Abstract
Background and Purpose
To identify the clinical and electrophysiological characteristics of temporal lobe epilepsy (TLE) with normal MRI.
Methods
Twenty-six patients were diagnosed with TLE with normal MRI by stereotaxically implanted depth electrode EEG (SEEG) and quantitative MRI. We divided the patients into anterior or diffuse temporal groups by interictal EEG, into localized, hemispheric or non-lateralized onset groups by ictal scalp EEG, and into focal or regional onset groups by SEEG. The clinical and electrophysiological characteristics were compared with those of 25 TLE patients with unilateral hippocampal atrophy (HA) on MRI. Four patients of TLE with unilateral HA also underwent SEEG.
Results
Patients in the normal MRI group showed a significantly higher frequency of secondarily generalization (225±235, median 160 vs 68±48, median 50, p<0.05), shorter duration of epilepsy (16±10 yrs vs 25.9±7.8 yrs, p<0.001), and less favorable surgical outcome (50% vs 88%, p <0.05) than patients in the unilateral HA group. Also, patients with normal MRI frequently showed diffuse temporal (50% vs 20%, p<0.05) discharges on interictal EEG. The ictal seizure patterns of patients with normal MRI showed less localization to one temporal lobe on scalp EEG (28% vs 65%, p<0.001) and a higher frequency of regional onset on SEEG (68% vs 8%, p<0.001) than patients with unilateral HA.
Conclusions
The characteristics of TLE with normal MRI compared with TLE with unilateral HA are shorter duration of epilepsy, higher frequency of secondarily generalization, and less favorable surgical outcome, suggesting wider areas of temporal lobe involved compared with patients with unilateral HA.
Keywords: Temporal lobe epilepsy, MRI, Intracranial EEG, Hippocampal atrophy, Temporal neocortex
INTRODUCTION
Temporal lobe epilepsy (TLE) with hippocampal sclerosis (HS) on MRI is a well-known condition of surgically remediable mesial TLE syndrome (MTLE). Diagnosis can be made by a combination of past history, clinical presentation, EEG and HS on MRI.1-5 It is estimated that at least 30% of patients with TLE have no evidence of HS on MRI. It is possible that some of these patients may have HS without HA (hippocampal atrophy) or T2-weighted signal changes that can be detectable using current technologies, and some may have bilateral HA that makes detection through volume asymmetry difficult. Similarly, small or subtle cortical temporal pathologies may be undetected with current procedures. Those patients have an older age of onset and less favorable surgical outcome compared with patients who have TLE with HS on MRI.6,7
TLE may result from the lateral temporal cortex or mesial temporal structures, or the interaction between the lateral and mesial structures.8-11 Compared with well-defined MTLE in which the atrophied hippocampus is nearly always the epileptic focus, TLE with normal MRI may be a heterogeneous condition either as a result of variable seizure onset or spread pattern.9,10
Although functional neuroimaging such as PET or MRSI are valuable tools for localizing epileptic foci in TLE with normal MRI,12-15 electrophysiological studies still have an important place in the detection of epileptic foci in the temporal lobe. In cases of TLE with normal MRI, extratemporal lobe epilepsy can be presented with temporal onset on scalp ictal EEG, and hence invasive intracranial investigation is necessary for demonstrating the ictal onset zone lies in the temporal lobe and not include other extratemporal lobes.10,16,17
In this study of TLE with normal MRI, the aims were to identify the clinical and electrophysiological characteristics of TLE with normal MRI compared with MTLE with unilateral HA.
MATERIALS AND METHODS
1. Subjects
We studied 26 intractable TLE patients with normal MRI. Investigations included stereotaxically implanted depth electrode EEG (SEEG) with quantitative and qualitative MRI. All of these 26 patients demonstrated their epileptic foci in the temporal lobe by SEEG and normal hippocampal volumes by quantitative measurement. All of these patients showed their habitual seizures during SEEG recording. We included the seizures only when the first electrographic ictal onset preceded the initial behavioral changes. We excluded if 1) electrographic seizures occurred along without clinical manifestation, 2) simultaneous temporal and extratemporal onset (multilobal onset) or definite extratemporal lobe onset occurred, 3) previous surgical treatment was performed, 4) significant FDG-PET hypometabolism or SPECT hyper- or hypoperfusion in the extratemporal area or other evidence of extratemporal onset was present, 5) either HA or structural lesions were noted on MRI. Of the 142 patients who had SEEG since 1995 when high resolution MRI became available in our institute, 116 patients were excluded by these criteria. The reasons for exclusions were as follows: 68 had extratemporal lobe or multilobal onset, 17 had HA on hippocampal volumetry, 10 had structural lesions on MRI, 5 had undergone a previous operation, 1 had electrographic seizures along, and 15 did not have available medical records.
2. Electrophysiological study
Scalp EEG telemetry was recorded in all patients using the international 10-20 systems. Interictal epileptiform discharges were divided into anterior or diffuse temporal groups based on the topography of discharges. The anterior temporal group was defined as having interictal epileptiform discharges confined to the Fp1/2, F7/8, and T3/4, T1/2 electrodes. The diffuse temporal group was defined as having interictal epileptiform discharges also involving the T5/6 with a wider area than that of the anterior temporal group. The ictal scalp EEG was divided into localized, hemispheric or non-lateralized groups by ictal scalp EEG onset patterns. The localized onset group was defined as the ictal onset being exclusively confined to one temporal lobe. The hemispheric onset group was defined as a diffuse onset between multiple lobes but clearly lateralized to one hemisphere. The non-lateralized onset group was defined as the onset being either bilaterally simultaneous or not clearly lateralized to one hemisphere. When non-invasive studies (i.e., clinical history and seizure semiology, scalp interictal or ictal EEG, MRI, FDG-PET, MRSI, SPECT, and neuropsychological results) were inconclusive for localizing the epileptic focus, SEEG was performed with orthogonally implanted depth electrodes. The number and location of the depth electrodes were individualized by the information from the non-invasive studies. The method of insertion and strategy of SEEG was previously described in detail.18 Briefly, three depth electrode strands were introduced horizontally through the second temporal gyrus. The two deepest contacts of the anterior, middle, and posterior depth electrode strands were located in the amygdalae, anterior hippocampus, and posterior portions of the hippocampus and parahippocampal gyrus, respectively. We divided SEEG into focal and regional groups. The focal onset group was defined as the ictal onset being exclusively confined to mesial or lateral temporal structures. The seizures of the focal onset group sometimes showed delayed spread to either contralateral or other temporal structures. The regional onset group was defined as having ictal onset that occurred simultaneously in mesial and lateral temporal structures, or where ictal onset was either in mesial or lateral temporal structures but with very early spread to other temporal structures. Early spread was defined as spreading to the other ipsilateral or contralateral mesial or lateral temporal structures within a quarter of the seizure. The seizures of bilaterally independent temporal onset were counted as either focal or regional onset groups regardless of the lateralization. In the case of multiple electrographic and spreading patterns in one patient, we counted the seizures that were socially disabling and habitual.
3. Clinical data
One of the authors (S.E.K.) retrospectively reviewed the medical records of the patients in the study. We were able to successfully document all of the clinical data from the medical records. The clinical data included age of onset, duration, initial precipitating insult (IPI), family history of epilepsy, history of status epilepticus, estimated seizure frequency, generalization fraction, surgical outcome, and surgical pathology. The following definitions were used. Duration was defined as the time from onset to SEEG; IPI as any significant events preceding onset of epilepsy, including neurological or medical illness; and generalization fraction as the fraction of secondarily generalized tonic-clonic seizures (SGTC) to total estimated seizure frequency. Surgical outcome was classified using Engel's classification.19
4. Qualitative and quantitative MRI
MRI scans were acquired on a 1.5-T Gyroscan (Philips Medical Systems, Eindhoven, The Netherlands). Visual analysis was performed using spin-echo sequences [field of view (FOV) 250 mm, 256×256 matrix] with sagittal T1-weighted [repetition time (TR) 550 ms, echo time (TE) 19 ms], transverse proton density (TR 2,000 ms, TE 20 ms), T2-weighted (TR 2,100 ms, TE 20, 78 ms), as well as coronal T1-weighted images. The MRI acquisitions for volumetric assessment of the hippocampus consisted of 1- or 3-mm contiguous slices perpendicular to the plane of the Sylvian fissure, obtained with either a three-dimensional gradient fast-field echo sequence or with inversion recovery using spin-echo sequences (FOV 250 mm, 256×256 matrix, TR 18 ms, TE 10 ms, 30° angle, 160 contiguous slices, 1 mm thick). The images were transferred to a computer workstation. Volumetric measurements were performed using an interactive software program. The regions of interest were outlined using manual contouring. Anatomic guidelines for outlining the amygdala and the hippocampus followed a previously reported method.20
5. TLE with HA on MRI as disease control
We randomly selected 25 patients who had TLE with unilateral HA on MRI and confirmed hippocampal neuronal loss and gliosis on surgical pathology as controls. All of these 25 patients had seizures originating from the temporal lobe ipsilateral to the atrophied hippocampus on MRI. Four patients with unilateral HA underwent SEEG because of either independent bitemporal onset on scalp EEG or contralateral temporal onset to HA on scalp EEG. These four patients showed habitual seizures during SEEG recording.
6. Statistical analysis
We analyzed the clinical differences between the two groups (TLE with normal MRI or TLE with HA) using Fisher's exact test or χ2 test (categorical variables) and Student's t-test or Mann-Whitney test (numerical data) using MedCalc®. For all calculations, the significance level was taken as 0.05.
RESULTS
1. Clinical characteristics
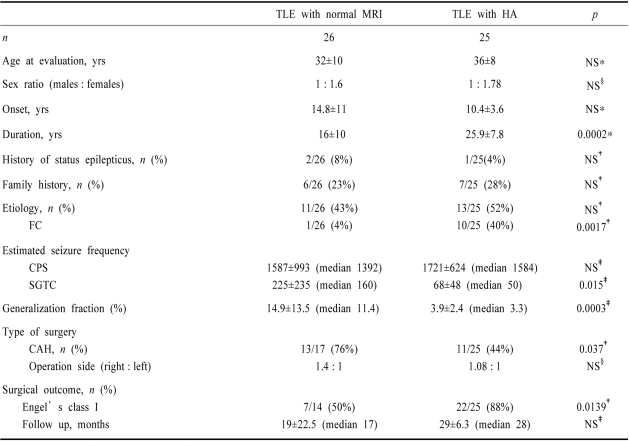
The clinical characteristics of TLE with normal MRI compared with TLE with unilateral HA are summarized in Table 1. There were no differences between the two groups with regard to age at evaluation, sex ratio, side of operation, and median postoperative follow up.
Table 1.
Clinical characteristics of temporal lobe epilepsy (TLE) with normal MRI
*Student's t-test, †Fisher's exact test, ‡Mann-Whitney test, §χ2 test
NS, not significant, p≥0.05; TLE, temporal lobe epilepsy; HA, hippocampal atrophy; FC, febrile convulsion; CPS, complex partial seizure; SGTC, secondarily generalized tonic-clonic seizure, CAH, cortico-amygdalo-hippocampectomy
The duration of active epilepsy in TLE with normal MRI was significantly shorter than that of TLE with unilateral HA (16±10 yrs vs 25.9±7.8 yrs, p<0.001) but there was a tendency toward being older at onset in TLE with normal MRI. Although the frequency of IPI as part of the etiology of epilepsy did not differ between the two groups, febrile convulsions were significantly less frequent in TLE with normal MRI than in TLE with unilateral HA (4% vs 40%, p<0.01). Of the 11 patients with TLE with normal MRI who had an IPI, 64% (7/11) had seizures related to encephalitis, hypoglycemia or accidental drug intoxication as the IPI. Two patients had a history of hypoxia at birth, one had a head trauma, and only one had a febrile convulsion. The frequencies of SGTC (225±235, median 160 vs 68±48, median 50, p<0.05) and generalization fraction (14.9±13.5, median 11.4 vs 3.9×2.4, median 3.3, p<0.001) were significantly higher in TLE with normal MRI than in TLE with unilateral HA, but the frequency of complex partial seizure (CPS) did not differ between the two groups.
The family history of epilepsy and the history of status epilepticus did not differ between the two groups.
Seventeen patients with TLE with normal MRI underwent surgery. Postoperative histopathology data of the temporal cortex were available for 13 of these 17 patients: 8 patients had neuronal loss and gliosis, 1 had focal cortical dysplasia, and only 4 patients had normal histopathology. HS was pathologically diagnosed in 2 of 4 patients available for histopathology. The postoperative outcome was available for 14 of 17 patients (range 8 months to 60 months, median 17 months). The operated side did not different between the two groups, but cortico-amygdalo-hippocampectomy was more frequently performed in TLE with normal MRI than in TLE with HA group (76% vs 44%, p<0.05). Also, a good surgical outcome (Engel's classification I) was significantly less common in TLE with normal MRI than in TLE with HA group (50% vs 88%, p<0.01)
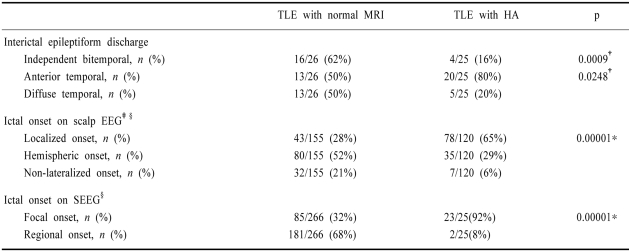
2. The electrophysiological characteristics
Table 2 compares the electrophysiological characteristics between TLE with normal MRI and TLE with unilateral HA. Compared with TLE with unilateral HA, the patient with normal MRI frequently showed diffuse temporal (50% vs 20%, p<0.05) or independent bitemporal (62% vs 16%, p<0.001) discharges on interictal EEG. On ictal scalp EEG, 61% (16/26) of patients with TLE with normal MRI showed independent bitemporal onset. A total of 155 seizures in the TLE with normal MRI group and 120 seizures in the TLE with unilateral HA group were counted and analyzed. The ictal seizure patterns on scalp EEG of patients with TLE with normal MRI were less localized to one temporal lobe than those in patients with TLE with unilateral HA (28% vs 65%, p<0.001). On SEEG, 61% (16/26) of patients with TLE with normal MRI showed ictal onset either in mesial or lateral temporal structures, but with very early spread to other temporal structures (12/16) or simultaneously in both mesial and lateral temporal structures (4/16). Of the other 10 patients, 2 patients had ictal onset confined to the lateral temporal neocortex, and 4 patients had ictal onset confined to either the amygdala or the hippocampus with occasional delayed spread to the contralateral or other temporal structures. The other four patients had independent bitemporal onset. A total of 266 seizures in patients with TLE with normal MRI and 25 seizures in patients with TLE with unilateral HA were counted and analyzed: 181 and 2 seizures were regional onset, respectively (68% vs 8%, p<0.001).
Table 2.
Electrophysiological characteristics of TLE with normal MRI
*Student's t-test, †Fisher's exact test, ‡Localized versus non-localized, namely hemispheric and non-lateralized onset, §The numbers of seizures in each patient were counted and analyzed
HA, hippocampal atrophy; SEEG, stereotaxically implanted depth electrode EEG
DISCUSSION
Since the introduction of MRI to the field of epilepsy, HS has been recognized in vivo.21-24 MTLE is a well-known homogeneous condition,1-5 whereas TLE with normal MRI, without HA or structural lesions on MRI, is not yet fully clarified. The temporal lobe contains a group of particularly epileptogenic structures. As a result, epileptogenic discharges within extratemporal areas can spread to the temporal lobe.10,16,17,25,26 When epileptogenic lesions in these areas can be identified on MRI the diagnosis is greatly simplified; when no visible lesions can be detected on MRI, accurate diagnosis is a challenge and must rely on clinical semiology, electrophysiological study or functional neuroimaging. To accurately assess cases of TLE with normal MRI in this study, we selected those cases where the ictal onset zone lied in the temporal lobe and did not include other extratemporal lobes on SEEG under very strict exclusion criteria. We included seizures only when the first electrographic ictal onset preceded the initial behavioral changes and also excluded electrographic seizures that appeared without clinical manifestations. In addition, three experienced epileptologists agreed on a diagnosis of TLE without visible lesions on MRI, strongly suggesting that the subjects of this study had TLE with normal MRI.
The age of onset is known to be higher for TLE with normal MRI than TLE with unilateral HA.6,7,27 In this study we found that the duration of active epilepsy was shorter in TLE with normal MRI than TLE with unilateral HA, and we attribute the absence of a significant difference between the groups in the age of onset to the small sample.
Van Paesschen et al7 and Holmes et al27 reported various etiologies in patients with TLE with normal MRI. Although all patients reported in both studies were not confirmed by SEEG, significant IPIs seemed to be related to TLE with normal MRI as part of their etiology. As reported above, we also found that the frequency of febrile convulsions was significantly lower in patients with TLE with normal MRI than in patients with TLE with unilateral HA. Febrile convulsion is a well-known etiology of MTLE with HA but is still one of the most controversial issues in epilepsy research.1-7 The finding in this study that the frequency of IPIs was as high in patients with TLE with normal MRI as in patients with TLE with unilateral HA allows the conclusion that significant IPIs could lead to epileptogenesis in patients with TLE with normal MRI. The same mechanism of epileptogenesis in MTLE with HA might also be involved in TLE with normal MRI, even though the area of active epileptic foci might be different in these two conditions.
TLE may originate from the lateral temporal cortex or mesial temporal structures, or from the interaction between both the lateral and mesial structures.8-11 Compared with well-defined MTLE in which the epileptic focus is nearly always in the atrophied hippocampus,28-30 TLE with normal MRI may be a heterogeneous condition that results from either a variable seizure onset or a spread pattern.9,10 One of the striking findings in this study was that many patients with TLE with normal MRI showed regional ictal onset on SEEG, diffuse temporal involvement on interictal EEG and were less well localized to one of temporal lobes on ictal scalp EEG. These electrophysiological characteristics imply that the area of active epileptic foci may constitute a wider areas of the temporal lobe in patients with TLE with normal MRI than in patients with TLE with unilateral HA. This assumption is also supported by the clinical characteristics, in that the frequency of SGTC and the generalization fraction were significantly higher in patients with TLE with normal MRI than in those with TLE with unilateral HA. The studies of electro-clinical patterns in TLE showed that each electro-clinical pattern gave the expression of the neuronal network involved by the ictal discharges for a given patient.11,31,32 It is known that the generalization tendency is much lower in MTLE with HA than in extratemporal epilepsy.1-3 The electro-clinical studies have shown that generalization in TLE usually occurs when ictal discharges reached other brain areas and spread beyond the area from which ictal onset originally occurs. The greater the number of involved neurons in the temporal lobe, the more frequent was the occurrence of generalization.11,31,32 Furthermore, a FDG-PET study also showed that more complex clinical patterns and generalization occurred when a wider area of hypometabolism in the temporal lobe was involved.33
In MTLE with unilateral HA, the mesial temporal structures are known as the main epileptic foci, but the anterior temporal cortical structures are also known to be involved during seizures.34-38 Our findings on SEEG support the point of view that the temporal cortex is more actively involved in TLE with normal MRI than in TLE with unilateral HA. On SEEG, 68% of seizures in TLE with normal MRI were of regional onset. The definition of regional onset in this study means that both the mesial and lateral temporal structures are actively involved during seizures. Sixty-nine percent (9/13) of postoperative histopathologies of the temporal cortex in this study showed evidence of neuronal loss even without visible lesions on MRI. In addition, a pathology study showed that neuronal and glial densities in the temporal lobe may influence the interictal-ictal transition and seizure propagation.39 Therefore, it is a reasonable conclusion that the temporal neocortex may be more actively and widely involved in TLE with normal MRI compared with TLE with unilateral HA.
The frequency of a favorable surgical outcome in TLE with normal MRI has been reported to be less than half that in TLE with unilateral HA.27,40,41 As reported above, the surgical outcome in our study was favorable in 50% of patients. Holmes et al27 suggested that more than 50% of patients would be seizure-free if seizures were proven to originate from a single basal-temporal region. Several reports that describe a poor surgical outcome after temporal resection in intractable TLE have suggested that the epileptogenic zone is not confined to be resected mesial temporal structures.42-46 After additional neocortical resections some poorly controlled seizures appear to be completely seizure free. The findings in this study may partly answer this question, in that the epileptic foci in the temporal neocortex may be diffuse enough to remain beyond the margin of resection. Alternatively, we could not completely exclude the presence of epileptic foci in the extratemporal areas, especially in four patients who had ictal onset simultaneously in both mesial and lateral temporal structures on SEEG. Despite thorough investigations, including SEEG, it remains possible that the epileptic foci exist in an area beyond the temporal lobe or in areas not accessed by depth electrodes.
TLE without HA on MRI does not necessarily mean that the hippocampus is histopathologically normal.47,48 As reported above, HS was pathologically diagnosed in 2 of 4 patients available for histopathology in this study. Although the sensitivity and specificity of MRI for the detection of HS in vivo is satisfactory,21-24 MRI cannot always detect HS in vivo. HS may exist as a spectrum between severely atrophied (and seen on MRI) and a normal volume on MRI such as endfolium sclerosis. Furthermore, functional neuroimaging studies have shown significant mesial and lateral temporal abnormalities despite no visible lesions or HA on MRI.12-15
This study is subject to several limitations. One of the weak points of using depth electrodes is that sampling only occurs in the localized areas where they are implanted. We implanted additional extratemporal depth electrodes, in addition to 3 depth electrodes in one temporal lobe, in 11 patients, and these patients were more convincingly localized to the temporal lobes. Although 15 of 26 patients had depth electrodes implanted that covered only the temporal lobes, only 33% of 9 patients available for postoperative follow up had a poor surgical outcome. Additionally, this study was retrospective and performed in a specialized tertiary epilepsy center, so we cannot completely exclude the bias of highly selected intractable TLE patients.
In conclusion, the characteristics of TLE with normal MRI compared with TLE with unilateral HA are shorter duration of epilepsy, higher frequency of secondarily generalized tonic-clonic seizures, and less favorable surgical outcome. These clinical characteristics of patients with TLE with normal MRI could be partially explained by the regional ictal onset on SEEG and diffuse temporal involvement on interictal EEG with wider areas of the temporal lobe involved compared with patients with unilateral HA.
Footnotes
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (A040155).
References
- 1.French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spensor SS, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;334:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- 2.Williamson PD, French JA, Thadani VM, Darcey TM, Novelly RA, Spensor SS, et al. Characteristics of medial temporal lobe epilepsy: II. Interictal and ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol. 1993;34:781–787. doi: 10.1002/ana.410340605. [DOI] [PubMed] [Google Scholar]
- 3.Wieser HG, Engel J, Jr, Williamson PD, Babb TL, Gloor P. Surgically remediable temporal lobe syndromes. In: Engel J Jr, editor. Surgical treatment of the epilepsies. New York: Raven Press; 1993. pp. 49–63. [Google Scholar]
- 4.Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK. The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain. 1995;118:105–118. doi: 10.1093/brain/118.1.105. [DOI] [PubMed] [Google Scholar]
- 5.Engel J., Jr Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26:141–150. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- 6.Van Paesschen W, Connelly A, King MD, Jackson GD, Duncan JS. The spectrum of hippocampal sclerosis: a quantitative magnetic resonance imaging study. Ann Neurol. 1997;41:41–51. doi: 10.1002/ana.410410109. [DOI] [PubMed] [Google Scholar]
- 7.Van Paesschen W, Connelly A, Johnson CL, Duncan JS. The amygdala and intractable temporal lobe epilepsy: a quantitative magnetic resonance imaging study. Neurology. 1996;47:1021–1031. doi: 10.1212/wnl.47.4.1021. [DOI] [PubMed] [Google Scholar]
- 8.Bartolomei F, Wendling F, Vignal JP, Kochen S, Bellanger JJ, Badier JM, et al. Seizures of temporal lobe epilepsy: identification of subtypes by coherence analysis using stereo-electro-encephalography. Clin Neurophysiol. 1999;110:1741–1754. doi: 10.1016/s1388-2457(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 9.Burgerman RS, Sperling MR, French JA, Saykin AJ, O'Connor MJ. Comparison of mesial versus neocortical onset temporal lobe seizures: neurodiagnostic findings and surgical outcome. Epilepsia. 1995;36:662–670. doi: 10.1111/j.1528-1157.1995.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 10.Walczak TS. Neocortical temporal lobe epilepsy: characterizing the syndrome. Epilepsia. 1995;36:633–635. doi: 10.1111/j.1528-1157.1995.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 11.Bartolomei F, Wendling F, Bellanger J, Regis J, Chauvel P. Neuronal networks involving the medial temporal structures in temporal lobe epilepsy. Clin Neurophysiol. 2001;112:1746–1760. doi: 10.1016/s1388-2457(01)00591-0. [DOI] [PubMed] [Google Scholar]
- 12.Koepp MJ, Hammers A, Labbe C, Woermann FG, Brooks DJ, Duncan JS. 11C-flumazenil PET in patients with refractory temporal lobe epilepsy and normal MRI. Neurology. 2000;54:332–339. doi: 10.1212/wnl.54.2.332. [DOI] [PubMed] [Google Scholar]
- 13.Lamusuo S, Jutila L, Ylinen A, Kalviainen R, Mervaala E, Haaparanta M, et al. [18F] FDG-PET reveals temporal hypometabolism in patients with temporal lobe epilepsy even when quantitative MRI and histopathological analysis show only mild hippocampal damage. Arch Neurol. 2001;58:933–939. doi: 10.1001/archneur.58.6.933. [DOI] [PubMed] [Google Scholar]
- 14.Connelly A, Van Paesschen W, Porter DA, Johnson CL, Duncan JS, Gadian DG. Proton magnetic resonance spectroscopy in MRI-negative temporal lobe epilepsy. Neurology. 1998;51:61–66. doi: 10.1212/wnl.51.1.61. [DOI] [PubMed] [Google Scholar]
- 15.Suhy J, Laxer KD, Capizzano AA, Vermathern P, Matson GB, Barbaro NM, et al. 1H MRSI predicts surgical outcome in MRI-negative temporal lobe epilepsy. Neurology. 2002;58:821–823. doi: 10.1212/wnl.58.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SK, Yun CH, Oh JB, Nam HW, Jung SW, Paeng JC, et al. Intracranial ictal onset zone in nonlesional lateral temporal lobe epilepsy on scalp ictal EEG. Neurology. 2003;61:757–764. doi: 10.1212/01.wnl.0000086377.94037.80. [DOI] [PubMed] [Google Scholar]
- 17.Andermann F. Pseudotemporal vs neocortical temporal epilepsy: things aren't always where they seem to be. Neurology. 2003;61:732–733. doi: 10.1212/wnl.61.6.732. [DOI] [PubMed] [Google Scholar]
- 18.Quesney LF. Clinical and EEG features of complex partial seizures of temporal lobe origin. Epilepsia. 1986;27(Suppl. 2):S27–S45. doi: 10.1111/j.1528-1157.1986.tb05738.x. [DOI] [PubMed] [Google Scholar]
- 19.Engel J, Jr, Van Ness PC, Rasmussen TB, Ojemann LM. Outcome with respect to epileptic seizures. In: Engel J Jr, editor. Surgical treatment of the epilepsies. 2nd edn. New York: Raven Press; 1993. pp. 609–621. [Google Scholar]
- 20.Cendes F, Andermann F, Gloor P, Evans A, Jones-Gotman M, Watson C, et al. MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology. 1993;43:719–725. doi: 10.1212/wnl.43.4.719. [DOI] [PubMed] [Google Scholar]
- 21.Jackson GD. The diagnosis of hippocampal sclerosis: other techniques. Magn Reson Imaging. 1995;13:1081–1093. doi: 10.1016/0730-725x(95)02016-m. [DOI] [PubMed] [Google Scholar]
- 22.Jackson GD. New techniques in magnetic resonance and epilepsy. Epilepsia. 1994;35(Suppl 6):S2–S13. doi: 10.1111/j.1528-1157.1994.tb05985.x. [DOI] [PubMed] [Google Scholar]
- 23.Jackson GD, Berkovic SF, Duncan JS, Connelly A. Optimizing the diagnosis of hippocampal sclerosis using MR imaging. AJNR Am J Neuroradiol. 1993;14:753–762. [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson GD, Berkovic SF, Tress BM, Kalnins RM, Fabinyi GC, Bladin PF. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990;40:1869–1875. doi: 10.1212/wnl.40.12.1869. [DOI] [PubMed] [Google Scholar]
- 25.Palmini A, Andermann F, Dubeau F, Gloor P, Olivier A, Quesney LF, et al. Occipitotemporal epilepsies: evaluation of selected patients requiring depth electrodes studies and rationale for surgical approaches. Epilepsia. 1993;34:84–96. doi: 10.1111/j.1528-1157.1993.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 26.Isnard J, Guénot M, Ostrowsky K, Sindou M, Mauguière F. The role of the insular cortex in temporal lobe epilepsy. Ann Neurol. 2000;48:614–623. [PubMed] [Google Scholar]
- 27.Holmes MD, Born DE, Kutsy RL, Wilensky AJ, Ojemann GA, Ojemann LM. Outcome after surgery in patients with refractory temporal lobe epilepsy and normal MRI. Seizure. 2000;9:407–411. doi: 10.1053/seiz.2000.0423. [DOI] [PubMed] [Google Scholar]
- 28.King D, Spencer SS, McCarthy G, Spencer DD. Surface and depth EEG findings in patients with hippocampal atrophy. Neurology. 1997;48:1363–1367. doi: 10.1212/wnl.48.5.1363. [DOI] [PubMed] [Google Scholar]
- 29.Cendes F, Dubeau F, Andermann F, Quesney LF, Gambardella A, Jones-Gotman M, et al. Significance of mesial temporal atrophy in relation to intracranial ictal and interictal stereo EEG abnormalities. Brain. 1996;119:1317–1326. doi: 10.1093/brain/119.4.1317. [DOI] [PubMed] [Google Scholar]
- 30.Cascino GD, Trenerry MR, Sharbrough FW, So EL, Marsh WR, Strelow DC. Depth electrode studies in temporal lobe epilepsy: relation to quantitative magnetic resonance imaging and operative outcome. Epilepsia. 1995;36:230–235. doi: 10.1111/j.1528-1157.1995.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 31.Bancaud J. Surgery of epilepsy based on stereotactic investigations. the plan of the SEEG investigation. Acta Neurochir Suppli (Wien) 1980;30:25–34. doi: 10.1007/978-3-7091-8592-6_4. [DOI] [PubMed] [Google Scholar]
- 32.Bancaud J, Bontis A, Munari C, Szikla G, Chodkievicz JP, Talairach J. Localizing value of the clinical manifestations of the partial seizures. Acta Neurochir Suppl (Wien) 1984;33:7–15. [Google Scholar]
- 33.Chassoux F, Semah F, Bouilleret V, Landre E, Devaux B, Turak B, et al. Metabolic changes and electro-clinical patterns in mesio-temporal lobe epilepsy: a correlative study. Brain. 2004;127:164–174. doi: 10.1093/brain/awh014. [DOI] [PubMed] [Google Scholar]
- 34.Ho SS, Berkovic SF, McKay WJ, Kalnins RM, Bladin PF. Temporal lobe epilepsy subtypes: differential patterns of cerebral perfusion on ictal SPECT. Epilepsia. 1996;37:788–795. doi: 10.1111/j.1528-1157.1996.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 35.Newton MR, Berkovic SF, Austin MC, Rowe CC, McKay WJ, Bladin PF. SPECT in the localisation of extratemporal and temporal seizure foci. J Neurol Neurosurg Psychiatry. 1995;59:26–30. doi: 10.1136/jnnp.59.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newton MR, Berkovic SF, Austin MC, Rowe CC, McKay WJ, Bladin PF. Ictal postictal and interictal single-photon emission tomography in the lateralization of temporal lobe epilepsy. Eur J Nucl Med. 1994;21:1067–1071. doi: 10.1007/BF00181061. [DOI] [PubMed] [Google Scholar]
- 37.Semah F. Temporoporal metabolic abnormalities in temporal lobe epilepsies. Epileptic Disord. 2002;4(Suppl 1):S41–S49. [PubMed] [Google Scholar]
- 38.Mitchell LA, Jackson GD, Kalnins RM, Saling MM, Fitt GJ, Ashpole RD, Berkovic SF. Anterior temporal abnor mality in temporal lobe epilepsy: a quantitative MRI and histopathologic study. Neurology. 1999;52:327–336. doi: 10.1212/wnl.52.2.327. [DOI] [PubMed] [Google Scholar]
- 39.Spencer SS, Kim J, de Lanerolle N, Spencer DD. Differential neuronal and glial relations with parameters of ictal discharge in mesial temporal lobe epilepsy. Epilepsia. 1999;40:708–712. doi: 10.1111/j.1528-1157.1999.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 40.Siegel AM, Jobst BC, Thadani VM, Rhodes CH, Lewis PJ, Roberts DW, Williamson PD. Medically intractable, localization-related epilepsy with normal MRI: presurgical evaluation and surgical outcome in 43 patients. Epilepsia. 2001;42:883–888. doi: 10.1046/j.1528-1157.2001.042007883.x. [DOI] [PubMed] [Google Scholar]
- 41.Suhy J, Laxer KD, Capizzano AA, Vermathern P, Matson GB, Barbaro NM, et al. 1H MRSI predicts surgical outcome in MRI-negative temporal lobe epilepsy. Neurology. 2002;58:821–823. doi: 10.1212/wnl.58.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyler AR, Hermann BP, Richey ET. Results of reoperation for failed epilepsy surgery. J Neurosurg. 1989;71:815–819. doi: 10.3171/jns.1989.71.6.0815. [DOI] [PubMed] [Google Scholar]
- 43.Awad IA, Nayel MH, Luders H. Second operation after the failure of previous resection for epilepsy. Neurosurgery. 1991;28:510–518. doi: 10.1097/00006123-199104000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Germano IM, Poulin N, Olivier A. Reoperation for recurrent temporal lobe epilepsy. J Neurosurg. 1994;81:31–36. doi: 10.3171/jns.1994.81.1.0031. [DOI] [PubMed] [Google Scholar]
- 45.Hennessy MJ, Elwes RD, Binnie CD, Polkey CE. Failed surgery for epilepsy. A study of persistence and recurrence of seizures following temporal resection. Brain. 2000;123:2445–2466. doi: 10.1093/brain/123.12.2445. [DOI] [PubMed] [Google Scholar]
- 46.Aghakhani Y, Rosati A, Dubeau F, Olivier A, Andermann F. Patients with temporoparietal ictal symptoms and inferomesial EEG do not benefit from anterior temporal resection. Epilepsia. 2003;45:230–237. doi: 10.1111/j.0013-9580.2004.43003.x. [DOI] [PubMed] [Google Scholar]
- 47.Bernasconi A, Bernasconi N, Caramanos Z, Reutens DC, Andermann F, Dubeau F, et al. T2 relaxometry can lateralize mesial temporal lobe epilepsy in patients with normal MRI. Neuroimage. 2000;12:739–746. doi: 10.1006/nimg.2000.0724. [DOI] [PubMed] [Google Scholar]
- 48.Jackson GD, Kuzniecky RI, Cascino GD. Hippocampal sclerosis without detectable hippocampal atrophy. Neurology. 1994;44:42–46. doi: 10.1212/wnl.44.1.42. [DOI] [PubMed] [Google Scholar]