Abstract
Background: Preclinical evidence of the preventive benefits of ω-3 (n–3) polyunsaturated fatty acids (PUFAs) in breast cancer continues to fuel interest in the potential role of dietary fat content in reducing breast cancer risk. The dose of fish-oil/ω-3 PUFAs needed to achieve maximal target tissue effects for breast cancer prevention remains undefined.
Objective: To determine the dose effects of ω-3 fatty acids on breast adipose tissue fatty acid profiles, we conducted a study of 4 doses of ω-3 PUFAs in women at high risk of breast cancer.
Design: In this 6-mo randomized open-label study, 48 women with increased breast cancer risk received 1, 3, 6, or 9 capsules/d of an ω-3 PUFA supplement that provided 0.84, 2.52, 5.04, and 7.56 g docosahexaenoic acid (DHA) + eicosapentaenoic acid (EPA) daily, respectively. Subjects made monthly visits, at which time pill counts were made and fasting blood samples were collected to determine fatty acid profiles; anthropometric measurements were made, breast adipose tissue samples were collected, and laboratory tests of toxicity (alanine aminotransferase, LDL cholesterol, and platelet function) were made at baseline and at 3 and 6 mo.
Results: All doses led to increased serum and breast adipose tissue EPA and DHA concentrations, but the response to 0.84 g DHA+EPA/d was less than the maximum possible response with ≥2.52 g/d. Body mass index attenuated the dose response for serum tissue DHA and EPA (P = 0.015 and 0.027, respectively) and breast adipose tissue DHA (P = 0.0022) in all of the treatment groups. The incremental increase in DHA and EPA correlated inversely with baseline fat and serum values. Compliance over 6 mo was 92.9 ± 9.2% and was unaffected by treatment arm. No severe or serious toxicities were reported.
Conclusions: Daily doses up to 7.56 g DHA+EPA were well tolerated with excellent compliance in this cohort at high risk of breast cancer. Body mass index and baseline fatty acid concentrations modulated the dose-response effects of ω-3 PUFA supplements on serum EPA and DHA and breast adipose tissue DHA.
INTRODUCTION
Evidence that the type of dietary fat may influence the development and progression of breast cancer is largely based on the findings of cell culture and rodent studies. In general, these experiments show the stimulatory effect of linoleic acid (LA), an ω-6 polyunsaturated fatty acid (PUFA), on tumor cell growth in contrast with inhibition by ω-3 marine fatty acids such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) (1, 2). Unlike the tumor-enhancing effects of diets containing elevated amounts of LA, diets enriched with EPA and DHA suppress tumor growth and metastasis in nude mice with human breast cancer xenografts (3).
In humans, case-control studies have explored differences in fatty acid profiles of subcutaneous or breast fat and its association with cancer risk, but a clear consensus has not emerged (4–7). A meta-analysis of case-control and cohort studies that used biomarkers of dietary fatty acid intake showed a significant protective effect of ω-3 PUFAs on breast cancer risk in the cohort studies (8). A recent review of epidemiologic studies of fish/ω-3 PUFA consumption did not find a significant association between ω-3 fatty acids and cancer incidence, which may relate in part to reliance on dietary data without concomitant biochemical correlates of fat intake (9, 10).
Several clinical trials have investigated the potential benefits of ω-3 PUFA/fish-oil supplements in cancer patients. Many studies have targeted cachexia in advanced end-stage malignancies such as pancreatic cancer, with results varying from lack of efficacy to the desired outcome of weight gain and/or stabilization (11, 12). Pilot studies in breast and prostate cancer patients reported adipose tissue uptake of ω-3 PUFAs after 3 mo of ≈3 g/d (13, 14).
A wide variety of doses and formulations of fish-oil/ω-3 fatty acids has been evaluated for antiinflammatory effects in healthy subjects and for therapeutic efficacy in various diseases mediated by inflammatory processes, such as rheumatoid arthritis, cardiovascular disease, and diabetes (15). Clinical trials of fish oil/ω-3 supplements in patients with active rheumatoid arthritis suggest that higher doses might prove more efficacious in alleviating symptoms, particularly with regard to changes in inflammatory biomarkers (16, 17). Cancer cachexia studies have also investigated high-dose therapy (eg, ≈8.5 g EPA+DHA/d for a 70-kg subject) (12, 18). Conversely, prospective secondary prevention trials indicate that lower doses of ω-3 fatty acids from supplements or fatty fish significantly reduce mortality from heart disease (eg, ≈1–3 g EPA+DHA/d) (19–21). Taken together, these prior studies provide parameters for consideration of different doses but fall short of defining an appropriate dose of ω-3 PUFAs for use in breast cancer survivors and women at high risk of breast cancer. We therefore conducted a randomized dose-finding study in women at increased risk of developing breast cancer to identify the optimal dose of ω-3 fatty acids for future studies of breast cancer prevention.
SUBJECTS AND METHODS
Subjects
Subjects at high risk of breast cancer and aged >18 y were recruited from the outpatient clinics of the James Cancer Hospital at The Ohio State University (OSU) Medical Center. Determinants of high-risk status included the following criteria: 1) one or more first-degree relatives with breast cancer, with at least one aged <60 y; 2) ≥2 second-degree relatives with breast cancer, with at least one aged <50 y; 3) prior biopsy diagnosis of atypical lobular hyperplasia, atypical ductal hyperplasia, lobular carcinoma in situ, or ductal carcinoma in situ in the past 10 y; 4) a Gail risk assessment with a 5-y Gail score ≥1.7% or a 10-y Gail score ≥3.4% (22); 5) prior diagnosis of T1 or T2 breast cancer diagnosed within the past 10 y; or 6) a known genetic mutation associated with hereditary breast cancer.
All patients had adequate hematopoietic, cardiac, metabolic, and hepatic function. Ineligibility criteria included <1 y from pregnancy, lactation, chemotherapy, bleeding tendency, current malignancy or history of metastatic malignancy, ongoing cancer-related treatment, use of anticoagulant medication, inability to undergo breast fat aspirations, known sensitivity or allergy to fish, chronic use of full-dose aspirin or nonsteroidal anti-inflammatory drugs, and a history of diabetes mellitus, hypertension, heart disease, or stroke. Subjects who used fish oil or ω-3 fatty acid supplements on a chronic basis within 3 mo of study enrollment were not eligible to participate.
Trial design
The study was conducted with after approval of the Institutional Review Board of The OSU and in accordance with the ethical standards of the institution and the Helsinki Declaration of 1975 as revised in 1983. Eligible high-risk women were recruited from May 2006 to April 2008, and the last subject completed the study in September 2008. After obtaining informed consent, the subjects were randomly assigned by the OSU Biostatistics Center to receive 1, 3, 6, or 9 capsules/d of an ω-3 fatty acid supplement that provided ≈0.84, 2.52, 5.04, or 7.56 g EPA+DHA/d (Omacor; Reliant Pharmaceuticals, Liberty Corner, NJ, and now available as Lovaza, GlaxoSmithKline, Brentford, Middlesex, United Kingdom) by using a stratified, fixed-block 1:1:1:1 randomization with a block size of 20. Each 1-g capsule of the supplement provided ≥0.9 g of the ethyl esters of ω-3 fatty acids, predominantly a combination of EPA (≈465 mg) and DHA (≈375 mg). Because the primary endpoint was to evaluate the dose effects in breast adipose tissue and serum for target organ and systemic responses, and given the lack of a completely inert dietary oil and safety concerns for use of an ω-6 PUFA placebo in a high-risk breast population, the trial was conducted as an open-label intervention without a placebo control. Patients used a daily diary to record all adverse events that occurred during the treatment period, the number of capsules taken each day, and the times of dosing. Pills were provided in monthly supplies, with collection of pill bottles/remaining capsules at each monthly visit for pill counts. Subjects were instructed to follow their usual diet. At enrollment and at 3 and 6 mo, the patients underwent physical examinations and measurements for body mass index (BMI) and waist-hip circumference ratio (WHR) calculations. Whereas participants and clinic personnel were not blinded to the treatment assignment, all laboratory personnel involved in sample processing and analysis were blinded to the allocation.
Breast adipose tissue samples were obtained by fine needle aspiration at enrollment and at 3 and 6 mo of study treatment and were immediately frozen and stored at −80°C. Fasting blood samples collected at enrollment and monthly, with storage of serum samples at −80°C for subsequent analysis of fatty acid profiles and serum biomarkers. At enrollment and at 3 and 6 mo, blood samples were also immediately processed for alanine transaminase (ALT), lipid profiles (total cholesterol, HDL and LDL cholesterol, and triglycerides), and platelet function (Platelet Function Analyzer – 100; Siemens, Deerfield, IL) at OSU clinical laboratories.
Fatty acid analysis
The fatty acid composition of breast adipose tissue and serum samples was determined by a 2-step procedure as described previously (23). Total lipids (both neutral and phospholipids) were analyzed. Total ω-3:ω-6 fatty acid ratios were calculated as the sum of 18:3n−3, 20:4n−3, 20:5n−3, 22:5n−3, 22:6n−3/sum of 18:2n−6, 18:3n−6, 20:2n−6, 20:3n−6, 20:4n−6, 22:2n−6, 22:4n−6.
Serum marker analyses
Serum samples were processed within 1 h of venipuncture and stored at −80°C for batch analysis at the conclusion of the study. Serum samples from baseline and 3 and 6 mo were analyzed in duplicate in the General Clinical Research Center at OSU for concentrations of adiponectin, leptin, glucose, insulin, C-peptide, insulin-like growth factor I (IGF-I), insulin-like growth factor binding protein 3 (IGFBP3), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), highly sensitive C-reactive protein (hsCRP), and sex hormone–binding globulin (SHBG). Specimens from postmenopausal patients were tested for estradiol concentrations. Adiponectin and leptin were measured by radioimmunoassay (Millipore, St Charles, MO); glucose by enzymatic assay (Yellow Springs International, Yellow Springs, OH); insulin, SHBG, and hsCRP by chemiluminescence (Siemens Medical Solutions Diagnositics, Duluth, MN); IGF-I and IGFBP3 by enzyme immunoassay (Beckman Coulter, Webster, TX); C-peptide and estradiol by radioimmunoassay (Beckman Coulter, Webster, TX); and IL-6 and TNF-α by electrochemiluminescence (Meso Scale Discovery, Gaithersburg, MD). The assay was repeated for any duplicate values with a CV of >8%.
Statistical analysis
The sample size was calculated by using an effect size of 0.5 in a one-factor analysis of variance to provide a power of 0.84 with a 2-sided α of 0.01. A potential dropout rate of 20% for compliance and/or tolerability issues was incorporated for a final sample size of 12 subjects randomly assigned to each of 4 dosage groups (n = 48).
The results are expressed as means ± SDs. Data were fitted to a linear mixed-effects model by using Proc Mixed in SAS (version 9.1; SAS Institute Inc, Cary, NC) and were analyzed by using Tukey's pairwise comparison method and Hsu's multiple comparison with the best (MCB) method (24). The MCB procedure was used to explore whether one or more doses achieved significantly higher serum or adipose response to ω-3 fatty acid supplementation. This method computes simultaneous CIs for the response of each dose compared with the best of the remaining doses. If the lower bound of a certain dose is positive, then it is the best dose, achieving a higher response than the other doses. If the upper bound of a certain dose is negative, then it is worse than the best of the other doses. If the CI covers 0, then that dose cannot be asserted to be the best dose nor can it be inferred to be worse than any other dose.
A linear mixed-effects model was used to model how each outcome variable (ω-3 fatty acid component) was affected by predictor variables of dose group, time, baseline measurement, and BMI. The subject was modeled as a random effect. If significant, the baseline level and BMI were kept in the model. The significance of the interaction between important predictor variables such as dose and time was tested at the 0.05 significance level. The method of Hsu and Berger (25) was used to find the minimal effective time to an increase in serum fatty acid concentrations. In contrast with the main results regarding serum and breast adipose fatty acid contents, the analyses of serum biomarkers were exploratory in nature.
RESULTS
Demographic characteristics
All 48 subjects were enrolled, and 100% of the subjects completed the intervention. The baseline characteristics of the study participants are shown in Table 1. This high-risk cohort consisted of 38 women with a family history of breast cancer and/or a Gail score ≥1.67, 7 women with a history of breast cancer, and 3 women with a history of high-risk histologic findings from a breast biopsy with (n = 2) and without (n = 1) a family history of breast cancer. Postmenopausal women accounted for 48% of the study group, as determined by clinical or surgical criteria.
TABLE 1.
Subject characteristics at baseline1
| All subjects (n = 48) | 1 capsule (n = 12) | 3 capsules (n = 12) | 6 capsules (n = 12) | 9 capsules (n = 12) | |
| Age (y) | 51 ± 7.72 (39–66) | 48.8 ± 8.1 (39–62) | 55 ± 7.7 (40–66) | 52 ± 7.2 (39–61) | 49 ± 6.3 (40–60) |
| Weight (kg) | 72.4 ± 14.9 (48.6–116.5) | 72.1 ± 11.7 (60.1–99.6) | 71.8 ± 20 (48.6–116.5) | 77.2 ± 16.2 (58.1–114.1) | 68.5 ± 10.5 (56.6–82.7) |
| Height (cm) | 165.6 ± 6.4 (151.9–182.9) | 166.4 ± 5.8 (154.48–172.7) | 163.6 ± 7.4 (151.9–177.8) | 166.4 ± 6.1 (157.5–176.5) | 168.9 ± 14.7 (160–182.9) |
| BMI (kg/m2) | 26.6 ± 6.5 (20.7–45.5) | 26.2 ± 4.7 (21.1–37.7) | 26.9 ± 7.8 (21.1–45.5) | 27.9 ± 5.9 (21.1–34.1) | 23.7 ± 6.8 (20.7–31.9) |
| Waist (cm) | 84.8 ± 11.9 (62–114) | 84.0 ± 11.4 (66–106) | 86 ± 15.6 (62–114) | 86.2 ± 12 (72–113) | 82.8 ± 9 (72–100) |
| WHR | 0.80 ± 0.05 (0.68–0.89) | 0.81 ± 0.07 (0.69–0.89) | 0.80 ± 0.06 (0.68–0.88) | 0.79 ± 0.05 (0.71–0.89) | 0.8 ± 0.02 (0.77–0.83) |
| Premenopausal (n) | 25 | 7 | 3 | 7 | 8 |
| Postmenopausal (n) | 23 | 5 | 9 | 5 | 4 |
There were no significant differences between groups other than randomly assigning more postmenopausal subjects to the 3 capsules/d treatment arm. WHR, waist-hip circumference ratio.
Mean ± SD; range in parentheses (all such values).
Compliance
Compliance was monitored by using 2 methods: 1) daily self-report diaries and 2) pill counts by study personnel at the monthly study visits. The average compliance for all 4 groups was 94.6 ± 6.7% and 92.9 ± 9.2% at 3 and 6 mo of the intervention, respectively. No significant differences in compliance were found between dose groups by analysis of variance. The average compliance at 6 mo was 96.2 ± 0.7% for the 1 capsule/d, 93.6 ± 0.2% for the 2 capsules/d, 92.5 ± 0.8% for the 6 capsules/d, and 89.5 ± 0.5% for the 9 capsules/d groups Only 8 subjects had a compliance rate <85%: 1 with 49% compliance (3 capsules/d group), 2 with 74% compliance, and 5 with >80% compliance. Fluctuations occurred in monthly compliance without an apparent decreasing trend over time.
Toxicity and lipid profiles
The ω-3 fatty acid supplements did not lead to serious adverse changes in the laboratory tests selected to assess toxicity, namely ALT, LDL cholesterol, and platelet function as tested by an in vitro clotting assay (PFA-100; Siemens) (Table 2). Eligibility criteria included an ALT ≤ 1.5 times the upper limit of normal of 35 U/L, and 6 subjects had slightly elevated values of 38–42 U/L (at baseline for a single patient randomly assigned to receive 3 capsules/d; at 6 mo for 3 subjects taking 1, 6, and 9 capsules/d, respectively; and at 6 mo for 2 subjects taking 1 and 3 capsules/d, respectively). At 6 mo, LDL cholesterol increased in 29 subjects within a range of 1 to 49 mg/dL. Of these increases, 13 resulted in a change in risk category to near optimal (100–129 mg/dL) for 1 subject taking 9 capsules/d, for 2 subjects taking 1 capsule/d to borderline high (130–159 mg/dL), for 4 subjects taking 6 capsules/d, and to high (160–189 mg/dL) for 1 subject taking 1 capsule/d, 1 taking 3 capsules/d, and 3 taking 6 capsules/d. However, for these subjects with increased LDL cholesterol, the ratio of total to HDL cholesterol remained <4.5 (low risk) in all but 3 subjects: 1 with a decrease from 9.3 to 6.4 (6 capsules/d group) and 2 with increases from 4.4 to 5.2 and 5.3–6 (1 capsule/d group). LDL cholesterol was unchanged or decreased in 34 subjects at 6 mo; a single subject taking 6 capsules/d had no data for LDL cholesterol at 6 mo, although LDL cholesterol had decreased by 3 mo.
TABLE 2.
Clinical laboratory test results for alanine aminotransferase (ALT), lipid profiles, and platelet function1
| Dose and time | ALT2 | LDL-C3 | Cholesterol4 | HDL-C5 | TGs6 | PFCT7 |
| 1 Capsule/d | ||||||
| 0 mo | 20.6 ± 7.7 | 114.2 ± 34.2 | 195.9 ± 41 | 55.8 ± 15.4 | 129.5 ± 69 | 93.4 ± 11.8 |
| 3 mo | 23.6 ± 9.3 | 114.8 ± 24.6 | 190.8 ± 20 | 58.8 ± 13.5 | 86.6 ± 50.2 | 99 ± 25.1 |
| 6 mo | 21.1 ± 8.5 | 126.8 ± 23.8 | 205.3 ± 24.6 | 57.6 ± 16.5 | 95.9 ± 49.8 | 103.4 ± 29.1 |
| 3 Capsules/d | ||||||
| 0 mo | 20.3 ± 7.3 | 133.3 ± 27 | 212.6 ± 30.5 | 57.4 ± 16.5 | 109.3 ± 70.9 | 103.3 ± 18.5 |
| 3 mo | 21.9 ± 6.3 | 139.8 ± 26.1 | 213.8 ± 28.5 | 57.5 ± 15.9 | 80.7 ± 51.1 | 106.4 ± 31.2 |
| 6 mo | 22.8 ± 8.6 | 135.8 ± 29.8 | 208.1 ± 30.4 | 56.4 ± 16.3 | 79.3 ± 46.1 | 95 ± 22.9 |
| 6 Capsules/d | ||||||
| 0 mo | 19.3 ± 3.8 | 135.6 ± 33.4 | 208.7 ± 33.2 | 56.8 ± 15.8 | 84.7 ± 47.7 | 120.8 ± 45.2 |
| 3 mo | 20.2 ± 7.6 | 130.1 ± 33.8 | 198.6 ± 32.9 | 54.9 ± 16.3 | 65.5 ± 27.5 | 93.8 ± 29.2 |
| 6 mo | 20.7 ± 6.0 | 136.8 ± 29.7 | 205 ± 27.5 | 55.8 ± 13.4 | 62.4 ± 31.2 | 106.2 ± 30.1 |
| 9 Capsules/d | ||||||
| 0 mo | 19.3 ± 4.8 | 113.3 ± 36.8 | 196.2 ± 34.1 | 66.3 ± 17.0 | 89.7 ± 49.3 | 109.8 ± 29.1 |
| 3 mo | 21.3 ± 7.9 | 104.4 ± 38.2 | 170.3 ± 42 | 56.5 ± 26.6 | 45.8 ± 24.4 | 109.8 ± 21.4 |
| 6 mo | 19.8 ± 5.2 | 111.2 ± 34.9 | 181.3 ± 34.9 | 60.7 ± 24.4 | 47.8 ± 16.7 | 99.5 ± 24.7 |
All values are means ± SDs; n = 12 subjects per treatment arm (total n = 48 subjects). LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; TGs, triglycerides; PFCT, platelet function closure time. No significant differences were found by repeated-measures ANOVA.
Range of normal values for ALT = 8–35 U/L.
Reference interval for LDL-C: optimal, <100 mg/dL; near optimal, 100–129 mg/dL; borderline high, 130–159 mg/dL; high, 160–189 mg/dL; and very high, ≥190 mg/dL.
Reference interval: desirable, <200 mg/dL; borderline high risk, 200–239 mg/dL; and high risk, ≥240 mg/dL.
Reference interval for HDL-C: low, <40 mg/dL; and high, >60 mg/dL.
Reference interval for TGs: desirable, <150 mg/dL; borderline, 150–199 mg/dL; high, 200–499 mg/dL; and very high, >500 mg/dL.
Reference range for PFCT as determined by the time for in vitro platelet plug formation: 88–198 s for clotting on collagen-adrenaline membranes.
None of the doses affected in vitro clotting times at 3 or 6 mo of treatment; one subject had a prolonged clotting time at baseline, and another subject had an increased value at 3 mo that returned to normal at 6 mo. Clinically significant bleeding did not occur based on reported events in the daily logs and updates at the monthly study visits. Reports of menorrhagia (n = 1) and rectal bleeding (n = 1) did not appear to be linked to the ω-3 PUFA supplements based on the sporadic and/or chronic nature of the events and potential for other more likely causes.
Dose-response effects of omega-3 supplements on serum fatty acids
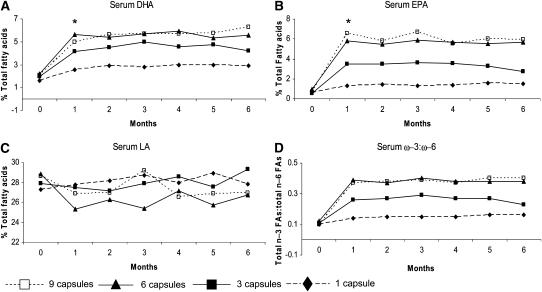
Serum DHA and EPA increased with each dose level (Figure 1, Table 3). Daily doses of 1 and 3 capsules (0.84 and 2.52 g EPA+DHA, respectively) resulted in a lower incremental change over time for both DHA and EPA compared with the maximum that can be achieved with 6 and 9 capsules (5.04 and 7.56 g EPA+DHA, respectively) from 2 to 6 mo by Hsu's MCB method (DHA: P < 0.0001 with 1 capsule/d for months 1 to 6 and P = 0.006, = 0.015, > 0.05, = 0.013, = 0.03, and <.0001 with 3 capsules/d for months 1 to 6, respectively; EPA: P < 0.0001 with 1 capsule/d for months 1 to 6 and P < 0.001, = 0.004, = 0.002, = 0.02, = 0.002, and <0.0001 with 3 capsules/d for months 1 to 6, respectively). The data do not support the superiority of either dose 6 or 9 capsules/d at achieving higher serum DHA or EPA concentrations.
FIGURE 1.
Mean serum concentrations of docosahexaenoic acid (DHA; A), eicosapentaenoic acid (EPA; B), and linoleic acid (LA; C) and ω-3:ω-6 ratios (n3 FAs:total n6 FAs; D) at monthly intervals from 0 mo (baseline) to 6 mo for 48 subjects (n = 12 per treatment arm). *A linear mixed-effects model that allowed for comparison of DHA and EPA at 2 different time points showed that the Bonferroni-adjusted P values were <0.012 for the difference between 1 mo and baseline for serum DHA at doses of 1, 3, 6, and 9 capsules/d and for baseline serum EPA at doses of 1, 3, 6, and 9 capsules/d.
TABLE 3.
Fatty acid composition of total serum lipids at baseline and at 6 mo by dose1
| Fatty acid and time | 1 capsule (n = 12) | 3 capsules (n = 12) | 6 capsules (n = 12) | 9 capsules (n = 12) |
| % of total fatty acids | % of total fatty acids | % of total fatty acids | % of total fatty acids | |
| 14:0 | ||||
| 0 mo | 0.96 ± 0.44 | 0.89 ± 0.51 | 0.77 ± 0.34 | 0.72 ± 0.34 |
| 6 mo | 0.94 ± 0.38 | 0.63 ± 0.39 | 0.71 ± 0.34 | 0.50 ± 0.19 |
| 16:0 | ||||
| 0 mo | 23.64 ± 3.26 | 22.79 ± 3.08 | 22.49 ± 1.74 | 22.58 ± 1.76 |
| 6 mo | 24.18 ± 3.58 | 21.31 ± 2.92 | 22.18 ± 2.47 | 22.14 ± 1.71 |
| 16:1ω-7 | ||||
| 0 mo | 1.96 ± 0.87 | 1.78 ± 0.71 | 2.33 ± 2.79 | 1.44 ± 0.46 |
| 6 mo | 1.91 ± 1.09 | 1.52 ± 0.71 | 1.13 ± 0.46 | 0.91 ± 0.22 |
| 18:0 | ||||
| 0 mo | 8.47 ± 0.86 | 9.33 ± 1.86 | 9.33 ± 1.77 | 9.33 ± 1.95 |
| 6 mo | 8.56 ± 1.08 | 8.79 ± 1.31 | 10.14 ± 1.32 | 10.01 ± 1.40 |
| 18:1ω-9 | ||||
| 0 mo | 20.10 ± 2.96 | 18.53 ± 2.68 | 16.41 ± 4.80 | 17.76 ± 3.21 |
| 6 mo | 18.18 ± 3.18 | 15.40 ± 2.76 | 14.13 ± 2.12 | 13.97 ± 2.14 |
| 18:1ω-7 | ||||
| 0 mo | 1.94 ± 0.37 | 2.10 ± 0.34 | 3.26 ± 4.52 | 1.94 ± 0.40 |
| 6 mo | 1.64 ± 0.16 | 1.94 ± 0.36 | 1.70 ± 0.27 | 1.69 ± 0.36 |
| LA | ||||
| 0 mo | 27.26 ± 5.30 | 27.93 ± 4.43 | 28.85 ± 4.04 | 28.63 ± 3.61 |
| 6 mo | 27.85 ± 6.49 | 29.32 ± 4.30 | 26.72 ± 4.22 | 26.95 ± 3.00 |
| 18:3ω-6 | ||||
| 0 mo | 0.50 ± 0.16 | 0.39 ± 0.18 | 0.44 ± 0.13 | 0.47 ± 0.18 |
| 6 mo | 0.43 ± 0.18 | 0.38 ± 0.26 | 0.29 ± 0.13 | 0.26 ± 0.14 |
| 18:3ω-3 | ||||
| 0 mo | 0.65 ± 0.27 | 0.74 ± 0.21 | 0.57 ± 0.21 | 0.57 ± 0.15 |
| 6 mo | 0.56 ± 0.18 | 0.68 ± 0.32 | 0.53 ± 0.22 | 0.50 ± 0.17 |
| 20:2ω-6 | ||||
| 0 mo | 0.29 ± 0.05 | 0.41 ± 0.39 | 0.26 ± 0.06 | 0.30 ± 0.04 |
| 6 mo | 0.25 ± 0.08 | 0.43 ± 0.37 | 0.26 ± 0.16 | 0.24 ± 0.06§ |
| 20:3ω-6 | ||||
| 0 mo | 2.05 ± 0.42 | 2.04 ± 0.44 | 2.06 ± 0.44 | 2.02 ± 0.48 |
| 6 mo | 1.77 ± 0.26 | 1.51 ± 0.33 | 1.35 ± 0.31 | 1.26 ± 0.46 |
| 20:4ω-3 | ||||
| 0 mo | 0.17 ± 0.14 | 0.16 ± 0.08 | 0.22 ± 0.15 | 0.13 ± 0.1 |
| 6 mo | 0.17 ± 0.13 | 0.22 ± 0.24 | 0.18 ± 0.14 | 0.18 ± 0.1 |
| AA | ||||
| 0 mo | 7.72 ± 1.72 | 8.27 ± 2.25 | 7.89 ± 1.49 | 9.07 ± 2.93 |
| 6 mo | 7.11 ± 1.64 | 7.04 ± 2.262 | 7.25 ± 2.102 | 6.88 ± 1.102 |
| EPA | ||||
| 0 mo | 0.63 ± 0.38 | 0.57 ± 0.26 | 0.96 ± 1.03 | 0.68 ± 0.40 |
| 6 mo | 1.52 ± 0.46 | 2.75 ± 1.072 | 5.65 ± 1.942 | 5.98 ± 2.032 |
| 22:2ω-6 | ||||
| 0 mo | 0.14 ± 0.21 | 0.03 ± 0.08 | 0.02 ± 0.07 | 0.001 ± 0.002 |
| 6 mo | 0.09 ± 0.14 | 0.03 ± 0.1 | 0.02 ± 0.03 | 0.03 ± 0.04 |
| 22:4ω-6 | ||||
| 0 mo | 0.28 ± 0.11 | 0.29 ± 0.13 | 0.26 ± 0.08 | 0.32 ± 0.16 |
| 6 mo | 0.24 ± 0.10 | 0.16 ± 0.11 | 0.09 ± 0.08 | 0.18 ± 0.19 |
| 22:5ω-3 | ||||
| 0 mo | 0.68 ± 0.30 | 0.65 ± 0.29 | 0.69 ± 0.30 | 0.68 ± 0.28 |
| 6 mo | 0.74 ± 0.27 | 1.01 ± 0.33 | 1.21 ± 0.33 | 1.26 ± 0.26 |
| DHA | ||||
| 0 mo | 1.59 ± 0.62 | 1.99 ± 0.84 | 2.20 ± 1.38 | 2.07 ± 0.77 |
| 6 mo | 2.90 ± 0.722 | 4.23 ± 0.862 | 5.57 ± 1.432 | 6.26 ± 1.642 |
| ω-3:ω-6 | ||||
| 0 mo | 0.10 ± 0.03 | 0.11 ± 0.04 | 0.12 ± 0.06 | 0.10 ± 0.03 |
| 6 mo | 0.16 ± 0.03 | 0.23 ± 0.062 | 0.38 ± 0.122 | 0.40 ± 0.122 |
All values are means ± SDs. LA, linoleic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid. A linear mixed-effects model was used to compare differences between 0 and 6 mo for DHA, EPA, AA, and LA and ω-3:ω-6 ratio. No significant differences between baseline and 6 mo were found for LA at all doses and for EPA and AA and ω-3:ω-6 ratio at 1 capsule/d. There was no evidence of a significant interaction between dose and time on the basis of current data for DHA, EPA, LA, and AA and ω-3:ω-6 ratio.
Significantly different from 0 mo (baseline), P < 0.012 (Bonferroni adjusted).
Higher baseline serum DHA was associated with a smaller change in DHA (P < 0.0001). There is not sufficient evidence to support an association between baseline serum EPA and the magnitude of the change with the supplements by the MCB analysis. Furthermore, higher BMI values were significantly associated with lower incremental increases in serum DHA and EPA across all doses (P = 0.015 and 0.027, respectively). Serum concentrations of EPA and DHA at study entry were not associated with BMI, although baseline arachidonic acid (AA) was significantly and negatively associated with BMI (P = 0.03). BMI was not found to be significantly associated with changes in serum AA, LA, or ω-3:ω-6 fatty acid ratios. WHR and menopausal status were not significantly associated with changes in DHA or EPA.
Serum DHA and EPA increased significantly at 1 mo of the study intervention compared with baseline (Figure 1). Although serum DHA continued to increase significantly after the first month with daily doses of 1, 3, and 9 capsules by Hsu's MCB method (P = 0.014, 0.0035, and 0.019, respectively), serum EPA was not found to differ significantly over time from 1 to 6 mo. Changes in the ratio of ω-3:ω-6 fatty acids relative to baseline values were not significant for dose or time effects. In addition, no significant effects by time, baseline level, dose, or interactions between these factors were found for changes in serum AA or LA at months 1–6 compared with baseline.
Dose-response effects of omega-3 supplements on breast adipose tissue fatty acids
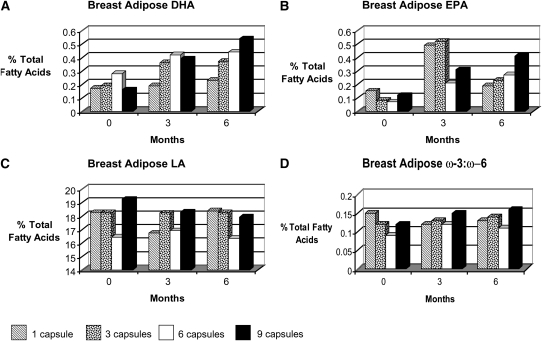
Breast adipose tissue EPA and DHA contents increased after 6 mo of ω-3 PUFA supplements (Figure 2, Table 4). Higher doses of EPA and DHA correlated positively with the breast adipose tissue content of EPA and DHA, with significant differences between the lowest dose of 1 capsule/d (0.9 g ω-3 PUFAs) and the maximum that could be achieved at the 95% confidence level with Hsu's MCB method. Doses of 3, 6, and 9 capsules/d led to similar incremental increases in DHA and EPA. Although the average values for breast adipose tissue EPA and DHA were successively higher with increased intakes of ω-3 PUFAs, the responses achieved with 3, 6, and 9 capsules did not differ significantly from the maximum that could be achieved at the 95% confidence level with Hsu's MCB method.
FIGURE 2.
Mean concentrations of breast adipose tissue docosahexaenoic acid (DHA; A), eicosapentaenoic acid (EPA; B), and linoleic acid (LA; C) and ω-3:ω-6 ratios (D) at baseline and at 3 and 6 mo for 48 subjects (n = 12 per treatment arm).
TABLE 4.
Fatty acid composition of total lipids in breast adipose tissue at baseline and at 6 mo by dose1
| Fatty acid and time | 1 capsule (n = 12) | 3 capsules (n = 12) | 6 capsules (n = 12) | 9 capsules (n = 12) |
| % of total fatty acids | % of total fatty acids | % of total fatty acids | % of total fatty acids | |
| 14:0 | ||||
| 0 mo | 3.14 ± 1.97 | 2.66 ± 1.39 | 2.2 ± 0.21 | 3.75 ± 1.99 |
| 6 mo | 3.03 ± 1.66 | 2.66 ± 1.71 | 2.42 ± 0.73 | 3.05 ± 1.52 |
| 16:0 | ||||
| 0 mo | 19.28 ± 2.16 | 18.61 ± 2.13 | 19.49 ± 1.55 | 18.35 ± 1.8 |
| 6 mo | 20.15 ± 2.07 | 18.65 ± 2.8 | 18.13 ± 3.91 | 18 ± 3.59 |
| 16:1ω-7 | ||||
| 0 mo | 3.28 ± 1.03 | 3.64 ± 1.05 | 3.15 ± 1.08 | 3.46 ± 1.05 |
| 6 mo | 3.41 ± 1.34 | 3.54 ± 1.38 | 3.31 ± 1.03 | 2.56 ± 0.38 |
| 18:0 | ||||
| 0 mo | 5.27 ± 1.58 | 4.84 ± 1.31 | 5.52 ± 1.32 | 4.65 ± 1.55 |
| 6 mo | 5.23 ± 1.68 | 4.66 ± 1.33 | 4.74 ± 1.92 | 5.34 ± 1.83 |
| 18:1ω-9 | ||||
| 0 mo | 38.68 ± 5.9 | 41.69 ± 4.12 | 42.5 ± 2.77 | 36.15 ± 6.94 |
| 6 mo | 39.87 ± 4.77 | 40.69 ± 5.16 | 38.53 ± 10.15 | 36.13 ± 8.41 |
| 18:1ω-7 | ||||
| 0 mo | 2.35 ± 0.34 | 2.62 ± 0.57 | 2.73 ± 0.67 | 2.32 ± 0.77 |
| 6 mo | 2.4 ± 0.4 | 2.41 ± 0.51 | 2.36 ± 0.86 | 2.19 ± 0.63 |
| LA | ||||
| 0 mo | 18.12 ± 2.442 | 18.41 ± 2.32 | 16.59 ± 1.823 | 19.17 ± 3.014 |
| 6 mo | 18.38 ± 2.95 | 18.25 ± 2.32 | 16.34 ± 1.94 | 17.92 ± 3.7 |
| 18:3ω-6 | ||||
| 0 mo | 0.29 ± 0.27 | 0.16 ± 0.18 | 0.1 ± 0.03 | 0.33 ± 0.28 |
| 6 mo | 0.23 ± 0.22 | 0.19 ± 0.11 | 0.22 ± 0.24 | 0.17 ± 0.24 |
| 18:3ω-3 | ||||
| 0 mo | 1.07 ± 0.49 | 1.13 ± 0.53 | 0.86 ± 0.21 | 1.36 ± 0.49 |
| 6 mo | 1.1 ± 0.51 | 1.16 ± 0.71 | 1.01 ± 0.45 | 1.18 ± 0.51 |
| 20:2ω-6 | ||||
| 0 mo | 0.38 ± 0.32 | 0.3 ± 0.09 | 0.28 ± 0.08 | 0.41 ± 0.39 |
| 6 mo | 0.24 ± 0.1 | 0.24 ± 0.09 | 0.28 ± 0.07 | 0.27 ± 0.14 |
| 20:3ω-6 | ||||
| 0 mo | 2.53 ± 3.32 | 1.51 ± 1.74 | 2.49 ± 4.15 | 5.69 ± 9.41 |
| 6 mo | 1.36 ± 0.99 | 1.91 ± 3.88 | 8.14 ± 15.45 | 8.42 ± 16.57 |
| 20:4ω-3 | ||||
| 0 mo | 0.78 ± 1.17 | 0.45 ± 0.67 | 0.22 ± 0.14 | 0.6 ± 0.57 |
| 6 mo | 0.54 ± 0.78 | 0.35 ± 0.44 | 0.32 ± 0.26 | 0.54 ± 0.56 |
| AA | ||||
| 0 mo | 0.67 ± 0.27 | 0.5 ± 0.1 | 0.55 ± 0.17 | 0.58 ± 0.28 |
| 6 mo | 0.59 ± 0.28 | 0.52 ± 0.23 | 0.54 ± 0.16 | 0.53 ± 0.33 |
| EPA | ||||
| 0 mo | 0.15 ± 0.17 | 0.07 ± 0.06 | 0.07 ± 0.03 | 0.13 ± 0.14 |
| 6 mo | 0.19 ± 0.21 | 0.23 ± 0.18 | 0.27 ± 0.095 | 0.41 ± 0.295 |
| 22:2ω-6 | ||||
| 0 mo | 0.04 ± 0.13 | 0.26 ± 0.73 | 0.38 ± 0.98 | 0 ± 0.01 |
| 6 mo | 0.29 ± 0.65 | 0.42 ± 1.03 | 0.19 ± 0.47 | 0.02 ± 0.05 |
| 22:4ω-6 | ||||
| 0 mo | 0.17 ± 0.13 | 0.27 ± 0.29 | 0.23 ± 0.09 | 0.11 ± 0.11 |
| 6 mo | 0.18 ± 0.1 | 0.6 ± 1.39 | 0.36 ± 0.49 | 0.09 ± 0.1 |
| 22:5ω-3 | ||||
| 0 mo | 1.21 ± 1.83 | 0.82 ± 0.92 | 0.4 ± 0.21 | 1.05 ± 0.88 |
| 6 mo | 0.9 ± 1.35 | 0.83 ± 1.08 | 0.54 ± 0.27 | 1.13 ± 0.92 |
| DHA | ||||
| 0 mo | 0.16 ± 0.196 | 0.21 ± 0.14 | 0.27 ± 0.16 | 0.15 ± 0.136 |
| 6 mo | 0.23 ± 0.11 | 0.37 ± 0.177 | 0.44 ± 0.165 | 0.54 ± 0.345 |
| ω-3:ω-68 | ||||
| 0 mo | 0.15 ± 0.15 | 0.12 ± 0.08 | 0.09 ± 0.03 | 0.13 ± 0.07 |
| 6 mo | 0.13 ± 0.11 | 0.14 ± 0.1 | 0.11 ± 0.04 | 0.16 ± 0.1 |
All values are means ± SDs. LA, linoleic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid. A linear mixed-effects model was used to compare differences between 0 and 6 mo for DHA, EPA, AA, and LA and ω-3:ω-6 ratio. There was no evidence of a significant interaction between dose and time on the basis of current data for DHA, EPA, LA, and AA and ω-3:ω-6 ratio. No significant differences between 0 mo (baseline) and 6 mo were found for breast adipose tissue AA and LA and ω-3:ω-6 ratio.
Significantly different from 3 capsules/d (Tukey's test): 2P = 0.04, 3P = 0.05, 4P = 0.0006.
Significantly different from baseline (Bonferroni adjusted): 5P < 0.004, 7P = 0.008.
Significantly different from 6 capsules/d, P = 0.04 (Tukey's method).
The ω-3:ω-6 ratio represents the sum of (18:3n−3, 20:4n−3, 20:5n−3, 22:5n−3, and 22:6n−3)/(sum of 18:2n−6, 18:3n−6, 20:2n−6, 20:3n−6, 20:4n−6, 22:2n−6, and 22:4n−6).
A higher baseline DHA content in breast adipose tissue was associated with smaller changes in the respective fatty acid value (P = 0.0002). Higher BMI values were also significantly associated with lower incremental changes in DHA (P = 0.0022); this effect did not significantly differ by dose or time. BMI did not significantly affect changes in EPA, LA, AA, or ω-3:ω-6 ratios. The baseline adipose tissue contents of DHA, EPA, AA, and LA and the ω-3:ω-6 ratio were not significantly associated with BMI. No significant associations with time, baseline, dose, or interactions between these factors were found for adipose tissue AA or LA contents or for the ω-3:ω-6 fatty acid ratio.
Dose-response effects of omega-3 supplements on serum biomarkers
A secondary endpoint of the study was to explore a panel of serum biomarkers of insulin resistance (C-peptide, insulin, and glucose), adiposity (adiponectin and leptin), inflammation (IL-6, C-reactive protein, and TNF-α), and IGF-I and IGFBP3 as potential surrogate measures of the effects of dietary ω-3 fatty acid supplements. Fasting serum samples collected at baseline and at 3 and 6 mo were assessed for these biomarkers. Estradiol concentrations were also measured in samples from postmenopausal women, and SHBG concentrations were measured in all subjects. No significant dose and time interactions were detected. Consideration of BMI or menopausal status did not alter this lack of effect.
These exploratory analyses showed an inverse association of BMI with serum adiponectin and SHBG and a concordant association of BMI with leptin, C-peptide, insulin, glucose, CRP, and IL-6 (Table 5). In postmenopausal subjects, a higher BMI was associated with increased estradiol concentrations (Table 5). Serum IGF-l, IGFBP3, and TNF-α did not appear to be linked to BMI or menopausal status.
TABLE 5.
Association of serum markers with BMI1
| Serum marker2 | Estimated value at a BMI (in kg/m2) of 21.8 (≈25th percentile in data set) and 95% CI | Estimated percentage change per one-unit increase in BMI | 95% CI for percentage change per one-unit increase in BMI | P value |
| % | % | |||
| Adiponectin (ng/mL) | 13.71 (11.38, 16.51) | −2.7 | (−4.9, −0.5) | 0.0189 |
| Leptin (ng/mL) | 7.20 (6.21, 8.35) | 8.3 | (6.2, 10.4) | <0.0001 |
| C-peptide (ng/mL) | 2.11 (1.84, 2.41) | 4.8 | (1.03, 1.066) | <0.0001 |
| Insulin (μIU/mL) | 4.29 (3.48, 5.30) | 5.3 | (1.025, 1.082) | 0.0002 |
| Glucose (μg/dL) | 73.58 (69.71, 77.68) | 1.1 | (0.4, 1.8) | 0.0035 |
| IGF-I (ng/mL) | 201.78 (180.66, 225.38) | −0.6 | (−2.1, 0.8) | 0.4036 |
| IGFBP3 (ng/mL) | 4729.15 (4510.67, 4857.71) | 0.30 | (0.3, 1.0) | 0.2392 |
| C-reactive protein (mg/dL) | 0.63 (0.47, 0.83) | 9.4 | (5.4, 13.7) | <0.0001 |
| IL-6 (pg/mL) | 2.75 (2.09, 3.60) | 4.6 | (0.8, 8.4) | 0.0161 |
| TNF-α (pg/mL) | 1.71 (1.52, 1.93) | 0.8 | (0.7, 2.3) | 0.3082 |
| Estradiol (pg/mL) | 10.31 (9.28, 11.44) | 2.6 | (1.2, 4.1) | 0.0004 |
| SHBG (nmol/L) | 13.71 (11.38, 16.51) | −4.2 | (−5.8, −2.5) | <0.0001 |
The results are secondary to the main analysis and represent the findings of exploratory model building. Outcomes were first natural-log transformed due to skewness, and then linear mixed-effects models were applied to the data, with BMI, time, dose, and menopausal status as potential predictors. From the models, we estimated the percentage change in outcome per one-unit change in BMI and 95% CIs. To give a sense of baseline outcome values in relation to BMI, we also used the model to estimate the outcome value at the 25th percentile of BMI (as calculated from our data set) and the 95% CI. We chose the 25th percentile rather than the minimum to avoid providing an estimate at what could potentially be an outlier value of BMI. IGF-I, insulin-like growth factor I; IGFB3, insulin-like growth factor binding protein 3; SHBG, sex hormone–binding globulin; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
n = 48 subjects for all serum markers, except for estradiol, which was assessed only in postmenopausal subjects (n = 23).
DISCUSSION
This study showed the effects of 4 different doses of an ω-3 PUFA supplement on breast adipose tissue fatty acid content over 6 mo of treatment. The dose range was designed to encompass a lower end of <1 g/d to a high dose approximating 8 g EPA+DHA/d based on maximally tolerated dosing data from prior clinical trials (12). Doses of 3, 6, or 9 capsules (ie, 2.52, 5.04, and 7.56 g EPA+DHA/d, respectively) resulted in greater increases of DHA and EPA in breast adipose tissue and serum than did 1 capsule/d (0.84 g/d) The larger doses of 2.52, 5.04, and 7.56 g EPA+DHA/d yielded incremental increases of EPA and DHA in breast fat with mean values that were progressively higher but not significantly different. On the basis of the current data, ≥2.52 g EPA+DHA/d represents the most appropriate dose for elevating EPA and DHA contents in the mammary microenvironment.
The lack of significant differences between the successively higher doses of 3, 6, and 9 capsules/d might indicate a maximal threshold for DHA and EPA in breast adipose tissue. Alternatively, additional time may be needed to distinguish between these doses, given the small but significant increase in breast adipose tissue DHA and EPA contents from 3 to 6 mo without evidence of a plateau. This outcome may also relate to variables such as the background diet, baseline DHA and EPA, and BMI, such that our sample size was insufficient to discriminate between the effects of the higher doses. Indeed, the data showed that a higher baseline serum or adipose DHA or EPA content was associated with a smaller incremental increase of that specific fatty acid. In our study, breast adipose tissue DHA and EPA at enrollment ranged from 0 to 0.65 and from 0 to 0.54, respectively, with a significantly higher DHA content at baseline in subjects who consumed 6 capsules/d and a higher LA content in subjects who consumed 3 capsules/d. Furthermore, a higher BMI attenuated the effect of the supplement on increases in the serum and adipose at all dose levels. In a prior report, post hoc analysis of a clinical trial of ω-3 fatty acids in children and young adults with IgA nephropathy showed that the dosage of Omacor per kilogram of body weight at 21–24 mo of treatment correlated significantly with plasma phospholipid EPA:AA and DHA:AA ratios and changes in the severity of proteinuria (26). Our data also support the need to consider body mass in future studies of ω-3 PUFAs as well as variations in usual dietary intake that might affect basal fatty acid concentrations.
Increases in DHA and EPA over time were noted in the breast adipose tissue samples, without evidence of a threshold level. Serum DHA and EPA concentrations rose after the first month of treatment, with general stability in the values per dose level after the first month of treatment. Other studies have made similar observations, including a randomized placebo-controlled study that assessed the dose effects of 0.97, 1.95, and 2.92 g EPA+DHA with a placebo of monounsaturated fatty acids in 58 monks that showed increasing EPA and DHA contents in gluteal fat samples without evidence of a plateau at 1 y of treatment and a relatively stable EPA content in serum cholesterol esters within weeks of treatment (27).
The current data did not show significant changes in the ω-3:ω-6 ratio on the basis of the sum of total ω-3 and ω-6 fatty acids in the adipose and serum samples. Although the EPA and DHA contents increased and the LA and AA contents remained essentially stable, shifts in the concentrations of other ω-3 and ω-6 fatty acids and the wide range of values within a given treatment arm may have accounted for the lack of significant dose effects. Other investigators have reported changes in the ω-3:ω-6 ratio with fish oil/ω-3 PUFA supplements; however, without a standardized method of determining this ratio, the seeming discordance with our results may relate to the specific ω-3 and ω-6 fatty acids included in the calculation, eg, totals based on fewer or more ω-6 and ω-3 fatty acids compared with our methods (13, 28). Homeostasis in the relative balance of ω-3 and ω-6 fatty acids may occur despite a shift in EPA and DHA, and consideration of the ratio of specific subsets of fatty acids may represent a better marker of response and/or disease susceptibility.
As a secondary, exploratory endpoint, we investigated the effect of the study intervention on serum hormones, growth factors, and cytokines that have been linked by either preclinical or clinical evidence to increased breast cancer risk. Although these serum markers were not affected by time or dose of the ω-3 PUFAs, the finding that BMI associated positively with insulin, glucose, C-peptide, leptin, CRP, IL-6, and estradiol concentrations and inversely with adiponectin and SHBG agrees with the known association of increasing BMI with insulin resistance, adiposity, inflammation, and peripheral aromatization and supports the validity of the results. The lack of effect of any dose on these selected serum factors may relate to the overall good health of the subjects because those with a history of diabetes and cardiovascular disease were excluded, such that serum indexes of inflammation and insulin resistance were already low, normal, or outside a modifiable range. Prior studies have also suggested a lack of effect of ω-3 PUFA supplements on inflammatory markers in a healthy population at lower doses (29). Cytokine release from peripheral blood mononuclear cells may represent a better measure of antiinflammatory response to dietary fatty acids such as EPA and/or DHA (30).
Limitations of the study include the lack of a placebo, which could have led to biased observations or actions by subjects and/or study personnel in the clinic. Future trials will need to address the issue of a fish-oil placebo, which is complicated by the potential for biologic effects with any dietary oil as well as the widespread interest of the general public in taking fish oil/ω-3 PUFA supplements for cardiovascular and overall health benefits. The dosing schedule was not prescribed, such that higher doses with multiple pills taken as a single daily dose could have led to inefficient absorption and/or metabolism resulting in an apparent lack of dose response with 3, 6, and 9 capsules/d. Indeed, subjects with the higher doses reported using a single daily dose of 6 or 9 capsules as the easiest to administer rather than multiple doses (data not shown). The study population was also somewhat varied, and there might have been differences in fatty acid uptake based on menopausal status that the current sample size could not detect. For example, sex differences in the conversion of α−linolenic acid to long-chain ω-3 PUFAs may relate to estrogen, with increased serum DHA in women receiving hormone replacement therapy or taking oral contraceptives (31, 32). Additionally, the baseline diet was not controlled for total fat or other components that might have affected fatty acid uptake.
Our study showed for the first time the dose-response effects of 4 different doses of an ω-3 fatty acid supplement in breast adipose tissue and serum samples of women at high risk of breast cancer. Daily doses of 3, 6, or 9 capsules yielded increased breast adipose tissue DHA and EPA relative to 1 capsule, and daily supplementation with 6 or 9 capsules achieved higher serum DHA and EPA concentrations than did lower doses of 1 or 3 capsules. The current data support a minimum dose of 2.52 g EPA+DHA/d to increase the concentrations of these fatty acids in breast adipose tissue. It is possible that a longer duration of treatment and/or larger sample size would help distinguish between the doses of 3, 6, or 9 capsules. Future studies will also need to consider the influence of BMI and usual diet on systemic and target tissue concentrations of DHA and EPA. Although prior clinical trials have evaluated dose responses of ω-3 fatty acid/fish-oil supplementation, the endpoints were often serum- or blood-based markers or clinical criteria for nonbreast cancer–related diseases (33, 34). Our study was the first to provide the basis for selection of a dose likely to elicit target tissue effects relevant to breast cancer based on modulation of EPA and DHA concentrations in breast adipose tissue.
Acknowledgments
We gratefully acknowledge the time, effort, and commitment of the study participants. We thank the nursing, laboratory, pharmacy, and Clinical Trials Office staff at JamesCare in Dublin and Donn C Young for invaluable assistance with the trial design.
The authors’ responsibilities were as follows—LDY and SKC: designed the trial; LDY: was responsible for the overall administration and direction of the project and all interpretations, dissemination of findings, and writing of the manuscript; JLL: was responsible for the accrual of subjects, clinical assessments, sample handling, and data collection; RMC and JRR: conducted the fatty acid analyses; LDY, JLL, JCH, YL, AL, and MAB: analyzed the data; and JLL, JCH, YL, AL, MAB, and SKC: edited and revised the manuscript. None of the authors had any personal or financial conflicts of interest.
REFERENCES
- 1.Pritchard GA, Jones DL, Mansel RE. Lipids in breast carcinogenesis. Br J Surg 1989;76:1069–73 [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez MJ, Schemmel RA, Gray JI, Dugan L, Sheffield LG, Welsch CW. Effect of dietary fat on growth of MCF-7 and MDA-MB-231 human breast carcinomas in athymic nude mice: relationship between carcinoma growth and lipid peroxidation product levels. Carcinogenesis 1991;12:1231–5 [DOI] [PubMed] [Google Scholar]
- 3.Rose DP, Connolly JM. Effects of dietary omega-3 fatty acids on human breast cancer growth and metastases in nude mice. J Natl Cancer Inst 1993;85:1743–7 [DOI] [PubMed] [Google Scholar]
- 4.Petrek JA, Hudgins LC, Levine B, Ho MN, Hirsch J. Breast cancer risk and fatty acids in the breast and abdominal adipose tissues. J Natl Cancer Inst 1994;86:53–6 [DOI] [PubMed] [Google Scholar]
- 5.London SJ, Sacks FM, Stampfer MJ, et al. Fatty acid composition of the subcutaneous adipose tissue and risk of proliferative benign breast disease and breast cancer. J Natl Cancer Inst 1993;85:785–93 [DOI] [PubMed] [Google Scholar]
- 6.Eid A, Berry EM. The relationship between dietary fat, adipose tissue composition, and neoplasms of the breast. Nutr Cancer 1988;11:173–7 [DOI] [PubMed] [Google Scholar]
- 7.Zhu ZR, Agren J, Maennistoe S, et al. Fatty acid composition of breast adipose tissue in breast cancer patients and in patients with benign breast disease. Nutr Cancer 1995;24:151–60 [DOI] [PubMed] [Google Scholar]
- 8.Saadatian-Elahi M, Norat T, Goudable J, Riboli E. Biomarkers of dietary fatty acid intake and the risk of breast cancer: a meta-analysis. Int J Cancer 2004;111:584–91 [DOI] [PubMed] [Google Scholar]
- 9.MacLean CH, Newberry SJ, Mojica WA, et al. Effects of omega-3 fatty acids on cancer risk. JAMA 2006;295:403–15 [DOI] [PubMed] [Google Scholar]
- 10.Chen YQ, Berquin IM, Daniel LW, et al. Omega-3 fatty acids and cancer risk. JAMA 2006;296:282. [DOI] [PubMed] [Google Scholar]
- 11.Wigmore SJ, Ross JA, Falconer JS, et al. The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition 1996;12:S27–30 [DOI] [PubMed] [Google Scholar]
- 12.Burns CP, Halabi S, Clamon G, et al. Phase II study of high-dose fish oil capsules for patients with cancer-related cachexia-a Cancer and Leukemia Group B Study. Cancer 2004;101:370–8 [DOI] [PubMed] [Google Scholar]
- 13.Bagga D, Capone S, Wang HJ, et al. Dietary modulation of omega-3/omega-6 polyunsaturated fatty acid ratios in patients with breast cancer. J Natl Cancer Inst 1997;89:1123–31 [DOI] [PubMed] [Google Scholar]
- 14.Aronson WJ, Glaspy JA, Reddy ST, Reese D, Heber D, Bagga D. Modulation of omega-3/omega-6 polyunsaturated ratios with dietary fish oils in men with prostate cancer. Urology 2001;58:283–8 [DOI] [PubMed] [Google Scholar]
- 15.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 2002;21:495–505 [DOI] [PubMed] [Google Scholar]
- 16.Geusens P, Wouters C, Nijs J, Jiang YD. J. Long-term effect of omega-3 fatty acid supplementation in active rheumatoid arthritis. a 12 month, double-blind, controlled study. Arthritis Rheum 1994;37:824–9 [DOI] [PubMed] [Google Scholar]
- 17.Kremer JM, Lawrence DA, Jubiz W, et al. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum 1990;33:810–20 [DOI] [PubMed] [Google Scholar]
- 18.Burns CP, Halabi S, Clamon G, et al. Phase I clinical study of fish oil fatty acid capsules for patients with cancer cachexia: Cancer and Leukemia Group B Study 9473. Clin Cancer Res 1999;5:3942–7 [PubMed] [Google Scholar]
- 19.Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 1989;2:757–61 [DOI] [PubMed] [Google Scholar]
- 20.Investigators of the GISSI-Prevenzione trial Dietary supplementation with n−3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999;354:447–55 [PubMed] [Google Scholar]
- 21.Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M. Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: the Indian experiment of infarct survival–4. Cardiovasc Drugs Ther 1997;11:485–91 [DOI] [PubMed] [Google Scholar]
- 22.Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989;81:1879–86 [DOI] [PubMed] [Google Scholar]
- 23.Belury MA, Kempa-Steczko A. Dietary conjugated linoleic acid modulates hepatic lipid composition in mice. Lipids 1997;32:199–204 [DOI] [PubMed] [Google Scholar]
- 24.Hsu JC.Multiple comparisons: theory and methods. London, United Kingdom: Chapman and Hall, 1996 [Google Scholar]
- 25.Hsu JC, Berger R. Stepwise confidence intervals without multiplicity adjustment for dose response and toxicity studies. J Am Stat Assoc 1999;94:468–82 [Google Scholar]
- 26.Hogg RJ, Fitzgibbons L, Atkins C, Nardelli N, Bay RC, Group NAINS. Efficacy of omega-3 fatty acids in children and adults with IgA nephropathy is dosage- and size-dependent. Clin J Am Soc Nephrol 2006;1:1167–72 [DOI] [PubMed] [Google Scholar]
- 27.Katan MB, Deslypere JP, van Birgelen APJM, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 1997;38:2012–22 [PubMed] [Google Scholar]
- 28.Kabir M, Skurnik G, Naour N, et al. Treatment for 2 mo with n−3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: a randomized controlled study. Am J Clin Nutr 2007;86:1670–9 [DOI] [PubMed] [Google Scholar]
- 29.Damsgaard CT, Frøkiaer H, Andersen AD, Lauritzen L. Fish oil in combination with high or low intakes of linoleic acid lowers plasma triacylglycerols but does not affect other cardiovascular risk markers in healthy men. J Nutr 2008;138:1061–6 [DOI] [PubMed] [Google Scholar]
- 30.Vedin I, Cederholm T, Levi YF, et al. Effects of docosahexaenoic acid-rich n−3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. Am J Clin Nutr 2008;87:1616–22 [DOI] [PubMed] [Google Scholar]
- 31.Giltay EJ, Gooren LJG, Toorians AWFT, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr 2004;80:1167–74 [DOI] [PubMed] [Google Scholar]
- 32.Giltay EJ, Duschek EJJ, Katan MB, Zock PL, Neele SJ, Netelenbos JC. Raloxifene and hormone replacement therapy increase arachidonic acid and docosahexaenoic acid levels in postmenopausal women. J Endocrinol 2004;182:399–408 [DOI] [PubMed] [Google Scholar]
- 33.Bønaa KH, Bjerve KS, Straume B, Gram IT, Thelle D. Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension. A population-based intervention trial from the Tromsø study. N Engl J Med 1990;322:795–801 [DOI] [PubMed] [Google Scholar]
- 34.Meyer BJ, Hammervold T, Rustan AC, Howe PR. Dose-dependent effects of docosahexaenoic acid supplementation on blood lipids in statin-treated hyperlipidaemic subjects. Lipids 2007;42:109–15 [DOI] [PubMed] [Google Scholar]