended.
INTRODUCTION
Childhood obesity rates in the United States have been on the rise since 1960. In 2003–2006, 17% of all 6–11-y-old children in the United States were considered obese, an increase from 15% in 1999–2000 (1). Obesity during childhood is associated with a high probability of obesity in adulthood (1–4) and is also associated with a large array of health problems later in life, including an increased risk of coronary heart disease (5), hypertension, and diabetes (6).
Whereas much research is being performed to identify the numerous factors that contribute to childhood as well as adult obesity, a great deal is also being done to try to reverse the increasing trends of weight gain and obesity. Of these is the use of conjugated linoleic acid (CLA) as an oral supplement and food ingredient. As recently reviewed, CLA supplementation has shown a beneficial effect on percentage body fat in healthy adults (7). The meta-analysis of the large number of adult studies found that the weight reduction observed during CLA supplementation consisted largely of fat and was dose dependent. The total weight loss was also relatively modest, reaching a group average of slightly more than 2 kg after treatments of ≥1 y (7).
CLA is a naturally occurring polyunsaturated 18-carbon compound most commonly present in dairy and beef products, but it is also found in other ruminant and nonruminant sources. It is naturally synthesized from linoleic acid by ruminant animals (8); however, human dietary intake from these sources is estimated at 0.1–1.1% of ruminant animal fat leading to possible total intakes of 130–440 mg/d (9). For larger doses, CLA can be commercially prepared from plant oils containing high amounts of linoleic acid. There are many CLA isomers. The most common naturally occurring in dairy fat and meat from ruminant animals is the cis-9, trans-11(c9,t11) isomer (9). The cis-9, trans-11 and trans-10, cis-12 (t10,c12) can also be commercially synthesized from linoleic acid–rich oils such as sunflower and safflower oil (10). These are considered the most physiologically active isomers, and the 50:50 mixture has been shown to be the most effective for weight management or body fat reduction (11). The first studies to identify the antiadiposity characteristics of CLA were performed in laboratory animals (12). The t10,c12 isomer was found to be more effective as an antiadiposity agent, but this isomer caused a slight increase in insulin resistance (13), which generally was not observed when both the c9,t11 and t10,c12 isomers were given together (14).
Not only did the studies in laboratory animals show the efficacy of CLA for fat loss, they also showed that the antiadiposity characteristics of CLA were stronger in young animals during growth than in mature animals (15, 16). Because of this, it is important to perform similar efficacy trials in children.
The hypothesis of the current study was that a 50–50 mixture of c9,t11 and t10,c12 CLA supplementation would improve measures of body mass index (BMI) and body composition in overweight and obese children in a randomized placebo-controlled trial. The aims of this study also included assessment of safety, including blood chemistry tests, and generalized well-being.
SUBJECTS AND METHODS
Subjects
Subjects were recruited from the Madison metropolitan area largely through recruitment flyers. Eligible participants were 6– 10-y-old children with a BMI at or above the 85th percentile (17) at the time of screening. The exclusion criteria included Tanner stage 2 and beyond, any history of metabolic disease, excessive fear of a blood draw, claustrophobia, and an extreme dislike of the taste of the treatment delivery beverage. Subjects were also excluded if their fasting blood chemistry values at screening exceeded the following: glucose >110 mg/dL, insulin >45 μIU/mL, LDL >160 mg/dL, total cholesterol >240 mg/dL, triglycerides >200 mg/dL, aspartate aminotransferase (AST) >40 U/L and >50 U/L (males age 10 y), alanine aminotransferase (ALT) >65 U/L, and γ-glutamyl transferase (GGT) >30 U/L. The study was approved by the Health Sciences Institutional Review Board of the University of Wisconsin–Madison. The parents or guardians signed an informed consent form, and the subjects signed a minor assent form before screening.
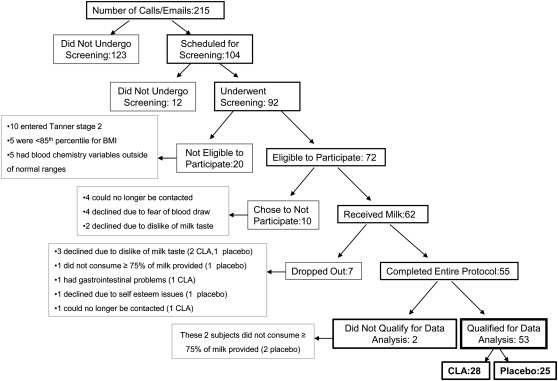
The parents of 92 subjects indicated a willingness to participate, and the children were screened for eligibility. Of the 92 screened subjects, 72 were eligible to participate. Of these, 62 (36 boys and 26 girls) agreed to participate and were randomly assigned to receive CLA or placebo (sunflower oil) (Figure 1). Randomized was achieved by using a stratified design according to sex (male or female), BMI percentile (<95th or ≥95th percentile), and then age (< 9.5 or ≥9.5 y) (Table 1). Criteria for completion included consumption of 75% of the milk provided and return for outcome measures scheduled at follow-up.
FIGURE 1.
Flow chart of subject enrollment and completion. CLA, conjugated linoleic acid.
TABLE 1.
Stratification of subjects at entry1
| <95th percentile of BMI |
≥95th percentile of BMI |
|||
| <9.5 y | ≥9.5 y | <9.5 y | ≥9.5 y | |
| Males (n) | ||||
| CLA | 3 | 3 | 8 | 4 |
| Placebo | 2 | 4 | 8 | 4 |
| Females (n) | ||||
| CLA | 4 | 1 | 7 | 2 |
| Placebo | 5 | 1 | 6 | 0 |
Total n = 62. All subjects were at or above the 85th percentile for BMI. CLA, conjugated linoleic acid.
Protocol
All procedures followed were in accordance with the ethical standards of the University of Wisconsin Health Sciences Institutional Review Board. During screening, BMI was calculated to determine eligibility. The subjects' parents or guardians provided a medical history, and subjects underwent a physical examination during a meeting with a University of Wisconsin Hospital pediatric endocrinologist, including the assessment of Tanner stage. A short nutrition education session was held with a registered dietitian. This session included describing a food guide pyramid, making healthy choices, trying new foods, and presenting ideas to parents on including children in the preparation of healthy meals.
The subjects were then seen at the Exercise Science Laboratory of the University of Wisconsin FitKids Clinic at Sports Medicine for body-composition measurements within 2 wk of passing screening. Body composition was measured by dual-energy X-ray absorptiometry (DXA). A baseline symptoms questionnaire was completed by the subjects' parents. A 3-mo supply of milk was distributed at this time with instructions for the child to drink one entire serving each day under parental supervision and instructions that only the child may consume the milk provided. A fasting blood sample was collected at the University of Wisconsin Hospital within 1 wk of the body-composition visit, and the subjects were informed to begin consumption of the milk after the blood draw. At 3 mo, the parents reported the amount of milk remaining to monitor compliance and were provided with an additional 3-mo supply. At 6 mo, the parents reported the amount of milk remaining and were scheduled for follow-up body-composition testing and survey completion and were instructed to return to the University of Wisconsin Hospital for a follow-up fasting blood draw. The parents were provided with enough milk for the child to consume until both body composition and the blood draw were completed. The parents were later contacted to again report the amount of milk remaining.
Both the CLA and placebo were added to skim milk to produce a 250-g chocolate milk beverage containing 1.4% fat and 183 kcal/serving. The CLA treatment milk had 3 g Clarinol (Lipid Nutrition BV, Wormerveer, Netherlands) added per serving. Clarinol contains 80% CLA manufactured from safflower oil and consists of a 50:50 mix of c9,t11 and t10,c12 isomers in triglyceride form, which resulted in 2.4 g active CLA per serving. The remaining 20% (0.6 g) consists of 14% linoleic acid, 4% palmitic acid, and 1% stearic acid. The placebo milk had 3 g sunflower oil added per serving, provided by Lipid Nutrition. The milk was packaged by Tetra Pak, Inc (Denton, TX). Fatty acid profiles were determined by the International Organization for Standardization method 15304 for determination of trans fatty acid isomers in animal and vegetable fats and oils. This method uses fatty acid methyl esterification followed by gas chromatography and is approved by the American Oil Chemists' Society.
Weight, height, and body composition
Weight, height, and body composition were measured within 2 wk of screening for pretreatment testing and at ≈7 mo for posttreatment testing while the subjects were still consuming the treatment. All measurements were made under standardized conditions by the same investigators. All participants were asked to void and defecate before beginning the procedures. Height used in the statistical analysis was measured with a wall-mounted stadiometer to the nearest 0.5 cm. Weight used for the DXA scan calculations and statistical analysis was measured with a calibrated beam balance platform scale to the nearest 0.1 kg while the subjects were wearing only a swim suit or T-shirt and gym shorts.
Body composition was measured with a Norland XR-36 Bone Densitometer (Norland Corporation, Fort Atkinson, WI), and tissue masses were analyzed by using host software version 3.7.4/2.1.0. On the basis of 18 scans in 6 subjects made by using XR-36 whole-body procedures, the total-body CVs were as follows: soft tissue mass, 0.2%; total body mass, 0.2%; lean body mass, 1.0%; fat mass (FM), 2.5%; percentage fat, 2.4%; and total BMC, 0.9%. The XR-36 uses the following calibration standards for measuring bone, fat, and lean mass from X-ray attenuation: bone, hydroxyapatite; fat, stearic acid; and lean mass, 0.6% NaCl in water. All body regions (head, trunk, abdomen, arms, and legs) were set manually after completion of the scan by the same trained investigator using predetermined bone landmarks for each subject. All subjects were scanned in the supine position. The subjects removed metal objects or clothing containing metal components and wore only a swim suit or gym shorts and a T-shirt for the scan procedure. The DXA-derived body density was converted to percentage body fat by using the Lohman equation. This calculation accounts for the physiologic differences in the water component of fat-free mass (FFM) in children, which changes with age and differs between sexes (18). This was calculated by using body density as measured by DXA and an adjustment on constants defined by Lohman (18). These constants are based on age (in y), and, because some of the subjects had birthdays between the baseline and follow-up measures, the constant that would apply to them changed. This approach creates a stepwise, nonlinear change with age, which is physiologically unlikely. To account for this, we used the linear relation between age and Lohman's constants and interpolated them to find the constant for follow-up measures based on their age at baseline and time elapsed by the time of follow-up. Percentage FFM was calculated as 100 minus percentage body fat. Fat and FFM were calculated as the percentages/100 multiplied by body weight. Abdominal and peripheral fat percentages were calculated as measured FM for those regions divided by total body mass multiplied by 100. DXA was also used to assess bone mineral content (BMC).
Safety
Fasting blood samples were collected within 1 wk of the first body-composition visit (before treatment began) and within 1 wk of the follow-up body-composition visit (during treatment). Fasting glucose and insulin, LDL cholesterol, HDL cholesterol, total cholesterol, triglycerides, AST, ALT, and GGT were measured in the samples. A pediatric endocrinologist reviewed all of the laboratory results and compared them with acceptable ranges as listed above.
Symptoms
The subjects' parents or guardians were asked to complete a health symptoms survey at the first body-composition visit and at the follow-up body-composition visit, which described their child's gastrointestinal and general health symptoms. They were asked to describe the incidence and intensity of their child's nausea, stomach pain, vomiting, diarrhea, constipation, heartburn, bloating, loss or increase of appetite, cold or flu, and any other health symptom over the previous 2 mo.
Statistics
The primary outcome variable was the change in percentage body fat as measured by DXA. A power analysis assuming an effect size of 2.3% fat, an SD of 4.4% fat, and a dropout rate of 25% indicated that 56 subjects completing the trial would provide a power of 80% and an α of 5%. Data from noncompliant participants were removed. All variables were tested for normality by using the Shapiro-Wilk test in Number Crunching Statistical System 2007 software, and none were different from a normal distribution. Next, the change scores for all variables were tested for outliers (measures beyond 3 SDs from the mean), which were removed from further analysis: BMC, head excluded (CLA, n = 4; placebo, n = 2), percentage peripheral fat (CLA, n = 1), and percentage abdominal fat (placebo, n = 2). Then, the between-group change was tested with a 2-tailed t test for comparison of intervention differences in BMI (in kg/m2), BMI z score, body-composition variables, and blood chemistry values; within-group changes were tested with a paired t test. This study was designed with an outcome analysis to be performed for subjects completing the trial with ≥75% milk compliance. Because of difficulties scheduling around the holidays and family schedules, treatment duration varied (average: 7.0 ± 0.5 mo). Thus, an analysis of covariance was performed to determine whether treatment duration had a significant effect on any outcome variables. No significant effects were found. In addition, age and sex had no significant effect as covariates. There was no main effect or interaction of sex with regard to treatment outcomes. Further statistical analyses were also performed on an intent-to-treat basis, with the last measure (baseline) carried forward (n = 62). Values are presented as means ± SDs. A P value ≤0.05 was used to identify statistical significance.
RESULTS
Subjects
There were 55 subjects who completed the study, 53 of whom are included in this data analysis (n = 28 CLA, n = 25 placebo). The 2 subjects who were not included in the data analysis did not meet the compliance rate of 75% treatment consumption. The 7 subjects who did not complete this study included 3 who grew to dislike the milk delivery vehicle, 1 who did not consume 75% of the milk in the first 3 mo, 1 who had gastrointestinal problems, 1 who left the study because of self-esteem issues, and 1 who could no longer be contacted (Figure 1).
Anthropometric measures and body composition
Baseline subject characteristics are presented in Table 2. In females, height was significantly different between groups at baseline (CLA: 136 ± 7 cm; placebo: 130 ± 3 cm; P = 0.01); however, when indexed with weight as BMI, there were no significant differences. In males, there were no significant differences in height or BMI between groups at baseline. In addition, there were no differences between either sex at baseline in weight, BMI percentile, or BMI z score. Females in the CLA group tended to be older (CLA: 8.6 ± 0.8 y; placebo: 8.1 ± 0.6 y; P = 0.09). There were no significant differences in body composition at baseline between groups for either sex, and there were no significant differences between treatment groups for any characteristics when sexes were combined.
TABLE 2.
Baseline characteristics of the subjects at entry1
| CLA |
Placebo |
|||
| Males | Females | Males | Females | |
| Race-ethnicity (n) | ||||
| White | 12 | 11 | 13 | 8 |
| Black | 2 | — | — | 2 |
| Asian | 1 | 1 | 2 | — |
| Hispanic | 1 | — | — | — |
| No. of subjects | 16 | 12 | 15 | 10 |
| Age (y) | 8.8 ± 1.32 | 8.6 ± 0.8 | 9.3 ± 0.8 | 8.1 ± 0.6 |
| Weight (kg) | 45.6 ± 16.5 | 43.8 ± 8.7 | 42.9 ± 6.8 | 38.1 ± 7.1 |
| Height (cm) | 139 ± 8 | 136 ± 73 | 140 ± 8 | 130 ± 3 |
| BMI (kg/m2) | 23.0 ± 5.7 | 23.3 ± 2.8 | 21.8 ± 1.9 | 22.5 ± 3.4 |
| BMI (%)4 | 95.1 ± 3.4 | 96.1 ± 2.8 | 94.6 ± 3.2 | 94.6 ± 5.2 |
| BMI z score | 1.7 ± 0.5 | 2.0 ± 0.4 | 1.5 ± 0.4 | 1.9 ± 0.5 |
CLA, conjugated linoleic acid.
Mean ± SD (all such values).
Significantly different from placebo within the same sex, P ≤ 0.05.
BMI percentile based on Centers for Disease Control and Prevention growth charts (19).
The increase in height from baseline to follow-up did not differ between the CLA (3 ± 1 cm) and placebo (3 ± 2 cm) (P = 0.3, Table 3) groups, nor did body weight gain (CLA: 3.2 ± 1.9 kg, placebo: 3.7 ± 2.3 kg; P = 0.4; Table 3). The increase in BMI in the CLA group (0.5 ± 0.8) was smaller than that in the placebo group (1.1 ± 1.1; P = 0.05; Table 3). When expressed as BMI z score, the value trended numerically downward in the CLA group (−0.03 ± 0.16) but was not statistically different from that of the placebo group (0.05 ± 0.23) (P = 0.2; Table 3).
TABLE 3.
Changes in anthropometric and body-composition measures from baseline to follow-up1
| CLA (n = 28) |
Placebo (n = 25) |
P for between-group change | |||||
| Month 0 | Follow-up | Change | Month 0 | Follow-up | Change | ||
| Height (cm) | 138 ± 8 | 141 ± 8 | 3 ± 12 | 136 ± 8 | 139 ± 7 | 3 ± 22 | 0.2 (NS) |
| Body weight (kg) | 45 ± 14 | 48 ± 14 | 3.2 ± 1.92 | 41 ± 7.2 | 45 ± 7.8 | 3.7 ± 2.32 | 0.4 (NS) |
| BMI (kg/m2) | 23.3 ± 4.6 | 23.8 ± 4.7 | 0.5 ± 0.82 | 22.6 ± 5.3 | 23.3 ± 2.9 | 1.1 ± 1.12 | 0.04 |
| BMI z score | 1.83 ± 0.48 | 1.81 ± 0.49 | −0.03 ± 0.16 | 1.70 ± 0.47 | 1.75 ± 0.50 | 0.05 ± 0.23 | 0.2 (NS) |
| Fat mass (kg) | 15.1 ± 7.0 | 16.0 ± 7.3 | 0.8 ± 1.42 | 13.0 ± 3.8 | 14.7 ± 4.3 | 1.8 ± 1.32 | 0.01 |
| Body fat (%) | 32.7 ± 5.0 | 32.2 ± 5.0 | −0.5 ± 2.1 | 31.1 ± 6.0 | 32.4 ± 6.0 | 1.3 ± 1.82 | 0.001 |
| Fat-free mass (kg) | 29.7 ± 6.9 | 32.1 ± 7.1 | 2.4 ± 1.22 | 28.3 ± 4.6 | 30.0 ± 4.8 | 1.9 ± 1.32 | 0.2 (NS) |
| Fat-free mass (%) | 67.3 ± 5.0 | 67.8 ± 5.0 | 0.5 ± 2.1 | 68.9 ± 6.0 | 67.6 ± 6.0 | −1.3 ± 1.82 | 0.001 |
| Peripheral fat (%) | 22.8 ± 2.3 | 21.8 ± 2.6 | −1.2 ± 1.02 | 21.7 ± 3.2 | 22.2 ± 3.1 | 0.5 ± 1.22 | <0.001 |
| Abdominal fat (%) | 9.1 ± 1.4 | 9.1 ± 1.4 | −0.1 ± 0.9 | 8.8 ± 1.9 | 9.1 ± 1.7 | 0.4 ± 0.63 | 0.02 |
| BMC, head excluded (kg) | 1.24 ± 0.23 | 1.28 ± 0.25 | 0.05 ± 0.03 | 1.17 ± 0.19 | 1.26 ± 0.20 | 0.07 ± 0.032 | 0.04 |
All values are means ± SDs. Subjects are those who completed follow-up measures with outliers beyond 3 SD from the mean removed. CLA, conjugated linoleic acid; BMC, bone mineral content.
Significant within-group change comparison: 2P ≤ 0.05, 3P ≤ 0.01.
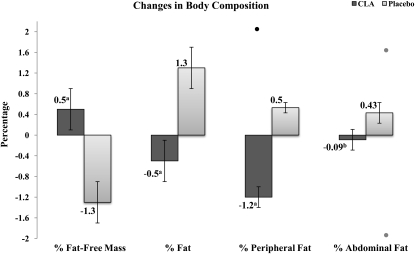
The increase in body FM, as measured by DXA, was smaller in the CLA group (0.8 ± 1.4 kg) than in the placebo group (1.8 ± 1.3 kg) (P = 0.01; Table 3). When taken as a percentage of total body weight, percentage body fat in the CLA group (−0.5 ± 2.1%) decreased numerically from baseline and differed significantly from that in the placebo (1.3 ± 1.8%; P = 0.001; Table 3; Figure 2). Abdominal body fat as a percentage of total body weight decreased numerically from baseline in the CLA group (−0.1 ± 0.9%) and differed significantly from that in the placebo group (0.4 ± 0.6%; P = 0.02; Table 3; Figure 2). Peripheral fat (including arms and legs) as a percentage of total body weight decreased from baseline in the CLA group (−1.2 ± 1.0%) and differed significantly from that in the placebo group (0.5 ± 1.2%; P ≤ 0.001; Table 3; Figure 2). There was a difference in change in BMC, excluding the head, during treatment as measured by DXA between the CLA (0.05 ± 0.03 kg) and placebo (0.07 ± 0.03 kg) groups (P = 0.04; Table 3). There was a trend, although not statistically significant, toward smaller increases in height-adjusted BMC (inferred bone strength) as measured by BMC/height in the CLA group (0.01 ± 0.09 g/mm) compared with placebo (0.04 ± 0.03 g/mm) (P = 0.09) (20).
FIGURE 2.
Mean (±SE) changes in body composition after 7 mo of treatment with 3 g/d of an 80% conjugated linoleic acid (CLA) 50:50 mixture of cis-9,trans-11 and trans-10,cis-12 isomers in triglyceride form compared with a sunflower-oil placebo. Results are expressed as the percentage of body weight, and the differences were determined by a 2-tailed t test. Includes subjects who completed follow-up measures with outliers beyond 3 SD from the mean removed. n = 27–28 in the CLA group, and n = 23–25 in the placebo group. Excluded outliers are indicated as data points above or below the bars. a,bSignificant between-group comparison: aP ≤ 0.001,bP ≤ 0.05.
Safety
There were no significant differences between groups in any of the blood chemistry variables at baseline (Table 4). After treatment, the only significant change was a decrease from baseline in HDL cholesterol in the CLA group, a change that also differed significantly from the placebo group (Table 4; P = 0.05).
TABLE 4.
Changes in blood chemistry values from baseline to follow-up1
| CLA (n = 27) |
Placebo (n = 25) |
||||||
| Variable | Month 0 | 6-mo Follow-up | Change | Month 0 | 6-mo Follow-up | Change | P |
| Glucose (mg/dL) | 90.3 ± 5.7 | 92.0 ± 5.2 | 1.8 ± 5.2 | 90.2 ± 4.63 | 90.6 ± 5.0 | 0.4 ± 3.9 | 0.3 |
| Insulin (μIU/mL) | 8.9 ± 7.1 | 9.5 ± 5.4 | 0.6 ± 4.4 | 9.4 ± 4.4 | 9.2 ± 4.8 | 0.04 ± 6.7 | 0.6 |
| LDL cholesterol (mg/dL) | 97.6 ± 25 | 103.9 ± 32 | 6.0 ± 19 | 97.2 ± 21 | 96.9 ± 29 | −0.3 ± 18 | 0.4 |
| HDL cholesterol (mg/dL) | 54.1 ± 11.6 | 49.0 ± 9.2 | −5.1 ± 7.32 | 53.6 ± 13.9 | 53.0 ± 13.2 | −0.7 ± 8.2 | 0.05 |
| Total cholesterol (mg/dL) | 164.7 ± 29.7 | 170.0 ± 34.1 | 5.3 ± 23 | 164.1 ± 26.7 | 164.8 ± 28.4 | 0.7 ± 18.2 | 0.7 |
| Triglycerides (mg/dL) | 63.6 ± 37 | 83.4 ± 45 | 19.8 ± 40 | 67.2 ± 39 | 76.5 ± 44 | 8.2 ± 34 | 0.3 |
| AST (U/L) | 26.8 ± 4.2 | 27.4 ± 7.3 | 0.2 ± 7.6 | 25.5 ± 3.63 | 25.0 ± 4.6 | −0.4 ± 2.7 | 0.8 |
| ALT (U/L) | 35.0 ± 4.1 | 37.3 ± 15.2 | 2.4 ± 16 | 34.2 ± 5.12 | 35.6 ± 8.0 | 1.4 ± 5.8 | 0.9 |
| GGT (U/L) | 16.3 ± 3.2 | 18.3 ± 4.4 | 2.1 ± 3.9 | 18.2 ± 4.0 | 19.0 ± 4.8 | 0.8 ± 4.2 | 0.3 |
| HOMA-IR | 2.0 ± 1.6 | 2.1 ± 1.2 | 0.2 ± 0.9 | 2.2 ± 1.0 | 2.1 ± 1.1 | −0.02 ± 2 | 0.4 |
All values are means ± SDs. One subject did not complete the follow-up laboratory tests; one subject in the conjugated linoleic acid (CLA) group did not have results for follow-up glucose or aspartate aminotransferase (AST) because of lysed cells. ALT, alanine aminotransferase; GGT, γ-glutamyltransferase; HOMA-IR, homeostasis model assessment of insulin resistance.
Significant between-group change comparison, P ≤ 0.05.
At follow-up, 3 subjects in the CLA treatment group had blood chemistry variables outside the normal ranges. One 9-y-old girl had an increase in AST (25–59 U/L) and ALT (34–112 U/L). One 10-y-old girl had a small increase in GGT (22–32 U/L). Finally, an 8-y-old boy had a total cholesterol concentration of 244 mg/dL at follow-up, but this reflected only a small change from the baseline value of 240 mg/dL. No subjects in the placebo group had follow-up blood chemistry values outside the normal range.
The reported general health and gastrointestinal symptoms did not differ between groups at baseline, and there was no difference in the change at follow-up. There were 22 reports of gastrointestinal symptoms at baseline and 29 at follow-up in the CLA group. In the placebo group, there were 14 reports at baseline and 17 reports at the follow-up visit.
Intent-to-treat analysis
The above analyses were completed by using inclusion criteria; however, the differences were also analyzed by intent-to-treat with the last measure carried forward. Thus, there was zero change for those who did not return for the 6-mo measurements. The CLA treatment outcomes that remained significant based on intent-to-treat were FM (P = 0.02) and percentage body fat (P = 0.002)
DISCUSSION
Our results indicate that CLA supplementation has an effect on fat gain during prepubertal growth. Compared with placebo, CLA significantly reduced total body fat gain in a 7-mo trial and regionally reduced the percentage of abdominal fat and peripheral fat as a percentage of total body weight. BMI increased less than control during the supplementation period, although the BMI z score did not.
In the current study, as indicated above, CLA was associated with a smaller rate of increase in BMI than was placebo. The change in BMI observed, however, was still numerically greater than the BMI value of 0.3 expected in 7 mo for both groups based on the 50th percentile of the 2001 National Center for Health Statistics growth charts (19), but was numerically less than the 95th percentile increase of 0.7 in 7 mo for the CLA group. Weight changes were not different between treatments, but both were similar to the 3- and 4-kg gains expected for the 95th percentile of this age group of boys and girls, respectively. This is still greater than the 2–2.5 kg expected at the 50th percentile (19). Thus, although CLA had a positive effect on the increase in BMI, the rates of increase did not appear to return to the 50th percentile averages for age. Results from individual studies of CLA supplementation in adults have been mixed with respect to whether or not CLA causes fat loss. Our recent meta-analysis, however, found that when taken as 50–50 mixture of c9,t11 and t10,c12 isomers, CLA decreased FM by 0.09 g/wk compared with placebo (7). A net FM loss, however, may not be an expectation in children of this age because, unlike adults, they are still growing. For example, in growing animals, CLA treatment has produced dramatic results, not in terms of loss of FM, but rather in terms of decreased accretion of FM. In the rat model, treatment with CLA as 2% of daily feed for 42 d resulted in a final body fat thickness of 18% less compared with placebo (21). Infant pigs supplemented with 1% CLA had a decrease in body fat accretion compared with no supplementation when given in either a low-fat (−3%) or a high-fat (25%) diet (16). Growing pigs had a dose-dependent lower back-fat thickness after 3 wk of supplementation (15). For dose comparison, we estimated the CLA treatment as 0.6% of dry matter in the current study based on prescribing 3 g/d of 80% CLA oil to children and assuming an intake of ≈2300 kcal/d from the estimated energy requirement prediction equations developed by the Institute of Medicine (22) and typical US macronutrient composition. It is not known whether the initiation of CLA treatment in younger children before excess weight gain and continuing throughout childhood would have the same effects on adiposity, as in animal models, or whether the effect would asymptotically approach a modest 2-kg effect as reported in adults (7). On the basis of the greater increase in FFM observed in our current pediatric study than observed in adults, it would appear that the effects of CLA on pediatric body composition may differ from those observed in adults (23). Longer-term studies, however, are needed in children to test this.
On the basis of the outcomes of many human studies, CLA was recently granted generally recognized as safe (GRAS) status in the United States (GRN no. 232; www.cfsan.fda.gov). During clinical trials, no severe adverse events have been reported. In some trials, a small incidence in minor gastrointestinal distress has been reported. Some of the shorter clinical studies using single isomer supplementation found increases in insulin resistance (13, 24). Several have reported a decrease in HDL cholesterol (13, 25–27).
CLA supplementation was relatively well tolerated in this study, which agrees with the study by Moya (28). One subject stopped taking the CLA supplement because of gastrointestinal distress; however, it was reported that this subject concurrently had influenza. Similar to this only other CLA trial in children (28), we found no change in fasting glucose, insulin, or homeostasis model assessment of insulin resistance. There has been only one report in adults of significant increases in insulin resistance in response to a 50:50 mixture of c9,t11 and t10,c12 (24). This study, however, was performed in subjects who already had a diagnosis of type 2 diabetes.
We did find that subjects taking CLA had a decrease from baseline in HDL, which was also significantly different from the change in subjects taking placebo. This negative finding is the only such report in children, but other studies in adults also reported a greater decrease in HDL cholesterol in the treatment group than in the placebo group (13, 25–27). Conversely, other studies found no decreases in HDL cholesterol with CLA supplementation (24, 29–31). The increases in insulin, LDL cholesterol, and total cholesterol in the CLA group at follow-up, although not statistically significant, were a little surprising in light of the reduction in percentage fat in the CLA group. The CLA-associated fat losses in this study, however, were smaller than those associated with the 7% body weight loss recommended to improve biomarkers of chronic disease risk (32). Our results with respect to chronic disease risk biomarkers were similar to those reported in many adult studies (33). Any longer-term studies of the efficacy of CLA in children conducted in the future should include more frequent measures of fasting glucose and insulin, liver function enzymes, lipid variables, BMC, BMI, and body composition.
Last, we found a slight trend toward a lower BMC accrual in the CLA group. This increase in BMC of 51 g in 7 ± 0.5 mo for the CLA treatment group is still typical for children of this age (34). The lesser rate of BMC increase in the CLA group is surprising and has not been seen in other studies, which tend to show no effect or small positive effects in animal studies (35). Studies in adults have also generally shown no effect of CLA on variables of bone or bone markers (36). We suggest that BMC be monitored in any future pediatric studies of CLA to resolve this issue.
Our study design had some limitations. For example, we recruited only overweight and obese children and thus did not obtain any data on the prevention of obesity in an average population. Consequently, we could not test a hypothesis of obesity prevention that has been shown in growing animal models. Because we did not determine Tanner stage at follow-up, we could not determine whether puberty-related changes in body composition might have influenced our results. An intent-to-treat analysis may have unusual effects in a pediatric study in terms of body composition compared with adults. In a group of adults, a follow-up measure of BMI, weight, height, and FM could reasonably result in zero change; however, in a group of growing children, it is reasonable to expect that any of these measures increase with time, and thus an intent-to-treat analysis in children is not as conservative an analysis as it is in adults. Specifically, the extrapolation of zero change for the intent-to-treat analysis may result in an unintended beneficial effect in a pediatric trial. In this regard, however, of the 8 subjects who did not complete follow-up, half were from the CLA group and the other half were from the placebo group, which should have minimized any possible dropout effect on the intent-to-treat outcome. In a similar vein, after randomization, the CLA group started with greater adiposity and, thus, some of the changes we observed could have been a result of a regression toward the mean. Finally, whereas the changes we observed were statistically significant, the question regarding clinical significance still remains. Because of this, a longer trial needs to be done to determine the maximal effect of CLA on body composition (7) and to further investigate the safety of its long-term use as a food ingredient or supplement as well as the long-term effects of CLA treatment on body weight and composition in children.
In conclusion, the CLA supplement was well tolerated, and no serious adverse events were reported; however, there was an adverse decrease in HDL and a small lowering of the accretion rate of BMC associated with CLA. Thus, safety and longer-term efficacy remain to be determined. Supplementation with 80% pure CLA containing equal amounts of c9,t11 and t10,c12 CLA was effective at reducing body fat accretion and percentage body fat in the first placebo-controlled trial of prepubescent overweight and obese children.
Acknowledgments
We thank the many members of the staff of the University of Wisconsin–Madison Sports Medicine FitKids Clinic and the Clinical and Translational Research Core for their invaluable support during clinic visits. We also thank Zhumin Zhang and Chad Cook of the University of Wisconsin for statistical support.
The authors' responsibilities were as follows—DAS and MO: conceived the study; DAS, MO, ACW, ALC, and DBA: participated in the study design; NMR, ACW, and ARO: participated in subject recruitment; NMR, ACW, ARO, and RRC: participated in data collection; JJM, ALC, and DBA: provided medical evaluation; DAS and NMR: participated in data analysis; NMR: wrote the first draft of the manuscript; and DAS, ACW, ALC, DBA, JJM, RRC, ARO, MO, and CES: revised the manuscript. MO and CES are employees of Lipid Nutrition, BV, Wormerveer, Netherlands, the manufacturer of the CLA used in this study. DAS has been named on a use patent application for CLA submitted by Lipid Nutrition. DAS, NMR, ACW, ALC, DBA, JJM, RRC, and ARO have had a portion of their salaries paid from the grant provided by Lipid Nutrition in proportion to the amount of effort each put toward designing, conducting, and documenting this study.
REFERENCES
- 1.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006. JAMA 2008;299:2401–5 [DOI] [PubMed] [Google Scholar]
- 2.Hartz AJ, Rimm AA. Natural history of obesity in 6,946 women between 50 and 59 years of age. Am J Public Health 1980;70:385–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham S, Collins G, Nordsieck M. Relationship of childhood weight status to morbidity in adults. HSMHA Health Rep 1971;86:273–84 [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham S, Nordsieck M. Relationship of excess weight in children and adults. Public Health Rep 1960;75:263–73 [PMC free article] [PubMed] [Google Scholar]
- 5.Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 2007;357:2329–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mossberg HO. 40-year follow-up of overweight children. Lancet 1989;2:491–3 [DOI] [PubMed] [Google Scholar]
- 7.Whigham LD, Watras AC, Schoeller DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr 2007;85:1203–11 [DOI] [PubMed] [Google Scholar]
- 8.Kepler CR, Hirons KP, McNeill JJ, Tove SB. Intermediates and products of the biohydrogenation of linoleic acid by Butyrinvibrio fibrisolvens. J Biol Chem 1966;241:1350–4 [PubMed] [Google Scholar]
- 9.Dhiman TR, Nam SH, Ure AL. Factors affecting conjugated linoleic acid content in milk and meat. Crit Rev Food Sci Nutr 2005;45:463–82 [DOI] [PubMed] [Google Scholar]
- 10.Christie WW, Dobson G, Adlof RO. A practical guide to the isolation, analysis and identification of conjugated linoleic acid. Lipids 2007;42:1073–84 [DOI] [PubMed] [Google Scholar]
- 11.Pariza MW, Park Y, Cook ME. Mechanisms of action of conjugated linoleic acid: evidence and speculation. Proc Soc Exp Biol Med 2000;223:8–13 [DOI] [PubMed] [Google Scholar]
- 12.Park Y, Albright KJ, Liu W. Effect of conjugated linoleic acid on body composition in mice. Lipids 1997;32:853–89270977 [Google Scholar]
- 13.Riserus U, Arner P, Brismar K, Vessby B. Treatment with dietary trans10cis12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care 2002;25:1516–21 [DOI] [PubMed] [Google Scholar]
- 14.Syvertsen C, Halse J, Hoivik HO, et al. The effect of 6 months supplementation with conjugated linoleic acid on insulin resistance in overweight and obese. Int J Obes (Lond) 2007;31:1148–54 [DOI] [PubMed] [Google Scholar]
- 15.Ostrowska E, Muralitharan M, Cross RF, Bauman DE, Dunshea FR. Dietary conjugated linoleic acids increase lean tissue and decrease fat deposition in growing pigs. J Nutr 1999;129:2037–42 [DOI] [PubMed] [Google Scholar]
- 16.Corl BA, Mathews Oliver SA, Lin X, et al. Conjugated linoleic acid reduces body fat accretion and lipogenic gene expression in neonatal pigs fed low- or high-fat formulas. J Nutr 2008;138:449–54 [DOI] [PubMed] [Google Scholar]
- 17.Must A, Dallal GE, Dietz WH. Reference data for obesity: 85th and 95th percentiles of body mass index (wt/ht2) and triceps skinfold thickness. Am J Clin Nutr 1991;53:839–46 [DOI] [PubMed] [Google Scholar]
- 18.Lohman TG. Applicability of body composition techniques and constants for children and youths. Exerc Sport Sci Rev 1986;14:325–57 [PubMed] [Google Scholar]
- 19.National Center for Health Statistics CDC growth charts. May 30, 2000 (modified 20 April 2001). Available from: http://www.cdc.gov/nchs/Default.htm (cited 11 March 2009)
- 20.Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone 2004;34:1044–52 [DOI] [PubMed] [Google Scholar]
- 21.Prais Botelho A, Santos-Zago LF, Costa de Oliveira A. Conjugated linoleic acid supplementation modified the body composition and serum leptin levels in weaning rats. Arch Latinoam Nutr 2008;58:156–63 [PubMed] [Google Scholar]
- 22.Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: The National Academies Press, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Schoeller D, Watras AC, Whigham LD. A meta-analysis of the effects of conjugated linoleic acid on fat-free mass in humans. Appl Physiol Nutr Metab 2009;34:975–8 [DOI] [PubMed] [Google Scholar]
- 24.Moloney F, Yeow TP, Mullen A, Nolan JJ, Roche HM. Conjugated linoleic acid supplementation, insulin sensitivity, and lipoprotein metabolism in patients with type 2 diabetes mellitus. Am J Clin Nutr 2004;80:887–95 [DOI] [PubMed] [Google Scholar]
- 25.Blankson H, Stakkestad JA, Fagertun H, Thom E, Wadstein J, Gudmundsen O. Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J Nutr 2000;130:2943–8 [DOI] [PubMed] [Google Scholar]
- 26.Mougios V, Matsakas A, Petridou A, et al. Effect of supplementation with conjugated linoleic acid on human serum lipids and body fat. J Nutr Biochem 2001;12:585–94 [DOI] [PubMed] [Google Scholar]
- 27.Song HJ, Grant I, Rotondo D, et al. Effect of CLA supplementation on immune function in young healthy volunteers. Eur J Clin Nutr 2005;59:508–17 [DOI] [PubMed] [Google Scholar]
- 28.Moya M. Utilizacion del acido linoleico conjugado en el nino y adolescente obesos. [The use of conjugated linoleic acid in obese children and adolescents.] Rev Esp Pediatr 2008;64:84–8 (in Spanish) [Google Scholar]
- 29.Noone EJ, Roche HM, Nugent AP, Gibney MJ. The effect of dietary supplementation using isomeric blends of conjugated linoleic acid on lipid metabolism in healthy human subjects. Br J Nutr 2002;88:243–51 [DOI] [PubMed] [Google Scholar]
- 30.Taylor JS, Williams SR, Rhys R, James P, Frenneaux MP. Conjugated linoleic acid impairs endothelial function. Arterioscler Thromb Vasc Biol 2006;26:307–12 [DOI] [PubMed] [Google Scholar]
- 31.Whigham LD, O'Shea M, Mohede IC, Walaski HP, Atkinson RL. Safety profile of conjugated linoleic acid in a 12-month trial in obese humans. Food Chem Toxicol 2004;42:1701–9 [DOI] [PubMed] [Google Scholar]
- 32.Ratner RE. An update on the Diabetes Prevention Program. Endocr Pract 2006;12(suppl 1):20–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riserus U, Smedman A, Basu S, Vessby B. CLA and body weight regulation in humans. Lipids 2003;38:133–7 [DOI] [PubMed] [Google Scholar]
- 34.Ellis KJ, Shypailo RJ, Hardin DS, et al. Z score prediction model for assessment of bone mineral content in pediatric diseases. J Bone Miner Res 2001;16:1658–64 [DOI] [PubMed] [Google Scholar]
- 35.Hur SJ, Park Y. Effect of conjugated linoleic acid on bone formation and rheumatoid arthritis. Eur J Pharmacol 2007;568:16–24 [DOI] [PubMed] [Google Scholar]
- 36.Gaullier JM, Halse J, Hoye K, et al. Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr 2005;135:778–84 [DOI] [PubMed] [Google Scholar]