Abstract
Background: The range of protein intakes for optimizing bone health among premenopausal women is unclear. Protein is a major constituent of bone, but acidic amino acids may promote bone resorption.
Objective: The objective was to examine cross-sectional and longitudinal associations between baseline dietary protein and bone mineral density (BMD) among 560 females aged 14–40 y at baseline enrolled in a Pacific Northwest managed-care organization. The role of protein source (animal or vegetable) and participant characteristics were considered.
Design: Dietary protein intake was assessed by using a semiquantitative food-frequency questionnaire in participants enrolled in a study investigating associations between hormonal contraceptive use and bone health. Annual changes in hip, spine, and whole-body BMD were measured by using dual-energy X-ray absorptiometry. Cross-sectional and longitudinal associations between baseline protein intake (% of energy) and BMD were examined by using linear regression analysis and generalized estimating equations adjusted for confounders.
Results: The mean (±SD) protein intake at baseline was 15.5 ± 3.2%. After multivariable adjustment, the mean BMD was similar across each tertile of protein intake. In cross-sectional analyses, low vegetable protein intake was associated with a lower BMD (P = 0.03 for hip, P = 0.10 for spine, and P = 0.04 for whole body). For every percentage increase in the percentage of energy from protein, no significant longitudinal changes in BMD were observed at any anatomic site over the follow-up period.
Conclusions: Data from this longitudinal study suggest that a higher protein intake does not have an adverse effect on bone in premenopausal women. Cross-sectional analyses suggest that low vegetable protein intake is associated with lower BMD.
INTRODUCTION
The effects of dietary protein intake on bone health are controversial. Protein is a major constituent of bone (1), so adequate protein intake is critical to maintaining bone health. Whereas a detrimental effect of insufficient protein intake on bone health has been documented (2), there also has been considerable concern over reports that higher-protein diets increase urinary calcium (3–6). Furthermore, acidifying amino acids such as cysteine and methionine, released after protein digestion, can stimulate osteoclastic bone resorption, thereby reducing bone mineral density (BMD) (7). Other data suggest the increased urinary calcium excretion due to higher protein intakes is compensated for by increased intestinal absorption of calcium (4), or that the adverse effects of high protein intake on bone are observed only among individuals with insufficient intakes of calcium (5).
The appropriate range of protein intakes for optimizing bone health among premenopausal women is unclear, as reflected by the width of the Acceptable Macronutrient Distribution Range for protein (10–35% of energy for adults aged >18 y) (8). To help inform public health recommendations for protein intake, we analyzed data from 2 longitudinal studies of bone health among females aged 14–40 y at baseline. We investigated the association of baseline total protein intake and baseline BMD and changes in BMD over time. We also evaluated whether the observed associations between protein and BMD varied by type of protein intake (animal compared with vegetable), age, BMI, physical activity, recent depot medroxyprogesterone acetate (DMPA) injectable contraceptive use, smoking, or calcium intake.
SUBJECTS AND METHODS
Subjects
This analysis used data from 2 cohort studies that enrolled a total of 631 participants to examine the effects of DMPA contraception on BMD, conducted at Group Health Cooperative, a Pacific Northwest nonprofit managed health care organization. The first study (1994–1999) enrolled 457 women aged 18–40 y and followed participants for 3 y. The second cohort (1999–2004) consisted of 174 females aged 14–18 y who were followed for 2 to 3 y. Women with conditions or using other medications known to affect bone mass were excluded from participation, and all adolescents had attained menarche before enrollment. For these analyses, additional exclusion criteria were invalid data on dual-energy X-ray absorptiometry (DXA) measurements (n = 6), misclassification of primary exposure (DMPA use; n = 21), or food-frequency questionnaires (FFQ) with implausible energy intakes (<500 or >5000 calories; n = 44). The Group Health Human Subjects Committee reviewed and approved all study procedures. All participants provided written informed consent or assent with parental approval for adolescents.
Data collection
At baseline, participants completed questionnaires querying for demographic information (age, race-ethnicity, and education), behaviors (smoking and physical activity), and medical history (age at menarche, family history of fracture, pregnancies, and hormonal contraceptive use). Height and weight were measured in the clinic, and BMI was calculated [weight (kg)/ height2 (m)]. Weight-bearing physical activity was assessed by adapting 2 validated measures (9, 10) for use in young women. To derive the weight-bearing physical activity score, the frequency (6-point scale) of 17 specific activities, up to 2 write-in sports activities, and up to 2 other regular activities was collected. Each activity was assigned a weight-bearing value of 0 (none), 1 (low/medium), or 2 (medium/high). The physical activity score was obtained by multiplying the weight-bearing value, and the frequency of each activity and summing up over all activities. Recent DMPA exposure was defined as current use, as reported by questionnaire and in-person interview by the clinic staff.
Outcome assessment
BMD and bone mineral content (BMC) of the total hip, the lumbar spine, and the whole body were measured semiannually by DXA. The Hologic 2000 and Hologic 4500 (Hologic Inc, Bedford, MA) were used for the cohort study conducted in 1994–1999, and the Hologic 4500 and Hologic Delphi (MNAP, Philadelphia, PA) were used for the study conducted in 1999–2004. A cross-calibration was performed in each study, in which measurements were taken on both machines for an independent sample and an adjustment was calculated to calibrate the BMD measures, as previously described (11). CVs ranged from 0.72% to 1.5%. Standard techniques were used by densitometrists who were trained by the manufacturer.
Exposure assessment
Protein intake was assessed at baseline and at annual follow-up visits by using an FFQ developed and evaluated in the Women's Health Initiative at the Fred Hutchinson Cancer Research Center (Seattle, WA) (12). Consistent with current dietary guidelines, protein was evaluated as a percentage of total energy. All nutrient measures were evaluated by using the FFQ.
Statistical analysis
Participant characteristics of women included and those excluded because of extreme caloric intakes on the FFQ (n = 44) were compared. Baseline characteristics and other dietary factors that can affect bone health were compared by tertile of baseline protein intake by analysis of variance for continuous variables and chi-square tests for categorical variables. Adjusted mean BMD at baseline was calculated by tertile of baseline protein intake by using linear regression models. Potential confounders of the association between protein intake and bone health were evaluated by testing bivariate associations based on the previous literature and the original study designs (race-ethnicity, age of menarche, time since menarche, family history of fracture, age, BMI, physical activity score, calories, dietary calcium, phosphorous, dietary vitamin D, magnesium, fluoride, alcohol, smoking, DMPA use, prior pregnancy, and education). The association of baseline protein intake with baseline BMD was examined by using models adjusted for variables associated with protein intake and BMD (age, BMI, physical activity, smoking, and recent DMPA use) as well as nutritional factors significantly associated with both the exposure and outcome in bivariate analyses (energy intake, phosphorous, and magnesium). Additional models estimated associations by type of protein intake by including separate terms for animal and vegetable protein in regression models.
To estimate longitudinal associations, we used linear regression to model absolute and percentage changes in BMD from baseline to each follow-up visit (12, 24, and 36 mo) as a function of baseline protein intake. Percentage energy from protein was modeled both continuously and categorically (tertiles) to allow for the possibility of nonlinear associations between protein intake and BMD. Likelihood ratio tests estimated P values for the main effect of categorized protein on BMD at each site (hip, spine, and whole body).
We also evaluated models that included baseline and annual data with protein exposure updated annually, accounting for the dependence of repeated observations within a study participant by using generalized estimating equations with an independent correlation structure. Cross-sectional analyses were repeated characterizing protein in absolute amounts (g/d) and expressed as a function of body weight (g/kg body wt). Cross-sectional and longitudinal analyses were repeated by using BMC as the outcome measure.
Statistical tests for interaction with baseline age category, BMI, physical activity, recent DMPA use, smoking, and calcium intake were conducted by using the likelihood ratio test statistic for cross-sectional analyses and the Wald test statistic for analyses of generalized estimating equations. Data were analyzed by using SAS (version 9.2; SAS Institute, Cary, NC), and statistical significance was defined as P < 0.05 by using 2-sided tests.
RESULTS
Baseline characteristics
Participants excluded because of invalid FFQs were more likely to be overweight (P = 0.005), nonwhite (P < 0.0001), and a recent DMPA user (P = 0.02). Overall, mean (±SD) protein intake as a proportion of energy was 15.5% ± 3.2%. Women consuming a higher proportion of energy from protein had a higher BMI and were less likely to smoke than were women consuming less protein (Table 1). Although the amount of vegetable protein was consistent across tertiles, the amount of animal protein intake was higher among women with a high total protein intake (Table 1). Higher protein intake was associated with lower carbohydrate (P < 0.0001) and caffeine (P = 0.01) intakes but with higher calcium (P < 0.0001), phosphorous (P < 0.0001), vitamin D (P < 0.0001), magnesium (P < 0.0001), and vegetable (P = 0.001) intakes (Table 1).
TABLE 1.
Baseline characteristics by tertile of protein intake1
| Tertile of protein intake (% of energy) |
||||
| Characteristic | Low (5.7–14.3) | Medium (14.4–17.1) | High (17.2–27.6) | P2 |
| No. of subjects | 186 | 187 | 187 | |
| Age (y) | 24.2 ± 6.63 | 24.3 ± 6.9 | 25.4 ± 7.4 | 0.2 |
| Ethnicity [n (%)] | ||||
| White | 143 (77) | 154 (82) | 142 (76) | |
| Black | 21 (11) | 18 (10) | 28 (15) | |
| Other | 22 (12) | 15 (8) | 17 (9) | 0.35 |
| BMI (kg/m2) | 24.9 ± 5.2 | 24.5 ± 5.9 | 25.9 ± 5.3 | 0.04 |
| Current smoker [n (%)] | 53 (28) | 46 (25) | 31 (17) | 0.02 |
| Physical activity4 | 78.2 ± 50.0 | 74.5 ± 47.9 | 81.6 ± 53.6 | 0.40 |
| Time since menarche (y) | 11.6 ± 0.48 | 12.1 ± 0.50 | 12.7 ± 0.6 | 0.33 |
| Family history of fracture [n (%)] | 37 (26) | 46 (31) | 49 (35) | 0.47 |
| Ever pregnant [n (%)] | 85 (46) | 82 (44) | 87 (47) | 0.87 |
| DMPA user [n (%)] | 81 (44) | 69 (37) | 83 (44) | 0.27 |
| Baseline diet | ||||
| Energy intake (kcal) | 1725 ± 733 (520–4183)5 | 1654 ± 633 (541–3975) | 1646 ± 681 (547–4817) | 0.47 |
| Animal protein (g) | 33.2 ± 18.1 (8.0–99.3) | 43.3 ± 18.8 (11.4–117.4) | 58.7 ± 25.8 (17.6–152.8) | <0.0001 |
| Vegetable protein (g) | 18.6 ± 10.2 (3.4–65.1) | 19.9 ± 9.2 (4.3–62) | 18.6 ± 8.2 (5.8–49.2) | 0.28 |
| Carbohydrate (% of energy) | 55 ± 9 (36–81) | 52 ± 9 (31–79) | 49 ± 9 (29–70) | <0.0001 |
| Fat (% of energy) | 33 ± 8 (8–52) | 33 ± 8 (10–55) | 32 ± 8 (11–51) | 0.58 |
| Alcohol (g) | 4.5 ± 10.4 (0–87) | 2.7 ± 5.6 (0–39) | 2.9 ± 6.8 (0–55) | 0.06 |
| Calcium (mg) | 673 ± 376 (132–2827) | 832 ± 416 (124–2362) | 1035 ± 636 (156–3666) | <0.0001 |
| Phosphorous (mg) | 953 ± 446 (215–3139) | 1136 ± 467 (321–2791) | 1348 ± 602 (362–3483) | <0.0001 |
| Vitamin D (μg) | 3.50 ± 2. 41 (0.34–15.91) | 4.56 ± 2.91 (0.61–16.40) | 6.45 ± 4.43 (1.27–27.00) | <0.0001 |
| Vitamin K (mg) | 66.68 ± 39.39 (15.55–223.58) | 66.20 ± 41.49 (15.75–232.16) | 60.71 ± 31.10 (22.82–169.96) | 0.68 |
| Magnesium (mg) | 216 ± 101 (49.0–651.4) | 239 ± 97 (68.7–619.9) | 262 ± 102 (66.7–582.4) | <0.0001 |
| Caffeine (mg) | 118 ± 131 (0–766) | 87 ± 107 (0–686) | 85 ± 108 (0–686) | 0.01 |
| Fruit (servings/d) | 1.8 ± 1.6 (0.1–8.0) | 1.7 ± 1.4 (0.1–8.0) | 1.7 ± 1.4 (0.1–7.0) | 0.89 |
| Vegetable (servings/d) | 1.4 ± 1.1 (0.1–5.7) | 1.5 ± 1.0 (0.1–5.1) | 1.8 ± 1.5 (0.1–11.5) | 0.001 |
| Bone mineral density (g/cm2) | ||||
| Hip | 0.93 ± 0.12 | 0.93 ± 0.12 | 0.96 ± 0.13 | 0.06 |
| Spine | 1.00 ± 0.13 | 1.01 ± 0.12 | 1.03 ± 0.12 | 0.25 |
| Whole body | 1.08 ± 0.09 | 1.08 ± 0.09 | 1.10 ± 0.10 | 0.10 |
DMPA, depot medroxyprogesterone acetate.
P values from ANOVA for continuous variables or chi-square tests for categorical variables.
Mean ± SD (all such values).
Weight-bearing physical activity was assessed by querying the frequency (6-point scale) of specific activities. Each activity was assigned a weight-bearing value of 0 (none), 1 (low/medium), or 2 (medium/high).
Range in parentheses (all such values).
Associations between protein intake and BMD
In unadjusted analyses, those in the highest tertile of protein had slightly higher baseline BMD at the hip and whole body compared with those in the lowest tertile of protein (P ≤ 0.10) (Table 1). After adjustment for age, BMI, physical activity, smoking, recent DMPA use, energy intake, phosphorous, and magnesium, mean baseline BMD was similar across each tertile of protein intake (Table 2). Women in the lowest tertile of vegetable protein intake had a lower BMD at each site than did women consuming more vegetable protein (P = 0.03 for hip, P = 0.10 for spine, and P = 0.04 for whole body).
TABLE 2.
Adjusted mean (95% CI) bone mineral density (BMD; in g/cm2), by protein intake at baseline (n = 560)1
| Tertile of protein intake (% of energy) |
||||
| BMD site | Low (5.7–14.3) | Medium (14.4–17.1) | High (17.2–27.6) | P value2 |
| Total protein (% of energy) | ||||
| Hip | 0.93 (0.91, 0.95) | 0.93 (0.92, 0.95) | 0.93 (0.91, 0.96) | 0.94 |
| Spine | 1.00 (0.98, 1.02) | 1.02 (1.00, 1.03) | 1.02 (1.00, 1.04) | 0.37 |
| Whole body | 1.08 (1.07, 1.10) | 1.08 (1.07, 1.09) | 1.08 (1.06, 1.10) | 0.98 |
| Animal protein (% of energy) | ||||
| Hip | 0.93 (0.91, 0.95) | 0.94 (0.92, 0.95) | 0.94 (0.92, 0.96) | 0.99 |
| Spine | 1.00 (0.98, 1.02) | 1.02 (1.00, 1.04) | 1.02 (1.00, 1.04) | 0.40 |
| Whole body | 1.08 (1.07, 1.10) | 1.08 (1.07, 1.09) | 1.08 (1.07, 1.10) | 0.80 |
| Vegetable protein (% of energy) | ||||
| Hip | 0.92 (0.90, 0.94) | 0.95 (0.94, 0.97) | 0.94 (0.92, 0.96) | 0.03 |
| Spine | 1.00 (0.98, 1.01) | 1.02 (1.01, 1.04) | 1.01 (0.99, 1.04) | 0.10 |
| Whole body | 1.07 (1.06, 1.09) | 1.09 (1.08, 1.11) | 1.08 (1.06, 1.09) | 0.04 |
Values were adjusted for age, BMI, physical activity, smoking, recent depot medroxyprogesterone acetate exposure, energy intake, phosphorous, and magnesium. Models estimating associations by type of protein intake (animal/vegetable) contain terms for each type of protein in multivariable linear regression models.
P values derived from a likelihood ratio test of significance of the categorical exposure.
Results modeling protein intake continuously were similar. For every percentage increase in the percentage of energy from protein, there were no significant longitudinal changes in BMD at any anatomic site at any time point (Table 3). Type of protein intake (animal/vegetable) was not associated with change in BMD at any site or time point (Table 3). No significant overall associations were observed when BMC was used as the outcome measure, modeling protein intake as g/d or as g/kg body wt, absolute change in protein as the exposure, or analyses by using generalized estimating equations (data not shown).
TABLE 3.
Adjusted mean absolute change (in g/cm2) from baseline in bone mineral density (BMD) by baseline protein intake1
| Year 1 (n = 423) |
Year 2 (n = 378) |
Year 3 (n = 224) |
||||
| BMD site | Per 1% of energy | P value | Per 1% of energy | P value | Per 1% of energy | P value |
| Total protein intake (% of energy) | ||||||
| Hip | −0.0002 | 0.64 | 0 | 0.99 | −0.0002 | 0.88 |
| Spine | −0.0004 | 0.51 | −0.0008 | 0.28 | 0.0004 | 0.71 |
| Whole body | 0.0005 | 0.29 | −0.0004 | 0.43 | −0.0012 | 0.19 |
| Animal protein (% of energy) | ||||||
| Hip | −0.0002 | 0.67 | 0.0002 | 0.83 | −0.0002 | 0.87 |
| Spine | −0.0004 | 0.54 | −0.0008 | 0.31 | 0.0005 | 0.69 |
| Whole body | 0.0004 | 0.36 | −0.0005 | 0.4 | −0.0011 | 0.21 |
| Vegetable protein (% of energy) | ||||||
| Hip | −0.0017 | 0.15 | −0.003 | 0.11 | −0.0023 | 0.4 |
| Spine | −0.0015 | 0.29 | −0.0016 | 0.37 | −0.0019 | 0.5 |
| Whole body | 0.0008 | 0.43 | −0.0011 | 0.4 | 0.0009 | 0.69 |
Values were adjusted for age, BMI, physical activity, smoking, recent depot medroxyprogesterone acetate exposure, energy intake, phosphorous, and magnesium. Models estimating associations by type of protein intake (animal/vegetable) contain terms for each type of protein in multivariable linear regression models.
Evaluation of effect modification
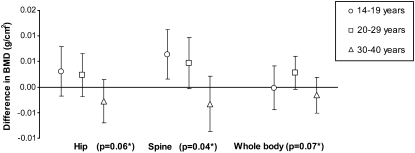
Evaluation of the relation between protein intake and BMD showed no statistically significant interactions for calcium, BMI, physical activity, smoking, recent DMPA exposure, or age category at baseline (all P > 0.05). In the longitudinal analyses, there was a suggestion of effect modification by age category (Figure 1; P < 0.10 at each site): among women younger than 30 y, a higher protein intake was associated with a higher BMD at each of the 3 anatomic sites, whereas for women aged ≥30 y, the association between protein intake and BMD was inverse. However, the only statistically significant stratum-specific estimate was among 14–19-y-olds at the spine.
FIGURE 1.
Adjusted mean difference in bone mineral density (BMD) per 1% total energy from protein, by anatomic site and age group. *P for interaction estimated from generalized estimating equation models adjusted for age, BMI, physical activity, smoking, recent depot medroxyprogesterone acetate injectable contraceptive exposure, energy intake, phosphorous, and magnesium.
DISCUSSION
Data from this large population-based study of premenopausal women suggest that women consuming more protein do not have a lower BMD than do women consuming less protein, irrespective of the site measured. In cross-sectional analyses, women consuming less vegetable protein had a lower BMD. The annual change in BMD was also similar across tertiles of protein intake, and there was no evidence that type of protein intake influenced associations. Although there were no statistically significant interactions across all 3 sites (hip, spine, and whole body), there was evidence of effect modification by age category, with higher protein intake being more beneficial for women aged <30 y than for women who have already achieved peak bone mass.
Studies to date of the association between protein intake and BMD report inconsistent results, with some finding beneficial associations (2, 13–16), others reporting no association (17, 18), and others finding adverse associations (19). A recent systematic review including 61 studies reported a small beneficial association between total protein intake and BMD and BMC, estimating the proportion of BMD attributable to protein was 1–2% (20). A longitudinal study reported that, with adequate calcium intake (>1000 mg/d), protein intake predicted BMC, BMD, and net gain in BMC in females followed from adolescence to young adulthood (21). A weight-loss feeding study in middle-aged adults found that a high-protein diet (1.4 g · kg−1 · d−1) with 3 dairy servings attenuated bone loss relative to a diet consistent with the current Recommended Dietary Allowance for protein (0.8 g · kg−1 · d−1) during both weight loss (4 mo) and maintenance of weight loss (8 mo) (22).
Studies with fracture as the outcome among women aged >50 y also report inconsistent results. Some studies of higher protein intake among women older than our study population found an increased risk of fracture (23, 24), whereas others show a decreased risk (25, 26).
Protein source (ie, animal or vegetable) may influence protein's effect on bone health. Because protein from animal sources is rich in acidifying amino acids, such as cysteine and methionine, and animal sources have fewer base precursors than vegetable sources, researchers have suggested that diets rich in protein from animal sources increase the risk of osteoporosis and sarcopenia (7). Studies to date investigating the role of protein source on bone health have been conducted primarily among postmenopausal women and report disparate findings. Among a cohort of adults aged ≥55 y, a higher animal protein intake was associated with a higher BMD, whereas vegetable protein intake was inversely correlated with BMD (27). A recent study found no overall association between protein intake and fracture risk, but did see a trend toward increased fracture risk with an increased intake of animal protein (28). A 2008 study in older women found increased odds of osteoporosis for total protein, but a decrease in odds with increased vegetable protein intake (29). An investigation of postmenopausal women in a large cohort study [the European Prospective Investigation into Cancer and Nutrition, Potsdam (EPIC)] found an adverse association between increased animal protein and bone structure assessed by ultrasound, but a beneficial association with a higher vegetable protein intake (30). Some recent feeding studies, one in postmenopausal females and 2 in young women, found no detrimental effect of animal-based protein on biochemical indicators of bone health (31–33).
In the current study, participants consumed similar amounts of vegetable protein in each tertile of protein intake; thus, much of the difference between protein intake groups was due to higher consumption of animal protein. Therefore, these data do not support a detrimental effect of animal protein on bone health in the consumption range that typified this study population.
Findings in young women could be different from those in older women because bone mass is still accruing in the young women. Peak bone density at the hip occurs at about age 19 y, and bone mass at age 30 y is used to define the “T-score,” which is the most frequently used measurement in reporting bone density results. We found suggestive, but not definitive, evidence of a qualitative interaction by age. Among women younger than 30 y, a higher protein intake was associated with a higher BMD at each of the 3 anatomic sites, whereas for women aged ≥30 y, the association between protein intake and BMD was inverse. Several mechanisms could explain age-related differences in the association between protein intake and BMD. Kerstetter et al (34) studied protein-induced effects on net bone balance in young women and showed increased gastrointestinal calcium absorption as well as a nonsignificant trend toward decreased bone resorption with a high-protein diet. Proteins likely increase the production of insulin-like growth factor I (35, 36), an important hormone that increases bone formation and that has positive effects on the growth plates. Renal function often decreases in older women, so the kidneys are less able to excrete the fixed acids from dietary protein and more of the fixed acids will be buffered by the bone, which causes bone loss.
Limitations should be considered in interpreting our findings. The FFQ has considerable measurement error and thus may have substantially attenuated diet-disease associations (37). However, calibration studies using urinary nitrogen as a biomarker of protein intake suggest that much of the measurement error lies in misreporting energy, rather than protein, intake, at least among postmenopausal women (38). Another limitation is that protein intake did not vary across the entire recommended range of 10% to 35% of protein intake, because the interquartile range of protein intake at baseline was 13.4–17.6% of energy. So, whereas these inferences apply to typical protein intake in the population, data are not available to evaluate lower and upper bounds of recommended ranges of intake. The study population was predominantly non-Hispanic white, so our findings may not be generalizable to other racial-ethnic groups with differences in bone metabolism. The sample consisted of a higher proportion of DMPA users compared with the general population, but recent DMPA use was controlled for in all models.
Strengths of the current study include the large sample size of population-based premenopausal women, allowing us to examine associations between dietary intake and bone health among a group of women who are still developing bone or have recently achieved peak bone mass. With 560 women, we had >80% power to detect a 2% difference in BMD with a 2-sided test at a level of significance of P < 0.05. Having longitudinal measures of BMD as measured by DXA provided us the opportunity to accurately and precisely detect changes in bone health over time. Data were collected on multiple exposures related to bone health in addition to dietary intake, such as physical activity and smoking, and these factors were accounted for in the analysis.
Data from this large population-based cohort provide evidence that protein intake in the upper range of typical consumption in the United States does not negatively affect bone mass in premenopausal women. These data suggest that the relation between protein intake and bone health among women who consume low amounts of vegetable protein or are still developing bone warrants further investigation. Additional studies in populations consuming protein in the upper end of the recommended range (25–35% of energy from protein) may be informative.
Acknowledgments
We thank Rebecca Hubbard for providing statistical expertise.
The authors' responsibilities were as follows—LEI, AZL, SMO, and DS: design and execution of the study; JMB and LEI: overall data management and statistical analysis; JMB, LEI, BAA, LS, AZL, SMO, and DS: data interpretation, critical review of the manuscript, and writing of manuscript; and DS: funding. The authors had no conflicts of interest to declare.
REFERENCES
- 1.Heaney RP, Layman DK. Amount and type of protein influences bone health. Am J Clin Nutr 2008;87(suppl):1567S–70S [DOI] [PubMed] [Google Scholar]
- 2.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 2000;15:2504–12 [DOI] [PubMed] [Google Scholar]
- 3.Feskanich D, Willett WC, Stampfer MJ, Colditz GA. Protein consumption and bone fractures in women. Am J Epidemiol 1996;143:472–9 [DOI] [PubMed] [Google Scholar]
- 4.Ginty F. Dietary protein and bone health. Proc Nutr Soc 2003;62:867–76 [DOI] [PubMed] [Google Scholar]
- 5.Kerstetter JE, Mitnick ME, Gundberg CM, et al. Changes in bone turnover in young women consuming different levels of dietary protein. J Clin Endocrinol Metab 1999;84:1052–5 [DOI] [PubMed] [Google Scholar]
- 6.Reddy ST, Wang CY, Sakhaee K, Brinkley L, Pak CY. Effect of low-carbohydrate high-protein diets on acid-base balance, stone-forming propensity, and calcium metabolism. Am J Kidney Dis 2002;40:265–74 [DOI] [PubMed] [Google Scholar]
- 7.Sellmeyer DE, Stone KL, Sebastian A, Cummings SR. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group. Am J Clin Nutr 2001;73:118–22 [DOI] [PubMed] [Google Scholar]
- 8.Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: National Academies Press, 2002:609. [DOI] [PubMed] [Google Scholar]
- 9.Kemper HC, Bakker I, Twisk JW, van Mechelen W. Validation of a physical activity questionnaire to measure the effect of mechanical strain on bone mass. Bone 2002;30:799–804 [DOI] [PubMed] [Google Scholar]
- 10.Kriska AM, Sandler RB, Cauley JA, LaPorte RE, Hom DL, Pambianco G. The assessment of historical physical activity and its relation to adult bone parameters. Am J Epidemiol 1988;127:1053–63 [DOI] [PubMed] [Google Scholar]
- 11.Ott SM, Ichikawa LE, LaCroix AZ, Scholes D. Navel jewelry artifacts and intravertebral variation in spine bone densitometry in adolescents and young women. J Clin Densitom 2009;12:84–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative Food Frequency Questionnaire. Ann Epidemiol 1999;9:178–87 [DOI] [PubMed] [Google Scholar]
- 13.Cooper C, Atkinson EJ, Hensrud DD, et al. Dietary protein intake and bone mass in women. Calcif Tissue Int 1996;58:320–5 [DOI] [PubMed] [Google Scholar]
- 14.Freudenheim JL, Johnson NE, Smith EL. Relationships between usual nutrient intake and bone-mineral content of women 35-65 years of age: longitudinal and cross-sectional analysis. Am J Clin Nutr 1986;44:863–76 [DOI] [PubMed] [Google Scholar]
- 15.Ilich JZ, Brownbill RA, Tamborini L. Bone and nutrition in elderly women: protein, energy, and calcium as main determinants of bone mineral density. Eur J Clin Nutr 2003;57:554–65 [DOI] [PubMed] [Google Scholar]
- 16.Teegarden D, Lyle RM, McCabe GP, et al. Dietary calcium, protein, and phosphorus are related to bone mineral density and content in young women. Am J Clin Nutr 1998;68:749–54 [DOI] [PubMed] [Google Scholar]
- 17.Nieves JW, Golden AL, Siris E, Kelsey JL, Lindsay R. Teenage and current calcium intake are related to bone mineral density of the hip and forearm in women aged 30-39 years. Am J Epidemiol 1995;141:342–51 [DOI] [PubMed] [Google Scholar]
- 18.Wang MC, Luz Villa M, Marcus R, Kelsey JL. Associations of vitamin C, calcium and protein with bone mass in postmenopausal Mexican American women. Osteoporos Int 1997;7:533–8 [DOI] [PubMed] [Google Scholar]
- 19.Metz JA, Anderson JJ, Gallagher PN., Jr Intakes of calcium, phosphorus, and protein, and physical-activity level are related to radial bone mass in young adult women. Am J Clin Nutr 1993;58:537–42 [DOI] [PubMed] [Google Scholar]
- 20.Darling AL, Millward DJ, Torgerson DJ, Hewitt CE, Lanham-New SA. Dietary protein and bone health: a systematic review and meta-analysis. Am J Clin Nutr 2009;90:1674–92 [DOI] [PubMed] [Google Scholar]
- 21.Vatanparast H, Bailey DA, Baxter-Jones AD, Whiting SJ. The effects of dietary protein on bone mineral mass in young adults may be modulated by adolescent calcium intake. J Nutr 2007;137:2674–9 [DOI] [PubMed] [Google Scholar]
- 22.Thorpe MP, Jacobson EH, Layman DK, He X, Kris-Etherton PM, Evans EM. A diet high in protein, dairy, and calcium attenuates bone loss over twelve months of weight loss and maintenance relative to a conventional high-carbohydrate diet in adults. J Nutr 2008;138:1096–100 [DOI] [PubMed] [Google Scholar]
- 23.Abelow BJ, Holford TR, Insogna KL. Cross-cultural association between dietary animal protein and hip fracture: a hypothesis. Calcif Tissue Int 1992;50:14–8 [DOI] [PubMed] [Google Scholar]
- 24.Meyer HE, Pedersen JI, Loken EB, Tverdal A. Dietary factors and the incidence of hip fracture in middle-aged Norwegians. A prospective study. Am J Epidemiol 1997;145:117–23 [DOI] [PubMed] [Google Scholar]
- 25.Munger RG, Cerhan JR, Chiu BC. Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr 1999;69:147–52 [DOI] [PubMed] [Google Scholar]
- 26.Wengreen HJ, Munger RG, West NA, et al. Dietary protein intake and risk of osteoporotic hip fracture in elderly residents of Utah. J Bone Miner Res 2004;19:537–45 [DOI] [PubMed] [Google Scholar]
- 27.Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E. Protein consumption and bone mineral density in the elderly: the Rancho Bernardo Study. Am J Epidemiol 2002;155:636–44 [DOI] [PubMed] [Google Scholar]
- 28.Dargent-Molina P, Sabia S, Touvier M, et al. Proteins, dietary acid load, and calcium and risk of postmenopausal fractures in the E3N French women prospective study. J Bone Miner Res 2008;23:1915–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J, Lim SY, Kim JH. Nutrient intake risk factors of osteoporosis in postmenopausal women. Asia Pac J Clin Nutr 2008;17:270–5 [PubMed] [Google Scholar]
- 30.Weikert C, Walter D, Hoffmann K, Kroke A, Bergmann MM, Boeing H. The relation between dietary protein, calcium and bone health in women: results from the EPIC-Potsdam cohort. Ann Nutr Metab 2005;49:312–8 [DOI] [PubMed] [Google Scholar]
- 31.Hunt JR, Johnson LK, Roughead ZF. Dietary protein and calcium interact to influence calcium retention: a controlled feeding study. Am J Clin Nutr 2009;89:357–65 [DOI] [PubMed] [Google Scholar]
- 32.Uenishi K, Ishida H, Toba Y, Aoe S, Itabashi A, Takada Y. Milk basic protein increases bone mineral density and improves bone metabolism in healthy young women. Osteoporos Int 2007;18:385–90 [DOI] [PubMed] [Google Scholar]
- 33.Zou ZY, Lin XM, Xu XR, et al. Evaluation of milk basic protein supplementation on bone density and bone metabolism in Chinese young women. Eur J Nutr 2009;48:301–6 [DOI] [PubMed] [Google Scholar]
- 34.Kerstetter JE, O'Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab 2005;90:26–31 [DOI] [PubMed] [Google Scholar]
- 35.Dawson-Hughes B, Harris SS, Rasmussen HM, Dallal GE. Comparative effects of oral aromatic and branched-chain amino acids on urine calcium excretion in humans. Osteoporos Int 2007;18:955–61 [DOI] [PubMed] [Google Scholar]
- 36.Schurch MA, Rizzoli R, Slosman D, Vadas L, Vergnaud P, Bonjour JP. Protein supplements increase serum insulin-like growth factor-I levels and attenuate proximal femur bone loss in patients with recent hip fracture. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1998;128:801–9 [DOI] [PubMed] [Google Scholar]
- 37.Subar AF, Kipnis V, Troiano RP, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol 2003;158:1–13 [DOI] [PubMed] [Google Scholar]
- 38.Neuhouser ML, Tinker L, Shaw PA, et al. Use of recovery biomarkers to calibrate nutrient consumption self-reports in the Women's Health Initiative. Am J Epidemiol 2008;167:1247–59 [DOI] [PubMed] [Google Scholar]