Abstract
The unique chemistry of oxygen has been both a resource and threat for life on Earth for at least the last 2.4 billion years. Reduction of oxygen to water allows extraction of more metabolic energy from organic fuels than is possible through anaerobic glycolysis. On the other hand, partially reduced oxygen can react indiscriminately with biomolecules to cause genetic damage, disease, and even death. Organisms in all three superkingdoms of life have developed elaborate mechanisms to protect against such oxidative damage and to exploit reactive oxygen species as sensors and signals in myriad processes. The sulfur amino acids, cysteine and methionine, are the main targets of reactive oxygen species in proteins. Oxidative modifications to cysteine and methionine can have profound effects on a protein’s activity, structure, stability, and subcellular localization. Non-reversible oxidative modifications (oxidative damage) may contribute to molecular, cellular, and organismal aging and serve as signals for repair, removal, or programmed cell death. Reversible oxidation events can function as transient signals of physiological status, extracellular environment, nutrient availability, metabolic state, cell cycle phase, immune function, or sensory stimuli. Because of its chemical similarity to sulfur and stronger nucleophilicity and acidity, selenium is an extremely efficient catalyst of reactions between sulfur and oxygen. Most of the biological activity of selenium is due to selenoproteins containing selenocysteine, the 21st genetically encoded protein amino acid. The most abundant selenoproteins in mammals are the glutathione peroxidases (five to six genes) that reduce hydrogen peroxide and lipid hydroperoxides at the expense of glutathione and serve to limit the strength and duration of reactive oxygen signals. Thioredoxin reductases (three genes) use nicotinamide adenine dinucleotide phosphate to reduce oxidized thioredoxin and its homologs, which regulate a plethora of redox signaling events. Methionine sulfoxide reductase B1 reduces methionine sulfoxide back to methionine using thioredoxin as a reductant. Several selenoproteins in the endoplasmic reticulum are involved in the regulation of protein disulfide formation and unfolded protein response signaling, although their precise biological activities have not been determined. The most widely distributed selenoprotein family in Nature is represented by the highly conserved thioredoxin-like selenoprotein W and its homologs that have not yet been assigned specific biological functions. Recent evidence suggests selenoprotein W and the six other small thioredoxin-like mammalian selenoproteins may serve to transduce hydrogen peroxide signals into regulatory disulfide bonds in specific target proteins.
Keywords: Selenium, Selenoprotein, Oxidation–reduction, Signaling
According to its position in the electrochemical series, molecular oxygen (O2) is a powerful oxidizing agent: O2 + 4H+ + 4e− → 2H2O, E 0 = +1.23 V. However, reactions with O2 generally proceed very slowly at typical in vivo temperatures due to the thermodynamically difficult addition of the first electron, which has a standard reduction potential of −0.32 V [1]. Once the first electron has been added to form the superoxide radical (O2⋅−), oxygen becomes much more reactive. Aerobic cells continuously encounter reactive oxygen species (ROS), such as O2⋅−, hydrogen peroxide (H2O2), and hydroxyl radical (OH⋅) from a variety of sources and counteract their effects with an array of antioxidants. ROS react with proteins—principally cysteine (Cys) and methionine residues—to modify or inactivate their function, and react with DNA and chromatin to cause mutations or double-stranded breaks—known collectively as “oxidative damage”. Excessive ROS exposure or inadequate antioxidant protection results in oxidative damage, cytotoxicity, or even death. However, moderate perturbations of oxidation–reduction (redox) homeostasis usually initiate a signaling response [2]. Such redox signaling is a well-recognized stress response with a variety of downstream effects, including upregulation of protective and repair enzymes [3]. Accumulating evidence suggests that redox signaling is also part of normal metabolism in non-stressed cells. There is an emerging understanding that H2O2 generation is an essential step in many receptor-mediated signaling events [4].
Mitochondria are the major source of ROS in most cells, due to side reactions during oxidative phosphorylation of adenosine diphosphate (ADP) to adenosine triphosphate (ATP). In the first step of the respiratory electron transport chain, one electron reduction of oxygen produces the O2⋅− radical. The production of O2⋅− by mitochondria has been localized to several enzymes of the electron transport chain, including Complexes I and III and glycerol-3-phosphate dehydrogenase [5]. In vitro studies indicate that 1% to 3% of the oxygen metabolized by a cell may escape the mitochondrial respiratory electron transport chain as O2⋅− although the figure in vivo is likely to be lower, probably around 0.2% [6]. Most of the released O2⋅− is disproportionated to H2O2 and molecular oxygen, either by manganese-dependent superoxide dismutase (Mn-SOD) in the mitochondrial matrix or by Cu/Zn-SOD in the cytoplasm (Fig. 1). The other main source of ROS is nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox). Originally described in phagocytic immune cells, where it produces prodigious amounts of O2⋅− to kill engulfed pathogenic cells, NADPH oxidase has subsequently been found at the inner surface of the plasma membrane in non-phagocytic cells, where it is associated with the cytoplasmic domains of cell surface receptors. Seven NADPH oxidase proteins are known: Nox1–Nox5 and dual oxidases 1 and 2. Upon binding of the receptor’s specific ligand, NADPH oxidase is activated and produces O2⋅−, which can be disproportionated to H2O2 by SOD. Recent studies also suggest that hydrogen peroxide may be directly generated by receptor–ligand interactions [7]. Through processes that have not been well-characterized, O2⋅− and/or H2O2 cause activation of the kinase activity of the complex, which initiates a series of protein phosphorylation events specific to each receptor.
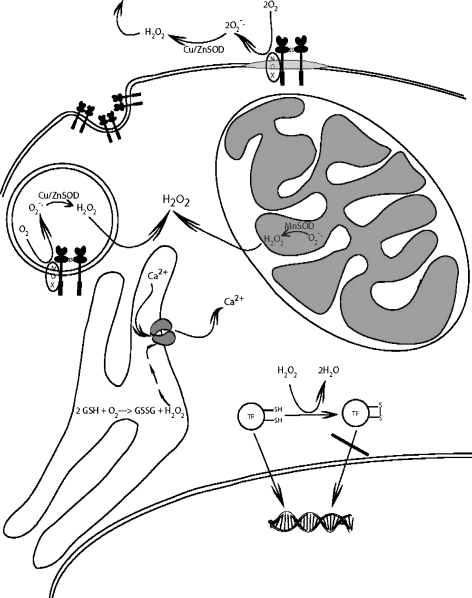
Fig. 1.
Reactive oxygen signaling. Schematic of eukaryotic cell showing intracellular compartmentalization of reactive oxygen species and interorganelle transport. TF transcription factor, NOX NADPH oxidase, SOD superoxide dismutase, O 2 − ⋅ superoxide radical, H 2 O 2 hydrogen peroxide, GSH glutathione, GSSG glutathione disulfide
Mild increases in intracellular O2⋅− or micromolar concentrations of exogenously added H2O2 cause growth responses in a wide variety of mammalian cells [2]. Slightly higher concentrations of H2O2 induce cell cycle arrest, senescence, and/or apoptosis, whereas millimolar concentrations of H2O2 can cause massive oxidative damage and necrotic cell death. Many studies have established that H2O2 is an essential signaling molecule involved in mediating the mitogenic effect of growth factor receptors [4]. Cell surface receptors producing ROS upon activation include those for epidermal growth factor, platelet-derived growth factor, insulin-like growth factor [8], vascular endothelial growth factor [9], toll-like receptor 4 [10], and various cytokines [11]. ROS regulate autophagy [12], calcium permeability in mitochondria [13], and calcium release from the endoplasmic/sarcoplasmic reticulum, which is critical to calcium signal propagation to the mitochondria [14].
ROS from mitochondrial respiration are released into the cytoplasm, where they can react with sensor or target molecules to initiate appropriate cellular responses. Paradoxically, NADPH oxidases produce O2⋅− on the extracellular side of the plasma membrane. Extracellular O2⋅− can diffuse back into the cell through anion channels, whereupon it is disproportionated to H2O2 by cytoplasmic Zn/Cu-SOD, or can diffuse into the cell as H2O2 after disproportionation by extracellular Zn/Cu-SOD (Fig. 1). Because of its relative stability, rapid diffusion, membrane permeability, and selective reactivity, H2O2 is well suited for a second messenger role. The half-life of H2O2 in lymphocytes is on the order of 10−3 s [15], allowing for extremely rapid signaling events.
Extracellular generation of O2⋅− and H2O2 is consistent with the cell–cell communication functions of ROS described in vascular cells [16], but is difficult to reconcile with its role as a second messenger in intracellular signaling pathways. The NADPH oxidase enzymes and dual oxidases that produce O2⋅− and H2O2 each have distinct subcellular distributions that impart a degree of spatial specificity to ROS generation [17]. In addition, these H2O2-producing enzymes, as well as endothelial nitric oxide synthase and heme oxygenase, localize with other signaling components in lipid rafts and caveolae [18]. Ligation of extracellular receptors leads to endocytosis of receptor-associated NADPH oxidase subunits localized in lipid rafts and caveolae to form redox-active endosomes containing redox-processing proteins capable of transmitting H2O2 signals from the endosome lumen (formerly extracellular space) to the cytoplasm. Processing of such “redoxosomes” can lead to changes in phospholipids, luminal pH, voltage, and osmotic pressure that modulate signaling events in myriad ways [19]. Membrane permeability of H2O2 is regulated by lipid composition, osmotic pressure, and selective transport through aquaporin channels, thus providing several mechanisms for control of H2O2 diffusion back into the cytoplasm [20]. Intracellular H2O2 signaling is also localized due to its rapid degradation by cytosolic glutathione peroxidase and peroxiredoxins that decrease its concentration tenfold within 4–6 µm [3]. Thus, receptor-mediated H2O2 signaling can be processed selectively, localized to a confined region, and terminated rapidly.
H2O2 reacts preferentially with thiols, although the reaction rate is greatly increased with activated “low pK a” Cys residues or by enzymatic catalysis. Oxidation of Cys by H2O2 produces an unstable sulfenic acid, which can be further oxidized to a sulfinic or sulfonic acid and is typically not reversible. Cysteine sulfenic acid will react rapidly with another Cys residue in the same or another protein to form intra- or interprotein disulfide bridges or react with the ubiquitous thiol tripeptide glutathione (GSH) to produce a protein–GSH mixed disulfide, a process known as S-glutathionylation. Recent evidence suggests that redox-sensitive regulatory disulfide bonds are predominately formed by H2O2 originating in mitochondria [21]. Cystine bridges formed by reaction with H2O2 can induce new protein conformations or formation of homodimers, heterodimers, and polymeric forms.
Reversible intraprotein disulfides that regulate protein functions are known as “redox switches”. One particularly well-known example is the Escherichia coli transcription factor OxyR that contains two conserved cysteines that form a disulfide in response to slight elevations in H2O2, which alters its DNA binding site and induces expression of, among other genes, glutathione reductase and glutaredoxin [22]. A similar pathway operates in yeast, where exposure to H2O2 forms a sulfenic acid in Orp1, which reacts with and forms an intramolecular disulfide in the Yap1 transcription factor causing its nuclear translocation and activation of antioxidant genes that reduce the Yap1 disulfide and shut off the signaling [23]. Another well-studied example is the CDC25 phosphatases that control the eukaryotic cell cycle, which are reversibly inhibited by formation of an intramolecular disulfide upon exposure to ROS [24].
Redox-regulated intermolecular disulfide bonds are less well studied. One example is turnover of the abundant chloroplast protein ribulose-1,5-bisphosphate carboxylase/oxygenase during leaf senescence. Oxidative stress-induced senescence results in cross-linking of ribulose-1,5-bisphosphate carboxylase/oxygenase via disulfide bridges, inhibition of enzyme activity, and rapid degradation [25]. Evidence has recently been obtained that KEAP1, the adaptor of a Cul3–ubiquitin ligase complex that marks the antioxidant response element-specific NRF2 transcription factor for proteasomal degradation, is regulated by dimerization via an intermolecular disulfide bridge induced by H2O2 [26]. Other examples include the apoptosis-inducing protein Bax, which is activated by oligomerization via disulfide bridges formed by ROS in colorectal cancer cells [27], and H2O2-mediated inactivation of receptor protein–tyrosine phosphatases by formation of disulfide-bridged dimers [28].
G protein-coupled receptors (GPCR), one of the largest and most diverse families of proteins in eukaryotes and the targets for many important classes of drugs, mediate responses to numerous extracellular stimuli such as hormones, odors, peptides, and neurotransmitters. ROS generated by NADPH oxidases following GPCR activation play an important role in propagating and modulating GPCR signaling [29]. Many GPCRs form homodimers and heterodimers, which enhance signaling and mediate transactivation between receptors [30]. Dimer formation in a model yeast GPCR has recently been shown to be accomplished by disulfide bridges [31], and ligand-induced disulfide-mediated dimerization is correlated with activation of growth hormone receptor [32] and the receptor tyrosine kinases for discoidin [33] and epidermal growth factor [34]. However, a role of ROS in receptor dimer formation has not been established.
Activation of cell surface receptors or exposure of cells to sublethal concentrations of H2O2 results in S-glutathionylation of specific Cys residues in cellular proteins, whose functions are thus significantly altered. Many signaling molecules and transcription factors fundamental for growth, differentiation, and apoptosis are regulated by S-glutathionylation, including protein kinase A, protein kinase C, mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), T cell p59fyn kinase, protein tyrosine phosphatase-1B, and MEKK1 (MAPK/ERK kinase kinase 1; MAP3K), an upstream activator of the SAPK/JNK (stress-activated protein kinase/c-Jun N-terminal kinase) pathway [35]. Transcription factors are also regulated by S-glutathionylation in response to ROS, including p53, AP-1, c-Jun, and NF-kappa B [36].
Protein S-glutathionylation is a pervasive post-translational modification that regulates protein function analogous to phosphorylation [35]. However, the Cys residues that become glutathionylated are frequently not the most reactive or the most sterically available residues [37], implying enzymatic processes control S-glutathionylation of these specific Cys residues [38]. Protein–GSH disulfides are thermodynamically unstable in the presence of the 10–20-mM reduced GSH concentration in cytosol and are deglutathionylated slowly by thiol–disulfide exchange with the reduced GSH pool or rapidly and specifically by the enzyme glutaredoxin (thioltransferase) [39], producing oxidized glutathione (GSSG). Thus, formation of protein–GSH disulfide bonds requires catalytic mechanisms that create kinetic barriers to thiol–disulfide exchange reactions. The putative enzymes controlling addition of GSH to specific Cys residues remain to be identified [40].
Selenium (Se) is a trace element required in nanomolar concentrations by organisms in all three superkingdoms of life, with higher plants and fungi being the only major taxa in which an essential biological function for Se has not been established [41]. The major functions of Se are attributable to proteins containing selenocysteine (Sec), the Se-containing homolog of Cys. Sec is synthesized from serine after aminoacylation to a unique transfer RNA, tRNASec. tRNASec is the longest known tRNA (90–100 nt), containing an extended acceptor stem and an extra loop compared to canonical tRNAs [42]. Sec is synthesized from seryl-tRNASec by a synthase that uses selenophosphate as the selenium donor and the cofactor pyridoxal phosphate in both eukarya/archaea and bacteria. In eukarya and archaea, the serine moiety is first phosphorylated by O-phosphoseryl-tRNA kinase, whereas bacteria form Sec directly from seryl-tRNASec. Sec is subsequently incorporated into growing polypeptide chains under control of the UGA codon (TGA in DNA), which usually codes for termination. Incorporation of Sec is directed by a specific stem-loop structure in the mRNA 3′ to the UGA codon known as a “selenocysteine insertion sequence” (SECIS). In bacteria, the SECIS element is located immediately after the UGA codon, whereas it is located some distance away from the UGA codon in eukarya and archaea [43]. The occurrence of this UGA-dependent co-translational pathway of selenoprotein synthesis has led to the recognition of Sec as the 21st protein amino acid specified in the universal genetic code [44].
All of the selenoproteins for which an enzymatic activity has been identified catalyze redox reactions involving oxidation of sulfhydryl groups and/or reduction of disulfides. The Se atom is isomorphous with sulfur, having the same outer electron structure, a slightly larger atomic radius and essentially identical electronegativity. Thus, Se homologs exist for most sulfur-containing molecules. Despite these structural similarities, Se is a much stronger nucleophile than sulfur, Se compounds have much more negative reduction potentials than homologous sulfur compounds, and hydrogens bonded to Se are much more acidic than those bonded to sulfur (pK a ∼5 vs. ∼9). Because of these properties, Sec is able to catalyze oxidation of thiols in the reducing environment of the cytoplasm, where the GSH to GSSG ratio of 30–100 to 1 results in a redox potential of approximately −230 mV [45]. The major cellular thiol/disulfide systems, including GSH/GSSG, thioredoxin-1 (-SH(2)/-SS-), and cysteine/cystine, are not in redox equilibrium and respond differently to chemical toxicants and physiologic stimuli [46]. Selenoproteins have the unique ability to trap H2O2 and use the resulting selenenic acid to form specific disulfide bonds in the presence of 10–20 mM GSH and are thus well suited for controlling the formation of disulfide bonds with specific Cys residues. Because of the rapid oxidation kinetics of selenols, H2O2 in the cytosol reacts completely with Sec-containing proteins to produce Sec selenenic acid before it can react with other potential targets [3]. Thus, rapid consumption of H2O2 by selenoproteins acts to limit the duration of H2O2 signals and the distance over which those signals are transmitted by diffusion (Table 1).
Table 1.
Human Selenoproteins
| Selenoprotein | Abbreviation | Subcellular localization |
|---|---|---|
| Cytosolic glutathione peroxidase | GPX1 | Cytosol, mitochondria |
| “Gastrointestinal” glutathione peroxidase | GPX2 | Cytosol, ER |
| Plasma glutathione peroxidase | GPX3 | Secreted |
| Phospholipid hydroperoxide glutathione peroxidase | GPX4 | Cytosol, mitochondria, nucleus |
| Olfactory glutathione peroxidase | GPX6 | Unknown |
| Thioredoxin reductase type I | TXNRD1, TR1 | Cytosol, nucleus |
| Thioredoxin reductase type II | TXNRD2, TR3 | Mitochondria |
| Thioredoxin reductase type III | TXNRD3, TR2, TGR | Cytosol, nucleus, ER |
| Iodothyronine deiodinase type I | DIO1, D1, 5DI | Plasma membrane |
| Iodothyronine deiodinase type II | DIO2, D2, 5DII | ER membrane |
| Iodothyronine deiodinase type III | DIO3, D3, 5DIII | Plasma membrane |
| Selenoprotein 15 | Sep15 | ER lumen |
| Selenoprotein H | SelH, BthD | Nucleus |
| Selenoprotein I | SelI, hEPT1 | Transmembrane |
| Selenoprotein K | SelK | ER and plasma membrane |
| Selenoprotein M | SelM | ER lumen |
| Selenoprotein N | SEPN1, SelN | ER membrane |
| Selenoprotein O | SelO | Unknown |
| Selenoprotein P | SelP | Secreted, cytosol |
| Methionine sulfoxide reductase B1 | MsrB1, SelR, SelX | Cytosol, nucleus |
| Selenoprotein S | SEPS1, SelS, VIMP | ER and plasma membrane |
| Selenoprotein T | SelT | ER membrane |
| Selenoprotein V | SelV | Unknown |
| Selenoprotein W | SelW, SEPW1 | Cytosol |
| Selenophosphate synthetase | SPS2 | Cytosol |
The first selenoenzyme identified—glutathione peroxidase (Gpx)—catalyzes the oxidation of cytosolic GSH by H2O2 (Table 1). Gpx1 was discovered in erythrocytes [47], where it removes H2O2 formed by dissociation of oxyhemoglobin into O2⋅− and methemoglobin [48]. The GPX1 gene codes for both the cytosolic and mitochondrial forms of the enzyme, which exist as a tetramer of four identical subunits, each containing a single Sec residue. Control of H2O2 in the insulin signaling pathway by cytosolic Gpx1 has been demonstrated in GPX1 overexpressing mice. GPX1 OE mice develop insulin resistance [49] due to excessively rapid degradation of H2O2 that normally causes glutathionylation and inhibition of protein tyrosine phosphatase (PTP) 1B responsible for terminating insulin receptor signaling. Thus, GPX1 overexpression causes sustained activation of the insulin signaling pathway, which leads to insulin resistance. Similarly, dietary Se supplementation of rats raised liver Gpx1 activity, decreased glutathionylation of PTP1B, and increased liver triglycerides and body weight, indicating impaired insulin signaling [50]. Conversely, GPX1 knockout mice have enhanced insulin sensitivity in muscle due to increased H2O2 signaling that is reversed by the antioxidant N-acetylcysteine [51].
Recent evidence suggests Gpx1 may also modulate redox-dependent cellular responses by regulating mitochondrial function. Substantial evidence indicates that ROS produced during respiration increase the activities of mitochondrial uncoupling proteins, presumably as a protective measure against accumulation of dangerous levels of ROS during respiration [52]. Superoxide activates uncoupling proteins 1, 2, and 3 [53], whereas H2O2 increases expression of uncoupling protein 5 [54]. Chang liver cells overexpressing GPX1 have decreased mitochondrial ROS production and blunted proliferative responses to growth factor stimulation that is attributed to dysregulated coupling of respiration to ADP phosphorylation [55]. Thus, mitochondrial GPX1 indirectly regulates cytosolic O−2⋅ and H2O2 by modulating respiratory ROS production.
At least four additional homologous GPX selenoproteins are known in humans [56]. Glutathione peroxidase 2 (GPX2) is a cytosolic GPX expressed only in epithelial cells and is thought to have a role detoxifying dietary lipid hydroperoxides in the gut. GPX3 is the extracellular form of the enzyme, found in blood plasma, lung alveolar fluid, and in the lumen of the thyroid gland. GPX3 has a role regulating extracellular H2O2 produced by receptor-activated NADPH oxidases that may be relevant to diabetes. ROS are involved in both the etiology [57] and pathology [58] of type II diabetes. Peroxisome proliferator-activated receptor gamma (PPARgamma) is the target of drugs that improve insulin sensitivity and acts in part by increasing antioxidant capacity. GPX3 expression is induced by activation of PPARgamma and is responsible for its extracellular antioxidant effects [59]. Thus, GPX3 not only modulates extracellular H2O2 signaling, it is itself the target of receptor-mediated signaling processes. GPX6 is a close homolog of GPX3 expressed in the olfactory system whose function is not yet known [56].
GPX4 is unique among the GPX enzymes: it is active as a monomer instead of a tetramer and it is the only form of the enzyme that can reduce phospholipid hydroperoxides in membranes without the prior action of phospholipase to liberate free fatty acid hydroperoxide. GPX4 is an important regulator of ROS activation of the NF-κB transcription factor system, which regulates the transcription of genes that code for cytokines, adhesion molecules, and other components of the inflammatory response [60]. Overexpression of GPX4 inhibits interleukin-1 induced NF-κB activation in human umbilical endothelial cells [61] and UV radiation-induced activation in human dermal fibroblasts [62]. GPX4 overexpression specifically inhibits expression of several NF-κB target genes, including VCAM-1 in smooth muscle cells, MMP-1 in fibroblasts, and COX-2 in mouse fibroblasts. In addition to the ubiquitously expressed 19-kDa form of GPX4 found in cytosol and mitochondria, a 34-kDa alternatively transcribed nucleolar form of GPX4 in testes uses H2O2 to form cystine bridges between protamine subunits as part of the chromatin condensation process during sperm maturation [63]. This specialized function of GPX4 is achieved by a slight modification of the ordinary GPX catalytic cycle (Fig. 2a), where the normal reductant GSH is replaced by Cys residues in protamine monomers, thus forming cystine bridges between protamine subunits instead of GSSG. GPX4 is the only GPX gene that causes embryonic lethality when knocked out in mice [64], suggesting that it may have an essential role in many cell types.
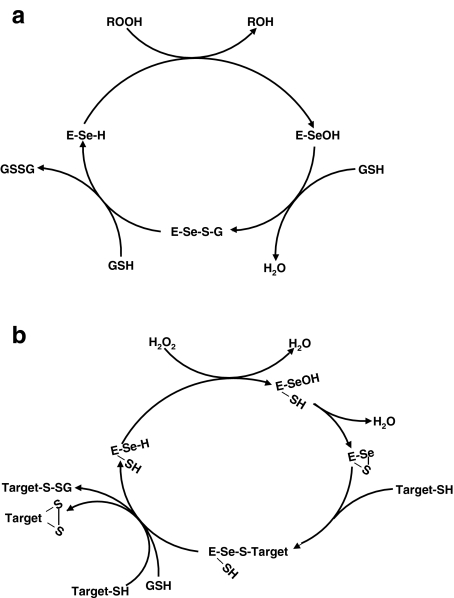
Fig. 2.
Glutathione peroxidase and thiol peroxidase reactions of selenoproteins. a Classical glutathione peroxidase catalytic cycle. When the thiols are provided by protein cysteines instead of GSH, this depicts the action of GPX4 in sperm DNA condensation. b Hypothetical thiol peroxidase catalytic cycle of thioredoxin-like selenoproteins
Thioredoxin reductase (TR) is a member of the pyridine nucleotide disulfide oxidoreductase family containing a C-terminal penultimate Sec residue that uses NADPH to maintain thioredoxin (Trx) in the reduced state. Three TRs exist in mammals: TR1 is the cytosolic enzyme and TR3 is the mitochondrial form, while TR2 contains an extra N-terminal glutaredoxin (Grx) domain and is expressed mainly in testes, where it may serve to couple the Trx and GSH redox systems [65]. Trx is a 12-kDa protein with two redox-active cysteines in a characteristic –CXXC– sequence motif with significant roles in cell–cell communication, DNA synthesis and repair, transcription regulation, protein folding and degradation, and signal transduction [64]. Trx was first described in E. coli as an essential cofactor for ribonucleotide reductase, the enzyme that synthesizes deoxyribonucleotides [66]. Trx’s two active site cysteines can act as a redox switch; for example, oxidation of Trx1 liberates ASK1 and activates the MAP kinase p38-dependent apoptosis pathway [67]. Human Trx was originally identified as a cytokine in human T-cell leukemia virus transformed cells [68], emphasizing its importance in cell–cell communications. More recently, extracellular Trx was shown to regulate the redox status and ligand binding of tumor necrosis factor receptor superfamily member 8 (CD30) on lymphocytes [69]. Trx regulates the redox status and activity of a number of transcription factors to control proliferation, hypoxic responses, and apoptosis [70]. Several isoforms and homologs of Trx are expressed in mammals and are reduced by TR and function as disulfide reductases directed at different target proteins to terminate ROS signals in specific subcellular compartments [71]. A recent example is blocking of nuclear import of class II histone deacetylases (HDAC) [72]. Class II HDACs are shuttled from the cytoplasm into the nucleus, where they negatively regulate transcription of genes involved in signal transduction, cell growth, apoptosis, cell cycle, and cancer. Oxidative modification of conserved cysteine residues in class II HDACs induces nuclear export and relieves inhibition of gene transcription. Trx1 inhibits nuclear export of class II HDACs by reducing the conserved cysteine residues, thus maintaining inhibition of gene transcription. Thus, TR controls a redox shuttle that modulates gene expression via redox regulation of HDAC nuclear translocation.
Inflammation and ROS are tightly linked. ROS are both a cause and a consequence of inflammation [73, 74]. Exogenously added ROS activate immune cells and activated immune cells produce ROS to kill invading pathogenic microbes. Activated tissue-resident macrophages produce a variety of ROS, including O2⋅−, nitric oxide, hydroxyl radical, peroxynitrite, hypochlorous acid, and H2O2, leading to signaling cascades that trigger production of inflammatory and chemotactic cytokines. Cytokines secreted by activated macrophages can cause profound changes in the cells of the surrounding tissues, including proliferation, apoptosis or differentiation into new phenotypes. Neutrophils, eosinophils, macrophages, and neighboring tissue cells produce pro-inflammatory ROS intermediates as part of the signaling cascades activated by released cytokines. Secreted chemokines attract other immune cells to the site of inflammation. Trx plays an important role in activation of the NLRP3 inflammasome responsible for activation of the cytokine interleukin 1β, deregulation of which is associated with several inflammatory diseases. Glucose-triggered inflammasome activation and IL-1β secretion are major factors in the pathogenesis of insulin resistance and type 2 diabetes [75]. ROS from receptor-linked NADPH oxidase or H2O2 treatment oxidizes Trx, releasing thioredoxin-interacting protein (TXNIP) that then binds with and activates the NLRP3 inflammasome to activate interleukin 1β [76]. Thus, thioredoxin reductase, by keeping Trx in the reduced state that binds to and inactivates TXNIP, regulates inflammasome activation and IL-1β secretion.
After cysteine, methionine is the amino acid most susceptible to modification by ROS. Oxidized methionine occurs in two diastereomers: methionine(R)sulfoxide and methionine(S)sulfoxide. Separate enzymes catalyze the reduction of each diastereomer back to methionine, using Trx as the reducing agent. Methionine sulfoxide reductase A (MsrA) is specific for methionine(S)sulfoxide and MsrB is specific for methionine(R)sulfoxide. Mammals have a single MsrA gene that produces the cytosolic, mitochondrial, and nuclear forms of the enzyme [77]. There are three MsrB genes in mammals, only one of which—MsrB1—is a selenoprotein [78] that is expressed in cytosol and the nucleus [79]. Thus, reduction of methionine(R)sulfoxide requires redox shuttling from NADPH through two selenoproteins, TR and MsrB1.
There is growing evidence that reversible oxidation of protein methionine residues regulates protein functions. Over 50 proteins have been reported to have altered activity due to formation of methionine sulfoxide [80]. For instance, the Ca2+/calmodulin-regulated phosphatase calcineurin regulates nuclear localization of nuclear factor of activated T cells (NFAT), a family of transcription factors upregulated during immune stimulus. Oxidation of a specific methionine in the nuclear localization sequence of calcineurin interferes with the proper NFAT nuclear localization and transcriptional activation in T cells [81]. Similarly, oxidation of a specific methionine in IκBα, which inhibits the activity of NF-κB, enhances its resistance to protein degradation and prevents NF-κB from activating transcription of pro-inflammatory genes [82]. Thus, reduction of methionine sulfoxide in these proteins may be an important mechanism of regulating the activities of immune cells. Another example is calcium/calmodulin (Ca2+/CaM)-dependent protein kinase II (CaMKII), which couples increases in Ca2+ to activation of ion channels, gene transcription, and apoptosis. Recent evidence suggests that CaMKII connects upstream oxidant stress and Ca2+ signals to downstream cellular responses. Oxidation of paired regulatory domain methionine residues by H2O2 sustains CaMKII activity in the absence of Ca2+/CaM and is reversed by methionine sulfoxide reductase, thus demonstrating a dynamic mechanism for CaMKII regulation by ROS and methionine sulfoxide reductase [83].
Selenoprotein S (SelS) is a glucose-regulated Sec-containing protein expressed in the endoplasmic reticulum (ER) membrane that participates in retrotranslocation of misfolded proteins from the ER to the cytosol, where they are ubiquitinated and degraded at the proteasome [84]. SelS expression is positively regulated by NF-κB and ER stress [85] and negatively regulates production of inflammatory cytokines [86], implicating a role in downregulating inflammatory processes involved in the development of chronic diseases such as obesity, type 2 diabetes, and cardiovascular disease. Disulfide bonds in the ER are formed by the action of protein disulfide isomerase, using H2O2 produced from O2 and GSH by the ER flavooxidase Ero1 [87]. ER stress is caused by aggregation of improperly folded proteins in the ER due to accumulation of underglycosylated proteins, formation of unspecific disulfide bonds, or depletion of Ca2+ in the ER. ER stress triggers the unfolded protein response (UPR), which is composed of both pro-adaptive and pro-apoptotic signaling pathways. ROS figure prominently both in causing ER stress and in UPR signaling, although the precise mechanisms require further investigation [88]. The mechanism by which SelS neutralizes ER stress and inhibits UPR signaling is not clear. However, SelS participates in the formation of multi-protein transmembrane channels composed of the membrane spanning Delrin-1, p97 ATPase, and E3 ubiquitin ligase that may be stabilized by intermolecular disulfide bridges [89].
GPX2 is known as “gastrointestinal GPX”. Although it is expressed at the highest level in intestine, GPX2 expression is not specific for the gastrointestinal tract and has been detected in epithelial cells in lung, skin, and breast [90]. GPX2 has been localized to the ER, where it suppresses cyclooxygenase 2 expression and pro-inflammatory prostaglandin E2 production, thus preventing inflammation [91]. Sep15 is a 15-kDa thioredoxin-like selenoprotein expressed in the ER lumen that is tightly associated with UDP-glucose:glycoprotein glucosyltransferase [92], which monitors the folding state of proteins released from the ER chaperone, calnexin. Recent work suggests that Sep15 assists oxidative folding of N-glycosylated proteins and is involved in UPR signaling in response to adaptive and acute ER stresses [93]. Thus, selenoproteins are involved in regulating multiple stress-related redox events in the oxidizing environment of the ER lumen.
Selenoprotein N (SelN) is another ER-resident Sec-containing protein and is the only selenoprotein directly associated with a human genetic disease. Mutations in the SelN gene cause SEPN1-related myopathy, an early onset muscle disorder affecting primarily the axial skeletal muscles and diaphragm. SelN metabolizes exogenously added H2O2 [94]; however, its selenocysteine is located in the ER lumen [95], suggesting that it may respond to H2O2 produced in the ER. SelN deficient muscle cells have increased cytosolic Ca2+ and decreased Ca2+ in the sarcoplasmic reticulum [94], suggesting a defect in Ca2+ release controlled by the ryanodine receptor. The ryanodine receptor Ca2+ release channel is regulated by redox modifications of cysteines, with oxidizing conditions favoring channel opening and Ca2+ release [96], suggesting SelN controls Ca2+ release by mediating oxidation of cysteines in the ryanodine receptor using sarcoplasmic reticulum H2O2 [97]. Thus, SelN may be viewed as a redox regulator of calcium in muscle [89]. Human selenoprotein T (SelT) is a 22-kDa Sec-containing protein expressed in ER and Golgi membranes and is implicated in redox regulation of Ca2+ homeostasis in response to a cAMP-stimulating neuropeptide, pituitary adenylate cyclase-activating polypeptide [98]. Thus, selenoproteins are involved in multiple processes at the intersection of cAMP and Ca2+ pathways and appear to mediate crosstalk between these important signaling systems.
The tumor suppressor protein p53 is a key molecular node in cellular signaling pathways, regulating the expression of specific sets of genes in response to different stresses [99]. The p53 protein is expressed constitutively, but levels are normally kept low by its rapid ubiquitination by the HDM2 protein, an E3 ubiquitin ligase, and subsequent proteasomal degradation. Phosphorylation of p53 in response to stress disrupts its binding with HDM2, blocks ubiquitination and proteolysis, and results in a rapid increase in p53 protein levels, allowing p53 to enter the nucleus, bind to DNA, and induce expression of genes controlling cell cycle, apoptosis, DNA repair, and cellular senescence [100]. In addition, p53 regulation of glucose metabolism and control of intracellular ROS levels are emerging as key functions of p53 [101]. p53 possesses five cysteines on the surface of the protein, at least one of which is readily susceptible to oxidation that inhibits tetramerization and DNA binding [102]. Oxidative stress or exogenous H2O2 cause S-glutathionylation of cysteines residues in the dimer–dimer interface and proximal DNA-binding domain of p53 leading to decreased DNA binding and loss of tetramer formation [103]. Inhibition of p53 tetramerization exposes a nuclear export signal that causes p53 to be retrotransported to the cytoplasm [104], where it is subject to rapid ubiquitination and proteasomal degradation.
Selenoprotein H (SELH) is a recently discovered 14-kDa thioredoxin-like nucleolar protein that reacts specifically with H2O2 [105]. Overexpression of SELH in neuronal cells prevents the increase of p53 tumor suppressor protein and caspase-mediated apoptosis normally induced by UVB radiation [106]. Thus, SELH is implicated in oxidative modifications of p53, such as S-glutathionylation and cysteine bridges, that inhibit DNA binding and lead to increased turnover and decreased steady-state protein levels. Selenoprotein W (SEPW1) is a 9-kDa thioredoxin-like protein where Sec is located two amino acids from a cysteine residue. Work in our laboratory has shown that SEPW1 expression is cell cycle regulated and that knockdown of SEPW1 inhibits cell cycle progression [107]. We have recently discovered that SEPW1 knockdown induces cell cycle arrest by stabilizing p53, implying a similar mode of action to that of SELH (manuscript in preparation). In addition to SELH and SEPW1, there are five small thioredoxin-like selenoproteins in humans (SELM, SELN, SELT, SELV, and Sep15) with active site Sec residues located one or two amino acids from a cysteine residue, thus allowing formation of intramolecular selenenyl–sulfide intermediates during redox catalysis. It is interesting to speculate that these small thioredoxin-like selenoproteins may have a general role as transducers of H2O2 signals. Because Sec is fully ionized at physiological pH, it will react with H2O2 until Sec is fully oxidized to the selenenic acid before H2O2 will react with cysteine [3]. Due to its proximity, the active site cysteine will react rapidly with the resulting selenenic acid to form an intramolecular selenenyl–sulfide bond that is resistant to reduction by cytosolic GSH. The relative stability of the selenenyl–sulfide bond allows a selenoprotein with a thioredoxin-like active site to act as a redox shuttle and carry the H2O2 signal from its site of formation to form a disulfide bond in a downstream target. This proposed mechanism of redox shuttling by thioredoxin-like selenoproteins is shown schematically in Fig. 2b.
The unique role of selenoproteins in regulating redox status of sulfur amino acids begs the question how this relationship came into being. It has been reasoned by several independent groups that the highly oxidizable Sec would have been more stable in the low oxygen environment that existed in the primordial atmosphere than it is today [108–110]. This line of reasoning assumes that the rise in atmospheric oxygen some 2.4 billion years ago and known as the Great Oxidation Event [111] created a selective pressure for replacement of Sec by cysteine [112]. This is consistent with the occurrence of Sec incorporation via the UGA codon in all three superkingdoms of life, implying selenoproteins existed in the last universal common ancestor as much as 3.5 billion years ago, long before the Great Oxidation Event [113]. Another line of evidence is based on phylogenomic analysis of nucleotide sequences and secondary structures in transfer RNAs, which concluded that the amino acid charging function for Sec is ancient and existed in archaea before diversification into the three superkingdoms of life [114]. This analysis of tRNA is complementary to a similar phylogenomic analysis of protein architectures that concluded the last universal common ancestor was molecularly complex and architecturally rich and gave rise to archaea, bacteria, and eukarya by reductive adaptation [115]. Thus, there is an emerging consensus that Sec-containing proteins existed in the last universal common ancestor and have been selectively lost and/or replaced by cysteine proteins during the diversification of life. Evolutionary biology implicates ROS as a profound constraint on evolution of fitness-related traits [116], inviting speculation that the unique ability of selenoproteins to regulate ROS is the indispensable feature responsible for their retention throughout the reductive evolution and diversification of life.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- Se
Selenium
- SEPW1
Selenoprotein W
- GPX
Glutathione peroxidase
Footnotes
The authors have no financial or other conflicting interest in any product or service mentioned in this article.
Contributor Information
Wayne Chris Hawkes, Email: wayne.hawkes@ars.usda.gov.
Zeynep Alkan, Email: zeynep.alkan@ars.usda.gov.
References
- 1.Fallab S. Reactions with Molecular Oxygen. Angew Chem Int Ed Eng. 1967;6:496–507. doi: 10.1002/anie.196704961. [DOI] [Google Scholar]
- 2.Day RM, Suzuki YJ. Cell proliferation, reactive oxygen and cellular glutathione. Dose Response. 2005;3:425–442. doi: 10.2203/dose-response.003.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Stone JR, Yang S. Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal. 2006;8:243–270. doi: 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- 5.Lambert AJ, Brand MD. Reactive oxygen species production by mitochondria. Methods Mol Biol. 2009;554:165–181. doi: 10.1007/978-1-59745-521-3_11. [DOI] [PubMed] [Google Scholar]
- 6.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 7.DeYulia GJ, Jr, Carcamo JM, Borquez-Ojeda O, Shelton CC, Golde DW. Hydrogen peroxide generated extracellularly by receptor–ligand interaction facilitates cell signaling. Proc Natl Acad Sci U S A. 2005;102:5044–5049. doi: 10.1073/pnas.0501154102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vardatsikos G, Sahu A, Srivastava A. The insulin-like growth factor family: molecular mechanisms, redox regulation and clinical implications. Antioxid Redox Signal. 2008;17:17. doi: 10.1089/ars.2008.2161. [DOI] [PubMed] [Google Scholar]
- 9.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 10.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purushothaman D, Sarin A. Cytokine-dependent regulation of NADPH oxidase activity and the consequences for activated T cell homeostasis. J Exp Med. 2009;206:1515–1523. doi: 10.1084/jem.20082851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Azad MB, Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009;16:1040–1052. doi: 10.1038/cdd.2009.49. [DOI] [PubMed] [Google Scholar]
- 13.Feissner RF, Skalska J, Gaum WE, Sheu SS. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front Biosci. 2009;14:1197–1218. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Csordas G, Hajnoczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim Biophys Acta. 2009;1787:1352–1362. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3:1129–1134. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 16.Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 17.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel HH, Insel PA. Lipid rafts and caveolae and their role in compartmentation of redox signaling. Antioxid Redox Signal. 2009;11:1357–1372. doi: 10.1089/ars.2008.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oakley FD, Abbott D, Li Q, Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal. 2009;11:1313–1333. doi: 10.1089/ars.2008.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Song Y, Loscalzo J. Regulation of the protein disulfide proteome by mitochondria in mammalian cells. Proc Natl Acad Sci U S A. 2007;104:10813–10817. doi: 10.1073/pnas.0702027104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng M, Aslund F, Storz G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science. 1998;279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 23.Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002;111:471–481. doi: 10.1016/S0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 24.Rudolph J. Redox regulation of the Cdc25 phosphatases. Antioxid Redox Signal. 2005;7:761–767. doi: 10.1089/ars.2005.7.761. [DOI] [PubMed] [Google Scholar]
- 25.Mehta RA, Fawcett TW, Porath D, Mattoo AK. Oxidative stress causes rapid membrane translocation and in vivo degradation of ribulose-1, 5-bisphosphate carboxylase/oxygenase. J Biol Chem. 1992;267:2810–2816. [PubMed] [Google Scholar]
- 26.Fourquet S, Guerois R, Biard D, Toledano MB. Activation of NRF2 by nitrosative agents and H2O2 involves KEAP1 disulfide formation. J Biol Chem. 2010;285:8463–8471. doi: 10.1074/jbc.M109.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang F, Nie C, Yang Y, Yue W, Ren Y, et al. Selenite induces redox-dependent Bax activation and apoptosis in colorectal cancer cells. Free Radic Biol Med. 2009;46:1186–1196. doi: 10.1016/j.freeradbiomed.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 28.van der Wijk T, Overvoorde J, den Hertog J. H2O2-induced intermolecular disulfide bond formation between receptor protein-tyrosine phosphatases. J Biol Chem. 2004;279:44355–44361. doi: 10.1074/jbc.M407483200. [DOI] [PubMed] [Google Scholar]
- 29.Ushio-Fukai M. Vascular signaling through G protein-coupled receptors: new concepts. Curr Opin Nephrol Hypertens. 2009;18:153–159. doi: 10.1097/MNH.0b013e3283252efe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milligan G. G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim Biophys Acta. 2007;1768:825–835. doi: 10.1016/j.bbamem.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Kim H, Lee BK, Naider F, Becker JM. Identification of specific transmembrane residues and ligand-induced interface changes involved in homo-dimer formation of a yeast G protein-coupled receptor. Biochemistry. 2009;48:10976–10987. doi: 10.1021/bi901291c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang N, Langenheim JF, Wang X, Jiang J, Chen WY, et al. Activation of growth hormone receptors by growth hormone and growth hormone antagonist dimers: insights into receptor triggering. Mol Endocrinol. 2008;22:978–988. doi: 10.1210/me.2007-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdulhussein R, Koo DH, Vogel WF. Identification of disulfide-linked dimers of the receptor tyrosine kinase DDR1. J Biol Chem. 2008;283:12026–12033. doi: 10.1074/jbc.M704592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macdonald J, Li Z, Su W, Pike LJ. The membrane proximal disulfides of the EGF receptor extracellular domain are required for high affinity binding and signal transduction but do not play a role in the localization of the receptor to lipid rafts. Biochim Biophys Acta. 2006;1763:870–878. doi: 10.1016/j.bbamcr.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free Radic Biol Med. 1996;21:335–348. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 37.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. Actin S-glutathionylation: evidence against a thiol-disulphide exchange mechanism. Free Radic Biol Med. 2003;35:1185–1193. doi: 10.1016/S0891-5849(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 38.Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, et al. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys. 2008;477:183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole LB, Nelson KJ. Discovering mechanisms of signaling-mediated cysteine oxidation. Curr Opin Chem Biol. 2008;12:18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lobanov AV, Hatfield DL, Gladyshev VN. Eukaryotic selenoproteins and selenoproteomes. Biochim Biophys Acta. 2009;1790:1424–1428. doi: 10.1016/j.bbagen.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itoh Y, Chiba S, Sekine S, Yokoyama S. Crystal structure of human selenocysteine tRNA. Nucleic Acids Res. 2009;37:6259–6268. doi: 10.1093/nar/gkp648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berry MJ. Knowing when not to stop. Nat Struct Mol Biol. 2005;12:389–390. doi: 10.1038/nsmb0505-389. [DOI] [PubMed] [Google Scholar]
- 44.Su D, Hohn MJ, Palioura S, Sherrer RL, Yuan J, et al. How an obscure archaeal gene inspired the discovery of selenocysteine biosynthesis in humans. IUBMB Life. 2009;61:35–39. doi: 10.1002/iub.136. [DOI] [PubMed] [Google Scholar]
- 45.Wouters MA, Fan SW, Haworth NL. Disulfides as redox switches: from molecular mechanisms to functional significance. Antioxid Redox Signal. 2010;12:53–91. doi: 10.1089/ars.2009.2510. [DOI] [PubMed] [Google Scholar]
- 46.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 47.Mills GC. The purification and properties of glutathione peroxidase of erythrocytes. J Biol Chem. 1959;234:502–506. [PubMed] [Google Scholar]
- 48.Schacter LP. Generation of superoxide anion and hydrogen peroxide by erythrocytes from individuals with sickle trait or normal haemoglobin. Eur J Clin Invest. 1986;16:204–210. doi: 10.1111/j.1365-2362.1986.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 49.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, et al. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51:1515–1524. doi: 10.1007/s00125-008-1055-3. [DOI] [PubMed] [Google Scholar]
- 50.Mueller AS, Bosse AC, Most E, Klomann SD, Schneider S, et al. Regulation of the insulin antagonistic protein tyrosine phosphatase 1B by dietary Se studied in growing rats. J Nutr Biochem. 2009;20:235–247. doi: 10.1016/j.jnutbio.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Loh K, Deng H, Fukushima A, Cai X, Boivin B, et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harper ME, Green K, Brand MD. The efficiency of cellular energy transduction and its implications for obesity. Annu Rev Nutr. 2008;28:13–33. doi: 10.1146/annurev.nutr.28.061807.155357. [DOI] [PubMed] [Google Scholar]
- 53.Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, et al. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 54.Santandreu FM, Valle A, Fernandez de Mattos S, Roca P, Oliver J. Hydrogen peroxide regulates the mitochondrial content of uncoupling protein 5 in colon cancer cells. Cell Physiol Biochem. 2009;24:379–390. doi: 10.1159/000257430. [DOI] [PubMed] [Google Scholar]
- 55.Handy DE, Lubos E, Yang Y, Galbraith JD, Kelly N, et al. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J Biol Chem. 2009;284:11913–11921. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, et al. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 57.Bashan N, Kovsan J, Kachko I, Ovadia H, Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol Rev. 2009;89:27–71. doi: 10.1152/physrev.00014.2008. [DOI] [PubMed] [Google Scholar]
- 58.Ha H, Hwang IA, Park JH, Lee HB. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82(Suppl 1):S42–S45. doi: 10.1016/j.diabres.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 59.Chung SS, Kim M, Youn BS, Lee NS, Park JW, et al. Glutathione peroxidase 3 mediates the antioxidant effect of peroxisome proliferator-activated receptor gamma in human skeletal muscle cells. Mol Cell Biol. 2009;29:20–30. doi: 10.1128/MCB.00544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brigelius-Flohe R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem. 2006;387:1329–1335. doi: 10.1515/BC.2006.166. [DOI] [PubMed] [Google Scholar]
- 61.Brigelius-Flohe R, Friedrichs B, Maurer S, Schultz M, Streicher R. Interleukin-1-induced nuclear factor kappa B activation is inhibited by overexpression of phospholipid hydroperoxide glutathione peroxidase in a human endothelial cell line. Biochem J. 1997;328:199–203. doi: 10.1042/bj3280199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wenk J, Schuller J, Hinrichs C, Syrovets T, Azoitei N, et al. Overexpression of phospholipid-hydroperoxide glutathione peroxidase in human dermal fibroblasts abrogates UVA irradiation-induced expression of interstitial collagenase/matrix metalloproteinase-1 by suppression of phosphatidylcholine hydroperoxide-mediated NFkappaB activation and interleukin-6 release. J Biol Chem. 2004;279:45634–45642. doi: 10.1074/jbc.M408893200. [DOI] [PubMed] [Google Scholar]
- 63.Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145–169. doi: 10.1016/S0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 64.Conrad M. Transgenic mouse models for the vital selenoenzymes cytosolic thioredoxin reductase, mitochondrial thioredoxin reductase and glutathione peroxidase 4. Biochim Biophys Acta. 2009;1790:1575–1585. doi: 10.1016/j.bbagen.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Gerashchenko MV, Su D, Gladyshev VN. CUG start codon generates thioredoxin/glutathione reductase isoforms in mouse testes. J Biol Chem. 2009;14:14. doi: 10.1074/jbc.M109.070532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laurent TC, Moore EC, Reichard P. Enzymatic synthesis of deoxyribonucleotides. IV. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli B. J Biol Chem. 1964;239:3436–3444. [PubMed] [Google Scholar]
- 67.Nadeau PJ, Charette SJ, Toledano MB, Landry J. Disulfide bond-mediated multimerization of Ask1 and its reduction by thioredoxin-1 regulate H(2)O(2)-induced c-Jun NH(2)-terminal kinase activation and apoptosis. Mol Biol Cell. 2007;18:3903–3913. doi: 10.1091/mbc.E07-05-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tagaya Y, Maeda Y, Mitsui A, Kondo N, Matsui H, et al. ATL-derived factor (ADF), an IL-2 receptor/Tac inducer homologous to thioredoxin; possible involvement of dithiol-reduction in the IL-2 receptor induction. EMBO J. 1989;8:757–764. doi: 10.1002/j.1460-2075.1989.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwertassek U, Balmer Y, Gutscher M, Weingarten L, Preuss M, et al. Selective redox regulation of cytokine receptor signaling by extracellular thioredoxin-1. EMBO J. 2007;26:3086–3097. doi: 10.1038/sj.emboj.7601746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mukherjee A, Martin SG. The thioredoxin system: a key target in tumour and endothelial cells. Br J Radiol. 2008;81(Spec No 1):S57–S68. doi: 10.1259/bjr/34180435. [DOI] [PubMed] [Google Scholar]
- 71.Jeong W, Jung Y, Kim H, Park SJ, Rhee SG. Thioredoxin-related protein 14, a new member of the thioredoxin family with disulfide reductase activity: implication in the redox regulation of TNF-alpha signaling. Free Radic Biol Med. 2009;47:1294–1303. doi: 10.1016/j.freeradbiomed.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 72.Oka S, Ago T, Kitazono T, Zablocki D, Sadoshima J. The role of redox modulation of class II histone deacetylases in mediating pathological cardiac hypertrophy. J Mol Med. 2009;87:785–791. doi: 10.1007/s00109-009-0471-2. [DOI] [PubMed] [Google Scholar]
- 73.Martinez JA. Mitochondrial oxidative stress and inflammation: an slalom to obesity and insulin resistance. J Physiol Biochem. 2006;62:303–306. doi: 10.1007/BF03165759. [DOI] [PubMed] [Google Scholar]
- 74.Swindle EJ, Metcalfe DD. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol Rev. 2007;217:186–205. doi: 10.1111/j.1600-065X.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 75.Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 76.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 77.Vougier S, Mary J, Friguet B. Subcellular localization of methionine sulphoxide reductase A (MsrA): evidence for mitochondrial and cytosolic isoforms in rat liver cells. Biochem J. 2003;373:531–537. doi: 10.1042/BJ20030443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim HY, Gladyshev VN. Methionine sulfoxide reduction in mammals: characterization of methionine-R-sulfoxide reductases. Mol Biol Cell. 2004;15:1055–1064. doi: 10.1091/mbc.E03-08-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee BC, Dikiy A, Kim HY, Gladyshev VN. Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta. 2009;1790:1471–1477. doi: 10.1016/j.bbagen.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levine RL, Moskovitz J, Stadtman ER. Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation. IUBMB Life. 2000;50:301–307. doi: 10.1080/15216540051081056. [DOI] [PubMed] [Google Scholar]
- 81.Agbas A, Moskovitz J. The role of methionine oxidation/reduction in the regulation of immune response. Curr Signal Transduct Ther. 2009;4:46–50. doi: 10.2174/157436209787048748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kanayama A, Inoue J, Sugita-Konishi Y, Shimizu M, Miyamoto Y. Oxidation of Ikappa Balpha at methionine 45 is one cause of taurine chloramine-induced inhibition of NF-kappa B activation. J Biol Chem. 2002;277:24049–24056. doi: 10.1074/jbc.M110832200. [DOI] [PubMed] [Google Scholar]
- 83.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kelly E, Greene CM, Carroll TP, McElvaney NG, O'Neill SJ. Selenoprotein S/SEPS1 modifies endoplasmic reticulum stress in Z variant alpha1-antitrypsin deficiency. J Biol Chem. 2009;284:16891–16897. doi: 10.1074/jbc.M109.006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao Y, Hannan NR, Wanyonyi S, Konstantopolous N, Pagnon J, et al. Activation of the selenoprotein SEPS1 gene expression by pro-inflammatory cytokines in HepG2 cells. Cytokine. 2006;33:246–251. doi: 10.1016/j.cyto.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, et al. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet. 2005;37:1234–1241. doi: 10.1038/ng1655. [DOI] [PubMed] [Google Scholar]
- 87.Frand AR, Kaiser CA. Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Molecular Cell. 1999;4:469–477. doi: 10.1016/S1097-2765(00)80198-7. [DOI] [PubMed] [Google Scholar]
- 88.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 89.Shchedrina VA, Zhang Y, Labunskyy VM, Hatfield DL, Gladyshev VN. Structure–function relations, physiological roles, and evolution of mammalian er-resident selenoproteins. Antioxid Redox Signal. 2010;12:839–849. doi: 10.1089/ars.2009.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banning A, Kipp A, Schmitmeier S, Lowinger M, Florian S, et al. Glutathione peroxidase 2 inhibits cyclooxygenase-2-mediated migration and invasion of HT-29 adenocarcinoma cells but supports their growth as tumors in nude mice. Cancer Res. 2008;68:9746–9753. doi: 10.1158/0008-5472.CAN-08-1321. [DOI] [PubMed] [Google Scholar]
- 91.Banning A, Florian S, Deubel S, Thalmann S, Muller-Schmehl K, et al. GPx2 counteracts PGE2 production by dampening COX-2 and mPGES-1 expression in human colon cancer cells. Antioxid Redox Signal. 2008;10:1491–1500. doi: 10.1089/ars.2008.2047. [DOI] [PubMed] [Google Scholar]
- 92.Labunskyy VM, Ferguson AD, Fomenko DE, Chelliah Y, Hatfield DL, et al. A novel cysteine-rich domain of Sep15 mediates the interaction with UDP-glucose:glycoprotein glucosyltransferase. J Biol Chem. 2005;280:37839–37845. doi: 10.1074/jbc.M508685200. [DOI] [PubMed] [Google Scholar]
- 93.Labunskyy VM, Yoo MH, Hatfield DL, Gladyshev VN. Sep15, a thioredoxin-like selenoprotein, is involved in the unfolded protein response and differentially regulated by adaptive and acute ER stresses. Biochemistry. 2009;48:8458–8465. doi: 10.1021/bi900717p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arbogast S, Beuvin M, Fraysse B, Zhou H, Muntoni F, et al. Oxidative stress in SEPN1-related myopathy: from pathophysiology to treatment. Ann Neurol. 2009;65:677–686. doi: 10.1002/ana.21644. [DOI] [PubMed] [Google Scholar]
- 95.Lescure A, Rederstorff M, Krol A, Guicheney P, Allamand V. Selenoprotein function and muscle disease. Biochim Biophys Acta. 2009;1790:1569–1574. doi: 10.1016/j.bbagen.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 96.Zissimopoulos S, Lai FA. Redox regulation of the ryanodine receptor/calcium release channel. Biochem Soc Trans. 2006;34:919–921. doi: 10.1042/BST0340919. [DOI] [PubMed] [Google Scholar]
- 97.Arbogast S, Ferreiro A. Selenoproteins and protection against oxidative stress: selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxid Redox Signal. 2010;12:893–904. doi: 10.1089/ars.2009.2890. [DOI] [PubMed] [Google Scholar]
- 98.Grumolato L, Ghzili H, Montero-Hadjadje M, Gasman S, Lesage J, et al. Selenoprotein T is a PACAP-regulated gene involved in intracellular Ca2+ mobilization and neuroendocrine secretion. FASEB J. 2008;22:1756–1768. doi: 10.1096/fj.06-075820. [DOI] [PubMed] [Google Scholar]
- 99.Staib F, Robles AI, Varticovski L, Wang XW, Zeeberg BR, et al. The p53 tumor suppressor network is a key responder to microenvironmental components of chronic inflammatory stress. Cancer Res. 2005;65:10255–10264. doi: 10.1158/0008-5472.CAN-05-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chumakov PM. Versatile functions of p53 protein in multicellular organisms. Biochemistry (Mosc) 2007;72:1399–1421. doi: 10.1134/S0006297907130019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bensaad K, Vousden KH. p53: new roles in metabolism. Trends Cell Biol. 2007;17:286–291. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 102.Sun XZ, Vinci C, Makmura L, Han S, Tran D, et al. Formation of disulfide bond in p53 correlates with inhibition of DNA binding and tetramerization. Antioxid Redox Signal. 2003;5:655–665. doi: 10.1089/152308603770310338. [DOI] [PubMed] [Google Scholar]
- 103.Velu CS, Niture SK, Doneanu CE, Pattabiraman N, Srivenugopal KS. Human p53 is inhibited by glutathionylation of cysteines present in the proximal DNA-binding domain during oxidative stress. Biochemistry. 2007;46:7765–7780. doi: 10.1021/bi700425y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Foo RS, Nam YJ, Ostreicher MJ, Metzl MD, Whelan RS, et al. Regulation of p53 tetramerization and nuclear export by ARC. Proc Natl Acad Sci U S A. 2007;104:20826–20831. doi: 10.1073/pnas.0710017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Novoselov SV, Kryukov GV, Xu XM, Carlson BA, Hatfield DL, et al. Selenoprotein H is a nucleolar thioredoxin-like protein with a unique expression pattern. J Biol Chem. 2007;282:11960–11968. doi: 10.1074/jbc.M701605200. [DOI] [PubMed] [Google Scholar]
- 106.Mendelev N, Witherspoon S, Li PA. Overexpression of human selenoprotein H in neuronal cells ameliorates ultraviolet irradiation-induced damage by modulating cell signaling pathways. Exp Neurol. 2009;220:328–334. doi: 10.1016/j.expneurol.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hawkes WC, Wang TTY, Alkan Z, Richter BD, Dawson K. Selenoprotein W modulates control of cell cycle entry. Biol Trace Elem Res. 2009;131:229–244. doi: 10.1007/s12011-009-8367-0. [DOI] [PubMed] [Google Scholar]
- 108.Bock A, Forchhammer K, Heider J, Baron C. Selenoprotein synthesis: an expansion of the genetic code. Trends Biochem Sci. 1991;16:463–467. doi: 10.1016/0968-0004(91)90180-4. [DOI] [PubMed] [Google Scholar]
- 109.Leinfelder W, Zehelein E, Mandrand BMA, Boeck A. Gene for a novel transfer RNA species that accepts l serine and cotranslationally inserts selenocysteine. Nature. 1988;331:723–725. doi: 10.1038/331723a0. [DOI] [PubMed] [Google Scholar]
- 110.Jukes TH. Genetic code 1990. Outlook. Experientia. 1990;46:1149–1157. doi: 10.1007/BF01936925. [DOI] [PubMed] [Google Scholar]
- 111.Sessions AL, Doughty DM, Welander PV, Summons RE, Newman DK. The continuing puzzle of the great oxidation event. Curr Biol. 2009;19:R567–R574. doi: 10.1016/j.cub.2009.05.054. [DOI] [PubMed] [Google Scholar]
- 112.Castellano S, Andres AM, Bosch E, Bayes M, Guigo R, et al. Low exchangeability of selenocysteine, the 21st amino acid, in vertebrate proteins. Mol Biol Evol. 2009;26:2031–2040. doi: 10.1093/molbev/msp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Glansdorff N, Xu Y, Labedan B. The last universal common ancestor: emergence, constitution and genetic legacy of an elusive forerunner. Biol Direct. 2008;3:29. doi: 10.1186/1745-6150-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun FJ, Caetano-Anolles G. Transfer RNA and the origins of diversified life. Sci Prog. 2008;91:265–284. doi: 10.3184/003685008X360650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang M, Yafremava LS, Caetano-Anolles D, Mittenthal JE, Caetano-Anolles G. Reductive evolution of architectural repertoires in proteomes and the birth of the tripartite world. Genome Res. 2007;17:1572–1585. doi: 10.1101/gr.6454307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dowling DK, Simmons LW. Reactive oxygen species as universal constraints in life-history evolution. Proc Biol Sci. 2009;276:1737–1745. doi: 10.1098/rspb.2008.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reeves MA, Hoffmann PR. The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci. 2009;66:2457–2478. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]