Abstract
Three experiments show that two associatively-activated stimulus representations may engage in excitatory or inhibitory learning, depending on their temporal relationship. Experiment 1a suggested that simultaneously-activated stimulus representations show evidence of inhibitory learning in an acquisition test. Experiment 1b showed similar evidence of inhibition in a summation test. Experiment 2 found that activation of two stimulus representations in a serial compound resulted in excitatory learning between the antecedent and the subsequent (forward), and inhibitory learning between the subsequent and the antecedent (backward). The results show the dynamic influence of temporal contiguity on mediated learning.
In a Pavlovian conditioning situation, a stimulus that is repeatedly paired with an unconditioned stimulus (US) comes to elicit a conditioned response. Learning theorists often assume that this learning involves the formation of associations between this stimulus (the conditioned stimulus, CS) and the US, such that subsequent presentations of the CS alone activate the representation of the US. It is this associatively-activated representation that produces much of the conditioned responding that is attributed to the CS. Although this concept is popular among theories of learning, and throughout associative models in psychology, there is some dispute concerning the properties possessed by associatively-activated stimulus representations. They are often assumed to represent at least a portion of the directly-experienced stimulus, but some theories suggest that an associatively-activated stimulus representation may differ from its directly-activated counterpart in many ways.
Investigations conducted by Holland and colleagues elucidated some of the associative properties possessed by associatively-activated stimulus representations. In a conditioned taste aversion preparation with rats, Holland (1981) showed that a taste aversion can be learned in a situation in which the taste CS and the illness-inducing US are never directly paired with each other. Holland (1981, Exp. 1) first paired a tone with a flavored food, and later paired that tone with an illness-inducing injection of LiCl. Rats that received this treatment showed an aversion to the flavored food, compared to rats that received a similar treatment, but with unpaired presentations of the tone and flavor or of the tone and LiCl. Holland (1981) asserted that learning of the food aversion was mediated by the pairing of an associatively-activated representation of that food with illness. There was no evidence of a directly-learned tone-LiCl association, which might otherwise have mediated the expression of the food aversion by a chain of food→tone and tone→illness associations. A related series of experiments conducted by Holland and Forbes (1982) showed that a previously-established flavor aversion could be extinguished if the flavor representation was associatively activated repeatedly in the absence of the illness US. In their experiments, a flavor was first paired with a tone, and then paired with LiCl. After this treatment, subsequent nonreinforced exposure to the tone produced extinction of the taste aversion.
Holland (1983) interpreted mediated acquisition and extinction as evidence that associatively-activated representations share some associative properties with their directly-activated counterparts. He suggested that an associatively-activated stimulus representation can form an excitatory association with a directly-experienced US, which accounts for the mediated-learning effect. Consistent with many traditional theories of direct extinction, Holland assumed that mediated extinction is driven by activation of the absent US representation by the CS. After these initial observations of mediated acquisition and extinction of aversions, Holland (1983) also observed mediated overshadowing and potentiation of aversions, and other researchers have observed mediated acquisition and extinction using different preparations (e.g., Dwyer, 2001; Dwyer, Mackintosh, & Boakes, 1998; Ward-Robinson & Hall, 1996).
Studies conducted by Dwyer and colleagues have shown that an associatively-activated CS can acquire excitatory strength not only when it is paired with a directly-experienced US, but also when it is paired with an associatively-activated US (Dwyer, 1999, 2000, 2001; Dwyer et al., 1998). This observation is well characterized by a conditioned taste preference experiment conducted by Dwyer et al. (1998). In their Experiment 1, two groups of rats were given access to a peppermint solution in one context, and an almond-sucrose solution in a second context. After this exposure, one group was given access to an almond solution in the first context, and the other group was given access to the almond solution in the second context. For subjects in the former group, exposure to the first context should have activated a representation of peppermint while exposure to almond activated a representation of sucrose. For subjects in the latter group, only the representation of sucrose should have been associatively activated. A subsequent consumption test revealed greater preference for peppermint in subjects that drank the almond solution in the context that was first associated with peppermint. Thus, the simultaneous activation of peppermint and sucrose representations appeared to produce excitatory learning between the two stimuli.
Considering the underlying associative mechanisms, Dwyer et al.’s (1998) findings appear to conflict with Holland and Forbes’s (1982) observation of mediated extinction. Specifically, a number of learning theories suggest that extinction occurs when a directly-activated CS representation is accompanied by an associatively-activated representation of an otherwise absent US (e.g., Rescorla & Wagner, 1972; Wagner, 1981). These circumstances also generate new inhibitory learning, which is generally accepted to underlie extinction, at least in part (e.g., Bouton, 2004; Calton, Mitchell, & Schachtman, 1996; Denniston & Miller, 2003; Myers & Davis, 2002). In Holland and Forbes’s (1982) experiments, an associatively-activated CS representation was extinguished. If extinction is a result of new inhibitory learning, and Holland and Forbes’s extinction was produced by pairing associatively-activated representations of both the CS and the US, then it must be assumed that inhibitory learning occurred between the two associatively-activated representations. This account of mediated extinction contrasts with Dwyer et al.’s observation of excitatory learning between two associatively-activated stimulus representations. Holland & Forbes’s view is also inconsistent with studies of retrospective revaluation that show an effect that is opposite to mediated extinction (e.g., Harris & Westbrook, 1998). Thus, the question of whether learning that occurs between two associatively-activated representations is excitatory or inhibitory remains open.
The experiments reported here were conducted to examine the nature of associations formed between two associatively-activated stimulus representations. We entertained the hypothesis that the nature of mediated learning may vary as a function of temporal contiguity, just as direct learning between a CS and US may manifest as excitation or inhibition, depending on the temporal arrangement of those events. To find common ground with the results of Dwyer and his colleagues, the initial impetus was to demonstrate excitatory learning between two associatively-activated representations. However, it will become apparent that excitatory learning was not so easily obtained.
In each of the following experiments, the representations of two neutral stimuli (a white noise and a tone) were paired with each other, by presenting together previously-established visual associates of those stimuli. The strength and nature of any association that was formed between the two stimuli was assessed by pairing the tone with sucrose, and then testing the noise stimulus. In this way, sensory preconditioning was used as a tool to diagnose the nature of learning that occurred between the associatively-activated representations, with the assumption that responding to noise would be based on a chain of associations between noise, tone, and sucrose and that the tone-sucrose association would be approximately equal among training groups.
Experiment 1a
Experiment 1a examined the nature of associations acquired between two behaviorally neutral events when their representations were simultaneously activated by their associates (see Table 1). In the first phase, all rats received pairings of a light CS (L1) with a tone, and pairings of a second light CS (L2) with a noise. In the second phase, the rats in Group Paired received simultaneous presentations of the two light CSs (L1+L2), whereas rats in Group Unpaired received separate presentations of L1 and L2. Thus, the rats in Group Paired might have been expected to receive pairings of tone and noise representations, whereas those in Group Unpaired would not. Finally, any association between noise and tone was assessed in all rats in a sensory-preconditioning procedure. All rats first received tone-sucrose pairings until the tone elicited a substantial conditioned response. Then, appetitive responding to the noise was assessed, first in a performance test of responding to the noise alone, and then in an acquisition test in which the noise was directly paired with sucrose. This method was based on the assumption that appetitive responding to the noise at test would be driven by excitatory noise-tone and tone-sucrose associations. We expected that excitatory associations between noise and tone in Group Paired would be revealed as more responding to the noise in the performance test and/or more rapid acquisition of responding to the noise in the acquisition test, relative to Group Unpaired.
Table 1.
The design of Experiment 1a
| Group | Phase 1 | Phase 2 | Phase 3 | Test | Acquisition Test |
|---|---|---|---|---|---|
| Paired | 16 L1→T/ 16 L2→N |
16 L1L2 | T→S | 4 N | 20 N→S |
| Unpaired | 16 L1→T/ 16 L2→N |
16L1/16L2 | T→S | 4 N | 20 N→S |
Note. L1 and L2 = flashing houselight and steady panel light, counterbalanced; T = tone; N = white noise; S = sucrose.
Method
Subjects
The subjects were 16 male Sprague-Dawley rats (Charles River Laboratories, Raleigh, North Carolina), which weighed 300–325 g when they arrived in the laboratory vivarium. Prior to experimental manipulations, the rats were divided into Groups Unpaired and Paired (ns = 8). The rats were maintained for 1 week with ad-lib access to food and water in individual cages before they were deprived to 85% of their ad-lib weights by limiting their access to food to a single daily meal. The vivarium was illuminated from 6 a.m. to 8 p.m.
Apparatus
The behavioral training apparatus consisted of eight individual chambers (22.9 × 20.3 × 20.3 cm) with aluminum front and back walls, clear acrylic sides and top, and a floor made of 0.48-cm stainless steel rods spaced 1.9 cm apart. A dimly illuminated liquid cup was recessed in the center of one end wall. A 6-w lamp was mounted behind a jeweled lens on the front panel, 10 cm above the food cup; illumination of this lamp served as one of the light CSs. An infrared photocell placed just inside the food cup was polled (1 kHz) by computer circuitry to measure food cup entry. Each chamber was enclosed in a sound-resistant shell. A 6-w house light (which served as another light CS) and a speaker for delivering auditory stimuli was mounted on the inside wall of the shell, 10 cm above the experimental chamber and even with the end wall opposite the food. Each of the shells was enclosed in another sound-resistant shell; ventilation fans mounted on the outer shells provided masking noise (70 dB).
Procedure
Training procedure
In each of the four 64-min daily sessions of Phase 1, the rats received four serial pairings of a 10-s presentation of L1 (an intermittent [4 Hz] illumination of the house light or the steady illumination of the panel light, counterbalanced) with a 1,500-Hz 78-dB (C-scale) tone, and four 10-s serial pairings of L2 (the other visual stimulus) with a 78-dB white noise. In these and all of the serial pairings in this series of experiments, the onset or delivery of the subsequent stimulus coincided with the termination of the antecedent stimulus. Next, in each of two daily 64-min Phase 2 sessions, the rats in Group Paired received eight 10-s presentations of a simultaneous compound of L1 and L2, and the rats in Group Unpaired received eight presentations each of L1 and L2.
All rats were then trained to drink from the recessed liquid cup in two daily 64-min sessions, each of which included 16 deliveries of the US, 0.3 ml of a 0.2-M sucrose solution. Next, all rats received four daily 64-min Phase 3 sessions, each of which included eight serial pairings of the tone with sucrose. Finally, responding to the noise was assessed in three daily 64-min sessions. The first half of the first of these sessions comprised a performance test of responding to the noise, consisting of four 10-s nonreinforced presentations of that CS. Twenty subsequent serial noise-sucrose pairing trials (four in the second half of the first test session and eight in each of the remaining two test sessions) provided an acquisition test of previously established tone-noise associations. To the extent that the noise and tone were associated in Group Paired, the noise would have access to tone-sucrose associations established in Phase 3 and so would evoke more responding than the noise in Group Unpaired.
Response measures
The primary response measure was the elevation in food cup responding produced by CSs over baseline responding. We recorded the percentage of time a rat spent with its head in the food cup during the last 5 s of each 10-s CS, and during the 5-s empty interval immediately before each CS, as indicated by disruption of the photocell beam. Previous data (Holland, 1977, 2000) show that food cup behaviors during 10-s CSs are more prevalent and less variable in the last 5 s of a 10-s cue. To reduce intra-group variability, elevation scores were constructed by subtracting a rat’s pre-CS times from its CS times. Pre-CS responding did not differ among the groups; we present those data in summary form as well. For purposes of data presentation and statistical analysis, we combined data from individual trials to form half-session (4-trial) blocks.
Results and discussion
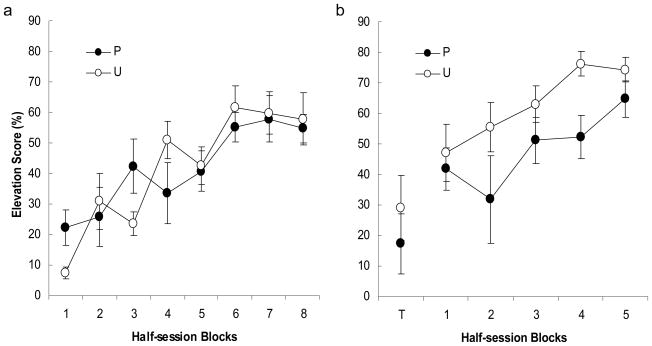
Little food cup behavior was observed in Phases 1 and 2, which occurred prior to food cup training. In Phase 3, the rats in Groups Paired and Unpaired acquired food cup responding in similar fashion over the course of tone-sucrose pairings (Figure 1, panel a). The primary results of Experiment 1a are those of the 3 test sessions (Figure 1, panel b). If excitatory associations were formed between tone and noise in Group Paired as a result of the simultaneous activation of noise and tone representations in Phase 2, then responding to the noise in these test sessions might be expected to be greater in that group than in Group Unpaired. However, in the initial, nonreinforced block of noise trials, the rats in Group Paired showed a nonsignificant tendency to respond less than the rats in Group Unpaired. Furthermore, over the course of the remaining five blocks of reinforced noise presentations, responding was significantly lower in Group Paired than in Group Unpaired. The following statistical analyses support these observations.
Figure 1.
Experiment 1a: (a) tone training and (b) noise test. The points depict elevation scores in response to tone in Phase 3 and the noise at test. The data are presented as 4-trial blocks. The noise test included one block of nonreinforced test trials (T) and the acquisition test trials (1–5). The error bars depict the standard error of the means.
First, to justify the use of elevation scores, we analyzed pre-CS responding. In Phase 3 training, a group × trial blocks analysis of variance (ANOVA) showed a significant effect of blocks, F(7, 98) = 2.65, p = .015, but no effect of groups, F(1, 14) = 0.28, p = .605. A significant group × blocks interaction, F(7, 98) = 2.15, p = .045, reflected random variations among the sessions. A similar analysis confined to the last block of training showed no significant effect of group, F(1, 14) = 0.10, p = .754. Mean (± SE) pre-CS responding was 11.7 ± 4.5% in Group Paired and 8.9 ± 3.8% in Group Unpaired on this block. In the test phase, we analyzed pre-CS responding separately for the initial nonreinforced block of trials, and the remaining 5 reinforced trial blocks. In the initial test block, mean pre-CS responding was 29.0 ± 7.5% in Group Paired and 9.2 ± 6.1% in Group Unpaired, but this difference was not statistically significant, F(1, 14) = 4.15, p = .061. Over the remaining blocks of test trials, pre-CS responding was similar in the two groups, with means of 12.4 ± 2.5% in Group Paired and 8.9 ± 3.8% in Group Unpaired. A groups × blocks ANOVA of those pre-CS scores showed a significant (declining) effect of blocks, F(4, 56) = 3.50, p = .013, but no effect of group, F(1, 14) = 1.32, p = .269, and no group × blocks interaction, F(4, 56) = 1.10, p = .365.
The primary analyses were those of elevation scores. A groups × blocks ANOVA over the last half of Phase 3 tone training showed a significant main effect of block, F(3, 42) = 4.58, p = .007, but no effect of group or group × block interaction, Fs < 1, ps > .629. The noise elevation scores from the nonreinforced test block did not differ between the groups, F(1, 14) = 0.67, p = .428, but a groups × block ANOVA of elevation scores over the reinforced test sessions showed significant effects of both group, F(1, 14) = 5.27, p = .038, and blocks, F(4, 56) = 5.33, p = .001. The group × blocks interaction was not significant, F(4, 56) = 0.72, p = .579.
The results of Experiment 1a were unexpected, and somewhat ambiguous. Clearly, the lower responding in Group Paired relative to Group Unpaired does not provide strong evidence for excitatory learning between two associatively-activated representations. Instead, retarded responding in Group Paired suggests that the noise acquired some sort of inhibitory potential with respect to sucrose in that group. However, it is possible that the training in the Group Paired retarded acquisition of the noise-sucrose association in testing for a reason other than inhibitory learning. If paired training produced a greater reduction in attention to the noise than unpaired training, for example, then one would expect slower acquisition of the noise-sucrose association (e.g., Lubow & Moore, 1959), regardless of the status of noise-tone associations. Although there is no obvious reason to expect such an effect on attention, this possibility was explored in Experiment 1b.
Experiment 1b
Experiment 1b used a summation test to assess inhibition after the training regimen described in Experiment 1a. By using Rescorla’s (1971) two-test strategy to assess inhibition, we sought to determine whether weak responding observed in Experiment 1a was due to a reduction in attention to the noise in Group Paired, or the expression of an inhibitory relationship between noise and sucrose that might have been mediated by the tone. If the paired treatment merely reduced attention to the noise, then the noise should have little impact when it is presented with an excitatory CS (i.e., the tone). Alternatively, if that treatment yielded an inhibitory association between noise and sucrose, then the noise should reduce responding when it is presented together with the tone.
Method
Subjects and apparatus
The subjects were 16 naïve rats similar to those used in Experiment 1a, maintained in the same manner. The apparatus was that used in Experiment 1a.
Procedure
The procedure was identical to Experiment 1a, except for the inclusion of a tone-noise compound test instead of an acquisition test. After Phase 3 tone-sucrose pairings, all subjects were administered a single 32-min test session with four simultaneous tone + noise compound presentations, and four tone-alone presentations. The order of trials was NT, T, T, NT, T, NT, NT, T.
Results and discussion
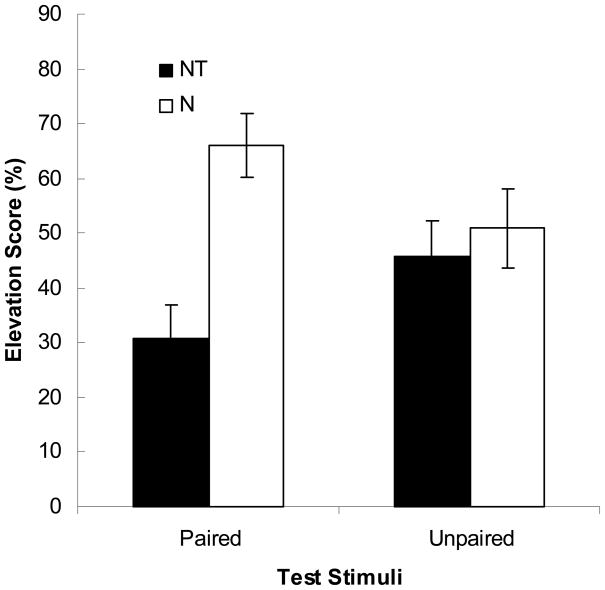
As in Experiment 1a, acquisition of responding to the tone in Phase 3 was similar in the two groups. Over the final half of training, mean elevation scores were 54.9 ± 2.9% in Group Paired and 55.8 ± 3.4% in Group Unpaired. Figure 2 shows the results of the summation test. The noise suppressed responding to the tone CS more in Group Paired than in Group Unpaired, indicating that the noise was a conditioned inhibitor in Group Paired but not in Group Unpaired. The following analyses support these assertions.
Figure 2.
Experiment 1b. The bars depict elevation scores in response to noise and the noise-tone compound. The data are presented as 4-trial blocks. The error bars depict the standard error of the means.
First, pre-CS responding did not differ across groups in either Phase 3 [11.9 ± 2.3% in Group Paired and 12.2 ± 2.0% in Group Unpaired, F(1, 14) = 0.51. p = .487] or testing [4.3 ± 1.7% and 7.9 ± 4.0%, respectively, F(1, 14) = 0.657, p = .431.] Next, a group × blocks ANOVA of elevation scores over the last half of Phase 3 training showed a significant effect of blocks, F(3, 42) = 12.42, p < .001, but no effect of group, F(1, 14) = 0.32, p = .581, or group × blocks interaction, F(3, 42) = 1.91, p = .143. Most important, a group × cue (tone or noise + tone compound) ANOVA of elevation scores during the test session showed an effect of cue, F(1, 14) = 64.86, p < .001, and a significant group × cue interaction, F(1, 14) = 36.39, p < .001, but no effect of group, F = 0.00, p = .999. Contrasts using the Tukey’s honestly-significant difference procedure showed that all individual comparisons were significant, ps < .004, except for the comparison of tone and tone + noise responding in Group U, p = .503.
The inhibition observed in Experiments 1a and 1b was unexpected, but not inexplicable. In designing Experiment 1a, we assumed that an excitatory association would form between noise and tone, and that this association would be expressed as enhanced responding to noise after the tone was paired with sucrose in Phase 3, as in standard sensory-preconditioning procedures. If we view the present data within that framework, then we must assume that an inhibitory association was formed between noise and tone in Phase 2, and that this association was expressed as inhibitory potential between noise and sucrose after tone-sucrose pairings in Phase 3. According to this account, the inhibition exhibited by the noise is a sort of sensory-preconditioned inhibition, like that observed by Espinet, Iraola, Bennett, and Mackintosh (1995) in a conditioned taste aversion preparation. In their experiments, alternating exposure of two flavor compounds that shared a common element (AX/BX) created inhibitory learning between the unique elements (A and B). This inhibitory learning was evidenced by pairing A with illness, and subsequently testing B for inhibitory potential. Notably, this effect has recently been observed in an appetitive learning preparation similar to the one employed here (Espinet, González, & Balleine, 2004). The specific treatment used in Experiments 1a and 1b was different from what was used by Espinet and colleagues, but the end result might have been similar. Inhibitory learning might have developed between two neutral stimuli, becoming apparent only when one stimulus is paired with a motivationally-significant outcome. One potential objection to this interpretation is that it demands that simultaneous pairings of two associatively-activated event representations resulted in inhibition. Although this possibility may seem unlikely, there is evidence that simultaneous first-order conditioning can yield inhibition after extensive training (e.g., Heth, 1976; Moscovitch & LoLordo, 1972). The results of Experiments 1a and 1b might stand as an analogous situation in which simultaneous activation of two representations led to a net inhibitory relationship.
Another account for these data is that inhibition developed between the noise and sucrose in Phase 3. According to this interpretation, an excitatory association formed between noise and tone in Phase 2, and an inhibitory association formed between the associatively-activated representation of noise, and the directly-activated representation of sucrose, in Phase 3. This interpretation can be derived from a modified version of Wagner’s (1981) SOP model of learning. Although the original SOP did not assume that learning would occur between two associatively-activated stimuli, Dickinson and Burke’s (1996; Aitken & Dickinson, 2005) modified SOP (MSOP) assumes that excitatory associations will be formed between two associatively-activated representations and inhibitory associations will be formed between an associatively-activated representation and a directly-activated representation. Applied to Experiments 1a and 1b, MSOP states that Phase 2 learning results in an excitatory association between the associatively-activated representations of noise and tone, but Phase 3 training allows an inhibitory association to be formed between the associatively-activated noise representation and the directly-activated sucrose representation. This explanation is similar to MSOP’s account of backward blocking (e.g., Dickinson & Burke, 1996), but perhaps less ambiguous, because in the current study the sucrose representation is not associatively activated during the early trials of Phase 3.
Experiment 2
In designing Experiment 2, we sought to compare the two accounts of Experiments 1a and 1b. If, as in our first account, we assume that simultaneous activation of noise and tone representations yielded inhibitory associations between those stimuli in Phase 2 of Experiments 1a and 1b, then excitatory noise-tone learning might be more readily obtained if the noise representation preceded the tone representation in a serial fashion. In contrast, if the tone preceded the noise, one might expect inhibitory learning similar to that observed in Experiments 1a and 1b. These expectations are based on the knowledge that consistent forward CS→US pairings result in excitatory learning (e.g., Pavlov, 1927), whereas backward US→CS conditioning typically results in the development of inhibition (e.g., Cole & Miller, 1999; Chang, Blaisdell, & Miller, 2003; Delamater, Lolordo, & Sosa, 2003; Heth, 1976; Williams & Overmier, 1988), often after a brief period of excitatory learning.
By contrast, within the MSOP account outlined previously, the temporal arrangement of noise and tone in Phase 2 might have little effect on whether noise forms an excitatory or inhibitory association with sucrose. In all cases, pairings of two associatively-activated event representations would result in excitatory learning between those representations. Furthermore, within this account, excitatory noise-tone learning that occurs in Phase 2 should only produce inhibitory associations between noise and sucrose in Phase 3.
Experiment 2 was designed to dissociate these two accounts and potentially attain the original goal of this series of experiments by demonstrating excitatory learning between associatively-activated representations. In Experiment 2 we compared two different types of Phase 2 paired treatments with an unpaired control (see Table 2). After L1→noise, L2→tone pairings as in Phase 1 of Experiments 1a and 1b, the rats in Group Forward received Phase 2 training with serial L1→L2 pairings, in which the noise representation should have been paired in a forward fashion with the tone representation. In Group Backward, the order of the Phase 2 stimulus presentations was reversed, so that the noise representation should have been paired with the tone representation in a backward fashion. In Group Unpaired, L1 and L2 were presented explicitly unpaired in Phase 2. Noise-tone learning was assessed with noise-alone and noise→sucrose acquisition tests, as in Experiment 1a. If the associatively-activated representations behave like their directly-activated counterparts, then forward pairings would result in excitatory learning and backward pairings would result in inhibitory learning, relative to the unpaired control condition. According to MSOP’s account of Experiments 1a and 1b, Groups Forward and Backward might ultimately produce differing levels of inhibition between noise and sucrose, but neither should produce net excitation.
Table 2.
The design of Experiment 2
| Group | Phase 1 | Phase 2 | Phase 3 | Test | Acquisition Test |
|---|---|---|---|---|---|
| Forward | 16 L1→N/ 16 L2→T |
16 L1→L2 | T→S | 4 N | 20 N→S |
| Unpaired | 16 L1→N/ 16 L2→T |
16L1/16L2 | T→S | 4 N | 20 N→S |
| Backward | 16 L1→N/ 16 L2→T |
16 L2→L1 | T→S | 4 N | 20 N→S |
Note. L1 and L2 = flashing houselight and steady panel light, counterbalanced; T = tone; N = white noise; S = sucrose.
Method
Subjects and apparatus
The subjects were 24 naïve rats similar to those used in Experiments 1a and 1b, maintained in the same manner. The rats were divided into three groups (ns = 8). The apparatus was that used in Experiments 1a and 1b.
Procedure
The procedures were identical to those of Experiment 1a, except that in Phase 2, the rats in Group Forward received serial L1→L2 pairings and the rats in Group Backward received L2→L1 pairings, rather than simultaneous L1+L2 presentations. In each compound trial, the onset of one visual stimulus occurred at the termination of the other. The rats in Group Unpaired were trained in the identical fashion as the rats in Group Unpaired in Experiment 1a. The rats were tested as in Experiment 1a.
Results and discussion
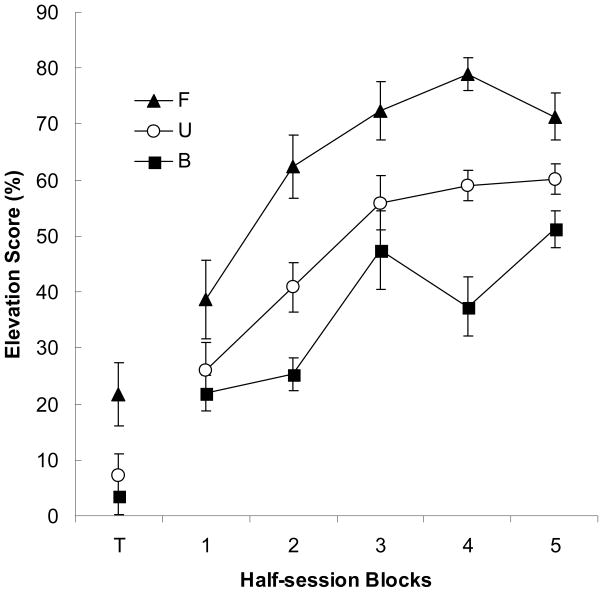
As in Experiments 1a and 1b, Phase 3 tone training progressed similarly for the three groups. Over the last half of Phase 3 training, the mean elevation scores were 51.5 ± 7.9%, 56.9 ± 6.5%, and 57.7 ± 4.7% in Groups Forward, Unpaired, and Backward, respectively. Most important (Figure 3), during both the nonreinforced test block and subsequent training blocks, elevation scores were greater in Group Forward than in the other 2 groups. Furthermore, over the course of reinforced training, Group Backward showed less responding than either of the other two groups. Thus, forward pairings of two associatively-activated event representations in Phase 2 apparently produced excitatory associations between those representations, and backward pairings produced inhibitory associations.
Figure 3.
Experiment 2. The points depict elevation scores in response to noise. The data are presented as 4-trial blocks from the nonreinforced test trials (T) and the acquisition test trials (1–5). The error bars depict the standard error of the means.
As in Experiments 1a and 1b, analyses of pre-CS responding revealed no significant group differences in either tone training or noise testing phases. Over the last half of Phase 3 training, mean pre-CS responding was 10.6 ± 2.2%, 7.6 ± 1.6%, and 6.3 ± 1.4%, in Groups Forward, Unpaired, and Backward, respectively, F(2, 21) = 1.58, p = .230. In the initial test block, mean pre-CS responding was 7.8 ± 2.2%, 10.2 ± 2.7%, and 7.0 ± 2.2% in those groups, F(2, 21) = 0.515, p = .605. Over the remaining 5 reinforced test blocks, pre-CS responding was similar in the three groups, with means of 4.2 ± 0.6%, 10.0 ± 3.0%, and 8.6 ± 2.0% in Groups Forward, Unpaired, and Backward, respectively. A groups × blocks ANOVA showed a significant effect of blocks, F(4, 84) = 5.33, p < .001, but no effect of group, F(2, 21) = 2.11, p = .146, and no group × blocks interaction, F(4, 84) = 1.41, p = .206.
A group × blocks ANOVA of tone elevation scores over the last half of Phase 3 showed an effect of blocks, F(3, 63) = 10.46, p < .001, but no effect of group or group × blocks interaction, Fs < 1, ps > .762. A one-way analysis of noise elevation scores in the nonreinforced test block showed a significant effect of group, F(2, 21) = 4.71, p = .020. Planned comparisons (using the pooled error term) confirmed that Group Forward showed higher responding to noise than either Group Unpaired, p = .032, or Group Backward, p = .008, but responding in Group Backward was not detectably lower than in Group Unpaired, p = .554. A group × block ANOVA of the elevation scores in the acquisition test revealed significant effects of group, F(2, 21) = 13.29, p < .001, and block, F(4, 84) = 69.18, p < .001, as well as the interaction between those two variables, F(8, 84) = 3.71, p < .001. Planned comparisons showed that each group differed significantly from each other group, ps < .044. Thus, relative to Group Unpaired, the acquisition test revealed excitatory learning in Group Forward and inhibitory learning in Group Backward.
The observation of both excitatory and inhibitory learning in Experiment 2 favors the idea that learning about two associatively-activated representations may be excitatory or inhibitory depending on the temporal relationship of the two associatively-activated representations. Similar to direct-learning situations, forward pairings yielded excitatory learning and backward pairings yielded inhibitory learning. MSOP, which provided an alternative account of Experiments 1a and 1b, correctly predicted inhibition in Group Backward, but incorrectly predicted that inhibition would also be apparent in Group Forward. Even if the temporal arrangement of tone and noise representations in Phase 2 caused differing levels of noise-tone excitation, the end result according to MSOP could only be differing levels of noise-sucrose inhibition, not noise-sucrose excitation.
Miller and colleague’s temporal coding hypothesis (e.g., Savastano & Miller, 1998) accurately predicts the results of Experiment 2. According to their theory, contiguity is sufficient to form associations, these associations include information about temporal relationships, the temporal information affects the nature of responding, and animals may integrate separate temporal maps that contain common stimuli. Applied to Experiment 2, the temporal coding hypothesis suggests that noise would have a predictive relationship with sucrose in Group Forward because noise precedes tone, which precedes sucrose. In Group Backward, the noise has a simultaneous relationship with sucrose (or backward considering the relative durations of the noise and sucrose stimuli) because the tone precedes both noise and sucrose. Thus, the temporal coding hypothesis could anticipate excitatory responding in Group Forward and inhibitory responding in Group Backward. However, the temporal coding hypothesis does not predict the results of Experiments 1a and 1b. In the Paired groups of those experiments, noise had a net predictive relationship with sucrose because it shared a simultaneous relationship with tone, which shared a serial relationship with sucrose. According to the temporal coding hypothesis, this should be expressed as excitation, regardless of the fact that the noise-tone relationship was not predictive (e.g., Cole, Barnet, & Miller, 1995).
General Discussion
The present data suggest that associatively-activated representations may enter into associations with each other, and that the nature of those associations may vary according to similar parameters that guide the formation of associations between directly-experienced stimuli. In Experiments 1a and 1b, simultaneous pairings of tone and noise representations followed by tone-sucrose pairings produced inhibitory potential in the noise stimulus. This inhibition could have been produced by inhibitory noise-tone learning in Phase 2, or by inhibitory noise-sucrose learning in Phase 3, as predicted by MSOP. In Experiment 2, backward pairings also produced inhibitory learning, but forward pairings produced excitatory learning. MSOP failed to account this result, because it does not simultaneously predict the excitation apparent in Group Forward and the inhibition apparent in Group Backward. Taken together, Experiments 1a, 1b, and 2 are more consistent with the idea that mediated learning produces net excitation or inhibition depending on the temporal relationship of the associates. Backward or simultaneous pairings can produce inhibition in both direct- and mediated-learning situations, whereas forward pairings produce excitation. These results demonstrate flexibility in representation-mediated conditioning, and raise basic theoretical issues concerning the conditions that support excitatory and inhibitory learning.
Although the empirical generalization that associations formed among associatively-activated event representations mimic those formed among directly-activated representations describes our data, it can not stand on equal footing with models such as MSOP, because it provides no mechanism by which excitatory or inhibitory learning occurs among associatively-activated event representations, nor how temporal contiguity influences that learning. On the surface at least, our findings may be more readily integrated into Harris’s (2006) recent elemental model of learning, which allows for both excitatory and inhibitory learning between associatively-activated CSs and USs based on the level of activation achieved by the US representation. Within that model CSs and USs enter into associations as representational elements. These representational elements are activated when a stimulus is presented, but they must compete with other elements for activation into a limited-capacity attention buffer. For CSs, activation into the attention buffer enhances acquisition and extinction, but learning will still occur for elements that are activated outside the buffer, albeit at a slower rate. For US elements, the level of activation dictates the nature of learning. US elements support excitatory learning when they are activated into the attention buffer, and inhibitory learning when they are activated outside of the buffer. Applied to the present studies, if we allow the noise to take the role of the CS and the tone to take the role of the US, then inhibitory learning would occur between the two if the tone elements were activated outside of the attention buffer, regardless of the level of activation of the noise elements. If the tone elements were activated into the attention buffer, then excitatory noise-tone learning would have occurred. Without a formal implementation, it is difficult to determine whether this model would predict the present results a priori, but some critical factors can still be identified. Depending on the capacity of the attention buffer, simultaneous L1L2 presentations in Experiments 1a and 1b might prevent tone elements from entering the buffer resulting in net inhibition between the noise and tone. Depending on the threshold of the attention buffer and the strength of activation of tone elements, serial forward presentations may alleviate some of the competition for attention, which could allow more tone elements to enter into the buffer. Serial backward presentations would produce similar activation, but the tone elements may decay out of the attention buffer before the tone is presented, depending upon the rate of decay.
Even in the context of the present data, the assertion of comparability between learning about directly- and associatively-activated event representations may be flawed. It is quite likely that the associative properties of indirectly-activated representations are not exactly like their directly-experienced counterparts. For example, in Experiments 1a and 1b inhibitory noise-tone learning was expressed after associatively-activated representations of noise and tone were simultaneously paired, and tone was later paired with food. Although we noted that many investigators have observed inhibitory learning when CS and US are presented simultaneously for many trials, we do not know if simultaneous direct presentations of noise and tone would produce evidence for inhibitory learning in a sensory preconditioning design such as that of Experiments 1a and 1b. This treatment might be viewed as a feature-negative training, which can result in net inhibition, but rarely does so when the nonreinforced compound trials entirely precede the reinforced trials. This form of phasic conditioned inhibition has been observed, but typically involves some sort of preexposure to the US (or outcome) through direct experience or instruction in those cases that involve human subjects (e.g., Amundson, Wheeler, & Miller, 2005; Chapman, 1991). Instead, it is probably more reasonable to anticipate excitatory sensory preconditioning (e.g., Rescorla, 1980; but see Holland & Ross, 1983; Thompson, 1972) in a direct-learning situation analogous to Experiments 1a and 1b, which indeed was our original expectation when designing Experiment 1a. Thus, the experiments presented here do show that mediated learning is sensitive to temporal contiguity, but it is not necessarily prudent to accept the strong assertion that mediated learning shares a one-to-one relationship with direct learning (also see Holland, 1983, 2006).
The results presented here are difficult to reconcile with Dwyer’s (2001) findings. In Experiments 3 and 4 of his series, excitatory learning between associatively-activated flavor and illness representations was more apt to occur if the representations were activated in a simultaneous compound rather than a serial compound. In fact, when the representations were activated in a serial compound, there was no evidence of any mediated learning. In our Experiments 1a and 1b, inhibitory learning occurred between two simultaneously-activated representations. Furthermore, in Experiment 2 excitatory learning occurred when the two representations were activated in a serial compound fashion. Prima facie, these results obviously stand in contrast to Dwyer’s. There are many differences between the two paradigms that could explain the discrepancy in the findings. Perhaps learning situations that involve discrete and relatively short cues are more sensitive to temporal relationships relative to situations involving extended exposure to diffuse contextual, gustatory, and visceral cues. Alternatively, the two different paradigms could engage different underlying mechanisms. For example, Dwyer (2003) indicated that simultaneous pairings of flavor and illness representations might engage the same processes that are involved in flavor-flavor taste aversion learning. He suggests that these processes are especially sensitive to temporal contiguity, such that little learning is observed if temporal contiguity is noticeably degraded. It is possible that the different effects of simultaneous conditioning observed here and by Dwyer reflect differences that are not specific to mediated learning, but are also apparent in direct-learning situations that involve these different procedures. Finally, our design was embedded withing a sensory-preconditioning preparation, which is known to differ in systematic ways from first-order conditioning situations (e.g., Miller & Matute, 1996). We are currently conducting research to determine whether the present observations are limited to sensory-preconditioning situations.
More research needs to be conducted to determine the associative rules that govern mediated learning in order to integrate the outcomes observed by different researchers. The present results suggest that learning between absent stimuli does not always result in excitatory associations (e.g., Dickinson & Burke, 1996), nor does it always produce inhibitory associations (e.g., Holland, 1983). Any comprehensive explanation of mediated learning will need to allow for dynamic processing of associatively-activated stimuli in order to account for changes in learning that can occur with shifts in variables such as temporal contiguity.
Acknowledgments
We thank Vanessa McKenna for her technical assistance. This research was supported in part by Grant MH65879 from the National Institutes of Health.
References
- Aitken MR, Dickinson A. Simulations of a modified SOP model applied to retrospective revaluation of human causal learning. Learning & Behavior. 2005;33:147–159. doi: 10.3758/bf03196059. [DOI] [PubMed] [Google Scholar]
- Amundson JC, Wheeler DS, Miller RR. Enhancement of Pavlovian conditioned inhibition achieved by posttraining inflation of the training excitor. Learning and Motivation. 2005;36:331–352. doi: 10.1016/j.lmot.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Brandon SE, Vogel EH, Wagner AR. A componential view of configural cues in generalization and discrimination in Pavlovian conditioning. Behavioural Brain Research. 2000;110:67–72. doi: 10.1016/s0166-4328(99)00185-0. [DOI] [PubMed] [Google Scholar]
- Calton JL, Mitchell KG, Schachtman TR. Conditioned inhibition produced by extinction of a conditioned stimulus. Learning and Motivation. 1996;27:335–361. doi: 10.1006/lmot.1996.0020. [DOI] [PubMed] [Google Scholar]
- Chang RC, Blaisdell AP, Miller RR. Backward conditioning: Mediation by the context. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:171–183. doi: 10.1037/0097-7403.29.3.171. [DOI] [PubMed] [Google Scholar]
- Cole RP, Barnet RC, Miller RR. Temporal encoding in trace conditioning. Animal Learning & Behavior. 1995;23:144–153. [Google Scholar]
- Cole RP, Miller RR. Conditioned excitation and conditioned inhibition acquired through backward conditioning. Learning and Motivation. 1999;30:129–156. [Google Scholar]
- Delamater AR, LoLordo VM, Sosa W. Outcome-specific conditioned inhibition in Pavlovian backward conditioning. Learning & Behavior. 2003;31:393–402. doi: 10.3758/bf03196000. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Miller RR. The role of temporal variables in inhibition produced through extinction. Learning & Behavior. 2003;31:35–48. doi: 10.3758/bf03195969. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Burke J. Within-compound associations mediate the retrospective revaluation of causality judgements. The Quarterly Journal of Experimental Psychology B. 1996;49:60–80. doi: 10.1080/713932614. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Retrospective revaluation or mediated conditioning? The effect of different reinforcers. The Quarterly Journal of Experimental Psychology B. 1999;52:289–306. doi: 10.1080/027249999393013. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Formation of a novel preference and aversion by simultaneous activation of the representations of absent cues. Behavioural Processes. 2000;48:159–164. doi: 10.1016/s0376-6357(99)00080-7. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Mediated conditioning and retrospective revaluation with LiCl then flavour pairings. The Quarterly Journal of Experimental Psychology B. 2001;54:145–165. doi: 10.1080/713932750. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Learning about cues in their absence: Evidence from flavour preferences and aversions. The Quarterly Journal of Experimental Psychology B. 2003;56:56–67. doi: 10.1080/02724990244000160. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Mackintosh NJ, Boakes RA. Simultaneous activation of the representations of absent cues results in the formation of an excitatory association between them. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:163–171. [Google Scholar]
- Espinet A, González F, Balleine BW. Inhibitory sensory preconditioning. The Quarterly Journal of Experimental Psychology B. 2004;57:261–272. doi: 10.1080/02724990344000105. [DOI] [PubMed] [Google Scholar]
- Espinet A, Iraola JA, Bennett CH, Mackintosh NJ. Inhibitory association between neutral stimuli in flavor- aversion conditioning. Animal Learning & Behavior. 1995;23:361–368. [Google Scholar]
- Harris JA. Elemental representations of stimuli in associative learning. Psychological Review. 2006;113:584–605. doi: 10.1037/0033-295X.113.3.584. [DOI] [PubMed] [Google Scholar]
- Harris JA, Westbrook F. Retroactive revaluation of an odor-taste association. Animal Learning & Behavior. 1998;26:326–335. [Google Scholar]
- Heth CD. Simultaneous and backward fear conditioning as a function of number of CS-UCS pairings. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:117–129. doi: 10.1037//0097-7403.2.2.117. [DOI] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. Acquisition of Representation-Mediated Conditioned Food Aversions. Learning and Motivation. 1981;12:1–18. doi: 10.1016/j.lmot.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Representation-mediated overshadowing and potentiation of conditioned aversions. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:1–13. [Google Scholar]
- Holland PC. Trial and intertrial durations in appetitive conditioning in rats. Animal Learning & Behavior. 2000;28:121–135. [Google Scholar]
- Holland PC. Limitations on representation-mediated potentiation of flavour or odour aversions. Quarterly Journal of Experimental Psychology. 2006;59:233–250. doi: 10.1080/17470210500242904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Forbes DT. Representation-mediated extinction of conditioned flavor aversions. Learning and Motivation. 1982;13:454–471. [Google Scholar]
- Holland PC, Ross RT. Savings test for associations between neutral stimuli. Animal Learning & Behavior. 1983;11:83–90. [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: The effect of nonreinforced pre-exposure to the conditional stimulus. Journal of Comparative and Physiological Psychology. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matute H. Biological significance in forward and backward blocking: Resolution of a discrepancy between animal conditioning and human causal judgment. Journal of Experimental Psychology: General. 1996;125:370–386. doi: 10.1037//0096-3445.125.4.370. [DOI] [PubMed] [Google Scholar]
- Moscovitch AB, LoLordo VM. Role of safety in the Pavlovian backward fear conditioning procedure. Journal of Comparative and Physiological Psychology. 1968;66:673–678. doi: 10.1037/h0026548. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and neural analysis of extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. New York: Dover; 1927. [Google Scholar]
- Rescorla RA. Summation and retardation tests of latent inhibition. Journal of Comparative and Physiological Psychology. 1971;75:77–81. doi: 10.1037/h0030694. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Simultaneous and successive associations in sensory preconditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1980;6:207–216. doi: 10.1037//0097-7403.6.3.207. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Savastano HI, Miller RR. Time as content in Pavlovian conditioning. Behavioural Processes. 2002;44:147–162. doi: 10.1016/s0376-6357(98)00046-1. [DOI] [PubMed] [Google Scholar]
- Thompson RF. Sensory preconditioning. In: Thompson RF, Voss JS, editors. Topics in learning and performance. New York: Academic Press; 1972. pp. 105–129. [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Ward-Robinson J, Hall G. Backward sensory preconditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1996;22:395–404. [Google Scholar]
- Williams DA, Overmier JB. Backward inhibitory conditioning with signaled and unsignaled unconditioned stimuli: Distribution of trials across days and intertrial interval. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:26–35. [Google Scholar]