Abstract
Recent progress in the measurement of the bioconversion of dietary provitamin A carotenoids to vitamin A is reviewed in this article. Methods to assess the bioavailability and bioconversion of provitamin A carotenoids have advanced significantly in the past 10 y, specifically through the use of stable isotope methodology, which includes the use of labeled plant foods. The effects of the food matrix on the bioconversion of provitamin A carotenoids to vitamin A, dietary fat effects, and the effect of genotype on the absorption and metabolism of β-carotene have been reported recently. A summary of the major human studies that determined conversion factors for dietary β-carotene to retinol is presented here, and these data show that the conversion efficiency of dietary β-carotene to retinol is in the range of 3.6–28:1 by weight. There is a wide variation in conversion factors reported not only between different studies but also between individuals in a particular study. These findings show that the vitamin A value of individual plant foods rich in provitamin A carotenoids may vary significantly and need further investigation.
INTRODUCTION
Dietary provitamin A carotenoids are a major source of our vitamin A needs. Vitamin A is an essential vitamin for the promotion of general growth, maintenance of visual function, regulation of differentiation of epithelial tissues, and embryonic development (1). Vitamin A can be obtained from food, either as preformed vitamin A in animal products, such as eggs and dairy products, or as provitamin A carotenoids, mainly β-carotene in plant products, such as green leafy and yellow-colored vegetables and orange-colored fruit.
In Western societies, the provitamin A carotenoids derived from plants provide <30% of daily vitamin A intake, whereas preformed vitamin A derived from animal products provides >70% daily vitamin A intake (2). In contrast, in developing countries, provitamin A carotenoids in vegetables and fruit provide >70% of daily vitamin A intakes (3).
Epidemiologic data have shown that diets rich in carotenoid-containing foods are associated with decreased risk of certain types of chronic diseases, such as cancer (4), cardiovascular disease (5), age-related macular degeneration (6, 7), and cataracts (8, 9). The disease-preventing activity of β-carotene and other provitamin A carotenoids could be ascribed either to their conversion into retinoid or to their activity as intact molecules. The results of several human intervention studies, however, indicate that high-dose supplementation with β-carotene, either alone (10) or with vitamin E (11) or vitamin A (12), does not decrease the risk of cancer or cardiovascular disease, and might even be harmful to smokers or former asbestos workers. Thus, it may be that β-carotene and other carotenoids promote health when taken at physiologic amounts in foods, but have adverse properties when given in high doses and under highly oxidative conditions. Furthermore, the health benefits of food β-carotene consumed at a physiologic amount as an intact molecule and/or its cleavage product (vitamin A) should be investigated to determine the relative importance of each potentially favorable property in various populations. The conversion of dietary β-carotene to vitamin A may relate to preformed vitamin A in the diet (13); that is, the conversion may be less efficient when vitamin A has been provided from other dietary sources. The issue of the efficiency of conversion of provitamin A carotenoids into retinol and other retinoids is therefore of interest for us to better understand the nutritional value of dietary provitamin A carotenoids.
It is well established that after an oral dose of β-carotene, both intact β-carotene and its metabolite retinol can be found in the circulation. In humans, conversion of β-carotene into vitamin A takes place predominantly in the intestine and less so in other tissues. The ratio of the amount of β-carotene given in an oral dose to the amount of vitamin A derived from this β-carotene dose is defined as the β-carotene-to-vitamin-A conversion factor or β-carotene equivalent to vitamin A.
For a healthy population, the major factors that affect the bioavailability of food carotenoids and the bioconversion of food provitamin A carotenoids to vitamin A in humans are food matrices, food preparation, and the fat content of a meal (14). Recent studies reported that the conversion efficiency of dietary β-carotene is in the range of 10 to 28:1 by weight (15–19). These data indicated that the bioconversion of β-carotene to vitamin A was not as efficient as expected, and, as a result, the Food and Nutrition Board recently revised the estimated efficiency factor for the conversion of dietary β-carotene to vitamin A from 6:1 by weight (20) to the new value of 12:1 by weight (21). However, this new conversion ratio must be regarded as temporary and could well change, as more data become available.
On the other hand, preformed vitamin A from animal origins or from supplements can be absorbed and stored in the human body very effectively. A recent report (22) showed that among women who did not take supplemental vitamin A, retinol from food was significantly associated with fracture risk (relative risk: 1.69; 95% CI: 1.05, 2.74; P for trend: 0.05, for ≥1000 compared with <400 μg/d). However, β-carotene did not contribute significantly to fracture risk (relative risk: 1.22; 95% CI: 0.90, 1.66; P for trend: 0.10, for ≥6300 compared with <2550 μg/d). That is, long-term intake of a diet high in retinol may promote the development of osteoporotic hip fractures in women. Because of such a narrow safe range of food retinol, it is important to study the vitamin A value of food provitamin A carotenoids for humans.
It is a challenge to study the conversion of β-carotene to vitamin A at physiologic doses and even more of a challenge to study this conversion at dietary intake amounts because of the inability to distinguish newly formed retinol from body reserves. Different methods have been developed and used to determine these bioconversion factors in various populations with different vitamin A nutritional status. The most widely used methods and the results obtained from the application of these methods are described and evaluated below.
DEPLETION-REPLETION METHOD
In early studies, a depletion-repletion method was used, which involved measurement of the repletion doses of β-carotene and vitamin A that were needed to reverse vitamin A deficiency in depleted adults. This approach is no longer acceptable. A depletion study (23) was conducted in 16 healthy subjects between the ages of 19 and 34 y (7 additional subjects served as positive controls). After 12 mo of depletion, only 3 of the subjects were vitamin A deficient; a blood concentration <0.35 μmol/L (10 μg/dL) and deterioration in dark adaptation were used to define “unmistakably deficient” subjects. Of the 3 subjects with vitamin A deficiency, 2 were given β-carotene and one was given preformed vitamin A. Daily doses of 1500 μg β-carotene or 390 μg retinol for 3 wk to 6 mo were sufficient to reverse vitamin A deficiency in these subjects. Therefore, from this human study, the β-carotene/vitamin A equivalence was determined to be 3.8:1 by weight. In 1974, another vitamin A depletion-repletion study in human subjects was reported (24). Eight healthy male subjects aged 31–43 y were depleted in vitamin A within 359–771 d. Five subjects were then given vitamin A and 3 subjects were given β-carotene. Daily doses of 600 μg retinol or 1200 μg β-carotene were required to cure vitamin A deficiency. In this study, the β-carotene-to-vitamin-A equivalence was, therefore 2:1 by weight. In these studies, all subjects had been made deficient in vitamin A, so it cannot be determined whether a 3.8- or 2-μg equivalence of β-carotene to 1 μg retinol is applicable in vitamin-A–sufficient individuals.
CHANGES IN CONCENTRATION OF SERUM VITAMIN A
The measurement of changes in serum vitamin A concentrations after the feeding of synthetic β-carotene or food rich in provitamin A carotenoids has been used in populations with lower vitamin A status. The conversion of β-carotene into vitamin A cannot be estimated accurately in well-nourished humans by the assessment of changes in serum retinol after supplementation with unlabeled β-carotene, because of the inability to distinguish newly formed retinol from retinol derived from body reserves. In addition, it is well known that blood retinol concentrations are homeostatically controlled in well-nourished individuals. Nevertheless, many investigations in populations with normally low vitamin A intakes have reported blood retinol responses to acute or chronic β-carotene supplements (25, 26). Changes in serum retinol concentrations were seen (16) in vitamin A–deficient (≈0.7 μmol/L) anemic school children aged 7–11 y, who were fed 1 of 4 supplements: 1) 556 retinol equivalents (RE)/d from retinol-rich foods (n = 48), 2) 509 RE/d from fruit (n = 49), 3) 684 RE/d from vegetables (n = 45), or 4) 44 RE/d from low-retinol and low-carotene foods (n = 46) 6 d/wk for 9 wk. The changes in serum retinol showed that the consumption of fruit (diet 2) or vegetables (diet 3) resulted in increases of 0.12 and 0.07 μmol/L, respectively, in serum retinol, whereas the group who consumed foods rich in preformed vitamin A (diet 1) showed an increase of 0.23 μmol/L. The relative mean conversion factor of vegetable β-carotene into retinol was calculated, by weight, as 26:1 and that of β-carotene from orange fruit as 12:1. Use of a similar approach (17) showed that, for breastfeeding women, the conversion factors of β-carotene into retinol were, by weight, 12:1 for fruit and 28:1 for green leafy vegetables.
CHANGES IN WHOLE-BODY STORES OF VITAMIN A (PAIRED DEUTERATED RETINOL DILUTION)
Advances in isotope technology have facilitated the measurement of changes in body stores of vitamin A after the feeding of dietary provitamin A carotenoids; specifically, paired deuterated-retinol-dilution (DRD) tests can be used to measure the conversion efficiency in food-based intervention studies. For populations with marginal-to-normal vitamin A status, changes in serum retinol concentrations may not be a sensitive indicator of vitamin A status. Instead, DRD can be used to measure changes of total body stores of vitamin A. A DRD method was used in a study of children with marginal-to-normal vitamin A status, who participated in a food-based intervention with either green-yellow vegetables or light-colored vegetables with low carotene content (18). The serum carotenoid concentrations of children fed green-yellow vegetables increased, whereas the serum concentration of vitamin A did not change. In contrast, the DRD tests carried out before and after the vegetable intervention showed that the body stores of vitamin A were stable in the group fed green-yellow vegetables (n = 10) but decreased in the group fed light-colored vegetables (n = 8). Over a 10-wk period, a loss of 7 mg vitamin A from body stores was seen in the children fed light-colored vegetables that contained little β-carotene, but 275 mg β-carotene from green-yellow vegetables prevented this loss. From this paired DRD test, it was calculated that 27 μg β-carotene from vegetables was equivalent to 1 μg retinol. This conversion factor is similar to that reported in another study, which measured changes in serum vitamin A concentration after consumption of carotenoids from vegetables (17).
The paired DRD technique has also been used (15) to measure change in the vitamin A pool size after 60-d supplementation with 750 RE/d as either retinyl palmitate, β-carotene, sweet potato, or Indian spinach, compared with a control that contained no retinol or carotene (n = 14/group). Vitamin A equivalency factors of 6:1 for β-carotene in oil, 10:1 for β-carotene in Indian spinach, and 13:1 for β-carotene in sweet potato were determined. A recent study used a mixed-vegetable intervention and the paired DRD test to measure changes in vitamin A pool size (27). The results showed that the conversion factors were better than 12:1 for β-carotene and 24:1 for other provitamin A carotenoids.
CONSUMPTION OF INTRINSICALLY LABELED DIETARY PROVITAMIN A CAROTENOIDS
To achieve an accurate assessment of carotenoid bioabsorption and a subsequent vitamin A value from a food source, food material is required in which the carotenoids have been endogenously or intrinsically labeled with a low-abundance stable isotope. Plant carotenoids can be intrinsically labeled either through the addition of a carbon-stable isotope presented in the gas atmosphere as 13CO2 or through the addition of a hydrogen-stable isotope presented to the roots in the form of heavy water, 2H2O. For 2H2O, plants can be labeled via hydroponic growth (28) on a nutrient solution composed of a fixed 2H2O percentage to achieve the labeling of the carotenoids. This allows presentation of the carotenoids in their normal cellular compartments, and the isotopic label enables identification of those serum carotenoids (or derived retinol), which come from the specific food in question. The deuteration of intrinsically labeled plant 2Hn-β-carotene has been shown to be distributed randomly throughout the carotenoid molecules (29).
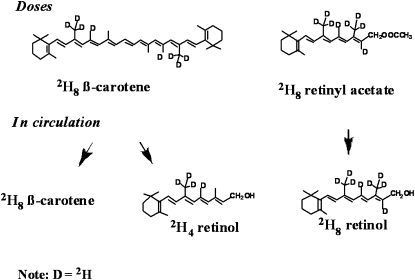
We have developed an isotope reference method to quantitatively determine the retinol equivalence of β-carotene (either synthetic pure β-carotene or β-carotene contained in a food). In the isotope reference method, through the use of a known amount of labeled vitamin A, such as retinyl acetate-d8, as a reference and the comparison of the amount of retinol formed in vivo from a vitamin A precursor (eg, 2H8 β-carotene), we may determine quantitatively the vitamin A value of the vitamin A precursor, as shown in Figure 1. Therefore, the retinol equivalence of the 2H8 β-carotene dose = (AUC2H4 retinol/AUC2H8 retinol) × Dose2H8 retinyl acetate, where AUC is the area under the curve of the circulating tracer measured compared with time. For example, in the serum of a volunteer, if, 21 d after a 6-mg 2H8 β-carotene oral dose and a 3-mg 2H8 retinyl acetate oral dose, the area under the 2H4 retinol curve (derived from the 2H8 β-carotene) is measured as 10 units and the area under the 2H8 retinol curve (from the 2H8 retinyl acetate) is measured as 10 units, we can say that 6 mg of synthetic β-carotene is nutritionally equivalent to 3 mg retinyl acetate. That is, we will be able to define the vitamin A activity of β-carotene or a food that contains β-carotene, as long as it is labeled properly. Such an isotope reference method (with a known amount of 2H8 retinyl acetate as the reference material) can be used to define the vitamin A activity of various vitamin A precursors, synthetic β-carotene (30), or provitamin A carotenoids in vegetables or other plants in humans.
FIGURE 1.
Conversion of 2H8 β-carotene and 2H8 retinyl acetate into 2H4 retinol and 2H8 retinol, respectively.
This method has been used to determine the vitamin A value of endogenously labeled spinach and carrot carotenoids (19). For these experiments on carotenoid absorbability and conversion, we recruited 7 healthy male adults who had normal vitamin A status and were nonsmokers. They were given pureed and cooked spinach (n = 14, 7 men and 7 women) and pureed and cooked carrot (n = 7 men) grown hydroponically in 25 atom % 2H2O. With the use of 3.0 mg labeled retinyl acetate as a reference dose, we observed that provitamin A carotenes in carrots have greater vitamin A potency than those in spinach. The 300 g labeled spinach and 100 g labeled carrots each contained ≈0.11 mg (all-trans)-β-carotene, and it was assumed, as usual, that α-carotene and (cis)-β-carotene, which were also present, had half the activity of (all-trans)-β-carotene (21). The retinol equivalences were determined to be 21 μg spinach β-carotene or 15 μg carrot β-carotene to 1 μg retinol.
With a similar approach, dried Spirulina powder was studied in humans. Spirulina is an alga rich in β-carotene. With the use of hydroponically grown and intrinsically deuterium-labeled Spirulina, and labeled retinyl acetate as a reference dose, a study was conducted in Chinese adults (n = 10 men) with normal vitamin A status (31). The volunteers (average age, 48 y) each took 5 g dried Spirulina powder that contained 4.3 mg β-carotene. When compared with a reference dose of 2.0 mg 13C10 retinyl acetate in oil administered in a capsule, 4.5 mg Spirulina β-carotene provided 1 mg retinol.
Very recently, a human study that used Golden Rice β-carotene was reported (32): 65–98 g (130–200 g cooked weight) of hydroponically grown Golden Rice that contained 0.99–1.53 mg of β-carotene was given to 5 healthy volunteers (3 women and 2 men) in the United States. With the use of the isotope reference method, in comparison with the 13C10 retinyl acetate reference dose, Golden Rice β-carotene provided 0.24–0.94 mg retinol. Thus, the conversion factor of Golden Rice β-carotene to retinol is 3.6:1 with a range of 1.6 to 6.4:1 by weight. Therefore, β-carotene derived from Golden Rice is effectively converted to vitamin A in humans.
OTHER METHODS
In the 1960s, a few studies were carried out in humans that investigated the vitamin A activity of β-carotene with the use of radioisotope-labeled β-carotene. Studies by Goodman et al (33) and Blomstrand and Werner (34) have provided most of our knowledge about how humans absorb and metabolize β-carotene. Such radioisotope methods can no longer be used for ethical reasons.
The measurement of β-carotene and retinyl esters in postprandial chylomicron fractions after the feeding of food rich in provitamin A carotenoids was developed to study a single dose of β-carotene. Postprandial chylomicron response curves of β-carotene and retinyl esters in blood were measured after a single dose of β-carotene supplement in oil or from vegetables (35–37). In these studies, triacylglycerol-rich lipoproteins (TRLs) with density <1.006 g/mL were separated and analyzed to evaluate the absorption efficiency of β-carotene (intact and, after central cleavage, as retinyl palmitate). The efficiency of absorption of β-carotene by each subject was calculated by measurement of the areas under the curve (AUC, nmol · h/L) of β-carotene and retinyl ester concentrations in postprandial TRL fractions collected hourly. These curves were compared with hypothetic AUC after an intravenous dose of the same amount of β-carotene, on the assumption that the β-carotene disappearance follows a first-order elimination from blood with a chylomicron remnant half-life of 11.5 min (35). To compensate for the variability of TRL recovery, deuterium-labeled vitamin A was used (38) as an extrinsic standard. A subject was given raw carrots that contained 9.8 μmol (5 mg) β-carotene and 5.2 μmol α-carotene (2.8 mg), together with 7 μmol (2 mg) [2H4]-retinyl acetate, and the concentrations of β-carotene, α-carotene, and labeled and unlabeled retinyl esters in the TRL were measured at various time points up to 7 h. With the assumption that absorption of labeled retinyl acetate was ≈80% of the dose, it was calculated that 0.8 μmol of the carrot β-carotene was absorbed intact and that 1.5 μmol of unlabeled retinyl esters were formed from the carrot dose. The mass equivalency of carrot β-carotene to vitamin A was, therefore, 13:1 by weight (without consideration of the contribution from 5.2 μmol of α-carotene to vitamin A). If the contribution of α-carotene is considered, the ratio is higher (16:1), with the assumption that α-carotene has half the activity of β-carotene.
FACTORS THAT AFFECT THE BIOCONVERSION OF beta-CAROTENE
Review articles that have evaluated the factors that affect the conversion of β-carotene to vitamin A have been published by Castenmiller and West in 1998 (39), van het Hof et al in 2000 (40), and Yeum and Russell in 2002 (41). For bioconversion of dietary β-carotene, the major factors are food matrix, food processing, and fat in the diet. As mentioned above, even though the spinach and carrots were both pureed and cooked, the in vivo study showed that spinach β-carotene had a conversion factor of 21:1, whereas carrot β-carotene had a conversion factor of 15:1. This was due to differences in the food matrix: β-carotene in spinach leaves is in the form of pigment proteins located in chloroplasts, whereas in carrots the β-carotene is in a crystal form in chromoplasts (42). In addition, the efficient conversion of Spirulina β-carotene may be due to its simple cell structure, which is composed of protein and peptidoglycans that are digested easily (43). Similarly, rice has a simple and easily digestible food matrix, which allows for a high bioavailability and bioconversion of β-carotene to vitamin A.
Intake of β-carotene at different dosage amounts will affect the conversion efficiency of β-carotene when given as β-carotene oil capsules (44). In this study a subject who took 6 mg β-carotene in oil capsules showed a conversion factor of 3.8:1, whereas the same subject who took a dose of 126 mg β-carotene in oil showed a conversion factor of 55:1 by weight. It is well known that dietary fat is a critical factor that affects bioavailability and bioconversion of β-carotene. However, the amount of fat needed in the diet to facilitate the absorption of carotenoids should be studied. A recent study on the influence of amounts of dietary fat on the bioavailability and bioconversion of provitamin A carotenoids in yellow and green leafy vegetable meals showed that carotenoid-rich yellow and green leafy vegetables need a certain minimum amount of fat (2.4 g fat/meal) to ensure the absorption of fat-soluble provitamin A carotenoids and to improve vitamin A nutritional status (27).
Our study also showed that there is a correlation of the β-carotene-to-vitamin-A conversion factor with the BMI in individual subjects (30). That is, conversion factors of the subjects who received synthetic β-carotene were significantly correlated with BMI. This suggests that subjects with more body fat have a lower capability to convert β-carotene to vitamin A.
We have observed large variations in the bioconversion of dietary β-carotene to vitamin A, which may be related to the genetic characteristics of the subjects. Because the enzyme responsible for β-carotene conversion into retinol is β-carotene 15,15'-monoxygenase (BCMO1), genetic polymorphisms in the BCMO1 gene may contribute to the poor converter phenotype. Very recently, it was reported (45) that 2 common nonsynonymous single nucleotide polymorphisms (R267S and A379V) had been identified and in vitro biochemical characterization of the recombinant 267S + 379V double mutant showed a decreased catalytic activity of BCMO1 by 57% (P < 0.001). A further assessment of the responsiveness to a pharmacologic dose of β-carotene in female volunteers confirmed that carriers of the 379V and 267S + 379V variant alleles showed a 69% decrease in their ability to convert β-carotene and a 240% increase in fasting plasma β-carotene concentration. Therefore, there is genetic variability in β-carotene metabolism. This may provide an explanation for the molecular basis of the poor converter phenotype within the population. Multiple single nucleotide polymorphisms and genes that might influence β-carotene status warrant further study.
CONCLUSIONS
Provitamin A carotenoids from various foods have been shown to have an almost 8-fold difference in β-carotene conversion factors (on a weight basis) that ranged from 3.6:1 to 28:1 with Golden Rice and leafy vegetables, respectively (Table 1), and thus have different values in terms of vitamin A nutrition. The major factor that affects the vitamin A value of plant provitamin A carotenoids is the food matrix. Stable isotope labeling has provided much of the technology to study the bioconversion of vegetable provitamin A carotenoids to vitamin A at a dietary level in various populations. Dietary provitamin-A-carotenoids-to-vitamin-A conversion factors can be used in the development of dietary guidelines in well-nourished populations, and ultimately used to help combat vitamin A deficiency worldwide. Future studies on various plant foods, which include staple foods rich in provitamin A carotenoids, will be needed to both discover and evaluate vitamin A–rich sources of plant foods.
TABLE 1.
Summary of studies to determine a conversion factor for β-carotene in food sources to vitamin A1
| Food matrix | Method | Dose | Conversion factor (by weight) | Reference |
| Fruit, n = 49; vegetables, n = 45; retinol-rich foods, n = 48 (ages 7–11 y) | Changes of serum retinol concentration in vitamin A–deficient (≈0.7 μmol/L) anemic schoolchildren | Fruit: 509 RE/d; vegetables: 684 RE/d; vitamin A–rich foods: 556 RE/d | 12:1 26:1 | 16 |
| Green/yellow vegetables, n = 10; light-colored vegetables, n = 8 | Total body stores of vitamin A before and after the vegetable intervention in schoolchildren (aged 5.3–6.5 y) with normal or marginal vitamin A status | Green/yellow vegetables (206 mg calculated trans-β-carotene) to prevent the decrease of 7.7 mg in liver stores | 27:1 | 18 |
| Sweet potato, Indian spinach, β-carotene capsule, or retinyl palmitate; all, n = 14 | Mean changes of total body stores of vitamin A before and after a 60-d intervention in adult men compared with the mean changes in the retinyl palmitate group | Sweet potato: 750 μg RE; Indian spinach: 750 μg RE; β-carotene capsule: 750 μg RE; retinyl palmitate: 750 μg RE | 13:1 10:1 6:1 | 15 |
| [2H]-Labeled spinach, and vitamin A in oil capsule, n = 14 | Comparison of AUC responses to the spinach and the vitamin A reference dose in adults | Calculated 11 mg trans-β-carotene from 300 g pureed, cooked spinach, and 3 mg [2H8]-vitamin A | 21:1 | 19 |
| [2H]-Labeled carrot, and vitamin A in oil capsule, n = 7 | Comparison of AUC responses to the carrot and the vitamin A reference dose in adults | Calculated 11 mg trans-β-carotene from 100 g pureed, cooked carrot, and 3 mg [2H8]-vitamin A | 15:1 | 19 |
| Fruit, n = 69; leafy vegetables, n = 70; retinol-rich foods, n = 70; control, n = 68 | Changes of serum retinol concentration in lactating women after they ate fruit or vegetables, or took preformed vitamin A | Fruit: 4.8 mg trans-β-carotene; vegetables: 5.6 mg trans-β-carotene; retinol-rich diet: 610 μg retinol; control: 0.6 mg β-carotene and 1 μg retinol | 12:1 28:1 | 17 |
| Spirulina powder, n = 10 | Comparison of AUC responses to the Spirulina and the vitamin A reference dose in adults | 4.3 mg Spirulina trans-β-carotene | 4.5:1 | 31 |
| Golden Rice, n = 5 | Comparison of AUC responses to a Golden Rice meal and the vitamin A reference dose in adults | Rice meal that contained 1–1.5 mg rice β-carotene | 3.6:1 | 32 |
RE, retinol equivalents; AUC, area under the curve.
It should be remembered that human subjects may have different abilities to convert provitamin A carotenoids to vitamin A. These differences in conversion efficiency may be due to the genetic variability in β-carotene metabolism of individual human subjects. Therefore, provitamin A carotenoids might not be a good vitamin A source for those subjects of the poor converter phenotype.
Acknowledgments
The author had no conflict of interest and gained no financial benefit from writing this manuscript.
REFERENCES
- 1.Underwood BA, Arthur P. The contribution of vitamin A to public health. FASEB J 1996;10:1040–8 [PubMed] [Google Scholar]
- 2.US Department of Agriculture, Agricultural Research Service. 2008. 2005-2006 What we eat in America, NHANES Tables 1-8. Available from: www.ars.usda.gov/Services/docs.htm?docid=17041 (cited 22 February 2010)
- 3. Press Conference of the State Council Information Office. The Nutrition and Health Status of the Chinese People. October 12, 2004. Table 2. Available from: http://www.chinacdc.net.cn/n272442/n272530/n273736/n273812/n293881/n293888/3748_2.html (cited 22 February 2010) [Google Scholar]
- 4.van Poppel G, Goldbohm G. Epidemiologic evidence for beta-carotene and cancer prevention. Am J Clin Nutr 1995;62:1393S–402S [DOI] [PubMed] [Google Scholar]
- 5.Gaziano JM, Hennekens CH. The role of beta-carotene in the prevention of cardiovascular disease. Ann N Y Acad Sci 1993;691:148–55 [DOI] [PubMed] [Google Scholar]
- 6.Goldberg J, Flowerdew GE, Smith E, Brody J, Tso M. Factors associated with age- related macular degeneration. An analysis of data from the first National Health and Nutrition Examination Survey. Am J Epidemiol 1988;128:700–10 [DOI] [PubMed] [Google Scholar]
- 7.Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA 1994;272:1413–20 [PubMed] [Google Scholar]
- 8.Hankinson SE, Stampfer MJ, Seddon JM, et al. Nutrient intake and cataract extraction in women: a prospective study. BMJ 1992;305:335–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacques PF, Chylack LT, McGandy RB, Hartz SC. Antioxidant status in persons with and without senile cataract. Arch Ophthalmol 1988;106:337–40 [DOI] [PubMed] [Google Scholar]
- 10.Hennekens CH, Buring UE, Peto R. Antioxidant vitamins-benefits not yet proved. N Engl J Med 1994;330:1080–1 [DOI] [PubMed] [Google Scholar]
- 11.The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med 1994;330:1029–35 [DOI] [PubMed] [Google Scholar]
- 12.Omenn GS, Goodman G, Thongquist MD, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 1996;334:1150–5 [DOI] [PubMed] [Google Scholar]
- 13.Lemke SL, Dueker SR, Follett JR, et al. Absorption and retinol equivalence of β-carotene in humans is influenced by dietary vitamin A intake. J Lipid Res 2003;44:1591–600 [DOI] [PubMed] [Google Scholar]
- 14.Furr HC, Clark RM. Intestinal absorption and tissue distribution of carotenoids. J Nutr Biochem 1997;8:364–77 [Google Scholar]
- 15.Haskell MJ, Jamil KM, Hassan F, et al. Daily consumption of Indian spinach (Basella alba) or sweet potatoes has a positive effect on total-body vitamin A stores in Bangladeshi men. Am J Clin Nutr 2004;80:705–14 [DOI] [PubMed] [Google Scholar]
- 16.de Pee S, West CE, Permaesih D, Martuti S, Muhilal S, Hautvast JG. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and beta-carotene in schoolchildren in Indonesia. Am J Clin Nutr 1998;68:1058–67 [DOI] [PubMed] [Google Scholar]
- 17.Khan NC, West CE, de Pee S, et al. The contribution of plant foods to the vitamin A supply of lactating women in Vietnam: a randomized controlled trial. Am J Clin Nutr 2007;85:1112–20 [DOI] [PubMed] [Google Scholar]
- 18.Tang G, Gu X, Xu Q, et al. Green and yellow vegetables can maintain vitamin A nutrition of Chinese children. Am J Clin Nutr 1999;70:1069–76 [DOI] [PubMed] [Google Scholar]
- 19.Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MG. Spinach or carrot can supply significant amounts of vitamin A as assessed by feeding with intrinsically deuterium-labeled vegetables. Am J Clin Nutr 2005;82:821–8 [DOI] [PubMed] [Google Scholar]
- 20.National Research Council. Food and Nutrition Board Recommended dietary allowances. Washington, DC: National Academy Press, 1989 [Google Scholar]
- 21.Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. A report of the Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Washington, DC: National Academy Press, 2001 [Google Scholar]
- 22.Feskanich D, Singh V, Willett WC, Colditz GA. Vitamin A intake and hip fractures among postmenopausal women. JAMA 2002;287:47–54 [DOI] [PubMed] [Google Scholar]
- 23.Hume EM, Krebs HA. Medical Research Council Special Report Series, No. 264 London: His Majesty's Stationery Office, 1949 [Google Scholar]
- 24.Sauberlich HE, Hodges RE, Wallace DL, et al. Vitamin A metabolism and requirements in the human studied with the use of labeled retinol. Vitam Horm 1974;32:251–75 [DOI] [PubMed] [Google Scholar]
- 25.de Pee S, West CE, Muhilal S, Karyadi D, Hautvast JGAJ. Lack of improvement in vitamin A status with increased consumption of dark-green leafy vegetables. Lancet 1995;346:75–81 [DOI] [PubMed] [Google Scholar]
- 26.Bulux J, Quan de Serrano J, Giuliano A, et al. Plasma response of children to short-term chronic β-carotene supplementation. Am J Clin Nutr 1994;59:1369–75 [DOI] [PubMed] [Google Scholar]
- 27.Ribaya-Mercado JD, Maramag CC, Tengco LW, Dolnikowski GG, Blumberg JB, Solon FS. Carotene-rich plant foods ingested with minimal dietary fat enhance the total-body vitamin A pool size in Filipino schoolchildren as assessed by stable-isotope-dilution methodology. Am J Clin Nutr 2007;85:1041–9 [DOI] [PubMed] [Google Scholar]
- 28.Grusak MA.Intrinsic stable isotope labeling of plants for nutritional investigations in humans. J Nutr Biochem 1997;8:164–71 [Google Scholar]
- 29.Putzbach K, Krucker M, Alert K, Grusak MA, Tang G, Dolnikowski GG. Structure determination of partially deuterated carotenoids from intrinsically labeled vegetables by HPLC-MS and 1H NMR. J Agric Food Chem 2005;53:671–7 [DOI] [PubMed] [Google Scholar]
- 30.Tang G, Qin J, Dolnikowski GG, Russell RM. Short-term (intestinal) and long-term (whole-body) conversion of β-carotene to vitamin A in adults as accessed by a stable isotope reference method. Am J Clin Nutr 2003;78:259–66 [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Wang Y, Wang Z, et al. Vitamin A equivalence of spirulina β-carotene in Chinese adults as assessed by using a stable-isotope reference method. Am J Clin Nutr 2008;87:1730–7 [DOI] [PubMed] [Google Scholar]
- 32.Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA. Golden Rice is an effective source of vitamin A. Am J Clin Nutr 2009;89:1776–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodman DS, Blomstrand R, Werner B, Huang HS, Shiratori T. The intestinal absorption and metabolism of vitamin A and β-carotene in man. J Clin Invest 1966;45:1615–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blomstrand R, Werner B. Studies on the intestinal absorption of radioactive β-carotene and vitamin A in man. Scand J Clin Lab Invest 1967;19:339–45 [DOI] [PubMed] [Google Scholar]
- 35.van Vliet T, Schreurs WHP, van den Berg H. Intestinal beta-carotene absorption and cleavage in men: response of beta-carotene and retinyl esters in the triglyceride-rich lipoprotein fraction after a single oral dose of beta-carotene. Am J Clin Nutr 1995;62:110–6 [DOI] [PubMed] [Google Scholar]
- 36.O'Neill ME, Thurnham DI. Intestinal absorption of β-carotene lycopene and lutein in men and women following a standard meal: response curves in the triacylglycerol-rich lipoprotein fraction. Br J Nutr 1998;79:149–59 [DOI] [PubMed] [Google Scholar]
- 37.van den Berg H, van Vliet T. Effect of simultaneous, single oral doses of beta-carotene with lutein or lycopene on the beta-carotene and retinyl ester responses in the triacylglycerol-rich lipoprotein fraction of men. Am J Clin Nutr 1998;68:82–9 [DOI] [PubMed] [Google Scholar]
- 38.Parker RS, Swanson JE, You CS, Edwards AJ, Huang T. Bioavailability of carotenoids in human subjects. Proc Nutr Soc 1999;58:155–62 [DOI] [PubMed] [Google Scholar]
- 39.Castenmiller JJM, West CE. Bioavailability and bioconversion of carotenoids. Annu Rev Nutr 1998;18:19–38 [DOI] [PubMed] [Google Scholar]
- 40.van het Hof KH, West CE, Weststrate JA, Hautvast JGAJ. Dietary factors that affect the bioavailability of carotenoids. J Nutr 2000;130:503–6 [DOI] [PubMed] [Google Scholar]
- 41.Yeum KJ, Russell RM. Carotenoids bioavailability and bioconversion. Annu Rev Nutr 2002;22:483–504 [DOI] [PubMed] [Google Scholar]
- 42.Goodwin T.The biochemistry of the carotenoids. London, United Kingdom: Chapman and Hall, 1980 [Google Scholar]
- 43.Ciferri O.Spirulina, the edible microorganism. Microbiol Rev 1983;47:551–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang G, Qin J, Dolnikowski GG, Russell RM. Vitamin A equivalence of β-carotene in a woman as determined by a stable isotope reference method. Eur J Nutr 2000;39:7–11 [DOI] [PubMed] [Google Scholar]
- 45.Leung WC, Hessel S, Méplan C, et al. Two common single nucleotide polymorphisms in the gene encoding β-carotene 15,15'-monoxygenase alter β-carotene metabolism in female volunteers. FASEB J 2009;23:1041–53 [DOI] [PubMed] [Google Scholar]