Abstract
Rationale
Mice lacking metabotropic glutamate receptors 5 (mGluR5) exhibit reduced glutamatergic function and behavioral abnormalities, including deficits in prepulse inhibition (PPI) of the startle response that may be relevant to schizophrenia. Thus, these mice are an animal model that may be used for preclinical evaluation of potentially new classes of antipsychotic compounds. Recent clinical studies have suggested several compounds that modulate glutamatergic transmission through distinct mechanisms, such as potentiation of the N-methyl-d-aspartate (NMDA) receptor glycine site, activation of group II mGluR, and activation of glutamate-cysteine antiporters, as being efficacious in the treatment of schizophrenia.
Objectives
The aim of this work is to evaluate the effects of sarcosine (a selective inhibitor of the glycine transporter 1 [GlyT1]), LY379268 (a group II mGluR agonist), and N-acetylcysteine (a cysteine prodrug that indirectly activates cystine-glutamate antiporters to increase glutamate levels in the extrasynaptic space) on PPI deficits in mGluR5 knockout mice.
Results
Sarcosine and N-acetylcysteine, but not LY379268, ameliorated PPI deficits in mGluR5 knockout mice. The ability of N-acetylcysteine to restore PPI deficits was not blocked by the group II mGluR antagonist LY341495, indicating that the effects of N-acetylcysteine were not attributable to activation of group II mGluRs by glutamate.
Conclusions
These findings provide evidence that the interactions between mGluR5 and NMDA receptors are involved in the regulation of PPI and suggest that activation of glutamate receptors, other than group II receptors, by increased endogenous glutamate transmission, may ameliorate the behavioral abnormalities associated with mGluR5 deficiency.
Keywords: Schizophrenia, Glycine transporter inhibitor, NMDA receptor, mGluR2 agonist
Introduction
The glutamatergic hypothesis of schizophrenia suggests that dysfunction in glutamatergic signaling contributes to the pathophysiology of this disorder (Coyle et al. 2003; Stahl 2007). This hypothesis derives from observations of a schizophrenia-like syndrome evoked by pharmacological blockade of N-methyl-d-aspartate (NMDA) receptor channels with phencyclidine (PCP) or ketamine (Adler et al. 1999; Krystal et al. 1994; Lindsley et al. 2006). Metabotropic glutamate receptor 5 (mGluR5) has an expression pattern mirroring that of NMDA receptors, indicating the interaction between these two receptor types (Awad et al. 2000; Pisani et al. 2001). Furthermore, mGluR5 is linked to the NMDA receptor via Homer, Shank, and postsynaptic density protein of 95 kDa (PSD-95; Tu et al. 1999). Such linkages allow for reciprocal potentiation of function and synergistic activity between these two receptors. Indeed, many of the outcomes of mGluR5 blockade or ablation may stem from loss of the cooperative effects of mGluR5 and NMDA receptors. The disturbances in hippocampal long-term potentiation (LTP) observed in mGluR5 knockout mice are restricted to NMDA receptor-dependent pathways (Jia et al. 1998; Lu et al. 1997). Moreover, the mGluR5 antagonists 2-methyl-6-(phenylethynyl)pyridine (MPEP) and 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine (MTEP) augment the behavioral effects induced by the NMDA receptor antagonists PCP and MK-801, including locomotor hyperactivity, prepulse inhibition (PPI) deficits, and learning impairments (Campbell et al. 2004; Henry et al. 2002; Homayoun et al. 2004; Kinney et al. 2003; Pietraszek et al. 2005). In contrast, the mGluR5 agonist (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) effectively reverses these behavioral effects of ketamine (Chan et al. 2008). Altogether, the data suggest that mGluR5 plays an important role in the dysregulation of NMDA-mediated neurotransmission in schizophrenia-related processes.
Sensorimotor gating, measured by PPI of the startle response, is a fundamental form of information processing that may be deficient in some schizophrenia patients and rodents treated with NMDA receptor antagonists (al-Amin and Schwarzkopf 1996). Therefore, PPI is widely used to study the neurobiology of schizophrenia (Braff et al. 2001; Geyer et al. 2001, 2002). Although PPI deficits have been found in schizophrenia (Braff et al. 2005), such PPI deficits are not diagnostic or unique to schizophrenia, because they are found in unaffected siblings (Wynn et al. 2004), and women with schizophrenia did not differ in PPI from healthy women (Kumari et al. 2004). Moreover, PPI deficits have been reported in many other nonpsychotic, psychiatric, and neurological disorders, suggesting that PPI deficits may reflect cognitive deficits in general (Geyer 2006). The mGluR5 antagonist MPEP has no effect on PPI by itself but potentiates PCP-induced disruptions of PPI (Henry et al. 2002). Mice lacking mGluR5 exhibit PPI deficits (Brody et al. 2004a; Kinney et al. 2003; Lipina et al. 2007) that are reversible by chronic (Gray et al. 2009), but not acute (Brody et al. 2004a), treatment with clozapine. This reversal of PPI deficits by clozapine has been attributed to upregulation of NMDA receptors (Gray et al. 2009). Moreover, a positive modulator of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors, CX546, restored PPI deficits in mGluR5 knockout mice (Lipina et al. 2007). PPI deficits seen in mGluR5 knockout mice are not mimicked by acute administration of mGluR5 antagonists, and these deficits are most likely attributable to compensatory alterations in neuronal circuitry occurring during development (Kinney et al. 2003). The reduced physiological responses associated with NMDA receptor function are suggested to play a critical role in the behavioral alterations seen in mGluR5 knockout mice. mGluR5 knockout mice, therefore, may represent a model of diseases characterized by PPI deficits and NMDA receptor hypofunction, such as schizophrenia. Thus, this genetic model may offer a unique opportunity to develop antipsychotic compounds with novel mechanisms of action working through the glutamatergic system.
To verify the predictive validity of this model (Geyer and Markou 1995; Markou et al. 2009), the effects of a variety of compounds that have been shown in earlier clinical trials of schizophrenia to modulate the glutamatergic system through distinct mechanisms and with therapeutic potential were evaluated on PPI deficits in mGluR5 knockout mice. The glutamate modulating agents included sarcosine, a glycine transporter-1 (GlyT-1) inhibitor, LY379268, an mGluR2/3 agonist, and N-acetylcysteine, a cysteine prodrug that indirectly activates cystine-glutamate antiporters located in the glial cells to increase glutamate levels in the extrasynaptic space. After demonstrating that sarcosine and N-acetylcysteine, but not the mGluR2/3 agonist LY379268, reversed PPI deficits in mGluR5 knockout mice, we examined whether the mGluR2/3 antagonist LY341495 can block the ameliorating effects of N-acetylcysteine on PPI deficits in mGluR5 knockout mice.
Materials and methods
Animals
mGluR5 knockout mice (Grm5tm1Rod; Jia et al. 1998) were backcrossed to C57BL/6J for at least 10 generations. Wild-type and knockout mice used in our study were generated by mating male and female heterozygous parents. Wild-type mice were littermates of the knockout animals, with the genotypes determined by polymerase chain reaction. Mice were housed in groups of four, with food and water available ad libitum. The animal holding room was maintained on a 12-h light/dark cycle (lights off at 0700 hours). All experiments were conducted in accordance with the guidelines of the American Association for the Accreditation of Laboratory Animal Care and the National Research Council's Guide for Care and Use of Laboratory Animals and were approved by the University of California, San Diego, Institutional Animal Care and Use Committee.
Prepulse inhibition apparatus and procedure
Three startle chambers were used to measure the startle response (SR-LAB, San Diego Instruments, San Diego, CA, USA). Each chamber consisted of a nonrestrictive Plexiglas cylinder mounted on a frame inside a lit, ventilated box (39 × 38 × 58 cm). Movements within the cylinder were detected by a piezoelectric accelerometer that was attached beneath the cylinder. Vibrations detected by the accelerometer were transduced into analog electrical signals that were subsequently digitized and stored by a computer. A total of 65 readings were recorded at 1-ms intervals, commencing at stimulus onset, and the average amplitude was used to describe the acoustic startle response. A high-frequency loudspeaker inside the box, mounted 24 cm above the chamber, generated the broadband background noise and acoustic stimuli, which were controlled by the SR-LAB software system and interface. The PPI chambers and cylinders were cleaned with 70% ethanol between mice.
The experimental session consisted of a 10-min acclimatization period in which only broadband background noise (65 dB) was presented. The acclimatization was followed by a PPI session that consisted of five different trial types: no stimulus trials (NOSTIM), a startle pulse alone trial of 40 ms at 120 dB (P120), and three prepulse + pulse trials of a 20-ms noise prepulse at 69, 73, or 77 dB, followed by a 80-ms delay, then a 40-ms 120 dB startle pulse. The NOSTIM trial consisted of only broadband background noise. All test sessions commenced and concluded with five presentations of the P120 trial, and the remainder of the session consisted of 10 presentations of each trial type in a pseudorandom order, with varying intertrial intervals (mean, 15 s; range, 12–30 s). Each animal was always tested in the same startle chamber. The startle magnitude was calculated as the average of all pulse alone trials, excluding the first and last five such trials in each session. PPI was calculated as the percent inhibition of the startle amplitude evoked by the pulse alone:  .
.
Drugs
Sarcosine and N-acetylcysteine were purchased from Sigma (St. Louis, MO, USA) and were dissolved in saline. LY379268 and LY341495 were purchased from ANAWA (Wangen, Switzerland) and Tocris (Bristol, UK), respectively, and were dissolved in sterile water, pH adjusted to 7.4 with sodium hydroxide. All drugs were administered intraperitoneally in a volume of 10 ml/kg.
Experimental design
Experiment 1: effect of sarcosine on PPI in wild-type and mGluR5 knockout mice
Wild-type and mGluR5 knockout mice (male, n = 4; female, n = 4) were pretreated with sarcosine (0, 100, or 300 mg/kg, i.p.) 30 min prior to the start of the PPI test in a counterbalanced order, using a within-subjects design.
Experiment 2: effect of LY379268 on PPI in wild-type and mGluR5 knockout mice
Wild-type and mGluR5 knockout mice (male, n = 4; female, n = 3) were administered with the mGluR2/3 agonist LY379268 (0, 3, or 10 mg/kg, i.p.) 30 min prior to the start of the PPI test in a counterbalanced order, using a within-subjects design.
Experiment 3: effect of N-acetylcysteine on PPI in wild-type and mGluR5 knockout mice
Wild-type and mGluR5 knockout mice (male, n = 5; female, n = 3) were pretreated with N-acetylcysteine (0, 50, or 100 mg/kg, i.p.) 90 min prior to the start of the PPI test in a counterbalanced order, using a within-subjects design.
Experiment 4: combined effects of LY341495 and N-acetylcysteine on impaired PPI in mGluR5 knockout mice
Eight mGluR5 knockout mice (male, n = 4; female, n = 4) received drug treatment according to a within-subjects Latin-square design. mGluR5 knockout mice were pretreated with N-acetylcysteine (100 mg/kg, 90 min) and the mGluR2/3 antagonist LY341495 (0, 1, or 5 mg/kg, 60 min) prior to the start of the PPI test.
The animals were only used for one experiment. Each test session was separated by at least 5 days.
Data analyses
Multiple factor analysis of variance (ANOVA) tests were employed to assess group variations across the data. Statistically significant effects in the ANOVA were followed with Student–Newman–Keuls post hoc comparisons. The level of significance was set at 0.05. Variation with sex was tested within each dataset (as a factor in the ANOVA) before combining groups for further analysis; in no case was a significant main effect or interactive effect of sex observed. However, the limited availability of animals of each sex does not give this study statistical power to identify subtle or complex sex-related variations.
Results
Experiment 1: effect of sarcosine on PPI in wild-type and mGluR5 knockout mice
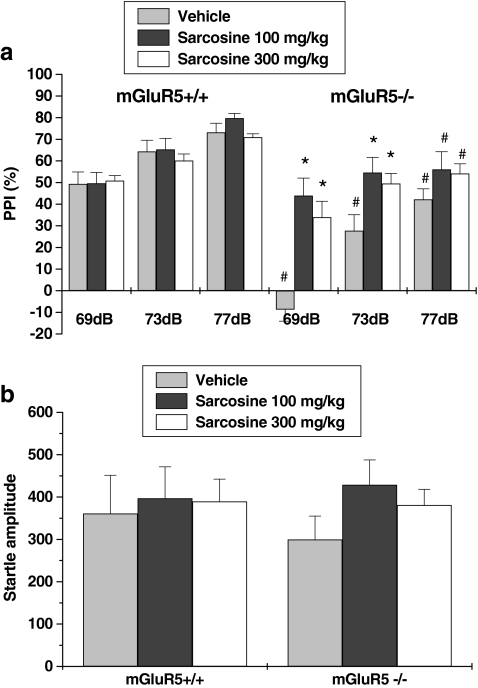
Four-way repeated measures ANOVA on PPI values revealed a main effect of sarcosine treatment (F 2,42 = 6.599, p < 0.01), genotype (F 1,42 = 35.97, p < 0.001), and prepulse intensity (F 1,42 = 82.385, p < 0.001), but no main effect of sex. Then, the sexes were collapsed, and a three-way ANOVA was run. A significant sarcosine treatment × genotype interaction (F 2,42 = 5.69, p < 0.01) was shown. Post hoc tests indicated that this interaction effect was attributable to statistically lower PPI in mGluR5 knockout mice compared with wild-type mice. Furthermore, sarcosine significantly increased PPI in knockout mice, but not in wild-type mice. Specifically, statistically significant differences were observed between wild-type and knockout mice treated with vehicle, but no differences between wild-type and knockout mice treated with sarcosine at prepulse intensities of 69 and 73 dB, indicating a reversal of PPI deficits in the knockout mice. Three-way ANOVA revealed that sarcosine treatment, genotype, and sex had no effect on startle amplitude (Fig. 1b).
Fig. 1.
Effects of sarcosine on PPI in mGluR5+/+ and mGluR−/− mice. Percentage of PPI (a) and startle amplitude (b) at each of three prepulse levels (69, 73, and 77 dB) are presented. Data are expressed as mean ± SEM (n = 8). * p < 0.05, compared with vehicle-treated group of the same genotype; # p < 0.05, compared with the same treatment group of wild-type mice
Experiment 2: effect of LY379268 on PPI in wild-type and mGluR5 knockout mice
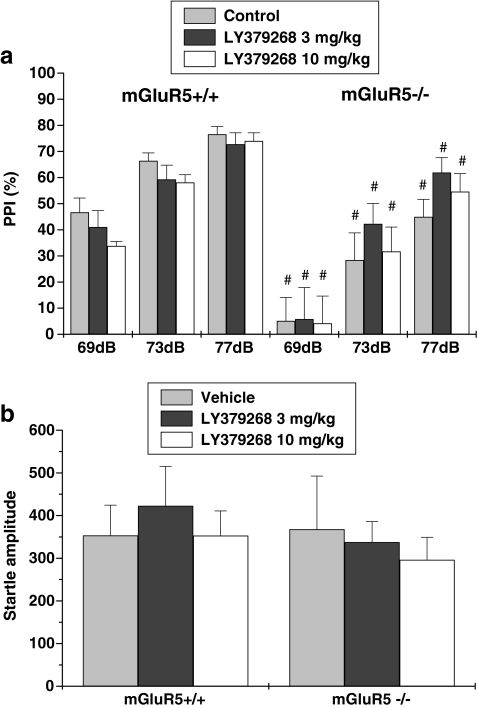
Four-way repeated-measures ANOVA on PPI values revealed a main effect of genotype (F 1,36 = 35.66, p < 0.001) and prepulse intensity (F 1,36 = 106.52, p < 0.001), indicating that the mGluR5 knockout mice exhibited a PPI deficit. There was no effect of sex or LY379268 treatment. After the sexes were collapsed and a three-way ANOVA was run, no LY379268 treatment × genotype interaction was found (Fig. 2a), and treatment (LY379268) and genotype did not affect startle amplitude (Fig. 2b).
Fig. 2.
Effects of the mGluR2/3 agonist LY379268 on PPI in mGluR5+/+ and mGluR−/− mice. Percentage of PPI (a) and startle amplitude (b) at each of three prepulse levels (69, 73, and 77 dB) are presented. Data are expressed as mean ± SEM (n = 7). # p < 0.05, compared with the same treatment group of wild-type mice
Experiment 3: effect of N-acetylcysteine on PPI in wild-type and mGluR5 knockout mice
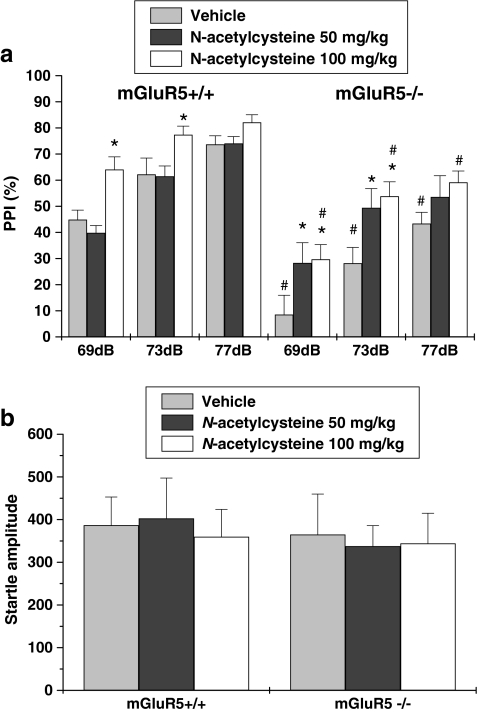
Four-way repeated-measures ANOVA indicated a main effect of N-acetylcysteine treatment (F 2,42 = 6.996, p < 0.01), genotype (F 1,42 = 46.71, p < 0.001), and prepulse intensity (F 2,42 = 107.56, p < 0.001), but no main effect of sex. After the sexes were collapsed and a three-way ANOVA was run, no N-acetylcysteine treatment × genotype interaction was found, which was attributable to N-acetylcysteine enhancing PPI in both wild-type and knockout mice at prepulse intensities of 69 and 73 dB (Fig. 3a). Treatment (N-acetylcysteine) and genotype did not affect startle amplitude (Fig. 3b).
Fig. 3.
Effects of N-acetylcysteine on PPI in mGluR5+/+ and mGluR−/− mice. Percentage of PPI (a) and startle amplitude (b) at each of three prepulse levels (69, 73, and 77 dB) are presented. Data are expressed as mean ± SEM (n = 8). * p < 0.05, compared with the vehicle-treated group of the same genotype; # p < 0.05, compared with the same treatment group of wild-type mice
Experiment 4: combined effect of LY341495 and N-acetylcysteine on impaired PPI in mGluR5 knockout mice
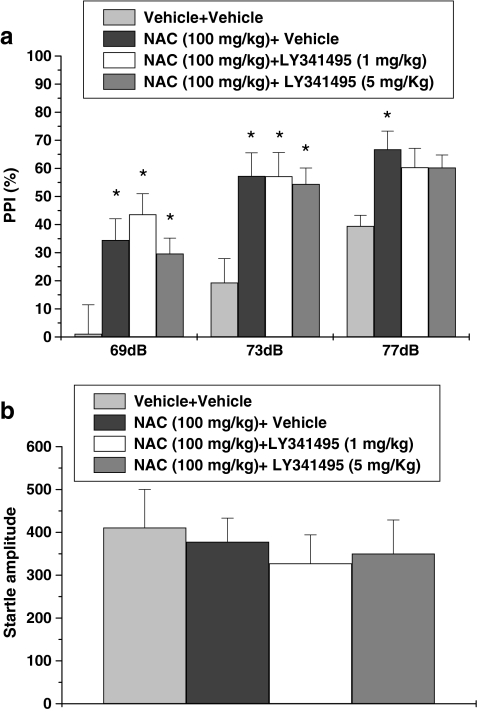
Three-way repeated-measures ANOVA revealed a main effect of drug treatment (F 3,42 = 8.37, p < 0.001) and prepulse intensity (F 2,42 = 41.36, p < 0.001), but no effect of sex. Post hoc analysis indicated that the LY341495 (1 and 5 mg/kg) plus N-acetylcysteine-treated groups were not different from the N-acetylcysteine only-treated group. This pattern of results demonstrates that the mGluR2/3 antagonist LY341495 did not block the beneficial effects N-acetylcysteine on PPI deficits in mGluR5 knockout mice (Fig. 4a). No significant difference was found between groups in startle amplitude (Fig. 4b).
Fig. 4.
Effects of co-treatment of LY341495 and N-acetylcysteine on PPI in mGluR−/− mice. Percentage of PPI (a) and startle amplitude (b) at each of three prepulse levels (69, 73, and 77 dB) are presented. Data are expressed as mean ± SEM (n = 8). * p < 0.05, compared with the vehicle-treated group
Discussion
Confirming previous findings (Brody et al. 2004a, b; Gray et al. 2009; Kinney et al. 2003; Lipina et al. 2007), our results showed that mGluR5 knockout mice exhibited pronounced deficits in PPI of the startle response. Importantly, the present data showed that these PPI deficits in mGluR5 knockout mice were ameliorated by acute treatment with the GlyT-1 inhibitor sarcosine and the cysteine prodrug N-acetylcysteine, but not by the group II mGluR agonist LY379268. Furthermore, the reversal of PPI deficits by N-acetylcysteine in mGluR5 knockout mice was not blocked by the group II mGluR antagonist LY341495.
mGluR5 knockout mice are resistant to acute treatment with typical and atypical antipsychotics (Brody et al. 2004a). However, our results showed that acute sarcosine treatment effectively ameliorated PPI deficits in mGluR5 knockout mice, consistent with the positive results reported in antipsychotic-naive acute schizophrenia patients (Lane et al. 2005, 2008), whereas other NMDA receptor-enhancing agents, including glycine and d-cycloserine, have not been effective antipsychotics (Buchanan et al. 2007). Sarcosine increases the availability of the NMDA receptor co-agonist glycine via inhibition of GlyT-1, as well as directly enhances NMDA receptor function as a co-agonist (Zhang et al. 2009). The dual mechanism by which sarcosine enhances NMDA receptor function might explain why sacrosine is superior to d-serine for the treatment of schizophrenia (Lane et al. 2005). Several studies have demonstrated the ameliorating effects of GlyT-1 inhibitors on neurochemical and behavioral disturbance related to NMDA receptor hypofunction. For example, N[3-(4′-flurophenyl)-3-(4′-phenylphenoxy) propyl]sarcosine (NFPS), a more potent Gly-T1 inhibitor than sarcosine, rescues LTP (Manahan-Vaughan et al. 2008) and cognitive impairment (Karasawa et al. 2008) induced by administration of the NMDA receptor antagonist MK-801 in rats, prevents dopaminergic dysregulation observed after subchronic or chronic administration of the NMDA receptor antagonist PCP (Javitt et al. 2004), and ameliorates cognitive deficits in mice chronically treated with PCP (Hashimoto et al. 2008). Sarcosine, at the same dose as we used (100 mg/kg), has been found to effectively reduce ketamine-induced PPI deficits and c-Fos expression in certain brain regions (Yang et al. 2009). Together with the observations that mGluR5 knockout mice are resistant to the disruptive effects of MK-801 on PPI and that a positive modulator of AMPA receptors restores PPI deficits in mGluR5 knockout mice (Lipina et al. 2007), our findings further support a hypofunctional state of NMDA receptors in mGluR5 knockout mice which leads to behavioral abnormalities with potential relevance to schizophrenia.
Our data showed that LY379268 had no effect on PPI deficits in mGluR5 knockout mice. At the same dose levels (3 and 10 mg/kg), LY379268 significantly reduced PCP-evoked hyperactivity and behavioral alterations (i.e., circling, falling, stereotypy, and ataxia, as well as amphetamine-evoked hyperactivity; Woolley et al. 2008). Additionally, LY379268 (1–3 mg/kg) reduced ketamine-evoked hyperlocomotion and ketamine-induced changes in glutamate in the dentate gyrus. However, this compound (3–12 mg/kg) did not restore PPI deficits induced by ketamine (Imre et al. 2006b). Similarly, other mGluR2/3 agonists, such as LY354740 or LY314582, did not ameliorate either PPI deficits induced by PCP (Henry et al. 2002; Ossowska et al. 2000; Schlumberger et al. 2009; Schreiber et al. 2000) or subchronic ketamine administration (Imre et al. 2006a). Although PPI cannot be considered to be a cognitive process per se, abnormalities in pre-attentive information processing may be predictive of, or lead to, complex cognitive deficits (Geyer 2006). Moreover, LY354740 alone produces cognitive impairments (Higgins et al. 2004). Thus, the therapeutic potential of mGluR2/3 agonists may be specific to the positive and negative symptoms, but not against sensorimotor and cognitive deficits in schizophrenia.
Clinically, N-acetylcysteine has been found to improve mismatch negativity, a deficit seen in schizophrenia patients (Lavoie et al. 2008), and to ameliorate positive and negative symptoms as an add-on treatment (Berk et al. 2008) in schizophrenia patients. Our results demonstrate that N-acetylcysteine significantly ameliorated the pronounced PPI deficits observed in mGluR5 knockout mice. Recently, N-acetylcysteine has been reported to block PCP-evoked extracellular glutamate in the prefrontal cortex and social withdrawal and memory deficits in rats, and these effects require cystine-glutamate exchange and group II mGluR activation (Baker et al. 2008). Thus, the present study sought to determine whether group II mGluR activation is involved in the ameliorating effects of N-acetylcysteine on PPI deficits in mGluR5 knockout mice. We found that the ability of N-acetylcysteine to restore PPI deficits in mGluR5 knockout mice was not blocked by the mGluR2/3 antagonist LY341495. Consistent with the lack of effect of the mGluR2/3 agonist LY379268 on PPI deficits, the mechanism underlying the reversal of PPI deficits by N-acetylcysteine in mGluR5 knockout mice probably does not involve mGluR2/3 activation by the enhanced glutamate release induced by N-acetylcysteine administration. Notably, a large portion of mGluR1 is distributed extrasynaptically (Hubert and Smith 2004). Moreover, recent evidence has linked activation of extrasynaptic NMDA receptors with astrocytic gliotransmission (Fellin et al. 2004; Le Meur et al. 2007). It is possible that extrasynaptic mGluR1 and NMDA receptors are implicated in the ameliorating effect of N-acetylcysteine on PPI deficits in mGluR5 knockout mice.
In addition to regulating the levels of extrasynaptic glutamate, N-acetylcysteine-evoked elevations of cysteine can trigger glutathione synthesis in neurons and glial cells. Glutathione is known to potentiate the NMDA receptor response to glutamate (Kohr et al. 1994), either by acting at redox modulatory sites (Sullivan et al. 1994) or by blocking high-affinity Zn2+ inhibition through Zn2+ chelation (Paoletti et al. 1997). The attenuation of PPI deficits in mGluR5 knockout mice by N-acetylcysteine might be attributable to the potentiation of NMDA receptors through glutathione. Several lines of evidence suggest that oxidative stress associated with impaired metabolism of the antioxidant glutathione plays a key role in the pathophysiology of schizophrenia. Glutathione levels in cerebrospinal fluid were significantly lower in drug-free patients with schizophrenia than in control subjects (Do et al. 2000). Furthermore, decreased levels of glutathione, glutathione-peroxidase, and glutathione reductase in the caudate region were observed in postmortem brain samples from patients with schizophrenia (Do et al. 2000). Whether the levels of glutathione are lower in mGluR5 knockout mice compared with wild-type animals remains to be determined.
In summary, the present results indicate that lack of mGluR5 during development and in adulthood is associated with PPI deficits relevant to schizophrenia that can be improved by acute treatment with sarcosine, a GlyT-1 inhibitor and NMDA receptor co-agonist, or N-acetylcysteine, a precursor of glutathione and also a prodrug of cysteine, but not with the mGluR2/3 agonist LY379268. Our results, together with an earlier report showing that the AMPA receptor modulator CX546 restored PPI deficits in mGluR5 knockout mice (Lipina et al. 2007), support the hypothesis that mGluR5 knockout mice represent an animal model to preclinically detect antipsychotic compounds with novel mechanisms of action that may involve potentiation of NMDA receptor function for the treatment of sensorimotor gating and cognitive impairments. Additionally, these findings provide evidence that interactions between mGluR5 and NMDA receptors, but not mGluR2/3, are involved in the regulation of PPI and suggest that positive modulation of NMDA receptor function may ameliorate the behavioral abnormalities associated with mGluR5 deficiency.
Acknowledgements
This work was supported by a grant (97-2918-I-320-001) from the National Science Council, Taiwan, to HHC, and by a grant (R01MH62527) from the US National Institute of Mental Health to AM. The authors would like to thank Mr. Mike Arends for editorial assistance.
Disclosures
AM declares that during the past 3 years, she has received contract research support from Intracellular Therapeutics, Inc., Lundbeck Research USA, Inc., Bristol-Myers Squibb Co, and F. Hoffman-La Roche Inc., received an honorarium from Abbott GmbH and Company, and has a consulting agreement with Astra-Zeneca.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156:1646–1649. doi: 10.1176/ajp.156.10.1646. [DOI] [PubMed] [Google Scholar]
- al-Amin HA, Schwarzkopf SB. Effects of the PCP analog dizocilpine on sensory gating: potential relevance to clinical subtypes of schizophrenia. Biol Psychiatry. 1996;40:744–754. doi: 10.1016/0006-3223(95)00485-8. [DOI] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Madayag A, Kristiansen LV, Meador-Woodruff JH, Haroutunian V, Raju I. Contribution of cystine-glutamate antiporters to the psychotomimetic effects of phencyclidine. Neuropsychopharmacology. 2008;33:1760–1772. doi: 10.1038/sj.npp.1301532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia—a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA, Ellwanger J, Sprock J, Swerdlow NR. Female schizophrenia patients have prepulse inhibition deficits. Biol Psychiatry. 2005;57:817–820. doi: 10.1016/j.biopsych.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Brody SA, Conquet F, Geyer MA. Effect of antipsychotic treatment on the prepulse inhibition deficit of mGluR5 knockout mice. Psychopharmacology (Berl) 2004;172:187–195. doi: 10.1007/s00213-003-1635-3. [DOI] [PubMed] [Google Scholar]
- Brody SA, Dulawa SC, Conquet F, Geyer MA. Assessment of a prepulse inhibition deficit in a mutant mouse lacking mGlu5 receptors. Mol Psychiatry. 2004;9:35–41. doi: 10.1038/sj.mp.4001404. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Lalwani K, Hernandez L, Kinney GG, Conn PJ, Bristow LJ. The mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates PCP-induced cognitive deficits in rats. Psychopharmacology (Berl) 2004;175:310–318. doi: 10.1007/s00213-004-1827-5. [DOI] [PubMed] [Google Scholar]
- Chan MH, Chiu PH, Sou JH, Chen HH. Attenuation of ketamine-evoked behavioral responses by mGluR5 positive modulators in mice. Psychopharmacology (Berl) 2008;198:141–148. doi: 10.1007/s00213-008-1103-1. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci. 2003;1003:318–327. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuenod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J NeuroSci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res. 2006;10:211–220. doi: 10.1007/BF03033358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Markou A. Animal models of psychiatric disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. New York: Raven; 1995. pp. 787–798. [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, McIlwain KL, Paylor R. Mouse genetic models for prepulse inhibition: an early review. Mol Psychiatry. 2002;7:1039–1053. doi: 10.1038/sj.mp.4001159. [DOI] [PubMed] [Google Scholar]
- Gray L, van den Buuse M, Scarr E, Dean B, Hannan AJ. Clozapine reverses schizophrenia-related behaviours in the metabotropic glutamate receptor 5 knockout mouse: association with N-methyl-D-aspartic acid receptor up-regulation. Int J Neuropsychopharmacol. 2009;12:45–60. doi: 10.1017/S1461145708009085. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Ishima T, Chaki S, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the glycine transporter-1 inhibitor NFPS and D-serine. Eur Neuropsychopharmacol. 2008;18:414–421. doi: 10.1016/j.euroneuro.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Henry SA, Lehmann-Masten V, Gasparini F, Geyer MA, Markou A. The mGluR5 antagonist MPEP, but not the mGluR2/3 agonist LY314582, augments PCP effects on prepulse inhibition and locomotor activity. Neuropharmacology. 2002;43:1199–1209. doi: 10.1016/S0028-3908(02)00332-5. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Ballard TM, Kew JN, Richards JG, Kemp JA, Adam G, Woltering T, Nakanishi S, Mutel V. Pharmacological manipulation of mGlu2 receptors influences cognitive performance in the rodent. Neuropharmacology. 2004;46:907–917. doi: 10.1016/j.neuropharm.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional interaction between NMDA and mGlu5 receptors: effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Smith Y. Age-related changes in the expression of axonal and glial group I metabotropic glutamate receptor in the rat substantia nigra pars reticulata. J Comp Neurol. 2004;475:95–106. doi: 10.1002/cne.20163. [DOI] [PubMed] [Google Scholar]
- Imre G, Fokkema DS, Ter Horst GJ. Subchronic administration of LY354740 does not modify ketamine-evoked behavior and neuronal activity in rats. Eur J Pharmacol. 2006;544:77–81. doi: 10.1016/j.ejphar.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Imre G, Salomons A, Jongsma M, Fokkema DS, Den Boer JA, Ter Horst GJ. Effects of the mGluR2/3 agonist LY379268 on ketamine-evoked behaviours and neurochemical changes in the dentate gyrus of the rat. Pharmacol Biochem Behav. 2006;84:392–399. doi: 10.1016/j.pbb.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Balla A, Burch S, Suckow R, Xie S, Sershen H. Reversal of phencyclidine-induced dopaminergic dysregulation by N-methyl-D-aspartate receptor/glycine-site agonists. Neuropsychopharmacology. 2004;29:300–307. doi: 10.1038/sj.npp.1300313. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem. 1998;5:331–343. [PMC free article] [PubMed] [Google Scholar]
- Karasawa J, Hashimoto K, Chaki S. D-Serine and a glycine transporter inhibitor improve MK-801-induced cognitive deficits in a novel object recognition test in rats. Behav Brain Res. 2008;186:78–83. doi: 10.1016/j.bbr.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Burno M, Campbell UC, Hernandez LM, Rodriguez D, Bristow LJ, Conn PJ. Metabotropic glutamate subtype 5 receptors modulate locomotor activity and sensorimotor gating in rodents. J Pharmacol Exp Ther. 2003;306:116–123. doi: 10.1124/jpet.103.048702. [DOI] [PubMed] [Google Scholar]
- Kohr G, Eckardt S, Luddens H, Monyer H, Seeburg PH. NMDA receptor channels: subunit-specific potentiation by reducing agents. Neuron. 1994;12:1031–1040. doi: 10.1016/0896-6273(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Sharma T. Sex differences in prepulse inhibition deficits in chronic schizophrenia. Schizophr Res. 2004;69:219–235. doi: 10.1016/j.schres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Lane HY, Chang YC, Liu YC, Chiu CC, Tsai GE. Sarcosine or D-serine add-on treatment for acute exacerbation of schizophrenia: a randomized, double-blind, placebo-controlled study. Arch Gen Psychiatry. 2005;62:1196–1204. doi: 10.1001/archpsyc.62.11.1196. [DOI] [PubMed] [Google Scholar]
- Lane HY, Liu YC, Huang CL, Chang YC, Liau CH, Perng CH, Tsai GE. Sarcosine (N-methylglycine) treatment for acute schizophrenia: a randomized, double-blind study. Biol Psychiatry. 2008;63:9–12. doi: 10.1016/j.biopsych.2007.04.038. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, Fornari E, Meuli R, Solida A, Vianin P, Cuenod M, Buclin T, Do KQ. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- Le Meur K, Galante M, Angulo MC, Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol. 2007;580:373–383. doi: 10.1113/jphysiol.2006.123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Jr, Sur C, Kinney GG. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem. 2006;6:771–785. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- Lipina T, Weiss K, Roder J. The ampakine CX546 restores the prepulse inhibition and latent inhibition deficits in mGluR5-deficient mice. Neuropsychopharmacology. 2007;32:745–756. doi: 10.1038/sj.npp.1301191. [DOI] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Wildforster V, Thomsen C. Rescue of hippocampal LTP and learning deficits in a rat model of psychosis by inhibition of glycine transporter-1 (GlyT1) Eur J NeuroSci. 2008;28:1342–1350. doi: 10.1111/j.1460-9568.2008.06433.x. [DOI] [PubMed] [Google Scholar]
- Markou A, Chiamulera C, Geyer MA, Tricklebank M, Steckler T. Removing obstacles in neuroscience drug discovery: the future path for animal models. Neuropsychopharmacology. 2009;34:74–89. doi: 10.1038/npp.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossowska K, Pietraszek M, Wardas J, Nowak G, Zajaczkowski W, Wolfarth S, Pilc A. The role of glutamate receptors in antipsychotic drug action. Amino Acids. 2000;19:87–94. doi: 10.1007/s007260070037. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietraszek M, Gravius A, Schafer D, Weil T, Trifanova D, Danysz W. mGluR5, but not mGluR1, antagonist modifies MK-801-induced locomotor activity and deficit of prepulse inhibition. Neuropharmacology. 2005;49:73–85. doi: 10.1016/j.neuropharm.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Pisani A, Gubellini P, Bonsi P, Conquet F, Picconi B, Centonze D, Bernardi G, Calabresi P. Metabotropic glutamate receptor 5 mediates the potentiation of N-methyl-D-aspartate responses in medium spiny striatal neurons. Neuroscience. 2001;106:579–587. doi: 10.1016/S0306-4522(01)00297-4. [DOI] [PubMed] [Google Scholar]
- Schlumberger C, Schafer D, Barberi C, More L, Nagel J, Pietraszek M, Schmidt WJ, Danysz W. Effects of a metabotropic glutamate receptor group II agonist LY354740 in animal models of positive schizophrenia symptoms and cognition. Behav Pharmacol. 2009;20:56–66. doi: 10.1097/FBP.0b013e3283242f57. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Lowe D, Voerste A, De Vry J. LY354740 affects startle responding but not sensorimotor gating or discriminative effects of phencyclidine. Eur J Pharmacol. 2000;388:R3–R4. doi: 10.1016/S0014-2999(99)00844-4. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Beyond the dopamine hypothesis to the NMDA glutamate receptor hypofunction hypothesis of schizophrenia. CNS Spectr. 2007;12:265–268. doi: 10.1017/s1092852900021015. [DOI] [PubMed] [Google Scholar]
- Sullivan JM, Traynelis SF, Chen HS, Escobar W, Heinemann SF, Lipton SA. Identification of two cysteine residues that are required for redox modulation of the NMDA subtype of glutamate receptor. Neuron. 1994;13:929–936. doi: 10.1016/0896-6273(94)90258-5. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/S0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 2008;196:431–440. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Dawson ME, Schell AM, McGee M, Salveson D, Green MF. Prepulse facilitation and prepulse inhibition in schizophrenia patients and their unaffected siblings. Biol Psychiatry. 2004;55:518–523. doi: 10.1016/j.biopsych.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Yang SY, Hong CJ, Huang YH, Tsai SJ. The effects of glycine transporter I inhibitor, N-methylglycine (sarcosine), on ketamine-induced alterations in sensorimotor gating and regional brain c-Fos expression in rats. Neurosci Lett. 2010;469:127–130. doi: 10.1016/j.neulet.2009.11.058. [DOI] [PubMed] [Google Scholar]
- Zhang HX, Hyrc K, Thio LL. The glycine transport inhibitor sarcosine is an NMDA receptor co-agonist that differs from glycine. J Physiol. 2009;587:3207–3220. doi: 10.1113/jphysiol.2009.168757. [DOI] [PMC free article] [PubMed] [Google Scholar]