Abstract
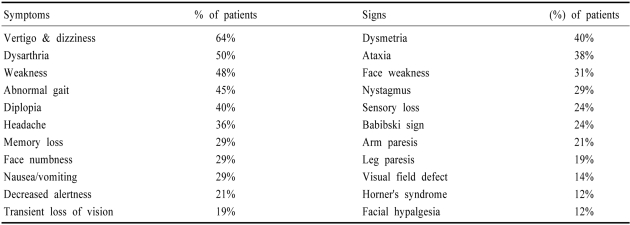
Among 407 New England Medical Center Posterior Circulation Registry (NEMC-PCR) patients, the extracranial (ECVA) and intracranial vertebral arteries (ICVA) were the commonest sites of severe occlusive disease followed by the basilar artery (BA). Severe occlusive lesions were found in >1 large artery in 148 patients; 134 had unilateral or bilateral severe disease at one arterial location. Single arterial site occlusive disease occurred most often in the ECVA (52 patients, 15 bilateral) followed by the ICVA (40 patients, 12 bilateral) and the BA (46 patients). Involvement of the ICVAs and the BA was very common and some patients also had ECVA lesions. Hypertension, smoking, and coronary and peripheral vascular disease were most prevalent in patients with extracranial disease while diabetes and hyperlipidemia were more common when occlusive lesions were only intracranial. Intra-arterial embolism was the most common mechanism of brain infarction in patients with ECVA and ICVA occlusive disease. ICVA occlusive lesions infrequently caused infarction limited to the proximal territory (medulla and posterior inferior cerebellum). BA lesions most often caused infarcts limited to the middle posterior circulation territory (pons and anterior inferior cerebellum). Posterior cerebral artery occlusive lesions were predominantly embolic. Penetrating artery disease caused mostly pontine and thalamic infarcts. Prognosis was poorest in patients with BA disease. The best prognosis surprisingly was in patients who had multiple arterial occlusive lesions; they often had position-sensitive transient ischemic attacks during months or years.
Keywords: Brain ischemia, Brain embolism, Posterior circulation, Vertebral arteries, Basilar artery, Posterior cerebral arteries, Brainstem, Cerebellum
INTRODUCTION
Less information is reported about the nature, distribution, and frequency of various cervicocranial arterial lesions in patients with posterior circulation ischemia than in those who have anterior circulation arterial lesions. Pathological, angiographic, and ultrasound studies of patients with carotid territory ischemia show that the most common occlusive lesions are in the internal carotid artery (ICA) near its origin.1-3 Occlusive lesions within the intracranial ICA, and within the middle and anterior cerebral arteries are much less common. In blacks, individuals of Asian descent, and women, intracranial occlusive lesions are commoner than in white men.4-7 Penetrating artery disease is also common.3,8 Series and registries of anterior circulation disease patients define the frequencies of arterial lesions and embolism.9-11
Pathological, angiographic, and ultrasound studies of patients with posterior circulation ischemia show that atherosclerotic occlusive lesions often involve the subclavian arteries, the extracranial vertebral artery (ECVA) origins, the intracranial vertebral arteries (ICVAs), the basilar artery (BA), and rarely, the posterior cerebral arteries (PCAs).12-15 Penetrating artery disease is also common. Important sex and racial differences occur in the distribution of posterior circulation lesions.6,7,16 Intracranial occlusive disease is more common in blacks, individuals of Asian origin, and women while ECVA origin lesions are commoner in white men. Dissections most often involve the distal portions of the ECVAs and the ICVAs.3,17
Autopsy studies select patients who die of stroke or its complications. Angiographic studies also contain selection bias since more severely ill patients have been studied. Unfortunately, no detailed reports from large registries of posterior circulation ischemia patients define the frequency and location of vascular occlusive lesions using modern vascular imaging techniques. We now report data from the 407 patients in the New England Medical Center Posterior Circulation Registry (NEMC-PCR) about the distribution of vascular lesions within the vertebrobasilar circulation.
BACKGROUND
Necropsy studies of the craniocerebral arteries during the third quarter of the twentieth century showed that atherosclerotic lesions predominantly involved the proximal ECVAs, the ICVAs, and the BA.1,13,14,18-21 Among 178 thorough necropsy examinations of the brainsupplying arteries during routine autopsies, Fisher et al showed that extracranial occlusive disease was more common in the anterior circulation but more posterior circulation occlusions were intracranial.1 The proximal BA was a favored site of intracranial atherosclerosis. Within the carotid arterial system all symptomatic lesions were extracranial while all posterior circulation symptomatic lesions were intracranial.1 Baker and Iannone found that intracranial atherosclerosis was most severe in the distal BA but also heavily involved the ICVAs and the proximal BA.18 Moossy found that the ICVAs were the most common site of atherosclerotic disease.21 Castaigne et al. found that most intracranial lesions among 44 necropsies involved the ICVAs (22 patients) and BAs (18 patients).22
Others reported arterial lesions found at necropsy in patients with particular clinical syndromes, particular infarct locations, or particular arterial sites. Hutchinson and Yates showed that atherosclerotic occlusive lesions were very common in the proximal ECVAs among Caucasians studied at autopsy in the UK; the frequency of these lesions paralleled ICA origin disease.19,20 Studies of cerebellar infarct patients, especially those within posterior inferior cerebellar arteries (PICAs) territory showed a very high frequency of occlusive ICVA lesions.23-25 The causative vascular occlusive lesions involved the ICVAs in patients with lateral medullary,26-29 and hemimedullary infarcts.29,30 BA occlusions were the usual cause of bilateral pontine infarcts.31-35 Distal BA occlusions or branch occlusions of arteries arising from the BA bifurcation were found in patients with midbrain and thalamic infarcts.36 Occlusion of the mouths of penetrating artery branches of the BA were found in patients with unilateral basal or basaltegmental pontine infarcts.37,38 Lipohyalinosis and segmental arterial disorganization were found within the pontine penetrating arteries in patients with hemiplegia,39 and in penetrating branches of the thalamogeniculate arteries in patients with pure sensory stroke and lateral thalamic infarcts.40 At necropsy, most PCA territory infarcts were caused by embolism from the heart or proximal posterior circulation arteries rather than intrinsic PCA atherosclerosis.22
The Joint Study of Extracranial Arterial Occlusion reported the frequency of arterial stenosis and occlusion among 4,748 patients who had angiography after transient ischemic attacks (TIAs) and strokes.2 The most common extracranial posterior circulation lesions were in the proximal ECVA; stenosis or occlusion was found in 22.4% of right ECVAs and in 28% of left ECVAs. The innominate artery was stenosed or occluded in 4.8% and the right subclavian artery in 9.1% of patients while the left subclavian artery was stenosed or occluded in 14.9%.2 Among 6,534 patients who had neck angiography, 1,114 (17%) had subclavian or innominate artery stenosis of >30%.41 Among these, 168 (15%) met criteria for subclavian steal. The extracranial ICAs showed occlusive abnormalities about twice as often as the ECVAs. Within the intracranial posterior circulation, 5.4% of right ICVAs were stenosed and 3.2% were occluded compared to 5.6% stenosis and 4.4% occlusion of the left ICVA. The BA was stenosed in 7.7% and occluded in 0.8% of angiograms.2
Other angiographic studies focused on specific arteries or selected patient groups.42-51 Most reported relatively good outcomes in patients with ECVA disease, and poor outcomes in patients with severe intracxranial disease.42-51 Some reports describe small groups of patients with severe ICVA or BA occlusive disease who survived strokes with little or no residual neurologic deficit.50-53 Meyer et al. reported vertebrobasilar angiography results noting "arteriography was not performed on critically ill patients but was only done on those with transient symptoms"; nevertheless they found several patients had severe BA occlusive disease.54
The frequencies of extracranial posterior circulation stenotic lesions was studied using cervical ultrasound.12 Hennerici et al. used continuous wave Doppler to study neck arteries in 2,009 patients. None had focal brain ischemia attacks or strokes; 1,030 (45%) also had angiography. Posterior circulation lesions were found in 183 patients (9%); 91 had subclavian steal; 34 had ECVA stenosis or hypoplasia, and 58 had ECVA occlusions. CW Doppler had a sensitivity of 90% in those patients who had angiography.12
Clinicians recently began to use magnetic resonance angiography (MRA) and CT angiography (CTA) to identify vascular lesions. In the Lausanne Stroke Registry, intracranial MRA effectively identified vascular lesions in patients with posterior circulation infarcts.55 Among 70 consecutive patients studied with MRA, the commonest lesions were BA stenosis (23 patients), BA occlusion (4 patients), unilateral ICVA stenosis (5 patients), and dolichoectatic intracranial arteries (10 patients).55 At NEMC, we relied on a combination of extracranial and transcranial ultrasound (TCD) and MRA to identify vascular lesions. Experience shows that these two techniques, when concordant, reliably define occlusive lesions.56-58 Rother et al. studied MRA utility among 41 patients with cerebellar and brainstem infarcts also studied by catheter angiography and TCD.57 Catheter angiography confirmed all 29 occlusive ICVA and BA lesions. MRA failed to detect one ECVA dissection. The sensitivity of MRA was 97% and the specificity 98%. For TCD, the sensitivity was 76.4% and the specificity 98.9%.57
At NEMC, we compared TCD and MRA with each other and with standard angiography in 60 patients with vertebrobasilar ischemia.56 In 170/180 (94%) arteries, TCD and MRA agreed on normal vs. stenotic vs. occluded ICVAs and BAs. In 5 arteries, TCD showed high grade stenosis while MRA suggested occlusion. In 5 other arteries TCD and MRA did not agree. There was 95% agreement between TCD and MRA and conventional angiography. No patient was misdiagnosed when TCD and MRA were both used.
Wentz et al. compared MRA with digital arterial angiography in 60 vertebrobasilar ischemia patients.58 MRA showed ICVA and BA disease with 100% sensitivity; MRA distinguished stenosis from occlusion but the severity of stenosis was overestimated in 63% of patients. MRA showed 117/120 superior cerebellar arteries (SCAs), 80/90 PICAs, but only 30/58 anterior inferior cerebellar arteries (AICAs).
No prior large series of consecutive prospectively collected posterior circulation ischemia patients have reported vascular lesions using modern diagnostic techniques. We initially relied on catheter angiography (about 80% of the patients had catheter angiography). More recently vascular lesions were often defined by a combination of MRA and ultrasound. Cardiac and hematologic evaluations were performed when clinically appropriate. We now report the nature, severity, and locations of occlusive lesions including an analysis of risk factors, and location of infarcts, Preliminary reports of specific posterior circulation vascular lesions were published previously.4,13-15,59-65
METHODS
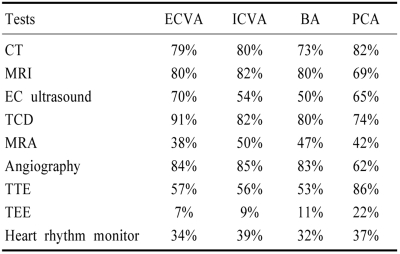
Criteria for the diagnosis of stroke mechanisms and etiology and localization of brain lesions to the proximal, middle, and distal intracranial posterior circulation territories are described in the companion paper. Each registry patient was thoroughly evaluated clinically and using modern neuroimaging. Table 1 shows the frequency of various investigations according to the vascular lesion site. Angiography was less often and cardiac investigations were more often performed in PCA territory infarct patients with cardiac embolic sources.
Table 1.
Investigations according to vascular lesions
EC; extracranial, TCD; transcranial doppler ultrasound, MRI; magnetic resonance imaging, MRA; magnetic resonance angiography, TTE; transthoracic echocardiography, TEE; transesophageal echocardiography, ECVA-VAO; extracranial vertebral artery-vertebral artery origin, ICVA; intracranial vertebral artery, BA; basilar artery, PCA; posterior cerebral artery
RESULTS
1. Vascular occlusive lesions
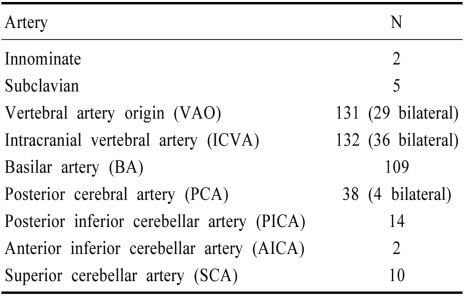
Table 2 lists occlusive lesions showing >50% stenosis. Table 3 lists the frequency of one arterial level involvement e.g, vertebral artery origin (VAO) or ICVA. Some patients had bilateral lesions of these arteries. The most common occlusive lesions involved the VAOs and ICVAs. At these sites, occlusive lesions were often bilateral. BA lesions were also common but subclavian and innominate artery lesions were rare.
Table 2.
Vascular lesions with >50% luminal stenosis
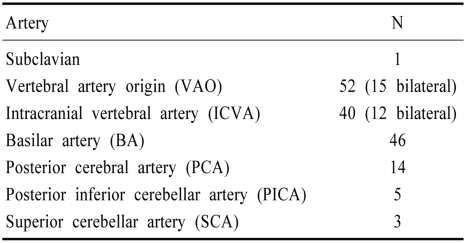
Table 3.
Isolated single artery involvement
A striking and unexpected finding was the prevalence of extensive occlusive disease. Single arteries were involved in 134 patients; 148 patients had multiple occlusive lesions (84 patients had 2 lesions, 53 had 3 lesions, and 11 patients had 4 or more occlusive large artery lesions). Extracranial arteries were involved in 117 patients and intracranial arteries in 213. The lesions were extracranial alone in 40, intracranial alone in 136, and both extracranial and intracranial in 77.
Anterior circulation arteries were sometimes also narrowed. Nineteen patients had unilateral and 13 had bilateral extracranial ICA occlusive lesions. Common carotid artery (CCA) occlusion or stenosis was present in 5 patients and 8 had severe middle cerebral artery (MCA) stenosis. Extracranial ICA disease was most prevalent in patients who had ECVA disease. Among 136 patients who had only intracranial occlusive disease, only 17 (12.5%) had ICA occlusive neck disease, and 4 (3%) had MCA disease.
Most non-embolic vascular lesions were related to atherosclerosis or penetrating artery disease. The next most common vascular lesion was arterial dissection. Dissections were found in 24 patients (6%). Sixteen patients had ECVA dissections; 6 involving the proximal artery (V1) and 10 involving the distal portion (V3). Extracranial dissections were bilateral in 4. Seven patients had ICVA dissections; 6 unilateral and 1 bilateral. One patient had an extracranial dissection that extended intracranially. Two patients had BA dissections. Brain infarction was attributed to migraine in 13 patients. Several patients had vertebrobasilar dolichoectasia. Only occasional patients had thrombi within extracranial and intracranial aneurysms (1 patient), coagulopathies (4 patients), drug related disease (1 patient), and systemic hypoperfusion (2 patient).
2. Stroke risk factors
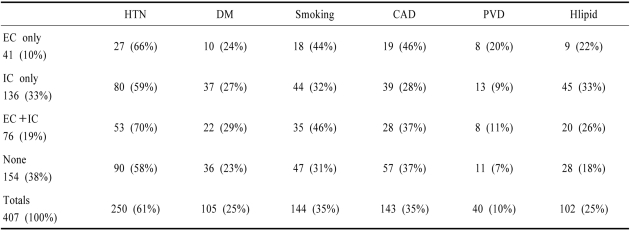
Hypertension was the commonest risk factor occurring in 250/407 (61%) patients. Diabetes Mellitus (26%), smoking (36%), and hyperlipidemia (25%) were also common. Coronary (35%) and peripheral vascular occlusive disease (10%) were frequent comorbidities. Table 4 shows risk factors in relation to location of occlusive vascular lesions. Hypertension, smoking, and coronary and peripheral vascular occlusive disease were more common in patients with extracranial disease - either alone or combined with intracranial disease, compared to those with only intracranial disease. This pattern of risk factors is the same as that reported in extracranial ICA disease.9-11,65 In contrast, diabetes and hyperlipidemia were more common when occlusive lesions were only intracranial.
Table 4.
Risk Factors in the NEMC-PCR in relation to the presence and location of large artery vascular occlusive lesions
HTN; hypertension, DM; Diabetes Mellitus, CAD; coronary artery disease, PVD; peripheral vascular occlusive disease, EC; extracranial occlusive disease (>50% stenosis), IC; intracranial occlusive disease (>50% stenosis), Hlipid; hyperlipidemia. Cholesterol >200
3. VAO disease
Occlusive lesions were often present within the first several centimeters of the ECVA origins. VAO stenosis (>50%) was found in 131 patients; bilateral in 29. In 6, the lesions were dissections and the remainder were atherosclerotic. Dissections always occurred above the origin and before the ECVA entered the vertebral column. Among patients with atherosclerotic bilateral ECVA disease, 3 also had bilateral ICVA occlusive lesions, and 8 had BA occlusive lesions. ICA neck lesions were also sometimes associated. Among patients with bilateral ECVA occlusive lesions, 1 also had ICA occlusion and 1 had innominate and CCA occlusions. One patient had unilateral VAO and bilateral ICA neck occlusions.
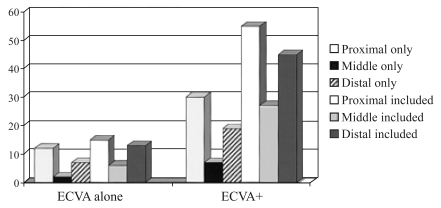
Fig. 1 displays the posterior circulation territories involved in patients with only ECVA lesions and in those who also had occlusive intracranial disease (ECVA +). The proximal intracranial territory (medulla and PICA cerebellum) was most often involved followed closely by the distal territory. ECVA lesions cause infarction primarily because of artery-to-artery embolism.63,65 The commonest recipient sites were the ipsilateral ICVAs causing proximal territory infarcts and the rostral BA causing distal territory infarcts. At times emboli caused both proximal and distal territory infarcts sparing the middle territory (13 patients, 11.5%).
Figure 1.
The distribution of intracranial posterior circulation infarcts in patients with proximal vertebral artery occlusive disease. ECVA; extracranial vertebral artery
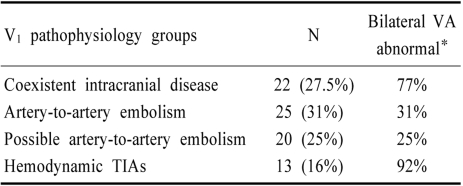
Among 102 VAO disease patients, 80 (78%) had very severe disease (37 occlusions and the remainder severe stenosis [>80% luminal narrowing]). Review of these 80 patients allowed separation into 4 groups (Table 5). More than 1/4th of patients had extensive coexistent intracranial disease and so the contribution of the ECVA disease was uncertain. The extracranial lesions could have been coincidental. Artery-to-artery embolism was the commonest mechanism of infarction accounting for more than half of the cases.
Table 5.
Pathophysiological groups of patients with severe proximal vertebral artery occlusive disease
Only 13 patients had a hemodynamic mechanism of ischemia. In 12 of these 13, there was severe bilateral VA occlusive disease. Six patients had severe bilateral ECVA disease and 6 had severe ICVA disease contralateral to severe disease of one ECVA. The only patient who did not have bilateral VA disease, had bilateral ICA occlusions. All had TIAs. Only two had brain infarcts, one affecting the occipital lobe and the other the temporal lobe and cerebellum. TIAs were multiple and recurred during one week to several months. Dizziness, often accompanied by veering to one side and gait ataxia, visual blurring, perioral paresthesias, and diplopia were the commonest TIA symptoms.
Patients with disease limited to the ECVA had better outcomes than those with ICVA and BA disease.66 They had a relative risk of 0.62 compared to all patients with poor outcomes (death or severe disability).
4. ECVA dissections
Sixteen patients had ECVA dissections (20 arteries). In 6 the dissections began in the V1 segment, often extending into the V2 segment. Ten patients had dissections involving the distal ECVAs (V2 and V3 segments). Dissections were bilateral in 4. Among 20 dissected arteries, 8 caused stenosis and 12 were occluded. Infarcts were due to embolism from thrombi within the ECVAs. Most emboli caused ischemia within the proximal territory including: 5 PICA cerebellar infarcts, 2 lateral medullary and 1 hemimedullary infarct, and 1 infarct that involved the lateral medulla and PICA-supplied cerebellum. One infarct involved the proximal, middle, and distal territories (PICA cerebellum, pons, and SCA cerebellum) and 1 patient had solely distal territory infarcts (SCA cerebellum and occipital lobe).
5. ICVA disease
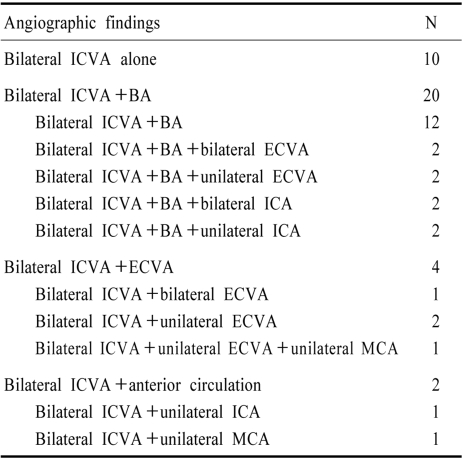
Occlusive lesions of >50% stenosis were present in 132 patients. Seven had ICVA dissections, 1 bilateral. The remainders were atherosclerotic. Bilateral ICVA atherosclerotic disease was present in 36 patients. The vascular lesions in these 36 patients are tabulated in Table 6. Twenty patients with bilateral ICVA disease also had BA disease. Risk factors were particularly prevalent in patients with bilateral ICVA disease in whom 76% were hypertensive, 52% had elevated cholesterol, 36% were diabetic, and 36% smoked cigarettes.62
Table 6.
lesions Lesions among 36 patients with bilateral intracranial vertebral artery (ICVA) occlusive disease
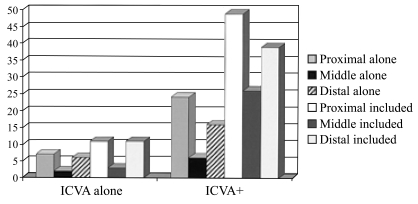
Fig. 2 shows the infarct distribution. Proximal and distal territories were about equally involved in patients who had only ICVA disease. Among patients with ICVA lesions + other occlusive disease the proximal territory was also more often involved, closely followed by distal territory. Only 1 in 5 patients with only ICVA disease had ischemia limited to the proximal territory. Prior reports of necropsy patients with ICVA disease were limited to patients with lateral medullary, hemimedullary, and PICA-cerebellar infarcts.23-30
Figure 2.
The distribution of intracranial posterior circulation infarcts in patients with intracranial vertebral artery occlusive disease. ICVA; intracranial vertebral artery
Proximal territory infarcts in patients with atherosclerotic ICVA disease were most often lateral medullary while infarcts limited to the PICA cerebellum were most often attributed to cardiac origin embolism and artery-to-artery embolism from the ECVAs. The lateral medulla and posterior inferior cerebellum have different branch artery supply. Penetrating arteries branch from the ICVA, course through the lateral medullary fossa, and penetrate into the lateral medulla. The medial-PICA branch of the ICVA supplies only a small portion of the dorsal medulla.67 The PICA branch of the ICVA usually arises more proximally than lateral medullary penetrators.
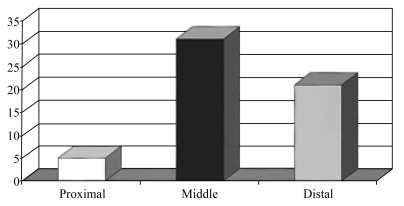
Occlusions and severe stenotic segments were most often located in the distal ICVAs. Most lesions were distal to the origin of PICA.61,62 The commonest ICVA segment involved was the distal third at or near the ICVA-BA junction. This segment was involved in about 1/3 of patients with unilateral ICVA disease and in half of patients with bilateral ICVA disease. We divided ischemia patterns into 6 groups (Table 7). The most frequent group, accounting for about half of the patients, was those with extensive occlusive disease involving the ICVAs bilaterally sometimes with other occlusive lesions. Table 6 lists the vascular lesions in patients with bilateral ICVA disease; many patients with unilateral ICVA disease also had BA and other occlusive lesions. The commonest clinical pattern in these patients was the presence of multiple TIAs. These attacks were often provoked by standing, hypovolemia, and drops in blood pressure. The commonest symptoms during attacks were faintness, visual blurring, diplopia, vertigo, and ataxia. TIAs continued to occur during months and even years. Table 8 lists the frequency of symptoms and signs in the patients with severe bilateral ICVA occlusive disease. These included 36 patients with bilateral ICVA occlusive disease and 5 who had unilateral severe disease and hypoplasia of the contralateral ICVA. Some patients in this group had distal territory infarcts due to intra-arterial embolism or hypoperfusion. Surprisingly, patients with extensive vascular posterior circulation occlusive disease who had predominantly multiple TIAs had excellent outcomes. Few had disabling brain infarction.
Table 7.
Subgroups of patients with ICVA severe occlusive disease (modified from Muller-Kuppers et al, 1997)
Some patients belonged to more than 1 group e.g those with widespread disease and TIAs who also had distal territory infarcts
Table 8.
Frequency of symptoms and signs in patients with bilateral severe ICVA occlusive disease
The next commonest subgroup of ICVA disease patients were those who presented with distal territory infarcts, accounting for about 1/3 of patients. Some patients in this group also had TIAs involving the vestibulo-cerebellar systems in the medulla and/or cerebellum. Proximal territory infarcts accounted for 1/5 of patients. Few patients presented with middle territory infarcts when thrombi within the distal ICVA extended into the BA, had embolism to the ICVA, or had infarcts attributable to other lesions (e.g, BA occlusion).
Among the 7 patients with ICVA dissection (1 bilateral), 4 arteries were stenotic and 4 occluded. Among the dissection patients with brain infarcts, 2 had lateral medullary infarcts and 3 had infarcts attributed to embolism to the distal territory (1 SCA cerebellum, midbrain; and thalamus, 1 PCA territory; and 1 SCA cerebellum and PCA).
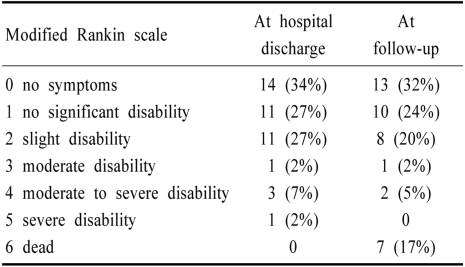
Mortality was highest among patients who had embolism to the distal territory arising from the ICVA 1/4th of these patients died. The mortality and morbidity in all other groups was very low. Upon hospital discharge 1/4 of the patients had moderate or severe disability but 1/2 had no disability. At follow-up examinations 3 years after discharge, 38% had no symptoms and 78% had no or only slight disability.61 Most surprising was the relatively good outcomes in patients with bilateral ICVA disease (Table 9). These patients with the most severe occlusive disease had the best outcomes, indicating the adequacy and durability of their collateral circulation.
Table 9.
Modified Rankin scale score at discharge and at follow-up among 41 patients with severe ICVA occlusive disease
6. BA disease
Among patients with BA occlusive disease (>50% stenosis), 2/3 were hypertensive, and about 1/3 had diabetes, smoked cigarettes, and /or had high cholesterol. 1/3 also had coronary artery disease. Nearly all patients had atherosclerotic lesions. Two had BA dissections. Dolichoectasia of the BA was common but was not included among patients with severe occlusive disease.
Fig. 3 shows the infarct distribution among the 109 patients with BA occlusive disease. Most infarcts were in the middle intracranial territory in patients with lesions limited to the BA and also when other arteries were also compromised. The distal territory was ofteninfarcted along with the middle territory indicating spread of disease to the rostral BA and its distal branches or embolism to these branches. Among the two patients with BA dissections, one had pontine infarction and the other had pontine, midbrain, and SCA cerebellar infarcts.
Figure 3.
The distribution of intracranial posterior circulation infarcts in patients with Basilar artery occlusive disease. BA; basilar artery
Many patients with BA occlusive disease (56 patients) also had severe disease of other posterior circulation arteries (Table 10). Patients who had bilateral ICVA occlusive disease were already included in Table 5. Among 109 patients in whom ischemia and infarction could be reasonably attributed to BA disease, 87 had moderate (50~80% stenosis), severe stenosis (>80% narrowing), or occlusion.68 We divided these patients into 3 subgroups (Table 11). Intrinsic occlusive disease limited to the BA and extensive multifocal occlusive disease including the BA were the commonest groups.
Table 10.
Patients with Basilar artery (BA) occlusive disease + occlusive disease in other arteries
Table 11.
Subgroups of patients with severe bBasilar artery occlusive disease
*includes two patients who had extension of threombus from one ICVA into the BA
The largest group of BA disease patients was those with pontine and often rostral brainstem infarction related to BA thrombosis. Two patients had extension of thrombus from an ICVA into the BA. Infarcts in this group usually involved the bilateral pons and signs were often motor and oculomotor. Hemiparesis was more common than tetraparesis but usually there were minor motor abnormalities on the non-hemiplegic side. Among all patients with severe BA disease, 44 had hemiparesis (51%) while only 5 (5.7%) were tetraparetic. Bulbar or pseudobulbar weakness was very common occurring in nearly 3/4ths of patients. The frequency of TIAs was similar to that in the group with extensive posterior circulation disease; 33% had only TIAs while 39% had TIAs preceding stroke and 28% had strokes without preceding TIAs. The average duration during which TIAs occurred was 2.1 months.
Thirty six patients had extensive occlusive disease including but not limited to the BA. These patients with extensive multifocal occlusive disease represented the second commonest patient group. The symptoms and signs in this group were those described in the ICVA section. TIAs were reported in 78% of these patients; 31% of patients had only TIAs while 47% had TIAs preceding stroke; 22% had only stroke without preceding TIAs. The average duration during which TIAs occurred was 7.8 months (excluding one patient whose TIAs lasted more than 5 years). Unexpectedly, these patients with extensive occlusive vascular disease had better outcomes than those with disease limited to the BA.
A small number of patients with BA disease had embolism to the BA with mostly distal territory infarcts. Emboli arose from the heart or ECVAs.
The frequency of poor outcome (mortality or severe disability at 30 days after hospital discharge) was higher among patients with BA disease than with ECVA and ICVA disease; 30% had poor outcomes yielding a relative risk of poor outcome of 3.64 (1.9~7.0 95% confidence interval).66 The worst outcomes occurred among patients with embolism to the BA; 58% of these had major deficits.
7. Penetrating and branch artery disease
In this category, brain infarcts are limited to the distribution of single branch penetrating arteries, clinical symptoms and signs are explained by involvement of this region, and vascular imaging shows no important compromise of the parent artery feeding the involved branch. Examples include: a paramedian pontine infarct in a patient with contralateral pure motor hemiplegia in whom MRA shows a normal BA; an infarct in the right ventrolateral thalamus in a patient with left hemisensory paresthesias and left arm incoordination and a normal right PCA. Using these strict criteria, 14% of patients (range 14~17%) were considered to have infarcts due to penetrating or branch artery disease. We can not be sure that some patients did not have small emboli that blocked branches. Fig. 4 shows the distribution of these branch lesions.
Figure 4.
The distribution of intracranial posterior circulation infarcts in patients with penetrating artery occlusive disease.
8. PCA territory infarcts
The vast majority of PCA territory infarcts were embolic. This finding corroborates prior reports.13,14,64 Most patients had cardiac or ECVA, ICVA, or BA disease that was the likely source of emboli to the PCA.
Among the 119 patients with PCA territory infarcts, 61% had infarcts limited to PCA-supplied regions, while 39% also had involvement of other supply zones (PCA +). Cortical infarcts were more common than cortical and deep infarcts but patients with PCA+ lesions had more cortical and deep infarcts indicating embolic occlusion of the proximal PCA. The occipital lobe was the most frequent cortical territory involved (92%) while the temporal lobe was involved in 39%; only 5 patients had temporal lobe infarcts without occipital lobe infarction. All patients with somatosensory symptoms and signs, usually hemisensory paresthesias, had lateral thalamic infarction or PCA occlusion before the thalamogeniculate pedicle origin.59 Visual field defects were present in 84% of patients while only 15% had sensory symptoms or signs. Motor signs, usually slight and contralateral were present in 29% and 25% had cognitive and/or behavioral abnormalities.64 Hemiplegia is attributable to proximal PCA disease causing ischemia to the cerebral peduncle.70
In only 7 patients could we be confident that the PCA occlusive lesion represented in-situ atherostenosis rather than embolic occlusion. One of these patients had stenosis of the distal BA and the PCA. Six patients with intrinsic PCA disease had TIAs preceding strokes. The TIAs occurred during 2 weeks to a year and were mostly visual or somatosensory. Four of these 7 patients had a prolonged period of fluctuating symptoms and signs that evolved during 2~17 days. All 7 patients had visual symptoms and visual field loss. PCA stenosis has been reported but is rare.71 The patient with distal BA and PCA stenosis had sensory symptoms and bilateral thalamic infarcts.
Four PCA territory infarcts were classified as migrainous. One of these patients also had a high serum calcium level, a factor known to promote cerebral vasoconstriction. Among patients with embolic PCA occlusion, 65 had a cardiac donor source, 38% had a proximal arterial source (equally divided among the ECVA, ICVA, and BA), and 15% were classified as cryptogenic embolism.
DISCUSSION
1. Risk factors
Hypertension was a prevalent risk factor being noted in 61% of patients. Diabetes mellitus was slightly more common in patients with intracranial compared to extracranial disease while coronary and peripheral vascular disease were more often associated with extracranial disease.
Prior studies showed that extracranial ICA lesions are highly associated with white race, male sex, hypertension, smoking, and coronary and peripheral artery occlusive disease.5,6,11,72,73 Studies of populations of white and black patients confirmed that this pattern was also true for VAO occlusive disease.15 NEMC-PCR patients with occlusive VAO disease were most often white and male and had risk factors similar to ICA disease patients. Hypertension was more common in patients with only ECVA disease compared to those with intracranial disease. ICA and VAO occlusive disease often coexist.19,20
Intracranial occlusive lesions are most common in blacks, individuals of Asian origins, and women.4-7,15,74,75-77 The NEMC-PCR includes more Asian patients, predominantly Chinese, than the Harvard and Lausanne Registries, and the Stroke Data Bank. The risk factor profile was consistent with that previously reported for intracranial disease; a relatively high frequency of diabetes and hyperlipidemia.
2. Frequency of various vascular lesions
Necropsy studies of posterior circulation vascular lesions differ in the nature of the patiets studied and on their focus of interest. Some studied unselected autopies.1,18,21 Castaigne et al. studied patients with posterior circulation infarcts and emphasized intracranial arteries.22 Hutchinson and Yates studied the ECVAs.19,20 These necropsy studies showed that although ECVA lesions were common, most posterior circulation infarcts were attributable to intracranial occlusive disease. This contrasted with anterior circulation occlusive disease in which the extracranial ICA more often caused brain infarction than intracranial disease.
In the NEMC-PCR, the VAs were the commonest vessels involved and the ECVAs and ICVAs were involved with about the same frequency. When only single vascular regions were involved, the VAO was more often severely stenotic or occluded than other vascular regions. BA disease was also very common. The most important and surprising finding was the frequency of multiple vascular involvement. Often extracranial and intracranial arteries were narrowed. In many patients multiple intracranial arteries were compromised. When one ICVA was narrowed or occluded by atherosclerosis, the contralateral VA was often hypoplastic or narrowed extracranially or intracranially, and the BA was also often severely compromised.
3. Subclavian and innominate artery disease rarely caused posterior circulation infarcts
In 1961, Reivich et al78 and Fisher79 described the "subclavian steal syndrome". Physicians were alerted and patients with the syndrome often had subclavian artery surgery. Subsequent studies showed that most patients with subclavian artery disease are asymptomatic, even those with subclavian steal shown by ultrasound or vascular imaging.4,41,80,81 Subclavian artery disease patients often have coexistent ICA disease that is usually responsible for brain ischemia. Patients with innominate artery disease, most often have anterior circulation infarcts related to thrombi within the right ICA innominate artery branch.4,82 Subclavian artery lesions may cause minor ischemic arm symptoms e.g. coolness, cramping, and inability to sustain prolonged arm exercise. These symptoms are important in athletes but are rarely important otherwise. Vertebro basilar territory ischemic symptoms are unusual and posterior circulation infarction attributable to subclavian or innominate artery disease is rare.
Two NEMC-PCR patients had innominate artery stenosis and 5 had subclavian arrtery disease, but only one patient with isolated subclavian artery disease had posterior circulation ischemia attributable to the extracranial occlusive lesion. The single patient with isolated subclavian artery disease had transient spells of dizziness and diplopia but no brain infarct. Subclavian artery disease is an important marker of atherosclerosis but a rare cause of posterior circulation infarction.
4. The importance of ECVA disease as a cause of posterior circulation infarction.
Fisher et al. concluded from their autopsy study that atherosclerosis affected the ECVA and the intracranial posterior circulation arteries equally but that extracranial lesions were seldom symptomatic.1 They did note that proximal ECVA atherosclerotic lesions occasionally became ulcerated, and that 3 of their patients may have had artery-to-artery emboli arising from the ECVA. Moossy also encountered an ulcerative ECVA plaque.21
Fisher commented on the usual benignity of ECVA disease.83 He reported 5 patients with bilateral ECVA occlusions who had transient symptoms but no persistent neurological signs. All had reconstitution of the distal ECVAs from collateral neck vessels. The benignity of atherosclerotic VAO lesions was attributed to: 1) the capacity to develop collateral reconstitution of the ECVAs; 2) the usual presence of two viable arteries that join together intracranially, so that if one became compromised, the contralateral artery could compensate adequately; and 3) the slow development of luminal compromise by atherosclerotic plaques allowing time for collateral development. A Cleveland Clinic analysis also supported benignity of ECVA atherosclerosis.43 None of their 89 VAO disease patients developed strokes and only 19 had TIAs, many not localizable.
Other studies showed that VAO lesions were more often the cause of posterior circulation infarcts than previously thought. Labauge et al. reported their experience among 100 patients in whom angiography had shown VAO lesions.42 Posterior circulation ischemia was the indication for angiography;42 59 patients had posterior circulation strokes and 29 vertebrobasilar territory TIAs. The predominant symptoms and signs were vestibulo-cerebellar. Embolism from the ECVA lesion was the presumed mechanism of medullary and cerebellar infarcts.42
Artery-to-artery embolism from a VAO donor source was also reported in small series of patients.84-87 A key observation was reported by Pelouze who showed that a VAO specimen removed from a patient with repeated posterior circulation TIAs, contained a typical ulcerated plaque, similar to plaques found within ICA surgical specimens.88 Caplan et al. reported 10 patients who had artery-to-artery embolism arising from occlusive VAO lesions; the commonest recipient site was the ICVA causing PICA-cerebellar infarcts.65
In the NEMC-PCR, VAO atherosclerosis was very common. Infarcts in patients with VAO disease were mostly attributable to artery-to-artery embolism arising from the ECVA. A thrombus-containing ECVA aneurysm and ECVA dissections were other donor sources of embolism. Extracranial aneurysms89 and dissections3,16 have been reported to be potential sources of artery-to-artery emboli. Only two patients considered to most likely have artery-to-artery emboli arising from proximal ECVA occlusive disease also had potential cardiac embolic sources. The commonest recipient region for emboli arising from the ECVAs was the ICVA causing PICA cerebellar infarcts. Recipient sites in some patients were in the distal territory or the proximal and distal territories.
Hypoperfusion related to VAO occlusive lesions was usually transient causing brief spells of dizziness, veering, visual blurring, and ataxia. Attacks were self-limited probably reflecting reconstitution of the ECVA distal to the occlusion. In 13 patients (12 with bilateral ECVA occlusive disease, and one with bilateral ICA and unilateral ECVA occlusions), hypoperfusion-related TIAs occurred over a prolonged period but did not cause brain infarcts. Unilateral ECVA stenosis or occlusion did not cause persistent TIAs.
ECVA disease is a very important cause of strokes and TIAs. A variety of treatments are available. Surgery, (direct endarterectomy or reconstruction using an ECVA to ICA bypass,)90 angioplasty with or without stents,91 and medical therapy using drugs that alter platelet functions or anticoagulants. Unfortunately, no trial to date compares the effectiveness and safety of these various strategies or defines indications for these treatments.
5. ICVA occlusive disease
There is general agreement that severe ICVA and BA occlusive lesions are not benign and frequently cause strokes. The closer an artery is to the brain, the more likely that occlusion will lead to brain infarction. Most reports of ICVA occlusive disease relate to necropsy and angiographic studies of patients with brain infarcts within the proximal intracranial territory. Patients with PICA cerebellar infarcts,23-25 lateral medullary infarcts,26-29 and hemimedullary infarcts29,30 show a very high frequency of occlusive ICVA lesions.
Few reports concern patients selected because they had ICVA disease discovered by vascular imaging., Moufarrij et al. reported findings among 44 patients with ICVA and/or BA stenosis; 16 with bilateral ICVA stenosis.44 The vertebro-basilar junction was a frequent disease site. Strokes and vertebro-basilar TIAs were common in follow-up. Many patients had multiple artery involvement. Caplan reported 9 patients with severe bilateral ICVA occlusive disease, 8 of whom died of brainstem infarction and the remaining patient survived with severe bulbar paralysis and quadriplegia.92 In contrast, Bogousslavsky et al. reported 10 patients who had bilateral severe ICVA occlusive lesions and a benign course.93 These patients were selected from candidates for anterior circulation extracranial to intracranial bypass.
In the NEMC-PCR, only 1/5th of patients with severe ICVA occlusive disease had infarcts limited to the proximal intracranial posterior circulation territory. More common were emboli arising from the ICVA causing distal territory infarction. Proximal territory infarcts were more often related to ECVA occlusive disease with artery-to-artery embolism to the ICVA than from intrinsic ICVA occlusive disease. The commonest group of patients with ICVA disease, accounting for more than half of the patients, had extensive atherosclerosis in multiple intracranial arteries (Table 6). These patients with severe multifocal disease most often had benign courses usually characterized by multiple TIAs but few serious strokes. The seven patients with ICVA dissections had proximal or distal territory infarcts.
Relatively few NEMC-PCR ICVA disease patients had isolated lateral medullary or PICA cerebellar infarcts. Most ICVA occlusive lesions involved the distal ICVA at or near the ICVA-BA junction beyond the ICVA branches that penetrate the lateral medullary fossa and beyond PICA. Occasional patients had thrombi originating in one distal ICVA and extended into the BA causing pontine, middle intracranial territory infarction. When an ICVA thrombus extended into the BA, the occluded ICVA was always dominant. Some patients with a single functional distal ICVA (the other ICVA being hypoplastic or ending in PICA) had middle and/or distal territory ischemia. Many patients with extensive combined bilateral ICVA and BA disease had TIAs or minor strokes within the middle and distal intracranial territories.
6. BA disease
BA occlusive disease has been considered a highly mortal condition since publication of the necropsy findings of Kubik and Adams.34 Labauge. et al. reviewed personal and previously reported cases and noted that only 31 among 348 patients with BA occlusion survived.47 Ferbert et al. reported 48 patients who had angiographically documented BA occlusion.94 Some had headache and episodic TIAs during the weeks preceding an acute stroke. Tetraparesis or tetraplegia and decreased consciousness were common findings on hospital presentation. Mortality was high despite thrombolytic therapy.49
Woolfenden et al. reported their findings among 28 patients with BA stenosis.95 TIAs were present in 19 (68%) patients, and 19 (68%) had posterior circulation infarcts on admission MRI. Hemiparesis was more common than tetraparesis. The annual stroke rate on follow-up was 10.3% and the annual death rate 6.2%. All patients were treated with anticoagulants and/or anti-platelet aggregants and 6 had angioplasty. All patients with recurrent strokes were either not treated with warfarin or had subtherapeutic INRs.95
A Lausanne Stroke Registry analysis compared 43 patients who had severe BA occlusive disease determined by vascular imaging (37 by MRA, 6 by catheter angiography) with 45 patients who had severe BA occlusive disease at necropsy.96 Among the vascular imaging group, 54% had mortality or severe disability, 34% had moderate disability, and 12% had no or minor disability. Poor outcome was predicted mostly by decreased level of consciousness on admission but dysarthria, abnormal pupils, and bulbar symptoms also significantly predicted poor outcome.96
BA disease was associated with the poorest outcome among all NEMC-PCR vascular occlusive lesions but outcomes were better than prior reports. Most patients had intrinsic atherosclerotic lesions in the proximal or middle BA or embolism from the heart or VA. Occasional patients had dolichoectasia, or dissection. BA aneurysms sometimes harbor thrombi that can cause middle and distal territory infarcts.97 Hemiparesis with minor motor abnormalities in the limbs contralateral to the hemiparesis was common. Stupor and coma were less frequent than in prior series as was tetraplegia. The case mix of our patients with BA disease likely included more patients with less severe ischemia than in other reports. We evaluated more patients with minor posterior circulation ischemia using presently available noninvasive vascular and brain imaging than was possible in the past. Undoubtedly this led to discovery of BA disease in many patients who would likely have not been exposed to catheter angiography in the past so that BA disease would have been missed.
Patients with BA disease mostly had infarcts involving the paramedian pontine base sometimes extending into the paramedian tegmentum. These infarcts are in the territory of the large median penetrators; flow in these penetrators is most compromised by BA thrombi and stenotic plaques. The tegmentum of the rostral pons derives supply from distal BA branches that descend into the tegmentum.35 Collateral blood flow through circumferential cerebellar arteries (PICA, AICA, and SCA) allows perfusion of the lateral pontine tegmentum. Flow from the distal BA allows perfusion of the rostral pontine tegmentum, the location of the reticular activating system. Survival is common when there is a short segment of occlusion and when the distal BA is spared.
This distal BA is occasionally the site of intrinsic atherosclerotic narrowing but more often distal occlusions are embolic.98,99 Decreased alertness, dysmemory, and oculomotor signs predominate and motor and bulbar symptoms are uncommon. The relatively good outcome from emboli to the top-of-the basilar likely relates to passage of small emboli.
7. Multiple intracranial large artery compromise
This report documents the frequent coexistence of multiple arterial constrictive lesions, most often involving the ICVAs bilaterally, often with accompanying BA and ECVA stenosis. Prior reports contain hints of this group of patients although none describes this group in detail.44,94 TIAs dominated the clinical findings in these NEMC-PCR patients. Few had severe strokes despite months or years of TIAs. The most frequent TIA components were dizziness, faintness, visual blurring, and ataxia. Ischemic attacks were usually multiple and brief and often were precipitated by rising from a supine or seated position, prolonged standing, defecating, and new or increased antihypertensive treatment. Despite severe vascular occlusive disease, these patients had surprisingly good outcomes. Adequate collateral circulation developed and stabilized. Patients with multiple occlusive vascular lesions might have been the basis of the early reports of so-called vertebrobasilar insufficiency in patients in whom vascular lesions were not studied.100-103
8. Penetrating artery disease
The pathology within penetrating arteries consists of lipohyalinotic disruption of the arterial lumen39 or atheromatous branch disease in which plaques within parent arteries obstruct the orifice of penetrating branches.37,38 Ischemia due to penetrating artery disease is important to recognize and to differentiate from larger artery disease since the causes, prognosis, and treatment are different. Nearly the entire brainstem is supplied by arteries that penetrate into the brainstem from the ICVAs, anterior spinal arteries, BA, and PCAs.
In the NEMC-PCR, as in other reports, most branch territory infarcts were located in the pons (middle intracranial territory) and the thalamus (distal intracranial territory). Paramedian pontine infarcts causing pure motor hemiparesis or ataxic hemiparesis and lateral thalamic infarcts causing contralateral hemisensory symptoms occasionally accompanied by contralateral limb ataxia and extrapyramidal abnormalities were the commonest syndromes encountered. Occasional patients had midbrain104,105 or medullary13,106 branch territory infarcts.
More than half of 70 Lausanne Stroke Registry patients with branch territory distribution infarcts had large artery occlusive lesions on MRA that could have obstructed branches.55 In contrast, in the NEMC registry, only 1 example of a protruding or stenotic large artery intracranial occlusive lesion was found in patients with infarcts limited to single penetrating branches.
9. Outcomes in relation to vascular lesions
Outcomes in the NEMC-PCR were better than in prior series of posterior circulation disease patients.4,13,17,66 Patients with embolism had the highest frequency of death and severe disability. Short-term outcomes were better in patients with non-cardioembolic infarcts than previously thought. Vascular occlusive disease caused more severe signs and persisting neurological deficits when artery-to-artery embolism was present, mimicking the experience in patients with anterior circulation large artery disease. Hemodynamic insufficiency rarely caused severe neurological deficits. Even patients with severe bilateral occlusive disease involving intracranial and/or extracranial large arteries had good short-term neurological outcomes. BA severe occlusive disease was the only arterial lesion that conveyed a more severe risk of disability or death compared to other arterial occlusive sites.66
References
- 1.Fisher CM, Gore I, Okabe N, White PD. Calcification of the carotid siphon. Circulation. 1965;32:538–548. doi: 10.1161/01.cir.32.4.538. [DOI] [PubMed] [Google Scholar]
- 2.Hass WK, Fields WS, North RR, Kircheff II, Chase NE, Bauer RB. Joint study of extracranial arterial occlusions. 11 Arteriography, techniques, sites, and complications. JAMA. 1968;203:961–968. [PubMed] [Google Scholar]
- 3.Caplan LR. Caplan's Stroke, a clinical approach. 3rd ed. Boston: ButterworthHeinemann; 2000. [Google Scholar]
- 4.Solberg LA, McGarry P. Cerebral atherosclerosis in Negroes and Caucasians. Atherosclerosis. 1972;16:141–154. doi: 10.1016/0021-9150(72)90048-2. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick PB, Caplan LR, Hier DB, Parker SL, Patel D. Racial differences in the distribution of anterior circulation occlusive disease. Neurology. 1984;34:54–59. doi: 10.1212/wnl.34.1.54. [DOI] [PubMed] [Google Scholar]
- 6.Caplan LR, Gorelick PB, Hier DB. Race, sex, and occlusive cerebrovascular disease: a review. Stroke. 1986;17:648–655. doi: 10.1161/01.str.17.4.648. [DOI] [PubMed] [Google Scholar]
- 7.Feldmann E, Daneault N, Kwan E, Ho KJ, Pessin MS, Langenberg P, Caplan LR. Chinese white differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990;40:1541–1545. doi: 10.1212/wnl.40.10.1540. [DOI] [PubMed] [Google Scholar]
- 8.Caplan LR. Intracranial branch atheromatous disease: a neglected, understudied and underused concept. Neurology. 1989;39:1246–1250. doi: 10.1212/wnl.39.9.1246. [DOI] [PubMed] [Google Scholar]
- 9.Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1000 consecutive patients with first stroke. Stroke. 1988;19:1083–1092. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- 10.Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke. 1988;19:547–554. doi: 10.1161/01.str.19.5.547. [DOI] [PubMed] [Google Scholar]
- 11.Mohr J, Caplan LR, Melski J, Duncan G, Goldstein R, Kistler JP, et al. The Harvard Cooperative Stroke Registry: a prospective registry. Neurology. 1978;28:754–762. doi: 10.1212/wnl.28.8.754. [DOI] [PubMed] [Google Scholar]
- 12.Hennerici M, Aulich A, Sandmann W, Freund HJ. Incidence of asymptomatic extracranial arterial d.isease. Stroke. 1981;12:750–758. doi: 10.1161/01.str.12.6.750. [DOI] [PubMed] [Google Scholar]
- 13.Caplan LR. Posterior circulation ischemia: then, now, and tomorrow. The Thomas Willis Lecture- 2000. Stroke. 2000;31:2011–2023. doi: 10.1161/01.str.31.8.2011. [DOI] [PubMed] [Google Scholar]
- 14.Caplan LR. Posterior circulation vascular disease: clinical features, diagnosis, and management. Boston: Blackwell; 1996. [Google Scholar]
- 15.Caplan LR, Wityk RJ, Glass TA, et al. The New England Medical Center Posterior Circulation Registry. Ann Neurol. 2004;56:389–398. doi: 10.1002/ana.20204. [DOI] [PubMed] [Google Scholar]
- 16.Gorelick PB, Caplan LR, Hier DB, Patel D, Langenberg P, Pessin MS, et al. Racial differences in the distribution of posterior circulation occlusive disease. Stroke. 1985;16:785–790. doi: 10.1161/01.str.16.5.785. [DOI] [PubMed] [Google Scholar]
- 17.Caplan LR, Tettenborn B. Vertebrobasilar occlusive disease: review of selected aspects. 1. spontaneous dissection of extracranial and intracranial posterior circulation arteries. Cerebrovasc Dis. 1992;2:256–265. [Google Scholar]
- 18.Baker AB, lannone A. Cerebrovascular disease: 1. the large arteries of the circle of Willis. Neurology. 1959;9:321–332. doi: 10.1212/wnl.9.5.321. [DOI] [PubMed] [Google Scholar]
- 19.Hutchinson EC, Yates PO. The cervical portion of the vertebral artery: a clincopathological study. Brain. 1956;79:319–331. doi: 10.1093/brain/79.2.319. [DOI] [PubMed] [Google Scholar]
- 20.Hutchinson EC, Yates PO. Carotico vertebral stenosis. Lancet. 1957;1:2–8. doi: 10.1016/s0140-6736(57)92431-5. [DOI] [PubMed] [Google Scholar]
- 21.Moossy J. Morphology, sites, and epidemiology of cerebral atherosclerosis. Res Publ Assoc Res Nerv Ment Dis. 1966;41:1–22. [PubMed] [Google Scholar]
- 22.Castaigne P, Lhermitte F, Gautier JC, Escourolle R, Derouesne C, Der Agopian P, et al. Arterial occlusions in the vertebrobasilar system. A study of 44 patients with postmortem data. Brain. 1973;96:133–154. doi: 10.1093/brain/96.1.133. [DOI] [PubMed] [Google Scholar]
- 23.Sypert GW, Alvord EC. Cerebellar infarction: a clinicopathological study. Arch Neurol. 1975;32:357–363. doi: 10.1001/archneur.1975.00490480023001. [DOI] [PubMed] [Google Scholar]
- 24.Amarenco P, Hauw JJ, Henin D, Duyckaerts C, Roullet E, Laplane D, et al. Les infarctus du territoire de l'artere cerebelleuse posteroinferieure, etude clinicopathologique de 28 cas. Rev Neurol. 1989;145:277–286. [PubMed] [Google Scholar]
- 25.Amarenco P, Hauw JJ, Gautier JC. Arterial pathology in cerebellar infarction. Stroke. 1990;21:1299–1305. doi: 10.1161/01.str.21.9.1299. [DOI] [PubMed] [Google Scholar]
- 26.Wallenberg A. Anatomischer Befund in einem als akute Bulbaraffektion (Embolie der arteria cerebelli inferior posterior sinistra) beschriebenen Falle. Arch fur Psychiatrie. 1901;34:923–959. [Google Scholar]
- 27.Wallenberg A. Verschluss der arteria cerebelli inferior posterior dextra (mit Sektion befund) Deutsche Zeitschrift fur Nervenheilkunde. 1922;73:189–212. [Google Scholar]
- 28.Fisher CM, Karnes W, Kubik CS. Lateral medullary infarction. The pattern of vascular occlusion. J Neuropath Exp Neurol. 1961;20:323–379. doi: 10.1097/00005072-196107000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Hauw JJ, Der Agopian P, Trelles L. Bulbar infarcts. Systematic study of lesion topography in 49 cases. J Neurol Sci. 1976;28:83–102. doi: 10.1016/0022-510x(76)90050-2. [DOI] [PubMed] [Google Scholar]
- 30.Babinski J, Nageotte J. Hemiasynergie, lateropulsion et myosis bulbaires avec hemianesthesie et hemiplegie croisees. Rev Neurol. 1902;10:358–365. [Google Scholar]
- 31.Leyden E. Uber die Thrombose der Basilar Arterie. Zeitschr Klin Med. 1882;5:165–182. [Google Scholar]
- 32.Pines L, Gilinsky E. Uber die Thrombose der Arteria Basilares und uber die Vaskularization der Brucke. Archiv f Psychiatrie Nervenkrank. 1932;97:380–387. [Google Scholar]
- 33.Lhermitte J, Trelles JO. L'arteriosclerose du tronc basilaire et ses consequence anatomocliniques. Jahrbucher f Psychiatr u Neurologie. 1934;51:91–107. [Google Scholar]
- 34.Kubik CS, Adams RD. Occlusion of the basilar artery a clinical and pathological study. Brain. 1946;69:73–121. doi: 10.1093/brain/69.2.73. [DOI] [PubMed] [Google Scholar]
- 35.Biemond A. Thrombosis of the basilar artery and the vascularization of the brain stem. Brain. 1951;74:300–317. doi: 10.1093/brain/74.3.300. [DOI] [PubMed] [Google Scholar]
- 36.Castaigne P, Lhermitte F, Buge A, Escourolle R, Hauw JJ, Lyon-Caen O. Paramedian thalamic and midbrain infarcts: clinical and neuropathological study. Ann Neurol. 1981;10:127–148. doi: 10.1002/ana.410100204. [DOI] [PubMed] [Google Scholar]
- 37.Fisher CM. Bilateral occlusion of basilar artery branches. J Neurol Neurosurg Psychiatry. 1977;40:1182–1189. doi: 10.1136/jnnp.40.12.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fisher CM, Caplan LR. Basilar artery branch occlusion: a cause of pontine infarction. Neurology. 1971;21:900–905. doi: 10.1212/wnl.21.9.900. [DOI] [PubMed] [Google Scholar]
- 39.Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1968;12:1–15. doi: 10.1007/BF00685305. [DOI] [PubMed] [Google Scholar]
- 40.Fisher CM. Thalamic pure sensory stroke: a pathological study. Neurology. 1978;28:1141–1144. doi: 10.1212/wnl.28.11.1141. [DOI] [PubMed] [Google Scholar]
- 41.Fields WS, Lemak NA. Joint Study of Extracranial Arterial Occusion. VII. Subclavian steala review of 168 cases. JAMA. 1972;222:1139–1143. [PubMed] [Google Scholar]
- 42.Labauge R, Boukobza M, Pages M, Blard JM, Dimitrijevic J, Salvaing P. Occlusion of the vertebral artery (100 personal cases) Rev Neurol. 1987;143:490–509. [PubMed] [Google Scholar]
- 43.Moufarrij NA, Little JR, Furlan AJ, Williams G, Marzewski DJ. Vertebral artery stenosis: long-term follow-up. Stroke. 1984;15:260–263. doi: 10.1161/01.str.15.2.260. [DOI] [PubMed] [Google Scholar]
- 44.Moufarrij NA, Little JR, Furlan AJ, Leatherman JR, Williams GW. Basilar or distal vertebral artery stenosis: long-term follow-up. Stroke. 1986;17:938–942. doi: 10.1161/01.str.17.5.938. [DOI] [PubMed] [Google Scholar]
- 45.Moscow NP, Newton TH. Angiographic implications in diagnosis and prognosis of basilar artery occlusion. Am J Roentgenol Radium Ther Nucl Med. 1973;119:597–604. doi: 10.2214/ajr.119.3.597. [DOI] [PubMed] [Google Scholar]
- 46.Archer C, Horenstein S. Basilar artery occlusion: clinical and radiologic correlation. Stroke. 1977;8:383–390. doi: 10.1161/01.str.8.3.383. [DOI] [PubMed] [Google Scholar]
- 47.Labauge R, Pages C, MartyDouble C, Blard JM, Boukobza M, Salvaing P. Occlusion du tronc basilaire. Rev Neurol. 1981;137:545–571. [PubMed] [Google Scholar]
- 48.Bruckmann H, Ferbert A, del Zoppo GJ, Hacke W, Zeumer H. Acute vertebrobasilar thrombosis: angiologicalclinical and therapeutic implications. Acta Radiol Suppl. 1986;369:38–42. [PubMed] [Google Scholar]
- 49.Hacke W, Zeumer H, Ferbert A, Bruckmann H, del Zoppo G. Intraarterial therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke. 1988;19:1216–1222. doi: 10.1161/01.str.19.10.1216. [DOI] [PubMed] [Google Scholar]
- 50.Fields WS, Ratinov G, Weibel J, Campos RJ. Survival following basilar artery occlusion. Arch Neurol. 1966;15:463–471. doi: 10.1001/archneur.1966.00470170017002. [DOI] [PubMed] [Google Scholar]
- 51.Labauge R, Pages M, Blard JM. Survie prolongee apres occlusion du tronc basilaire. Rev Neurol. 1989;145:789–795. [PubMed] [Google Scholar]
- 52.Caplan LR. Occlusion of the vertebral or basilar artery. Followup analysis of some patients with benign outcome. Stroke. 1979;10:277–282. doi: 10.1161/01.str.10.3.277. [DOI] [PubMed] [Google Scholar]
- 53.Caplan LR, Rosenbaum AE. Role of cerebral angiography in vertebrobasilar occlusive disease. J Neurol Neurosurg Psychiatry. 1975;38:601–612. doi: 10.1136/jnnp.38.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer JS, Sheehan S, Bauer R. An arteriographic study of cerebrovascular disease in man. I. Stenosis and occlusion of the vertebral basilar arterial system. Arch Neurol. 1960;2:27–45. [Google Scholar]
- 55.Bogousslavsky J, Regli F, Maeder P, Meuli R, Nader J. The etiology of posterior circulation infarcts: a prospective study using magnetic resonance angiography. Neurology. 1993;43:1528–1533. doi: 10.1212/wnl.43.8.1528. [DOI] [PubMed] [Google Scholar]
- 56.Rosengart A, DeWitt LD, Caplan LR, Pessin MS, Wolpert S. Noninvasive tests in vertebrobasilar occlusive disease. Ann Neurol. 1992;32:265. [Google Scholar]
- 57.Rother J, Wentz KU, Rautenberg W, Schwartz A, Hennerici M. Magnetic resonance angiography in vertebrobasilar ischemia. Stroke. 1993;24:1310–1315. doi: 10.1161/01.str.24.9.1310. [DOI] [PubMed] [Google Scholar]
- 58.Wentz KU, Rother J, Schwartz A, Mattle HP, Suchalla R, Edelman RR. Intracranial vertebrobasilar system: MR angiography. Radiology. 1994;190:105–110. doi: 10.1148/radiology.190.1.8259384. [DOI] [PubMed] [Google Scholar]
- 59.Georgiadis AI, Yamamoto Y, Kwan ES, Pessin MS, Caplan LR. Anatomy of sensory findings in patients with Posterior Cerebral Artery Territory Infarction. Arch Neurol. 1999;56:835–838. doi: 10.1001/archneur.56.7.835. [DOI] [PubMed] [Google Scholar]
- 60.Graf KJ, Pessin MS, DeWitt LD, Caplan LR. Proximal intracranial territory posterior circulation infarcts in the New England Medical Center Posterior Circulation Registry. Eur Neurol. 1997;37:157–168. doi: 10.1159/000117428. [DOI] [PubMed] [Google Scholar]
- 61.Muller-Kuppers M, Graf KJ, Pessin MS, DeWitt LD, Caplan LR. Intracranial vertebral artery disease in the New England Medical Center Posterior Circulation Registry. Eur Neurol. 1997;37:146–156. doi: 10.1159/000117427. [DOI] [PubMed] [Google Scholar]
- 62.Shin H-K, Yoo K-M, Chang H-M, Caplan LR. Bilateral intracranial vertebral artery disease in the New England medical Center Posterior Circulation Registry. Arch Neurol. 1999;56:1353–1358. doi: 10.1001/archneur.56.11.1353. [DOI] [PubMed] [Google Scholar]
- 63.Wityk RJ, Chang H-M, Rosengart A, Han W-C, DeWitt LD, Pessin MS, et al. Proximal extracranial vertebral artery disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 1998;55:470–478. doi: 10.1001/archneur.55.4.470. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto Y, Georgiadis AI, Chang H-M, Caplan LR. Posterior Cerebral Artery territory Infarcts in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 1999;56:824–832. doi: 10.1001/archneur.56.7.824. [DOI] [PubMed] [Google Scholar]
- 65.Caplan LR, Amarenco P, Rosengart A, Lafranchise EF, Teal P, Belkin M, et al. Embolism from vertebral artery origin disease. Neurology. 1992;42:1505–1512. doi: 10.1212/wnl.42.8.1505. [DOI] [PubMed] [Google Scholar]
- 66.Glass TA, Hennessey PM, Pazdera L, Chang H-M, Wityk RJ, et al. Outcome at 30 days in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 2002;59:369–376. doi: 10.1001/archneur.59.3.369. [DOI] [PubMed] [Google Scholar]
- 67.Duvernoy HM. Human Brainstem Vessels. Berlin: Springer-Verlag; 1978. [Google Scholar]
- 68.Voetsch B, DeWitt LD, Pessin MS, Caplan LR. Basilar artery occlusive disease in the New England medical Center Posterior Circulation Registry. Arch Neurol. 2003;61:496–504. doi: 10.1001/archneur.61.4.496. [DOI] [PubMed] [Google Scholar]
- 69.Pessin MS, Lathi ES, Cohen MB, Kwan ES, Hedges TR, lll, Caplan LR. Clinical features and mechanism of occipital infarction. Ann Neurol. 1987;21:290–299. doi: 10.1002/ana.410210311. [DOI] [PubMed] [Google Scholar]
- 70.Hommel M, Besson G, Pollak P, Kahane P, Le Bas JF, Perret J. Hemiplegia in posterior cerebral artery occlusion. Neurology. 1990;40:1496–1499. doi: 10.1212/wnl.40.10.1496. [DOI] [PubMed] [Google Scholar]
- 71.Pessin MS, Kwan ES, DeWitt LD, Hedges TR, 3rd, Gale D, Caplan LR. Posterior cerebral artery stenosis. Ann Neurol. 1987;21:85–89. doi: 10.1002/ana.410210115. [DOI] [PubMed] [Google Scholar]
- 72.Bauer RB, Sheehan S, Wechsler N, Meyer JS. Arteriographic study of the sites, incidence, and treatment of arteriosclerotic cerebrovascular lesions. Neurology. 1962;12:698–711. [Google Scholar]
- 73.Fields WS, North RR, Hass WK, Galbraith JG, Wyler EJ, Ratinov G, et al. Joint Study of Extracranial Arterial Occlusion as a cause of stroke. I. Organization of study and survey of patient population. JAMA. 1968;203:955–960. [PubMed] [Google Scholar]
- 74.Kieffer SA, Takeya Y, Resch JA, Amplatz K. Racial differences in cerebrovascular disease: Angiographic evaluation of Japanese and American populations. Am J Roentgenol Radium Ther Nucl Med. 1967;101:94–99. [PubMed] [Google Scholar]
- 75.Heyden S, Heyman A, Goree JA. Nonembolic occlusion of the middle cerebral and carotid arteries: a comparison of predisposing factors. Stroke. 1970;1:363–369. doi: 10.1161/01.str.1.5.363. [DOI] [PubMed] [Google Scholar]
- 76.Heyman A, Fields WS, Keating RD. Joint Study of Extracranial Arterial Occlusions VI. Racial differences in hospitalized patients with ischemic stroke. JAMA. 1972;222:285–289. [PubMed] [Google Scholar]
- 77.Leung SY, Ng THK, Yuen ST, Lauder IJ, Ho FCS. Pattern of cerebral atherosclerosis in Hong Kong Chinese. Severity in Intracranial and extracranial vessels. Stroke. 1993;24:779–786. doi: 10.1161/01.str.24.6.779. [DOI] [PubMed] [Google Scholar]
- 78.Reivich M, Holling HE, Roberts B, Toole JF. Reversal of blood flow through the vertebral artery and its effect on cerebral circulation. N Engl J Med. 1961;265:878–885. doi: 10.1056/NEJM196111022651804. [DOI] [PubMed] [Google Scholar]
- 79.Fisher CM. Editorial. A new vascular syndrome: "the subclavian steal". N Engl J Med. 1961;265:912–913. [Google Scholar]
- 80.Baker RA, Rosenbaum AE, Caplan LR. Subclavian steal syndrome. Contemporary Surgery. 1974;4:96–104. [Google Scholar]
- 81.Hennerici M, Klemm C, Rautenberg W. The subclavian steal phenomenon: a common vascular disorder with rare neurologic deficits. Neurology. 1988;38:669–673. doi: 10.1212/wnl.38.5.669. [DOI] [PubMed] [Google Scholar]
- 82.Brewster DC, Moncure AC, Darling RC, Ambrosino JJ, Abbott WM. Innominate artery lesions: problems encountered and lessons learned. J Vasc Surg. 1985;2:99–112. doi: 10.1067/mva.1985.avs0020099. [DOI] [PubMed] [Google Scholar]
- 83.Fisher CM. Occlusion of the vertebral arteries causing transient basilar symptoms. Arch Neurol. 1970;22:13–19. doi: 10.1001/archneur.1970.00480190017003. [DOI] [PubMed] [Google Scholar]
- 84.Fisher CM, Karnes WE. Local embolism. J Neuropathol Exp Neurol. 1965;24:174–175. [Google Scholar]
- 85.George B, Laurian C. Vertebro-basilar ischemia with thrombosis of the vertebral artery: report of 2 cases with embolism. J Neurol Neurosurg Psychiatry. 1982;45:91–93. doi: 10.1136/jnnp.45.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koroshetz WJ, Ropper AH. Artery- to -artery embolism causing stroke in the posterior circulation. Neurology. 1987;37:292–296. doi: 10.1212/wnl.37.2.292. [DOI] [PubMed] [Google Scholar]
- 87.Pessin MS, Daneault N, Kwan ES, Eisengart M, Caplan LR. Local embolism from vertebral artery occlusion. Stroke. 1988;19:112–115. doi: 10.1161/01.str.19.1.112. [DOI] [PubMed] [Google Scholar]
- 88.Pelouze GA. Plaque Ulceree emboligene de l'ostium de l'artere vertebrale. Rev Neurol. 1989;145:478–481. [PubMed] [Google Scholar]
- 89.Catala M, Rancurel G, Koskas F, Martin-Delassalle E, Kieffer E. Ischemic stroke due to spontaneous extracranial vertebral giant aneurysm. Cerebrovasc Dis. 1993;3:322–326. [Google Scholar]
- 90.Berguer R, Flynn LM, Kline RA, Caplan LR. Surgical reconstruction of the extracranial vertebral artery: management and outcome. J Vasc Surg. 2000;31:9–18. doi: 10.1016/s0741-5214(00)70063-2. [DOI] [PubMed] [Google Scholar]
- 91.Piotin M, Spelle L, Martin J-B, Weill A, Rancurel G, Ross IB, et al. Percutaneous transluminal angioplasty and stenting of the proximal vertebral artery for symptomatic stenosis. AJNR Am J Neuroradiol. 2000;21:727–731. [PMC free article] [PubMed] [Google Scholar]
- 92.Caplan LR. Bilateral distal vertebral artery occlusion. Neurology. 1983;33:552–558. doi: 10.1212/wnl.33.5.552. [DOI] [PubMed] [Google Scholar]
- 93.Bogousslavsky J, Gates PC, Fox AJ, Barnett HJM. Bilateral occlusion of vertebral artery: clinical pattern and long-term prognosis. Neurology. 1986;36:1309–1315. doi: 10.1212/wnl.36.10.1309. [DOI] [PubMed] [Google Scholar]
- 94.Ferbert A, Bruckmann H, Drummen R. Clinical features of proven basilar artery occlusion. Stroke. 1990;21:1135–1142. doi: 10.1161/01.str.21.8.1135. [DOI] [PubMed] [Google Scholar]
- 95.Woolfenden AR, Tong DC, Norbash AM, Ali AO, Marks MP, O'Brien MW, et al. Basilar artery stenosis: clinical and neuroradiographic features. J Stroke Cerebrovasc Dis. 2000;9:57–63. doi: 10.1053/jscd.2000.0090057. [DOI] [PubMed] [Google Scholar]
- 96.Devuyst G, Bogousslavsky J, Meuli R, Moncayo J, de Freitas G, van Melle G. Stroke or transient ischemic attacks with basilar artery stenosis or occlusion,: clinical patterns and outcome. Arch Neurol. 2000;59:567–573. doi: 10.1001/archneur.59.4.567. [DOI] [PubMed] [Google Scholar]
- 97.DeGeorgia M, Belden J, Pao L, Pessin MS, Kwan E, Caplan LR. Thrombus in vertebrobasilar dolichoectatic artery treated with intravenous urokinase. Cerebrovasc Dis. 1999;9:28–33. doi: 10.1159/000015892. [DOI] [PubMed] [Google Scholar]
- 98.Caplan LR. Top of the basilar syndrome: selected clinical aspects. Neurology. 1980;30:72–79. doi: 10.1212/wnl.30.1.72. [DOI] [PubMed] [Google Scholar]
- 99.Mehler MF. The rostral basilar artery syndrome: diagnosis, etiology, prognosis. Neurology. 1989;39:9–16. doi: 10.1212/wnl.39.1.9. [DOI] [PubMed] [Google Scholar]
- 100.Millikan CH, Siekert RG. Studies in cerebrovascular disease; I. The syndrome of intermittent insufficiency of the basilar arterial system. Proc Staff Meet Mayo Clin. 1955;30:61–68. [PubMed] [Google Scholar]
- 101.Millikan CH, Siekert RG, Shick R. Studies in cerebrovascular disease. 3. The use of anticoagulant drugs in the treatment of insufficiency or thrombosis within the basilar arterial system. Proc Staff Meet Mayo Clin. 1955;30:116–126. [PubMed] [Google Scholar]
- 102.Williams D. Vertebrobasilar ischaemia. Brit Med J. 1964;1:84–86. doi: 10.1136/bmj.1.5375.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williams D, Wilson TG. The diagnosis of the major and minor syndromes of basilar insufficiency. Brain. 1962;85:741–774. doi: 10.1093/brain/85.4.741. [DOI] [PubMed] [Google Scholar]
- 104.Bogousslavsky J, Maeder P, Regli F, Meuli R. Pure midbrain infarction: clinical syndromes, MRI, and etiologic patterns. Neurology. 1994;44:2032–2040. doi: 10.1212/wnl.44.11.2032. [DOI] [PubMed] [Google Scholar]
- 105.Martin PJ, Chang H-M, Wityk R, Caplan LR. Midbrain infarction: associations and aetiologies in the New England Medical Center Posterior Circulation Registry. J Neurol Neurosurg Psychiatry. 1998;64:392–395. doi: 10.1136/jnnp.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim JS, Kim HG, Chung CS. Medial medullary syndrome: report of 18 new patients and review of the literature. Stroke. 1995;26:1548–1552. doi: 10.1161/01.str.26.9.1548. [DOI] [PubMed] [Google Scholar]