Abstract
Matrix metalloproteinases (MMPs) are involved in the pathophysiology of several central nervous system diseases that share common pathogeneses, such as disruption of the blood-brain barrier (BBB), neuroinflammation, oxidative stress, and remodeling of the extracellular matrix (ECM). In early ischemic injury, MMPs participate in disruption of the BBB by digesting the basal lamina of capillaries and ECM, leading to vasogenic edema and hemorrhagic transformation. However, ECM degradation and remodeling are essential for tissue recovery, with MMPs having a key role as modulators of homeostasis between neuronal death and tissue regeneration. Thus, MMPs may be a double-edged sword that has a deleterious or beneficial role depending on the stage of brain injury.
Keywords: Matrix metalloproteinase, Extracellular matrix, Cerebral ischemia, Stroke
INTRODUCTION
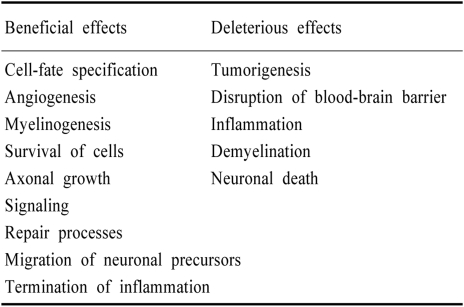
Extracellular proteases comprise serine proteases, tissue-type and urokinase-type plasminogen activator (PA), and the families of matrix metalloproteinases (MMPs). MMPs are families of enzymes that require a zinc ion for catalytic activity. The role of MMPs has classically been described as strict regulators of extracellular matrix (ECM) remodeling in tissue morphogenesis and wound healing. MMPs regulate cellular activity in various ways, including ECM degradation, cell adhesion, proteolytic release of ECM-sequestrated molecules, and shedding of cell surface proteins that transduce signals from the extracellular environment (Table 1).1,2 Recently, a deleterious role of MMPs in several diseases of the nervous system has been widely studied. However, some beneficial functions of MMPs during the recovery phase after brain injury have also been suggested. This review focuses on the role of MMPs in the acute and recovery phases of cerebral ischemia.
Table 1.
Effects of MMPs in the central nervous system
STRUCTURE AND EXPRESSION OF MMPs
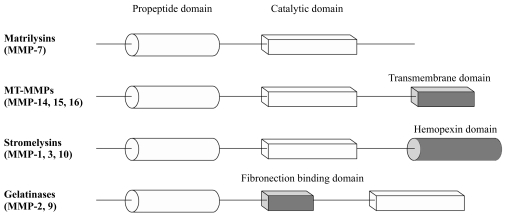
According to the basic protein domain and substrate preference, there are five major classes of MMPs: matrilysins, membrane-type MMPs (MT-MMPs), stromelysins, gelatinases, and other recently discovered MMP families.3 The protein structures of MMPs share a basic common pattern (Fig. 1) of propeptide and catalytic domains. However, they differ in more specific domains. The matrilysin MMP-7 has the simplest domain structure, with the propeptide domain and zinc containing catalytic domain, but it degrades most ECM components. The MT-MMPs (MMP-14, -15, and -16) have an additional transmembrane domain, whereas the stromelysins (MMP-1, -3, and -10) have a hemopexin domain. The gelatinases (MMP-2 and -9) have a unique fibronectin-binding domain, allowing them to bind to basement membranes. Thus, gelatinases specifically attack type IV collagen, laminin, and fibronectin. The common propeptide domain maintains the latency state of the enzyme, with removal of the signal peptide of this domain activating enzymes. Activation can also occur through a change in configuration. Proteolytic removal or reconfiguration of the propeptide domain activates the enzymes. Exposure of the catalytic site is referred to as the "cysteine switch".4 The various MMPs exist as secreted or membrane-bound enzymes that require conversion from zymogen to active forms through proteolytic processing, thus ensuring that MMP expression is tightly regulated at the transcriptional and posttranslational levels. Additionally, MMPs are also regulated extracellularly by proteins known as tissue inhibitors of metalloproteinases (TIMPs).5 The strict control of the activity of MMPs at multiple levels, such as transcription, posttranslational modification, and interaction with TIMPs is important, because MMPs play sophisticated roles and are extremely deleterious if they are erroneously activated.
Figure 1.
MMP families.
INCREASED MMPs IN EXPERIMENTAL CEREBRAL ISCHEMIA
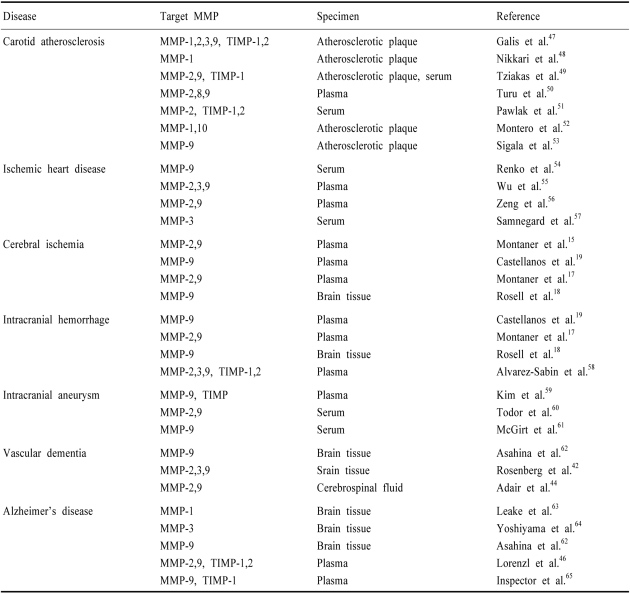
Recent studies have revealed the involvement of MMPs with clinical vascular diseases (Table 2). Ischemic stroke is often associated with disruption of the blood-brain barrier (BBB), leading to vasogenic edema and hemorrhage. Numerous animal studies suggest that disruption of the BBB and hemorrhagic transformation in ischemic stroke results from MMP activation in pathologic conditions.6-9 A deleterious role of MMPs associated with BBB injury has been demonstrated by a reduced infarct size and less BBB damage after focal ischemia in MMP-9 knockout mice,7,9 and by both MMP activation and BBB injury being reduced in mice with superoxide dismutase overexpression.10 In nonhuman primates, early opening of the BBB after ischemic stroke correlates with elevated MMP-2, whereas hemorrhagic transformation is associated with elevated MMP-9.11,12
Table 2.
Clinical vascular diseases, target MMPs, and specimens in various studies
CLINICAL IMPLICATION OF MMPs IN ISCHEMIC STROKE
Gelatin zymography has been used to compare MMP activity between infarcted and matched noninfarcted cerebral tissue of acute or chronic ischemic stroke patients.13 MMP-9 activity was markedly elevated in the acute ischemic stroke tissue, whereas increases in MMP-2 activity were subtle at 2~5 days after ischemic stroke.13 An association between elevated MMPs and BBB disruption is also supported by the significant correlation between the level of MMP-9 and stroke severity being observed in stroke patients.14,15 The MMP-9 levels measured by ELISA after acute cardioembolic stroke and the final NIHSS score are closely related,15 and MMP-9 appears also to be especially associated with hemorrhagic transformation.16-18 One shortcoming of analyzing the correlation between biomarkers and the clinicoradiologic outcome is the uncertainty of the cause-and-effect relationship. An increase in plasma MMP-9 levels may result from an acute-phase reaction or prior systemic causes. However, some studies have found that high plasma MMP-9 levels were related to hemorrhagic transformation, because increased MMP-9 levels were detected on admission in patients who subsequently developed hemorrhagic transformation in whom the initial stroke severity and final infarct volume were similar to those without hemorrhagic transformation.16,19 Interestingly, a correlation between the baseline MMP-9 level and hemorrhagic complications after thrombolysis treatment has been observed, with the baseline MMP-9 level being a possible predictor of hemorrhagic complications after tPA thrombolysis.17 Measurement of the pretreatment level of MMP-9 was suggested as a possible safety measure when selecting candidates for thrombolysis. These data suggest a role for MMP-2 and MMP-9 in both the acute and chronic states of cerebral ischemia, BBB disruption, and efficacy and complication rate of tPA treatment. Future studies should focus on the temporal profiles of activation, selective activation among MMPs families, and the source of released MMPs.
tPA TREATMENT AND THE PA AXIS
PA and MMPs are the major proteases that modulate the ECM in the brain. Emerging data show important linkages between tPA, MMPs, vasogenic edema, and hemorrhage after stroke. Thrombolysis using tPA is the only FDA-approved treatment modality in acute stroke patients. The beneficial effect of tPA is intravascular thrombolysis restoring the blood flow to the ischemic parenchymal tissue. However, when tPA gains access to the extravascular neural parenchyma through a compromised BBB, any interaction between the NMDA receptors and exogenous tPA is a potential threat to the neural parenchyma. In addition to the direct neurotoxicity of tPA,20,21 vasogenic edema or parenchymal hemorrhage induced by BBB disruption is another deleterious complication. MMP-9 may attack components of the basal lamina of cerebral vessels, and its activation can cause neurovascular damage of the BBB, leading to edema and hemorrhagic complications.6,22,23 The increased risk of edema and hemorrhagic complications in stroke patients following tPA therapy may therefore be due to tPA-induced upregulation of MMP-9. Plasmin is a potent protease that cleaves blood fibrin and activates other proteases, and thus is implicated in neurovascular and ECM degradation. Plasmin has an important role in the mechanism of tPA-induced hemorrhage by activating MMP-9 and MMP-2.24,25 Whilst tPA can resolve intravascular blood clots, it can also produce abnormally high levels of plasmin that can trigger excessive activation of MMPs, leading to uncontrollable BBB disruption and, finally, intracranial hemorrhagic complications. MMP-9 levels are increased by tPA after experimentally induced focal ischemic stroke,26,27 and MMP inhibitors reduce tPA-induced hemorrhagic transformation.27,28 These data suggest that the activation of MMP-9 by administered tPA induces ischemic injury in the neurovascular unit. Therefore, modulating tPA and MMP-9 activity may provide a new approach for reducing complications of tPA thrombolysis. Once the clinical effects of MMPs are determined, clinical trials to modulate their activity could be started. Coadministration of MMP inhibitor or TIMPs with thrombolytic agents could avoid BBB disruption or ECM degradation. However, in clinical settings, such a drug should be highly tissue specific and avoid disturbing the physiologic ECM-rebuilding properties. Simple downregulation of MMP levels could evoke other side effects. Many MMP inhibitors have been tested for their efficacy in preventing cancer and rheumatoid arthritis,29,30 but their side effects have so far precluded clinical trials. Thus, balanced modulation of MMP represents the major challenge for future stroke therapies.
MMPs IN STROKE RECOVERY
MMPs are known to be rapidly upregulated at 24~48 hours after ischemia,12,25,31 but little is known about their role during the subsequent recovery phase. The mechanism of stroke recovery mainly involves neuronal plasticity, neurogenesis, and angiogenesis.32,33 MMPs may be critical for this recovery process because they can remodel components of the ECM. MMPs participate in dendritic and axonal extension as well as blood vessel formation.34 A recent study showed a beneficial role of MMP-9 in the recovery phase of experimental stroke,35 with surrogate markers of neuronal plasticity and vascular remodeling being correlated with MMP-9 signals in neurons and astrocytes in the peri-infarction area. Delayed inhibition of MMPs at 7 days poststroke worsened outcomes at 14 days by decreasing neurovascular remodeling, increasing infarction volumes, and blunting behavioral recovery.35 MMPs may in fact play beneficial roles during delayed stroke recovery, despite their deleterious effects in the acute phase of stroke. MMPs, and in particular MMP-9, have emerged as a target for stroke therapy in recent years.36 Pathophysiologic data from cell and animal models have been increasingly validated by the correlations between MMP-9 biomarkers and clinical outcomes in patients.16,17 Therefore, there is growing momentum toward the development of MMP inhibitors for stroke therapy. In contrast to the therapeutic potential of MMP inhibition for acute stroke therapy, approaches to modulating or even enhancing MMPs in the late stages of stroke may represent novel techniques for improving stroke recovery and enhancing the long-term functional outcome. However, future studies should focus on how to modulate such a delicate and orchestrated role of MMPs according to the different phases of stroke.
MMPs IN SUBCORTICAL ISCHEMIC VASCULAR DEMENTIA
The significance of the white matter lesions that appear in various neurodegenerative diseases is still debated. The pathophysiology of subcortical ischemic vascular dementia or CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy) is significantly associated with white matter lesions.37-40 Demyelination around affected subcortical blood vessels, activated microglia, and macrophages can also be found in white matter lesions.38,41 Brain tissue in patients with vascular dementia show fibrosis of small blood vessels with adjacent demyelination demyelination. Increased levels of several MMPs have also been observed in some patients with vascular dementia, with MMP-3 observed in reactive astrocytes and microglial cells.42 After controlling for the variation of MMP expression with age,43 the level of active MMP-9 in the cerebrospinal fluid or plasma of patients with vascular dementia still varies from that in Alzheimer's disease and other neurodegenerative conditions.44-46 MMPs may play an important role in nearly all kinds of dementia, especially in vascular dementia, but whether they are more important for the disease progress or are only an epiphenomenon of tissue adjustment is not clear. The profile of MMPs may in the future be a helpful marker for differentiating between subtypes of dementia.
CONCLUSIONS
MMPs are implicated in all cerebrovascular diseases and play a deleterious role in the pathophysiology of BBB disruption, neuroinflammation, oxidative stress, and delayed neuronal cell death. MMPs also have a role in the subacute or recovery phase through glial scarring, neuronal cell migration, and brain tissue recovery. Therapeutic strategies designed to modulate the activity of MMPs should consider these pleiotropic roles, and hence oversimplified experimental approaches with MMP inhibitors are likely to produce disappointing results. Carefully considering the various effects of MMPs is a challenge for future studies.
Footnotes
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (no. A050079).
References
- 1.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 2.Yong VW, Power C, Forsyth P, Edwards DR. Metalloproteinases in biology and pathology of the nervous system. Nat Rev Neurosci. 2001;2:502–511. doi: 10.1038/35081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg GA. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. [DOI] [PubMed] [Google Scholar]
- 4.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metallopro teinase gene family. Proc Natl Acad Sci U S A. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- 7.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Asahi M, Sumii T, Fini ME, Itohara S, Lo EH. Matrix metalloproteinase 2 gene knockout has no effect on acute brain injury after focal ischemia. Neuroreport. 2001;12:3003–3007. doi: 10.1097/00001756-200109170-00050. [DOI] [PubMed] [Google Scholar]
- 9.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Chang DI, Hosomi N, Lucero J, Heo JH, Abumiya T, Mazar AP, et al. Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J Cereb Blood Flow Metab. 2003;23:1408–1419. doi: 10.1097/01.WCB.0000091765.61714.30. [DOI] [PubMed] [Google Scholar]
- 12.Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR. Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett. 1997;238:53–56. doi: 10.1016/s0304-3940(97)00859-8. [DOI] [PubMed] [Google Scholar]
- 14.Horstmann S, Kalb P, Koziol J, Gardner H, Wagner S. Profiles of matrix metalloproteinases, their inhibitors, and laminin in stroke patients: influence of different therapies. Stroke. 2003;34:2165–2170. doi: 10.1161/01.STR.0000088062.86084.F2. [DOI] [PubMed] [Google Scholar]
- 15.Montaner J, Alvarez-Sabin J, Molina C, Angles A, Abilleira S, Arenillas J, et al. Matrix metalloproteinase expression after human cardioembolic stroke: temporal profile and relation to neurological impairment. Stroke. 2001;32:1759–1766. doi: 10.1161/01.str.32.8.1759. [DOI] [PubMed] [Google Scholar]
- 16.Montaner J, Alvarez-Sabin J, Molina CA, Angles A, Abilleira S, Arenillas J, et al. Matrix metalloproteinase expression is related to hemorrhagic transformation after cardioembolic stroke. Stroke. 2001;32:2762–2767. doi: 10.1161/hs1201.99512. [DOI] [PubMed] [Google Scholar]
- 17.Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- 18.Rosell A, Ortega-Aznar A, Alvarez-Sabin J, Fernandez-Cadenas I, Ribo M, Molina CA, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 19.Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. [PubMed] [Google Scholar]
- 20.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 21.Matys T, Strickland S. Tissue plasminogen activator and NMDA receptor cleavage. Nat Med. 2003;9:371–372. doi: 10.1038/nm0403-371. [DOI] [PubMed] [Google Scholar]
- 22.Lo EH, Wang X, Cuzner ML. Extracellular proteolysis in brain injury and inflammation: role for plasminogen activators and matrix metalloproteinases. J Neurosci Res. 2002;69:1–9. doi: 10.1002/jnr.10270. [DOI] [PubMed] [Google Scholar]
- 23.Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995;26:2120–2126. doi: 10.1161/01.str.26.11.2120. [DOI] [PubMed] [Google Scholar]
- 24.Murphy G, Atkinson S, Ward R, Gavrilovic J, Reynolds JJ. The role of plasminogen activators in the regulation of connective tissue metalloproteinases. Ann N Y Acad Sci. 1992;667:1–12. doi: 10.1111/j.1749-6632.1992.tb51590.x. [DOI] [PubMed] [Google Scholar]
- 25.Mun-Bryce S, Rosenberg GA. Matrix metalloproteinases in cerebrovascular disease. J Cereb Blood Flow Metab. 1998;18:1163–1172. doi: 10.1097/00004647-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Aoki T, Sumii T, Mori T, Wang X, Lo EH. Blood-brain barrier disruption and matrix metalloproteinase-9 expression during reperfusion injury: mechanical versus embolic focal ischemia in spontaneously hypertensive rats. Stroke. 2002;33:2711–2717. doi: 10.1161/01.str.0000033932.34467.97. [DOI] [PubMed] [Google Scholar]
- 27.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- 28.Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–3040. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]
- 29.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 31.Gasche Y, Fujimura M, Morita-Fujimura Y, Copin JC, Kawase M, Massengale J, et al. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19:1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Cramer SC, Chopp M. Recovery recapitulates ontogeny. Trends Neurosci. 2000;23:265–271. doi: 10.1016/s0166-2236(00)01562-9. [DOI] [PubMed] [Google Scholar]
- 33.Chmielnicki E, Goldman SA. Induced neurogenesis by endogenous progenitor cells in the adult mammalian brain. Prog Brain Res. 2002;138:451–464. doi: 10.1016/s0079-6123(02)38093-2. [DOI] [PubMed] [Google Scholar]
- 34.Kaczmarek L, Lapinska-Dzwonek J, Szymczak S. Matrix metalloproteinases in the adult brain physiology: a link between c-Fos, AP-1 and remodeling of neuronal connections? EMBO J. 2002;21:6643–6648. doi: 10.1093/emboj/cdf676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 36.Zlokovic BV. Remodeling after stroke. Nat Med. 2006;12:390–391. doi: 10.1038/nm0406-390. [DOI] [PubMed] [Google Scholar]
- 37.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 38.Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry. 1997;63:749–753. doi: 10.1136/jnnp.63.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singhal S, Rich P, Markus HS. The spatial distribution of MR imaging abnormalities in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy and their relationship to age and clinical features. AJNR Am J Neuroradiol. 2005;26:2481–2487. [PMC free article] [PubMed] [Google Scholar]
- 40.van Gijn J. Leukoaraiosis and vascular dementia. Neurology. 1998;51:S3–S8. doi: 10.1212/wnl.51.3_suppl_3.s3. [DOI] [PubMed] [Google Scholar]
- 41.Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H. Alterations in glia and axons in the brains of Binswanger's disease patients. Stroke. 1997;28:1423–1429. doi: 10.1161/01.str.28.7.1423. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg GA, Sullivan N, Esiri MM. White matter damage is associated with matrix metalloproteinases in vascular dementia. Stroke. 2001;32:1162–1168. doi: 10.1161/01.str.32.5.1162. [DOI] [PubMed] [Google Scholar]
- 43.D'Armiento FP, Bianchi A, de Nigris F, Capuzzi DM, D'Armiento MR, Crimi G, et al. Age-related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis. Stroke. 2001;32:2472–2479. doi: 10.1161/hs1101.098520. [DOI] [PubMed] [Google Scholar]
- 44.Adair JC, Charlie J, Dencoff JE, Kaye JA, Quinn JF, Camicioli RM, et al. Measurement of gelatinase B (MMP-9) in the cerebrospinal fluid of patients with vascular dementia and Alzheimer disease. Stroke. 2004;35:e159–e162. doi: 10.1161/01.STR.0000127420.10990.76. [DOI] [PubMed] [Google Scholar]
- 45.Lorenzl S, Albers DS, LeWitt PA, Chirichigno JW, Hilgenberg SL, Cudkowicz ME, et al. Tissue inhibitors of matrix metalloproteinases are elevated in cerebrospinal fluid of neurodegenerative diseases. J Neurol Sci. 2003;207:71–76. doi: 10.1016/s0022-510x(02)00398-2. [DOI] [PubMed] [Google Scholar]
- 46.Lorenzl S, Albers DS, Relkin N, Ngyuen T, Hilgenberg SL, Chirichigno J, et al. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer's disease. Neurochem Int. 2003;43:191–196. doi: 10.1016/s0197-0186(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 47.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikkari ST, O'Brien KD, Ferguson M, Hatsukami T, Welgus HG, Alpers CE, et al. Interstitial collagenase (MMP-1) expression in human carotid atherosclerosis. Circulation. 1995;92:1393–1398. doi: 10.1161/01.cir.92.6.1393. [DOI] [PubMed] [Google Scholar]
- 49.Tziakas DN, Lazarides MK, Tentes IK, Georgiadis GS, Eleftheriadou E, Chalikias GK, et al. Gelatinases [matrix metalloproteinase-2 (MMP-2) and MMP-9] induce carotid plaque instability but their systemic levels are not predictive of local events. Ann Vasc Surg. 2005;19:529–533. doi: 10.1007/s10016-005-5018-6. [DOI] [PubMed] [Google Scholar]
- 50.Turu MM, Krupinski J, Catena E, Rosell A, Montaner J, Rubio F, et al. Intraplaque MMP-8 levels are increased in asymptomatic patients with carotid plaque progression on ultrasound. Atherosclerosis. 2006;187:161–169. doi: 10.1016/j.atherosclerosis.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 51.Pawlak K, Pawlak D, Mysliwiec M. Serum matrix metalloproteinase-2 and increased oxidative stress are associated with carotid atherosclerosis in hemodialyzed patients. Atherosclerosis. 2007;190:199–204. doi: 10.1016/j.atherosclerosis.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 52.Montero I, Orbe J, Varo N, Beloqui O, Monreal JI, Rodriguez JA, et al. C-reactive protein induces matrix metalloproteinase-1 and -10 in human endothelial cells: implications for clinical and subclinical atherosclerosis. J Am Coll Cardiol. 2006;47:1369–1378. doi: 10.1016/j.jacc.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 53.Sigala F, Georgopoulos S, Papalambros E, Chasiotis D, Vourliotakis G, Niforou A, et al. Heregulin, cysteine rich-61 and matrix metalloproteinase 9 expression in human carotid atherosclerotic plaques: relationship with clinical data. Eur J Vasc Endovasc Surg. 2006;32:238–245. doi: 10.1016/j.ejvs.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 54.Renko J, Kalela A, Jaakkola O, Laine S, Hoyhtya M, Alho H, et al. Serum matrix metalloproteinase-9 is elevated in men with a history of myocardial infarction. Scand J Clin Lab Invest. 2004;64:255–261. doi: 10.1080/00365510410006054. [DOI] [PubMed] [Google Scholar]
- 55.Wu TC, Leu HB, Lin WT, Lin CP, Lin SJ, Chen JW. Plasma matrix metalloproteinase-3 level is an independent prognostic factor in stable coronary artery disease. Eur J Clin Invest. 2005;35:537–545. doi: 10.1111/j.1365-2362.2005.01548.x. [DOI] [PubMed] [Google Scholar]
- 56.Zeng B, Prasan A, Fung KC, Solanki V, Bruce D, Freedman SB, et al. Elevated circulating levels of matrix metalloproteinase-9 and -2 in patients with symptomatic coronary artery disease. Intern Med J. 2005;35:331–335. doi: 10.1111/j.1445-5994.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 57.Samnegard A, Silveira A, Tornvall P, Hamsten A, Ericsson CG, Eriksson P. Lower serum concentration of matrix metalloproteinase-3 in the acute stage of myocardial infarction. J Intern Med. 2006;259:530–536. doi: 10.1111/j.1365-2796.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 58.Alvarez-Sabin J, Delgado P, Abilleira S, Molina CA, Arenillas J, Ribo M, et al. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke. 2004;35:1316–1322. doi: 10.1161/01.STR.0000126827.69286.90. [DOI] [PubMed] [Google Scholar]
- 59.Kim SC, Singh M, Huang J, Prestigiacomo CJ, Winfree CJ, Solomon RA, et al. Matrix metalloproteinase-9 in cerebral aneurysms. Neurosurgery. 1997;41:642–666. doi: 10.1097/00006123-199709000-00027. [DOI] [PubMed] [Google Scholar]
- 60.Todor DR, Lewis I, Bruno G, Chyatte D. Identification of a serum gelatinase associated with the occurrence of cerebral aneurysms as pro-matrix metalloproteinase-2. Stroke. 1998;29:1580–1583. doi: 10.1161/01.str.29.8.1580. [DOI] [PubMed] [Google Scholar]
- 61.McGirt MJ, Lynch JR, Blessing R, Warner DS, Friedman AH, Laskowitz DT. Serum von Willebrand factor, matrix metalloproteinase-9, and vascular endothelial growth factor levels predict the onset of cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2002;51:1128–1134. doi: 10.1097/00006123-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Asahina M, Yoshiyama Y, Hattori T. Expression of matrix metalloproteinase-9 and urinary-type plasminogen activator in Alzheimer's disease brain. Clin Neuropathol. 2001;20:60–63. [PubMed] [Google Scholar]
- 63.Leake A, Morris CM, Whateley J. Brain matrix metalloproteinase 1 levels are elevated in Alzheimer's disease. Neurosci Lett. 2000;291:201–203. doi: 10.1016/s0304-3940(00)01418-x. [DOI] [PubMed] [Google Scholar]
- 64.Yoshiyama Y, Asahina M, Hattori T. Selective distribution of matrix metalloproteinase-3 (MMP-3) in Alzheimer's disease brain. Acta Neuropathol (Berl) 2000;99:91–95. doi: 10.1007/pl00007428. [DOI] [PubMed] [Google Scholar]
- 65.Inspector M, Aharon-Perez J, Glass-Marmor L, Miller A. Matrix metalloproteinase-9, its tissue inhibitor (TIMP)-1 and CRP in Alzheimer's disease. Eur Neurol. 2005;53:155–157. doi: 10.1159/000086124. [DOI] [PubMed] [Google Scholar]