Abstract
Purpose
To describe a case of homogeneous macro vacuolar formation in oocytes from a patient undergoing IVF/ICSI over 3 cycles and implications thereof.
Methods
Case report and analysis of cycle outcomes including spindle visualization to determine severity of internal disruption caused.
Results
Characteristic macro vacuoles (>25 μm diameter) were identified in the vast majority of oocytes. Spindle visualization identified that macro vacuoles were not associated with spindle displacement. IVF resulted in failed fertilization. Low numbers of fertilized oocytes were obtained through ICSI. The vast majority of embryos exhibited maturation arrest on day 2.
Conclusions
It is likely that persistent Oocyte Macro Vacuolization is the cause of infertility in this patient. It probably has a biological basis and possibly a genetic cause, resulting in either uncontrollable endocytosis or poor exocytosis and consequent vacuolar formation.
Keywords: ICSI, Infertility, Oocyte, Spindle, Vacuole
Introduction
Oocyte developmental competence, the ability to undergo successful fertilization and embryogenesis, is highly dependent on successful maturation. During assisted reproductive treatments oocyte maturation is induced via the administration of exogenous hCG following follicle stimulation using gonadotrophins.
Accumulation of RNA molecules and proteins within the ooplasm is crucial as the oocyte prepares to undergo fertilization and embryogenesis. One of the most fundamental determinants of oocyte developmental competence is the attainment of nuclear maturity. This complex process involves: germinal vesicle breakdown, chromosome segregation, asymmetric meiotic division, expulsion of the first polar body and organisation of oocyte cytoskeletal structure. The MII spindle, constructed of microtubules arranged around centrosomic poles, plays an essential role in fertilization. Sperm penetration through the zona pellucida triggers resumption of meiosis and expulsion of the second polar body. Once the male and female pronuclear envelopes fuse, mixing maternal and paternal DNA, the chromosomes align along the newly formed mitotic spindle in preparation for segregation into separate blastomeres during embryonic cleavage.
Cytoplasmic abnormalities that occur during oocyte maturation, especially those that interfere with the meiotic spindle and intricate cytoskeletal structure, can thus adversely affect the ability of oocytes to undergo normal fertilization and embryo development.
Oocyte vacuolization is a common observation in clinical IVF. Ebner et al. [1] found vacuoles in 3.9% of oocytes at collection, of which 66% had single, 21.3% had double and 12.7% had multiple vacuoles. Other studies [2, 3] suggest a slightly higher oocyte vacuolization rate (5.7% & 12.4% respectively).
Van Blerkom and colleagues identified the fluid within such large membrane-bound vacuoles to be virtually identical to that found within the periviteline space [4]. Therefore, it is likely that these structures form either due to uncontrollable endocytosis or via fusion of pre-existing vesicles produced by the smooth endoplasmic reticulum and golgi apparatus that would usually be exocytosed [1]. Otsuki et al. [5] used transmission electron microscopy to identify 2 different types of vacuole, smooth endoplasmic reticulum clusters (sERCs), formed by accumulation of smooth endoplasmic reticulum and ‘fluid filled’ vacuoles, at the same time noting that sERC positive cycles resulted in significantly lower clinical pregnancy rates. In contrast Ebner et al. [6] found no significant difference in clinical pregnancy rate but significantly higher miscarriage rates, lower birth weights and greater obstetric problems in pregnancies from cycles where some oocytes collected exhibited sERCs. Rienzi and colleagues [7] identified significantly lower fertilization rates in vacuoled oocytes. It has previously been demonstrated that extremely large cytosolic vacuoles physically displace the MII spindle from its usual polar position and thus might cause fertilization failure, as well as zygotic and embryonic arrest [4]. Other groups have observed lower than normal fertilization rates if vacuoles are up to 14 μm (51.6% for single and 43.8% for multiple vacuoles) and suggest that fertilization cannot be expected if vacuoles are >14 μm [1].
Case report
A couple presented with a history of 2 years primary infertility. The female partner, aged 29, was diagnosed with polycystic ovarian syndrome (PCOS) with elevated LH and low TSH. Day 2 FSH was normal (between 7.2 and 10 iu/L). Periods were irregular (35–60 days). She had previously smoked 10 cigarettes per day. The male partner, aged 31, presented with a relatively normal semen profile. On the basis of the investigations and diagnosis, the couple were initially given two cycles of clomiphene citrate treatment, which resulted in ovulation but not pregnancy. In addition, the PCOS failed to respond to metformin treatment or ovarian drilling. The couple were subsequently referred for IUI treatment and underwent two unsuccessful cycles.
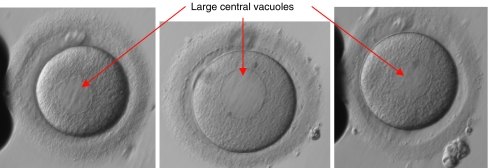
Four years after initial referral the couple received IVF treatment. Standard ‘step-down’ ‘long protocol’ stimulation was performed using GnRH agonist (Gonapeptyl 3.75 mg depot, Ferring) administered for down regulation and ovarian stimulation with urinary gonadotrophins (Menopur, Ferring) (starting dose 300 iu/day, reducing to 150 iu/day on day 7). hCG (Pregnyl, Organon) was administered 36 h prior to oocyte collection on day 14. Fifteen oocytes were collected with ease from a cohort of follicles including 13 >14 mm diameter. The sperm sample was deemed suitable for IVF with parameters of: 244 million/ml, 26% rapid and 63% progressive motility, however all 15 oocytes failed to fertilize. On investigation all oocytes were mature (Metaphase II stage). Twenty-four hour sperm survival was normal but sperm-zona pellucida binding was minimal. Visualization of the oocytes at x200 magnification identified the presence of large central vacuoles in the ooplasm of the majority of oocytes.
Following failed fertilization at IVF the couple returned for a cycle of ICSI. Standard ‘step-down’ ‘long protocol’ stimulation was performed using a GnRH agonist (Gonapeptyl 3.75 mg depot, Ferring) administered for down regulation and ovarian stimulation with urinary gonadotrophin (Menopur, Ferring) (starting dose 600 iu/day, reducing to 300 iu/day on day 6). hCG (Pregnyl, Organon) was administered 36 h prior to oocyte collection on day 14. Twenty oocytes were collected with ease from a cohort of follicles including 18 >14 mm diameter. Three oocytes were identified as Metaphase I, and 17 as Metaphase II and thus suitable for injection. When performing the ICSI procedure it was noted that more than 90% of the oocytes contained very large central vacuoles (See Fig. 1), the largest of which were estimated to be over 30% of the volume of the oocyte. These vacuoles were similar in appearance to the ‘sERCs’ described by other authors [5]. Extreme care was taken not to rupture the vacuoles during the injection procedure. However, due to the size of the structures this was not always possible. Whilst it appeared at first that all 17 oocytes had failed to fertilize, on closer inspection under ×200 magnification faint pronuclei-like structures were observed. The oocytes were cultured for a further 2 h and then re-checked. Eight oocytes were identified as having 2 very faint pronuclei with 2 visible polar bodies and thus assumed to have fertilized successfully. A routine check for early cleavage 25 h post-injection revealed that one of the fertilized oocytes had cleaved into a 2-cell embryo. On day 2 all 8 fertilized oocytes had cleaved, with 5 at the 2-cell stage, 2 at the 3-cell stage, and one cleaving into 4-cells. This appeared to be a promising sign of normal development following successful fertilization. On day 3, however, 6 out of the 8 embryos had arrested development and the 2 that had divided had only progressed by 1 cell.
Fig. 1.
Examples of patient’s oocytes exhibiting consistently large central vacuoles
Whereas 6–8 cell embryos would usually be expected on day 3, the embryos transferred for this couple were a 4-cell and 3-cell of ‘grade 1’ quality (See Table 1 for embryo grading criteria). A urinary pregnancy test 2 weeks after the embryo transfer was negative.
Table 1.
In-house embryo grading system
| Grade 1 | Clear cells with even shape and size. No fragmentation. |
| Grade 1- | Clear cells with even shape and size. <5% fragmentation. |
| Grade 2 | Fairly clear cells with mostly even shape and size (<10% difference). Slight fragmentation. 6–10% |
| Grade 2- | Fairly clear cells with mostly even shape and size (<10% difference). Slight fragmentation. 10%–15% |
| Grade 3 | Cells are unclear and uneven in shape. Considerable fragmentation 16–25%. |
| Grade 3- | Cells are unclear and uneven in shape. Considerable fragmentation 26–35%. |
| Grade 4 | Undistinguishable cells with large amounts of fragmentation >35% |
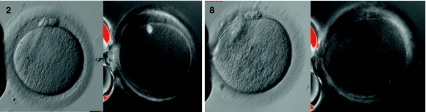
Subsequently the couple underwent a second cycle of ICSI treatment. Standard ‘step-down’ ‘long protocol’ stimulation was performed using a GnRH agonist (Gonapeptyl 3.75 mg depot, Ferring) administered for down regulation, ovarian stimulation with urinary gonadotrophin (Menopur, Ferring) (starting dose 300 iu/day reducing to 150 iu/day on day 7 and 112.5 iu/day on day 9). hCG (Pregnyl, Organon) was administered 36 h prior to oocyte collection on day 14. Twenty-seven oocytes were collected with ease from a cohort of follicles including 18 >14 mm diameter. Of these, 13 were MI, 13 were MII and 1 was at the GV stage. On this occasion we were able to use ‘polscope’ apparatus (Research Instruments Ltd, UK) in order to visualize the meiotic spindle prior to ICSI injection (See Fig. 2). Of the 13 oocytes injected, 7 were identified as having fertilized the next day, 4 at 18.5 h post injection and 3 at 23 h post injection (late fertilization). Again, all pronuclei were only faintly visible. Embryo transfer occurred on day 3 when 2 embryos were transferred (6 cell grade 3 & 5 cell grade 3), all other embryos had arrested at the 4-cell stage or earlier. The morphology and progress of each individual oocyte was tracked throughout their development, as summarised in Table 2.
Fig. 2.
Oocytes 2 & 8 pictured normally and using polscope apparatus to identify the meiotic spindle
Table 2.
The morphology and subsequent development of each oocyte following ICSI
| Oocyte | Morphology | Spindle position | Fertilisation check (pronuclear number) | Day 2 Morphology | Day 3 Morphology | Fate |
|---|---|---|---|---|---|---|
| 1 | Large central vacuole | None seen | 3PN | – | – | – |
| 2 | Large central vacuole | Correct position | 0PN | – | – | – |
| 3 | Medium central vacuole | Correct position | 2PN | 2C② | 6C③ | ET |
| 4 | Massive PB, Central fragments | None seen | 2PN (late) | U/C | 4C④ | D/C |
| 5 | 2 PBs, Highly viscous cytoplasm | None seen | 2PN | 3C② | 2C③ | D/C |
| 6 | Large central vacuole | Correct position | 0PN | – | – | – |
| 7 | Fragmented PB | Correct position | 2PN (late) | 4C② | 5C③ | ET |
| 8 | Multiple central vacuoles, Misshapen | Displaced (5’O clock) | Necrotic | – | – | – |
| 9 | Large central vacuole | Correct position | 2PN | 2C③ | 3C③ | D/C |
| 10 | Misshapen, Large central fragments | Correct position | 0PN | – | – | – |
| 11 | Granular | Displaced (2’O clock) | 0PN | – | – | – |
| 12 | Large central vacuole | Correct position | 2PN (late) | 1C③ | 4C③ | D/C |
| 13 | Large PB | Displaced (5’O clock) | 2PN | 1C④ | 4C④ | D/C |
Key: D/C = discarded, ET = Embryo transferred, U/C = Uncleaved
Unfortunately, this cycle did not result in a pregnancy. By using ‘polscope’ apparatus (Research Instruments Ltd, UK) it became clear that the large central vacuoles were not displacing the meiotic spindle to a large extent in this case, in contrast to previously published reports [4]. Interestingly, in the few oocytes with displaced meiotic spindles there were either no vacuoles present or multiple small vacuoles, similar to those described by other authors as ‘fluid filled’ vacuoles [5] rather than single macro vacuoles. Those oocytes that did achieve fertilization and cleavage resulted in very poor quality embryos with presumed poor prognosis.
Discussion
Sperm factor infertility can reasonably be excluded as a contributory factor as the male partner repeatedly had normal sperm parameters. The need for sperm-egg signalling, sperm binding and penetration of the zona pellucida, which were assumed to be the cause of failed fertilization at IVF, were all bypassed by the ICSI procedure. At 35 with a normal day 2 FSH, female age is not a likely underlying factor.
The diagnosis of PCOS might be considered important as it is associated with abnormal circulating hormones, peri-follicular vascularity and granulosa cell abnormality [8]. Some have suggested a decreased fertilization rate in PCOS patients but this reduced rate does not appear to be attributable to cytogenetic immaturity or chromosomal aberrations [9]. In addition there is little evidence in the literature that PCOS results in lower than normal pregnancy or live birth rates [10]. It is therefore not possible to attribute the vacuolization, poor fertilization and lack of pregnancy to PCOS in this case.
Poor fertilization in this case appears to be due to poor oocyte quality as a result of the central macro vacuoles present within the ooplasm. As these massive cytosolic structures were observed prior to ICSI it can be assumed that they were present at oocyte collection and thus formed in vivo during oocyte maturation under exogenous gonadotrophin and hCG control. It is thought that vacuoles that arise during the transition from MI to MII form around the time of polar body extrusion from the site at which it was abstricted, appearing rapidly within a matter of minutes [4].
Interestingly, in this case it appears that the large central vacuoles did not completely prevent fertilization or embryo cleavage, but resulted in a poor fertilization rate and embryonic arrest on day 2. This arrested development under these circumstances was probably due to the inability to activate the embryonic genome, which should occur between the 4–8-cell stages [11]. An alternative explanation for arrest at this stage of development could be that the vacuolar structures affected spindle positioning, preventing successful fertilization, whilst mechanical injury from ICSI initiated parthenogenetic activation, mimicking normal development up to the point of embryonic genome activation. However ‘polscope’ visualization of the spindle location showed little or no spindle displacement in the majority of oocytes. It should be noted that the developmental consequences of spindle location have been discussed by other authors [12] who observe that the positioning of the spindle in relation to the first polar body may not be the most important factor in normal embryo development, whilst some groups have failed to find significant correlations between spindle retardance and oocyte developmental competence [13]. Such developmental patterns have been reported following sham-injection of bovine oocytes, and compared to that following normal ICSI procedures, showing parthenotes to exhibit embryonic retardation between the 4–8-cell stages [14]. However, this scenario is highly unlikely in this case due to the large numbers of injected oocytes that exhibited faint pronuclei and 2 polar bodies, which would not occur upon parthenogenetic activation.
Observation of similar vacuolar structures following fertilization failure at IVF suggests this is a persistent problem experienced by this patient and thus the reason that previous attempts at clomiphene stimulation and IUI were unsuccessful. Although ovarian stimulation with exogenous gonadotrophins has been associated with aberrations in oocyte cellular organization, this is due to recruitment of a large and heterogenous oocyte population [1]. What is unusual in this case is the homogeneity of vacuole formation (most oocytes displayed vacuolization in successive egg collections) and the sheer size of the vacuoles (we estimate the majority to be over 25 μm in diameter), which is not consistent with abnormalities caused by superovulation. In addition, poor oocyte quality as a result of hyperstimulation would not explain the couple’s inability to conceive naturally.
Conclusion
Poor fertilization rate followed by embryogenic arrest in this case was probably due to an extreme and rare oocyte abnormality. Macro vacuoles present within the ooplasm appear to have distorted oocyte cytoskeletal structure to such an extent that physiological processes involved in fertilization and embryogenesis were impaired, including: sperm-oocyte signalling, sperm binding, meiotic resumption and embryonic cleavage. It is possible that there is an underlying genetic problem within the female patient’s oogonia cohort that developed during foetal oogenesis, resulting in either uncontrollable endocytosis or fusion of pre-existing vesicles that would normally be exocytosed [4, 5]. A genetic element would help to explain the relative homogeneity of vacuole formation within successive oocyte cohorts.
The initial assumption in this case was that impaired developmental competence was likely to be caused by physical displacement of the meiotic spindle and cytoplasmic organelles, which are essential for successful fertilization and embryogenesis. Whilst we did not identify greater than normal spindle displacement, it is logical that structures this large would interfere with the cytoskeletal structure of the oocyte. A novel solution to this problem could be to attempt removal of the massive cytosolic vacuoles using modern micromanipulation techniques similar to those employed in nuclear transfer. Puncturing the vacuoles with a fine ICSI or biopsy needle and draining the intra-vacuolar fluid might alleviate the distortion of the spindle and cytoskeletal structure, thus allowing normal development to progress. However vacuole drainage could result in a reduction in cytoplasmic volume, which in itself could be detrimental to development. A study by Goud et al. [15] on oocyte cytoplasmic biopsy demonstrated this was not the case when removing 12–20 µm ooplasmic fragments from mouse oocytes, as this did not affect the viability of the oocytes, impair fertilization or affect spindle integrity and developmental ability, with only a 1.8% damage rate. However, vacuolar volume in our patient’s oocytes greatly exceeds that of the cytoplasm removed in this study. Puncture and drainage of the vacuoles could also result in the leakage of intra-vacuolar fluid, which could contain toxic waste products that should have been exocytosed, into the surrounding ooplasm. Male pronuclei aspiration has been successfully performed by Kattera & Chen [16] in tripronuclear human zygotes, using a specially designed enucleation needle, resulting in live birth [6]. The microsurgical technique implemented in this study could be suitably adapted for complete vacuole removal/ drainage.
The above-mentioned techniques have not been widely used in clinical treatment, and their adaptation for vacuolar removal has not previously been proposed or performed. In the UK special licensing from the Human Fertilisation & Embryology Authority (HFEA) would have to be granted in order to perform this suggested procedure. Therefore, until this technique is permitted for clinical use the most likely treatment progression for this couple exhibiting homogeneous oocyte macro vacuolisation would be the use of donor oocytes in an IVF cycle.
Footnotes
Capsule
A woman exhibited repeated homogeneous oocyte macro vacuolization over 3 cycles of IVF/ICSI. Spindle displacement did not occur. Embryonic arrest observed on day 2.
References
- 1.Ebner T, Moser M, Sommergruber M, Gaiswinkler U, Shebl O, Jesacher K, et al. Occurrence and developmental consequences of vacuoles throughout preimplantation development. Fert Steril. 2005;83:1635–40. doi: 10.1016/j.fertnstert.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Sutter P, Dozortzev D, Qian C, Dhont M. Oocyte morphology does not correlate with fertilisation rate and embryo quality after intra-cytoplasmic sperm injection. Hum Reprod. 1996;11:595–7. doi: 10.1093/humrep/11.3.595. [DOI] [PubMed] [Google Scholar]
- 3.Alikani M, Palermo G, Adler A, Bertoli M, Blake M, Cohen J. Intra-cytoplasmic sperm injection in dysmorphic human oocytes. Zygote. 1995;3:283–8. doi: 10.1017/S0967199400002707. [DOI] [PubMed] [Google Scholar]
- 4.Blerkom J. Occurrence and developmental consequences of aberrant cellular organization in meiotically mature human oocytes after exogenous ovarian hyperstimulation. J Electron Microsc Tech. 1990;16:324–46. doi: 10.1002/jemt.1060160405. [DOI] [PubMed] [Google Scholar]
- 5.Otsuki J, Okada A, Morimoto K, Nagai Y, Kubo H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum Reprod. 2004;19:1591–7. doi: 10.1093/humrep/deh258. [DOI] [PubMed] [Google Scholar]
- 6.Ebner T, Moser M, Shebl O, Sommerguber M, Tews G. Prognosis of oocytes showing aggregation of smooth endoplasmic reticulum. RBM Online. 2007;16:113–8. doi: 10.1016/s1472-6483(10)60563-9. [DOI] [PubMed] [Google Scholar]
- 7.Rienzi L, Ubaldi FM, Iacobelli M, Minasi MG, Romano S, Ferrero S, et al. Significance of metaphase II human oocyte morphology on ICSI outcome. Fertil Steril. 2008;90:1692–700. doi: 10.1016/j.fertnstert.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 8.Franks S, Roberts R, Hardy K. Gonadotrophin regimens and oocyte quality in women with polycystic ovaries. Reprod Biomed Online. 2003;6:181–4. doi: 10.1016/s1472-6483(10)61708-7. [DOI] [PubMed] [Google Scholar]
- 9.Sengoku K, Tamate K, Takuma N, Yoshida T, Goishi K, Ishikawa M. The chromosomal normality of unfertilised oocytes from patients with polycystic ovarian syndrome. Hum Reprod. 1997;12:474–7. doi: 10.1093/humrep/12.3.474. [DOI] [PubMed] [Google Scholar]
- 10.Heijnen EMEW, Eijkemans MJC, Hughes EG, Laven JSE, Macklon NS, Fauser BCJM. A meta-analysis of outcomes of conventional IVF in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:13–21. doi: 10.1093/humupd/dmi036. [DOI] [PubMed] [Google Scholar]
- 11.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332:449–61. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 12.Cooke S, Tyler JPP, Driscoll GL. Meiotic spindle location and identification and its effect on embryonic cleavage plane and early development. Hum Reprod. 2003;18:2397–405. doi: 10.1093/humrep/deg447. [DOI] [PubMed] [Google Scholar]
- 13.Santis L, Cino I, Rabellotti E, Calzi F, Persico P, Borini A, et al. Polar body morphology and spindle imaging as predictors of oocyte quality. RBM Online. 2005;11:36–42. doi: 10.1016/s1472-6483(10)61296-5. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Hamano K, Qian X, Funauchi K, Furudate M, Minato Y. Oocyte activation and parthenogenetic development of bovine oocytes following intracytoplasmic sperm injection. Zygote. 1999;7:233–7. doi: 10.1017/S0967199499000611. [DOI] [PubMed] [Google Scholar]
- 15.Goud AP, Goud PT, Oostveldt P, Hughes MR. Mictrotubule turnover in ooplasm biopsy reflects ageing phenomena in the parent oocyte. Reprod Biomed Online. 2005;11:43–52. doi: 10.1016/s1472-6483(10)61297-7. [DOI] [PubMed] [Google Scholar]
- 16.Kattera S, Chen C. Normal birth after microsurgical enucleation of tripronuclear human zygotes: case report. Hum Reprod. 2003;18:1319–22. doi: 10.1093/humrep/deg262. [DOI] [PubMed] [Google Scholar]