Abstract
Purpose
To ask whether distinct kinase signaling pathways mediate cytoplasmic or nuclear maturation of mouse oocytes and if in vitro maturation influences the distribution and timing of these phosphorylation events.
Methods
Mouse cumulus oocyte complexes (COCs) were matured under conditions known to influence oocyte quality (basal or supplemented media) and assayed with epitope specific antibodies that would distinguish between Cdk1 or tyrosine kinase targets at 0, 2, 4, 8, and 16 hrs. Semi-quantitative image analysis was used to assess the topographical patterns of protein phosphorylation during in vitro maturation. In vitro fertization and embryo culture were used to examine the effects of culture conditions on developmental potential.
Results
Protein tyrosine phosphorylation increased during meiotic progression from methaphase-I to metaphase-II. Levels were significantly higher in the oocyte cortex. Levels of cortical staining are enhanced in oocytes matured in supplemented media that displayed higher developmental competence. In contrast, bulk substrates for Cdk1 kinase localize to the meiotic spindle while cytoplasmic levels of kinase activity increase throughout meiotic progression; culture media had no measurable effect. Ablation of the tyrosine kinase Fyn significantly reduced cortical levels of tyrosine phosphorylation.
Conclusions
The findings indicate that distinct signaling pathways mediate nuclear and cytoplasmic maturation during in vitro maturation in a fashion consistent with a role for tyrosine kinases in cortical maturation and oocyte quality.
Keywords: Oocyte maturation, Phosphorylation, Fertilization, Cytoplasmic maturation
Introduction
Interacting cascades of signaling pathways dependent upon protein phosphorylation drive many aspects of cellular metabolism. These pathways are essential in the life cycle of an oocyte and may be used discriminately during the growth and maturation stages of oogenesis [1]. In the case of oocyte maturation, regional modifications in protein phosphorylation contemporaneously support the maturation of both the nucleus and cytoplasm during meiotic cell cycle progression and are required for developmental competence following fertilization [2–7]. Despite growing interest in human in vitro maturation, few studies have focused on the impact of culture conditions on cytoplasmic quality in human or other animal models with the focus typically being on the fidelity of nuclear maturation [8].
Alterations in the timing or topographical distribution of protein phosphorylation during meiotic maturation can impose detrimental effects on embryonic, fetal or offspring health. For instance, in vitro culture of oocytes and embryos contributes to fetal wastage due to aneuploidy or abnormalities in imprinting and gene expression [9]. Disruption of kinase signaling pathways in oocytes results in defects in chromosome condensation and segregation that have been linked to embryonic aneuploidy [1]. Moreover, failure to correctly localize and activate kinases cause disruptions in fertilization and embryo development [4]. While ser/thr kinases are well known to drive the meiotic cell cycle, only recently has it become apparent that tyrosine kinases are also essential components in the process of oocyte maturation and early development in mammals. For example, Src-family kinases (SFKs) appear to mediate meiotic spindle and chromatin modifications in rodent oocytes [10–14]. Until the interplay between discrete protein kinase signaling pathways can be resolved in mammalian oocytes, the role of these factors in determining the quality of oocyte nuclear and cytoplasmic maturation will remain unclear. Knowledge of the regional patterns of phosphorylated proteins during maturation may help in the diagnosis of egg quality. Indicators of oocyte quality would be useful in the development of in vitro systems for ART and may provide insight into the regulatory mechanisms that are required to obtain and express developmental competence.
In somatic cells it is well accepted that global changes in protein phosphorylation are triggered in response to extracellular cues that activate growth factor and integrin receptors and stimulate entry into the cell cycle. Ligand receptor interactions and the ensuing signaling events are often regionalized to achieve tighter temporal control. Oocytes present a unique set of problems with respect to signaling and cell cycle regulation. For example, a strict localization of protein kinase-A (PKA) to the cytoplasm is essential for the maintenance of G2/M meiotic arrest. To reinitiate the meiotic cell cycle PKA migrates from the cytoplasm to the mitochondrial membrane where it is sequestered by one of the many forms of A-Kinase anchoring proteins (AKAP1) [15]. And as mentioned above, tyrosine kinase activity has recently been shown to increase significantly following fertilization [16] but the origins of these changes during meiotic maturation have not been rigorously studied. Thus, the importance of kinase regionalization and the lack of information on the relative contributions of tyr or ser/thr kinases has prompted this investigation.
Materials and methods
Oocyte collection
Cumulus-Oocyte-Complexes (COC) were collected from 6 to 7 week old female mice. Most experiments used CF1 female mice (Harlan Sprague-Dawley, Indianapolis IN USA or Charles River Laboratories, Wilmington MA). Fyn knock-out mice (B6/129S7-Fyntm1Sor/J) and the recommended control (B6/129SF2/J) mice were purchased from Jackson Laboratories (Bar Harbor, ME USA) and a homozygous knock-out colony was maintained at KUMC. Mice were housed in a temperature and light-controlled room on a 14 L:10D light cycle and experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences 1996). Mice were euthanized by isofluorothane inhalation anesthesia followed by cervical dislocation. Females were stimulated with 5 IU equine chorionic gonadotropin (eCG; Calbiochem, San Diego CA USA). Ovaries were collected at 42–46 h (h) post-eCG. COC were released from large antral follicles into HEPES-buffered KSOM (FHM, Chemicon) and 4 mg/ml BSA (mFHM) with 300 µM dbcAMP to prevent meiosis resumption during the collections. COC were cultured in either KSOMAA (basal IVM media; IVMb; (Chemicon-Millipore, Billerica MA) or KSOMAA medium supplemented with components previously shown to improve cumulus expansion and oocyte maturation (1 mM glycyl-glutamine, 0.23 mM pyruvate, 4 mg/ml BSA, 0.6 mM L-cysteine, 0.5 mg/ml D-glucosamine, 0.02 µM ascorbate, 1% insulin-transferrin-selenium (ITS; Sigma Corp, St Louis MO), 0.2 IU/ml recombinant human FSH (Serono Reproductive Biological Institute, Rockland, MA) and 10 ng/ml EGF (Calbiochem, San Diego CA); IVMh). IVM culture media (500 µl) were overlayed with 1 ml of embryo tested mineral oil (0.2µ m sterile filtered and stored in the dark, Sigma Chemical Corp) For the IVF experiments, females were given 5 IU human chorionic gonadotrophin (hCG) at 48 h post-eCG and ovulated oocytes recovered from the oviducts 16 h later IVO.
In vitro fertilization and embryo culture
Methods of in vitro fertilization (IVF) and embryo culture were modifications of those previously published [17]. Briefly, COC that were either matured IVMb or IVMh for 17 h or IVO and collected from the oviducts at 16 h post-hCG. To prevent zona hardening, 1 mg/ml of dialyzed Fetuin [18, 19] and 1% FBS were added to the FHM collection medium and the IVM culture media. IVM oocytes were gently pipetted to loosen cumulus cells before transfer to IVF dishes. Sperm were collected from the cauda epidydimus of B6D2 F1 males and capacitated in modified Tyrodes medium for 90–120 min prior to fertilization. COC were transferred into mKSOMaa medium (KSOM medium with 1/2× essential and non-essential amino acids, 5.5 mM glucose and 4 mg/ml BSA [20]) and capacitated sperm were added. Eggs were removed from the fertilization dishes after 6 h of sperm exposure and checked for pronuclei, gently washed to remove loose sperm and cultured in mKSOMaa. After 24 h of culture, eggs were examined for cleavage to 2 cells. All 2-cell embryos were washed and transferred to fresh culture plates of mKSOMaa to remove the dead sperm. Embryo development was evaluated at 120 h post-IVF for number of embryos that developed to at least compacted morula, blastocyst formation and zona hatching.
Fixation and immunohistochemical labeling
Methods for fixation and immunohistochemistry were similar to those previously reported [10]. Briefly, oocytes and COC were fixed for 10–20 min at room temperature in FHM medium with 3% paraformaldehyde followed by 30 min at 35°C in microtubule stabilization buffer with 2% formalin and 0.5% triton-X100 (MTSB-XF [21]. After fixation, eggs and embryos were transferred into wash solution [10] and held overnight at 4°C. All fixatives and wash solutions were supplemented with 40 µM phenylarsine oxide, 100 µM sodium orthovanadate and 10 µM calyculin-A to inhibit phosphatase activity. Antibodies used included mouse monoclonal anti-phosphotyrosine antibody (clone 4G10, Upstate, Temecula CA) and MPM2 (Upstate, Temecula CA). Secondary antibodies were Alexa 488 goat anti-mouse (Molecular Probes, Eugene OR). Oocytes were labeled with primary antibodies overnight at 4C followed by secondary antibody for 2 h at 35C. After secondary labeling, oocytes were transferred to a wash solution containing 10 ug/ml Hoechst 33258 (DNA) and stored in the dark overnight at 4°C. Oocytes that were not part of the fluorescence intensity pools were co-labeled with rat monoclonal tubulin antibody (YOL1/34; Abcam, Cambridge MA) followed by secondary antibody goat anti-rat or goat anti-rabbit Alexa-568; primary antibodies labeled for 1 h at 35C followed by secondary for 1 h at 35C). Oocytes and COC were mounted the following morning and imaged (mounting medium consisted of 1:1 glycerol: PBS supplemented with 5 mg/ml sodium azide and 10 µg/ml Hoechst 33258). All blastocysts were fixed for 30 min in 2% PFA in PBS (pH 7.4) and labeled for 30 min with 10 µg/ml Hoechst 33258 (DNA) and phalloidin-Alexa 568 (Molecular Probes-Invitrogen, Carlsbad, CA). All chemicals, hormones and reagents were purchased from Sigma Chemical Company, St. Louis, MO unless otherwise stated.
Oocyte processing
Oocytes were matured in vitro for 0, 2, 4, 8 or 16 h in IVMb or IVMh media. At specific times, a random pool of COC were removed from culture, cumulus cells were stripped manually by pipetting with a fine tipped glass pipet followed by brief washing in hyaluronidase (0.3 mg/ml) and fixation. Following fixation, oocytes were sorted into groups containing 2 oocytes from each time point selected at random from the pools of fixed oocytes. This permitted the labeling and imaging of oocytes from all time points together in one cohort and eliminated any signal variability due to labeling or imaging conditions; in this way, we were able to determine variations in staining intensity attributable to phosphoepitope abundance between oocytes within each set.
Imaging and data analysis
Serial z-sections (1 μm depth) were obtained for each oocyte using a 40× lens on a Zeiss Pascal confocal microscope. These data sets were used to measure 3-dimensional relationships between the oolema, chromatin, meiotic spindle and first polar body. Fluorescence intensity levels at the central plain of each oocyte (6 oocytes/ time/ treatment) were considered to represent the overall amount of phospho-protein at each stage of maturation. A single TIFF image from the central plain of each oocyte set was exported for Metamorph image processing. This method allowed us to compare the localized patterns of phospho-proteins at each time point and between the types of maturation conditions. Line-scans were produced for each oocyte to allow for comparisons of localized fluorescence intensity. Measurements taken for each oocyte included: (1) a line-scan (7 um thick) covering the maximum diameter of the oocyte; (2) linescan around the circumference (7 µm thick) that included the oolema and cortex; and (3) the central cytoplasm that excluded the oolema/cortical regions of the original linescan. Blastocysts were also imaged by serial sections on the Zeiss Pascal confocal microscope. The number of inner cell mass cells (ICM), trophectoderm cells (TE), mitotic and picnotic (dead) cells in each blastocyst were counted.
Average fluorescence intensity was statistically compared between stages of maturation and culture media treatment. Blastocysts from IVO & IVM-IVF oocytes were compared for total number of cells, number of ICM, TE, mitotic and pyknotic cells using SPSS software (SPSS Inc, Chicago IL). Data were analyzed by ANOVA followed by Sidak post-hoc comparisons. P-value of less than 0.05 was considered significant.
Results
Tyrosine phosphorylation increases during oocyte maturation
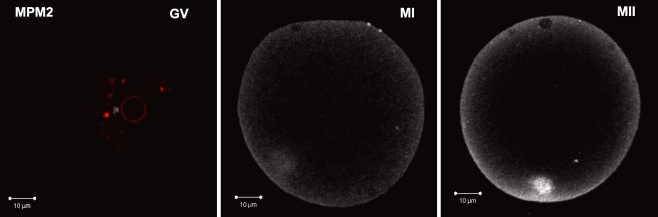
Oocytes were matured in vitro for 0, 2, 4, 8 or 16–17 h. Two oocytes from each time point were pooled, labeled and imaged together to enable a direct comparison of fluorescence intensity between oocytes during maturation. Images taken from the central plain of each oocyte were compared visually (confocal images, Figs. 1 and 2), and used for linescan comparisons of fluorescence intensity across the median and around the cortex of each oocyte (Figs. 3 and 4). Image intensity in Fig. 1 has been increased to provide a global representation of pTyr epitope distribution. In contrast, Figure 2 shows oocytes from the same experiments represented at their original fluorescence intensity levels as used for quantitative image analysis. Intense cortical labeling associated with the oolema and large cytoplasmic patches were seen at all stages (Figs. 1 and 2). Figure 1 demonstrates pTyr levels in oocytes at the plane containing the chromatin and shows the diversity of cytoplasmic pTyr labeling. Most cytoplasmic patches were subcortical however, in GV stage oocytes one or two large cytoplasmic patches were localized adjacent to the GV in both maturation incompetent (non-surrounded nucleolus; NSN) and competent (surrounded nucleolus; SN) (Fig. 1GV)oocytes. By 2 h of maturation (GVBD) pTyr labeling was more diffuse throughout the cytoplasm, but still exhibited cytoplasmic patches (Fig. 1h2). Oocytes at metaphase-II were easily distinguished from immature oocytes by increased pTyr proteins throughout the cytoplasm (Fig. 2). Intense label was evident at the spindle poles of metaphase-II (Fig. 1h16 arrow) of IVMh and IVMb oocytes (5/5 and 3/6, respectively), which is similar to our previous studies with oocytes matured in vivo [10]. Tyrosine phosphorylated proteins were also evident at the spindle poles of most metaphase-I (fig MI arrow) oocytes (4/5 IVMh and 4/6 IVMb). Cytoplasmic pTyr epitope staining was similar between oocytes at the GV (fig GV) and metaphase-I (Fig. 2 MI) stages. Notably, total cytoplasmic pTyr epitope levels increased between MI-MII (Fig. 2 MI & MII).
Fig. 1.
Phospho-tyrosine proteins increase at MII and localize primarily to the oocyte cortex and within large cytoplasmic patches. Complete serial z-sections of oocytes labeled with pTyr and imaged in sets were examined to identify patterns of pTyr proteins associated with cellular structures at each time point of maturation (H0=GV, H2=GVBD, H4=pre-MI, H8=MI, H16=MII). pTyr labeled oocytes are shown here in the plane where chromatin was found. Two to three sections have been compressed together to enable visualization of entire structures such as both poles of the spindles. Image intensity has been increased in this figure to improve visualization of labeled structures. (white=pTyr, red=DNA)
Fig. 2.
Patterns of tyrosine phospho-proteins change during meiotic maturation. The oocytes in this figure were selected from a single set of oocytes imaged together as a set to demonstrate the actual differences of fluorescence intensity between GV, MI and MII. Each image shown here is a single section (1 µm thick) at the central plane of the oocyte. The intensity of these images has not been modified in order to demonstrate actual fluorescence intensity differences between oocytes at different stages of maturation. This is the same plane used for later measurements for linescans and fluorescence intensity comparisons. (white=pTyr, red=DNA)
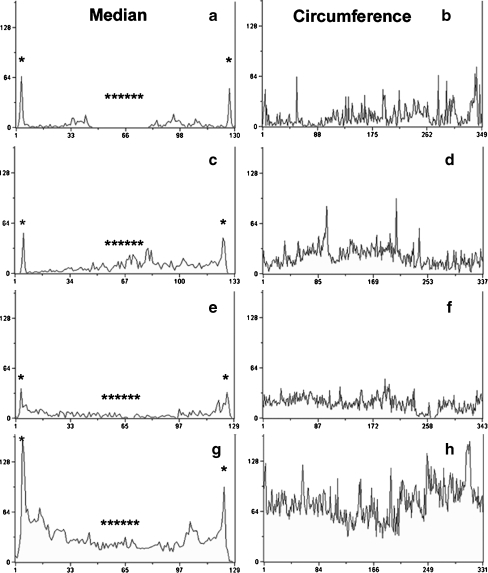
Fig. 3.
Linescans demonstrate regional localization of tyrosine phospho-proteins. Metamorph imaging software was used to produce linescans of fluorescently labeled oocytes. Linescans were drawn on the image from the central plane (from serial z-stacks) of each oocyte from the original pTyr sets (Fig. 2). Two linescans (each 7 µm thick) were taken from each oocyte including (1) a straight line across the entire diameter of each oocyte at the median and (2) circumferential scan around the entire surface of each oocyte to include the oolema and cortical region. (a, c, e & g) are linescans taken across the median; (b, d, f & h) are linescans taken around the circumference. (* oolema/cortex; ***** central cytoplasm; A–B=GV, C–D=pre-MI, E–F=MI, G–H=MII)
Fig. 4.
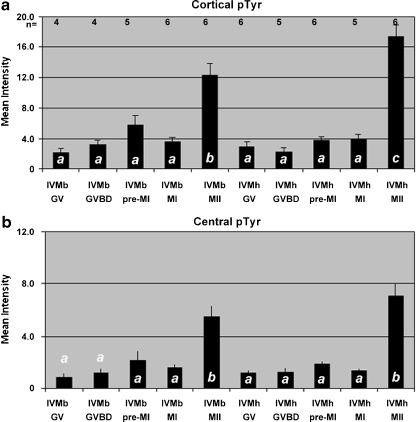
Levels of pTyr proteins is increased significantly at metaphase-II. The same oocytes used for the linescan analysis were used for statistical comparisons of average fluorescent intensity. This included the cortical (a) measurements from linescans seen in Fig. 3 plus the central cytoplasm (b), ie that region of the cytoplasm not included in the cortical scans & not including the region of the nucleus and/or spindle. A significant rise in pTyr levels was detected in both the cytoplasm and the egg cortex. Cortical pTyr levels were higher in oocytes matured in IVMh than IVMb (bars labeled with different letters (a, b, c) are significantly different, P < 0.05)
Stage specific Tyrosine phosphorylation at the oocyte cortex
To quantify protein tyrosine phosphorylation, images from oocytes in the previous experiment were analyzed by linescan comparisons and statistical analysis of fluorescence intensity levels. Linescan tracings graphically demonstrate alterations evident in fluorescence images. As seen in photographs (Figs. 1 and 2), pTyr epitope is highly localized in the oocyte cortex at all stages of maturation. During maturation, the transition from metaphase-I to metaphase-II was associated with an increase in both cytoplasmic and cortical pTyr epitope (Figs. 3 and 4). In addition to showing high levels of pTyr localized at the cortex (Fig. 3*), levels were increased as oocytes transitioned from MI to MII. Although present at much lower levels (Fig. 3***), cytoplasmic pTyr epitope also increased during the MI to MII transition (Fig. 3). Statistical analysis of cortical and cytoplasmic pTyr levels confirmed significance at the MII stage (Fig. 4). Moreover, cortical pTyr levels (Fig. 4a) were also statistically higher in oocytes matured in IVMh medium (17.4 ± 1.53; n = 6) as compared to IVMb (12.3 ± 1.49; n = 6; P < 0.05) suggesting that culture conditions influence the magnitude of cortical protein tyrosine phosphorylation. However, no such difference was observed between levels of cytoplasmic pTyr (Fig. 4b) in either culture treatments (7.12 ± 0.87 and 5.47 ± 0.87, respectively; n = 6 each). Thus stage and culture conditions are associated with differential quantity of pTyr epitopes during meiotic maturation in the mouse. We next examined epitopes detected by the monoclonal antibody MPM2 that is known to mark many relevant substrates for M-phase specific kinases.
Patterns of MPM2
Sets of oocytes from the same cohorts that were labeled for pTyr proteins were labeled for mitosis-associated ser/thr phospho-proteins (MPM2). This antibody has been used by our laboratory in previous studies to identify meiosis-specific phosphorylation patterns associated with the meiotic spindles and MTOCs, but overall cytoplasmic patterns of localization have not been reported. Figure 5 compares oocytes from three stages of maturation. These images were taken at the central focal plane under the same conditions as described above. Little MPM2 epitope is detectable in the cytoplasm of GV stage oocytes (fig GV) but levels progressively increased from GV to MI (fig GV & MI) to metaphase-II (fig MII). A gradient of epitope distribution is apparent in an eccentric pattern characterized by higher levels near the cortex as relative to the center of the oocyte. Unlike pTyr epitope, which aligned with the cortex and/or oolema, MPM2 epitope is concentrated in a subcortical zone (Fig. 5). Fluorescence intensity measurements were performed as described for pTyr proteins. Because of the intense labeling of MPM2 at the meiotic spindles (see below), the spindle region was excluded from measurement of the cytoplasmic fluorescence intensity. Linescans confirmed a continuous increase in MPM2 epitope both in the sub-cortical region (Fig. 6*) and cytoplasmic region (Fig. 6***) as maturation progressed from GV to MII (Fig. 6). Increased MPM2 levels were statistically significant in both subcortical (Fig. 7a) and cytoplasmic zones (Fig. 7b) at MI and MII when compared to earlier stages (Fig 7; a, b and c are statistically different, P < 0.05). There was an additional significant increase between MI and MII. Interestingly, and unlike the pTyr results presented earlier, culture conditions had no effect on the levels of MPM2 epitope present at all cell cycle stages that were assayed (P > 0.05).
Fig. 5.
Cytoplasmic MPM2 proteins increased steadily during maturation. Oocytes from the same cohorts as were used for the pTyr experiments were also sorted into sets, labeled and imaged for MPM2 epitope proteins (phosphorylation targets of Cdk1/MPF). Oocytes in this figure represent single sections at the central plane of oocytes from a single set all imaged together in order to show the changes in fluorescence intensity at different stages of maturation to demonstrate the original images similar to Fig. 2 for pTyr
Fig. 6.
MPM2 proteins increase during maturation and concentrate in the cytoplasm beneath the oolema. Oocytes labeled for MPM2 proteins were compared by linescans similar to pTyr images (see Fig. 3). Linescans across the median (a, c, e and f) demonstrate the steady increase of MPM2 proteins in both the central cytoplasm (*****) and the cortex (*) as oocytes mature from GV to MII. Fluorescence intensity was too low to measure at GV stage (a–b). The cortical (b, d, f and h) scans further demonstrate the increase in MPM2 proteins especially at MI (f) and MII (h). (* oolema/cortex; ***** central cytoplasm; A–B=GV, C–D=pre-MI, E–F=MI, G–H=MII)
Fig. 7.
The rise in MPM2 proteins is significant at MI and MII stages of maturation. The average fluorescence intensity of MPM2 labeled oocytes were analyzed similar to pTyr protein analysis in Fig. 4. MPM2 protein concentration increased significantly in both the cytoplasm (a) and cortical (b) regions. Levels at MI and MII were significantly higher than earlier times. MII levels were significantly higher than MI. No differences were seen between culture treatments (IVMh, IVMb). (a, b, c are significantly different, P < 0.05)
Fyn knock-out oocytes fail to increase cortical phosphorylation of pTyr proteins
Our studies of SFKs during meiotic maturation and fertilization have demonstrated an essential function for SFKs for proper organization of chromosomes and spindles [10, 11, 14]. Of the nine SFKs produced in mammalian cells, FYN kinase is expressed at very high levels in oocytes. YES and SRC kinases are also expressed, but at lower levels (BioGPS.gnf.org, mouse, GeneAtlas GNF1M, gcrma). To determine if Fyn kinase may be responsible for the patterns of tyrosine phosphorylation demonstrated above, Fyn (-/-) oocytes and B6/129 wildtype control oocytes were in vitro cultured for 17 h (IVMh), fixed, labeled and imaged as in our earlier pTyr and MPM2 experiments. MPM2 proteins localized to the spindles and spindle poles as seen in previous experiments and no difference could be detected between Fyn (-/-) and wildtype (Fig. 8a and b, respectively). However, a difference was seen in pTyr epitope localization. Most (11/12; 92%) wildtype oocytes exhibited the expected cortical localization of pTyr proteins (Fig. 8c), however only 4/28 (14%) of the Fyn (-/-) oocytes exhibited cortical pTyr phosphorylation as seen in wildtype oocytes. The remaining 21/28 (75%) Fyn (-/-) oocytes displayed barely detectable and patchy cortical pTyr while 3/28 (11%) had no detectable pTyr at the cortex (8D). It was also apparent that Fyn (-/-) oocytes had no detectable spindle associated pTyr epitope at either MI or MII spindle poles (0/25) whereas all wildtype control oocytes exhibited prominent spindle pole staining (10/10; not shown). Thus, genetic ablation of Fyn kinase causes reduced cortical and spindle associated tyrosine phosphorylation. Given the discrete spindle patterns shown by pTyr (spindle poles) or MPM2 (entire spindle and poles), we explored the origins of these differences by examining earlier stages of meiotic cell cycle progression in CF1 oocytes.
Fig. 8.
Fyn (-/-) oocytes exhibit reduced levels of pTyr proteins in the cortex while MPM2 proteins localize normally the spindle and spindle poles. Fyn (-/-) and wildtype oocytes were matured in IVMh for 17 h, fixed and labeled for pTyr and MPM2 epitopes. The images of MPM2 labeled spindles (a–b) are compressions of 2–3 sections to show the entire spindle region however, pTyr images (c–d) are single sections from the central plane of each oocyte. There was no difference in MPM2 labeling on MII spindles or spindle poles of wildtype (a) or Fyn (-/-) (b) oocytes. However, Fyn (-/-) oocytes often failed to develop intense pTyr at the egg cortex or the spindle poles. As reported previously, Fyn (-/-) oocytes often produce MII oocytes with disorganized chromosomes (d) [14]. (A–B; white=MPM2, red=DNA: C–D; white=pTyr, red=tubulin, white on red=DNA)
MPM2 proteins localize near to chromatin and meiotic spindles
At the GV stage, intense labeling of MPM2 was detectable on MTOCs near the cell cortex and adjacent to the GV as previously reported (not shown [21]). In addition, novel localization patterns were detected that we believe are due to the inclusion of phosphatase inhibitors in our fixation cocktail. Thus MPM2 epitope formed dense clusters near chromatin within the GV (Fig. 9 GV) and occasionally was localized at the surface of nucleoli but was never detected within nucleoli based upon z-section analysis. Aggregates of MPM2 epitope associated with condensing chromosomes and developing spindles (Fig. 9 GVBD & pre-MI). And, as noted earlier, MPM2 decorates MI and MII spindles as well as spindle pole MTOCs (Fig. 9 MI & MII).
Fig. 9.
MPM2 proteins aggregate near to chromatin within the GV and associate with spindle microtubules and spindle poles. Oocytes from the MPM2 fluorescence intensity comparisons were examined for localization of MPM2 proteins to intracellular structures. Images in this figure were extracted from these data sets including compressions of 2–3 sections to enable visualization of the entire structure. Dense patches of MPM2 proteins were seen to associate with chromatin within the GV stage oocytes. The specific pattern of this labeling was diverse. As chromosomes condensed at GVBD and began to organize at pre-MI, MPM2 patches were seen to remain near to the chromosomes. At MI and MII, MPM2 localized to the spindle microtubules and the spindle poles. (green=MPM2, red=DNA)
Basal conditions during maturation produce oocytes with lower developmental potential
Patterns of phosphorylation were significantly affected by IVM conditions therefore we fertilized oocytes in vitro and cultured for 120 h to compare fertilization and embryonic developmental potential between the two IVM conditions compared to ovulated oocytes (IVO). Fertilization rates (% 2cells) and the percentage of fertilized embryos that developed to the blastocyst stage were the same for IVO (100% & 93%), IVMb (87% & 65%) and IVMh (94% & 85%; Table 1). Oocytes that matured in IVMb were less likely to begin hatching at the blastocyst stage (27%; P < .003) when compared to either IVMh (60%) or IVO (88%; Table 1). IVO and IVMh oocytes produced blastocysts with more total cells (101 & 85), including a higher number of ICM (26 & 18) and TE (75 & 68) cells than oocytes matured in IVMb (62, 9 & 52, respectively; P < .05; Table 2). While IVO and IVMh blastocysts had similar numbers of TE cells, IVO embryos had significantly more total cells and ICM cells as compared to IVMh blastocyts (P < .05).
Table 1.
Rates of fertilization, blastocyst development and zona hatching
| Treatment | 1cell (n) | 2cells (%) | ≥BL (%) | ≥Hg (%) |
|---|---|---|---|---|
| IVMb | 71 | 87a | 65a | 27a |
| IVMh | 83 | 94a | 85a | 60b |
| IVO | 40 | 100a | 93a | 88b |
a, bvalues in columns with different superscripts are statistically different at P < 0.05
BL blastocysts (percent from 2cells); Hg hatching blastocysts (percent from blastocysts)
Table 2.
Comparison of the number of cells in blastocyts following IVM, IVF & 120 h culture
| Treatment | N | Total cells | ICM | TE | Mito | Pic |
|---|---|---|---|---|---|---|
| IVMb | 42 | 62a | 9a | 52a | 2a | 5a |
| IVMh | 42 | 85b | 18b | 68b | 2a | 5a |
| IVO | 35 | 101c | 26c | 75b | 4b | 6a |
a, b, cvalues in columns with different superscripts are statistically different at P < 0.05
ICM inner cell mass cells; TE trophectoderm cells; mito mitotic cells; pic picnotic (dead) cells
Discussion
The present studies address the question of how the topography of kinases and their targets is regulated during the meiotic cell cycle progression as exhibited by the mouse oocyte. Two points emerge. Semi-quantitative measurements of both M-phase related (MPM2) and tyrosine kinase targets revealed discrete temporal and spatial patterns of phosphorylation during the rather synchronous cell cycle progression exhibited by in vitro matured mouse oocytes. In addition,culture media supplements improve oocyte quality under conditions that are associated with increased tyrosine kinase activity.
Intracellular localization and dynamics of protein phosphorylation
The current studies have demonstrated regional changes of protein phosphorylation during oocyte maturation. Clone 4G10 antibody is a well characterized antibody that specifically recognizes a diverse array of tyrosine phosphorylated proteins. We used this reagent to identify changes in pTyr proteins during in vitro maturation. Tyrosine phosphorylated proteins increased in both the cell cortex and the cytoplasm as the oocyte matured (Figs. 1, 2, 3 and 4). This disagrees with previous studies showing no change in overall levels of pTyr proteins between GV and MII [16]. Interestingly, a recent study examining changes in protein phosphorylation and the kinome of porcine oocytes during maturation found that the number of tyrosine phosphorylated proteins increased was equal to the number that decreased during transitions from GV-MI and MI-MII [22]. This may explain why traditional global protein measurement methods are unable to detect small changes in protein phosphorylation.
Immunohistochemical labeling and confocal microscopy combined with image and statistical analysis has enabled us to further define localized changes at an intracellular level and with high spatial resolution. That pTyr epitopes concentrate in cytoplasmic patches, MI and MII spindle poles [10] and the oocyte cortex are novel observations that will inform future studies into the identity and function of subcellular domains (Fig. 1). While pTyr levels rose in all regions of the oocyte during the MI-MII transition, the change in cortical intensity was the most dramatic. Interestingly, culture media had a significant effect on the concentration of pTyr proteins in the cortex. Supplemented IVMh media resulted in higher levels of cortical tyrosine phosphorylation that basal IVMb (Fig. 4a). Due to difficulties in timing of IVO versus IVM it is not currently known whether this higher level signifies improved oocyte quality. Since cortical pTyr levels further increase at fertilization, the levels of pTyr proteins present during MII arrest may be important to proper sperm incorporation, fertilization success and zygotic development [23]. Previous studies have demonstrated an increase in embryo survival from oocytes matured in supplemented media [24]. Further studies will be necessary to determine whether fertilization and embryo developmental rates between oocytes matured under the current culture conditions are due to effects on the efficiency of tyrosine kinases or phosphatases. As shown here, Fyn kinase is a very likely candidate for such regulation and may be modified under less than optimal culture conditions.
MPM2 antibody was deployed here to track serine and threonine phosphorylated protein epitopes known to be phosphorylated by M-phase kinases during mitosis [25, 26]. This antibody has been used in our laboratory for the identification of M-phase protein phosphorylation in oocytes as they acquire meiotic competence and undergo meiotic progression [21, 27], transition states in the cell cycle that are known to be accompanied by gradual activation of H1 kinase that occurs during GVBD [28]. These studies extend that work identifying unreported dynamics of the MPM2 labeled phosphoproteins. The incorporation of phosphatase inhibitors in the fixation solutions and minimization of storage time is likely to be partly responsible for improved phosphoprotein preservation given the notorious stability of phosphatases. As reported previously, MPM2 labeled MTOCs in the cytoplasm and near the GV at all stages of maturation. MPM2 proteins also formed aggregates surrounding the condensing chromosomes as well as the meiotic spindle microtubules and spindle poles (Fig. 9). Of particular interest were aggregates of MPM2 proteins found within the GV that were closely associated with chromatin (Fig 9GV). The diversity detected between oocytes in the abundance, location and size of MPM2 aggregates within GV suggests the presence of protein assemblies, such as centrosomes are transient and subject to changes during meiotic arrest of GV intact oocytes. Interestingly, our methodology also demonstrates the cytoplasmic localization of MPM2 proteins. Unlike pTyr proteins which were localized directly at the cell cortex (Figs. 1, 2 and 3), MPM2 proteins were concentrated sub-cortically and in a pattern that decreased in intensity towards the center of the oocyte (Fig. 5). The concentration of MPM2 proteins increased steadily from GV to MII both in the cortical region and the central cytoplasm (Fig. 6). This was strikingly different from the changes in pTyr which remained relatively constant from GV to MI then significantly increased by metaphase-II. This suggests a steady increase in MPM2 phosphoproteins as maturation progresses. However, a significant increase in tyrosine phosphorylation is associated specifically with metaphase-II arrest and preparation for fertilization. A division of labor between targets of tyr versus ser/thr kinases would be consistent with these observations although the identity of these components remained ill-defined with one exception as shown here.
Our recent studies have focused on the role of SFKs during oocyte maturation and fertilization [10, 11, 14]. The present data reinforce a role for Fyn kinase in what we suggest is an example of cortical maturation. Fyn kinase is associated with changes in cortical actin dynamics of cultured cells [29–31] and sertoli cells [32]. Since pTyr proteins localize specifically at the oocyte cortex, we examined the localization of pTyr in oocytes from Fyn (-/-) mice to determine whether Fyn kinase participated in this phosphorylation event. Interestingly, oocytes from wildtype control mice exhibited intense cortical pTyr localization as seen in the earlier studies. However, pTyr localization in Fyn (-/-) oocytes was heterogeneous wherein some oocytes showed no cortical staining while others displayed extremely low level and patchy staining at the cortex. Expression of mitotic/meiotic cell cycle specific MPM2 epitopes was not visibly affected by the lack of Fyn kinase (Fig. 8a–b). However, Fyn (-/-) oocytes lacked pTyr spindle pole labeling at MI or MII. This distinct spatial change in spindle pole foci helps explain our previous data showing spindle and chromosome dysfunction during meiosis of Fyn (-/-) oocytes [10, 14]. Collectively, these findings implicate a role for Fyn kinase in establishing spindle pole integrity and chromosome segregation at meiosis I and II. Furthermore, the loss of cortical epitope in Fyn null oocytes suggests a role for Fyn kinase in oocyte cortical dynamics and preparation for fertilization. It is tempting to speculate that Fyn driven cortical maturation may be linked to the recent discovery of a subcortical maternal complex that in mouse oocytes harbors maternal proteins that are essential for embryonic development [33].
Since IVM conditions had a significant effect on regional kinase activities as evidenced by differences in pTyr and MPM2 patterns, we next compared fertilization and blastocyst development of these oocytes to determine if changes in phosphorylation patterns may correlate with developmental potential.
Ingredients of oocyte maturation media affect embryo developmental potential
Oocytes matured in vivo (ovulated) and in vitro were similarly able to be fertilized and cleave to the 2-cell stage. This suggests that variations of pTyr epitopes at the egg cortex induced by variations in culture media did not significantly affect sperm binding, penetration or progression through the first mitotic cell cycle. Interestingly, ovulated eggs and those matured in supplemented IVMh media developed to blastocysts with significantly more cells and higher rates of hatching suggesting a higher developmental potential of these oocytes over those matured in basal conditions. These findings highlight the importance of carrying developmental assessments to at least the blastocyst stage where cell allocation parameters can be accurately evaluated. In contrast, the prognostic value of early cleavage events remains uncertain given the fact that no difference was detectable between fertilization and cleavage rates between experimental groups that clearly were distinguishable at the blastocyst stage. This prompts several questions about the role that differential phosphorylation plays in establishing cytoplasmic maturation and oocyte quality after in vitro maturation.
The main finding in these studies is that optimizing conditions for in vitro maturation leads to an enhanced level of cortical tyrosine phosphorylation that correlated with a measurable increase in developmental potential. We suggest that this may be an important dimension of cytoplasmic maturation that has not been fully appreciated in animal or human models of in vitro maturation. In fact, while we anticipated that events associated with the remodeling of the oocyte cortex during fertilization and early cleavage might have been affected by in vitro maturation conditions, we instead observed that the consequences of such modifications (i.e. tyrosine phosphorylation) was not apparent until later stages of development. Identifying the specific signaling intermediates and their protein targets remains an important challenge for future work in oocyte maturation.
In conclusion, our evidence suggests a separation of function in kinases that operate through distinct signaling pathways. That there is clear topographical segregation in epitope disposition suggests further that these pathways serve to integrate the progression of nuclear and cytoplasmic maturation in the mouse oocyte.
Acknowledgements
The authors would like to thank Dr. William H. Kinsey for providing the Fyn knock-out mice from his breeding colony. Supported by NICHD 42076, The Hall Family Foundation and the ESHE fund.
Footnotes
Capsule Tracking of distinct kinase targets during meiotic progression of mouse oocytes suggests that oocyte quality is determined by the action of tyrosine kinases on the cortex illustrating a novel aspect of cytoplasmic maturation in mammalian oocytes.
References
- 1.Swain JE, Smith GD. Reversible phosphorylation and regulation of mammalian oocyte meiotic chromatin remodeling and segregation. Soc Reprod Fertil Suppl. 2007;63:343–358. [PubMed] [Google Scholar]
- 2.Besterman B, Schultz RM. Regulation of mouse preimplantation development: inhibitory effect of genistein, an inhibitor of tyrosine protein phosphorylation, on cleavage of one-cell embryos. J Exp Zool. 1990;256:44–53. doi: 10.1002/jez.1402560107. [DOI] [PubMed] [Google Scholar]
- 3.Harrouk W, Clarke HJ. Mitogen-activated protein (MAP) kinase during the acquisition of meiotic competence by growing oocytes of the mouse. Mol Reprod Dev. 1995;41:29–36. doi: 10.1002/mrd.1080410106. [DOI] [PubMed] [Google Scholar]
- 4.Ito J, Yoon S-Y, Lee B, Vanderheyden V, Vermassen E, Wojcikiewicz R, Alfandari D, Smedt HD, Parys JB, Fissore RA. Inositol 1, 4, 5-trisphosphate receptor 1, a widespread CA2+ channel, is a novel substrate of polo-like kinase 1 in eggs. Dev Biol. 2008;320:402–413. doi: 10.1016/j.ydbio.2008.05.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee B, Vermassen E, Yoon S-Y, Vanderheyden V, Ito J, Alfandari D, Smedt H, Parys JB, Fissore RA. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development. 2006;133:4355–4365. doi: 10.1242/dev.02624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohsugi M, Butz S, Kemler R. Beta-Catenin is a major tyrosine-phosphorylated protein during mouse oocyte maturation and preimplantation development. Dev Dyn. 1999;216:168–176. doi: 10.1002/(SICI)1097-0177(199910)216:2<168::AID-DVDY7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Puscheck EE, Lewis JJ, Trostinskaia AB, Wang F, Rappolee DA. Increases in phosphorylation of SAPK/JNK and p38MAPK correlate negatively with mouse embryo development after culture in different media. Fertil Steril. 2005;83:1144. doi: 10.1016/j.fertnstert.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 8.Jurema MW, Nogueira D. In vitro maturation of human oocytes for assisted reproduction. Fertil Steril. 2006;86:1277–1291. doi: 10.1016/j.fertnstert.2006.02.126. [DOI] [PubMed] [Google Scholar]
- 9.Roberts R, Iatropoulou A, Ciantar D, Stark J, Becker DL, Franks S, Hardy K. Follicle-stimulating hormone affects metaphase I chromosome alignment and increases aneuploidy in mouse oocytes matured in vitro. Biol Reprod. 2005;72:107–118. doi: 10.1095/biolreprod.104.032003. [DOI] [PubMed] [Google Scholar]
- 10.McGinnis LK, Albertini DF, Kinsey WH. Localized activation of Src-family protein kinases in the mouse egg. Dev Biol. 2007;306:241–254. doi: 10.1016/j.ydbio.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng L, Luo J, Li C, Kinsey WH. Role of Src homology 2 domain-mediated PTK signaling in mouse zygotic development. Reproduction. 2006;132:413–421. doi: 10.1530/rep.1.01151. [DOI] [PubMed] [Google Scholar]
- 12.Zheng K-G, Meng X-Q, Yang Y, Yu Y-S, Liu D-C, Li Y-L. Requirements of Src family kinase during meiotic maturation in mouse oocyte. Mol Reprod Dev. 2007;74:126–131. doi: 10.1002/mrd.20613. [DOI] [PubMed] [Google Scholar]
- 13.Talmor-Cohen A, Tomashov-Matar R, Tsai WB, Kinsey WH, Shalgi R. Fyn kinase-tubulin interaction during meiosis of rat eggs. Reproduction. 2004;128:387–393. doi: 10.1530/rep.1.00266. [DOI] [PubMed] [Google Scholar]
- 14.McGinnis LK, Kinsey WH, Albertini DF. Functions of Fyn kinase in the completion of meiosis in mouse oocytes. Dev Biol. 2009;327:280–287. doi: 10.1016/j.ydbio.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newhall KJ, Criniti AR, Cheah CS, Smith KC, Kafer KE, Burkart AD, McKinght GS. Dynamic anchoring of PKA is essential during oocyte maturation. Curr Biol. 2006;16:321–327. doi: 10.1016/j.cub.2005.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Yosef D, Talmor A, Shwartz L, Granot Y, Shalgi R. Tyrosyl-phosphorylated proteins are involved in regulation of meiosis in the rat egg. Mol Reprod Dev. 1998;149:176–185. doi: 10.1002/(SICI)1098-2795(199802)49:2<176::AID-MRD8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 17.Summers MC, McGinnis LK, Lawitts JA, Biggers JD. Mouse embryo development following IVF in media containing either L-glutamine or glycyl-L-glutamine. Hum Reprod. 2005;20:1364–1371. doi: 10.1093/humrep/deh756. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder AC, Schultz RM, Kopf GS, Taylor FR, Becker RB, Eppig JJ. Fetuin inhibits zona pellucida hardening and conversion of ZP2 to ZP2f during spontaneous mouse oocyte maturation in vitro in the absence of serum. Biol Reprod. 1990;43:891–897. doi: 10.1095/biolreprod43.5.891. [DOI] [PubMed] [Google Scholar]
- 19.Spiro RG. Studies on fetuin, a glycoprotein of fetal serum. I. Isolation, chemical composition, and physiochemical properties. J Biol Chem. 1960;235:2860–2869. [PubMed] [Google Scholar]
- 20.Summers MC, McGinnis LK, Lawitts JA, Raffin M, Biggers JD. IVF of mouse ova in a simplex optimized medium supplemented with amino acids. Hum Reprod. 2000;15:1791–1801. doi: 10.1093/humrep/15.8.1791. [DOI] [PubMed] [Google Scholar]
- 21.Messinger SM, Albertini DF. Centrosome and microtubule dymanics during meiotic progression in the mouse oocyte. J Cell Sci. 1991;100:289–298. doi: 10.1242/jcs.100.2.289. [DOI] [PubMed] [Google Scholar]
- 22.Pelech S, Jelinkova L, Susor A, Zhang H, Shi X, Pavlok A, Kubelka M, Kovarova H. Antibody microarray analyses of signal transduction protein expression and phosphorylation during porcine oocyte maturation. J Proteome Res. 2008;7:2860–2871. doi: 10.1021/pr800082a. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, McGinnis LK, Kinsey WH. Fyn kinase activity is required for normal organization and functional polarity of the mouse oocyte cortex. Mol Reprod Dev. 2009;76:819–831. doi: 10.1002/mrd.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eppig JJ, Hosoe M, O’Brien MJ, Pendola FM, Requena A, Watanabe S. Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Mol Cell Endocrinol. 2000;163:109. doi: 10.1016/S0303-7207(99)00247-6. [DOI] [PubMed] [Google Scholar]
- 25.Vandre DD, Davis FM, Rao PN, Borisy GG. Phosphoproteins are components of mitotic microtubule organizing centers. Proc Natl Acad Sci U S A. 1984;81:4439–4443. doi: 10.1073/pnas.81.14.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis FM, Tsao TY, Fowler SK, Rao PN. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci U S A. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wickramasinghe D, Ebert KM, Albertini DF. Meiotic competence acquisition is associated with the appearance of M-phase characteristics in growing mouse oocytes. Dev Biol. 1991;143:162–172. doi: 10.1016/0012-1606(91)90063-9. [DOI] [PubMed] [Google Scholar]
- 28.Reis A, Madgwick S, Chang H-Y, Nabti I, Levasseur M, Jones KT. Prometaphase APCcdh1 activity prevents non-disjunction in mammalian oocytes. Nat Cell Biol. 2007;9:1192. doi: 10.1038/ncb1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapus A, Ciano C, Sun J, Zhan X, Kim L, Wong T, Rotstein OD. Cell volume-dependent phosphorylation of proteins of the cortical cytoskeleton and cell-cell contact sites. THE ROLE OF Fyn AND FER KINASES. J Biol Chem. 2000;275:32289–32298. doi: 10.1074/jbc.M003172200. [DOI] [PubMed] [Google Scholar]
- 30.Thomas SM, Soriano P, Imamoto A. Specific and redundant roles of Src and Fyn in organizing the cytoskeleton. Nature. 1995;376:267. doi: 10.1038/376267a0. [DOI] [PubMed] [Google Scholar]
- 31.Tournaviti S, Hannemann S, Terjung S, Kitzing TM, Stegmayer C, Ritzerfeld J, Walther P, Grosse R, Nickel W, Fackler OT. SH4-domain-induced plasma membrane dynamization promotes bleb-associated cell motility. J Cell Sci. 2007;120:3820–3829. doi: 10.1242/jcs.011130. [DOI] [PubMed] [Google Scholar]
- 32.Maekawa M, Toyama Y, Yasuda M, Yagi T, Yuasa S. Fyn tyrosine kinase in sertoli cells is involved in mouse spermatogenesis. Biol Reprod. 2002;66:211–221. doi: 10.1095/biolreprod66.1.211. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Baibakov B, Dean J. A subcortical maternal complex essential for preimplantation mouse embryogenesis. Dev Cell. 2008;15:416–425. doi: 10.1016/j.devcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]