Abstract
Atherothromboembolism and intracranial small vessel disease are considered to be the main causes of cerebrovascular injury, which may lead to cognitive impairment and vascular dementia (VaD). VaD appears to be the second most common type of dementia with prevalence estimates of 10-15%. Cortical or multi-infarct dementia and subcortical vascular dementia (SVD) are suggested to be the two main forms of VaD. The main clinical features of SVD comprise decreased motor performance, early impairment of attention and executive function with slowing of information processing. SVD results from lacunar infarcts or multiple microinfarcts in the basal ganglia, thalamus, brainstem and white matter and are associated with more than 50% of the VaD cases. White matter changes including regions of incomplete infarction are usually widespread in VaD but their contribution to impairment of subcortical regions is unclear. While most of VaD occurs sporadically only a small proportion of cases bear clear familial traits. CADASIL is likely the most common form of hereditary VaD, which arises from subcortical arteriopathy. SVD needs unambiguous definition to impact on preventative and treatment strategies, and critical for selective recruitment to clinical trials.
Keywords: Cerebrovascular disease, CADASIL, Cognitive impairment, Dementia, Ischaemia, Lacunes, Small vessel disease, Stroke
INTRODUCTION
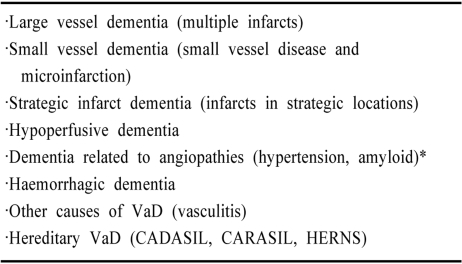
About a century ago Alois Alzheimer and Emil Kraeplin among other neuropsychiatrists had ascribed arteriosclerotic dementia resulting from gradual strangulation of the blood supply to the brain as the main cause of dementia.1 Otto Binswanger should be recognized for introducing the notion of subclasses of vascular dementia (VaD). He described subcortical arteriosclerotic encephalopathy upon pathological verification of cerebral white matter disorder in a group of eight patients.1 Currently we accept that cerebrovascular disease causes the second most common form of age-related dementia. VaD can, however, result from all forms of cerebrovascular lesions that include post-stroke syndromes.2 The challenge of defining the pathological substrates of VaD is complicated by the heterogeneous nature of cerebrovascular disease and co-existence of other pathologies including Alzheimer type of lesions. Blood vessel size, origin of vascular occlusion and genetic factors are critical factors in defining subtypes of VaD (Table 1). Multi-infarct dementia is caused by large vessel disease whereas Binswanger type of VaD involving subcortical regions including the white matter results from small vessel changes. Subcortical ischaemic VaD appears the most significant subtype of VaD.3 Other factors that may define the subtype and degree of impairment include multiplicity, size, anatomical location, laterality and age of the lesion. For the purposes of prevention strategies and treatments it is imperative to recognise subtypes of VaD yet not devise numerous categories to make the task unnecessarily complicated.4
Table 1.
VaD subtypes defined by blood vessel size and pathological process
Hereditary forms of cerebral amyloid angiopathy involving ischaemic strokes and intracerebral haemorrhages may also lead to cognitive impairment and stroke.
CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CARASIL, cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopahty; HERNS, hereditary endotheliopathy, retinopathy, nephropathy and stroke.77
DIAGNOSTIC CRITERIA: VaD VERSUS MIXED DEMENTIA
There is now a general consensus that the clinical diagnosis of VaD in demented patients with evidence of cerebrovascular lesions applies only when other causes of dementia are ruled out.5 The diagnosis of VaD continues to be a challenge in the face of pathological outcomes (Fig. 1). As with diagnosis of other causes of dementia criteria may be derived from a range of investigations including a detailed clinical history, timing of event, neuropsychometric tests, neuroimaging and neuropathological reports in accord with the DSM criteria. Since the 1970s several clinical criteria for VaD have been put forward.5-7 Historically all the criteria have largely adopted the Alzheimer type disease model despite the fact that vascular cognitive impairment (VCI) or VaD is a cluster of clinical syndromes related to complex interactions between cerebrovascular disease (CVD), vascular risk factors, brain changes and host factors. The widely used criteria for VaD include the DSM-IV,8 the ICD-10,9 the ADDTC criteria10 and the NINDS-AIREN criteria.4 The two cardinal elements implemented in the clinical criteria for VaD are the nature of the cognitive syndrome11 and the type of vascular injury in the cause of the dementia.5,7,12 Variation in definitions of these two elements has resulted in different prevalence estimates, vulnerable groups of subjects and consequently different types and distribution of brain lesions7,11,13-15
Figure 1.
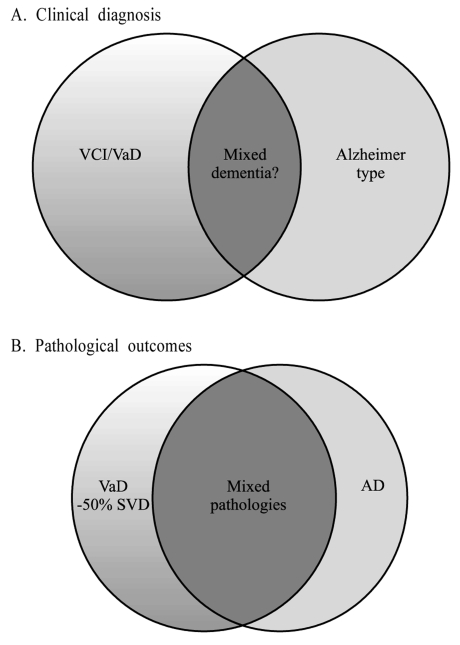
Overlap of VaD and AD. Clinical diagnosis (A) criteria predict overlap of 20-30% whereas autopsy findings (B) suggest a greater overlap where cases with mixed pathologies including cerebrovascular lesions and Alzheimer pathology up to Braak stage V are frequent. Several studies suggest this to be particularly true in the oldest old (i.e. >85 years old).
The NINDS-AIREN criteria4 are the most widely used in randomised clinical trials. These criteria adequately (1) emphasise the heterogeneity of VaD syndromes and pathologic subtypes including not only ischaemic stroke but also other causes of CVD such as cerebral hypoxicischaemic events, white matter lesions (WMLs) and hemorrhagic strokes, (2) recognise the variability in clinical course, which may be static, remitting or progressive, (3) highlight the location of ischaemic lesions and the need to establish a causal relationship between vascular brain lesions and cognition, (4) recognise the need to establish a temporal relationship between stroke and dementia onset, (5) include specific findings early in the course that support a vascular rather than a degenerative cause, (6) emphasise the importance of brain imaging to support clinical findings, and (7) recognise the value of neuropsychological testing to document impairment in multiple cognitive domains. Thus the NINDS-AIREN criteria define VaD as a syndrome with different aetiologies and clinical manifestations rather than a single entity. The recognition of subtypes are potentially important for clinical trials. These criteria also incorporate three different levels of certainty of clinical diagnosis, namely probable, possible and definite.4
In a previous neuropathological series, the sensitivity of the NINDS-AIREN criteria for probable and possible VaD was determined to be 58% and the specificity 80%.16 The criteria successfully excluded AD in 91% of cases, and the proportion of mixed cases misclassified as probable VaD was 29%. Interestingly, compared to the ADDTC criteria,10 the NINDS-AIREN criteria were more specific and better in excluding mixed cases (54% vs. 29%). Despite these limitations, the frequencies of VaD in autopsy series have been reported to be as low as 0.03% and as high as 58% with an overall mean rate of 17%17,18 that is consistent with estimates from clinical studies. In cases where currently accepted criteria have been applied the frequencies are even lower with a mean of 7%. Whereas the incidence of VaD cases in Japan was reported to be 35%19 and 22%.20 Taking these into account the worldwide frequency of VaD in autopsy verified cases might be estimated to be between 10 and 15%.
Clinical and pathological evidence indicates combined neurodegenerative and vascular pathologies worsen presentation and outcome of dementia.21-24 A high proportion of individuals fulfilling the neuropathological diagnosis of AD have significant cerebrovascular lesions including silent infarcts upon imaging and extensive white matter disease.25-28 Conversely, clinically diagnosed VaD patients frequently show extensive AD-type neuropathological changes.28-30 For example, in one study31 87% of the patients clinically diagnosed by NINDS-AIREN criteria were found to have AD either alone (58%) or in combination with cerebrovascular disease (42%) at post-mortem (Fig. 1). Hulette et al30 noted that "pure" VaD is very uncommon because VaD cases without co-existing neuropathological evidence of AD are rarely found. Therefore current clinical diagnostic criteria serve to detect pathology but not "pure" pathology. Early validation studies indicated that while mixed dementia could be distinguished from AD, it could not be separated from VaD.32 Recent studies suggest that 30-50% of mixed AD and VaD cases are misclassified as VaD16,33 and the neuroimaging component of the NINDS-AIREN criteria does not distinguish between people with and without dementia in the context of cerebrovascular disease. The potential overlap of pathologies is therefore complex, with different types of cerebrovascular lesions including subcortical infarction and small vessel disease,22,34 and accompanying neurodegenerative changes including tau, amyloid and α-synuclein pathology (Fig. 1). While evidence-based pathological criteria for the diagnosis of mixed dementia remains to be perfected, the diagnosis35,36 should be made when a primary neurodegenerative disease known to cause dementia exists with one or more of the pathological lesions defining the VaD subtypes (Table 1). The diagnosis of mixed dementia (AD and VaD or less frequently dementia with Lewy bodies and VaD) particularly among the oldest old (>85 years of age) therefore also remains a challenge.
TYPES OF LESIONS IN VaD
Atherothromboembolism may be responsible for up to 50% of all ischaemic strokes involving both cortical and subcortical infarctions. Intracranial small vessel disease causes 25% of the infarcts.37 Small vessel alternations involve arteriosclerosis, hyalionsis and are associated with lacunar infarcts and lacunes predominantly occurring in the subcortical structures. White matter disease or subcortical leukoencephalopathy with incomplete infarction and small vessel disease are common pathological changes in cerebrovascular disease. Others features include borderzone (watershed) infarctions, laminar necrosis and amyloid angiopathy (Table 2). Complicated angiopathies such as fibromuscular dysplasia, arterial dissections, granulomatous angiitis, collagen vascular disease and giant-cell arteritis are rarer causes of cerebrovascular disease37 and VaD.
Table 2.
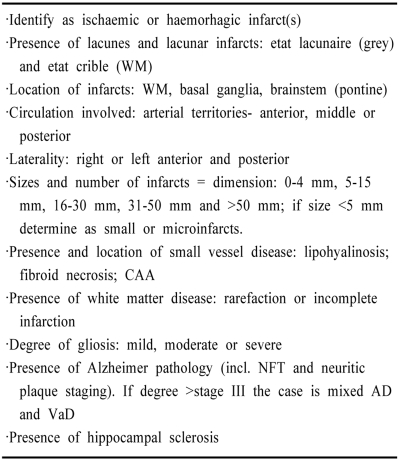
Key variables to define pathology of SV Dementia
For reporting purposes each of above features can be scored numerically to provide a summary. For example, 0 is absent and 1 means present. Less frequent lesions including watershed infarcts and laminar necrosis. Increasing numerical value may also be assigned to the infarcts.
CAA, cerebral amyloid angiopathy; NFT, neurofibrillary tangles; WM, white matter.
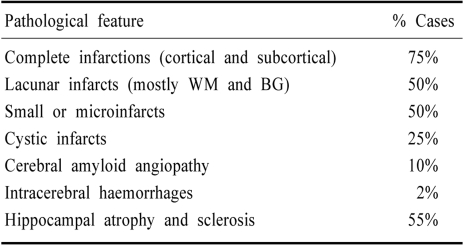
Previous studies have recorded ischaemic, oedematous and haemorrhagic lesions affecting the brain circulation or perfusion to be associated with VaD (Table 3). In four studies where VaD was diagnosed 75% of the cases revealed cortical and subcortical infarcts suggesting other vascular pathologies involving incomplete infarction or borderzone infarcts could be important factors. Among other lesions 25% of the cases had cystic infarcts whereas 50% of the cases had lacunar infarcts or microinfarcts. Lacunar infarcts, however, appear to be a common category of infarcts, and currently recognised as the most frequent cause of stroke (dissections, granulomatous angiitis, collagen vascular disease and giant-cell arteritis are rarer causes of cerebrovascular disease).37 Severe amyloid angiopathy was present in 10% of the cases. Most interestingly, in one study 55% of the cases revealed hippocampal atrophy.38 Ischaemic vascular disease appears to correlate with widespread small ischaemic lesions distributed throughout the CNS.38
Table 3.
Types of vascular and hippocampal lesions reported in VaD
Data compiled from 70 cases reported in previous studies22,30,34,38 (RN Kalaria et al., unpublished observations). % cases are averaged from 2 or more reported studies. Cystic infarcts (possibly also lacunar) with typically ragged edges were admixed in both cortical and subcortical structures. BG, basal ganglia; WM, white matter.
SUBTYPES OF VaD AND SUBCORTICAL VASCULAR SYNDROME
The main subtypes of VaD included in current classifications are cortical VaD or MID (also referred as post-stroke VaD) and subcortical vascular dementia (SVD) or small vessel disease related dementia.4,39-42 Strategic infarct dementia, hypoperfusive dementia resulting from global cerebrovascular insufficiency are less common types.4,39,41,43 Further, subtypes include hemorrhagic dementia and hereditary vascular dementias. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy or CADASIL is probably the most common form of familial SVD.
Not surprisingly, the clinical presentation of cortical and subcortical forms of VaD show remarkable differences. Large vessel occlusions resulting in large cortical infarcts produce cognitive and other deficits that depend on the location of the infarcts, while SVD may have a relatively characteristic neuropsychological profile that includes early impairment of attention and executive function, with slowing of motor performance and information processing.2,3,44
Clinically, SVD is characterised by pure motor hemiparesis, bulbar signs and dysarthria, gait disorder, variable depressive illness, emotional lability, and deficits in executive functioning.45-49 The early clinical phase of SVD includes episodes of mild upper motor neuron signs (drift, reflex asymmetry, incordination), gait disorder (apractic-atactic or small-stepped), imbalance and falls, urinary frequency and incontinence, dysarthria, dysphagia as well as extrapyramidal signs such as hypokinesia and rigidity.44,47,48,50 However, these focal neurological signs are often subtle.51,52 Upon imaging patients with SVD have multiple lacunes and extensive WMLs and often reveal clinical history of "prolonged TIA" or "multiple TIAs" which mostly are small strokes without residual symptoms and only mild focal findings (e.g. drift, reflex asymmetry, gait disturbance). This supports the importance of neuroimaging requirements in the criteria.44
The early cognitive syndrome of SVD is characterized by a dysexecutive syndrome with slowed information processing, usually mild memory deficit and behavioural symptoms.3 The dysexecutive syndrome includes impairment in goal formulation, initiation, planning, organising, sequencing, executing, set-shifting and set-maintenance, as well as in abstraction.40,47,53 The memory deficit in SVD is usually milder than in AD, and is specified by impaired recall, relative intact recognition, less severe forgetting and better benefit from cues.53 Episodic memory may be relatively spared, compared with AD. There is more learning impairment that can be partially corrected by providing salient cues to encourage learning and promote recognition.54 Therefore, memory deficits in VaD appear to be caused by problems in retrieval of information;55-57 in turn, memory retrieval deficits are due to aberrant frontal and subcortical mechanisms. In contrast, in AD involvement of the hippocampus by neurofibrillary tangles (Braak Stage III) prevents storage of new information causing amnesic mild cognitive impairment. The SVD syndrome may be readily confused with AD in view of the neuronal loss and coexisting vascular factors. The onset can be variable as suggested previously.45 Sixty percent of the patients had a slow, less abrupt onset, and only 30% an acute onset of cognitive symptoms. The course was gradual without (40%) and with (40%) acute deficits, and fluctuating in only 20%.45
Behavioural and psychological symptoms in SVD include depression, personality change, emotional lability, loss of volition (apathy),42 incontinence, as well as inertia, emotional bluntness and psychomotor retardation.4,40,47 These may include greater tendencies of aggression and agitation.2 Such symptomatology is attributed to damage to the prefrontal subcortical circuits.44,58
PATHOLOGICAL LESIONS IN SVD
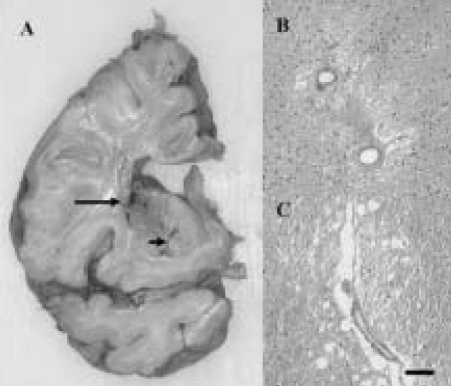
SVD incorporates two entities "the lacunar state" and "Binswanger's disease."3 SVD is accredited to small vessel disease and is characterised by lacunar infarcts, focal and diffuse ischaemic WMLs, and incomplete ischaemic injury.47-50,59 The infarcts and WMLs are expected consequences of small vessel disease. Small vessels including intracerebral end-arteries and arterioles undergo progressive age-related changes,59,60 which may result in lacunar or microinfarcts. These range from wall thickening by hyalinosis, reduction or increment of the intima to severe arteriosclerosis and fibroid necrosis (Fig. 2). Uncomplicated hyalinosis is characterised by almost complete degeneration of vascular smooth muscle cells (becomes acellular) with concentric accumulation of extracellular matrix components like the collagens and fibroblasts.60 These changes are most common in the small vasculature of the white matter (Fig. 2-B). Small vessel changes likely promote occlusion or progressive stenosis with consequent acute or chronic ischaemia of the tissue behind it.60 Alternatively, arteriolsclerotic changes located in small vessels in the deep white matter and basal ganglia (lenticulostriate) may loose their elasticity to dilate and constrict in response to variations of systemic blood pressure or loss of auto-regulation. This in turn causes fluctuations in blood flow response and changes in cerebral perfusion. The deep cerebral structures would be rendered most vulnerable because the vessels are end arteries almost devoid of anastomoses. Small vessel pathology could lead also oedema and damage of the blood-brain barrier (BBB) with chronic leakage of fluid and macromolecules in the white matter.61
Figure 2.
Pathological lesions associated with small vessel disease. A small infarct (arrow) and lacunes (arrowhead) in the basal ganglia of a 78 year old man with cognitive impairment. B and C, hyalinsed vessels with perivascular rarefaction and microinfarct in the white matter of a 78 year old man. Moderate gliosis in the surrounding region is also evident in both cases. Magnification Bar: A=2 cm; B, C=50 µm.
Lacunes as complete or cavitating infarcts as defined above, measuring up to 15 mm in diameter, are seen radiologically and upon gross examination at autopsy (Table 2; Fig. 2). These lesions are largely confined to the cerebral white matter and subcortical structures including the thalamus, basic ganglia and brainstem. Most lacunes, remnants of small infarcts, are detected in the cystic or chronic stage with no viable central tissue but could have perifocal regions with incomplete infarction, particularly in the white matter. A few lacunes may represent healed or re-absorbed as minute or petechial haemorrhages. Microlacunes have also been described which essentially should be thought of as large cystic microinfarcts. To distinguish perivascular cavities it has been suggested that lacunes be classed into three subtypes: lacunar infarcts, lacunar haemorrhages and dilated perivascular spaces.63 Lacunar infarcts usually result from progressive small vessel disease manifested as hypertensive angiopathy that may involve stenosis caused by hyalinosis. Small vessel disease in a perforating artery for example, may also reveal regions of incomplete infarction, attenuation or rarefaction usually recognised by pallor upon microscopic examination. However, lacunar lesions can also be caused by infections and neoplasms. Lacunes are associated with small perivascular cavities up to 2 mm in diameter often found in the basal ganglia and the white matter.59,61 Perivascular cavities or empty spaces resulting from distortion or elongation of small arteries collectively referred to as état lacunaire in the grey matter and état crible in the white matter may be numerous in older subjects.62
Microinfarcts have been variably described but widely thought to be small ishaemic lesions visible only upon light microscopy (Fig. 2-A and B). These lesions of up to 5 mm diameter may or may not involve a small vessel at its centre but are foci with pallor, neuronal loss, axonal damage (white matter) and gliosis. Sometimes these may include regions of incomplete infarction or rarefied (subacute) change. Microinfarcts have also been described as attenuated lesions of indistinct nature occurring in both cortical or subcortical regions. Microinfarcts and lacunar infarcts appear central to the most common cause of VaD and predict poor outcome in the elderly.37,38,63 Interestingly, in the autopsied older Japanese-American men the importance of microvascular lesions as a likely explanation for dementia was nearly equal to that of Alzheimer lesions.64 Microinfarction in the subcortical structures has been emphasised as substrate of cognitive impairment, and correlated with increased Alzheimer type of pathology but cortical microinfarcts also appear to contribute to significantly to the progression of cognitive deficits in brain aging.65 Furthermore microinfarcts even in borderzone (watershed) regions may aggravate the degenerative process as indicated by worsening impairment in AD.66 Thus multiple microinfarction appears strongly correlated with dementia indicated by several studies.17
White matter lesions (or subcortical leucoencephalopathy) incorporating myelin loss are considered a consequence of vascular disease. The frequency of white matter changes is increased in patients with cerebrovascular disease and those at risk for vascular disease including arterial hypertension, cardiovascular disease and diabetes mellitus.2 Lesions in the deep white matter have been correlated with dementia including VaD.2,28 Conflicting data with respect to periventricular lesions may depend on definition of the boundaries between the periventricular and deep white matter if the coursing of the fibres are used as markers. Lacunar infracts are produced when the ischaemic damage is focal and of sufficient severity to result in a small area of necrosis whereas diffuse white matter change is considered a form of rarefaction or incomplete infarction where there may be selective damage to some cellular components. While the U-fibres are usually spared white matter disease comprises several patterns of alterations including pallor or swelling of myelin, loss of oligodendrocytes, axons and myelin fibres, cavitations with or without presence of macrophages and areas of reactive astrogliosis,61 where the astrocytic cytoplasm and cell processes may be visible with standard stains. Lesions in the white matter also include spongiosis i.e. vacuolisation of the white matter structures and widening of the perivascular spaces.
Cerebral amyloid angiopathy (CAA) also falls in to the category of small vessel diseases and contributes to the SVD syndrome. Current evidence shows age rather than gender, history of hypertension or other vascular disease to be the strongest risk factor for sporadic occurrence of CAA.67,68 Several familial forms of CAA presenting with ischaemic and haemorrhagic infarcts or oligaemia are perhaps the most studied among the hereditary cerebrovascular disorders.69,70 The clinical features comprise focal neurological signs including spasticity, ataxia, facial paralysis, occasional seizures and cognitive impairment often leading to dementia.71-73 Consideration of the hereditary cerebral haemorrhage with amyloidosis of the Dutch type (HCHWA-D) disease provides certain clues to a link between CAA and stroke. It is thought that the first stroke-like episode triggers multiple cerebral bleeds which may be preceded by diffuse white matter changes that in turn lead to rapid decline of cognitive functions.71,74 The degree of CAA is strongly correlated with the presence or absence of dementia while this was not true for diffuse plaques or neurofibrillary tangles. This suggests CAA alone causes dementia in HCHWA-D,73 a notion likely to be the case in other hereditary amyloid angiopathies.71 However, cognitive impairment is also a consistent feature in sporadic CAA cases in the absence of other pathologies as verified by MRI and neuropathological assessment.69
Ischaemic WMLs associated with lipohyalinosis and narrowing of the lumen of the small perforating arteries and arterioles, which nourish the deep white matter, often occur in AD and are common in VaD. Upon MRI these correspond best with deep with matter hyperintensities, which may increases the likelihood of correct diagnosis.69 In a few studies biopsy proven CAA was associated with extensive diffuse hyperintensities presenting as multifocal non-haemorrhagic leukoariosis.67,71 Pathological studies also supported the robust relationship between CAA and WMLs irrespective of the presence of Alzheimer type of pathology.75,76
CADASIL AND HEREDITARY SMALL VESSEL DEMENTIA
Several early reports suggest the existence of several familial stroke disorders unrelated to atherosclerotic disease. Most of these disorders may be classed as SVD involving small vessels of the subcortical structures.77 CADASIL is perhaps the most common form of these hereditary CVDs leading to cognitive decline and dementia.78-81 It is difficult to estimate the world-wide prevalence of CADASIL but in western countries CADASIL cases occur ~5 in 100,000. There are estimated to be at least 500 CADASIL families worldwide suggesting that this disorder is much more common than familial AD.77 CADASIL begins with migraine as the first symptom in up to 40% of the patients.38,78,79 The age of onset is usually ascribed by the age at which the first ever stroke occurs rather than the migraine attack. CADASIL may be manifest well before the first stroke on the basis of characteristic white matter hyperintensities upon MRI.82,83 Migraine usually with aura may begin even before the age of 10 years, but more commonly during the third decade.38,79 More severe manifestations including TIAs, recurrent strokes and depressive illness follow quickly. Motor deficits, an ataxic hemiparesis, hemianopsia and dysarthria may accompany these principal events. Other features include seizures, incomplete cerebellar ataxia, pseudobulbar palsy and unexplained coma. Neocortical strokes are rare and they usually do not cover a wide territory.83 Large artery infarcts such as those of the middle or posterior cerebral artery are uncommon.77 Dichgans and colleagues80 have suggested that the men are at greater risk for early immobilisation and death. However, smoking and high homocysteine (>15 µmol/l) can increase risk for more strokes and migraine.81 There is not a clear consensus for genotype-phenotype correlations80 and the apolipoprotein E ε4 allele does not influence disease progression81 (RK et al, unpublished observations).
Pathological features include severe arteriopathy with the presence of granular osmiophilic material in the arterial walls of both the brain and systemic organs.84 Loss of brain vascular smooth muscle cells leads to wall thickening and fibrosis in small and medium-sized penetrating arteries.77,84 This would reduce both cerebral blood flow and blood volume in affected white matter with effects on the haemodynamic reserve by decreasing the vasodilatory response. Affected vessels presumably progress to obliteration or thrombose as evident by the appearance of lacunar infarcts, mainly in the basal ganglia and fronto-temporal white matter.84 These pathologies initiate cognitive deficits, which progress to dementia of the subcortical vascular type.
CADASIL is caused by single missense mutations or exon deletions in the Notch 3 gene.85 The gene encodes a type 1 transmembrane protein (Notch 3), which is essential during development and regulating cellular differentiation. In adults Notch 3 appears to be expressed only in vascular smooth muscle cells and may promote cell survival by inhibiting apoptosis but the exact function remains to be elucidated.77 Notch 3 mutations consistently result in either a gain or loss of one (or more odd number of) cysteine residue(s) in one of the 34 epidermal growth factor-like repeats in the amino-terminal region of the molecule.85 It is not entirely clear which step in the Notch signalling pathway leads to the charactersitic vascular pathology of CADASIL. The clinical diagnosis of CADASIL is relied upon positive family history and hyperintense lesions upon T2 MRI particularly in the temporal pole, whereas confirmed by Notch 3 gene screening or the presence of GOM in skin or nerve-muscle biopsies.77
CONCLUSIONS
The heterogeneous nature of cerebrovascular disease compels better understanding of VaD presentation and prevalence. The two main subtypes of the VaD syndrome include large cortical infarction or MID and SVD or small vessel disease related dementia. SVD is clinically characterised by pure motor hemiparesis, bulbar signs and dysarthria, gait disorder, variable depressive illness, emotional lability, and deficits in executive functioning. The pathological features of SVD involve small vessel degeneration, lacunes, multiple microinfarcts in the subcortical structures and white matter lesions extending into the deep white matter. CADASIL is an example of familial SVD that presents with recurrent subcortical strokes and slowly progressing course leading to cognitive impairment and dementia. The SVD syndrome may be readily confused with AD in view of the neuronal loss and coexisting vascular factors. However, the symptomatology in SVD is attributed to damage to the frontal- subcortical neuronal circuits.
Footnotes
Timo Erkinjuntti MD PhD is a visiting professor of Neurology at the Institute for Ageing and Health, Newcastle upon Tyne. Our work has been supported by grants from the Medical Research Council (UK), the EC grant QLK6-CT-1999-02112, the B Overstreet fund of the Alzheimer's Association (USA), the Alzheimer's Research Trust (UK) and the CADASIL Trust (UK).
References
- 1.Berrios GE, Freeman HL. Alzheimer and the dementias. London: Royal Society of Medicine Services; 1991. pp. 69–76. Eponymists in Medcine series. [Google Scholar]
- 2.O'Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 3.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1:426–436. doi: 10.1016/s1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 4.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular Dementia: Diagnostic Criteria for Research Studies. Report of the NINDS-AIREN International Work Group. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 5.Erkinjuntti T. Clinical criteria for vascular dementia: The NINDS-AIREN criteria. Dementia. 1994;5:189–192. doi: 10.1159/000106721. [DOI] [PubMed] [Google Scholar]
- 6.Rockwood K, Parhad I, Hachinski V, Erkinjuntti T, Rewcastle B, Kertesz A, et al. Diagnosis of vascular dementia: Consortium of Canadian Centres for Clinical Cognitive Research consensus statement. Can J Neurol Sci. 1994;21:358–364. doi: 10.1017/s0317167100040968. [DOI] [PubMed] [Google Scholar]
- 7.Wetterling T, Kanitz RD, Borgis KJ. Comparison of different diagnostic criteria for vascular dementia (ADDTC, DSM-IV, ICD-10, NINDS-AIREN) Stroke. 1996;27:30–36. doi: 10.1161/01.str.27.1.30. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4 ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 9.World Health Organization . ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for research. Geneva: WHO; 1993. [Google Scholar]
- 10.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischaemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 11.Erkinjuntti T, Ostbye T, Steenhuis R, Hachinski V. The effect of different diagnostic criteria on the prevalence of dementia. N Engl J Med. 1997;337:1667–1674. doi: 10.1056/NEJM199712043372306. [DOI] [PubMed] [Google Scholar]
- 12.Wetterling T, Kanitz RD, Borgis KJ. The ICD-10 criteria for vascular dementia. Dementia. 1994;5:185–188. doi: 10.1159/000106720. [DOI] [PubMed] [Google Scholar]
- 13.Erkinjuntti T, Bowler JV, DeCarli C, Fazekas F, Inzitari D, O'Brien JT, et al. Imaging of static brain lesions in vascular dementia: implications for clinical trials. Alzheimer Dis Assoc Disord. 1999;13 Suppl 3:S81–S90. [PubMed] [Google Scholar]
- 14.Pohjasvaara T, Erkinjuntti T, Vataja R, Kaste M. Dementia three months after stroke. Baseline frequency and effect of different definitions of dementia in the Helsinki Stroke Aging Memory Study (SAM) cohort. Stroke. 1997;28:785–779. doi: 10.1161/01.str.28.4.785. [DOI] [PubMed] [Google Scholar]
- 15.Skoog I, Nilsson L, Palmertz B, Andreasson LA, Svanborg A. A population-based study of dementia in 85-year-olds. N Engl J Med. 1993;328:153–158. doi: 10.1056/NEJM199301213280301. [DOI] [PubMed] [Google Scholar]
- 16.Gold G, Giannakopoulos P, Montes-Paixao JC, Herrmann FR, Mulligan R, Michel JP, et al. Sensitivity and specificity of newly proposed clinical criteria for possible vascular dementia. Neurology. 1997;49:690–694. doi: 10.1212/wnl.49.3.690. [DOI] [PubMed] [Google Scholar]
- 17.Jellinger KA. Vascular-ischemic dementia: an update. J Neural Transm Suppl. 2002;62:1–23. doi: 10.1007/978-3-7091-6139-5_1. [DOI] [PubMed] [Google Scholar]
- 18.Markesbery W. Vascular dementia. In: Markesbery WR, editor. Neuropathology of dementing disorders. London: Arnold; 1998. pp. 293–311. [Google Scholar]
- 19.Seno H, Ishino H, Inagaki T, Iijima M, Kaku K, Inata T. A neuropathological study of dementia in nursing homes over a 17-year period in Shimane Prefecture, Japan. Gerontology. 1999;45:44–48. doi: 10.1159/000022054. [DOI] [PubMed] [Google Scholar]
- 20.Akatsu H, Takahashi M, Matsukawa N, Ishikawa Y, Kondo N, Sato T, et al. Subtype analysis of neuropathologically diagnosed patients in a Japanese geriatric hospital. J Neurol Sci. 2002;196:63–69. doi: 10.1016/s0022-510x(02)00028-x. [DOI] [PubMed] [Google Scholar]
- 21.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 22.Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiat. 1997;63:749–753. doi: 10.1136/jnnp.63.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossi R, Joachim C, Geroldi C, Combrinck M, Esiri MM, Smith AD, et al. Association between subcortical vascular disease on CT and neuropathological findings. Int J Geriatr Psychiatry. 2004;19:690–695. doi: 10.1002/gps.1144. [DOI] [PubMed] [Google Scholar]
- 24.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 25.Premkumar DR, Cohen DL, Hedera P, Friedland RP, Kalaria RN. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer's disease. Am J Pathol. 1996;148:2083–2095. [PMC free article] [PubMed] [Google Scholar]
- 26.Heyman A, Fillenbaum GG, Welsh-Bohmer KA, Gearing M, Mirra SS, Mohs RC, Peterson BL, Pieper CF. Cerebral infarcts in patients with autopsy-proven Alzheimer's disease. CERAD Part XVIII. Neurology. 1998;51:159–162. doi: 10.1212/wnl.51.1.159. [DOI] [PubMed] [Google Scholar]
- 27.Heyman A, Fillenbaum GG, Welsh-Bohmer KA, Gearing M, Mirra SS, Mohs RC, et al. Comparison of pathological diagnostic criteria for Alzheimer disease. Alzheimer Dis Assoc Disord. 1998;12:182–189. doi: 10.1097/00002093-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999;13:S115–S123. doi: 10.1097/00002093-199912003-00017. [DOI] [PubMed] [Google Scholar]
- 29.Erkinjuntti T, Haltia M, Palo J, Sulkava R, Paetau A. Accuracy of the clinical diagnosis of vascular dementia: a prospective clinical and post-mortem neuropathological study. J Neurol Neurosurg Psychiatry. 1988;51:1037–1044. doi: 10.1136/jnnp.51.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hulette C, Nochlin D, McKeel D, Morris JC, Mirra SS, Sumi SM, et al. Clinical-neuropathologic findings in multi-infarct dementia: a report of six autopsied cases. Neurology. 1997;48:668–672. doi: 10.1212/wnl.48.3.668. [DOI] [PubMed] [Google Scholar]
- 31.Nolan KA, Lino MM, Seligmann AW, Blass JP. Absence of vascular dementia in an autopsy series from a dementia clinic. J Am Geriatr Soc. 1998;46:597–604. doi: 10.1111/j.1532-5415.1998.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 32.Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 33.Gold G, Bouras C, Canuto A, Bergallo MF, Herrmann FR, Hof PR, et al. Clinicopathological validation study of four sets of clinical criteria for vascular dementia. Am J Psychiatry. 2002;159:82–87. doi: 10.1176/appi.ajp.159.1.82. [DOI] [PubMed] [Google Scholar]
- 34.Ballard C, McKeith I, O'Brien J, Kalaria R, Jaros E, Ince P, et al. Neuropathological substrates of dementia and depression in vascular dementia, with a particular focus on cases with small infarct volumes. Dement Geriatr Cogn Disord. 2000;11:59–65. doi: 10.1159/000017215. [DOI] [PubMed] [Google Scholar]
- 35.Kalaria RN, Ballard CG. Stroke and Cognition. Curr Atheroscler Rep. 2001;3:334–339. doi: 10.1007/s11883-001-0028-5. [DOI] [PubMed] [Google Scholar]
- 36.Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet. 2002;359:1283–1290. doi: 10.1016/S0140-6736(02)08267-3. [DOI] [PubMed] [Google Scholar]
- 37.Kalimo H, Kaste M, Haltia M. In: Vascular diseases. Graham D, Lantos P, editors. Greenfield's Neuropathology Arnold Press; 2002a. pp. 281–255. [Google Scholar]
- 38.Vinters HV, Ellis WG, Zarow C, Zaias BW, Jagust WJ, Mack WJ, et al. Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol. 2000;59:931–945. doi: 10.1093/jnen/59.11.931. [DOI] [PubMed] [Google Scholar]
- 39.Brun A. Pathology and pathophysiology of cerebrovascular dementia: pure subgroups of obstructive and hypoperfusive etiology. Dementia. 1994;5:145–147. doi: 10.1159/000106712. [DOI] [PubMed] [Google Scholar]
- 40.Cummings JL. Vascular subcortical dementias: clinical aspects. Dementia. 1994;5:177–180. doi: 10.1159/000106718. [DOI] [PubMed] [Google Scholar]
- 41.Wallin A, Blennow K. The clinical diagnosis of vascular dementia. Dementia. 1994;5:181–184. doi: 10.1159/000106719. [DOI] [PubMed] [Google Scholar]
- 42.Wallin A, Milos V, Sjögren M, Pantoni L, Erkinjuntti T. Classification and subtypes of vascular dementia. Int Psychogeriatr. 2003;15:27–37. doi: 10.1017/S1041610203008937. [DOI] [PubMed] [Google Scholar]
- 43.Sulkava R, Erkinjuntti T. Vascular dementia due to cardiac arrhythmias and systemic hypotension. Acta Neurol Scand. 1987;76:123–128. doi: 10.1111/j.1600-0404.1987.tb03555.x. [DOI] [PubMed] [Google Scholar]
- 44.Erkinjuntti T, Inzitari D, Pantoni L, Wallin A, Scheltens P, Rockwood K, et al. Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Suppl. 2000;59:23–30. doi: 10.1007/978-3-7091-6781-6_4. [DOI] [PubMed] [Google Scholar]
- 45.Babikian V, Ropper AH. Binswanger's disease: a review. Stroke. 1987;18:2–12. doi: 10.1161/01.str.18.1.2. [DOI] [PubMed] [Google Scholar]
- 46.Ishii N, Nishihara Y, Imamura T. Why do frontal lobe symptoms predominate in vascular dementia with lacunes? Neurology. 1986;36:340–345. doi: 10.1212/wnl.36.3.340. [DOI] [PubMed] [Google Scholar]
- 47.Mahler ME, Cummings JL. The behavioural neurology of multi-infarct dementia. Alzheimer Dis Assoc Disord. 1991;5:122–130. doi: 10.1097/00002093-199100520-00009. [DOI] [PubMed] [Google Scholar]
- 48.Roman G.C. Senile dementia of the Binswanger type. A vascular form of dementia in the elderly. JAMA. 1987;258:1782–1788. doi: 10.1001/jama.1987.03400130096040. [DOI] [PubMed] [Google Scholar]
- 49.Wallin A, Blennow K, Gottfries CG. Subcortical symptoms predominate in vascular dementia. Int J Geriatr Psychiatry. 1991;6:137–146. [Google Scholar]
- 50.Erkinjuntti T. Types of multi-infarct dementia. Acta Neurol Scand. 1987;75:391–399. doi: 10.1111/j.1600-0404.1987.tb05467.x. [DOI] [PubMed] [Google Scholar]
- 51.Fischer P, Gatterer G, Marterer A, Simanyi M, Danielczyk W. Course characteristics in the differentiation of dementia of the Alzheimer type and multi-infarct dementia. Acta Psychiatr Scand. 1990;81:551–553. doi: 10.1111/j.1600-0447.1990.tb05497.x. [DOI] [PubMed] [Google Scholar]
- 52.Skoog I. Blood pressure and dementia. In: Hansson L, Birkenhäger WH, editors. Handbook of hypertension. Vol 18. Amsterdam: Elsevier Science BV; 1997. pp. 303–331. Assessment of hypertensive organ damage. [Google Scholar]
- 53.Desmond DW, Erkinjuntti T, Sano M, Cummings JL, Bowler JV, Pasquier F, et al. The cognitive syndrome of vascular dementia: implications for clinical trials. Alzheimer Dis Assoc Disord. 1999;13 Suppl 3:S21–S29. [PubMed] [Google Scholar]
- 54.Pillon B, Deweer B, Agid Y, Dubois B. Explicit memory in Alzheimer's, Huntington's, and Parkinson's diseases. Arch Neurol. 1993;50:374–379. doi: 10.1001/archneur.1993.00540040036010. [DOI] [PubMed] [Google Scholar]
- 55.Kertesz A, Clydesdale S. Neuropsychological deficits in vascular dementia vs Alzheimer's disease. Frontal lobe deficits prominent in vascular dementia. Arch Neurol. 1994;51:1226–1231. doi: 10.1001/archneur.1994.00540240070018. [DOI] [PubMed] [Google Scholar]
- 56.Traykov L, Baudic S, Thibaudet MC, Rigaud AS, Smagghe A, Boller F. Neuropsychological deficit in early subcortical vascular dementia: comparison to Alzheimer's disease. Dement Geriatr Cogn Disord. 2002;14:26–32. doi: 10.1159/000058330. [DOI] [PubMed] [Google Scholar]
- 57.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Looi JCL, Wen W, et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004;62:912–919. doi: 10.1212/01.wnl.0000115108.65264.4b. [DOI] [PubMed] [Google Scholar]
- 58.Cummings JL. Fronto-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 59.Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T. Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci. 2004;226:75–80. doi: 10.1016/j.jns.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 60.Lammie G. Pathology of small vessel stroke. Br Med Bull. 2000;56:296–306. doi: 10.1258/0007142001903229. [DOI] [PubMed] [Google Scholar]
- 61.Ho K-L, Garcia JH. Neuropathology of the small blood vessels in selected disease of the cerebral white matter. In: Pantoni L, Inzitari D, Wallin A, editors. The matter of white matter. Vol. 10. Utrecht, The Netherlands: Academic Pharmaceutical Productions; 2000. pp. 247–273. Current Issues in Neurodegenerative diseases. [Google Scholar]
- 62.Kalaria RN, Hedera P. Differential degeneration of the endothelium and basement membrane of capillaries in Alzheimer's disease. Neuroreport. 1995;6:477–480. doi: 10.1097/00001756-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 63.Benhaiem-Sigaux N, Gray F, Gherardi R, Roucayrol AM, Poirier J. Expanding cerebellar lacunes due to dilatation of the perivascular space associated with Binswanger's subcortical arteriosclerotic encephalopathy. Stroke. 1987;18:1087–1092. doi: 10.1161/01.str.18.6.1087. [DOI] [PubMed] [Google Scholar]
- 64.White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia aging study participants. Ann NY Acad Sci. 2002;977:9–23. doi: 10.1111/j.1749-6632.2002.tb04794.x. [DOI] [PubMed] [Google Scholar]
- 65.Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, et al. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004;35:410–414. doi: 10.1161/01.STR.0000110791.51378.4E. [DOI] [PubMed] [Google Scholar]
- 66.Suter OC, Sunthorn T, Kraftsik R, Straubel J, Darekar P, Khalili K, et al. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002;33:1986–1992. doi: 10.1161/01.str.0000024523.82311.77. [DOI] [PubMed] [Google Scholar]
- 67.Kalaria RN, Thomas A, Oakley A, Ince P, Tamaoka A, Mori H, et al. Cerebrovascular amyloidosis and dementia. Curr Med Chem Immun Endoc & Metab Agents. 2003;4:317–327. [Google Scholar]
- 68.Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 69.Greenberg SM. Cerebral amyloid angiopathy and vessel dysfunction. Cerebrovasc Dis. 2002;13:42–47. doi: 10.1159/000049149. [DOI] [PubMed] [Google Scholar]
- 70.Kalaria RN. Advances in molecular genetics and pathology of cerebrovascular disorders. Trends Neurosci. 2001;24:392–400. doi: 10.1016/s0166-2236(00)01836-1. [DOI] [PubMed] [Google Scholar]
- 71.Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004;35:2616–2619. doi: 10.1161/01.STR.0000143224.36527.44. [DOI] [PubMed] [Google Scholar]
- 72.Haan J, Lanser JBK, Zijderveld I, van der Does IGF, Roos RAC. Dementia in hereditary cerebral hemorrhage with amyloidosis-Dutch type. Arch Neurol. 1990;47:965–967. doi: 10.1001/archneur.1990.00530090035010. [DOI] [PubMed] [Google Scholar]
- 73.Bornebroek M, Haan J, Roos RA. Hereditary cerebral hemorrhage with amyloidosis--Dutch type (HCHWA-D): a review of the variety in phenotypic expression. Amyloid. 1999;6:215–224. doi: 10.3109/13506129909007331. [DOI] [PubMed] [Google Scholar]
- 74.Natte R, Maat-Schieman ML, Haan J, Bornebroek M, Roos RA, van Duinen SG. Dementia in hereditary cerebral hemorrhage with amyloidosis-Dutch type is associated with cerebral amyloid angiopathy but is independent of plaques and neurofibrillary tangles. Ann Neurol. 2001;50:765–772. doi: 10.1002/ana.10040. [DOI] [PubMed] [Google Scholar]
- 75.Haglund M, Englund E. Cerebral amyloid angiopathy, white matter lesions and Alzheimer encephalopathy - a histopathological assessment. Dement Geriatr Cogn Disord. 2002;14:161–166. doi: 10.1159/000063606. [DOI] [PubMed] [Google Scholar]
- 76.Sarazin M, Amarenco P, Mikol J, Dimitri D, Lot G, Bousser MG. Reversible leukoencephalopathy in cerebral amyloid angiopathy presenting as subacute dementia. Eur J Neurol. 2002;9:353–358. doi: 10.1046/j.1468-1331.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- 77.Kalimo H, Ruchoux MM, Viitanen M, Kalaria RN. CADASIL: a common form of hereditary arteriopathy causing brain infarcts and dementia. Brain Pathol. 2002;12:371–384. doi: 10.1111/j.1750-3639.2002.tb00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chabriat H, Vahedi K, Iba-Zizen MT, Joutel A, Nibbio A, Nagy TG, et al. Clinical spectrum of CADASIL: a study of 7 families. Lancet. 1995;346:934–939. doi: 10.1016/s0140-6736(95)91557-5. [DOI] [PubMed] [Google Scholar]
- 79.Dichgans M, Mayer M, Uttner I, Bruning R, Muller-Hocker J, Rungger G, et al. The phenotypic spectrum of CADASIL: Clinical findings in 102 cases. Ann Neurol. 1998;44:731–739. doi: 10.1002/ana.410440506. [DOI] [PubMed] [Google Scholar]
- 80.Opherk C, Peters N, Herzog J, Luedtke R, Dichgans M. Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain. 2004;127:2533–2539. doi: 10.1093/brain/awh282. [DOI] [PubMed] [Google Scholar]
- 81.Singhal S, Bevan S, Barrick T, Rich P, Markus HS. The influence of genetic and cardiovascular risk factors on the CADASIL phenotype. Brain. 2004;127:2031–2038. doi: 10.1093/brain/awh223. [DOI] [PubMed] [Google Scholar]
- 82.Chabriat H, Levy C, Taillia H, Iba-Zizen MT, Vahedi K, Joutel A, et al. Patterns of MRI lesions in CADASIL. Neurology. 1998;51:452–457. doi: 10.1212/wnl.51.2.452. [DOI] [PubMed] [Google Scholar]
- 83.Lesnik Oberstein SA, van den Boom R, van Buchem MA, van Houwelingen HC, Bakker E, Vollebregt E, et al. The Dutch CADASIL Research Group. Cerebral microbleeds in CADASIL. Neurology. 2001;57:1066–1070. doi: 10.1212/wnl.57.6.1066. [DOI] [PubMed] [Google Scholar]
- 84.Ruchoux MM, Maurage CA. CADASIL: Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy. J Neuropathol Exp Neurol. 1997;56:947–964. [PubMed] [Google Scholar]
- 85.Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssiere C, et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 1997;350:1511–1515. doi: 10.1016/S0140-6736(97)08083-5. [DOI] [PubMed] [Google Scholar]