Abstract
Purpose
We attempted clinical application of a plastic blade, which is a novel cryopreservation device, for vitrification of human embryos and blastocysts.
Methods
Between February 2003 and December 2007, a total of 4,430 Day 3 embryos from 898 patients (Day 3 group) and 55 blastocysts from 29 patients (blastocyst group) were vitrified and cryopreserved with a plastic device, and subsequently thawed for embryo transfer. Clinical outcomes after thawing and transfer of vitrified embryos and blastocysts were evaluated.
Results
In the Day 3 group, all embryos resulting from 1,441 oocyte retrieval cycles were recovered, and the thawed embryo survival rate was 98.4%. In the blastocyst group, the survival rate after thawing was 100%. A total of 3,026 day 3 embryos and 46 blastocysts were transferred. The pregnancy and implantation rates in the Day 3 group were 25.0% and 15.5%, respectively, and in the blastocyst group the rates were 24.2% and 26.1%, respectively. The miscarriage rates in the Day 3 and blastocyst groups were 18.3% and 50.0%, respectively.
Conclusions
A plastic blade is a useful novel device in cryopreservation of vitrified human embryos.
Keywords: Blastocyst, Cryopreservation, Cryoloop, Cryotop, Embryo, Vitrification
Introduction
The first report documenting a successful pregnancy resulting from the transfer of cryopreserved human embryos, was published approximately 25 years ago [1, 2]. Since this landmark publication, cryopreservation of human embryos has played an important role in assisted reproductive technology (ART). For several years, reduction in the number of transferred embryos has been suggested as a way to reduce the incidence of multi-fetal pregnancy. Moreover, elective single embryo transfer (SET), which was first reported in 1999, is frequently used to prevent twin pregnancy [3]. As the number of redundant embryos has increased, so has the demand for cryopreservation of human embryos. Therefore, a simple and convenient method for cryopreservation of human embryos is needed.
Worldwide, the most common method for cryopreservation of human embryos is the slow-cooling method. However, the entire slow-cooling procedure takes more than 2 h to complete, and requires both an expensive programmable freezer for freezing embryos and large amounts of liquid nitrogen. Recently, the vitrification method, which is a common method for cryopreservation of oocytes and embryos in veterinary medicine, has been used for cryopreservation of human embryos. Subsequently, a vitrification method that uses conventional cryostraws was established [4], and successful pregnancies resulting from human blastocysts that had been vitrified in cryostraws was reported in 2000 [5]; however, the survival rates associated with this procedure have been disappointing. A new vitrification procedure that uses a cryoloop, which enables facile manipulation of embryos during both vitrification and thawing, has been reported. Mukaida et al. have successfully used the cryoloop vitrification procedure for cryopreservation of human blastocysts in a clinical setting [6].
Currently, the vitrification method for cryopreservation of human embryos is becoming increasingly common and cryopreservation devices, such as the cryoloop and cryotop, are commercially available. These devices facilitate embryo manipulation and make vitrification easier to perform compared with the conventional cryostraw. However, information about the embryos, such as the patient’s name, ID number and date of cryopreservation, cannot be written directly on these devices, which is also not possible using the cryostraws, and the cryoloop and cryotop are more expensive than the cryostraw.
Recently, we developed a novel device for use in the vitrification of human embryos. It is made from a thin plastic board and, because it is shaped like a small blade, we call it a “plastic blade.” Since February 2003, we have been using this plastic blade for cryopreservation of human embryos in our clinic. In this paper, we evaluate the clinical outcomes resulting from the transfer of embryos that were vitrified using the plastic blade and subsequently thawed.
Materials and methods
Patients and ovarian stimulation
Among the patients who underwent ART treatment at the Division of Reproductive Medicine, Sugiyama Clinic between February 2003 and December 2007, a total of 4,430 Day 3 embryos from 898 patients (Day 3 group) and 55 blastocysts from 29 patients (blastocyst group) were vitrified and cryopreserved with a plastic device. During this period, a total of 4,572 oocyte retrievals in 1,892 patients were performed in our clinics. These vitrified embryos or blastocysts were derived from the patients who had redundant embryos on day 3 or blastocysts day 5 during these period. The patients who had not performed fresh embryo transfer due to avoidance of ovarian hyperstimulation or early progesterone rise before oocyte retrieval, also were included, but the patients who did not have no redundant embryos or blastocysts for cryopreservation were not included for this evaluation. The number of previous IVF cycles was not used as a participant-selection criterion. All patients who enrolled in this evaluation agreed to the use of the plastic blade during vitrification to cryopreserve their embryos. Informed consent was obtained from each participant. The cryopreserved embryos included both redundant embryos that remained unused after embryo transfer and all embryos that were not transferred for prevention of ovarian hyperstimulation syndrome.
Patients were treated according to three ovarian stimulation protocols as follows: GnRH agonist and human menopausal gonadotropin (hMG; Humegon, Organon, Osaka); recombinant follicle-stimulating hormone (rec-FSH; Follistim, Organon, Osaka) in either a long- or a short-treatment protocol; or, clomiphen citrate (Serophen, Merck Serono, Tokyo) and rec-FSH [7]. When dominant follicles reached ≥18 mm in diameter without an LH-surge, 10,000 IU of human chorionic gonadotropin (hCG; Fuji Pharma, Tokyo) were administered. Oocyte retrieval was performed 35 h after hCG administration.
IVF/ICSI procedure and embryo transfer
The IVF procedure used in this study has been previously described [8]. Oocytes were retrieved transvaginally using a needle-guided technique, aided by ultrasonography. All follicles with a mean diameter >15 mm were aspirated individually, using a 17-gauge needle connected to a tube and a machine for suction (Flush pomp/oocyte Incubator; AIREY Co. Ltd, Fukushima, Japan). The needle was removed after aspiration of each follicle. The aspiration was interrupted and a new syringe was used if blood appeared in the tube connected to the syringe, thus avoiding contamination by blood. All follicles were washed with culture media. Semen was produced by masturbation and, after washing, motile sperm were separated using a 30–60 min swim-up period. In vitro insemination was performed by incubation of each oocyte with 50–100 × 103 motile sperm within 5 h to 6 h of collection. When there was evidence of male factor infertility, in vitro insemination was not performed. Instead, intracytoplasmic sperm injection (ICSI) was performed as previously described [9]. Oocytes were examined using a dissecting microscope 16–18 h after insemination or ICSI. The presence of two pronuclei with extrusion of the second polar body was taken as evidence of successful fertilization. All embryos were cultured in culture media (HUCUM ,Nipro, Osaka) for 72 h after insemination or ICSI. Embryos were assessed 72 h after insemination or ICSI and either one or two embryos were transferred into the uterus of each patient. Embryos containing seven or eight mononucleated blastomeres that were regular, approximately equal in size, with no fragmentation, were classified as grade 1. Those with similar mononucleated blastomeres and less than 10% fragmentation were evaluated as grade 2. Embryos containing less than seven or unequal blastomeres, with no fragmentation, were grade 3. While those with unequal blastomeres and more than 15 % fragmentation were assigned to grade 4. This system of embryo assessment was modified from the classification described by Veek [10]. The embryos which had more than seven blastomeres and showed grade 1 or 2 were classified as good embryo on day 3.
Residual embryos, of sufficient quality, were cryopreserved using the vitrification method. The blastocysts were cultured in HUCUM for 72 h, at which time the culture medium was removed and replaced with Blastocyst Medium (Complete MultiBlast Medium, Irvine Scientific, CA). The blastocysts were then cultured in Blastocyst Medium for an additional 48 h, i.e., until 120 h after insemination or ICSI. The blastocysts were assessed 120 h after insemination or ICSI according to the Gardner’s classification [11].
Either one or two embryos were placed transcervically into the uterus of each patient 72 h or 120 h after in vitro insemination or ICSI, respectively. The residual embryos or blastocysts were cryopreserved using the vitrification method as follows. The patients who showed high estradiol levels on the day of hCG administration agreed to forgo embryo transfer and cryopreserve all embryos 72 h after insemination or ICSI to prevent the onset of ovarian hyperstimulation syndrome. On the day of embryo transfer, and 3 days and 7 days following embryo transfer, each patient received an injection of 100 mg of progesterone for luteal support. A combination of estrogen and progesterone was administered orally for 12 days after embryo transfer.
Vitrification of human embryos and blastocysts
The protocol for the vitrification of human embryos and blastocyst using a plastic blade was adopted from the report by Kuwayama et al. [12] with slight modifications. The vitrification solution used was a commercially avairable kit (Vitirification kit, Kitazato Tokuo). Briefly, Human 8-cell-stage embryos or blastocysts were vitrified by the use of a two-step loading process with a cryoprotectant. Initially, embryos or blastocysts were placed in vitrification solution-ES, which was the base medium (TCM199 medium) containing 7.5% (vol/vol) ethylene glycol (EG) + 7.5% dimethylsulfoxide (DMSO) + 20% synthetic serum substitute (SSS), at room temperature for 5–15 min before being suspended in vitrification solution-VS, which was the same as vitrification solution-ES except that the concentrations were 15% ethylene glycol + 15% DMSO + 0.5 M sucrose. The duration of placing embryos or blastocysts was decided depending on the degree of dehydration of each embryo or blastocyst. After 1 min in vitrification solution-VS, embryos or blastocysts were placed on a plastic blade that was then put into a serum tube filled with filtered liquid nitrogen. The serum tube was immediately submerged into filtered liquid nitrogen.
The plastic blade
IRB approval for use of this tool for cryopreservation of human embryos was secured before its use in a clinical setting. A serum Tube (Sumitomo Bakelite, Tokyo) was employed as a vessel for cryopreservation. A clear polyethylene telephthalate film (50 mm in thick) was cut into a T-shaped piece. As shown in Fig. 1, the horizontal arm of the “T” shape was rolled and fit securely to the inner wall of the cap (Fig. 1). After equilibration with cryo-medium, the embryo for vitrification was placed at the center of a plastic blade, the vertical limb projected from the cap, and five embryos were the maximum allowed on one blade. The width of the plastic blade was significantly wider than that of the cryotp which was commercially available tool for the storage of the vitrified human embryo (Fig. 2). The blade was submerged directly into filtered liquid nitrogen and inserted into the tube that was pre-cooled with liquid nitrogen, then the cap was fastened on the serum tube, which accomplished preservation in the liquid nitrogen container. Information such as name and ID of patient, the embryos and date of cryopreservation could be written directly on the surface of the serum tube.
Fig. 1.
The plastic blades were handmade in our laboratory as follows. A thin plastic board (0.05 mm in thick), which is readily available at a hardware store, was cut into a T-shaped piece using scissors (center picture in the Fig. 1a). The “T” was the securely attached to the cap of a serum tube (right picture in the Fig. 1a). The plastic blade remained tightly attached to the cap of the serum tube because both halves of the wing of the plastic blade were secured in the cap of the serum tube, and the picture of 1b is ready to use. Information about the embryos, such as name of holder, date of cryopreservation, and ID number of patients, was written directly on the surface of the serum tube (left picture in the Fig. 1a)
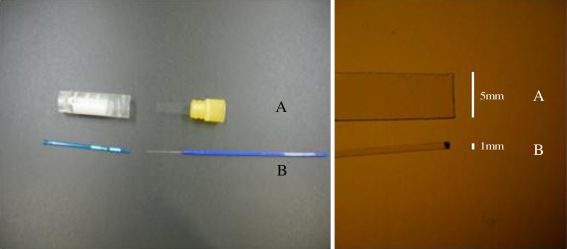
Fig. 2.
This picture compares between the plastic blade (a) with the cryotop® (b). The width of the plastic blade was 5 mm, which is significantly wider than that of the cryotop®
Warming of embryos/blastocysts and assessment of survival
The embryos or blastocysts were warmed using a two-step dilution with sucrose. The serum tube that contained the plastic blade submerged under liquid nitrogen, and serum tube was opened and the plastic blade containing vitrified embryos or blastocysts was removed from the liquid nitrogen and placed directly into the well of the base medium containing 1.0 M sucrose and 20% SSS at 37°C. The embryos or blastocysts immediately fell from the plastic blade into the solution. After 1 min, embryos or blastocysts were transferred to the base medium containing base medium containing 0.5 M sucrose and 20% SSS at room temperature for 3 min. Finally, warmed embryos or blastocysts were washed twice for 5 min in the base medium containing 20% SSS at room temperature and then were returned to either HUCUM medium or Blastocyst Medium for further culture until embryo transfer.
Two hours after thawing, the appearance of the embryos was examined using an inverted microscope at 400× magnification. Survival was assessed based on morphological integrity of the blastomeres and proportion of fragmentation, and the embryo which had at least one intact blastomere was defined as survival. Assessment of blastocysts also included evaluation of inner cell mass, trophectoderm and re-expansion of the blastocoele, and the blastocyst which had an intact inner cell mass was defined as survival. All solutions used in both vitrification and thawing are commercially available (Vitrification kit, KITAZATO BioPhama, Sizuoka, Japan).
Warmed embryo transfer and assessment of pregnancy
Patients underwent embryo transfer during either a natural cycle or a hormone replacement (HR) cycle. All patients who selected HR received transdermal estradiol (Estraderm plaster 0.72 mg, Kissei, Matsumoto) and conjugated estrogens (Premarin 0.625 mg, Wyeth, Tokyo) from the third day of the menstrual cycle until the day of the urinary pregnant test. Administration of progesterone (100 mg in oil) was initiated on the twelfth day of the menstrual cycle. Three days after initiation of progesterone treatment, embryos were thawed and surviving embryos were used in uterine transfer. Blastocysts were thawed and transferred 5 days after initiation of progesterone treatment. A pregnancy was recognized when the development of a gestational sac was detected by transvaginal ultrasound imaging on the 21st day after oocyte retrieval.
Results
The clinical outcomes resulting from transfer of embryos and blastocysts that were vitrified using the plastic blade are summarized in Table 1. During this period, a total of 4,572 oocyte retrievals in 1,892 patients were performed and 18,511 oocytes were retrieved in our clinics. A total of 4,430 Day 3 embryos derived from 1,441 cycles of oocyte retrieval in 898 patients (Day 3 Embryo group) and 55 blastocysts derived from 33 cycles of oocyte retrieval in 29 patients (blastocyst group) were vitrified and thawed.
Table 1.
The background for both groups
| Day 3 embryo group | Blastocyst group | |
|---|---|---|
| Patient number | 898 | 29 |
| OPU cyclesa | 1,689 | 45 |
| Age (years)b | 35.4 ± 4.4 | 34.6 ± 3.8 |
| Indications for ART | ||
| Tubal factor | 118 | 4 |
| Male factor | 298 | 11 |
| Endometriosis | 68 | 1 |
| Unexplained infertility | 414 | 13 |
| IVF/ICSI | 791/898 | 21/24 |
| Ovarian stimulation (cycles)c | ||
| CC with recFSH | 1267 | 34 |
| Rec-FSH with GnRH-antagonist | 132 | 4 |
| Long protocol/Short protocol | 172/118 | 5/2 |
aOPU: oocyte pick up
bAge: mean age
cCC: clomiphen citrate, recFSH; recombinant follicle-stimulating hormone, GnRH; gonadotropin-releasing hormone
The mean number of Day 3 embryos in the Day 3 embryos and blastocysts in the blastocyst groups per patients were 4.1 ± 0.2 and 2.2 ± 0.2, respectively (mean±S.E.). In the Day 3 Embryo group, all embryos resulting from 1,441 oocyte retrieval cycles were recovered, and the thawed embryo survival rate was 98.4%. In the Blastocyst group, all blastocysts resulting from 33 cycles of oocytes retrieval were recovered and survival after thawing was 100%. Because all embryos survived, cancellation of embryo transfer was not required. A total of 3,026 day 3 embryos and 46 blastocysts were transferred. For the warmed embryo transfer, natural cycle was used in 925 cycles of the Day 3 embryo group and in 21 cycles of blastocyst group. On the other hand, hormone replacement cycles were used in 516 cycles of the day 3 groups and 12 cycles in the blastocyst group. The mean number of transferred embryos in the Day 3 Embryo and Blastocyst groups were 2.1 ± 0.2 and 1.2±0.1, respectively, and the percentage of good embryo before cryopreservation was 75.9% and 57.8%, respectively (Table 2).
Table 2.
Clinical outcomes resulting from the transfer of vitrified, and subsequently thawed, 3-day old embryos and blastocysts using a plastic blade
| Day 3 embryo group | Blastocyst group | Total | |
|---|---|---|---|
| Estradiol level on hCG in OPU cycle (pg/ml)a | 976.1 ± 92.2 | 897 ± 53.8 | 951 ± 57.2 |
| No. of retrieved oocytes per OPU cyclea | 5.2 ± 0.3 | 4.8 ± 0.3 | 5.0 ± 0.3 |
| No. of embryos created OPU cyclea | 4.1 ± 0.2 | 2.2 ± 0.2 | 4.1 ± 0.2 |
| No.of transferred embryo in fresh cyclea | 1.4 ± 0.2 | 1.0 ± 0.2 | 1.3 ± 0.2 |
| Average age (years)a | 35.4 ± 4.4 | 34.6 ± 3.8 | 35.0 ± 4.0 |
| No. of cycles for vitrification | 1,441 | 33 | 1,474 |
| No. of cycles for thawing and transfer | 1,441 | 33 | 1,474 |
| No. of embryos vitrified | 4,430 | 55 | 4,485 |
| Mean number of embryos vitrified (per OPU cycle)a | 2.6 ± 0.2 | 1.2 ± 0.1 | 2.6 ± 0.2 |
| No. of embryos recovered | 4,430 | 55 | 4.485 |
| Recovery rate (%) | 100 | 100 | 100 |
| No. of surviving embryos | 4,357 | 55 | 4,412 |
| Survival rate (%) | 98.4 | 100 | 98.4 |
| No. of transferred embryos | 3,026 | 46 | 3,072 |
| Mean number of warmed transferred embryosa | 2.1 ± 0.2 | 1.2 ± 0.1 | 1.9 ± 0.2 |
| No. of embryos implanted | 468 | 12 | 480 |
| No. of pregnant cycles | 360 | 8 | 368 |
| Pregnancy rate (%) | 25.0 | 24.2 | 25.0 |
| Miscarriage rate (%) | 18.3 | 50 | 19.0 |
| Good embryob rate on cryopreserved (%) | 75.9 | 57.8 | |
| No. of live birth | 294 | 4 | 298 |
OPU oocyte pick up
aMean±S.E.M
bThe embryos which had more than seven blastomeres and showed grade 1 or 2 were classified as good embryo on day 3. The blastocysts which showed more than 3AA of Gardner’s classification were defined as good blastocyst
The pregnancy and implantation rates in the Day 3 Embryo group were 25.0% and 15.5%, respectively, and in blastocyst group the rates were 24.2% and 26.1%, respectively. To evaluate pregnancy rate classifying all patients into the four subgroups according to maternal age, the pregnancy rates in the groups with a maternal age of less than 30 years of age, from 30 to 34, from 35 to 39, and more than 40 years of ages were 45.2%, 33.3%, 32.6% and 19.3%, respectively. The pregnancy rate in the group with more than 40 years of age was significantly lower than it was for the group of less than 30 years of age (p < 0.01). The miscarriage rates in each group were 18.1%, 16.7%, 23.8%, and 48.8%, respectively. Again, the rate for the group with more than 40 years of age was significantly higher than that of the youngest group (p < 0.01) (Table 3).
Table 3.
The pregnancy and miscarriage rates according to maternal age in the Day 3 groups
| Maternal age (years) | Pregnancy rate (%) | Miscarriage rate (%) |
|---|---|---|
| <30 | 45.2 | 18.1 |
| 30–34 | 33.3 | 16.7 |
| 35–39 | 32.6 | 23.8 |
| ≥40 | 19.3 | 48.8 |
The miscarriage rates in the Day 3 Embryo and blastocyst groups were 18.3% and 50.0%, respectively; from a total of 368 pregnancies, 298 healthy babies were born.
Discussion
Recently, vitrification, which was originally developed using mouse embryos [13], has been applied to the cryopreservation of human embryos. Vitrification is defined as the solidification of a solution at a temperature below the glass transition temperature of the solution, by an extreme increase in viscosity rather than by ice crystallization, using high cooling rates ranging from −15,000 to −30,000°C per minute [14]. This ice-free cryopreservation method has been modified to achieve optimal results with human embryos. However, there are two major shortcomings of the vitrification technique: (1) high concentrations of cryoprotectants are required, which may be toxic to oocytes and embryos; and, (2) if the cooling rates applied are insufficient, intracellular ice formation and freezing may occur [9]. To overcome these problems, several methods have been devised, in which very small volumes of vitrification solution are used. For example, use of 1 μL of solution and carrier systems of minimal capacity increase the rate at which heat dissipates from the sample, so that the sample cools very rapidly. Various techniques to overcome the limitations of vitrification have been described in the literature [15] and applied with varying degrees of success in several mammalian species, including humans.
Mukaita et al. described a simple method for vitrification of human embryos in the four- to eight-cell stage that uses an ethylene glycol-based solution and conventional cryostraws3. Prior to the clinical application of a cryoloop, the clinical outcomes of several methods of cryopreservation, including slow freezing and vitrification, were inconsistent [16]. Recently, however, techniques that use the cryoloop and therefore require substantially samller volumes of vitrification solution have improved the clinical success of vitrification [17, 18]. A major difference between use of the cryoloop and of the conventional straw in vitrification is that the cryoloop allows for ultra-rapid cooling and warming, thereby preventing intracellular ice formation.
A significant increase in the cooling rate allows for reduction in the cryoprotectant concentration. As a result, a high cooling rate both reduces freeze injury and allows for a reduction in cryoprotectant concentration, thereby preserving the cells at nontoxic concentrations of cryoprotectant. The most recently developed vitrification system, the cryotop, which was designed by Kuwayama et al. [19], allows the samples to be loaded into a very small volume of vitrification solution.
Since the start of our clinic stared in 2003, we have used only the vitrification method for embryo cryopreservation and have not performed slow freezing method. Moreover, this plastic blade has been used since the start of our clinic. Therefore, we have no data for cryopreservation through the use of either the slow freezing method or for the other devices used for vitrification. During this study period, the pregnancy rate in the fresh day 3 embryo transferred cycles in our clinic was 25.2%, and this rate in the warmed Day 3 embryo group was 25.0% and comparable to the fresh one, however, these pregnancy rates for both fresh and warmed embryo transfer cycles were somewhat lower because of advanced maternal ages. However, the pregnancy rate for a younger age of the warmed embryo transfer cycle in our clinic was 45.2% and, which is comparable to the previous report [5].
Devices such as the cryoloop and cryotop are designed both to reduce the volume of cryoprotectant required for vitrification and to simplify the procedure. Although both cryoloop and cryotop achieve these goals, they are difficult to produce in-house and the commercially available kit, are rather expensive (cryoloops cost approximately $20 (US) per device). In contrast, the plastic blade is easily made in the laboratory at a significantly lower cost (plastic blades approximately $ 0.7 (US) per device. The fact that this new device used in the cryopreservation for human embryos in our clinic was widely announced to the patients by the booklet. The clinical outcomes resulting from the transfer of embryos and blastocysts that were vitrified using the plastic blade were comparable to those resulting from the transfer of embryos that were vitrified using the either the cryotop or the cryoloop methods [12, 16]. In total, approximately 300 healthy babies were born as a result of the transfer of embryos that were vitrified using the plastic blade and subsequently thawed. Based on these results, we conclude that the plastic blade is a very convenient and useful device for cryopreservation of human embryos.
There were two major concerns associated with this method. First is the use of relatively high concentrations of cryoprotectants, which might harm the embryo and interfere with subsequent intrauterine growth. However, the results of the present study indicate that vitrification of human blastocysts using a plastic blade is safe for clinical use based on the perinatal outcomes evaluated for vitrification using a cryoloop [20]. The second concern is the possibility of viral contamination during storage in liquid nitrogen (LN), because embryos in a cryoloop are directly exposed to LN during vitrification where the cryovial is not completely sealed. Transmission of hepatitis B virus to patients via transplantation of bone marrow cells stored in contaminated LN has been reported [21]. Viral contamination was detected in zona pellucida of bovine embryos stored in unsealed containers in LN. The bovine virus was present in high concentrations-equivalent to the viremic stage [22]. The possibility of viral contamination using the plastic blade is extremely low, even for embryos from viremic patients, because the plastic blade on which the embryo is placed is put into a serum tube and the cap is fastened tightly. Therefore, liquid nitrogen should not enter the sealed tube. Moreover, all patients are routinely screened for viral infections, such as syphilis, HIV, hepatitis B and hepatitis C. The embryos derived from patients who test positive for these viral diseases are not cryopreserved. Thus, it is unlikely that the storage tank would be contaminated by virus-infected embryos.
In conclusion, the plastic blade is a simple device for use in cryopreservation of human embryos and blastocysts that can be made in most laboratories. The cost of making a plastic blade is much less than the expense of purchasing a commercially available kit, such as the cryoloop or cryotop. Therefore, a plastic blade is a useful novel device in cryopreservation of vitrified human embryos.
Footnotes
Capsule
A plastic blade that can easily be made in the laboratory for use is a simple and useful device in cryopreservation of vitrified human embryos.
References
- 1.Trounson A, Mohr L. Human pregnancy following cryopreservation; thawing and transfer of an eight-cell embryo. Nature. 1983;305:707–9. doi: 10.1038/305707a0. [DOI] [PubMed] [Google Scholar]
- 2.Zeilmaker GH, Alberda AT, Gent I, Rijkmans CM, Drogendijk AC. Two pregnancies following transfer of intact frozen-thawed embryos. Fertil Steril. 1984;42:293–6. doi: 10.1016/s0015-0282(16)48029-5. [DOI] [PubMed] [Google Scholar]
- 3.Vilska S, Tiitinen A, Hyden-Granslog C, Hovatta O. Elective transfer of one embryo results in an acceptable pregnancy rate and eli, imates the risk of multiple births. Hum Reprod. 1999;14:2392–5. doi: 10.1093/humrep/14.9.2392. [DOI] [PubMed] [Google Scholar]
- 4.Mukaida T, Wada S, Takahashi K, Pedro PB, An TZ, Kasai M. Vitrification of human embryos based on the assessment of suitable conditions for 8-cell mouse embryos. Hum Reprod. 1998;13:2874–9. doi: 10.1093/humrep/13.10.2874. [DOI] [PubMed] [Google Scholar]
- 5.Yokota Y, Sato S, Yokota M, Ishikawa Y, Mukaida M, Asada T, et al. Successful pregnancy following blastocyst vitrification. Hum Reprod. 2000;15:1802–3. doi: 10.1093/humrep/15.8.1802. [DOI] [PubMed] [Google Scholar]
- 6.Mukaida T, Nakamura S, Tomiyama T, Wada S, Kasai M, Takahashi K. Successful birth after transfer of vitrified human blastocysts with use of a cryoloop containerless thechnique. Fertil Steril. 2001;76:618–20. doi: 10.1016/S0015-0282(01)01968-9. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama R, Nakagawa K, Nishi Y, Sugiyama R, Ezaki K, Inoue M. The dilemma faced by patients who undergo single embryo transfer. Reprod Med Biol 2009;8:33–37. [DOI] [PMC free article] [PubMed]
- 8.Nakagawa K, Ohgi S, Kojima R, Ito M, Horikawa T, Irahara M, Saito H. Reduction of perifollicular arterial blood flow resistance after hCG administration is a good indicator of the recovery of mature oocytes in ART treatment. J Assist Reprod Genet. 2006;23:433–8. doi: 10.1007/s10815-006-9087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa K, Yamano S, Moride N, Yamashita M, Yoshizawa M, Aono T. Effect of activation with Ca ionophore A23187 and puromycin on the development of human oocytes that failed to fertilize after intracytoplasmic sperm injection. Fertil Steril. 2001;76:148–52. doi: 10.1016/S0015-0282(01)01839-8. [DOI] [PubMed] [Google Scholar]
- 10.Veeck LL. Atlas of the Human Oocyte and Early Conceptus. Vol. 2, Williams & Wilkins Co, 1991.
- 11.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–8. doi: 10.1016/S0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 12.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300–8. doi: 10.1016/s1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 13.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryo at -196 egrees C by vitrification. Nature. 1985;313:573–5. doi: 10.1038/313573a0. [DOI] [PubMed] [Google Scholar]
- 14.Liebermann J, Dietl J, Vanderzwalmen P, Tucker MJ. Recent developments in human oocyte, embryo and blastocyst vitrification: were are we now? Reprod Biomed Online. 2003;7:622–33. doi: 10.1016/s1472-6483(10)62084-6. [DOI] [PubMed] [Google Scholar]
- 15.Liebermann J, Nawroth F, Isachenko V, Isachenko E, Rahimi G, Tucker MJ. Potential importance vitrification in reproductive medicine. Biol Reprod. 2002;67:1674–80. doi: 10.1095/biolreprod.102.006833. [DOI] [PubMed] [Google Scholar]
- 16.Mukaida T, Nakamura S, Tomiyama T, Wada S, Oka C, Kasai M, Takahashi K. Vitrification of huma blastocysts using cryoloop: clinical outcome if 223 cycles. Hum Reprod. 2003;18:384–91. doi: 10.1093/humrep/deg047. [DOI] [PubMed] [Google Scholar]
- 17.Lane M, Schoolcraft WB, Gardner DK. Vitrification of mouse and human blastocysts using a novel cryoloop container-less technique. Fertil Steril. 1999;72:1073–8. doi: 10.1016/S0015-0282(99)00418-5. [DOI] [PubMed] [Google Scholar]
- 18.Lane M, Bavister BD, Lyona EA, Forest KT. Container-less vitrification of mammalian oocytes and embryos. Nat Biotechnol. 1999;17:1234–6. doi: 10.1038/70795. [DOI] [PubMed] [Google Scholar]
- 19.Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod Biomed Online. 2005;11:608–14. doi: 10.1016/s1472-6483(10)61169-8. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi K, Mukaida T, Goto T, Oka C. Perinatal outcome of blastocyst transfer with vitrification using cryoloop: a 4-year follow-up study. Fertil Steril. 2005;84:88–92. doi: 10.1016/j.fertnstert.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 21.Tedder RS, Zuckerman MA, Goldstone AH, Hawkins AE, Fielding A, Briggs EM, Irwin D, Blair S, Gorman MA, Patterson KG. Hepatitis B transmission from contaminated cryopreservation tank. Lancet. 1995;346:137–49. doi: 10.1016/S0140-6736(95)91207-X. [DOI] [PubMed] [Google Scholar]
- 22.Bielanski A, Nadin-Davis S, Sapp T, Lutez-Wallance C. Viral contamination of embryos cryopreserved in liquid nitrogen. Cryobiol. 2004;40:110–6. doi: 10.1006/cryo.1999.2227. [DOI] [PubMed] [Google Scholar]