Abstract
Among 407 New England Medical Center Posterior Circulation Registry (NEMC-PCR) patients, 59% had strokes without transient ischemic attacks (TIAs), 24% had TIAs before strokes, and 16% had only posterior circulation TIAs. Embolism was the commonest stroke mechanism accounting for 40% of cases (24% cardiac origin, 14% arterial origin, 2% had potential cardiac and arterial sources). In 32%, large artery occlusive lesions caused hemodynamic brain infarction. Stroke mechanisms in the posterior and anterior circulation are very similar. Infarcts most often included the distal posterior circulation territory (rostral brainstem, superior cerebellum and occipital and temporal lobes), while the proximal (medulla and posterior inferior cerebellum) and middle (pons and anterior inferior cerebellum) territories were equally involved. Infarcts that included the distal territory were twice as common as those that included the proximal or middle territories. Most distal territory infarcts were attributable to embolism. Thirty day mortality was low (3.6%). Embolic stroke mechanism, distal territory location, and basilar artery occlusive disease conveyed the worst prognosis.
Keywords: Brain ischemia, Brain embolism, Posterior circulation, Vertebral arteries, Basilar artery
INTRODUCTION
Clinical information about posterior circulation ischemia has lagged behind that for anterior circulation ischemia.1-4 The causes of brain infarcts and ischemia has in the past been posited to be different in the two cervico-cranial circulations. Posterior circulation disease is attributed to hemodynamically significant vertebral (VA), basilar artery (BA), and penetrating artery disease. Anterior circulation ischemia is most often attributed to embolism from the heart or extracranial internal carotid arteries (ICAs) and penetrating artery disease.2,3 In the past, patients with carotid territory ischemia usually had brain imaging and cardiac and ICA evaluations while patients with vertebrobasilar territory ischemia usually had neuroimaging but seldom had cardiac or vascular investigations. Because of these different clinical practices, much more is known about anterior circulation than about posterior circulation disease.
Before the mid-1980s, posterior circulation brain and vascular imaging required catheter angiography and computed tomogram (CT). Precise definition of brain lesions was not possible before magnetic resonance imaging (MRI). Inability to safely and quickly define causative vascular lesions limited therapy. The lack of treatment options contributed to disinterest in vascular evaluation of patients with suspected vertebro-basilar disease. From 1988~1996, at the New England Medical Center (NEMC), we thoroughly evaluated all posterior circulation ischemia patients using brain imaging and vascular studies at first angiography and later magnetic resonance angiography (MRA), extracranial and transcranial ultrasound (TCD), and appropriate cardiac and hematological investigations. We collected the data in a prospective computerized registry. The 407 NEMC-PCR patients serve as the data base for this and other reports.2-11 We now report the frequency of various infarct locations, stroke mechanisms, clinical findings, vascular lesions, and outcomes in consecutive patients.
BACKGROUND
Reports of the classic brainstem syndromes during the 19th and early 20th centuries were stimulated by fascination with brainstem anatomy and functions.3,12-20 The causative arterial lesions and vascular pathology were only occasionally discussed. Before 1950, reports had defined clinical syndromes of the medulla,17,18 pons,24,25 thalamus22,26-28 and temporal and occipital lobes.22,29 Although vascular anatomy and blood supply regions were studied by Duret,30,31 Foix et al,23,27-29,32 and Stopford,33 there was little interest in the nature of the cerebrovascular disease. Probably the first major inquiry into vascular mechanisms was by Foix et al.34 Their preliminary report concerned vascular pathology in patients with brain softenings.34 Among 56 patients, arteries supplying infarcts were completely occluded in only 12, partially occluded in 14, and widely patent in 30. Posited explanations for the lack of arterial occlusions at necropsy were: (1) occlusion might follow brain softening; (2) embolism with passage of embolic material before autopsy; (3) proximal circulatory failure ("insuffisance cardioarterielle"); and (4) vasospasm ("spasme arterielle").34
In 1946 Kubik and Adams reported clinical and pathological findings in patients who had BA occlusion at necropsy.25 BA occlusions were mostly due to intrinsic atherosclerosis often with superimposed thrombosis, but were embolic in 7 of 18 patients. The location and extent of vascular occlusion was correlated with clinical findings during life and the location and extent of brainstem infarction. BA occlusion was potentially recognizable during life.25
Clinical interest in cerebrovascular disease was stimulated during the early 1950s by Fisher's reports on the clinical findings in patients with ICA occlusions.35,36He emphasized that the occlusive process was in the neck and not the head, was often heralded by transient ischemic attacks (TIAs), and was potentially amenable to surgery. Hutchinson and Yates reported that occlusive posterior circulation disease also often involved the neck VAs.37,38 Clinicians began to report series of patients with transient attacks of brainstem, cerebellar, and posterior cerebral hemispheral ischemia.39-45 Symptoms and signs were similar to those noted in the fatal cases of BA occlusion reported by Kubik and Adams.25 These ischemic events were the posterior circulation equivalents of TIAs reported by Fisher in ICA disease.35,36 Reasoning that posterior circulation TIAs (dubbed "vertebrobasilar insufficiency"44,45) presaged fatal brainstem infarction (Kubik and Adams patients all died), warfarin anticoagulation, a popular 1950s therapy, was given to posterior circulation TlA patients to prevent strokes. Fisher reported good outcomes among 58 TIA anticoagulant-treated patients, 29 with vertebrobasilar attacks.46 Mayo Clinic physicians reported that many patients with vertebrobasilar insufficiency survived, sometimes without strokes, after warfarin anticoagulation.45,47 Although the results were anecdotal and most patients did not have angiography showing vascular lesions, anticoagulation was considered effective treatment for vertebrobasilar occlusive disease, and warfarin was, and still is, widely used for this indication.
During the third quarter of the twentieth century, angiography and diagnostic ultrasound of anterior circulation ischemia patients showed a variety of occlusive and embolic lesions. Various treatments were given depending on vascular lesions and mechanisms of brain ischemia. During the same years, physicians caring for patients with posterior circulation ischemia debated whether to anticoagulate but seldom investigated vascular lesions.
MRI, ultrasound (cervical and TCD), CT angiography (CTA), and MRA have recently allowed safe and accurate definition of posterior circulation infarcts and cervico-cranial vascular lesions.2,3,48-50 Echocardiography has helped define cardiac and aortic embolic sources and identify coexistent heart disease.
Necropsy studies, modern neuroimaging, and retrospective case series have clarified many posterior circulation vascular syndromes including: brainstem lacunes,51-55 intracranial branch atheromatous disease,56-58 medullary59-61 cerebellar62-70 pontine25 thalamic and midbrain infarcts,71 embolism,72-75 extracranial and intracranial dissections,76-80 dolichoectasia,81-84 "top of the BA"85,86 and PCA territory infarcts.87,88 Large stroke registries89-95 reported stroke causes and mechanisms, risk factors, and outcomes in unselected stroke patient populations. We now report data from 407 NEMC-PCR patients regarding risk factors, stroke mechanisms, and outcomes, comparing the results with other registries. A previous report summarized a portion of the data from the registry.5
METHODS
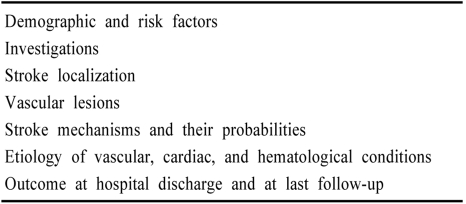
The NEMC-PCR (1988-1996) had 3 major inclusion criteria: 1) all patients must be examined by Drs. Caplan, Pessin, or Dewitt; 2) patients must have had posterior circulation TIAs or strokes within the prior six months. Stroke patients must have had CT or MRI documented infarction. TIAs had to be clearly vertebrobasilar and brain imaging and vascular tests must have shown vertebrobasilar territory infarction or occlusive lesions; 3) Investigations must have been adequate. Table 1 lists categories of data. All clinical data and neuroimages were reviewed by the senior clinicians, often in a conference where all were present. All 407 cases were rereviewed at least twice to ensure that data entered and diagnoses were complete and accurate. We used the following criteria to classify stroke mechanisms:2,4
Table 1.
Categories of data collected in the NEMC-PCR
1. Large artery occlusive disease hemodynamic mechanism (LAH)
Laboratory: Necessary: Vascular tests showing occlusion or severe stenosis (>50% luminal narrowing) of the VA, BA or PCAs. Abrupt cutoff of vessels or branches was considered more likely embolic and not intrinsic disease. Supportive: a) CT or MRI showing infarction in the territory of the occlusion larger than a single branch artery territory, or in distal fields and/or b) hematologic tests suggesting hypercoagulability. When infarction occurred in the same intracranial territory as a large artery occlusion or severe stenosis, the mechanism was designated as LAH. For example: left intracranial vertebral artery (ICVA) occlusion and left lateral medullary or left posterior inferior cerebellar (PICA) infarction; BA occlusion and bilateral pontine base infarction. Supportive clinical features included: a) TIAs, especially multiple and in the same vascular territory, b) ischemia developing repeatedly after standing, c) gradual, stepwise, or stuttering onset and course, and d) unaccustomed headaches out of proportion to neurological signs.
2. Branch artery occlusive disease (Br A)
This category includes penetrating arteries (lacunar or atheromatous branch disease) (BrAP) infarcts ((BrAC).
(1) Penetrating arteries (lacunar or atheromatous branch disease) (BrAP).
Laboratory: Necessary: CT or MRI showing infarcts limited to single penetrating or circumferential branch territory. Supportive: no occlusive parent artery disease and no proximal arterial or cardiac embolic source. Clinical features: Necessary: Neurological deficit compatible with a single branch territory. Supportive: a) gradual, stepwise, or stuttered onset and course during hours to a few days, and b) hypertension, diabetes, or polycythemia, and c) stereotyped TIAs over hours to 2~3 days.
(2) Circumferential branch arteries (BrA-C)
Laboratory: Necessary: (1) CT or MRI showing infarcts limited to territory of a single circumferential artery; (2) MRA or angiography showing stenosis, occlusion, or absence of filling of involved branch [PICA, anterior inferior cerebellar artery (AICA), or superior cerebellar artery (SCA)] without in situ lesion of the parent artery; and (c) ultrasound, MRA, angiography, and cardiac test results showing the absence of proximal arterial occlusive disease and the absence of cardiac embolic sources. Clinical features: Supportive: Individuals of black or Asian origin especially women, and 1, 2, 3 listed as supportive clinical features for penetrating artery disease.
3. Embolism (Emb)
Recipient site. Laboratory Supportive: a) CT or MRI showing an infarct in the territory of a main intracranial artery or superficial branch(es) and b) vascular imaging showing abrupt cutoff of branch(es), or main artery(ies), or patency of arteries known to supply infarcts. Clinical Supportive: sudden or stepwise (one step only) onset and course.
Donor sources:
Cardiac (EmbC). Necessary: cardiac studies showing a cardiac embolic source. Stroke Data Bank criteria are used.96 High risk sources include: prosthetic valves; atrial fibrillation; ventricular aneurysm; mural thrombi; cardiomyopathy; global left ventricular hypokinesis; akinetic ventricular regions; endocarditis (infective and marantic); intracardiac tumors; and patent foramen ovale with atrial septal aneurysms. Medium risk sources are: myocardial infarction within 6 months; valvular heart disease without atrial fibrillation; atrial flutter; sicksinus syndrome; mitral valve prolapse; mitral annulus calcification; atrial septal defect or patent foramen ovale; hypokinetic ventricular segment. Supportive: Laboratory: CT or MRI showing infarcts in multiple vascular territories. Clinical: systemic embolism.
Aorta (Emb-Aor) Necessary: TEE or surgical inspection showing a >4 mm protruding mobile plaque or complex ulcerated plaques in the ascending aorta or aortic arch.
Artery-to-artery (EmbA-A). Necessary: Ultrasound, MRA, CTA or contrast catheter angiography showing occluded, severely stenotic (>50% luminal narrowing), or ulcerated proximal artery or an arterial dissection. Infarction in intracranial territories distal to occlusive lesions, e.g. ICVA stenosis and SCA territory infarction, left ECVA occlusion and left PICA cerebellar infarct
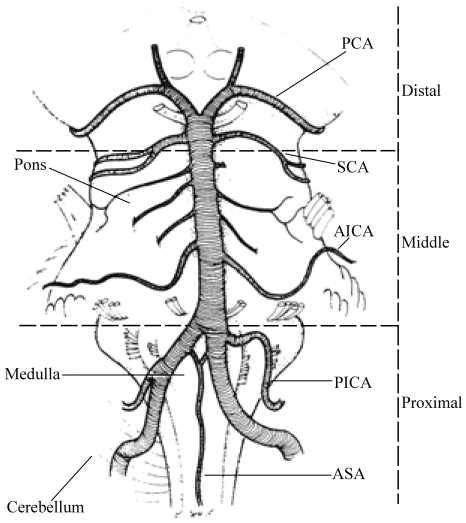
In the NEMC-PCR, brain lesions were categorized as involving proximal, middle, and distal intracranial posterior circulation territories (Fig. 1). Proximal posterior circulation territory included ICVAs supplied regions-the medulla and PICA cerebellum. The middle intracranial posterior circulation territory included brain regions supplied by the BA up to its SCA branches-the pons and AICA cerebellum. The distal territory included regions supplied by the rostral BA, SCA, PCA and their penetrating artery branches-midbrain, thalamus, SCA cerebellum, and PCA territories.
Figure 1.
Sketch of the base of the brain showing the intracranial vertebral and basilar arteries and their branches. The brain is divided into proximal, middle, and distal intracranial territories. ASA; anterior spinal artery, PICA; posteroinferior cerebellar artery, AICA; anteroinferior cerebellar artery, SCA; superior cerebellar artery, PCA; posterior cerebral artery. Redrawn by Laurel Cook-Lowe with permission from Duvernoy HM. Human Brainstem Vessels. Berlin: Springer-Verlag, 1978.
Each registry patient was thoroughly evaluated clinically and with modern neuroimaging. Brain imaging (CT and/or MRI) was performed on all patients (>80% had MRI). Vascular imaging was also performed in all patients (80% had contrast catheter angiography). Ultrasound was widely used (>80% of patients had TCD). Echocardiography and heart rhythm monitoring were performed when clinically indicated.
RESULTS
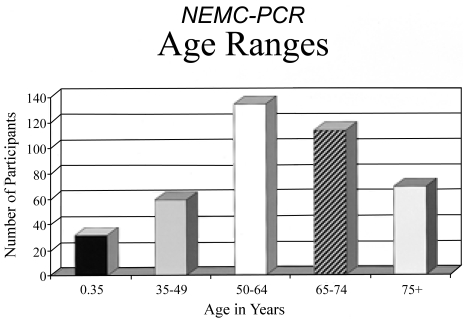
The 256 men (63%) and 151 women (37%) had an average age of 60.5 years. The age distribution is shown in Fig. 2. There were 343 (84%) white patients; non-whites included 39(9.5%) Asian origin, 18 (4%) black, and 7 (2%) hispanic patients.
Figure 2.
Age ranges of patients in the NEMC-PCR.
1. Ischemia tempo
Stroke without TIAs developed in 240 patients (59%). Four patients (1%) had strokes followed by TIAs; 63 patients (16%) had only TIAs, while 98 (24%) had TIAs before stroke.
2. Stroke mechanisms and related outcomes
Table 2 records the frequency of the various stroke mechanisms. The most likely single stroke mechanism was decided by consensus. The table shows all potential mechanisms as a range of frequencies. In a patient with atrial fibrillation and a left atrial thrombus, who had sudden onset hemianopia and an occipital lobe infarct, cardiac origin embolism was the most likely stroke mechanism. If that patient also had ECVA, stenosis then artery-to-artery embolism was a possible but less likely mechanism.
Table 2.
Stroke mechanisms in the NEMC-PCR
BrA-P; branch artery-penetrating, BrA-C; branch artery-circumferential
Embolism was the commonest stroke mechanism accounting for 40~54% of cases. Cardiac-origin embolism accounted for 24~33% of strokes while artery-to-artery embolism accounted for 14~18%. Artery-to-artery embolism was the designated stroke mechanism when an infarct was beyond the donor artery e.g, occipital lobe infarction in a patient with an ECVA occlusion. If infarcts were in regions directly perfused by the involved arteries e.g, lateral medullary infarction in a patient with a proximal ipsilateral ICVA occlusion then the designated mechanism was large artery hemodynamic.
Cardiac disease was a very common potential source of embolism. Coronary artery disease (angina pectoris, myocardial infarction or investigations showing coronary artery disease) was present in 143 patients (35%). Myocardial infarcts were present in 75 patients. Among 231 registry patients who had thorough cardiac evaluations, 147 (64%) had cardiac abnormalities. Among 34 patients with atrial fibrillation, 15 also had myocardial infarcts, 3 of whom also had congestive heart failure. Valvular heart disease was present in 25/147 (17%); 6 had cardiomyopathies. We did not routinely investigate the aorta as a potential donor embolic source so that aortic-source embolism is undoubtedly underestimated.
Patients with cardiac-origin embolism were more likely to have poor outcomes (death or severe disability) than patients with other stroke mechanisms. The relative risk of poor outcome in cardiogenic embolism patients was 1.89 compared with artery-to-artery embolism (relative risk of 0.82). Large artery occlusive disease accounted for 32~35% of cases and branch artery disease for 14~17%. Patients with large artery hemodynamic and those with penetrating artery disease had relatively low risk of poor outcome (0.59 and 0.58 relative risks respectively).4 Migraine and other mechanisms were unusual.
3. Brain lesion distribution
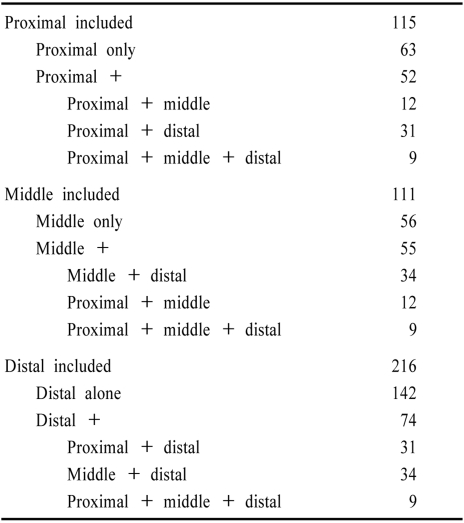
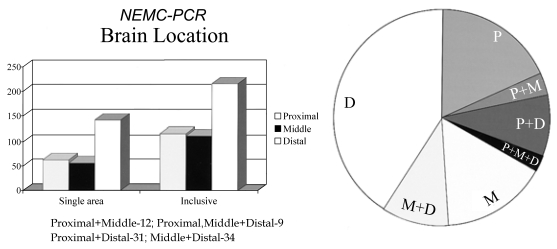
Territorial infarcts were present in 339 (83%) patients and 8 others had signs localizable to one intracranial territory. The remaining patients had no infarcts or only anterior circulation or border-zone infarcts. Among 347 patients with localizable posterior circulation infarcts, the distribution of brain locations is tabulated in Table 3 and displayed in Fig. 3a and 3b. Shown are the frequency of patients in whom one posterior circulation territory was involved alone as well as those in whom that territory was included. The distal territory was most often involved. Emboli are known to preferentially reach the most distal posterior circulation arteries.2,74,85,86,97 The proximal and middle territories were about equally involved.
Table 3.
Distribution of ischemia within the posterior circulation
Figure 3.
(A) Brain territory locations in the NEMC-PCR. (B) Pie chart showing distribution of brain locations. P; proximal, M; middle, d; distal posterior circulation territories
Among patients with more than one territory involved, the middle and distal (34 patients), and the proximal and distal (31 patients) territories were most often involved. Proximal and middle (12 patients), and proximal, middle, and distal (9 patients) territories were less common combinations.
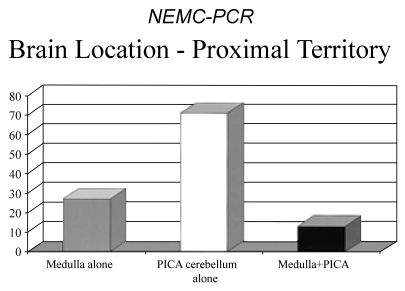
There were 115 patients with proximal territory infarction. Among these patients, the PICA supplied cerebellum was most often involved (71 patients, 64%) while the medulla was involved alone in 27 (24%). The medulla and cerebellum were both involved in only 1/8 of patients with proximal territory infarcts. The distribution of proximal territory infarcts is shown in Fig. 4.
Figure 4.
Locations of infarcts within the proximal intracranial posterior circulation territory. PICA; Posterior inferior cerebellar artery territory
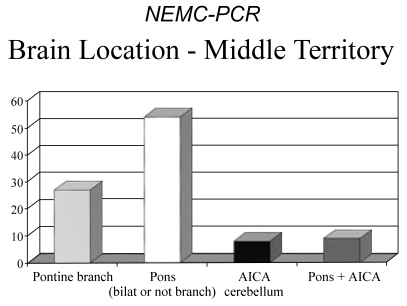
Among 111 patients with middle territory ischemia, most lesions involved the pons more extensively than a single penetrating artery branch-54 patients (55%). Single branch pontine infarcts (27 patients, 28%) were next most common. AICA territory cerebellar infarcts were found in 8 patients (8%), and combined pons and AICA territory infarcts in 9 (9%). Fig. 5 shows the distribution of middle territory infarcts.
Figure 5.
Locations of infarcts within the middle intracranial posterior circulation territory. AICA; Anterior Inferior cerebellar artery territory
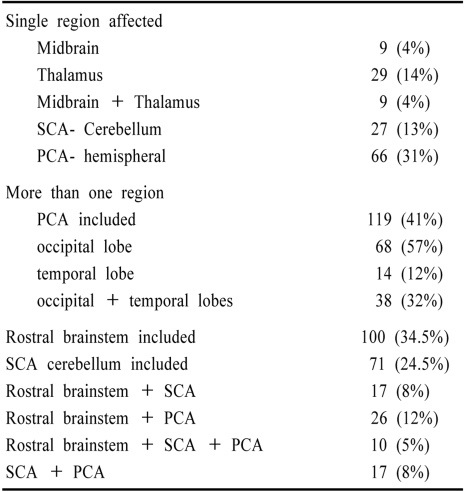
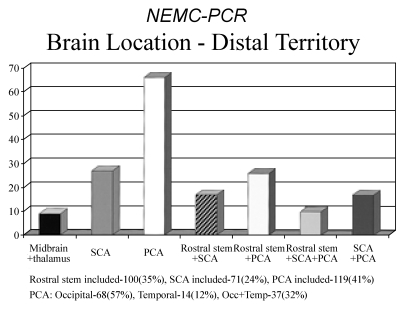
Among 216 patients who had distal territory infarction, the occipital and temporal lobe PCA territories were most often involved (119 patients, 41%), while the rostral brainstem (thalamus and midbrain) was infarcted in 100 patients (34.5%). The SCA-supplied cerebellum was involved in 71 patients (24.5%). When considering only single region involvement, PCA hemispheral territory was most common followed by the thalamus and SCA cerebellum (Table 4 and Fig. 6).
Table 4.
Location of infarcts within the distal intracranial posterior circulation territory (n=216 patients)
Figure 6.
Locations of infarcts within the distal intracranial posterior circulation territory. SCA; superior cerebellar artery (SCA) territory of the cerebellum, PCA; occipital and temporal lobe territories of the posterior cerebral arteries (PCAs), Rostral stem; midbrain and thalamus
Patients with infarcts limited to the proximal territory had better outcomes than patients with infarcts limited to other territories.4 Only 3 of 59 (5%) patients with infarcts involving only the proximal territory had poor outcomes (death or severe disability). The highest frequency of poor outcomes (32 of 125, 26%) among patients with single territory infarcts were those with distal territory infarction; these patients accounted for 42% of poor outcomes. Ten of 51 (20%) patients with infarcts limited to the middle territory had poor outcomes accounting for 13% of poor outcomes. More than one territory involvement predicted poorer outcome than single territory infarction. Distal territory included and middle territory included had more poor outcomes than patients with proximal territory included.4
4. Brain locations in relation to stroke mechanisms
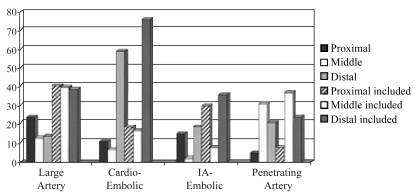
Fig. 7 shows the distribution of brain lesions according to stroke mechanisms. About half of the proximal territory infarcts are caused by cardiac-origin and artery-to-artery emboli arising from the ECVAs, while the other half are explained by hypoperfusion related to ICVA occlusive disease.7 Middle territory infarcts are explained by occlusive lesions of the BA or its branches. Most distal territory infarcts are attributable to cardiogenic and artery-to-artery embolism (donor sites mainly the ECVAs and ICVAs) while most of the remainder are related to penetrating artery disease.
Figure 7.
Brain locations within the posterior circulation in patients with various stroke mechanisms. IA; Intraarterial
Cardiogenic embolism caused predominantly distalonly or distal-included infarcts; infarcts limited to or including the proximal and middle territories were much less common. Patients with PCA, SCA, and top-of-the BA infarcts had a very high likelihood of cardiac or artery-to-artery embolism. Cardiac-origin embolism causing solely middle territory infarction was rare (7% of patients). Artery-to-artery embolism caused more even distribution of infarction. ICVA occlusive disease caused infarction locally (proximal territory) and embolized to recipient distal territory arteries. BA occlusive disease often caused middle territory infarcts and spread or embolization to the distal territory. Some patients with ECVA occlusive disease had emboli to the ipsilateral ICVA causing proximal territory ischemia which and then went distally. Proximal and distal territory infarcts resulted. Curiously this often produced infarction limited to the cerebellum involving PICA and SCA cerebellar territories.
The distribution of large artery hemodynamic-related infarcts was even reflecting almost equal involvement of the ICVAs and the BA and frequent involvement of multiple intracranial arteries. The distribution of infarcts in relation to specific arteries is analyzed in an accompanying report.5
DISCUSSION
1. Stroke Mechanisms-do posterior and anterior circulation ischemia patients differ?
Modern investigations show that most anterior circulation infarcts are attributable to embolism from the heart, aorta, and proximal arteries. TCD monitoring of the middle cerebral artery shows that microembolic signals are common after acute brain infarcts and in patients with high risk embolic sources.98
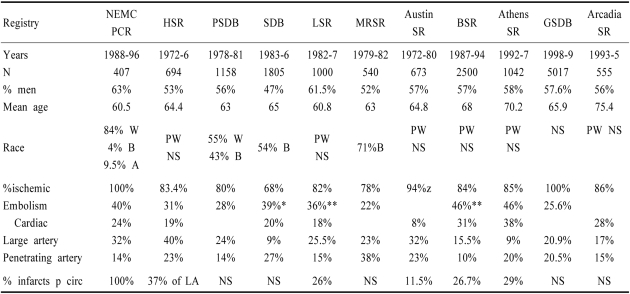
Stroke registries used different diagnostic criteria and had available different technologies to determine stroke mechanisms. (Table 5). The Harvard Stroke Registry (HSR)89 was the first to report a high rate of embolism. In this prospective registry, 694 stroke patients were thoroughly investigated using available technology. Embolism was diagnosed when symptoms, signs, and investigations showed sudden occlusion of large intracranial arteries or branches. Emboli arose from the heart or a cervico-cranial artery. Sudden onset of focal neurological signs and angiography showing blockage of an intracranial artery not due to local atherosclerosis was the commonest evidence for embolism. Echocardiography was not available and only half the patients had CT which became available in the mid 1970s. In the HSR, 215 of 694 (31%) strokes were considered embolic. Cardiac disease was present in 112 of 215 (52%) embolic stroke patients -73 had atrial fibrillation and others had recent myocardial infarction or valvular heart disease. The HSR did not report anterior and posterior circulation localization.
Table 5.
Stroke mechanisms in various stroke registries
NEMC-PCR; New England Medical Center Posterior Circulation Registry, HSR; Harvard Stroke Registry-Ref 89, PSDB; Pilot Stroke Data Bank- Ref 99, SDB; Stroke Data Bank-Ref 91, LSR; Lausanne Stroke Registry Ref 92, MRSR; Michael Reese Stroke Registry Ref 90, BSR; Besancon Stroke Registry Ref 94,100, SR; Stroke registry, Austin Stroke Registry Ref 116; Arcadia Stroke Registry 101, Athens Styroke Registry- Ref 95, GSDB; German Stroke data Bank Ref 102, *embolism recalculated using HSR criteria (Caplan, 1995) †cardiac+large artery without stenosis, PW; predominantly white, LA; large artery
The HSR diagnosed embolism mostly in reference to recipient arteries. Previously, embolism was defined as cardiac, and was diagnosed only when rheumatic valvular disease or acute myocardial infarction were present. The frequency of diagnosed embolism ranged from 3~8%.73,97 The Pilot Stroke Data Bank (PSDB)99 and the Stroke Data Bank (SDB)91 characterized cases as embolic only when there was a high-risk cardiac embolic source. The SDB had a category for intra-arterial embolism called "tandem arterial pathology". When there was no defined cardiac source and the stroke was not lacunar, patients were classified as "infarcts of unknown cause" (IUC). If the HSR criteria for embolism are applied to SDB data the frequency of embolism and cardiac source embolism are comparable. Neither the PSDB or the SDB reported anterior and posterior circulation localization within stroke mechanism categories.
The Lausanne Stroke Registry (LSR) categorized patients as having potential cardiac sources of embolism (PCSE), atherosclerosis with stenosis, and atheroscerosis without stenosis.92 Atherosclerosis without stenosis was comparable to artery-to-artery embolism in the HSR and tandem arterial pathology in the SDB. The frequency of embolism and cardiac origin embolism are comparable to those in the HSR and the SDB (Table 5). The LSR reported the frequencies of stroke mechanisms in patients with anterior and posterior circulation infarcts. More posterior circulation patients had presumed intra-arterial embolism (large artery disease without stenosis), and penetrating artery disease. More anterior circulation patients had large artery disease with stenosis. The frequency of cardiac origin embolism was comparable (19% anterior vs 16% posterior circulation) (Table 5).
In the Besancon,94,100 Arcadia101 and Athens95 registries, slightly more patients had cardiac origin embolism (reflecting availability of improved echocardiography) compared to prior registries (Table 5). In the German Stroke Data Bank102 cardioembolism was diagnosed when there was a high or medium risk cardiac source and no large artery atherosclerosis. The large artery atherosclerosis ("macroangiopaathy") category was used for >50% stenosis or occlusion of a large artery supplying the region of brain ischemia; hemodynamic and artery-to-artery embolism were not distinguished.102
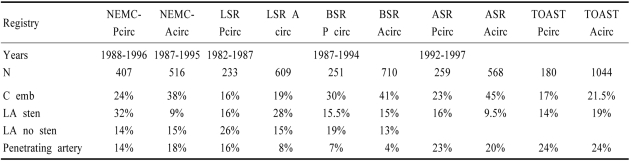
Limited data are available comparing stroke mechanisms in patients with anterior and posterior circulation disease (Table 6). Comparing NEMC-PCR data with that from prospective patients with anterior circulation ischemia collected at NEMC during the same time, there were more cardiogenic emboli (38% vs 24%), and fewer large artery occlusive lesions (9% vs 32%) in anterior circulation patients but the frequency of intra-arterial embolism and penetrating artery lesions were similar. The Lausanne,92 Besancon,94,100 and Athens95 registries and the TOAST trial103,104 reported stroke mechanisms in anterior and posterior circulation patients. (Table 6). Cardiac origin embolism was more common among anterior circulation cases in all registries but the frequencies were close in the TOAST trial and the LSR. Large artery disease with stenosis was more common among posterior circulation patients in the Athens Stroke Registry but was more common among anterior circulation patients in the LSR and the TOAST trial, and was about equal in the Besancon Registry. Penetrating artery disease was slightly more common among posterior circulation patients in all registries.
Table 6.
Stroke mechanisms in registries that compared posterior and anterior circulations
C Emb; cardioembolic, LA; large artery, Sten; stenosis, NEMC; New England MedicaL Center, LSR; Lausanne Stroke Registry- Ref 92, BSR; Besancon Stroke Registry Ref 94, 100, ASR; Athens Stroke registry- Ref 95, TOAST; Trial of ORG 10172 in Acute Stroke Treatment Ref 103,104
Differences in stroke mechanisms are explainable considering anatomical and blood flow differences. About 2/5ths of brain blood flow goes into each ICA and only 1/5ths into the vertebro-basilar arteries.105 By chance alone 1/5th of cardiac-origin emboli should go to the posterior circulation. The posterior circulation consists of relatively more brainstem and thalamic tissue supplied by penetrating arteries compared to the anterior circulation. Relatively more lacunes and branch territory infarcts are expected in the posterior circulation.
The anterior and posterior circulation arteries have the same vascular coats, are fed by the same heart, and contain the same blood constituents under the same arterial pressures. The frequencies of stroke mechanisms should be similar. The data from the NEMC-PCR and other registries shows that the frequencies of stroke mechanisms are more alike than dissimilar. Historical differences are explained by the phenomenon of self-fullfilling prophecy. Vertebrobasilar angiography was considered dangerous and was performed only in patients with severe brainstem signs. The non-study of posterior circulation cases led to continuation of early diagnostic biases while at the same time patients with carotid territory ischemia were more thoroughly investigated. The causes of posterior and anterior circulation disease are similar. All brain ischemia patients deserve full brain and vascular imaging and appropriate cardiac, aortic, and coagulation evaluation. There is no reason to investigate or treat patients with anterior and posterior circulation disease differently.
2. Concurrent cardiac disease
Cardiac and aortic lesions are well recognized and accepted sources of embolism to anterior circulation intracranial arteries. Coronary artery and ICA disease often coexist. Mortality in anterior circulation stroke patients is often cardiac. Patients with ICA disease are often investigated for important coronary artery disease. However, it has not been customary to examine the heart and aorta in vertebro-basilar disease patients.2,3 The NEMC-PCR data confirms that a substantial proportion of patients with cardioembolic strokes will have poor outcomes. We also show a relatively frequent coexistence of coronary artery disease and myocardial infarction. Cardiac and aortic evaluation is just as important in posterior circulation disease as it is in the anterior circulation.
3. Posterior circulation territories and stroke mechanisms
Dividing the posterior circulation into vascular supply territories helps predict stroke mechanisms, arterial lesions, and outcomes.2-4 In the NEMC-PCR, distal territory lesions were most common. Distal territory infarcts were also common in other registries. In the LSR, 164/401 (41%) posterior circulation patients had thalamic or PCA territory infarcts.93 Among 70 LSR patients who had MRA and NEMC-PCR divisions were used, 37% had distal territory infarcts while 27% had proximal territory and 23% had middle territory infarcts,49 a frequency matching the NEMC-PCR. The Besancon94,100 and Athens registries95 characterized localizations as brainstem, cerebellum, and PCA territories. Among 251 patients with posterior circulation ischemia in the Besancon Registry, 34% were PCA, 39% brainstem, and 27% cerebellar.100 Among 259 posterior circulation ischemia patients in the Athens Registry, 27% included PCA territory, 28% were brainstem, and 24% cerebellar.95 In the NEMC-PCR, PCA territories were most often infarcted but distal territory rostral brainstem and SCA infarcts were also common. If rostral brainstem and SCA cerebellar infarcts are added to PCA infarcts in the Lausanne, Besancon, and Athens Registries, the frequency of distal territory infarction parallels the NEMC-PCR.
The proximal intracranial territory was next most commonly affected in the NEMC-PCR. The middle territory was least often involved. This distribution cannot be compared to that found in the Lausanne, Athens, and Besancon registries since they did not divide the brainstem or cerebellum into regions. Among 70 LSR patients classified according to NEMC-PCR rules, the distribution was comparable to that found in the NEMC-PCR.49 Multiple territories were often involved in all registries.
No registry contains data about the frequency of stroke mechanisms according to the NEMC-PCR territorial classification. However several registries report information about mechanisms in regard to brainstem, cerebellar, and PCA territory localizations. In the LSR, cerebellar and PCA territory infarcts were commoner in patients with PCSEs compared to those with no PCSE.93 In the Besancon100 and Athens95 registries, PCA territory and cerebellar infarcts were predominantly attributed to cardiac origin embolism. In the Athens Registry 52% of 71 PCA territory infarcts were cardioembolic and 26% were IUC.95 The Athens Stroke registry attributed 40% of cerebellar infarcts to cardioembolism, 26% to large artery atherosclerosis, and 26% to IUC. In the Besancon registry, 41% of PCA territory infarcts were cardioembolic and 26% were undetermined and 33% of cerebellar infarcts were cardioembolic while 20% were undetermined.100 In these registries, IUC and undetermined meant no large artery occlusive disease and non-lacunar infarction. These two classifications are usually due to unidentified cardiac, aortic, or proximal arterial donor embolic sources.97 In all series of PCA territory infarction, cardiac origin and artery-to-artery embolism are the predominant causes.2,3,11,72,74 PICA and SCA territory cerebellar infarcts are also predominantly embolic.2,3,64,69,72 Few infarcts involve AICA cerebellum. Most likely cerebellar infarcts in the Lausanne, Besancon, and Athens registries were predominantly in PICA and SCA supplied regions explaining the predominance of embolism as the major cause. In the Lausanne,49,93 Besancon,100 and Athens95 registries, brainstem infarcts were less often attributed to embolism; atherosclerosis and penetrating artery disease predominated. In the LSR, brainstem infarct patients most often had no PCSEs.49 In the Athens Registry, only 9.5% of patients with brainstem infarcts had cardiac-origin embolism95 and in the Besancon Registry 17.5% of brainstem infarcts were cardioembolic.100 These frequencies of cardioembolism are much lower than among patients with cerebellar and PCA territory infarcts in these registries. Lateral medullary infarction (proximal territory) is most often caused by atherosclerotic ICVA disease. Pontine infarction (middle territory) is most often related to BA or penetrating artery disease. Isolated thalamic infarcts (distal territory) are most often attributable to penetrating artery disease while distal territory infarcts that include the thalamus and PCA supplied regions are mostly caused by cardiac origin and intra-arterial embolism. The predominant mechanisms of isolated brainstem infarcts are large artery hemodynamic disease and penetrating artery disease. Embolism is a much less common mechanism underlying brainstem infarction.
4. Outcomes according to mechanism and location
NEMC-PCR 30 day mortality was very low, 3.6%;4 21% of patients died or had major disability. Nearly 4/5ths of patients had no or little disability. Vertebrobasilar territory disease has an undeserved reputation for causing high morbidity and mortality. Prior series were biased towards including mostly patients with severe neurological signs and did not include the broad spectrum of posterior circulation ischemia patients. Data from the NEMC-PCR and other data banks should dispell this false belief.
The NEMC-PCR may contain a referral bias. Patients entering other hospitals in coma or with severe disabling deficits might not have been referred to NEMC. The NEMC stroke center was well known for interest in caring for patients with vertebrobasilar territory strokes, so that the case mix might be different from that in the community.
We cannot determine whether treatment in the NEMC-PCR was responsible for the relatively good outcomes. Therapies were chosen by stroke service clinicians and were not randomized. Anticoagulants were given to 221 (54%) NEMC-PCR patients; 137 (34%) patients received intravenous heparin and 185 (45%) were given warfarin. Antiplatelet agents, mostly aspirin, were prescribed at discharge or in the hospital to 86 (19%) patients. Intra-arterial thrombolysis was used in only 3 patients. Surgery was seldom pursued; 4 cerebellar infarct patients had ventriculostomies or shunts, and 3 patients had vascular surgery (1 ECVA to common carotid artery bypass, 2 occipital to PICA bypasses). Another 5 patients (1 with BA stenosis, and 4 with bilateral ICVA stenosis) had intracranial angioplasties.
Mortality in the LSR was 5.9%,92 comparable to that in the NEMC-PCR. Among 70 LSR patients with vertebrobasilar infarcts studied with MRA examinations, 3 patients (4%) died within 3 weeks, a figure identical to that in the NEMC-PCR.49 In the Besancon registry, 10% of patients with posterior circulation infarcts died compared to a mortality of 18.6% among anterior circulation patients.100 Among posterior circulation stroke survivers, 79% had good outcomes while 21% had poor outcomes (Rankin scales 4 and 5). The comparable anterior circulation figures were 50.3% good and 49% poor.100 In the TOAST study,103 152/180 (84%) patients with posterior circulation disease had good outcomes compared with 749/1039 (72%) anterior circulation infarct patients.104
Contrasting with the relatively good outcomes in the NEMC-PCR were results reported in a study of 404 consecutive patients with brainstem and/or cerebellar infarcts in Giesen Germany.106 In this study 75 patients (18.6%) died and another 64 (15.8% were disabled, a third were dead or disabled by the end of the hospitalization (average hospital stay 28 days). In this study 177/404 (44%) were somnolent or comatose on admission and 86 (21%) were tetraplegic.106 The severe neurological signs and inclusion of only brainstem and cerebellar infarcts meant that the patient mix studied was different from the NEMC-PCR. Similarly the patient mix chosen for angiography by Archer and Horenstein107 and those evaluated for intra-arterial thrombolysis by Hacke et al108 had more severe neurological signs than those in the NEMC-PCR.
In the NEMC-PCR, cardioembolic stroke mechanism increased the risk of poor outcome. Embolic mechanism conveyed similar higher risks in other studies. Petty et al reported Mayo Clinic data that showed the following figures for mortality and severe disability (Rankin 4 and 5) at 90 days after stroke: cardioembolism 56.8%, atherosclerosis with stenosis 32.4%, lacunar stroke 4.2%, and IUC 35.8%.109 An earlier Mayo Clinic report also showed that patients with cardioembolic strokes had worse outcomes than patients who had no cardiac embolic sources.110 In the German Stroke Data Bank, cardioembolic stroke patients had the poorest outcomes at 90 days (22.6%).102
In the NEMC-PCR patients with distal intracranial territory ischemia had more poor outcomes compared to patients with other territory lesions. Patients with multiple intracranial territory infarcts had higher risks than patients with single territory lesions.4 The relative risk of distal territory localization for poor outcome was 3.12 (95% CI 1.92~5.07; p=.001), compared with middle territory relative risk 1.88 (95% CI 1.88; p=.002) and proximal territory relative risk of 0.81 (95% CI 0.5~1.3; p=.37). The most likely explanation for higher risk is that most distal territory infarcts are embolic. Middle territory infarcts patients have more severe morbidity because of a high frequency of BA occlusive disease. Nadeau et al also found that multiple sector lesions carried a higher risk of poor outcomes than single sector lesions.111 Among 236 patients with posterior circulation strokes followed in the LSR, 25% of patients with multiple acute infarcts had died or were dependent a month after stroke onset.112
5. Therapeutic implications
The frequency of embolism and the relatively poor outcome of patients with embolic strokes makes these patients important targets for treatment. To date no study of intravenous thrombolysis has reported results in patients with embolism to the ICVAs or BA and its branches. Three studies report results of intra-arterial thrombolysis in patients with BA occlusions.108,113,114 In thrombolytic series, patients with embolism had more successful recanalization and better outcomes than patients with in-situ thrombosis.115 In a study of thrombolysis of BA occlusions, 23 of 51 patients had distal BA occlusions and occlusions were embolic in 35.114 Posterior circulation embolism is a potentially important therapeutic target for future thrombolytic trials. Stenting and angioplasty will become important therapeutic considerations in patients with severe occlusive disease.
References
- 1.Barnett HJM. A modern approach to posterior circulation ischemic stroke. Arch Neurol. 2002;59:359–360. doi: 10.1001/archneur.59.3.359. [DOI] [PubMed] [Google Scholar]
- 2.Caplan LR. Posterior circulation disease: clinical features, diagnosis, and management. Boston: Blackwell Science; 1996. [Google Scholar]
- 3.Caplan LR. Posterior circulation ischemia: then, now, and tomorrow. The Thomas Willis Lecture 2000. Stroke. 2000;31:2011–2023. doi: 10.1161/01.str.31.8.2011. [DOI] [PubMed] [Google Scholar]
- 4.Glass TA, Hennessey PM, Pazdera L, Chang H-M, Wityk RJ, DeWitt LD, et al. Outcome at 30 days in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 2002;59:369–376. doi: 10.1001/archneur.59.3.369. [DOI] [PubMed] [Google Scholar]
- 5.Caplan LR, Wityk RJ, Glass TA. The New England Medical Center Posterior Circulation Registry. Ann Neurol. 2004;56:389–398. doi: 10.1002/ana.20204. [DOI] [PubMed] [Google Scholar]
- 6.Georgiadis AI, Yamamoto Y, Kwan ES, Pessin MS, Caplan LR. Anatomy of sensory findings in patients with Posterior Cerebral Artery Territory Infarction. Arch Neurol. 1999;56:835–838. doi: 10.1001/archneur.56.7.835. [DOI] [PubMed] [Google Scholar]
- 7.Graf KJ, Pessin MS, DeWitt LD, Caplan LR. Proximal intracranial territory posterior circulation infarcts in the New England Medical Center Posterior Circulation Registry. Eur Neurol. 1997;37:157–168. doi: 10.1159/000117428. [DOI] [PubMed] [Google Scholar]
- 8.Muller-Kuppers M, Graf KJ, Pessin MS, DeWitt LD, Caplan LR. Intracranial vertebral artery disease in the New England Medical Center Posterior Circulation Registry. Eur Neurol. 1997;37:146–156. doi: 10.1159/000117427. [DOI] [PubMed] [Google Scholar]
- 9.Shin H-K, Yoo K-M, Chang H-M, Caplan LR. Bilateral intracranial vertebral artery disease in the New England medical Center Posterior Circulation Registry. Arch Neurol. 1999;56:1353–1358. doi: 10.1001/archneur.56.11.1353. [DOI] [PubMed] [Google Scholar]
- 10.Wityk RJ, Chang H-M, Rosengart A, Han W-C, DeWitt LD, Pessin MS, Caplan LR. Proximal extracranial vertebral artery disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 1998;55:470–478. doi: 10.1001/archneur.55.4.470. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto Y, Georgiadis AI, Chang H-M, Caplan LR. Posterior Cerebral Artery territory Infarcts in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 1999;56:824–832. doi: 10.1001/archneur.56.7.824. [DOI] [PubMed] [Google Scholar]
- 12.Benedikt M. Tremblement avec paralysie croisee du moteur oculaire commun. Bull Med Paris. 1889;3:547–548. [Google Scholar]
- 13.Claude H. Syndrome pedonculaire de la region du noyau rouge. Rev Neurol. 1912;23:311–313. [Google Scholar]
- 14.Claude H, Levy-Valensi J. Maladies des pedoncules cerebraux in Maladies du cervelet et de l'isthme de l'encephale (pedoncule, protuberance, bulbe) Paris: Bailliere; 1922. pp. 184–211. [Google Scholar]
- 15.Babinski J, Nageotte J. Hemiasynergie, lateropulsion et myosis bulbaires avec hemianesthesie et hemiplegie croisees. Rev Neurol. 1902;10:358–365. [Google Scholar]
- 16.Foville A. Note sur une paralysie peu connue de certains muscles de l'oeil. Bull Soc Anat Paris. 1858;3:393–405. [Google Scholar]
- 17.Wallenberg A. Anatomischer befund in einern als akute bulbaraffection (Embolie der arteria cerebelli inferior posterior sinistra) bescriebenen falle. Arch fur Psychiatrie. 1901;34:923–959. [Google Scholar]
- 18.Wallenberg A. Verschluss der arteria cerebelli inferior posterior dextra (mit Sektion befund) Deutsche Zeitschrift fur Nervenheilkunde. 1922;73:189–212. [Google Scholar]
- 19.Weber H. A contribution to the pathology of the crura cerebri. Medico Chirurg Trans. 1863;46:121–139. doi: 10.1177/095952876304600112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfe JK. The classical brainstem syndromes. Springfield, Illinois: Charles C Thomas Publ; 1971. [Google Scholar]
- 21.Liu GT, Crenner CW, Logoigian EL, Charness ME, Samuels MA. Midbrain syndromes of Benedikt, Claude, and Nothnagel. Setting the record straight. Neurology. 1992;42:1820–1822. doi: 10.1212/wnl.42.9.1820. [DOI] [PubMed] [Google Scholar]
- 22.Dejerine J. Serniologie des affections du systeme nerveux. Paris: Masson et cie; 1926. [Google Scholar]
- 23.Foix C, Hillemand P, Schalit 1. Sur le syndrome lateral du bulbe et irrigation du bulbe superieur. Rev Neurol. 1925;41:160–179. [Google Scholar]
- 24.Dana CL. Acute bulbar paralysis due to hemorrhage and softening of the pons and medulla with reports of cases and autopsies. Medical Record. 1903;64:361–374. [Google Scholar]
- 25.Kubik CS, Adams RD. Occlusion of the basilar artery a clinical and pathological study. Brain. 1946;69:73–121. doi: 10.1093/brain/69.2.73. [DOI] [PubMed] [Google Scholar]
- 26.Dejerine J, Roussy G. Le syndrome thalamique. Rev Neurol. 1906;14:521–532. [Google Scholar]
- 27.Foix C, Hillemand P. Les arteres de I'axe encephalique jusqu'au diencephale inclusivement. Rev Neurol. 1925;41:705–739. [Google Scholar]
- 28.Foix C, Hillemand P. Les syndromes de la region thalamique. Presse Med. 1925;33:113–117. [Google Scholar]
- 29.Foix C, Masson A. Le syndrome de l'artere cerebrale posterieure. Presse Med. 31923;31:361–365. [Google Scholar]
- 30.Duret H. Sur la distribution des arteres nourricieres du bulbe rachidien. Arch Physiol Norm Pathol. 1873;2:97–113. [Google Scholar]
- 31.Duret H. Reserches anatomiques sur la circulation de 1'encephale. Arch Physiol Norm Pathol. 1874;3:6091, 316–353, 664–693, 919–957. [Google Scholar]
- 32.Foix C, Hillemand P. Contribution a 1'etude des ramollissements protuberantiels. Rev Med. 1926;43:287–305. [Google Scholar]
- 33.Stopford JSB. The arteries of the pons and medulla oblongata. J Anat Physiol (London) 1916;50:131–164. 225–280. [PMC free article] [PubMed] [Google Scholar]
- 34.Foix C, Hillemand P, Ley J. Relativement au ramollissement cerebral a sa frequence et a son siege et a l'importance relative des obliterations arterielles, completes ou incompletes dans sa pathogenie. Rev Neurol. 1927;43:217–218. [Google Scholar]
- 35.Fisher CM. Occlusion of the internal carotid artery. Arch Neurol Psychiatr. 1951;65:346–377. doi: 10.1001/archneurpsyc.1951.02320030083009. [DOI] [PubMed] [Google Scholar]
- 36.Fisher CM. Occlusion of the internal carotid arteries: further experiences. Arch Neurol Psychiat. 1954;72:187–204. doi: 10.1001/archneurpsyc.1954.02330020055006. [DOI] [PubMed] [Google Scholar]
- 37.Hutchinson EC, Yates PO. The cervical portion of the vertebral artery: clincopathological study. Brain. 1956;79:319–331. doi: 10.1093/brain/79.2.319. [DOI] [PubMed] [Google Scholar]
- 38.Hutchinson EC, Yates PO. Caroticovertebral stenosis. Lancet. 1957;1:28. [Google Scholar]
- 39.Bradshaw P, McQuaid P. The syndrome of vertebrobasilar insufficiency. Quarterly J Med. 1963;32:279–296. [PubMed] [Google Scholar]
- 40.Williams D. Vertebrobasilar ischaemia. Brit Med J. 1964;1:84–86. doi: 10.1136/bmj.1.5375.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams D, Wilson TG. The diagnosis of the major and minor syndromes of basilar insufficiency. Brain. 1962;85:741–774. doi: 10.1093/brain/85.4.741. [DOI] [PubMed] [Google Scholar]
- 42.DennyBrown D. Basilar artery syndromes. Bull N Engl Med Center. 1953;15:53–60. [PubMed] [Google Scholar]
- 43.Fang HC, Palmer JL. Vascular phenomena involving brainstern structures: a clinical and pathological correlation study. Neurology. 1956;6:402–419. doi: 10.1212/wnl.6.6.402. [DOI] [PubMed] [Google Scholar]
- 44.Millikan CH, Siekert RG. Studies in cerebrovascular disease; I The syndrome of intermittent insufficiency of the basilar arterial system. Proc Staff Meet Mayo Clin. 1955;30:61–68. [PubMed] [Google Scholar]
- 45.Millikan CH, Siekert RG, Shick R. Studies in cerebrovascular disease. 3. The use of anticoagulant drugs in the treatment of insufficiency or thrombosis within the basilar arterial system. Proc Staff Meet Mayo Clin. 1955;30:111–126. [PubMed] [Google Scholar]
- 46.Fisher CM. The use of anticoagulants in cerebral thrombosis. Neurology. 1958;8:311–332. doi: 10.1212/wnl.8.5.311. [DOI] [PubMed] [Google Scholar]
- 47.Millikan CH, Siekert RG, Whisnant JP. Anticoagulant therapy in cerebrovascular disease: current status. JAMA. 1958;166:587–592. doi: 10.1001/jama.1958.02990060025006. [DOI] [PubMed] [Google Scholar]
- 48.Rosengart A, DeWitt LD, Caplan LR, et al. Noninvasive tests in vertebrobasilar occlusive disease. Ann Neurol. 1992;32:265. [Google Scholar]
- 49.Bogousslavsky J, Regli F, Maeder P, Meuli R, Nader J. The etiology of posterior circulation infarcts: a prospective study using magnetic resonance angiography. Neurology. 1993;43:1528–1533. doi: 10.1212/wnl.43.8.1528. [DOI] [PubMed] [Google Scholar]
- 50.Wentz KU, Rother J, Schwartz A. Intracranial vertebrobasilar system: MR angiography. Radiology. 1994;190:105–110. doi: 10.1148/radiology.190.1.8259384. [DOI] [PubMed] [Google Scholar]
- 51.Fisher CM. Pure sensory stroke involving face, arm, and leg. Neurology. 1965;15:76–80. doi: 10.1212/wnl.15.1.76. [DOI] [PubMed] [Google Scholar]
- 52.Fisher CM. A lacunar stroke: the dysarthria clumsy hand syndrome. Neurology. 1967;17:614–617. doi: 10.1212/wnl.17.6.614. [DOI] [PubMed] [Google Scholar]
- 53.Fisher CM. Ataxic hemiparesis. Arch Neurol. 1978;35:126–128. doi: 10.1001/archneur.1978.00500270008002. [DOI] [PubMed] [Google Scholar]
- 54.Fisher CM. Thalamic pure sensory stroke: a pathological study. Neurology. 1978;28:1141–1144. doi: 10.1212/wnl.28.11.1141. [DOI] [PubMed] [Google Scholar]
- 55.Mohr JP. Lacunes. Stroke. 1982;13:311. doi: 10.1161/01.str.13.1.3. [DOI] [PubMed] [Google Scholar]
- 56.Caplan LR. lntracranial branch atheromatous disease; a neglected, understudied, and underused concept. Neurology. 1989;39:1246–1250. doi: 10.1212/wnl.39.9.1246. [DOI] [PubMed] [Google Scholar]
- 57.Fisher CM, Caplan LR. Basilar artery branch occlusion: a cause of pontine infarction. Neurology. 1971;21:900–905. doi: 10.1212/wnl.21.9.900. [DOI] [PubMed] [Google Scholar]
- 58.Fisher CM. Bilateral occlusion of basilar artery branches. J Neurol Neurosurg Psychiatry. 1977;40:1182–1189. doi: 10.1136/jnnp.40.12.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hauw JJ, Der Agopian P, Trelles L, Escourolle R. Bulbar infarcts. Systematic study of lesion topography in 49 cases. J Neurol Sci. 1976;28:83–102. doi: 10.1016/0022-510x(76)90050-2. [DOI] [PubMed] [Google Scholar]
- 60.Escourolle R, Hauw JJ, Agopian PD, Trelles L. Bulbar infarcts. Vascular lesions in 26 observations. J Neurol Sci. 1976;28:103–113. doi: 10.1016/0022-510x(76)90051-4. [DOI] [PubMed] [Google Scholar]
- 61.Fisher CM, Karnes W, Kubik CS. Lateral medullary infarction. The pattern of vascular occlusion. J Neuropath Exp Neurol. 1961;20:323–379. doi: 10.1097/00005072-196107000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Sypert GW, Alvord EC. Cerebellar infarction: a clinicopathological study. Arch Neurol. 1975;32:357–363. doi: 10.1001/archneur.1975.00490480023001. [DOI] [PubMed] [Google Scholar]
- 63.Amarenco P. Cerebellar stroke syndromes. In: Bogousslavsky J, Caplan LR, editors. Stroke syndromes. 2nd edition. Cambridge University Press; 2001. pp. 540–556. [Google Scholar]
- 64.Amarenco P, Caplan LR. Vertebrobasilar occlusive disease: review of selected aspects. 3. Mechanisms of cerebellar infarctions. Cerebrovasc Dis. 1993;3:66–73. [Google Scholar]
- 65.Amarenco P, Hauw JJ. Cerebellar infarction in the territory of the anterior and inferior cerebellar artery. Brain. 1990;113:139–155. doi: 10.1093/brain/113.1.139. [DOI] [PubMed] [Google Scholar]
- 66.Amarenco P, Hauw JJ. Cerebellar infarction in the territory of the superior cerebellar artery: a clinicopathologic study of 33 cases. Neurology. 1990;40:1383–1390. doi: 10.1212/wnl.40.9.1383. [DOI] [PubMed] [Google Scholar]
- 67.Amarenco P, Hauw JJ, Henin D, et al. Les infarctus du territoire de artere cerebelleuse posteroinferieure, etude clinicopathologique de 28 cas. Rev Neurol. 1989;145:277–286. [PubMed] [Google Scholar]
- 68.Amarenco P, Hauw JJ, Gautier JC. Arterial pathology in cerebellar infarction. Stroke. 1990;21:1299–1305. doi: 10.1161/01.str.21.9.1299. [DOI] [PubMed] [Google Scholar]
- 69.Amarenco P, Levy C, Cohen A, et al. Causes and mechanisms of territorial and nonterritorial cerebellar infarcts in 115 consecutive patients. Stroke. 1994;25:105–112. doi: 10.1161/01.str.25.1.105. [DOI] [PubMed] [Google Scholar]
- 70.Amarenco P, Rosengart A, DeWitt LD, Pessin MS, Caplan LR. Anterior inferior cerebellar artery territory infarcts. Mechanisms and clinical features. Arch Neurol. 1993;50:154–161. doi: 10.1001/archneur.1993.00540020032014. [DOI] [PubMed] [Google Scholar]
- 71.Castaigne P, Lhermitte F, Buge A, Escourolle R, Hauw JJ, Lyon-Caen D. Paramedian thalamic and midbrain infarcts: clinical and neuropathological study. Ann Neurol. 1981;10:127–148. doi: 10.1002/ana.410100204. [DOI] [PubMed] [Google Scholar]
- 72.Caplan LR. The 1991 E Graeme Robertson lecture: Vertebrobasilar embolism. Clin Exp Neurol. 1991;28:123. [PubMed] [Google Scholar]
- 73.Caplan LR. Brain embolism. In: Caplan LR, Hurst JW, Chimowitz M, editors. Clinical Neurocardiology. New York: Marcel Decker; 1999. pp. 35–185. [Google Scholar]
- 74.Caplan LR, Tettenborn B. Vertebrobasilar occlusive disease: review of selected aspects. 2: Posterior circulation embolism. Cerebrovasc Dis. 1992;2:320–326. [Google Scholar]
- 75.Caplan LR, Amarenco P, Rosengart A, Lafranchise EF, Teal PA, Belkin M, et al. Embolism from vertebral artery origin occlusive disease. Neurology. 1992;42:1505–1512. doi: 10.1212/wnl.42.8.1505. [DOI] [PubMed] [Google Scholar]
- 76.Caplan LR, Tettenborn B. Vertebrobasilar occlusive disease: review of selected aspects. 1. Spontaneous dissection of extracranial and intracranial posterior circulation arteries. Cerebrovasc Dis. 1992;2:256–265. [Google Scholar]
- 77.Caplan LR, Zarins C, Hemmatti M. Spontaneous dissection of the extracranial vertebral artery. Stroke. 1985;16:1030–1038. doi: 10.1161/01.str.16.6.1030. [DOI] [PubMed] [Google Scholar]
- 78.Caplan LR, Baquis G, Pessin MS, D'Alton J, Adelman LS, DeWitt LD, et al. Dissection of the intracranial vertebral artery. Neurology. 1988;38:867–877. doi: 10.1212/wnl.38.6.868. [DOI] [PubMed] [Google Scholar]
- 79.Mas JL, Bousser MG, Hasboun D, Laplane D. Extracranial vertebral artery dissection. A review of 13 cases. Stroke. 1987;18:1037–1047. doi: 10.1161/01.str.18.6.1037. [DOI] [PubMed] [Google Scholar]
- 80.Mokri B, Houser OW, Sandok BA, Piepgras DG. Spontaneous dissections of the vertebral arteries. Neurology. 1988;38:880–885. doi: 10.1212/wnl.38.6.880. [DOI] [PubMed] [Google Scholar]
- 81.Moseley IP, Holland IM. Ectasia of the basilar artery: the breadth of the clinical spectrum and the diagnostic value of computed tomography. Neuroradiolgy. 1979;18:83–91. doi: 10.1007/BF00344828. [DOI] [PubMed] [Google Scholar]
- 82.Nishizaki T, Tamaki N, Takeda N, et al. Dolichoectatic basilar artery: a review of 23 cases. Stroke. 1986;17:1277–1281. doi: 10.1161/01.str.17.6.1277. [DOI] [PubMed] [Google Scholar]
- 83.Pessin MS, Chimowitz MI, Levine SR, Kwan ES, Adelman LS, Earnest MP, et al. Stroke in patients with fusiform vertebrobasilar aneurysms. Neurology. 1989;39:16–21. doi: 10.1212/wnl.39.1.16. [DOI] [PubMed] [Google Scholar]
- 84.Schwartz A, Rautenberg W, Hennerici M. Dolichoectatic intracranial arteries: review of selected aspects. Cerebrovasc Dis. 1993;3:273–279. [Google Scholar]
- 85.Caplan LR. Top of the basilar syndrome: selected clinical aspects. Neurology. 1980;30:72–79. doi: 10.1212/wnl.30.1.72. [DOI] [PubMed] [Google Scholar]
- 86.Mehler MF. The rostral basilar artery syndrome: diagnosis, etiology, prognosis. Neurology. 1989;39:916. doi: 10.1212/wnl.39.1.9. [DOI] [PubMed] [Google Scholar]
- 87.Pessin MS, Kwan ES, DeWitt LD, Gale D, Caplan LR. Posterior cerebral artery stenosis. Ann Neurol. 1987;21:85–89. doi: 10.1002/ana.410210115. [DOI] [PubMed] [Google Scholar]
- 88.Pessin MS, Lathi E, Cohen MB, Kwan ES, Hedges TR, 3rd, Caplan LR. Clinical features and mechanisms of occipital infarction in the posterior cerebral artery territory. Ann Neurol. 1987;21:290–299. doi: 10.1002/ana.410210311. [DOI] [PubMed] [Google Scholar]
- 89.Mohr JP, Caplan LR, Melski JW. The Harvard Cooperative Stroke Registry: a prospective registry. Neurology. 1978;28:754–762. doi: 10.1212/wnl.28.8.754. [DOI] [PubMed] [Google Scholar]
- 90.Caplan LR, Hier DB, D'Cruz 1. Cerebral embolism in the Michael Reese Stroke Registry. Stroke. 1983;14:530–536. doi: 10.1161/01.str.14.4.530. [DOI] [PubMed] [Google Scholar]
- 91.Foulkes MA, Wolf PA, Price TR, et al. The Stroke Data Bank: design, methods, and baseline data. Stroke. 1988;19:547–554. doi: 10.1161/01.str.19.5.547. [DOI] [PubMed] [Google Scholar]
- 92.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1000 consecutive patients with first stroke. Stroke. 1988;19:1083–1092. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 93.Bogousslavsky J, Cachin C, Regli F, Despland P-A, Van Melle G, Kappenberger L Lausanne Stroke registry Group. Cardiac sources of embolism and cerebral infarction- clinical consequences and vascular concomitants: the Lausanne Stroke Registry. Neurology. 1991;41:855–859. doi: 10.1212/wnl.41.6.855. [DOI] [PubMed] [Google Scholar]
- 94.Moulin T, Tatu L, Crepin-Leblond T, Chavot D, Berges S, Rumbach L. The Besancon Stroke Registry: an acute stroke registry of 2500 consecutive patients. Eur Neurol. 1997;38:10–20. doi: 10.1159/000112896. [DOI] [PubMed] [Google Scholar]
- 95.Vemmos K, Takis C, Georgilis K, Zakopoulos N, Lekakis J, Papamichael CM, et al. The Athens Stroke Registry: results of a five-year hospital-based study. Cerebrovasc Dis. 2000;10:133–141. doi: 10.1159/000016042. [DOI] [PubMed] [Google Scholar]
- 96.Kittner SJ, Sharkness CM, Price TR, et al. Infarcts with a cardiac source of embolism in the NINCDS Stroke Data Bank; historical features. Neurology. 1990;40:281–284. doi: 10.1212/wnl.40.2.281. [DOI] [PubMed] [Google Scholar]
- 97.Caplan LR. Clinical diagnosis of brain embolism. Cerebrovasc Dis. 1995;5:79–88. [Google Scholar]
- 98.Caplan LR, Hennerici M. Hypothesis: Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55:1475–1482. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 99.Kunitz S, Gross CR, Heyman A, Kase CS, Mohr JP, Price TR, et al. The Pilot Stroke Data Bank: definition, design, and data. Stroke. 1984;15:740–746. doi: 10.1161/01.str.15.4.740. [DOI] [PubMed] [Google Scholar]
- 100.Moulin T, Tatu L, Vuillier F, Berger E, Chavot D, Rumbach L. Role of a stroke data bank in evaluating cerebral infarction subtypes: patterns and outcome of 1776 consecutive patients from the Besancon Stroke Registry. Cerebrovasc Dis. 2000;10:261–271. doi: 10.1159/000016068. [DOI] [PubMed] [Google Scholar]
- 101.Vemmos K, Bots M, Tsibouris P, Zis V, Grobbee D, Stranjalis G, et al. Stroke incidence and case fatality in Southern Greece. The Arcadia Stroke Registry. Stroke. 1999;30:363–370. doi: 10.1161/01.str.30.2.363. [DOI] [PubMed] [Google Scholar]
- 102.Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German Stroke Data Bank. Stroke. 2001;32:2559–2566. doi: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 103.Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) investigators. Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke. A randomized controlled trial. JAMA. 1998;279:1265–1272. [PubMed] [Google Scholar]
- 104.Libman RB, Kwiatkowski TG, Hansen MD, Clarke WR, Woolson RF, Adams HP. Differences between anterior and posterior circulation stroke in TOAST. Cerebrovasc Dis. 2001;11:311–316. doi: 10.1159/000047659. [DOI] [PubMed] [Google Scholar]
- 105.Boyajian RA, Schwend RB, Wolfe MM, Bickerton RE, Otis SM. Measurement of anterior and posterior circulation flow contributions to cerebral blood flow. J Neuroimag. 1995;5:1–3. doi: 10.1111/jon1995511. [DOI] [PubMed] [Google Scholar]
- 106.Hornig CR, Buttner T, Hoffman O, Dorndorf W. Short term prognosis of Vertebrobasilar ischemic stroke. Cerebrovasc Dis. 1992;2:273–281. [Google Scholar]
- 107.Archer C, Horenstein S. Basilar artery occlusion: clinical and radiologic correlation. Stroke. 1977;8:383–390. doi: 10.1161/01.str.8.3.383. [DOI] [PubMed] [Google Scholar]
- 108.Hacke W, Zeumer H, Ferbert A, Bruckmann H, del Zoppo G. Intraarterial therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke. 1988;19:1216–1222. doi: 10.1161/01.str.19.10.1216. [DOI] [PubMed] [Google Scholar]
- 109.Petty GW, Brown RD, Whisnant JP, Sicks JD, O'Falon WM, Wiebers DO. Ischemic stroke subtypes. A population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–1068. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 110.Broderick JP, Phillips SJ, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1992;23:1250–1256. doi: 10.1161/01.str.30.12.2513. [DOI] [PubMed] [Google Scholar]
- 111.Nadeau S, Jordan J, Mishra S. Clinical presentation as a guide to early prognosis in vertebrobasilar stroke. Stroke. 1992;23:165–170. doi: 10.1161/01.str.23.2.165. [DOI] [PubMed] [Google Scholar]
- 112.Bernasconi A, Bogousslavsky J, Bassetti C, Regli F. Multiple acute infarcts in the posterior circulation. J Neurology Neurosurg Psychiatry. 1996;60:289–296. doi: 10.1136/jnnp.60.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wijdicks EF, Nichols DA, Thielen KR, Fulgham JR, Brown RD, Meissner I, et al. Intra-arterial thrombolysis in acute basilar artery thromboembolism: the initial Mayo Clinic experience. Mayo Clin Proc. 1997;72:1005–1013. doi: 10.4065/72.11.1005. [DOI] [PubMed] [Google Scholar]
- 114.Brandt T, von Kummer R, Muller-Kuppers M, Hacke W. Thrombolytic therapy of acute basilar artery occlusion. Variables affecting recanalization and outcome. Stroke. 1996;27:875–881. doi: 10.1161/01.str.27.5.875. [DOI] [PubMed] [Google Scholar]
- 115.Caplan LR. Caplan's Stroke, a clinical approach. 3rd ed. Boston: ButterworthHeinemann; 2000. [Google Scholar]
- 116.Chambers BR, Donnan GA, Bladin PF. Patterns of stroke. An analysis of the first 700 consecutive admissions to the Austin Hospital stroke unit. Aust NZ J Med. 1983:13–64. doi: 10.1111/j.1445-5994.1983.tb04552.x. [DOI] [PubMed] [Google Scholar]