Abstract
Background: Leukocyte telomere length is associated with diseases of aging, but there is limited knowledge of diet and lifestyle determinants.
Objective: The objective was to examine cross-sectionally the association between diet, body composition, and lifestyle factors on leukocyte telomere length in women.
Design: Leukocyte telomere length was measured by quantitative polymerase chain reaction in 2284 female participants from the Nurses’ Health Study, who were selected as controls for an investigation of biological predictors of cancer. Diet, lifestyle, and anthropometric data were assessed by questionnaire.
Results: After multivariate adjustment, dietary fiber intake was positively associated with telomere length (z score), specifically cereal fiber, with an increase of 0.19 units between the lowest and highest quintiles (P = 0.007, P for trend = 0.03). Although total fat intake was not associated with telomere length, polyunsaturated fatty acid intake (−0.26 units, quintile 5 compared with quintile 1: P = 0.002, P for trend = 0.02), specifically linoleic acid intake, was inversely associated with telomere length after multivariate adjustment (−0.32 units; P = 0.001, P for trend = 0.05). Waist circumference was inversely associated with telomere length [0.15-unit difference in z score in a comparison of the highest (≥32 in, 81.28 cm) with the lowest (≤28 in, 71.12 cm) category (P = 0.01, P for trend = 0.02) in the multivariate model]. We found no association between telomere length and smoking, physical activity, or postmenopausal hormone use.
Conclusion: Although the strength of the associations was modest in this population of middle- and older-age women, our results support the hypothesis that body composition and dietary factors are related to leukocyte telomere length, which is a potential biomarker of chronic disease risk.
INTRODUCTION
Telomeres are critical in maintaining the structural integrity of the genome and in protecting chromosomes from degradation and end-to-end fusion (1). They undergo erosion with each cycle of replication, and this shortening may trigger cellular senescence or apoptosis (2, 3), a process that is accelerated by oxidative stress and inflammation both in vitro (2–8) and in vivo (9–12). Telomere length progressively shortens with age in various mitotic tissues and cell types (2, 13–17). Leukocyte telomere length (LTL) may serve as a potential biomarker of biological age, reflecting the cumulative burden of oxidative stress and inflammation (18).
A growing body of epidemiologic and clinical data suggest that accelerated telomere attrition is associated with diseases of aging (19–21), including an increased risk of bladder cancer (22, 23), osteoporosis (24), coronary heart disease, diabetes, and heart failure (12, 25–32). Telomere length is a complex trait that is shaped by a combination of genetic, epigenetic, and environmental determinants (33–36); however, the range of factors that influence telomere dynamics is not fully established. Of the biological factors, a growing body of evidence suggest that heredity plays an important role. Several genes influence telomere length (37–39), and the reported heritability ranges from 36% to 90% (40, 41). Furthermore, genome-wide linkage studies provide evidence of linkage to autosomal regions (40, 41). Environmental and lifestyle factors may also play a key role, and shortened telomere length has been associated with, psychological stress (11), low physical activity levels (42), and equivocal data on the effects of body size (43, 44), smoking (43, 45), and socioeconomic status (46, 47) have been reported. However, to date, limited studies have investigated the relative importance of dietary intake on LTL (48, 49). Given that telomere shortening is accelerated by oxidative stress and inflammation and that diet affects both of these processes, the objective of our study was to determine the potential relation between dietary factors and LTL and also to further examine the importance of other lifestyle factors. Although this was a cross-sectional study, and therefore exploratory in nature, we hypothesized that 1) a diet high in fruit and vegetables and rich in dietary fiber and whole grains would be associated with longer LTL because these dietary factors exert antioxidant and antiinflammatory effects, and 2) a diet high in polyunsaturated and trans fatty acids, which are associated with inflammation and oxidative stress, may accelerate the biological aging process and be associated with shorter LTL.
SUBJECTS AND METHODS
The study population comprised 2284 female participants from the Nurses’ Health Study who were selected as control subjects in nested case-control studies of biological predictors of breast, skin and endometrial cancer (50, 51). The Nurses’ Health Study is a prospective cohort investigation involving 121,700 female US nurses who were 30–55 y of age at baseline in 1976. Information about health and disease is assessed biennially, and dietary information was obtained every 4 y through self-administered questionnaires (52, 53). From 1989 to 1990, a blood sample was requested from all participants and was provided by 32,826 women. Women who provided blood samples were similar to those who did not (54), and the controls who participated in the current study were randomly selected by using a risk-set sampling (55) at a ratio of 1:1 to 1:3. Controls were matched to cases on age, menopausal status, blood collection variables (time of day, season, year of blood collection, and fasting status), and recent (<3 mo) postmenopausal hormone use from the subgroup of participants who were free of diagnosed cancer at the time cancer was diagnosed in the cases. We did not include cancer cases in our analyses because case status may be related to telomere length and also to specific lifestyle characteristics that predict cancer and, thus, lead to biased results from the oversampling of women with cancer.
Anthropometric, lifestyle, self-reported blood pressure measurements, and dietary data were derived from the questionnaire administered in 1990, with missing information substituted from previous questionnaires. Body mass index (BMI) was calculated as weight (in kg) divided by the square of the height (in m). Average nutrient intakes were computed by using a validated semiquantitative food-frequency questionnaire (52, 53, 56). Physical activity, expressed in terms of metabolic equivalent task (MET) hours, was assessed by using a previously validated questionnaire (57).
Measurement of LTL
A quantitative real-time polymerase chain reaction method was used to measure relative telomere length in genomic DNA extracted from peripheral blood leukocytes (50), and the ratio of telomere repeat copy number to a single gene copy number (T:S) was determined as previously described (50). Each sample was analyzed in triplicate, and the relative telomere length was calculated as the exponentiated T:S ratio. CVs of the telomere and single-gene assay ranged from 0.57% to 3.07% and 0.56% to 2.07%, respectively. The CVs for the exponentiated T:S ratio of quality control samples ranged from 14% to 16% (50, 51).
Statistical analyses
Control subjects were derived from 3 ongoing nested case-control studies examining the relation between LTL and skin, breast, and endometrial cancer (50, 51). Given the interrun differences in assessment of relative LTL, we derived the log relative telomere length for each set of controls separately after deleting extremes (1%). We then derived a z score for each set of controls, and, after excluding participants with missing covariates, the z scores were pooled for all subsequent analyses (n = 2284).
We calculated age-adjusted participant demographic and lifestyle characteristics and age- and energy- adjusted nutrient intake data across quintiles of the LTL z score. Spearman's age-adjusted partial correlation coefficients were derived to examine the correlation of LTL with all variables. To evaluate the associations between diet and lifestyle variables and LTL we used 2 multivariable linear regression models with robust variance estimates (52). In model 1 we adjusted for age (5-y categories) and smoking status (never, past, nonsmoker with unknown past history, current smoker, and unknown), and in model 2 we additionally adjusted for postmenopausal hormone use (never, past <5 y, past ≥5 y, current <5 y, and current ≥5 y), physical activity (quintiles), and BMI (in kg/m2: <25, 25–29, 30–34, and ≥35). We also included total energy intake and energy-adjusted protein, individual fatty acids [polyunsaturated (PUFA), saturated, trans, and monounsaturated], cereal fiber (for carbohydrate analysis protein was removed from the model) in model 2. We calculated P trends across categories by including the independent variable as a continuous predictor in the regression models.
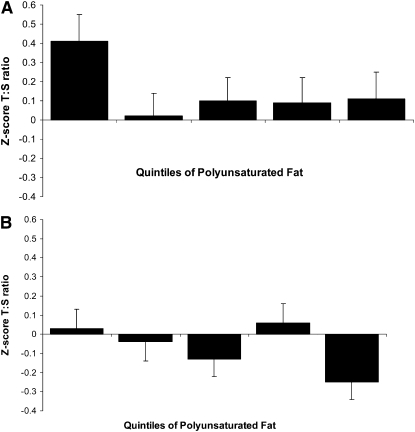
To assess the joint and independent effects of PUFAs and age, we created categorical interaction variables by cross-classifying tertiles of PUFA and age (Figure 1). Statistical analyses were conducted by using SAS software (version 9; SAS Institute, Cary, NC). All P values are 2-tailed.
FIGURE 1.
Joint associations of age, <60 y (A; n = 922) compared with ≥60 y (B; n = 1362), and polyunsaturated fatty acids on telomere length in the Nurses’ Health Study. The median intakes of polyunsaturated fatty acids were 7.4, 9.0, 10.3, 11.8, and 14.1 g/d for quintiles 1–5, respectively. Multivariable linear regression was performed, with adjustment for age, smoking, postmenopausal hormone use, BMI, physical activity, and intakes of polyunsaturated fatty acids, saturated fatty acids, trans fatty acids, monounsaturated fatty acids, energy, cereal fiber, and protein. P for interaction = 0.07. T:S, ratio of telomere repeat copy number to a single gene copy number.
RESULTS
Age was inversely correlated with LTL (r = −0.11, P < 0.0001). Subjects in the lowest quintile of LTL had a higher BMI, higher total fat intake (specifically from monounsaturated fatty acids and PUFAs), and a lower fiber intake, specifically from cereal fiber and whole grains (Table 1). The strongest age-adjusted Spearman correlations were for body composition (BMI: r = −0.06, P = 0.009; waist: r = −0.06, P = 0.01), total fat intake, polyunsaturated fatty acids and linoleic acid (all r = −0.06, P = 0.004), and fiber, specifically cereal fiber (r = 0.06, P = 0.004).
TABLE 1.
Characteristics of the 2284 women (controls) who participated in the Nurses’ Health Study by quintile of telomere length1
| Quintile of z score |
||||||
| 1 | 2 | 3 | 4 | 5 | P for trend | |
| Age (y) | 59.3 ± 6.4 | 59.7 ± 6.3 | 59.2 ± 6.5 | 58.1 ± 6.5 | 58.0 ± 6.9 | — |
| Postmenopausal (%) | 87.6 ± 0.32 | 85.9 ± 0.32 | 88.2 ± 0.31 | 87.7 ± 0.35 | 87.2 ± 0.37 | 0.82 |
| HRT use (%) | 64.7 ± 0.48 | 62.3 ± 0.49 | 65.2 ± 0.48 | 61.8 ± 0.48 | 64.4 ± 0.48 | 0.96 |
| BMI (kg/m2) | 26.0 ± 4.9 | 25.7 ± 4.8 | 25.4 ± 4.6 | 25.3 ± 4.5 | 25.3 ± 4.3 | 0.005 |
| Waist (cm) | 80.3 ± 11.5 | 80.0 ± 10.8 | 79.3 ± 10.3 | 78.7 ± 9.9 | 78.7 ± 10.1 | 0.009 |
| Hip (cm) | 101.9 ± 10.2 | 101.4 ± 9.8 | 100.8 ± 9.8 | 101.1 ± 8.9 | 100.8 ± 9.5 | 0.1 |
| Waist:hip ratio | 0.79 ± 0.12 | 0.79 ± 0.10 | 0.79 ± 0.08 | 0.78 ± 0.06 | 0.78 ± 0.12 | 0.14 |
| Weight (kg) | 70.2 ± 13.9 | 68.9 ± 13.1 | 68.3 ± 13.2 | 67.4 ± 12.6 | 68.2 ± 11.2 | 0.004 |
| Height (cm) | 164.1 ± 5.6 | 163.6 ± 5.8 | 163.8 ± 6.0 | 163.3 ± 5.9 | 164.1 ± 5.8 | 0.59 |
| Alcohol (g/d) | 6.1 ± 10.5 | 6.4 ± 10.2 | 6.1 ± 9.2 | 6.4 ± 11.2 | 6.4 ± 9.7 | 0.59 |
| Energy intake (kcal/d) | 1795 ± 494 | 1774 ± 492 | 1771 ± 488 | 1789 ± 507 | 1774 ± 474 | 0.62 |
| Total fatty acids (g/d) | 56.2 ± 10.3 | 56.0 ± 11.6 | 54.6 ± 10.2 | 55.6 ± 10.3 | 54.1 ± 11.1 | 0.003 |
| Saturated fatty acids (g/d) | 18.8 ± 4.4 | 18.7 ± 4.4 | 18.5 ± 4.5 | 18.6 ± 4.1 | 18.4 ± 4.5 | 0.11 |
| Monounsaturated fatty acids (g/d) | 21.5 ± 4.8 | 21.5 ± 5.0 | 20.7 ± 4.3 | 21.4 ± 4.8 | 20.6 ± 4.9 | 0.006 |
| Polyunsaturated fatty acids (g/d) | 10.9 ± 2.9 | 10.9 ± 3.7 | 10.5 ± 2.8 | 10.7 ± 2.8 | 10.2 ± 2.8 | 0.0008 |
| trans Fatty acids (g/d) | 2.6 ± 1.0 | 2.7 ± 1.0 | 2.5 ± 0.93 | 2.6 ± 0.94 | 2.5 ± 1.0 | 0.05 |
| Omega-3, marine (g/d) | 0.25 ± 0.19 | 0.26 ± 0.34 | 0.25 ± 0.26 | 0.25 ± 0.25 | 0.25 ± 0.21 | 0.96 |
| Linolenic acid (g/d) | 0.90 ± 0.30 | 0.94 ± 0.40 | 0.90 ± 0.33 | 0.90 ± 0.30 | 0.88 ± 0.29 | 0.13 |
| Linoleic acid (g/d) | 9.4 ± 2.7 | 9.4 ± 3.1 | 9.0 ± 2.5 | 9.2 ± 2.7 | 8.8 ± 2.6 | 0.0009 |
| Protein (g/d) | 75.9 ± 13.3 | 75.4 ± 13.7 | 75.1 ± 12.9 | 76.1 ± 12.8 | 76.8 ± 13.1 | 0.19 |
| Carbohydrates (g/d) | 200.1 ± 30.7 | 199.6 ± 35.2 | 203.4 ± 33.2 | 201.0 ± 30.9 | 204.0 ± 33.1 | 0.06 |
| Fiber (g/d) | 18.5 ± 5.3 | 18.5 ± 6.1 | 18.8 ± 5.6 | 18.6 ± 5.2 | 19.3 ± 5.7 | 0.03 |
| Cereal fiber (g/d) | 5.0 ± 2.7 | 5.5 ± 4.0 | 5.5 ± 3.5 | 5.5 ± 3.2 | 5.7 ± 3.3 | 0.006 |
| Whole grains (g/d) | 19.9 ± 16.8 | 21.2 ± 18.6 | 21.2 ± 15.0 | 21.5 ± 15.8 | 22.6 ± 17.5 | 0.01 |
| Fruit and vegetable intake | 6.0 ± 2.5 | 5.7 ± 2.4 | 5.9 ± 2.4 | 5.8 ± 2.6 | 6.0 ± 2.4 | 0.77 |
| Vitamin E (IU/ d) | 78.7 ± 179.9 | 84.3 ± 192.6 | 82.3 ± 175.8 | 104.7 ± 211.6 | 93.9 ± 190.3 | 0.07 |
| Vitamin C (mg/d) | 319.0 ± 349.6 | 310.1 ± 336.7 | 340.6 ± 392.1 | 368.5 ± 414.8 | 324.9 ± 344.2 | 0.24 |
| Vitamin D (IU/d) | 332.1 ± 220.6 | 350.8 ± 259.7 | 365.7 ± 252.3 | 374.0 ± 309.6 | 367.9 ± 242.9 | 0.01 |
| Multivitamin users (%) | 34.2 ± 0.47 | 37.6 ± 0.49 | 39.6 ± 0.49 | 43.6 ± 0.50 | 37.7 ± 0.49 | 0.09 |
| Physical activity (METs/wk) | 19.8 ± 20.9 | 20.2 ± 21.8 | 19.9 ± 20.0 | 19.2 ± 21.6 | 19.6 ± 20.7 | 0.69 |
| Smoking status | ||||||
| Never smoker (%) | 45.9 ± 0.50 | 45.2 ± 0.50 | 48.4 ± 0.50 | 48.4 ± 0.50 | 46.8 ± 0.50 | 0.49 |
| Past smoker (%) | 43.5 ± 0.50 | 41.8 ± 0.49 | 40.4 ± 0.49 | 39.6 ± 0.49 | 42.8 ± 0.50 | 0.63 |
| Current smoker (%) | 10.1 ± 0.30 | 12.6 ± 0.33 | 10.5 ± 0.31 | 11.8 ± 0.32 | 10.0 ± 0.30 | 0.76 |
| Pack-years | 12.5 ± 19.5 | 12.6 ± 18.2 | 12.1 ± 19.2 | 11.0 ± 17.2 | 11.7 ± 17.4 | 0.25 |
All values (except age) are age-adjusted means ± SDs. HRT, hormone replacement therapy; METs, metabolic equivalent tasks.
Compared with women in the lowest category of waist circumference (≤28 in, 71.12 cm), women in the top category of waist circumference (≥32 in, 81.28 cm) had a −0.15-unit difference in z score (P for trend = 0.02). In analyses stratified by age, increased waist circumference in the younger age group was negatively associated with LTL (−0.27 change in z score in the group aged <60 y, P for trend = 0.05; −0.07 in the older age group, P for trend = 0.12). The highest compared with lowest BMI category (≥35 compared with <25) was also negatively associated with LTL (P = 0.03) (Table 2).
TABLE 2.
Lifestyle determinants of telomere length (change in z score) in 2284 women from the Nurses’ Health Study1
| Mean ± SE | P | Mean ± SE | P | Mean ± SE | P | Mean ± SE | P | Mean ± SE | P | Mean ± SE | P | P for trend | |
| Pack-years | None: 0.01 ± 0.06 | Ref | <10: −0.02 ± 0.07 | 0.64 | 10–25: 0.05 ± 0.08 | 0.58 | 25–30: −0.09 ± 0.10 | 0.30 | 30–35: 0.04 ± 0.13 | 0.83 | >35: −0.004 ± 0.08 | 0.83 | 0.77 |
| Smoking (%) | Never: 0.002 ± 0.06 | Ref | Past quit <10 y: 0.05 ± 0.07 | 0.46 | Past quit >10 y: −0.007 ± 0.07 | 0.87 | Current 1–14: 0.04 ± 0.11 | 0.71 | Current 15–24: −0.06 ± 0.11 | 0.58 | Current ≥25: 0.005 ± 0.15 | 0.99 | — |
| Postmenopausal hormone use (%) | Never: 0.05 ± 0.06 | Ref | Past <5 y: −0.01 ± 0.07 | 0.33 | Past ≥5 y: 0.09 ± 0.17 | 0.84 | Current <5 y: −0.004 ± 0.06 | 0.30 | Current ≥5 y: −0.06 ± 0.10 | 0.20 | — | — | — |
| BMI (kg/m2) | 18.5 to <25: 0.10 ± 0.06 | Ref | 25–29: −0.01 ± 0.06 | 0.02 | 30–34: 0.07 ± 0.09 | 0.66 | ≥35: −0.14 ± 0.12 | 0.03 | — | — | — | — | 0.07 |
| Waist (inches)(n = 1837) | ≤28 (71.1 cm): 0.13 ± 0.06 | Ref | 28–32 (71.1–81.3 cm): 0.06 ± 0.07 | 0.27 | ≥32 (81.3 cm): −0.02 ± 0.07 | 0.01 | — | — | — | — | — | — | 0.02 |
| Waist:hip ratio (n = 1830) | <0.74: 0.14 ± 0.07 | Ref | 0.74–0.79: 0.06 ± 0.06 | 0.20 | ≥0.80: −0.05 ± 0.07 | 0.003 | — | — | — | — | — | — | 0.14 |
| Physical activity (METs/wk) | Q1: −0.03 ± 0.07 | Ref | Q2: 0.02 ± 0.07 | 0.39 | Q3: 0.07 ± 0.08 | 0.13 | Q4: 0.05 ± 0.08 | 0.22 | Q5: −0.09 ± 0.07 | 0.43 | — | — | 0.13 |
n = 2284 except for waist (n = 1837) and waist:hip ratio (n = 1830). Multivariable linear regression with adjustment for age, smoking, postmenopausal hormone use, BMI, physical activity, and intakes of polyunsaturated fatty acids, saturated fatty acids, trans fatty acids, monounsaturated fatty acids, energy, cereal fiber, and protein. METs, metabolic equivalent tasks; Ref, reference; Q, quintile.
Total fat intake was only inversely associated with LTL after adjustment for age and smoking, but this association did not remain statistically significant after multivariate adjustment. However, results from analyses of individual fatty acid intake suggest that a higher PUFA intake (−0.26 z score units, P for trend =0.02), specifically linoleic acid intake, was inversely associated with LTL in the multivariate model (−0.32 z score unit difference, P for trend = 0.05; Table 3). As shown in Figure 1, LTL was lowest among the older women in the highest category of PUFA intake (P = 0.004, P for interaction = 0.07).
TABLE 3.
Dietary determinants of telomere length (change in z score) in 2284 women from the Nurses’ Health Study1
| Quintile 1 |
Quintile 2 |
Quintile 3 |
Quintile 4 |
Quintile 5 |
|||||||
| Mean ± SE | P | Mean ± SE | P | Mean ± SE | P | Mean ± SE | P | Mean ± SE | P | P for trend | |
| Energy intake (kcal/d) | 0.01 ± 0.07 | Ref | −0.01 ± 0.07 | 0.71 | −0.02 ± 0.07 | 0.59 | 0.03 ± 0.08 | 0.88 | 0.02 ± 0.07 | 0.93 | 0.76 |
| Total fatty acids (g/d) | 0.10 ± 0.08 | Ref | 0.05 ± 0.07 | 0.42 | −0.02 ± 0.07 | 0.07 | −0.06 ± 0.07 | 0.02 | −0.01 ± 0.07 | 0.12 | 0.05 |
| Total protein (g/d) | −0.03 ± 0.08 | Ref | 0.01 ± 0.08 | 0.55 | −0.04 ± 0.07 | 0.94 | 0.07 ± 0.08 | 0.15 | 0.02 ± 0.07 | 0.49 | 0.34 |
| Carbohydrate (g/d) | −0.01 ± 0.08 | Ref | 0.03 ± 0.07 | 0.56 | 0.00009 ± 0.07 | 0.84 | 0.008 ± 0.08 | 0.79 | 0.007 ± 0.09 | 0.82 | 0.88 |
| Fiber (g/d) | −0.006 ± 0.07 | Ref | −0.10 ± 0.08 | 0.21 | −0.03 ± 0.07 | 0.68 | 0.007 ± 0.08 | 0.87 | 0.12 ± 0.08 | 0.11 | 0.03 |
| Cereal fiber (g/d) | −0.08 ± 0.07 | Ref | 0.03 ± 0.08 | 0.11 | −0.003 ± 0.07 | 0.22 | −0.02 ± 0.08 | 0.40 | 0.11 ± 0.08 | 0.007 | 0.03 |
| Whole grains (g/d) | −0.08 ± 0.07 | Ref | 0.02 ± 0.07 | 0.19 | −0.04 ± 0.07 | 0.65 | 0.04 ± 0.08 | 0.11 | 0.08 ± 0.08 | 0.04 | 0.46 |
| Vitamin E (IU/d) | −0.10 ± 0.08 | Ref | −0.02 ± 0.07 | 0.25 | 0.05 ± 0.07 | 0.05 | 0.06 ± 0.08 | 0.04 | 0.03 ± 0.07 | 0.06 | 0.49 |
| Saturated fatty acids (g/d) | −0.008 ± 0.09 | Ref | −0.04 ± 0.08 | 0.62 | −0.005 ± 0.08 | 0.98 | 0.004 ± 0.07 | 0.89 | 0.08 ± 0.08 | 0.38 | 0.29 |
| Polyunsaturated fatty acids (g/d) | 0.15 ± 0.08 | Ref | −0.04 ± 0.07 | 0.02 | −0.04 ± 0.07 | 0.01 | 0.06 ± 0.08 | 0.28 | −0.11 ± 0.08 | 0.002 | 0.02 |
| Monounsaturated fatty acids (g/d) | 0.04 ± 0.09 | Ref | 0.05 ± 0.08 | 0.88 | −0.02 ± 0.07 | 0.48 | −0.02 ± 0.08 | 0.56 | −0.03 ± 0.08 | 0.49 | 0.42 |
| trans Fatty acids (g/d) | 0.02 ± 0.08 | Ref | −0.02 ± 0.07 | 0.52 | −0.01 ± 0.08 | 0.63 | 0.02 ± 0.07 | 0.95 | 0.03 ± 0.08 | 0.94 | 0.70 |
| Linolenic acid (g/d) | −0.03 ± 0.08 | Ref | 0.07 ± 0.08 | 0.18 | −0.08 ± 0.08 | 0.51 | 0.01 ± 0.07 | 0.60 | 0.10 ± 0.08 | 0.1 | 0.23 |
| Linoleic acid (g/d) | 0.19 ± 0.08 | Ref | 0.02 ± 0.07 | 0.03 | −0.04 ± 0.07 | 0.007 | 0.04 ± 0.08 | 0.09 | −0.13 ± 0.08 | 0.001 | 0.046 |
| Vitamin D (IU) | −0.03 ± 0.08 | Ref | 0.03 ± 0.07 | 0.45 | −0.05 ± 0.07 | 0.74 | 0.03 ± 0.07 | 0.40 | 0.04 ± 0.07 | 0.38 | 0.11 |
| Fruit and vegetables (g/d) | 0.01 ± 0.07 | Ref | 0.05 ± 0.07 | 0.60 | −0.02 ± 0.08 | 0.68 | −0.04 ± 0.08 | 0.54 | 0.03 ± 0.08 | 0.83 | 0.75 |
n = 2284. Multivariable linear regression with adjustment for age, smoking, postmenopausal hormone use, BMI, physical activity, and intakes of polyunsaturated fatty acids, saturated fatty acids, trans fatty acids, monounsaturated fatty acids, energy, cereal fiber, and protein (for carbohydrate analyses, protein was removed from model). Ref, reference.
In contrast with PUFAs, dietary fiber intake was positively associated with LTL, specifically cereal fiber, with an increase of 0.19 z score units between the highest and lowest quintiles (quintile 5 compared with quintile 1: P for trend = 0.03), and this was in part explained by whole-grain intake. In our analyses of other factors, there was a trend toward a relation between increased vitamin E intake and LTL, but it was not statistically significant. No significant associations were observed for vitamin D intake, fruit and vegetable intake, smoking, physical activity, or postmenopausal hormone use.
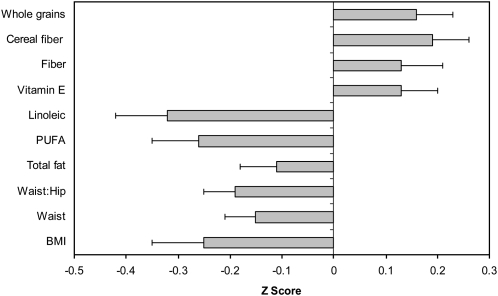
The relative effects of many dietary and anthropometric measures on LTL are summarized in Figure 2. These data suggest that the relative magnitude of the association of body composition and diet on LTL are similar; a difference of 0.24 SDs between the average T:S ratio when the extremes of BMI categories were compared and similar effects when the highest and lowest quintiles of PUFA intake were compared.
FIGURE 2.
Relative effect of body composition and dietary factors on telomere length (change in z score) in the Nurses’ Health Study (comparison of quintile 5 with quintile 1). Multivariable linear regression was performed, with adjustment for age, smoking, postmenopausal hormone use, BMI, physical activity, and intakes of polyunsaturated fatty acids, saturated fatty acids, trans fatty acids, monounsaturated fatty acids, energy, cereal fiber, and protein. n = 2284, except for waist (n = 1837) and waist:hip ratio (n = 1830). PUFA, polyunsaturated fatty acids.
DISCUSSION
In this cross-sectional study, waist circumference and polyunsaturated fatty acid intake were inversely associated with LTL, whereas a diet high in fiber, specifically cereal fiber, was positively associated with longer LTL. Although the strength of the associations was modest, our results support the hypothesis that body composition and dietary factors are related to LTL in women, a potential mediator of chronic disease risk.
Accelerated telomere attrition is not only associated with diseases of aging (12, 19–32), but telomere shortening may more accurately predict the biological aging process than the chronological age (2). To date, few studies have examined lifestyle determinants of LTL (42–47), and to our knowledge even fewer studies have investigated the relative effect of diet on LTL in humans (48, 49, 63). Our finding that dietary factors and other lifestyle factors are associated with LTL may in part explain potential pathways by which diet and body composition affect the risk of developing type 2 diabetes, cardiovascular disease, and some cancers (58–62).
We observed an inverse association between waist circumference, BMI, and the waist:hip ratio and LTL after adjusting for age and smoking; however, after adjustment for many known lifestyle factors, this relation only remained significant for waist circumference (Table 2). Previous data on the effects of body composition on LTL have been equivocal (43, 44), with one study showing that women with a BMI > 30 had shorter telomeres than those with a BMI < 20 after adjustment for age, but this relation did not remain significant after further adjustment for smoking (43). In the Cardiovascular Health Study, associations between BMI and LTL were observed only in overweight men (BMI > 27) and not in women (BMI > 25) (29). Biologically, an inverse relation between obesity and LTL is plausible given that accumulating adiposity increases oxidative stress and causes deregulation of inflammatory cytokines (64). Compared with genomic DNA, the G-rich telomeric sequence is not only a potential target for acute oxidative damage, but telomeric DNA is relatively less capable of DNA repair, resulting in accelerated telomere loss during the cell cycle and subsequent replicative senescence (4)
To date, there are limited data on the influence of dietary factors on LTL; however, in a recent pilot study, adoption of a healthy lifestyle was associated with an increase in telomerase activity in peripheral blood mononuclear cells—an important finding because telomerase activity is normally low or undetectable in most adult somatic cells (48). The study involved following a 3-mo comprehensive lifestyle change, including dietary change (low-fat, high-plant based), moderate aerobic exercise, stress management, and supplementation with soy, selenium, fish oil, and vitamin C; this intervention resulted in significant reductions in BMI and blood pressure and decreases in LDL cholesterol and psychological stress (48). Although these findings are important, it is difficult to disentangle the relative importance of the individual factors in modifying telomerase activity or to determine whether changes were due specifically to the interventions or to the changes in the clinical characteristics that resulted from the interventions.
Two previous small cross-sectional studies have examined the relation between some dietary components and LTL. In a multiethnic cross-sectional study, the only significant relation between food groups and LTL was an inverse association between consumption of processed meat, which was independent of intake of other food groups (49). In a second study of British women, serum 25-hydroxyvitamin D was significantly related to LTL (63). We were unable to replicate these findings in our large cross-sectional study of women.
In our study, the individual fatty acids were more strongly associated with LTL than with total fat. Specifically, intake of the n−6 PUFA linoleic acid was inversely associated with LTL (Table 2). Available evidence on the effects of n−6 PUFA interventions on cardiovascular disease risk markers suggest that consumption of ≥5–10% energy from omega-6 (n−6) PUFAs has the greatest cardiovascular benefit (65). Furthermore, no association between breast and prostate cancer risk and n−6 PUFAs or linoleic acid has been reported (66–69). n−6 PUFAs are involved in a range of biological pathways, and, although there is evidence suggesting that the mediators formed from n−6 fatty acids may exert proinflammatory and pro-arrhythmic effects (70) and result in modulation of gene expression (71), the modest observed effects of n−6 PUFAs on LTL may be outweighed by the strong inverse association of linoleic acid with LDL cholesterol and other chronic disease risk biomarkers and beneficial metabolic processes (65). Our findings merit further investigation in other studies.
Given that shorter telomeres may represent a potential marker of the cumulative burden of oxidative stress and inflammation, our finding that dietary fiber intake is positively associated with LTL, specifically cereal fiber and whole-grain intake, suggests that a diet high in plant-based foods may favorably influence telomere length via antiinflammatory and antioxidant mechanisms. Growing evidence supports the influence of whole grains and diets high in fruit and vegetables on inflammatory processes (72–75), and a high intake of plant-based diets and whole grains is inversely associated with total mortality and risk factors for chronic disease (76–79). This finding warrants further investigation, particularly to examine the relative importance of specific plant bioactivities such as dietary lignans on telomere biology.
Several previous studies have observed an association between smoking and telomere length (23, 43). In one study, adjusted telomere length was lower in women smokers than in nonsmokers (43) However, in our study and others, smoking status was not associated with LTL (29, 30, 80, 81)—a surprising finding given that telomere attrition is accelerated by oxidative stress (4). In agreement with Nettleton et al (49), we observed no association between physical activity (active leisure time) and telomere length. In one previous study, physical activity was associated with telomere length; however, diet was not included in the multivariate model (42).
Our study had several limitations. The cross-sectional design limits causal inference, and there is the possibility of unmeasured confounding, although we controlled for many lifestyle and dietary factors previously associated with telomere length. Our study relies on a single measure of telomere length; therefore, we cannot examine interindividual variability in telomere length over longer periods of time, and the lack of serial measurements may have limited our ability to detect associations. Our telomere length data were derived from 3 ongoing nested case-control studies and to pool the data z scores were derived. As a result, although we were able to show the magnitude of the associations, we were unable to quantify the T:S ratio or to determine the approximate age-related changes associated with the various lifestyle and dietary factors (Figure 2). We only had data available for healthy, primarily white women; therefore, the findings may not be generalizable to men or other ethnicities. As in any observational study, measurement error in self-reported variables is inevitable; however, misclassification in this prospective study would underestimate the true relation. Although we attempted to control for any potential confounding variables, the possibility of residual confounding remains.
In conclusion, we found that waist circumference and polyunsaturated fatty acid intake were negatively associated, and dietary fiber, specifically cereal fiber, was positively associated with LTL in a large cross-sectional study of middle-aged and older women. Although the strength of the associations was modest, our results support the hypothesis that body composition and dietary factors are related to LTL in women—a potential mediator of chronic disease risk.
Acknowledgments
The authors’ responsibilities were as follows—AC, YL, and EBR: conducted the statistical analysis; AC and EBR: interpreted the data and drafted the manuscript; IDV and JP: performed the telomere length measurements; and DJH, IDV, and JP: critically reviewed the manuscript. All authors contributed to the manuscript and agreed to the final version. None of the authors had a conflict of interest.
REFERENCES
- 1.Blackburn EH. Telomere states and cell fates. Nature 2000;408:53–6 [DOI] [PubMed] [Google Scholar]
- 2.Harley CB, Vaziri H, Counter CM, Allsopp RC. The telomere hypothesis of cellular aging. Exp Gerontol 1992;27:375–82 [DOI] [PubMed] [Google Scholar]
- 3.Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 2005;579:859–62 [DOI] [PubMed] [Google Scholar]
- 4.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci 2002;27:339–44 [DOI] [PubMed] [Google Scholar]
- 5.Serra V, von Zglinicki T, Lorenz M, Saretzki G. Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slows telomere shortening. J Biol Chem 2003;278:6824–30 [DOI] [PubMed] [Google Scholar]
- 6.Forsyth NR, Evans AP, Shay JW, Wright WE. Developmental differences in the immortalization of lung fibroblasts by telomerase. Aging Cell 2003;2:235–43 [DOI] [PubMed] [Google Scholar]
- 7.Haendeler J, Hoffmann J, Brandes RP, Zeiher AM, Dimmeler S. Hydrogen peroxide triggers nuclear export of telomerase reverse transcriptase via Src kinase family-dependent phosphorylation of tyrosine 707. Mol Cell Biol 2003;23:4598–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Zglinicki T, Serra V, Lorenz M, et al. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab Invest 2000;80:1739–47 [DOI] [PubMed] [Google Scholar]
- 9.Cattan V, Mercier N, Gardner JP, et al. Chronic oxidative stress induces a tissue-specific reduction in telomere length in CAST/Ei mice. Free Radic Biol Med 2008;44:1592–8 [DOI] [PubMed] [Google Scholar]
- 10.Tarry-Adkins JL, Ozanne SE, Norden A, Cherif H, Hales CN. Lower antioxidant capacity and elevated p53 and p21 may be a link between gender disparity in renal telomere shortening, albuminuria, and longevity. Am J Physiol Renal Physiol 2006;290:F509–16 [DOI] [PubMed] [Google Scholar]
- 11.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA 2004;101:17312–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell 2006;5:325–30 [DOI] [PubMed] [Google Scholar]
- 13.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ 2006;174:801–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crespo CJ, Keteyian SJ, Heath GW, Sempos CT. Leisure-time physical activity among US adults. Results from the Third National Health and Nutrition Examination Survey. Arch Intern Med 1996;156:93–8 [PubMed] [Google Scholar]
- 15.Kirkwood TB, Austad SN. Why do we age? Nature 2000;408:233–8 [DOI] [PubMed] [Google Scholar]
- 16.Hastie ND, Dempster M, Dunlop MG, Thompson AM, Green DK, Allshire RC. Telomere reduction in human colorectal carcinoma and with ageing. Nature 1990;346:866–8 [DOI] [PubMed] [Google Scholar]
- 17.Ishii A, Nakamura K, Kishimoto H, et al. Telomere shortening with aging in the human pancreas. Exp Gerontol 2006;41:882–6 [DOI] [PubMed] [Google Scholar]
- 18.Aviv A. Telomeres and human aging: facts and fibs. Sci Aging Knowledge Environ 2004;2004:pe43. [DOI] [PubMed] [Google Scholar]
- 19.Aviv A. Telomeres and human somatic fitness. J Gerontol A Biol Sci Med Sci 2006;61:871–3 [DOI] [PubMed] [Google Scholar]
- 20.Fuster JJ, Andres V. Telomere biology and cardiovascular disease. Circ Res 2006;99:1167–80 [DOI] [PubMed] [Google Scholar]
- 21.Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med 2008;44:235–46 [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Amos CI, Zhu Y, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst 2003;95:1211–8 [DOI] [PubMed] [Google Scholar]
- 23.McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 2007;16:815–9 [DOI] [PubMed] [Google Scholar]
- 24.Valdes AM, Richards JB, Gardner JP, et al. Telomere length in leukocytes correlates with bone mineral density and is shorter in women with osteoporosis. Osteoporos Int 2007;18:1203–10 [DOI] [PubMed] [Google Scholar]
- 25.Benetos A, Okuda K, Lajemi M, et al. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension 2001;37:381–5 [DOI] [PubMed] [Google Scholar]
- 26.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol 2003;23:842–6 [DOI] [PubMed] [Google Scholar]
- 27.Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 2006;29:283–9 [DOI] [PubMed] [Google Scholar]
- 28.Fuster JJ, Diez J, Andres V. Telomere dysfunction in hypertension. J Hypertens 2007;25:2185–92 [DOI] [PubMed] [Google Scholar]
- 29.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol 2007;165:14–21 [DOI] [PubMed] [Google Scholar]
- 30.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet 2007;369:107–14 [DOI] [PubMed] [Google Scholar]
- 31.van der Harst P, van der Steege G, de Boer RA, et al. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol 2007;49:1459–64 [DOI] [PubMed] [Google Scholar]
- 32.Zee RY, Michaud SE, Germer S, Ridker PM. Association of shorter mean telomere length with risk of incident myocardial infarction: a prospective, nested case-control approach. Clin Chim Acta 2009;403:139–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munoz P, Blanco R, Flores JM, Blasco MA. XPF nuclease-dependent telomere loss and increased DNA damage in mice overexpressing TRF2 result in premature aging and cancer. Nat Genet 2005;37:1063–71 [DOI] [PubMed] [Google Scholar]
- 34.Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nat Cell Biol 2005;7:712–8 [DOI] [PubMed] [Google Scholar]
- 35.Steinert S, Shay JW, Wright WE. Modification of subtelomeric DNA. Mol Cell Biol 2004;24:4571–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benetti R, Garcia-Cao M, Blasco MA. Telomere length regulates the epigenetic status of mammalian telomeres and subtelomeres. Nat Genet 2007;39:243–50 [DOI] [PubMed] [Google Scholar]
- 37.Gatbonton T, Imbesi M, Nelson M, et al. Telomere length as a quantitative trait: genome-wide survey and genetic mapping of telomere length-control genes in yeast. PLoS Genet 2006;2:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saldanha SN, Andrews LG, Tollefsbol TO. Assessment of telomere length and factors that contribute to its stability. Eur J Biochem 2003;270:389–403 [DOI] [PubMed] [Google Scholar]
- 39.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 2005;19:2100–10 [DOI] [PubMed] [Google Scholar]
- 40.Andrew T, Aviv A, Falchi M, et al. Mapping genetic loci that determine leukocyte telomere length in a large sample of unselected female sibling pairs. Am J Hum Genet 2006;78:480–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasa-Nicotera M, Brouilette S, Mangino M, et al. Mapping of a major locus that determines telomere length in humans. Am J Hum Genet 2005;76:147–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med 2008;168:154–8 [DOI] [PubMed] [Google Scholar]
- 43.Valdes AM, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet 2005;366:662–4 [DOI] [PubMed] [Google Scholar]
- 44.Nordfjall K, Eliasson M, Stegmayr B, Melander O, Nilsson P, Roos G. Telomere length is associated with obesity parameters but with a gender difference. Obesity (Silver Spring) 2008;16:2682–9 [DOI] [PubMed] [Google Scholar]
- 45.Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet 2004;363:507–10 [DOI] [PubMed] [Google Scholar]
- 46.Cherkas LF, Aviv A, Valdes AM, et al. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell 2006;5:361–5 [DOI] [PubMed] [Google Scholar]
- 47.Adams J, Martin-Ruiz C, Pearce MS, White M, Parker L, von Zglinicki T. No association between socio-economic status and white blood cell telomere length. Aging Cell 2007;6:125–8 [DOI] [PubMed] [Google Scholar]
- 48.Ornish D, Lin J, Daubenmier J, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol 2008;9:1048–57 [DOI] [PubMed] [Google Scholar]
- 49.Nettleton JA, Diez-Roux A, Jenny NS, Fitzpatrick AL, Jacobs DR., Jr Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2008;88:1405–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Vivo I, Prescott J, Wong JY, Kraft P, Hankinson SE, Hunter DJ. A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev 2009;18:1152–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han J, Qureshi AA, Prescott J, et al. A prospective study of telomere length and the risk of skin cancer. J Invest Dermatol 2009;129:415–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willett WC, Reynolds RD, Cottrell-Hoehner S, Sampson L, Browne ML. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. J Am Diet Assoc 1987;87:43–7 [PubMed] [Google Scholar]
- 53.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer 2005;5:388–96 [DOI] [PubMed] [Google Scholar]
- 54.Hankinson SE, Colditz GA, Hunter DJ, et al. Reproductive factors and family history of breast cancer in relation to plasma estrogen and prolactin levels in postmenopausal women in the Nurses’ Health Study (United States). Cancer Causes Control 1995;6:217–24 [DOI] [PubMed] [Google Scholar]
- 55.Prentice RL, Breslow NE. Retrospective studies and failure time models. Biometrika 1978;65:153–8 [Google Scholar]
- 56.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26, discussion 1127–36 [DOI] [PubMed] [Google Scholar]
- 57.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9 [DOI] [PubMed] [Google Scholar]
- 58.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105–20 [DOI] [PubMed] [Google Scholar]
- 59.Willett W. Nutrition and cancer: the search continues. Nutr Cancer 2008;60:557–9 [DOI] [PubMed] [Google Scholar]
- 60.Hu FB. Plant-based foods and prevention of cardiovascular disease: an overview. Am J Clin Nutr 2003;78:544S–51S [DOI] [PubMed] [Google Scholar]
- 61.Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 2008;31:1311–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fung TT, McCullough M, van Dam RM, Hu FB. A prospective study of overall diet quality and risk of type 2 diabetes in women. Diabetes Care 2007;30:1753–7 [DOI] [PubMed] [Google Scholar]
- 63.Richards JB, Valdes AM, Gardner JP, et al. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in women. Am J Clin Nutr 2007;86:1420–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harris WS, Mozaffarian D, Rimm E, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009;119:902–7 [DOI] [PubMed] [Google Scholar]
- 66.Giovannucci E, Rimm EB, Colditz GA, et al. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst 1993;85:1571–9 [DOI] [PubMed] [Google Scholar]
- 67.Mannisto S, Pietinen P, Virtanen MJ, et al. Fatty acids and risk of prostate cancer in a nested case-control study in male smokers. Cancer Epidemiol Biomarkers Prev 2003;12:1422–8 [PubMed] [Google Scholar]
- 68.Harvei S, Bjerve KS, Tretli S, Jellum E, Robsahm TE, Vatten L. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. Int J Cancer 1997;71:545–51 [DOI] [PubMed] [Google Scholar]
- 69.Kim EH, Willett WC, Colditz GA, et al. Dietary fat and risk of postmenopausal breast cancer in a 20-year follow-up. Am J Epidemiol 2006;164:990–7 [DOI] [PubMed] [Google Scholar]
- 70.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci USA 2003;100:1751–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmitz G, Ecker J. The opposing effects of n−3 and n−6 fatty acids. Prog Lipid Res 2008;47:147–55 [DOI] [PubMed] [Google Scholar]
- 72.Nettleton JA, Steffen LM, Mayer-Davis EJ, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2006;83:1369–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Esposito K, Marfella R, Ciotola M, et al. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 2004;292:1440–6 [DOI] [PubMed] [Google Scholar]
- 74.Lopez-Garcia E, Schulze MB, Fung TT, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2004;80:1029–35 [DOI] [PubMed] [Google Scholar]
- 75.Fung TT, Rimm EB, Spiegelman D, et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr 2001;73:61–7 [DOI] [PubMed] [Google Scholar]
- 76.Newby PK, Maras J, Bakun P, Muller D, Ferrucci L, Tucker KL. Intake of whole grains, refined grains, and cereal fiber measured with 7-d diet records and associations with risk factors for chronic disease. Am J Clin Nutr 2007;86:1745–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Munter JS, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole grain, bran, and germ intake and risk of type 2 diabetes: a prospective cohort study and systematic review. PLoS Med 2007;4:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qi L, Hu FB. Dietary glycemic load, whole grains, and systemic inflammation in diabetes: the epidemiological evidence. Curr Opin Lipidol 2007;18:3–8 [DOI] [PubMed] [Google Scholar]
- 79.Heidemann C, Schulze MB, Franco OH, van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation 2008;118:230–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Broberg K, Bjork J, Paulsson K, Hoglund M, Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis 2005;26:1263–71 [DOI] [PubMed] [Google Scholar]
- 81.Bischoff C, Petersen HC, Graakjaer J, et al. No association between telomere length and survival among the elderly and oldest old. Epidemiology 2006;17:190–4 [DOI] [PubMed] [Google Scholar]