Abstract
BACKGROUND
Both depression and diabetes have been found to be risk factors for dementia. This study examined whether comorbid depression in patients with diabetes increases the risk for dementia compared to those with diabetes alone.
METHODS
We conducted a prospective cohort study of 3,837 primary care patients with diabetes (mean age 63.2 ± 13.2 years) enrolled in an HMO in Washington State. The Patient Health Questionnaire (PHQ-9) was used to assess depression at baseline, and ICD-9 diagnoses for dementia were used to identify cases of dementia. Cohort members with no previous ICD-9 diagnosis of dementia prior to baseline were followed for a 5-year period. The risk of dementia for patients with both major depression and diabetes at baseline relative to patients with diabetes alone was estimated using cause-specific Cox proportional hazard regression models that adjusted for age, gender, education, race/ethnicity, diabetes duration, treatment with insulin, diabetes complications, nondiabetes-related medical comorbidity, hypertension, BMI, physical inactivity, smoking, HbA1c, and number of primary care visits per month.
RESULTS
Over the 5-year period, 36 of 455 (7.9%) patients with major depression and diabetes (incidence rate of 21.5 per 1,000 person-years) versus 163 of 3,382 (4.8%) patients with diabetes alone (incidence rate of 11.8 per 1,000 person-years) had one or more ICD-9 diagnoses of dementia. Patients with comorbid major depression had an increased risk of dementia (fully adjusted hazard ratio 2.69, 95% CI 1.77, 4.07).
CONCLUSIONS
Patients with major depression and diabetes had an increased risk of development of dementia compared to those with diabetes alone. These data add to recent findings showing that depression was associated with an increased risk of macrovascular and microvascular complications in patients with diabetes.
KEY WORDS: depression, dementia, diabetes
Depression is associated with the development of dementia, including vascular dementia1 and Alzheimer's disease (AD)2,3. Although depression may occur in a prodromal phase of dementia or as secondary to dementia4, two recent meta-analyses found that a history of depression approximately doubled the risk of subsequent AD and dementia in general.2,3 Several studies have reported that depression was associated with faster cognitive decline in carriers of the APOE-ε4 allele, a genetic marker for AD, compared to those without this genetic marker.5,6 Depression may raise the risk of dementia because of biologic abnormalities associated with this affective illness, such as hypothalamic-pituitary axis dysregulation and high cortisol levels7, decreased homeostasis of the autonomic nervous system resulting in decreased heart-rate variability8, increased platelet activation9, and an increase in pro-inflammatory factors.10 Depression may also increase the risk of dementia because of its association with a higher risk for behavioral risk factors such as smoking, obesity, and sedentary lifestyle and vascular risk factors such as hypertension, hyperlipidemia, and diabetes.11
Diabetes is also a risk factor for development of vascular dementia and may accelerate cognitive decline in AD.12–14 Population-based studies have shown that the risk of dementia, AD, and vascular dementia ranged from 40% to two-fold higher in patients with diabetes compared to controls without diabetes.12–14 Some, but not all, studies also showed that diabetes modifies the association between the APOE-ε4 allele and AD, with those with both diabetes and the APOE-ε4 allele at the greatest risk of AD.14 Multiple factors associated with diabetes could increase the risk of dementia, including inflammatory factors, hyperglycemia-induced tissue damage, vascular complications, hypoglycemic episodes, insulin resistance, oxidative stress, and microvascular and macrovascular disease.15
Patients with diabetes have a high prevalence of comorbid depression16. Among patients with diabetes, depression has been associated with poor self-care (adherence to diet, exercise, smoking cessation, and disease control medications)17, more cardiovascular risk factors,18 hyperglycemia, and macrovascular and microvascular diabetic complications,19,20 all of which raise the risk of both vascular dementia and AD. It is not known whether patients with comorbid depression and diabetes have a higher risk of subsequent development of dementia compared to those with diabetes alone.
In this prospective study, we examined the risk of development of dementia among a large sample of primary care patients with diabetes followed over a 5-year period. We hypothesized that patients with comorbid major depression and diabetes compared to those with diabetes alone would have a greater risk of development of dementia.
METHODS
Setting
Group Health (GH) is a mixed model capitated health plan serving over half a million members in Washington State. Most GH members receive health care within the integrated health practice, which includes approximately 30 primary care clinics in Western Washington State. The GH enrollment is demographically similar to the area population. All study procedures were approved by the institutional review board of GH.
Study Cohort Selection
The cohort for this prospective study, the Pathways Epidemiologic Follow-Up Study, was initially sampled between 2000 and 2002 from adults in the GH diabetes registry who received care from nine primary care clinics in the Puget Sound region. The clinics were chosen because of the geographic proximity to Seattle and the socioeconomic, racial/ethnic diversity of patients they serve. Patients were selected for the GH diabetes registry based on meeting any of the following eligibility criteria in the prior 12 months: two fasting plasma glucose levels ≥126 mg/dl, two random plasma glucose levels ≥200 mg/dl, a prescription for insulin or an oral hypoglycemic agent, two outpatient diagnoses or any inpatient diagnosis of diabetes.21 Surveys were mailed to 9,063 patients, but 1,222 were later found to be ineligible due to death, disenrollment, erroneous diagnosis of diabetes, or cognitive impairment. Among 7,841 eligible patients, 4,839 (61.7% of the eligible patients) returned the baseline mail questionnaire.
Permission to review medical records was obtained for 4,128 of 4,839 (85%) patients enrolled in the original Pathways study. Nonconsenting and consenting patients had similar rates of major depression, being married, type 1 diabetes, and smoking. Nonconsenting patients were younger (61.6 ± 12.9 vs. 63.4 ± 13.4), p < 0.001), more often non-white (35.8% vs. 19.5%, p < 0.0001), female (52.5% vs. 48.1%, p < 0.05), less educated (31.7% vs. 24.1% with ≤12 years of education, p < 0.0001), had a shorter duration of diabetes (8.1 ± 8.0 vs. 9.6 ± 9.4 years, p < 0.0001), lower mean BMI (30.5 ± 6.7 vs. 31.5 ± 7.3, p < 0.001), lower mean HbA1c (7.6 ± 1.4 vs. 7.8 ± 1.6, p < 0.01, and less hypertension (38.6% vs. 44.0%, p < 0.01) and lower RxRisk scores (2,614 ± 2,313 vs. 3,136 ± 2,444, p < 0.001).
Pathways Epidemiologic Follow-Up Study
Approximately 5 years post-enrollment, surviving members of the cohort were re-contacted by mail and follow-up telephone calls during 2005 to 2007. Consenting patients received a 20-minute telephone interview and were asked for permission to review their medical records. Waiver of consent to review medical records was approved by the GH Human Subjects Committee for patients who had died during follow-up of the cohort.
Predictor of Interest
The predictor of interest was major depression as determined by the Patient Health Questionnaire (PHQ-9).22 The PHQ-9 is a self-report measure based on the American Psychiatric Association Diagnostic and Statistical Manual, Version IV (DSM-IV) criteria for major depression. A systematic review has shown that the PHQ-9 has a 77% sensitivity and 94% specificity to the diagnosis of major depression based on structured psychiatric interviews.23 The PHQ-9 includes the nine DSM-IV major depressive symptoms with each item scored with a 0 to 3 Likert scale; criteria for major depression require at least five symptoms endorsed more than half the time, including at least one of the cardinal symptoms: depressed mood or anhedonia.22
Potential Confounders
Potential confounders obtained from the mailed survey included sociodemographic characteristics (age, gender, race/ethnicity, and education), diabetes duration, and height, weight, and health habits including smoking and physical activity from the Summary of Diabetes Self-Care Questionnaire.24 Physical inactivity was defined as 0 to 1 days of exercise in the prior week based on research showing that ≥2 h per week of exercise was cardio-protective in patients with diabetes.25 The number of diabetes complications and intensity of treatment were obtained from automated medical and laboratory records. This diabetes complication measure is associated with hospitalizations and mortality in the subsequent year.26 Other non-diabetes-related medical comorbidity was ascertained by using the RxRisk score, a computerized pharmacy measure of medical comorbidity, which is associated with costs and utilization.27 Hypertension diagnosis at baseline was determined by review of paper and electronic medical records. The HbA1c level drawn closest to baseline enrollment was obtained from automated clinical laboratory databases. Because depression has been shown to increase health utilization in patients with diabetes28 and potentially provide more opportunities for the physician to diagnose dementia, we used GH automated data to develop a measure of primary care visits per month over the 5-year period.
Outcome of Interest: Dementia
Incident cases of dementia were identified from both outpatient and inpatient databases based on the presence of one or more ICD-9-CM diagnosis codes of senile dementia uncomplicated (290.0), Alzheimer’s disease (331.01), vascular dementia (290.4), or dementia not otherwise specified (290.1) over the 5-year period after baseline.29 We excluded patients with evidence of ≥1 dementia diagnoses prior to baseline. These ICD-9 codes have been used successfully in recent studies to identify patients with dementia among those with diabetes.29,30 Based on a recent GH study, having one of the above ICD-9 diagnoses for dementia was found to have a sensitivity of 77% and a specificity of 95% compared to a consensus diagnosis of dementia based on a neuropsychiatric battery, physical examination, structured interview with informants, and review of medical records (personal communication, P. Crane). A similar battery of ICD-9 codes from 5 years of Medicare claims data had sensitivity of 87% for identifying cognitive impairment compared to a neuropsychiatric battery among patients in an AD registry, and patients with more severe cognitive impairment were more likely to be identified.31
Statistical Analyses
We described baseline demographic and clinical characteristics of the depressed and nondepressed groups. We estimated the association between major depression and incident dementia diagnosis using proportional hazards models. Patients with evidence of ≥1 dementia diagnoses prior to baseline were excluded. Because the length of follow-back (i.e., years of observation prior to baseline survey) varied across individuals, analyses were stratified by years of follow-back to account for variations in the amount of prior data available, essentially matching by years of follow-back without restricting the number of matches.
We censored individuals at the time of development of dementia, disenrollment, death from any cause, or the end of follow-up, whichever came first. We fit two proportional hazards models to the dementia outcome, with the first model including only baseline depression (major vs. no depression) and the second model including demographic characteristics (age, gender, race/ethnicity), clinical characteristics (diabetes duration, insulin use, RxRisk score, baseline evidence of hypertension, and diabetes complications) and baseline self-care variables (BMI, smoking, physical inactivity, and HbA1c) and a health utilization measure (the number of primary care visits per month over the 5-year period).
To address the possibility that depression was a prodrome of dementia or secondary to dementia, we completed a sensitivity analysis that repeated the above proportional hazards models, excluding patients who had an ICD-9 dementia diagnosis during the 2-year period after baseline. In a second sensitivity analysis, we defined dementia more conservatively using ≥2 ICD-9 dementia diagnoses, excluding patients with ≥2 ICD-9 dementia diagnoses prior to baseline and with time to second ICD-9 dementia diagnoses as the post-baseline dependent variable. A final sensitivity analysis added two additional cognitive impairment codes to the four used in the current study as one previous study did (ICD codes 331.83, mild cognitive impairment and 780.93, memory loss NOS)29 for both dementia pre-baseline dementia exclusion diagnoses and post-baseline case ascertainment.
Our primary cause-specific analyses assume that death and dementia are independent events. These analyses censor individuals at death, removing them from the risk set. If death and dementia are positively correlated, then removing individuals with other events from the risk set systematically removes individuals at higher risk for the event of interest. We explored whether there were competing risks for dementia and death by carrying out cause-specific analyses that keep individuals with the competing event (death) in the risk set.32
All analyses were carried out using SAS statistical software.
RESULTS
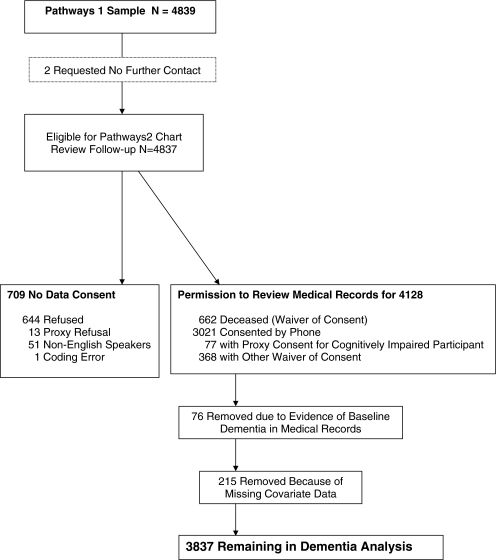
Of the 4,128 consenting patients, 76 had a dementia diagnosis prior to screening and were excluded (see Fig. 1). Another 215 patients were excluded because of missing covariate data, leaving 3,837 participants for the current study.
Figure 1.
Pathways flow diagram for analysis of dementia onset.
Compared to those without major depression, patients with major depression were younger and more likely to be female, unmarried, smokers, physically inactive, and treated with insulin. They also had higher RxRisk scores, higher HbA1c levels, more diabetes complications, and higher mean BMI (Table 1).
Table 1.
Baseline Characteristics of the Pathways sample Without Cognitive Impairment by Depression Status
| Characteristic | Overall N = 3837 | No major depression N = 3382 | Major depression N = 455 | p-value |
|---|---|---|---|---|
| Age, mean (SD), y | 63.2 (13.2) | 63.7 (13.1) | 59.0 (13.5) | <0.0001 |
| Women, N (%) | 1,837 (47.9%) | 1,568 (46.4%) | 269 (59.1%) | <0.0001 |
| Non-white, N (%) | 740 (19.3%) | 647 (19.1%) | 93 (20.4%) | 0.51 |
| ≥1+ year(s) of college, N (%) | 2,924 (76.2%) | 2,592 (76.6%) | 332 (73.0%) | 0.08 |
| Single marital status, N (%) | 1,297 (33.8%) | 1,096 (32.4%) | 201 (44.2%) | <0.0001 |
| Duration of diabetes, mean (SD), years | 9.6 (9.4) | 9.6 (9.5) | 9.6 (8.3) | 0.97 |
| Diabetes treatment, N (%) | <0.0001 | |||
| None or diet | 941 (24.5) | 864 (25.6) | 77 (16.9) | |
| Oral hypoglycemic | 1,726 (45.0) | 1,547 (45.7) | 179 (39.3) | |
| Any insulin | 1,170 (30.5) | 971 (28.7) | 199 (43.7) | |
| RxRisk score, mean (SD), $ | 3,120 (2,444) | 3,078 (2,384) | 3,426 (2,836) | 0.01 |
| Hypertension, N (%) | 2,392 (62.3%) | 2,105 (62.2%) | 287 (63.1%) | 0.73 |
| Body mass index, mean (SD), kg/m2 | 31.6 (7.3) | 31.2 (6.8) | 34.9 (9.3) | <0.0001 |
| Physical inactivity (0 to 1 days of exercise per week) | 1,247 (32.5%) | 1,036 (30.6%) | 211 (46.4%) | <0.0001 |
| Current smoking, N (%) | 331 (8.6%) | 264 (7.8%) | 67 (14.7%) | <0.0001 |
| HbA1c, mean (SD), % | 7.8 (1.6) | 7.7 (1.5) | 8.2 (1.7) | <0.0001 |
| No. of baseline diabetes complications/ automated data (0–6) | 1.4 (1.3) | 1.3 (1.3) | 1.6 (1.4) | <0.0001 |
During the follow-up period, a total of 199 participants (5.2%), representing over 15,468 person-years of follow-up, met our primary definition of dementia with ≥1 ICD-9 dementia diagnosis for an incidence rate of 12.9 per 1,000 person-years. Of the 455 patients with major depression at baseline, 36 (7.9%) had a new diagnosis of dementia during follow-up over 1,671 person-years for an incidence rate of 21.5 per 1,000 person-years, compared to 163 of 3,382 (4.8%) patients without major depression over 13,797 person-years for an incidence rate of 11.8 per 1,000 persons-years.
Multivariable analyses were conducted with major depression as the predictor of interest and dementia as the primary outcome (Table 2). Baseline comorbid major depression in patients with diabetes was associated with a 2.69-fold greater risk of dementia (95% CI 1.77, 4.07) compared to diabetes alone after adjustment for sex, age, race, education, diabetes duration, treatment intensity, diabetes complications, other medical comorbidity (RxRisk), hypertension, self-care and health utilization variables (BMI, smoking, physical inactivity, HbA1c, and number of primary care visits per month).
Table 2.
Hazard Ratios with 95% Confidence Intervals in Patients With Major Depression Compared to Controls Without Major Depression
| Covariate adjustment | Major depression hazard ratio (95% confidence interval) |
|---|---|
| Unadjusted | 1.83 (1.27, 2.62) |
| Demographic characteristicsa and clinical characteristicsb, self-care, and health care utilization variablesc | 2.69 (1.77, 4.07) |
aDemographic characteristics at baseline: age, gender, education, ethnicity/racial status
bClinical characteristics at baseline: diabetes duration, treatment intensity (insulin or no insulin treatment), expected costs (RxRisk), diabetes complications, hypertension (at baseline)
cSelf-care and health care utilization variables at baseline: BMI, smoking, HbA1c, physical inactivity, number of primary care visits per month
In a sensitivity analysis excluding patients who developed dementia in the 2-year period after baseline, we found that 17 (3.9%) of 436 participants with major depression versus 106 (3.2%) of 3,325 participants without major depression had evidence of a dementia diagnosis in years 2 to 5. The results of the fully adjusted model were very similar (HR = 2.05, 95% CI 1.19, 3.53). In a second sensitivity analysis, a total of 128 (3.3%) patients met the more conservative definition of dementia using ≥2 ICD-9 codes, including 23 of 459 (5.0%) patients with major depression and 105 of 3,407 (3.1%) patients without depression. Although depressed and nondepressed groups had lower rates by this definition, the fully adjusted hazard ratio associated with depression was similar (HR = 2.77, 95% CI, 1.71, 4.49). The final sensitivity analyses that added two additional dementia codes to the four used in the current study found 48 of 455 (10.6%) of depressed versus 237 of 3,382 (7.0%) nondepressed developed dementia with only a slight change in the adjusted hazard ratio (HR = 2.38, 95% CI 1.71, 3.32)
The cause-specific analysis produced similar results. The fully adjusted hazard ratio associated with depression was 2.18 (95% CI 1.49, 3.19).
DISCUSSION
In this prospective study, comorbid major depression in patients with diabetes was associated with a 2.7-fold increase in risk of dementia compared to patients with diabetes alone. Prior systematic reviews have found that both diabetes12,13 and depression2,3 are risk factors for dementia, but to our knowledge, this is the first study that has found a higher risk of incident dementia associated with comorbid major depression among patients with diabetes.
Depression may add to the risk of dementia associated with diabetes in several ways. Comorbid depression in patients with diabetes is associated with poor glucose control33, higher BMI34, higher rates of smoking34, physical inactivity, and lapses in adherence to hypoglycemic, lipid-lowing, and antihypertensive medications17. These adverse self-care characteristics could increase risk of macrovascular and microvascular complications. However, in our adjusted proportional hazard model, none of the baseline self-care variables were significant covariates (i.e., BMI, smoking, physical inactivity, or HbA1c), suggesting that these health risk factors may play a minor role in the risk of dementia among patients with depression and diabetes. Biologic effects of depression include hypothalamic pituitary axis dysregulation, which results in chronic elevation of glucocorticoid production, and impaired negative feedback.4 High cortisol levels may damage brain regions involved in cognition, such as the hypothalamus,35,36 as well as decrease neurogenesis in key brain areas37. Chronic and recurrent depression have been shown to be associated with hippocampal atrophy.38–40 Elevated cortisol levels also independently predict several components of the metabolic syndrome, including abdominal obesity, low levels of high density lipoproteins (HDL), and hypertriglyceridemia, all of which are thought to be risk factors for AD and vascular dementia.4
Depressed persons with diabetes are twice as likely to have three or more cardiovascular risk factors compared to nondepressed people with diabetes.18 Higher numbers of cardiovascular risk factors are associated with higher risk of vascular dementia and AD.41,42 Depression is associated with increased risk of myocardial infarction43 and stroke44 in community populations and a 35% increased risk for macrovascular complications in patients with diabetes.45 Diabetes is also associated with a significant risk of macrovascular complications.14 These vascular complications associated with both depression and diabetes may lead to additive risk of both AD and vascular dementia.14,46–48
Another possible interpretation of our data is that depression at baseline was secondary to dementia or was a prodromal symptom of early stage dementia.4 Both vascular dementia and AD are associated with a higher prevalence of depression.49,50 Depression frequently occurs in the early stages of AD and may result from both cell loss in key areas of the brain controlling emotions as well as a psychological reaction to cognitive loss.4 Vascular changes such as white matter hyperintensities are a risk factor for both depression51,52 and dementia,53 and are associated with a high prevalence of depression in cross-sectional studies54. In our sensitivity analysis that excluded patients with an ICD-9 diagnosis of dementia in the 2 years after baseline, depression remained associated with an increased risk of dementia, suggesting that depression was less likely to be a prodromal symptom or secondary to dementia. The patients in the current study also had to accurately fill out a 20-page mailed questionnaire at baseline, suggesting that dementia prior to baseline measurement of depression was unlikely.
Limitations of this study include that identification of dementia was based on ICD-9-CM codes and thus physician recognition of dementia. A prior study at GH has shown ICD-9-CM codes of dementia have high specificity (i.e., 95%), but only about 77% sensitivity compared to prospective comprehensive case ascertainment, including cognitive testing, physical examination, informant interviews, and medical records review (personal communication, Paul Crane).31 This suggests low rates of false positives. Because depression has been shown to be associated with increased health utilization in patients with diabetes28, patients with depression could have more opportunities for a physician to diagnose depression. However, controlling for primary care visits over the 5-year period had a negligible effect on risk of dementia associated with depression in our study. A second limitation is that diagnosis of probable major depression was made by the PHQ-9, not a structured psychiatric interview, and was only measured at baseline. However, a PHQ-9 score of ≥10 has moderate sensitivity and high specificity for the diagnosis of depression compared to structured interview22, suggesting few false positives. The patients in our study who were depressed at baseline also had a high rate of chronic depression with approximately 75% stating they had been depressed for ≥2 years.55 This study was observational and, while our models adjusted for potential confounding by including covariates related to non-response, it is possible that there was some residual confounding. A final limitation is that self-report of physical inactivity, BMI, and smoking status at baseline may not reflect subsequent health behaviors over the 5-year follow-up period.
CONCLUSION
Comorbid major depression in patients with diabetes was associated with more than double the risk for development of dementia. Until more research is available, it seems prudent for clinicians to add effective treatment of depression to other preventive measures such as increasing exercise, weight control and glycemic control to protect against the development of cognitive deficits in patients with diabetes.
Acknowledgements
This study was supported by NIMH R01 MH073686 (Von Korff) and NIMH K24 MH069741 (Katon). The authors would like to acknowledge Jürgen Unützer, MD, MPH, for providing critiques for this manuscript.
Conflicts of Interest Dr. Katon had received honorariums for lectures from Wyeth, Eli Lilly, Forest, and Pfizer pharmaceutical companies and serves on the Advisory Board for Eli Lilly and Wyeth. Dr. Von Korff has submitted a grant to Johnson & Johnson, but its award status is still pending. Dr. Paul Ciechanowski is CEO and founder of Same Page, Inc., a consulting company providing services for improving patient-provider relationships. Dr. Lin has received honorariums from Health Star Communications and Prescott Medical.
References
- 1.Cankurtaran M, Yavuz BB, Cankurtaran ES, Halil M, Ulger Z, Ariogul S. Risk factors and type of dementia: vascular or Alzheimer? Arch Gerontol Geriatr. 2008;47:25–34. doi: 10.1016/j.archger.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Jorm AF. Is depression a risk factor for dementia or cognitive decline? A review. Gerontology. 2000;46:219–227. doi: 10.1159/000022163. [DOI] [PubMed] [Google Scholar]
- 3.Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10:345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corsentino EA, Sawyer K, Sachs-Ericsson N, Blazer DG. Depressive symptoms moderate the influence of the apolipoproteine epsilon4 allele on cognitive decline in a sample of community dwelling older adults. Am J Geriatr Psychiatry. 2009;17:155–165. doi: 10.1097/JGP.0b013e31818f3a6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irie F, Masaki KH, Petrovitch H, et al. Apolipoprotein ε4 allele genotype and the effect of depressive symptoms on the risk of dementia in men: the Honolulu-Asia aging study. Arch Gen Psychiatry. 2008;65:906–912. doi: 10.1001/archpsyc.65.8.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/S0006-3223(01)01157-X. [DOI] [PubMed] [Google Scholar]
- 8.Taylor CB, Conrad A, Wilhelm FH, et al. Psychophysiological and cortisol responses to psychological stress in depressed and nondepressed older men and women with elevated cardiovascular disease risk. Psychosom Med. 2006;68:538–546. doi: 10.1097/01.psy.0000222372.16274.92. [DOI] [PubMed] [Google Scholar]
- 9.Musselman DL, Marzec U, Davidoff M, et al. Platelet activation and secretion in patients with major depression, thoracic aortic atherosclerosis, or renal dialysis treatment. Depress Anxiety. 2002;15:91–101. doi: 10.1002/da.10020. [DOI] [PubMed] [Google Scholar]
- 10.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32:1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 11.Wassertheil-Smoller S, Shumaker S, Ockene J, et al. Depression and cardiovascular sequelae in postmenopausal women. The Women's Health Initiative (WHI) Arch Intern Med. 2004;164:289–298. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- 12.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 13.Kloppenborg RP, Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol. 2008;585:97–108. doi: 10.1016/j.ejphar.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 14.Messier G, Gagnon M. Cognitive decline assocaited with dementia and type 2 diabetes: the interplay on risk factors. Diabetologia. 2009;52:2471–2474. doi: 10.1007/s00125-009-1533-2. [DOI] [PubMed] [Google Scholar]
- 15.Watson GS, Craft S. The role of insulin resistance in the pathogenesis of Alzheimer's disease: implications for treatment. CNS Drugs. 2003;17:27–45. doi: 10.2165/00023210-200317010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 17.Lin EH, Katon W, Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 18.Katon WJ, Lin EH, Russo J, et al. Cardiac risk factors in patients with diabetes mellitus and major depression. J Gen Intern Med. 2004;19:1192–1199. doi: 10.1111/j.1525-1497.2004.30405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black SA, Markides KS, Ray LA. Depression predicts increased incidence of adverse health outcomes in older Mexican Americans with type 2 diabetes. Diabetes Care. 2003;26:2822–2828. doi: 10.2337/diacare.26.10.2822. [DOI] [PubMed] [Google Scholar]
- 20.Lin EH, Rutter CM, Katon W, et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care. Nov 23, 2009 [Epub]. [DOI] [PMC free article] [PubMed]
- 21.McCulloch DK, Price MJ, Hindmarsh M, Wagner EH. A population-based approach to diabetes management in a primary care setting: early results and lessons learned. Eff Clin Pract. 1998;1:12–22. [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wittkampf KA, Naeije L, Schene AH, Huyser J, Weert HC. Diagnostic accuracy of the mood module of the patient health questionnaire: a systematic review. Gen Hosp Psychiatry. 2007;29:388–395. doi: 10.1016/j.genhosppsych.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from seven studies and a revised scale. Diabetes Care. 2000;23:943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- 25.Hu FB, Stampfer MJ, Solomon C, et al. Physical activity and risk for cardiovascular events in diabetic women. Ann Intern Med. 2001;134:96–105. doi: 10.7326/0003-4819-134-2-200101160-00009. [DOI] [PubMed] [Google Scholar]
- 26.Young BA, Lin E, Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care. 2008;14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 27.Fishman PA, Goodman MJ, Hornbrook MC, Meenan RT, Bachman DJ, O'Keeffe Rosetti MC. Risk adjustment using automated ambulatory pharmacy data: the RxRisk model. Med Care. 2003;41:84–99. doi: 10.1097/00005650-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Simon G, Katon W, Lin E, et al. Diabetes complications and depression as predictors of health care costs. Gen Hosp Psychiatry. 2005;27:344–351. doi: 10.1016/j.genhosppsych.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor DH, Jr, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer's disease. J Clin Epidemiol. 2002;55:929–937. doi: 10.1016/S0895-4356(02)00452-3. [DOI] [PubMed] [Google Scholar]
- 32.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 33.Lustman PJ, Anderson RJ, Freedland KE, Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23:934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 34.Katon W, Korff M, Ciechanowski P, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914–920. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 35.Peavy GM, Lange KL, Salmon DP, et al. The effects of prolonged stress and APOE genotype on memory and cortisol in older adults. Biol Psychiatry. 2007;62:472–478. doi: 10.1016/j.biopsych.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee BK, Glass TA, McAtee MJ, et al. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch Gen Psychiatry. 2007;64:810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- 37.Elder GA, Gasperi R, Gama Sosa MA. Research update: neurogenesis in adult brain and neuropsychiatric disorders. Mt Sinai J Med. 2006;73:931–940. [PubMed] [Google Scholar]
- 38.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 39.Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bell-McGinty S, Butters MA, Meltzer CC, Greer PJ, Reynolds CF, 3rd, Becker JT. Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am J Psychiatry. 2002;159:1424–1427. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- 41.Skoog I, Lernfelt B, Landahl S, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/S0140-6736(96)90608-X. [DOI] [PubMed] [Google Scholar]
- 42.Breteler MM, Claus JJ, Duijn CM, Launer LJ, Hofman A. Epidemiology of Alzheimer's disease. Epidemiol Rev. 1992;14:59–82. doi: 10.1093/oxfordjournals.epirev.a036092. [DOI] [PubMed] [Google Scholar]
- 43.Kooy K, Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysis. Int J Geriatr Psychiatry. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- 44.Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke: the Framingham Study. Stroke. 2007;38:16–21. doi: 10.1161/01.STR.0000251695.39877.ca. [DOI] [PubMed] [Google Scholar]
- 45.Lin E, Rutter C, Katon W, et al. Depression and adverse outcomes of diabetes. Diabetes Care. In Press.
- 46.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. doi: 10.1001/jama.277.10.813. [DOI] [PubMed] [Google Scholar]
- 47.Lim A, Tsuang D, Kukull W, et al. Clinico-neuropathological correlation of Alzheimer's disease in a community-based case series. J Am Geriatr Soc. 1999;47:564–569. doi: 10.1111/j.1532-5415.1999.tb02571.x. [DOI] [PubMed] [Google Scholar]
- 48.Heyman A, Fillenbaum GG, Welsh-Bohmer KA, et al. Cerebral infarcts in patients with autopsy-proven Alzheimer's disease: CERAD, part XVIII. Consortium to establish a registry for Alzheimer's disease. Neurology. 1998;51:159–162. doi: 10.1212/wnl.51.1.159. [DOI] [PubMed] [Google Scholar]
- 49.Korcizyn A, Haperin I. Depression and dementia. J Neurol Sci. April 3, 2009 (epub). [DOI] [PubMed]
- 50.Zubenko GS, Zubenko WN, McPherson S, et al. A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer's disease. Am J Psychiatry. 2003;160:857–866. doi: 10.1176/appi.ajp.160.5.857. [DOI] [PubMed] [Google Scholar]
- 51.Steffens DC, Krishnan KR, Crump C, Burke GL. Cerebrovascular disease and evolution of depressive symptoms in the cardiovascular health study. Stroke. 2002;33:1636–1644. doi: 10.1161/01.STR.0000018405.59799.D5. [DOI] [PubMed] [Google Scholar]
- 52.Teodorczuk A, O'Brien JT, Firbank MJ, et al. White matter changes and late-life depressive symptoms: longitudinal study. Br J Psychiatry. 2007;191:212–217. doi: 10.1192/bjp.bp.107.036756. [DOI] [PubMed] [Google Scholar]
- 53.Pantoni L, Leys D, Fazekas F, et al. Role of white matter lesions in cognitive impairment of vascular origin. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S49–S54. doi: 10.1097/00002093-199912003-00008. [DOI] [PubMed] [Google Scholar]
- 54.Krishnan MS, O'Brien JT, Firbank MJ, et al. Relationship between periventricular and deep white matter lesions and depressive symptoms in older people. The LADIS Study. Int J Geriatr Psychiatry. 2006;21:983–989. doi: 10.1002/gps.1596. [DOI] [PubMed] [Google Scholar]
- 55.Katon WJ, Korff M, Lin EH, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry. 2004;61:1042–1049. doi: 10.1001/archpsyc.61.10.1042. [DOI] [PubMed] [Google Scholar]