Abstract
Background
The membrane permeability transition of mitochondria has been suggested to be involved in toxic and oxidative forms of cell injury. Mitochondrial dysfunction is considered to play a critical role in neurodegeneration in Parkinson's disease. Despite the suggestion that indole β-carbolines may be neurotoxic, these compounds provide a protective effect against cytotoxicity of other neurotoxins. In addition, the effect of indole β-carbolines on change in the mitochondrial membrane permeability due to reactive nitrogen species (RNS), which may lead to cell death, has not been clarified.
Methods
Differentiated PC12 cells were used as the experimental culture model for the investigation of neuronal cell injury, which occurs in Parkinson's disease. The effect of indole β-carbolines (harmalol and harmine) on differentiated PC12 cells against toxicity of S-nitroso-N-acetyl-DL-penicillamine (SNAP) was determined by measuring the effect on the change in transmembrane potential, cytochrome c release, formation of ROS, GSH contents, caspase-3 activity and cell viability, and was compared to that of R-(-)-deprenyl.
Results
Specific inhibitors of caspases (z-LEHD.fmk, z-DQMD.fmk) and antioxidants (N-acetylcysteine, dithiothreitol, melatonin, carboxy-PTIO and uric acid) depressed cell death in PC12 cells due to SNAP. β-Carbolines and R-(-)-deprenyl attenuated the SNAP-induced cell death and GSH depletion concentration dependently with a maximal inhibitory effect at 25-50 µM. The compounds inhibited the nuclear damage, decrease in mitochondrial transmembrane potential, cytochrome c release and formation of reactive oxygen species caused by SNAP in PC12 cells. β-Carbolines and R-(-)-deprenyl attenuated the H2O2-induced cell death and depletion of GSH.
Conclusions
The results suggest that indole β-carbolines attenuate the SNAP-induced viability loss in PC12 cells by inhibition of change in the mitochondrial membrane permeability, which may be caused by free radicals. Indole β-carbolines appear to exert a protective effect against the nitrogen species-mediated neuronal cell injury in Parkinson's disease comparable to R-(-)-deprenyl.
Keywords: Indole β-carbolines, Nitrogen species, Mitochondrial membrane permeability, Cell death, Differentiated PC12 cells
INTRODUCTION
Mitochondrial dysfunction and increased formation of free radicals have been shown to be involved in neuronal cell death.1-3 Many lines of evidences reveal that oxidative damage of dopaminergic neurons occurring in Parkinson's disease may be mediated by the actions of nitric oxide (NO).2 The substantia nigra of patients with Parkinson's disease shows increased immunoreactivity for inducible nitric oxide synthase.4 In addition, the presence of 3-nitrotyrosine in the core of Lewy bodies, striatum and substantia nigra supports the role of NO in neurodegeneration.5 The 7-nitroindazole, an inhibitor of neuronal nitric oxide synthase, reduces toxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on the baboons.6 Toxicity of NO is mainly ascribed to peroxynitrite (ONOO-), a product of reaction with superoxide.7 NO inhibits the mitochondrial respiratory chain, leading to increase in the production of reactive oxygen species (ROS)8 and induces the membrane permeability change in mitochondria.9 It has been suggested that NO donors, including S-nitroso-N-acetyl-penicillamine (SNAP), may trigger apoptosis in PC12 cells by opening of the mitochondrial permeability transition pore or cause necrotic cell death in PC12 cells by respiratory inhibition.10 The pathologic feature in Parkinson's disease shows the striking degenerative loss of dopaminergic neurons in the nigrostriatal system. Although rat PC12 cells are not brain dopaminergic neurons, these cells are able to produce dopamine and express dopamine transporter.11 Upon nerve growth factor stimulation, PC12 cells not only display abundant neuritic growth, but also adopt a neurochemical dopaminergic phenotype. In this study, therefore differentiated PC12 cells were used as the experimental culture model to investigate the nitrogen species-induced injury of dopaminergic neurons, which can occur in Parkinson's disease.
R-(-)-deprenyl is considered to provide a beneficial effect in the treatment of Parkinson's disease by inhibition of monoamine oxidase (MAO)-B.12 However, it has been also suggested that deprenyl provides a protective effect on nigral neurons against a variety of insults independent of the inhibition of MAO-B.13,14 Indole-derived β-carbolines and its metabolites, methylated β-carboliniums, are known as endogenous substances and are suggested to act as neurotoxins because of their structural similarity to MPTP.15,16 N-Methyl-β-carbolinium shows a cytotoxic effect on PC12 cells,17 and the precursor harmaline causes a degeneration of Purkinje cells in rat cerebellum.18 In contrast, several reports indicate that indole β-carbolines have effective antioxidant abilities19 and an inhibitory effect on activities of MAO-A and -B.20 Indole β-carbolines, including harman, are found to inhibit lipid peroxidation of liver microsomes.19 These compounds inhibit the oxidative toxicity of glutamate on HT-22 hippocampal cell line21 and attenuate the catecholamine-induced mitochondrial dysfunctions and cell death in PC12 cells.22
Despite the suggestion that indole β-carbolines may be neurotoxic, these compounds provide an inhibitory effect against cytotoxicity of other neurotoxins. In addition, the effect of indole β-carbolines on change in the mitochondrial membrane permeability due to reactive nitrogen species (RNS), which may lead to cell death, has not been clarified. We therefore assessed the effect of indole β-carbolines (expressed as β-carbolines in the text, harmalol and harmine) on differentiated PC12 cells against the toxicity of SNAP by measuring the effect on the change in transmembrane potential, cytochrome c release, formation of ROS, GSH contents, caspase-3 activity and cell viability. The effect of β-carbolines on the cytotoxicity of SNAP was compared to that of R-(-)-deprenyl.
MATERIALS AND METHODS
1. Materials
R-(-)-deprenyl, harmalol (3,4-dihydro-1-methyl-9H-pyrido[3,4-b]indol-7-ol), harmine (7-methoxy-1-methyl-9H-pyridol[3,4-β]indole, S-nitroso-N-acetyl-DL-penicillamine (SNAP), z-Asp-(OMe)-Gln-Met-Asp(OMe) fluoromethyl ketone, z-Leu-Glu-(O-ME)-His-Asp(O-Me) fluoromethyl ketone, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Hoechst 33258, 3,3'-dihexyloxacarbocyanine iodide (DiOC6(3)), 2',7-dichlorofluorescin diacetate (DCFH2-DA), 5,5'-dithio-bis-(2-nitrobenzoic acid) (DTNB), phenylmethylsulfonyl-fluoride (PMSF), tyrosine, 3-nitrotyrosine, nerve growth factor 7S and RPMI 1640 medium were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Quantikine® M rat/mouse cytochrome c assay kit was obtained from R&D systems (Minneapolis, MN, USA), and ApoAlert™ CPP32/caspase-3 assay kit was from CLONTECH Laboratories Inc. (Palo Alto, CA, USA). Protein concentration was determined by the method of Bradford according to the manufacturer's instructions (Bio-Rad Laboratories, Hercules, CA, USA).
2. Cell culture
Rat PC12 cells (adrenal gland; pheochromocytoma) were obtained from Korean cell line bank (Seoul, Korea). PC12 cells were cultured in RPMI medium supplemented with 10% heat-inactivated horse serum, 5% fetal bovine serum (FBS), 100 units/ml of penicillin and 100 µg/ml of streptomycin as described in the manual of the cell line bank. Cells were differentiated by treating with 100 ng/ml 7S nerve growth factor in the 60×15 mm cell culture dishes coated with poly-L-lysine for 9 days.23 Cells were washed with RPMI medium containing 1% FBS 24 hours before experiments and replated onto the 96- and 24-well plates.
3. Cell viability assay
Cell viability was measured by using the MTT assay, which is based on the conversion of MTT to formazan crystals by mitochondrial dehydrogenases.24 PC12 cells (4×104) were treated with SNAP for 24 hours at 37℃. The medium (200 µl) was incubated with 10 µl of 10 mg/ml MTT solution for 2 hours. Culture medium was removed and 100 µl of dimethyl sulfoxide was added to each well to dissolve the formazan. Absorbance was measured at 570 nm using a microplate reader (Molecular Devices Co., Spectra MAX 340, Sunnyvale, CA, USA). Cell viability was expressed as a percentage of the control culture value.
4. Morphological observation of nuclear change
PC12 cells (1×106) were treated with SNAP for 24 hours, and the nuclear morphological change was assessed using the Hoechst dye 33258.25 Cells were incubated with 1 µg/ml Hoechst 33258 for 3 minutes at room temperature and nuclei were visualized using an Olympus Microscope with a WU excitation filter (Tokyo, Japan).
5. Measurement of apoptosis in cells
Apoptosis was assessed by measuring the DNA fragmentation, which occurs following the activation of endonucleases. PC12 cells (1×105) were treated with SNAP for 24 hours, washed with PBS and fixed with formaldehyde solution. Nucleotide (dNTP) was incorporated at the 3'- ends of DNA fragments using terminal deoxynucleotidyl transferase (TdT) and this nucleotide was detected using a horseradish-peroxidase and TACS-Sapphire according to TiterTACS protocol. Data were expressed as absorbance at 450 nm.
6. Measurement of mitochondrial transmembrane potential
Change in the mitochondrial transmembrane potential during apoptosis in PC12 cells was quantified by flow cytometry with the cationic lipophilic dye DiOC6(3).26 Cells (1×106) were treated with SNAP for 24 hours, DiOC6(3) (40 nM) added to the medium and cells were incubated for 15 minutes at 37℃. After centrifugation at 1500 g for 5 minutes, the supernatants were removed and the pellets were resuspended in PBS containing 0.5 mM EDTA. For analysis, a FACScan cytofluorometer (Becton Dickinson, San Jose, CA, USA) with argon laser excitation at 501 nm was used to assess 10,000 cells from each sample.
7. Measurement of cytochrome c release
The release of cytochrome c into the cytosol of PC12 cells was assessed by using a solid phase, enzyme-linked immunosorbent assay kit for the detection of rodent cytochrome c. PC12 cells (5×105) were harvested by centrifugation at 800 g for 10 minutes, washed twice with PBS, resuspended in 250 mM sucrose, 20 mM HEPES-KOH (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 0.1 mM dithiothreitol and 0.1 mM PMSF and homogenized further by successive passages through a 26-G hypodermic needle. The homogenates were centrifuged at 100,000 g for 30 minutes and the supernatant was used for analysis of cytochrome c. The supernatants were added into the 96-well microplates coated with monoclonal antibody specific for rat/mouse cytochrome c that contain cytochrome c conjugate. The procedure was performed as described in the assay kit. Absorbance of samples was measured at 450 nm in a microplate reader. The standard curve was constructed by adding diluted solutions of cytochrome c standard, handled like samples, to the microplates coated with monoclonal antibody. The amount was expressed as nanograms/ml by reference to the standard curve.
8. Measurement of caspase-3 activity
Apoptosis in PC12 cells was assessed by measuring activity of caspase-3, an apoptosis executor.1 Cells (2×106) were treated with SNAP for 24 hours, and the effect of β-carbolines on apoptosis in the SNAP-treated cells was determined according to the user's manual for the ApoAlert™ CPP32/caspase-3 assay kit. The supernatant obtained by a centrifugation of cells dissolved was added to the reaction mixture containing dithiothreitol and caspase-3 substrate (N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide) and was incubated for 1 hour. Absorbance of the chromophore p-nitroanilide produced was measured at 405 nm. The standard curves were obtained from absorbances in the p-nitroanilide standard reagent diluted with cell lysis buffer (up to 20 nM). One unit of the enzyme was defined as the activity producing 1 nM of p-nitroanilide.
9. Measurement of intracellular ROS formation
The dye DCFH2-DA, which is oxidized to fluorescent 2',7'-dichlorofluorescin (DCF) by hydroperoxides, was used to measure relative levels of cellular peroxides.27 After exposure to SNAP for 24 hours, PC12 cells (4×104) were incubated with 50 µM dye for 30 minutes and were then washed with PBS. The cell suspensions were centrifuged at 412 g for 10 minutes, and medium was removed. Cells were dissolved with 1% triton X-100 and fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm using a fluorescence microplate reader (SPECTRAFLUOR, TECAN, Salzburg, Austria).
10. Measurement of total glutathione
The total glutathione (GSH+GSSG) was determined using glutathione reductase.28 PC12 cells (4×104) were treated with SNAP for 24 hours. The cell suspensions were centrifuged at 412 g for 10 minutes in a microplate centrifuge and medium was removed. Cells were dissolved with 2% 5-sulfosalicylic acid (100 µl) and then incubated in 100 µl of the solution containing 22 mM sodium EDTA, 600 µM NADPH, 12 mM DTNB and 105 mM Nah2PO4, pH 7.5 at 37℃. Glutathione reductase (20 µl, 100 units/ml) was added and the mixture incubated further for 10 min. Absorbance was measured at 412 nm using a microplate reader. The standard curve was obtained from absorbance of the diluted commercial GSH that was incubated in the mixture as in samples.
11. Statistical analysis
The values given in the text are expressed as means±SEM. Data were analyzed by one-way analysis of variance. When significance was detected, post hoc comparisons between the different groups were made using the Duncan's test for multiple comparisons. A probability less than 0.05 was considered to be statistically significant. Depiction and expression of data in the text were based on the previous reports and protocol as described in Materials and Methods.
RESULTS
1. Effect of β-carbolines and R-(-)-deprenyl on SNAP-induced cell viability loss
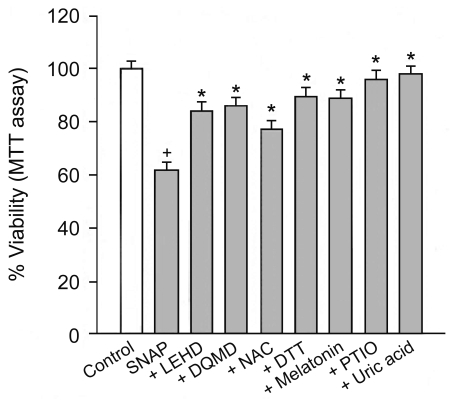
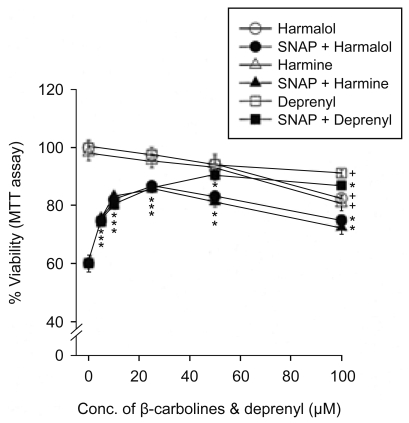
The effect of β-carbolines and R-(-)-deprenyl on RNS-induced cell death was assessed using the PC12 cells differentiated by nerve growth factor. In various experiments, PC12 cells were treated with 500 µM SNAP (a donor of NO) in the presence of 25 µM of β-carbolines or R-(-)-deprenyl for 24 hours at 37℃. When PC12 cells were treated with 500 µM SNAP for 24 hours, the cell viability decreased to 62% (Fig. 1). The cytotoxic effect of SNAP was inhibited by 40 µM z-LEHD.fmk (a cell-permeable inhibitor of caspase-9), 40 µM z-DQMD.fmk (a cell-permeable inhibitor of caspase-3) and antioxidants (1 mM N-acetylcysteine, 1 mM dithiothreitol, 100 µM melatonin [scavenger of ROS and RNS], 25 µM carboxy-PTIO [a NO scavenger] and 250 µM uric acid [a ONOO- scavenger]). β-Carbolines (harmalol and harmine) and R-(-)-deprenyl differentially depressed cell death due to SNAP depending on concentration (Fig. 2). The maximum inhibition of cell death due to SNAP was observed with 25 µM β-carbolines and 50 µM R-(-)-deprenyl; beyond these concentrations the inhibitory effect declined. We investigated the cytotoxic effect of compound alone on PC12 cells. β-Carbolines or R-(-)-deprenyl (100 µM) alone for a treatment of 24 hours caused cell viability loss in PC12 cells by 9~19%.
Figure 1.
Effect of NO scavengers on cell death due to SNAP. PC12 cells were treated with 500 µM SNAP in the presence of caspase inhibitors (40 µM z-LEHD.fmk [LEHD], 40 µM z-DQMD.fmk [DQMD]) or antioxidants (1 mM N-acetylcysteine [NAC], 1 mM dithiothreitol [DTT], 100 µM melatonin, 25 µM carboxy-PTIO [PTIO] and 250 µM uric acid) for 24 hours at 37℃ and mixtures were treated with 0.5 mg/ml MTT for 2 hours. Data are expressed as the percentage of control and represent means±SEM of 6 replicate values in two separate experiments. †P<0.05, compared to control. *P<0.05, compared to SNAP alone.
Figure 2.
Effect of β-carbolines and R-(-)-deprenyl on SNAP-induced loss of cell viability. PC12 cells were treated with 500 µM of SNAP in the presence of 5-100 µM β-carbolines (harmalol and harmine) or R-(-)-deprenyl for 24 hours. Data are expressed as the percentage of control and represent means±SEM of 6 replicate values in two separate experiments. †P<0.05, compared to control. *P<0.05, compared to SNAP alone.
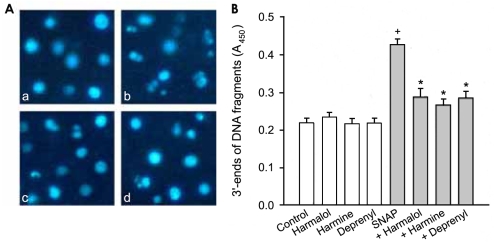
To clarify the inhibitory effect of β-carbolines on cytotoxicity of SNAP, we investigated the effect on the nuclear damage occurred in the SNAP-treated cells. Nuclear staining with Hoechst 33258 demonstrated that control PC12 cells had regular and round-shaped nuclei. In contrast, the condensation and fragmentation of nuclei characteristic of apoptotic cells were evident in cells treated with SNAP (Fig. 3A). Harmine reduced the SNAP-induced nuclear damage while the nuclear morphology in cells exposed to harmine alone was similar to that in the control cells.
Figure 3.
Effect of β-carbolines and R-(-)-deprenyl on SNAP-induced nuclear damage. PC12 cells were treated with 500 µM SNAP in the presence of 25 µM β-carbolines or R-(-)-deprenyl for 24 hours. In the experiment (A) cells were observed by fluorescence microscopy after nuclei staining with Hoechst 33258. a: control cells, b: cells treated with SNAP alone, c: cells treated with SNAP and harmine, (d) cells treated with harmine alone. All the subparts are representative of four different experiments. In the experiment (B) the 3'-ends of DNA fragments were detected as described in Materials and Methods. Data are expressed as absorbance and represent the means±SEM of 6 replicate values in two separate experiments. †P<0.05, compared to control. *P<0.05, compared to SNAP alone.
During the process of apoptosis, DNA fragmentation is caused by activation of endonucleases. Fragmented DNA was assessed by measuring the binding of dNTP to the 3'-ends of DNA fragments and detection by a quantitative colorimetric assay. Control cells showed an absorbance of 0.219±0.017 (means±SEM of 6 replicates values), whilst exposure to 500 µM SNAP for 24 hours increased the absorbance about twofold (Fig. 3B). β-Carbolines and R-(-)-deprenyl inhibited the SNAP-induced increase in absorbance, while absorbance in cells treated with β-carbolines alone was not significantly different from that in control cells.
2. Effect of β-carbolines on SNAP-induced change in mitochondrial membrane permeability and caspase-3 activation
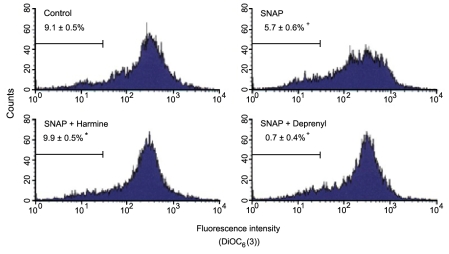
The cytotoxic effect of SNAP was assessed by measuring the effect on the mitochondrial transmembrane potential. Change in the mitochondrial transmembrane potential in PC12 cells treated with SNAP was quantified by flow cytometry with the dye DiOC6(3). When PC12 cells were treated with SNAP, the increase in the percentage of cells with depolarized mitochondria (characterized by low values of the transmembrane potential) was observed. Harmine and R-(-)-deprenyl inhibited the SNAP-induced increase in cells with depolarized mitochondria, while the mitochondrial transmembrane potential in cells treated with compounds alone was similar to that in the control (Fig. 4).
Figure 4.
Effect of harmine and R-(-)-deprenyl on loss of the mitochondrial transmembrane potential due to SNAP. PC12 cells were treated with 500 µM SNAP in the presence of 25 µM harmine for 24 hours and mixtures were treated with 40 nM DiOC6(3). Data represent means±SEM of the percentage-depolarized cells in three independent experiments. †P<0.05, compared to control. *P<0.05, compared to SNAP alone.
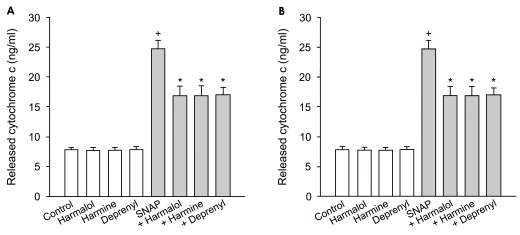
The SNAP-induced change in mitochondrial membrane permeability was assessed by measuring the cytochrome c, released into the cytosol. Treatment of PC12 cells with SNAP showed a 3.4-fold increase in the cytosolic cytochrome c (Fig. 5A). Harmalol, harmine and R-(-)-deprenyl significantly inhibited the SNAP-induced release of cytochrome c, while β-carbolines alone did not significantly affect cytochrome c release. The activation of caspase-3, which appears to be mediated by the cytochrome c accumulation, was assessed in PC12 cells treated with SNAP. PC12 cells treated with SNAP showed a significant increase in caspase-3 activity, which was depressed by β-carbolines and R-(-)-deprenyl (Fig. 5B). The compounds alone did not significantly affect the caspase-3 activity in PC12 cells.
Figure 5.
Effect of β-carbolines on cytochrome c release and caspase-3 activation. PC12 cells were treated with 500 µM SNAP in the presence of β-carbolines or R-(-)-deprenyl (each 25 µM) for 24 hours. Data are expressed as nanograms/ml for cytochrome c release (A) and units for caspase-3 activity (B). The values represent means±SEM of 4-6 replicate values in two separated experiments. †P<0.05, compared to control. *P<0.05, compared to SNAP alone.
3. Effect of R-(-)-deprenyl and β-carbolines on formation of ROS and decrease in GSH contents due to SNAP
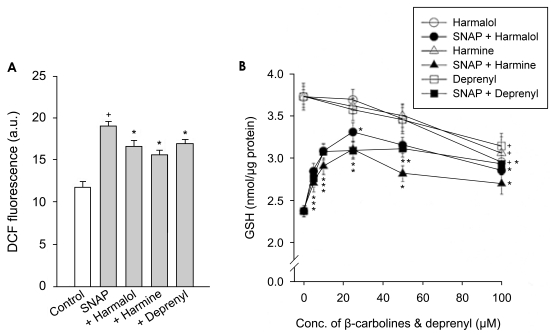
The involvement of ROS in the SNAP-induced cell death in PC12 cells was determined by monitoring a conversion of DCFH2-DA to DCF. PC12 cells exposed to SNAP caused a significant increase in DCF fluorescence. Harmalol, harmine and R-(-)-deprenyl depressed the increase in DCF fluorescence due to SNAP (Fig. 6A).
Figure 6.
Effect of β-carbolines and R-(-)-deprenyl on ROS formation and GSH depletion. PC12 cells were treated with 500 µM of SNAP in the presence of 5-100 µM β-carbolines (harmalol and harmine) or R-(-)-deprenyl for 24 hours. The values are expressed as arbitrary units of fluorescence for ROS formation (A) and nmol for GSH contents (B). Data represent means±SEM of 5-6 replicate values in two separated experiments. †P<0.05, compared to control. *P<0.05, compared to SNAP alone.
We investigated the effect of β-carbolines on the decrease in GSH contents due to SNAP. The thiol content in the control PC12 cells was 3.73±0.12 nmol/µg protein. Addition of SNAP decreased the GSH contents in cells by 36%. As observed in cell death, β-carbolines and R-(-)-deprenyl differentially inhibited the SNAP-induced GSH depletion depending on concentration. β-Carbolines and R-(-)-deprenyl had the maximum inhibitory effect on GSH depletion at 25 and 50 µM, respectively; beyond these concentrations the inhibitory effect declined (Fig. 6B). The compounds alone at 100 µM for 24 hours significantly decreased the GSH contents in cells by 16~18%.
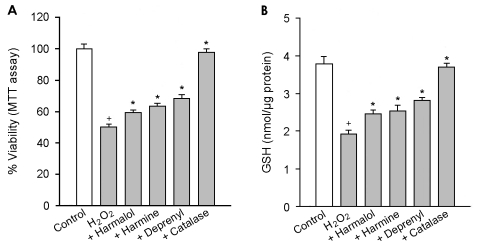
To investigate further the effect of β-carbolines on the ROS-induced cell death, we examined their effects against cell death due to a cell-permeable H2O2. β-Carbolines and 10 µg/ml catalase (a scavenger of hydrogen peroxide) significantly attenuated the H2O2-induced cell death and depletion of GSH. The inhibitory effect of β-carbolines on the cytotoxicity of H2O2 was less than that of R-(-)-deprenyl (Fig. 7).
Figure 7.
Effect of β-carbolines and R-(-)-deprenyl on the H2O2-induced cell death and GSH depletion. PC12 cells were treated with 100 µM of H2O2 in the presence of compounds (25 µM β-carbolines, 25 µM R-(-)-deprenyl and 10 µg/ml catalase) for 24 hours. Data are expressed as the percentage of control for cell viability and nmol/µg protein for the GSH contents. The values represent means±SEM of 6 replicate values in two separate experiments. †P<0.05, compared to control. *P<0.05, compared to H2O2 alone.
DISCUSSION
Opening of the mitochondrial permeability transition pore cause depolarization of the transmembrane potential, release of Ca2+ and cytochrome c, and loss of oxidative phosphorylation, which results in the loss of cell viability.29 Apoptotic cell death is suggested to be mediated by interaction of ligand with cell surface CD95 receptor followed by caspase-8 activation or by mitochondrial dysfunction, which results in the release of cytochrome c and subsequent activation of caspase-9 and -3.1,29 One of the aims of this study was to elucidate cytotoxicity of SNAP in relation to the mitochondrial membrane permeability transition, leading to cell death. In the present study, the inhibitory effect of specific caspase inhibitors (z-DQMD and z-LEHD.fmk) suggests that SNAP induces apoptotic cell death in differentiated PC12 cells by causing mitochondrial dysfunction, leading to activation of caspase-9 and -3. The fragmentation of nuclei and a significant increase in caspase-3 activity further indicated apoptotic death of PC12 cells due to SNAP.
NO and ONOO- inhibit the mitochondrial respiratory chain, leading to energy failure and induce the mitochondrial membrane permeability transition, which result in neuronal cell death.8,10 Mitochondrial damage, followed by the release of cytochrome c and subsequent casapse-3 activation, were observed in PC12 cells treated with SNAP. In PC12 cells depleted of GSH, the increased formation of ROS and a subsequent rise of Ca2+ level may cause a cell death.30 Therefore, the present study suggests that change in the mitochondrial membrane permeability due to SNAP in PC12 cells may be mediated by the enhanced ROS formation and reduction in GSH contents. This suggestion could be supported by the inhibitory effect of various antioxidants on cell death and depletion of GSH.
Indole β-carbolines are found endogenously in mammalian tissues, including the central nervous system, liver and platelets and in plasma and urine.31,32 Indole β-carbolines and their precursors are suggested to be involved in idiopathic Parkinson's disease.16 It has been shown that the concentrations of β-carbolines and their N-methylated cations localized in the substantia nigra of the nonparkinsonian human brains were higher than that in the cortex.33 The contents of β-carbolines and β-carbolinium cations in the lumbar cerebrospinal fluid of patients with Parkinson's disease (norharmane, 331±48 nmol; harmane, 206±66 nmol) were 1.9 times higher than that in the nonparkinsonian patients.33 However, in contrast to the low level of endogenous β-carbolines, the intraperitoneal administration of high concentrations (0.5 mmol/kg of norharman or 0.25 mmol/kg of 9-mono-N'-methylnorharman) is found to induce parkinsonism in C57BL/6 mice.34 2-Methylharmaline at 232 µM of IC50 inhibits the NAD+-linked respiration in isolated liver mitochondria.15 Harmaline (40~75 mg/kg, approximately 150~280 µM) injected in rats induces selective cerebellar neurodegeneration.18 Therefore, the results indicate that in vivo and in vitro studies, high concentrations of β-carboline precursors and metabolites are necessary to show a toxic effect. In this study, β-carbolines and R-(-)-deprenyl at 5~50 µM did not significantly cause cell death in differentiated PC12 cells, while the compounds at 100 µM showed a cytotoxic effect.
Indole β-carbolines inhibit the catecholamines- or glutamate-induced cell death in PC12 cells and HT-22 hippocampal cells.21,22 However, it has not been elucidated whether β-carbolines exert a protective effect on neuronal cells against toxicity of RNS. The concentrations of β-carbolines used in the present study were based on the previous reports.19,21,22 The present results suggest that β-carbolines (harmalol and harmine) and R-(-)-deprenyl reduce the SNAP-induced cell death in differentiated PC12 cells by suppressing the loss of mitochondrial transmembrane potential, cytochrome c release and subsequent caspase-3 activation. The enhanced ROS formation and GSH depletion has considered to induce change in the mitochondrial membrane permeability. Therefore, the inhibitory effect of β-carbolines and R-(-)-deprenyl on the mitochondrial membrane permeability change due to SNAP may be accomplished by inhibition of ROS formation and recovery of GSH contents. Compare to R-(-)-deprenyl, the inhibitory effect of β-carbolines was accomplished by its defending action on the toxicity of nitrogen species rather than ROS. The present study showed that 25 µM harmaline and harmalol did not exhibit a significant cytotoxicity on differentiated PC12 cells and showed a maximum inhibitory effect against the toxicity of SNAP. Indole β-carbolines are distributed wide in medicinal plants35 and found in a variety of foods: grain flower, soy sauce, milk, beer and wine.36,37 Despite the protective effect, the inhibitory effect of β-carbolines against the cytotoxicity of SNAP appears to be decreased by the cytotoxic effect, occurs in the concentrations more than 100 µM. Although the level of indole β-carbolines in the body may be affected by the intake of foods containing β-carbolines precursors, it uncertain how much of β-carbolines and toxic metabolite β-carboliniums, are produced. Therefore, the study for the evaluation of change in the β-carboline levels according to the intake is required. In addition, for clinical application as neuroprotective agents, the detection and synthesis of the analogues to indole β-carbolines, which have a wide margin of safety, are necessary.
Deprenyl appears to exert a neuroprotective effect on Parkinson's disease through a selective inhibition of MAO-B.12 Deprenyl at high concentrations inhibits MAO-A as well as MAO-B.13 However, it has been shown that deprenyl may exert a protective effect on neuronal cells against a variety of insults by a mechanism that does not involve the inhibition of MAO.13,14 Therefore, although β-carbolines show a non-selective inhibition on mitochondrial MAO, these compounds appear to depress the toxicity of SNAP against PC12 cells without intervention of MAO inhibition.
Overall, therefore, we conclude that β-carbolines may reduce the nitrogen species-induced viability loss in PC12 cells by attenuation of the mitochondrial membrane permeability change that seems to be mediated by free radicals. β-Carbolines appear to exhibit a neuroprotective effect against the nitrogen species-mediated neuronal cell injury in Parkinson's disease comparable to R-(-)-deprenyl.
References
- 1.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29:323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 2.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003;53(suppl 3):S26–S38. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- 3.Lee CS, Song EH, Park SY, Han ES. Combined effect of dopamine and MPP+ on membrane permeability in mitochondria and cell viability in PC12 cells. Neurochem Int. 2003;43:147–154. doi: 10.1016/s0197-0186(02)00214-0. [DOI] [PubMed] [Google Scholar]
- 4.Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y, et al. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience. 1996;72:355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- 5.Good PF, Hsu A, Wener P, Perl DP, Olanow CW. Protein nitration in Parkinson's disease. J Neuropathol Exp Neurol. 1998;57:338–342. doi: 10.1097/00005072-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Hantraye P, Brouillet E, Ferrante R, Palfi S, Dolan R, Matthews RT, et al. Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat Med. 1996;2:1017–1021. doi: 10.1038/nm0996-1017. [DOI] [PubMed] [Google Scholar]
- 7.Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide and superoxide. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 8.Stewart VC, Heales SJR. Nitric oxide-induced mitochondrial dysfunction: implications for neurodegeneration. Free Radic Biol Med. 2003;34:287–303. doi: 10.1016/s0891-5849(02)01327-8. [DOI] [PubMed] [Google Scholar]
- 9.Ghafourifar P, Schenk U, Klein SD, Richter C. Mitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formation. J Biol Chem. 1999;274:31185–31188. doi: 10.1074/jbc.274.44.31185. [DOI] [PubMed] [Google Scholar]
- 10.Bal-Price A, Brown GC. Nitric-oxide-induced necrosis and apoptosis in PC12 cells mediated by mitochondria. J Neurochem. 2000;75:1455–1464. doi: 10.1046/j.1471-4159.2000.0751455.x. [DOI] [PubMed] [Google Scholar]
- 11.Kadota T, Yamaai T, Saito Y, Akita Y, Kawashima S, Moroi K, et al. Expression of dopamine transporter at the tips of growing neurites of PC12 cells. J Histochem Cytochem. 1996;44:989–996. doi: 10.1177/44.9.8773564. [DOI] [PubMed] [Google Scholar]
- 12.Birkmayer W, Knoll J, Riederer P, Youdim MB, Hars V, Marton J. Increased life expectancy resulting from addition of L-deprenyl to Madopar treatment in Parkinson's disease: a longterm study. J Neural Transm. 1985;64:113–127. doi: 10.1007/BF01245973. [DOI] [PubMed] [Google Scholar]
- 13.Tatton WG, Chalmers-Redman RM. Modulation of gene expression rather than monoamine oxidase inhibition: (-)-deprenyl-related compounds in controlling neurodegeneration. Neurology. 1996;47:S171–S183. doi: 10.1212/wnl.47.6_suppl_3.171s. [DOI] [PubMed] [Google Scholar]
- 14.Wu RM, Chen RC, Chiueh CC. Effect of MAO-B inhibitors on MPP+ toxicity in Vivo. Ann N Y Acad Sci. 2000;899:255–261. doi: 10.1111/j.1749-6632.2000.tb06191.x. [DOI] [PubMed] [Google Scholar]
- 15.Albores R, Neafsey EJ, Drucker G, Fields JZ, Collins MA. Mitochondrial respiratory inhibition by N-methylated β-carboline derivatives structurally resembling N-methyl-4-phenylpyridine. Proc Natl Acad Sci USA. 1990;87:9368–9372. doi: 10.1073/pnas.87.23.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gearhart DA, Toole PF, Beach JW. Identification of brain proteins that interact with 2-methylnorharman. An analog of the parkinsonian-inducing toxin, MPP+ Neurosci Res. 2002;44:255–265. doi: 10.1016/s0168-0102(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 17.Cobuzzi RJ, Jr, Neafsey EJ, Collins MA. Differential cytotoxicities of N-methyl-β-carbolinium analogues of MPP+ in PC12 cells: insights into potential neurotoxicants in Parkinson's disease. J Neurochem. 1994;62:1503–1510. doi: 10.1046/j.1471-4159.1994.62041503.x. [DOI] [PubMed] [Google Scholar]
- 18.O'Hearn E, Molliver ME. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience. 1993;55:303–310. doi: 10.1016/0306-4522(93)90500-f. [DOI] [PubMed] [Google Scholar]
- 19.Tse SYH, Mak IT, Dickens BF. Antioxidative properties of harman and β-carboline alkaloids. Biochem Pharmacol. 1991;42:459–464. doi: 10.1016/0006-2952(91)90305-o. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez de Arriva A, Lizcano JM, Balsa MD, Unzeta M. Inhibition of monoamine oxidase from bovine retina by β-carbolines. J Pharm Pharmacol. 1994;46:809–813. doi: 10.1111/j.2042-7158.1994.tb03735.x. [DOI] [PubMed] [Google Scholar]
- 21.Maher P, Davis JB. The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci. 1996;16:6394–6401. doi: 10.1523/JNEUROSCI.16-20-06394.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Jang YY, Han ES, Lee CS. Protective effect of harmaline and harmalol against dopamine- and 6-hydroxydopamine-induced oxidative damage of brain mitochondria and synaptosomes, and viability loss of PC12 cells. Eur J Neurosci. 2001;13:1861–1872. doi: 10.1046/j.0953-816x.2001.01563.x. [DOI] [PubMed] [Google Scholar]
- 23.Tatton WG, Chalmers-Redman RME, Ju WJH, Mammen M, Carlile GW, Pong AW, et al. Propargylamines induce antiapoptotic new protein synthesis in serum-and nerve growth factor (NGF)-withdrawn, NGF-differentiated PC-12 cells. J Pharmacol Exp Ther. 2002;301:753–764. doi: 10.1124/jpet.301.2.753. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Oberhammer FA, Pavelka M, Sharma S, Tiefenbacher R, Purchio AF, Bursch W, et al. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor β1. Proc Natl Acad Sci USA. 1992;89:5408–5412. doi: 10.1073/pnas.89.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lizard G, Miguet C, Bessede G, Monier S, Gueldry S, Neel D, et al. Impairment with various antioxidants of the loss of mitochondrial transmembrane potential and of the cytosolic release of cytochrome c occurring during 7-ketocholesterol-induced apoptosis. Free Radic Biol Med. 2000;28:743–753. doi: 10.1016/s0891-5849(00)00163-5. [DOI] [PubMed] [Google Scholar]
- 27.Fu W, Luo H, Parthasarathy S, Mattson MP. Catecholamines potentiate amyloid β-peptide neurotoxicity: involvement of oxidative stress, mitochondrial dysfunction, and perturbed calcium homeostasis. Neurobiol Dis. 1998;5:229–243. doi: 10.1006/nbdi.1998.0192. [DOI] [PubMed] [Google Scholar]
- 28.van Klaveren RJ, Hoet PH, Pype JL, Demedts M, Nemery B. Increase in gamma-glutamyltransferase by glutathione depletion in rat type II pneumocytes. Free Radic Biol Med. 1997;22:525–534. doi: 10.1016/s0891-5849(96)00375-9. [DOI] [PubMed] [Google Scholar]
- 29.Polster BM, Fiskum G. Mitochondrial mechanisms of neuronal cell apoptosis. J Neurochem. 2004;90:1281–1289. doi: 10.1111/j.1471-4159.2004.02572.x. [DOI] [PubMed] [Google Scholar]
- 30.Jurma OP, Hom DG, Andersen JK. Decreased glutathione results in calcium-mediated cell death in PC12. Free Radic Biol Med. 1997;23:1055–1066. doi: 10.1016/s0891-5849(97)00134-2. [DOI] [PubMed] [Google Scholar]
- 31.Airaksinen MM, Kari I. β-Carbolines, psychoactive compounds in the mammalian body. Part I; Occurrence, origin and metabolism. Med Biol. 1981;59:21–34. [PubMed] [Google Scholar]
- 32.Beck O, Faull KF. Concentrations of the enantiomers of 5-hydroxymethtryptoline in mammalian urine: implications for in vivo biosynthesis. Biochem Pharmacol. 1986;35:2636–2639. doi: 10.1016/0006-2952(86)90067-5. [DOI] [PubMed] [Google Scholar]
- 33.Matsubara K, Kobayashi S, Kobayashi Y, Yamashita K, Koide H, Hatta M, et al. β-Carbolinium cations, endogenous MPP+ analogs, in the lumbar cerebrospinal fluid of patients with Parkinson's disease. Neurology. 1995;45:2240–2245. doi: 10.1212/wnl.45.12.2240. [DOI] [PubMed] [Google Scholar]
- 34.Matsubara K, Gonda T, Sawada H, Uezono T, Kobayashi Y, Kawamura T, et al. Endogenously occurring β-carboline induces parkinsonism in nonprimate animals: a possible causative protoxin in idiopathic Parkinson's disease. J Neurochem. 1998;70:727–735. doi: 10.1046/j.1471-4159.1998.70020727.x. [DOI] [PubMed] [Google Scholar]
- 35.Holmstedt B, Lingren JE. Chemical constituents and pharmacology of south American snuffs. In: Efron DH, Holmstdet B, Kline NS, editors. Ethnopharmacologic search for psychoactive drugs. New York: Raven Press; 1967. pp. 339–373. [Google Scholar]
- 36.Ogawa Y, Adachi J, Tatsuno Y. Accumulation of 1-methyl-tetrahydro-β-carboline-3-carboxylic acid in blood and organs of rat. A possible causative substance of eosinophilia-myalgia syndrome associated with ingestion of L-tryptophan. Arch Toxicol. 1993;67:290–293. doi: 10.1007/BF01974349. [DOI] [PubMed] [Google Scholar]
- 37.Pari K, Sundari CS, Chandani S, Balasubramanian D. β-Carbolines that accumulate in human tissues may serve a protective role against oxidative stress. J Biol Chem. 2000;275:2455–2462. doi: 10.1074/jbc.275.4.2455. [DOI] [PubMed] [Google Scholar]