Abstract
Zinc has earned recognition recently as a micronutrient of outstanding and diverse biological, clinical, and global public health importance. Regulation of absorption by zinc transporters in the enterocyte, together with saturation kinetics of the absorption process into and across the enterocyte, are the principal means by which whole-body zinc homeostasis is maintained. Several physiologic factors, most notably the quantity of zinc ingested, determine the quantity of zinc absorbed and the efficiency of absorption. Other factors are age and the time over which zinc is ingested. Zinc from supplements has not been shown to be absorbed differently from that taken with meals that lack inhibitors of zinc absorption. The principal dietary factor known to impair zinc bioavailability is inositol hexa- (and penta-) phosphate or phytate. Modeling of zinc absorption as a function of dietary zinc and phytate accounts for >80% of the variability in the quantity of zinc absorbed. Fitting the model to new data has resulted in continual improvement in parameter estimates, which currently indicate a maximal absorption in adults of ≈6 mg Zn/d and that the average estimated dietary requirement doubles with 1000 mg dietary phytate/d. Intestinal excretion of endogenous zinc is regulated in response to recent absorption and to zinc status. The quantitative relation of intestinal excretion of endogenous zinc to zinc absorption is currently considered to be of major importance in the determination of zinc requirements. The effects of phytate on intestinal losses of endogenous zinc merit further investigation but are probably not of the same magnitude as its inhibitory effects on absorption of exogenous zinc.
INTRODUCTION
This article assumes a very broad definition of bioavailability that encompasses the physiologic adaptations that maintain whole-body zinc homeostasis, as well as phytate, which is the one outstanding dietary factor that impairs the bioavailability of ingested zinc. The entire focus is on the gastrointestinal tract, which reflects the vital importance of this organ in the maintenance of whole-body zinc homeostasis. Consideration of physiologic, genetic, or acquired conditions that impair the bioavailability of absorbed zinc to, or within, specific organs and cells, which may attain greater prominence in the future, is beyond the scope of this article, as is a discussion of the excretion of endogenous zinc via the kidneys and integument. Homeostatic changes in the former are, at most, minor, except in circumstances of severe zinc restriction (1). Variations in the latter may be of greater importance, but accurate measurements are especially challenging and data remain limited.
Despite the hopes and expectations fostered by recent rapid advances in our knowledge of zinc metabolism at a molecular level, new approaches to the estimation of dietary zinc requirements or Dietary Reference Intakes (DRIs) (2) have not yet emerged, and estimates remain dependent on a factorial approach. However, this should not be regarded as a stalemate because there have been notable advances in our understanding of whole-body zinc homeostasis that can now result in further advances in the refinement of DRIs (2). These advances have been both conceptual and quantitative, both dependent especially on the application of zinc stable isotope techniques and on advances in modeling of experimental data. It is this progress that provides the principal basis for this article.
SATURATION RESPONSE MODELING OF ZINC ABSORPTION DATA
The active absorption of zinc into and across the enterocyte was shown to be a saturable process in experimental animals a quarter of a century ago (3), but application of this knowledge to our concept of human zinc homeostasis was slow to emerge. Hints of its application were evident in the 1973 World Health Organization (WHO) publication on micronutrients (4). Although the micronutrient panel of the Food and Nutrition Board recognized that zinc absorption as a function of ingested zinc is fit well by an asymptotic nonlinear regression, the underlying physiologic explanation was ignored. A little later it was noted that these data were best fit with saturation response modeling (5) and that this is in harmony with the physiology of absorption. As was the case with the DRIs (2), this initial work, and most of the subsequent work, has been undertaken with total diet studies that have provided quantitative data on both ingested zinc and the quantity of zinc absorbed for the entire test day during which meals were extrinsically labeled with a zinc stable isotope or radioisotope. Modeling of the quantity (rather than the fraction) of zinc ingested and absorbed has been important in the advancement of concepts of human zinc homeostasis. Measurement of fractional absorption of zinc with isotope, typically stable isotope, techniques is essential to determine the quantity of zinc absorbed and the efficiency of zinc absorption of any quantity of ingested zinc. In our experience, extrinsic labeling with zinc stable isotopes provides a reliable technique to determine the absorption of intrinsic zinc from a wide range of total diets (6).
Remarkably, in consideration of the molecular complexities of the process of zinc absorption into and across the enterocyte and basolateral membrane, the fit to the data for the entire process is as good with a simple specific binding model as with any more complex saturation response modeling (and superior to that achieved with any other nonlinear relation). The fit for the means of the 10 sets of young adult male data identified by the Food and Nutrition Board has an R2 value of 0.72. One of the useful parameters of this modeling is the maximal absorption of zinc (AMAX), and these data had an AMAX of ≈7 mg Zn/d. The most recent estimate of AMAX, derived from estimates of absorption at zero phytate intakes from trivariate modeling of data from 72 studies, and described below, is a little lower. AMAX is the maximal attainable amount of absorption per day. If all the zinc (and phytate) is ingested from a single meal, maximal absorption is considerably lower (7).
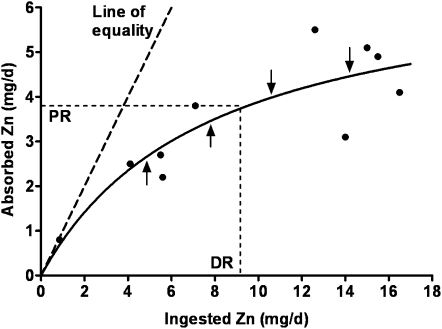
If zinc homeostasis were 100% efficient, the quantity of zinc absorbed would coincide with the line of equality for absorbed zinc compared with ingested zinc (Figure 1) until the physiologic requirement is met, from which point the line would be horizontal. Saturation response modeling indicates that the quantity of zinc absorbed approaches this line of equality only at very low amounts of ingested zinc, followed by a progressive decline in the efficiency of zinc absorption (ie, fractional absorption at any given amount of ingested zinc) as the quantity of ingested zinc per day increases. Nevertheless, absorption is favorable up to the quantity of ingested zinc that meets the estimated physiologic requirements [ie, the Estimated Average Requirement (EAR)], and the fractional absorption over this range of ingested zinc averages ≈0.4 from phytate-free meals. Beyond the EAR, the efficiency of absorption declines rapidly with progressive increases in ingested zinc, which thus provides a major contribution to the maintenance of zinc homeostasis by the minimization of the absorption of excessive zinc. Incidentally, whereas regulation of zinc absorption is very effective in response to changes in the quantities of bioavailable zinc ingested, regulation of absorption in response to changes in zinc status is questionable and, at most, plays a minor role in the maintenance of zinc homeostasis (8, 9). This minor role is in sharp contrast to earlier notions of the maintenance of zinc homeostasis.
FIGURE 1.
Saturation response model of total absorbed zinc per day as a function of total ingested zinc. Data modeled are the means of the 10 sets of healthy young adults used in the estimation of Dietary Reference Intakes for zinc by the Institute of Medicine (2). Also depicted is the line of equality for absorbed zinc compared with ingested zinc. Arrows depict theoretic up- and down-regulation of zinc transporters in the enterocytes. PR, physiologic requirement for men estimated by the Institute of Medicine (2); DR, average dietary zinc requirement estimated from this model, an estimate that is essentially identical to the Estimated Average Requirement (2).
It is now apparent that saturation kinetics plays a major role in the homeostatic modulation of zinc absorption. However, up- and down-regulation of zinc transporters and other proteins involved in the transport of zinc across the apical and basolateral membranes and within the enterocyte underlies this modulation, as depicted in Figure 1 (10, 11). It is regulation to maintain this optimal relation between the quantities of absorbed and ingested zinc or to restore this relation over a few hours, and possibly days, that determines the parameter values of the saturation response modeling, which includes the AMAX. A priority for future research is to achieve a quantitative understanding of the role of up- and down-regulation of these transporters in the maintenance of whole-body human zinc homeostasis.
One factor that has a major effect on the AMAX, and on the entire saturation response model, is age. The AMAX for infants has been calculated as being less than one-third that of adults, which is in accord with the difference in physiologic requirements for zinc (12).
ABSORPTION OF ZINC FROM SUPPLEMENTS
Although not necessary for the establishment of dietary zinc requirements, zinc supplements are widely used, and knowledge of the quantity of zinc absorbed is, therefore, of considerable practical import. The specific choice of zinc salt does not appear to be of practical importance, at least in a normal host environment (13–15). However, the efficiency of absorption of zinc from supplements appears to be much higher than it is from meals (16), even in the absence of a dietary inhibitor of zinc absorption in the meals, which led to the earlier conclusion that absorption of zinc from supplements is via a different mechanism. Although an important practical area for further detailed research, it now appears that the explanation lies in study design. Typically, absorption from a supplement has been measured by one-time administration of a tracer-labeled supplement. This design does not allow for any time for down-regulation of transporters in response to this relatively high intake of zinc. Support for this explanation has been derived from the measurement of zinc absorption from the supplement on day 2 or day 6 after commencement of daily supplement administration, rather than on day 1. Not only is fractional absorption much lower (16), but it appears to decline to approximately that value expected from a phytate-free meal.
EFFECT OF DIETARY PHYTATE ON THE BIOAVAILABILITY OF INGESTED ZINC
Apart from the physiologic effect of the quantity of ingested zinc discussed above, phytate is the one outstanding dietary factor that impairs the bioavailability of zinc. The first major attempt to determine the quantitative effect of phytate on dietary zinc requirements, which continues to provide the basis for the current Recommended Nutrient Intakes of the WHO/Food and Agriculture Organization, was based on the results of single test meals (4). This study design appeared to overestimate the combined effects of dietary phytate and zinc over an entire day (7).
More recently, a trivariate model was developed (17) that is based on all identified total diet studies that include phytate intake data and is designed to predict the quantity of zinc absorbed per day as a function of dietary zinc and phytate. This is a mathematic model, but it is also based on a simple concept of the physiology of zinc absorption and of zinc chelation by phytate in the small intestine. It is, in essence, a more complex example of saturation response modeling than that used to define the relation between ingested and absorbed zinc, as described above.
After publication of the detailed description of the development of this model (17), it was used to estimate the quantitative effect of dietary phytate on dietary requirement for zinc by men and women (18). These estimates showed, for example, that the EAR is doubled in the presence of an evenly distributed intake of 1000 mg phytate/d. It currently requires extrapolation of this model to determine the effect of phytate on the Tolerable Upper Intake Levels for zinc. With phytate-free meals or zinc supplements, the most current model predicts that the adult Tolerable Upper Intake Level of the DRI corresponds to absorption of 5.6 mg Zn/d. Note that this figure approaches the AMAX per day for adults. It is, therefore, difficult to exceed this maximal absorption level even without phytate in the diet. As dietary phytate increases, the estimated ingested zinc necessary to achieve absorption of 5.6 mg Zn/d by adults increases rapidly and progressively. For example, with only 900 mg phytate intake/d, the model predicts that it will require 100 mg ingested Zn to achieve this quantity of absorbed zinc (18). This model has also been used to develop recommendations for the zinc fortification of flour from a range of cereal grains (19); to predict the increase in absorbed zinc from a zinc-biofortified cereal (20, 21); and to assist in the evaluation of data derived from experimental human studies in which quantities of ingested zinc and phytate were manipulated (22). The last research was also then used to evaluate this trivariate model, an evaluation that proved to be strongly supportive of the model in contrast to another purely mathematic model (23).
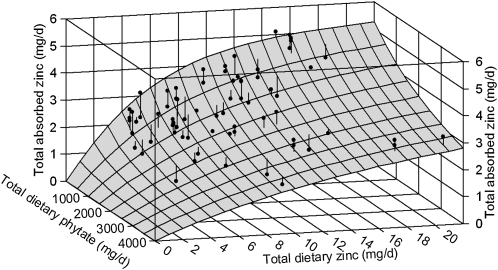
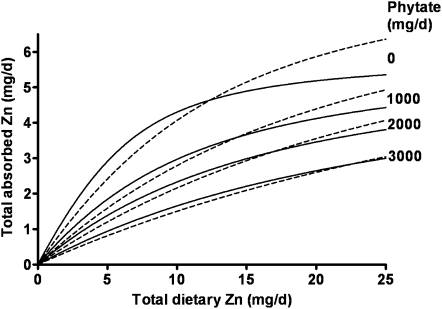
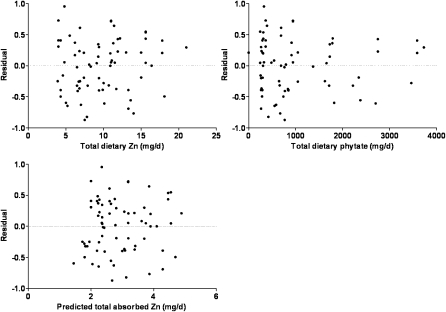
As additional total diet data are identified and new data are published that include phytate intake, these data have been added to the original data set of 21 to give a current total of 72 data sets (means) (Figure 2) (6, 9, 17, 18, 20, 22, 24). The parameters of the current model have been compared with the corresponding values for the original model (Table 1) and indicate further improvement in the certainty of the estimates, which include a lower AMAX. When the data are weighted according to numbers of subjects per study, the model does not change and the parameter values reported here are not derived from the regression of weighted data. The differences between the original and current model are depicted in Figure 3. The validity of the model is further confirmed by the normal distribution, constant variance, and independence of the regression residuals relative to quantity of dietary zinc, quantity of dietary phytate, and predicted quantity of zinc absorbed (Figure 4).
FIGURE 2.
Trivariate model of absorbed zinc as a function of dietary zinc and phytate. A 3-dimensional surface of the model is shown. Each black dot represents the mean for one set of data from all identified research projects that include measurements of quantity of zinc absorbed, dietary zinc, and dietary phytate. Lines associated with each dot represent residuals.
TABLE 1.
Current parameter values compared with original values1
| Parameter | Original values | Current values |
| AMAX | 0.130 ± 0.047 | 0.091 ± 0.007 |
| KP | 1.20 ± 0.74 | 0.68 ± 0.22 |
| KR | 0.100 ± 0.083 | 0.033 ± 0.01 |
All values are means ± SEs. AMAX, maximal absorption of zinc; KP, zinc-phytate equilibrium constant; KR, zinc-transport receptor equilibrium dissociation constant.
FIGURE 3.
Comparison of current and original model parameters. Saturation response curves for selected phytate concentrations are shown as dashed lines for the original model (18) and as continuous lines for the current model.
FIGURE 4.
Regression residuals relative to quantities of dietary zinc and phytate and predicted quantities of zinc absorbed.
INTESTINAL EXCRETION OF ENDOGENOUS ZINC
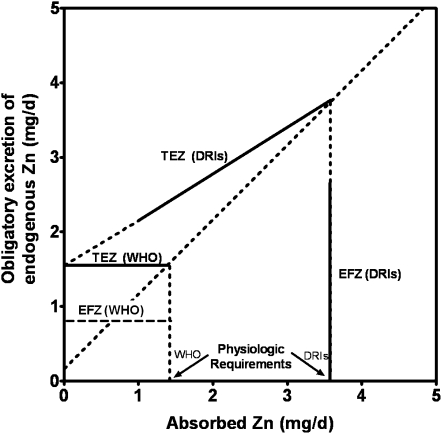
Excretion of endogenous zinc, primarily intestinal excretion, also merits attention with respect to zinc bioavailability. The quantity of endogenous fecal zinc (EFZ) plays an important role in the maintenance of zinc homeostasis and is determined by both the quantity of recently absorbed zinc and zinc status (8, 25). Deliberations that led to the DRIs for zinc resulted in a major paradigm shift with the recognition of the significance of the positive correlation between the quantity of zinc absorbed and EFZ over a range that extends well below any estimate of physiologic requirements (26). This recognition resulted in a 2–3-fold increase in the estimate of physiologic requirements for men when contrasted, for example, with those estimated by the WHO (Figure 5) (4). At the level of absorption estimated to be required to meet physiologic requirements by the micronutrient DRI panel, EFZ is 3–4-fold higher than the “normative” (unadapted) estimate of the WHO (4). Data were and remain insufficient for other subgroups of the population, which include women, and this resulted in the requirement for extrapolation from the adult male data on a body weight basis.
FIGURE 5.
Estimates of endogenous fecal zinc (EFZ) at amounts of absorbed zinc required to meet physiologic requirements by the Institute of Medicine (IOM) compared with those of the World Health Organization (WHO). Shown are estimated quantities of total endogenous zinc excretion (TEZ) and EFZ as a function of absorbed zinc at amounts of absorbed zinc below and up to quantities estimated to be necessary to meet physiologic requirements of men. WHO “normative” (unadapted) (17) and IOM calculations used in the estimation of Dietary Reference Intakes (DRIs) are compared (2). The intercept of TEZ excretion lines with the line of equality shows the estimated physiologic requirement. Continuous vertical lines indicate EFZ at amounts of absorption estimated to be necessary to just meet physiologic requirements. Dotted vertical lines indicate endogenous excretion by other routes.
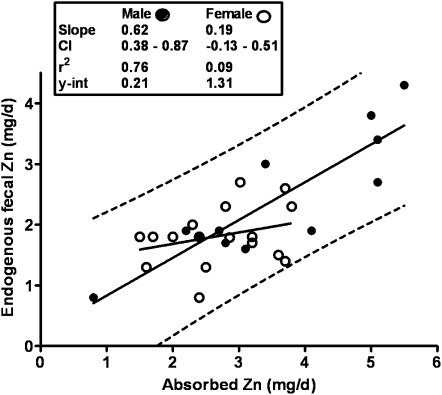
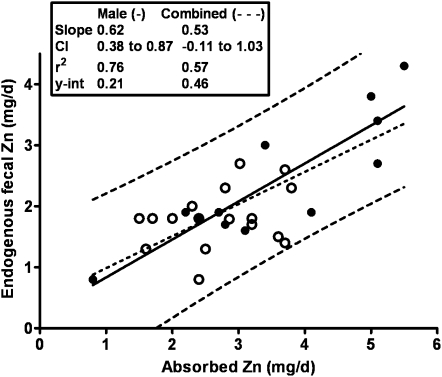
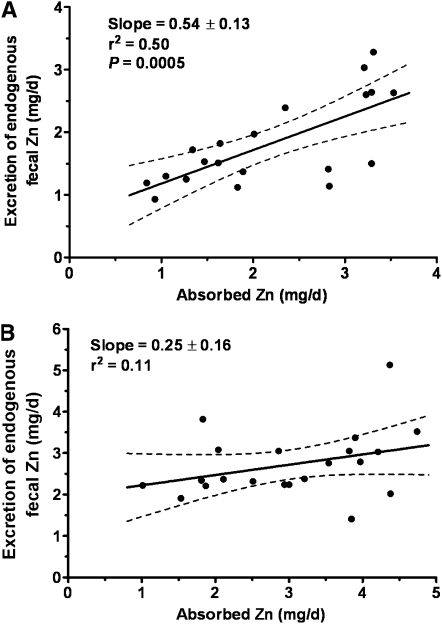
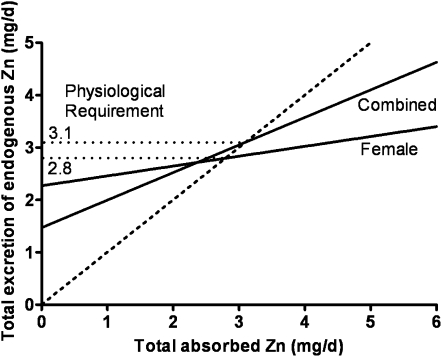
The data for women have recently been revisited (27). The available data (study means) for women have increased from 7 to17 during the interim since the DRIs were determined. The most common techniques used for the determination of these female data differed from those, mainly earlier techniques, used for the male studies (Table 2), but there is no evidence that this difference had any important effect on the comparison of data between sexes (Figure 6). Reasons for this sex difference are only speculative at this time, but one explanation resides in the limited range of female means. Consistent with this explanation is the linear regression obtained for combined sexes for which both the slope and y-intercept are quite close to the male-only data (Figure 7). In our own experience, linear regression of individual data from a single study of young Chinese women (26, 28), which extended to very low amounts for absorbed zinc, had a slope and y-intercept very similar to that for men (Figure 8A). In contrast, for a parallel study undertaken in women in the United States, the slope for EFZ compared with quantity of absorbed zinc was nonsignificant and similar to that for mean female data (Figure 8B). In this particular instance, there is reason to conjecture that a partial explanation resides in underestimates of habitual dietary intake for those subjects with the lowest absorbed zinc. Whereas additional female EFZ data over a wide range of zinc absorption are required, it is perhaps useful to note that there is only a minor difference in our current estimate of physiologic requirement for zinc by adult women, whether estimated with the use of the linear regression for women alone or that for combined sexes (Figure 9). Lack of a positive slope appears to be counterbalanced by the high y-intercept for the female data. In both cases, the estimated physiologic requirement is ≈3 mg Zn/d, which is slightly lower than that estimated in the development of the DRIs (2).
TABLE 2.
Number of studies that used each technique for measurement of endogenous fecal zinc
| Technique | Men | Women |
| Apparent tracer absorption | 9 | 2 |
| Whole-body counting | 1 | 5 |
| Isotope dilution | 1 | 8 |
| Compartmental model | 1 | 2 |
| Total | 12 | 17 |
FIGURE 6.
Relation of endogenous fecal zinc to absorbed zinc by sex. Linear regressions of sex-specific data for endogenous fecal zinc compared with absorbed zinc. Female and male regression lines are are shown. y-int, y-intercept.
FIGURE 7.
Linear regression slopes for endogenous fecal zinc compared with absorbed zinc in combined sexes and men only. y-int, y-intercept.
FIGURE 8.
Linear regression for endogenous fecal zinc compared with absorbed zinc in 2 individual data sets: young Chinese women (A) (29) and young American women (B).
FIGURE 9.
Estimated total endogenous zinc losses compared with absorbed zinc in women and combined sexes. Note similarity in intercepts with the line of equality (- - -), which gives an estimate of physiologic requirements.
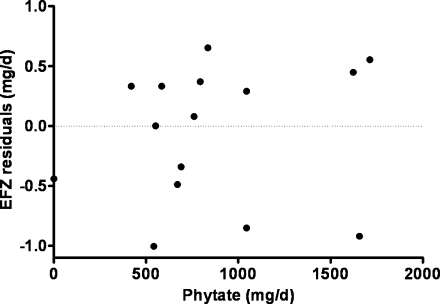
Theoretically, phytate in the lumen of the intestine may decrease EFZ secondarily to a decrease in absorbed zinc, or may increase EFZ by chelating endogenous zinc secreted into the gut lumen and, therefore, inhibiting reabsorption (29). In our experience, it has not been possible to identify any phytate effect (8, 30). Plotting the residuals of the linear regression of EFZ compared with absorbed zinc has not shown any relation between these residuals and the phytate intakes (Figure 10). However, the numbers are quite small (n = 14).
FIGURE 10.
Residuals from linear regression of endogenous fecal zinc (EFZ) on absorbed zinc, plotted against dietary phytate.
In conclusion, recognition of the physiologic appropriateness and statistical advantages of the analysis of zinc absorption data as a function of dietary zinc intake by saturation response modeling has advanced our understanding of the physiology that underlies total body zinc homeostasis and provides both a concept and an approach that will facilitate refinements of estimates of DRIs. Conceptually and quantitatively linking saturation kinetic data with data on the up- and down-regulation of human zinc proteins in the enterocyte is a more ambitious prospect. For absorption of zinc, this regulation occurs in hours or days. For intestinal excretion of endogenous zinc, regulatory changes are rapid in response to changes in the quantity of zinc absorbed and more prolonged in response to zinc status. Phytate is the only substantial dietary factor that inhibits zinc absorption. A trivariate model of zinc absorption as a function of dietary zinc and dietary phytate now allows quantitative prediction of this inhibitory effect in adults and indicates that dietary zinc and phytate explain >80% of the variance in the quantity of zinc absorbed as a function of quantity of ingested zinc. Additional experimental data are needed before similar predictive modeling is available for young children. Advances in our concepts of human zinc homeostasis and its relation to the quantity of bioavailable zinc ingested allow us to initiate the development of evidence-based strategies for the global prevention of zinc deficiency.
Acknowledgments
The authors' responsibilities were as follows—KMH: presentation at the Micronutrient Bioavailability workshop in Barcelona, Spain, in June 2009, and preparation of the manuscript; LVM: preparation of the workshop, which included the updated trivariate model of zinc absorption as a function of dietary zinc and phytate; and JEW: preparation of the PowerPoint presentation and editing of manuscript. All authors participated in the research and discussions that resulted in the updated perspective of zinc bioavailability and homeostasis presented in this article. None of the authors had any conflicts of interest in the preparation of this manuscript.
REFERENCES
- 1.Baer MT, King JC. Tissue zinc levels and zinc excretion during experimental zinc depletion in young men. Am J Clin Nutr 1984;39:556–70 [DOI] [PubMed] [Google Scholar]
- 2.Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for Vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc. Washington, DC: National Academy Press, 2001 [PubMed] [Google Scholar]
- 3.Steel L, Cousins RJ. Kinetics of zinc absorption by luminally and vascularly perfused rat intestine. Am J Physiol 1985;248:G46–53 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Trace elements in human nutrition. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1973;532:9–15 [PubMed] [Google Scholar]
- 5.Hambidge KM, Miller LV, Tran CD, Krebs NF. Measurements of zinc absorption: application and interpretation in studies designed to improve human zinc nutriture. Int J Vitam Nutr Res 2005;75:385–93 [DOI] [PubMed] [Google Scholar]
- 6.Sheng X, Hambidge KM, Miller LV, Westcott JE, Sian L, Krebs NF. Measurement of zinc absorption from meals: comparison of extrinsic zinc labeling and independent measurements of dietary zinc absorption. Int J Vitam Nutr Res (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hambidge KM, Miller LV, Westcott JE, Krebs NF. Modeling zinc (Zn) absorption (AZ) from single test meals (SM) as a function of dietary zinc (DZ) and phytate (DP). FASEB J 2008;22:697.4 [Google Scholar]
- 8.Krebs NF, Hambidge KM. Zinc metabolism and homeostasis: the application of tracer techniques to human zinc physiology. Biometals 2001;14:397–412 [DOI] [PubMed] [Google Scholar]
- 9.Chung CS, Stookey J, Dare D, et al. Current dietary zinc intake has a greater effect on fractional zinc absorption than does longer term zinc consumption in healthy adult men. Am J Clin Nutr 2008;87:1224–9 [DOI] [PubMed] [Google Scholar]
- 10.Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr 2004;24:151–72 [DOI] [PubMed] [Google Scholar]
- 11.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci USA 2004;101:14355–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hambidge KM, Krebs NF, Westcott JE, Miller LV. Changes in zinc absorption during development. J Pediatr 2006;149:S64–8 [DOI] [PubMed] [Google Scholar]
- 13.Hotz C, DeHaene J, Woodhouse LR, Villalpando S, Rivera JA, King JC. Zinc absorption from zinc oxide, zinc sulfate, zinc oxide + EDTA, or sodium-zinc EDTA does not differ when added as fortificants to maize tortillas. J Nutr 2005;135:1102–5 [DOI] [PubMed] [Google Scholar]
- 14.Lopez de Romana D, Lonnerdal B, Brown KH. Absorption of zinc from wheat products fortified with iron and either zinc sulfate or zinc oxide. Am J Clin Nutr 2003;78:279–83 [DOI] [PubMed] [Google Scholar]
- 15.Herman S, Griffin IJ, Suwarti S, et al. Cofortification of iron-fortified flour with zinc sulfate, but not zinc oxide, decreases iron absorption in Indonesian children. Am J Clin Nutr 2002;76:813–7 [DOI] [PubMed] [Google Scholar]
- 16.Tran CD, Miller LV, Krebs NF, Lei S, Hambidge KM. Zinc absorption as a function of the dose of zinc sulfate in aqueous solution. Am J Clin Nutr 2004;80:1570–3 [DOI] [PubMed] [Google Scholar]
- 17.Miller LV, Krebs NF, Hambidge KM. A mathematical model of zinc absorption in humans as a function of dietary zinc and phytate. J Nutr 2007;137:135–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hambidge KM, Miller LV, Westcott JE, Krebs NF. Dietary reference intakes for zinc may require adjustment for phytate intake. J Nutr 2008;138:2363–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown KH, Hambidge KM, Ranum P, Tyler V. Zn fortification of cereal flours: current recommendations and research needs. Food Nutr Bull (in press) [DOI] [PubMed] [Google Scholar]
- 20.Rosado JL, Hambidge KM, Miller LV, et al. The quantity of zinc absorbed from wheat in adult women is enhanced by biofortification. J Nutr 2009;139:1920–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hambidge KM, Rosado JL, Miller LV, et al. Absorption of zinc (Zn) from high Zn and control wheat. FASEB J 2008;22:149.5 [Google Scholar]
- 22.Hunt JR, Beiseigel JM, Johnson LK. Adaptation in human zinc absorption as influenced by dietary zinc and bioavailability. Am J Clin Nutr 2008;87:1336–45 [DOI] [PubMed] [Google Scholar]
- 23.International Zinc Nutrition Consultative Group Assessment of the risk of zinc status in populations and options for the control of zinc deficiency. Boston, MA: International Nutrition Foundation for United Nations University Press, 2004 [PubMed] [Google Scholar]
- 24.Hunt JR, Beiseigel JM. Dietary calcium does not exacerbate phytate inhibition of zinc absorption by women from conventional diets. Am J Clin Nutr 2009;89:839–43 [DOI] [PubMed] [Google Scholar]
- 25.Krebs NF, Hambidge KM, Westcott JE, et al. Exchangeable zinc pool size in infants is related to key variables of zinc homeostasis. J Nutr 2003;133:1498S–501S [DOI] [PubMed] [Google Scholar]
- 26.Hambidge M, Krebs NF. Interrelationships of key variables of human zinc homeostasis: relevance to dietary zinc requirements. Annu Rev Nutr 2001;21:429–52 [DOI] [PubMed] [Google Scholar]
- 27.Hambidge KM, Miller LV, Krebs NF. Relationship between endogenous fecal zinc and zinc absorbed revisited. FASEB J 2009;23:216.8 [Google Scholar]
- 28.Sian L, Mingyan X, Miller LV, Tong L, Krebs NF, Hambidge KM. Zinc absorption and intestinal losses of endogenous zinc in young Chinese women with marginal zinc intakes. Am J Clin Nutr 1996;63:348–53 [DOI] [PubMed] [Google Scholar]
- 29.Oberleas D, Harland BF. Phytate content of foods: effect on dietary zinc bioavailability. J Am Diet Assoc 1981;79:433–6 [PubMed] [Google Scholar]
- 30.Hambidge KM, Mazariegos M, Solomons NW, et al. Intestinal excretion of endogenous zinc in Guatemalan school children. J Nutr 2007;137:1747–9 [DOI] [PubMed] [Google Scholar]