Abstract
Considerable evidence indicates that associations may be formed between two events even when one or both of them is absent at the time of learning. Previously, some researchers asserted that excitatory associations are formed when associatively-activated representations for two events are paired, whereas others claimed that inhibitory associations are formed. In three experiments we investigated the nature of tone-sucrose learning when associatively-activated representations of those events were paired in the absence of either of the events themselves. Experiment 1 found substantial excitatory learning when the tone surrogate preceded the sucrose surrogate in training. Experimental 2 evaluated other accounts for the results of Experiment 1, and Experiment 3 found evidence for inhibitory tone-sucrose learning when the tone and sucrose surrogates were presented in simultaneous or backward order. The results indicated that the nature of representation-mediated learning is influenced by some of the same variables as more standard associative learning.
Most learning theorists agree that through associative learning, conditioned stimuli (CSs) can come to activate representations of their unconditioned stimulus (US) referents. These associatively-activated event representations may serve many functions, including mediating the performance of conditioned responses (CRs) (Rescorla, 1973), interacting with real events in overshadowing and potentiation (Holland, 1983), and participating in new learning about represented, but physically absent events (Holland, 1981). The experiments reported here examined the nature of tone-sucrose learning produced when associatively-activated representations of those events were paired in the absence of either of the events themselves.
Holland (1981) found that rats could acquire a food aversion when an associatively-activated representation of food was paired with toxin-induced illness. In one experiment, rats first received pairings of one auditory CS with wintergreen-flavored sucrose and another CS with peppermint-flavored sucrose. Then, the rats were given injections of lithium chloride (LiCl) after presentations of one of those CSs in the absence of either sucrose reinforcer. In a subsequent test, the rats consumed less of the flavored sucrose whose CS partner had been paired with LiCl. However, there was no evidence for a direct CS-LiCl association. Holland (1981) concluded that excitatory flavor-illness associations could be established when an associatively-activated (imaginary) representation of the flavor was paired with the actual induction of illness.
Similarly, Holland and Forbes (1982) found that extinction of a previously-established food aversion could be facilitated if an associatively-activated representation of that food was presented repeatedly in the absence of illness. In one experiment, two auditory CSs were first paired with two flavored sucrose reinforcers, as in Holland’s (1981) study. Then, aversions were established to both flavored reinforcers by pairing each of them directly with LiCl. Next, the rats were given repeated presentations of one of the auditory CSs alone. Finally, all rats received test presentations of each of the two flavors. Although initially consumption of both flavors was suppressed, over the course of continued testing, all rats consumed more of the flavor whose CS partner had been presented in extinction. Thus, the flavor representation activated by the CS alone substituted for the flavor itself in extinction learning. Many current theories of extinction posit that it involves the acquisition of inhibitory associations between the extinguished cue and the reinforcer with which it was originally paired. The implication of this position for Holland and Forbes’s (1982) results is that inhibitory flavor-illness associations were formed when associatively-activated representations of both flavor and illness were paired.
To accommodate Holland’s (1981) and Holland and Forbes’s (1982) results within contemporary learning theory, Holland (1983) suggested modifications of Wagner’s (1981) SOP model of learning. In that model, memory representations (nodes) may be in any of three activity states, A1, A2 or I. An event enters the focal A1 state only by direct activation by the physical event itself. By contrast, an event enters the more marginal A2 state either by spontaneous decay from the A1 state or by retrieval by an A1- or A2-state node that is associated with that event. Finally, events enter the inactive, resting I state by spontaneous decay from the A2 state. Within this model, excitatory associations between a CS and US are formed when their representations are processed simultaneously in the A1 state, and inhibitory CS-US associations are formed when the CS node is in the A1 state and US node is in the A2 state. Thus, excitatory associations are formed between CS and US only when they are both physically presented, whereas inhibitory associations are formed when the CS is physically presented together with an associatively-activated representation of the US.
SOP made no provision for learning when the CS representation is in the A2 state, rather than in the A1 state, and thus could not readily account for either mediated learning or mediated extinction. Holland (1983) suggested that representations in the A2 state, such as associatively-activated representations, could also enter into associations as CSs, albeit with a lower probability than A1-state (directly-presented) CSs. Thus, consistent with his mediated learning and extinction results, Holland (1983) suggested that “A2-Al” learning (in which the CS and US are in the A2 and A1 states, respectively) would be excitatory and “A2-A2” learning would be inhibitory. In keeping with the original SOP model, the establishment of excitatory or inhibitory associations with the US would be determined by the status of the US node (A1-state or A2-state, respectively), regardless of whether the CS representation was directly- or associatively activated. Thus, both A1-A1and A2-A1 learning would be excitatory and both A1-A2 and A2-A2 learning would be inhibitory.
However, Dwyer and his colleagues have amassed considerable evidence that the pairing of two associatively-activated event representations produces excitatory associations between those events (Dwyer, 1999, 2000, 2001, 2003; Dwyer, Mackintosh, & Boakes, 1998), rather than the inhibitory A2-A2 learning Holland (1983) might have anticipated. For example, in one experiment, Dwyer et al. (1998) first gave rats access to a peppermint solution in context one and to an almond + sucrose solution in context two. Next, almond alone was administered in context one in one group of rats and in context two for another group. A final consumption test revealed a greater preference for peppermint in the rats that received almond-alone in context one. Dwyer et al. (1998) asserted that this preference reflected the establishment of A2-A2 peppermint-sucrose associations: in those rats the associative activation of the peppermint flavor by context one cues was accompanied by the associative activation of sucrose by almond. By contrast, the rats in the other group had no opportunity for peppermint-sucrose learning because context two would not have activated a representation of peppermint. Dwyer et al. (1998) interpreted their results within another modification of SOP, offered by Dickinson and Burke (1996), which was originally intended to deal with observations of retrospective revaluation in studies of human causality judgments. Within this framework, excitatory associations are formed between two nodes when they are both activated in the same state (i.e., A1-A1 or A2-A2) and inhibitory associations are formed when the two nodes are activated in different states (i.e., A1-A2 and A2-A1).
Clearly, for more thorough understanding of the rules of association, Dwyer’s observation of excitatory A2-A2 learning and Holland’s observation of inhibitory A2-A2 learning need to be reconciled. A simple assumption motivating the present experiments was that A2-A2 learning might be either excitatory or inhibitory, depending on the nature of relations arranged between them, especially temporal relations. Indeed, Dwyer (2003) noted the potential importance of temporal contiguity in determining the outcome of mediated learning experiments. However, the use of unspecified contextual cues and agents that induce illness with delayed, gradual onsets makes an analysis of the role of temporal factors difficult.
Recently, Wheeler, Sherwood, and Holland (2008) investigated the nature of A2-A2 learning between two auditory stimuli when visual associates of those stimuli were paired. Using designs based on sensory preconditioning procedures, Wheeler et al. (2008) found evidence for both excitatory and inhibitory learning between associatively-activated event representations, depending on the temporal arrangement of cues. They first administered light1→ noise and light2→ tone pairings, followed by light1-light2 compound presentations, designed to establish A2-A2 associations between noise and tone. The presence of those associations was evaluated by first pairing the tone with sucrose and then testing responding to the noise. Wheeler et al. (2008) found evidence for excitatory noise-tone associations when the elements of the compound were administered in the order light1→ light2, but inhibitory associations when the elements were presented simultaneously or in the opposite order (light2→ light1). Wheeler et al. (2008) concluded that the nature of A2-A2 learning is neither always excitatory nor always inhibitory, but rather depends on task parameters, such as the temporal arrangement of stimuli.
Miller and Matute (1996) noted that the results of conditioning experiments may often depend on the biological significance of the events involved. It is possible that Wheeler et al.’s (2008) observations depended on their use of relatively neutral A2-state events. In the present experiments, we extended Wheeler et al.’s (2008) findings by examining the nature of tone-sucrose learning when associatively-activated representations of those events were presented jointly. In Experiment 1 we found evidence for strong excitatory A2-A2 learning, in Experiment 2 we evaluated alternative accounts for those results, and in Experiment 3 we examined the effects of variation in the temporal arrangement of the two associatively-activated event representations on the nature of learning obtained.
Experiment 1
In Experiment 1 we examined the nature of A2-A2 learning between a tone and sucrose when associatively-activated representations of those two events were presented jointly, in the absence of either of those events themselves. In the first phase, rats in Group PP received one visual CS (light1) paired with a tone, and a second visual CS (light2) paired with sucrose (see Table 1). In the second phase, these rats received light1→ light2 pairings, which might be expected to produce A2-state pairings of tone and sucrose. Control rats received training designed to preclude such pairings of A2-state events but which equated presentations of the four overt (A1-state) events. These rats received light2-sucrose pairings, and either light1→ tone (Group PU) or light1→ light2 (Group UP) pairings, but not both. Finally, the rats’ responding to the tone was evaluated by examining responding to the tone alone, and the rate of acquisition of tone-sucrose learning with explicit tone-sucrose pairings. If the A2-state pairings of tone and sucrose established excitatory associations between those events, then responding to the tone should be initially higher in Group PP than in the other groups, and the acquisition of CRs with tone-sucrose pairings in that group would be faster (i.e., show savings). By contrast, if these A2-state pairings established inhibitory associations between tone and sucrose, then little or no initial responding to the tone would be anticipated in any of the groups, and rats in Group PP would show slower tone-sucrose learning (retardation) relative to the control groups.
Table 1.
Outline of procedures of experiments 1–3
| Experiment 1 | ||||
|---|---|---|---|---|
| Group | Phase 1 | Phase 2 | Test | |
| PP | Light1→tone Light2→sucrose |
Light1→light2 | tone? tone→sucrose | |
| PU | Light1→tone Light2→sucrose |
Light1, light2 | tone? tone→sucrose | |
| UP | Light1, tone Light2→sucrose |
Light1→light2 | tone? tone→sucrose | |
| Experiment 2 | ||||
| Group | Phase 1 | Phase 2 | Phase 3 | Test |
| PP-No | Light1→tone Light2→sucrose |
Light1→light2 | nothing | tone? tone→sucrose |
| PP-L1 | Light1→tone Light2→sucrose |
Light1→light2 | Light1- | tone? tone→sucrose |
| PP-L2 | Light1→tone Light2→sucrose |
Light1→light2 | Light2- | tone? tone→sucrose |
| PU-No | Light1→tone Light2→sucrose |
Light1, light2 | nothing | tone? tone→sucrose |
| Experiment 3 | ||||
| Group | Phase 1 | Phase 2 | Test | |
| Simultaneous | Light1→tone Light2→sucrose |
Light1+light2 | tone? tone→sucrose | |
| Backward | Light1→tone Light2→sucrose |
Light2→light1 | tone? tone→sucrose | |
| Unpaired | Light1→tone Light2→sucrose |
Light1, light2 | tone? tone→sucrose | |
Notes. In Experiments 1 and 2 the group labels refer to whether the critical events in Phases 1 (first letter) and 2 (second letter) were paired (P) or unpaired (U); in Experiment 2 the final group labels refer to the events that were repeatedly presented in Phase 3 (light1, light2, or no event).
Methods
Subjects
The subjects were 32 male albino rats (CD strain; Charles River Laboratories, Raleigh, North Carolina), which weighed 300–325 g when they arrived in the laboratory vivarium. The rats were maintained for 1 week with ad lib access to food and water in individual cages before they were deprived to 85% of their ad lib weights by limiting their access to food to a single daily meal. The vivarium was illuminated from 6 a.m. to 8 p.m.
Apparatus
The behavioral training apparatus consisted of eight individual chambers (22.9 × 20.3 × 20.3 cm) with aluminum front and back walls, clear acrylic sides and top, and a floor made of 0.48-cm stainless steel rods spaced 1.9 cm apart. A dimly illuminated liquid cup was recessed in the center of one end wall. A 6-w lamp was mounted behind a jeweled lens on the front panel, 10 cm above the food cup; illumination of this lamp served as the panel light CS. An infrared photocell placed just inside the food cup and an infrared motion detector (Coulbourn model EH-61) placed on the top of the chamber were polled (1 kHz) by computer circuitry to measure food cup entry and general activity, respectively. Each chamber was enclosed in a sound-resistant shell. A 6-w lamp (which served as the house light CS), and a speaker for delivering a 1,500-hz, 78-db tone CS, were mounted on the inside wall of the shell, 10 cm above the experimental chamber and even with the end wall opposite the food. Each of the shells was enclosed in another sound-resistant shell; ventilation fans mounted on the outer shells provided masking noise (70 dB).
Training procedures
The rats were first trained to drink from the recessed liquid cup in two 64-min sessions, each of which included 16 deliveries of the US, 0.3 ml of 0.2 M sucrose solution. In each of the 10 64-min daily sessions of Phase 1, the rats received four 10-s presentations of light2 (intermittent [4 hz] house light or steady panel light, counterbalanced) followed immediately by sucrose, and 4 10-s presentations each of light1 (steady panel light or intermittent house light) and the tone. In Groups PP and PU (ns = 12), light1 and the tone were paired, with tone onset following immediately on light1 termination. In Group UP (n=8) those stimuli were explicitly unpaired. Next, in each of two daily 64-min sessions, the rats received eight 10-s presentations each of light1 and light2. In Group PP and UP, the two light CSs were paired (light1→ light2), whereas in Group PU, they were explicitly unpaired. Finally, the rats received four 64-min test sessions, each with 8 10-s tone presentations, to evaluate the formation of tone-sucrose associations. In the first of these sessions, the tone presentations were nonreinforced, and in the remaining three sessions each tone presentation was followed immediately by sucrose.
Response measures
The primary response measure was the percentage of time the rat spent with its head in the food cup during the last 5 s of each CS, and during the 5-s empty interval immediately before each CS, as indicated by disruption of the photocell beam. Previous data (Holland, 1977) show that food cup behaviors during 10-s CSs are more prevalent and less variable in the last 5 seconds of the cue. In previous studies (e.g., Wheeler, Sherwood, & Holland, 2008), to reduce intra-group variability we constructed elevation scores by subtracting a rat’s pre-CS times from its during-CS times. However, this derived measure is less appropriate for the current experiments because we occasionally found significant between-group differences in pre-CS responding.
Results
Phase 1
The rats in all groups acquired food cup behavior to the reinforced light2, but after initial generalization, showed considerably less responding during the nonreinforced light1. Table 2 shows responding in the final session of Phase 1. A group X cue X sessions (session 1 was excluded due to a recording failure) ANOVA showed significant main effects of cue, F(1, 29) = 129.73, p < 0.001, and session, F(8, 232) = 3.18, p = 0.002, and a significant cue X session interaction, F(8, 232) = 13.45, p < 0.001, reflecting discrimination learning. However, the 3-way interaction, which would indicate differences among the groups in discrimination learning, was not significant, F < 1.
Table 2.
Mean (±SEM) Percentage time in liquid cup in the final session of Phases 1 and 2 of Experiments 1–3
| Phase 1 | Phase 2 | ||||||
|---|---|---|---|---|---|---|---|
| Group | light1 | light2 | tone | pre-CS | light1 | light2 | pre-CS |
| Experiment 1 | |||||||
| PP | 12.7±5.1 | 37.8±4.6 | 10.6±5.7 | 4.3±2.0 | 4.1±1.1 | 16.6±2.6 | 3.5±1.6 |
| PU | 6.2±1.8 | 36.5±6.6 | 12.0±3.6 | 6.1±2.0 | 2.1±1.0 | 21.2±4.1 | 4.2±1.6 |
| UP | 10.4±4.4 | 30.3±5.3 | 9.8±3.5 | 3.9±1.7 | 1.6±1.2 | 15.7±3.9 | 2.4±1.6 |
| Experiment 2 | |||||||
| PP-L1 | 2.1±1.0 | 28.6±7.1 | 8.3±2.2 | 2.8±1.3 | 2.1±1.7 | 10.0±2.8 | 1.7±1.6 |
| PP-L2 | 4.2±1.6 | 37.1±5.9 | 10.6±3.4 | 3.7±3.0 | 2.2±1.4 | 10.6±3.1 | 0.9±0.3 |
| PP-No | 7.2±3.5 | 36.7±6.7 | 17.1±5.2 | 11.5±8.8 | 1.6±0.7 | 14.4±4.4 | 5.0±2.2 |
| PU-No | 2.2±0.7 | 32.1±2.9 | 7.9±1.2 | 3.0±1.1 | 1.7±0.7 | 15.6±1.3 | 1.4±0.7 |
| Experiment 3 | |||||||
| Simultaneous | 8.6±3.3 | 35.6±7.4 | 11.6±5.9 | 12.8±3.9 | 9.8±1.5* | 9.8±1.5* | 5.4±1.4 |
| Backward | 5.9±4.4 | 32.7±5.3 | 13.7±4.2 | 8.6±5.9 | 10.7±1.4 | 18.2±1.9 | 5.6±1.2 |
| Unpaired | 9.6±3.5 | 34.8±6.7 | 10.0±3.9 | 13.1±5.4 | 4.6±1.0 | 18.2±1.7 | 4.3±0.6 |
Note.
For ease of comparison, in Experiment 3, Phase 2 light1 and light2 entries for Group Simultaneous both refer to responding evoked by the simultaneous light1+light2 compound.
Through much of Phase 1, the rats in Groups PP and PU displayed significantly more responding to the tone (which followed light1 in those groups) than in the rats in Group UP (in which the tone and light1 were unpaired). It is likely that this responding (which occurred mostly during the first 5-s of the 10-s tone) reflected carryover of generalized responding controlled by light1. However, the differences between groups declined steadily over the course of Phase 1, and were insignificant, F<1, on the final session (Table 2). Finally, pre-CS responding in Phase 1 (Table 2) did not differ among the groups; a group X sessions ANOVA showed only a significant effect of sessions, F(8, 232) = 3.16, p = 0.002; other ps > 0.17.
Phase 2
Although responding to light2 declined over the course of the two nonreinforced Phase 2 sessions, the light2 vs light1 discrimination was maintained in all groups (Table 2). A group X cue X sessions ANOVA showed a significant effect of cue, F(2, 29) = 55.79, p < 0.001, and sessions, F(1, 29) = 11.82, p = 0.002. Except for the cue X session interaction, which approached significance, F(1, 29) = 3.21, p = 0.083, none of the other effects or interactions was close to significance, Fs < 1.
It is worth noting is passing that light1→ light2 pairings received by Groups PP and UP in phase 2 constituted a second-order conditioning procedure, contrasting with the unpaired light1 and light2 presentations received by Group PU. A corresponding planned comparison of food cup responding to light1 in Groups PP and UP with that in Group PU was not significant, p > 0.50. However, activity counts/min, recorded by the activity monitors, were higher in Groups PP (44.2 ± 5.5) and Groups UP (43.3 ± 7.3) than in Group PU (27.1 ± 5.8). A group X sessions ANOVA of activity to light1 showed a significant effect of group, F(2, 29) = 3.54, p = 0.042, and the aforementioned contrast was significant, F(1, 29) = 6.88, p = 0.014. Finally, groups X sessions ANOVAs of pre-CS responding in Phase 2 showed no significant effects or interactions for either food cup (Table 2) or activity measure, ps > 0.18. Mean (± SEM) pre CS activity counts/min were 20.0 ± 4.2, 17.0 ±3.6, and 14.0 ± 4.4 in Groups PP, PU, and UP, respectively.
Test
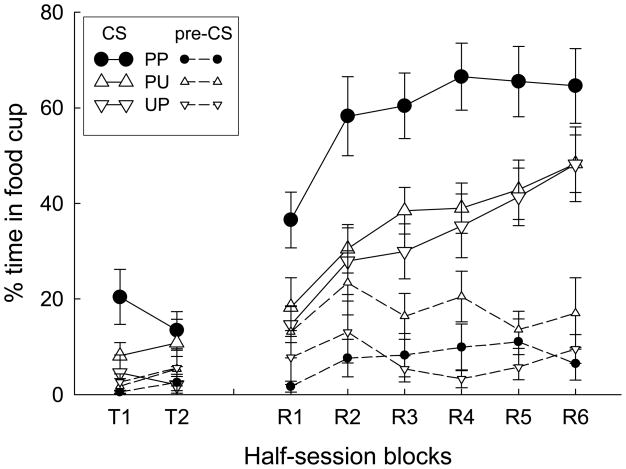
Figure 1 shows the results of the tone CS test sessions. In both the initial, nonreinforced test and the more extended reinforced test, responding to the tone was higher in Group PP than in either of the other two groups. Notably, in Group PP, an associate of the tone (light1) had previously been paired with an associate of sucrose (light2). By contrast, in Group PU the tone surrogate (light1) had been explicitly unpaired with the sucrose surrogate, and in Group UP, light1 was not associated with the tone, and so again, a tone surrogate had not been paired with a sucrose surrogate prior to the test. Thus, these results demonstrate the formation of excitatory associations between two events as a result of pairings of their associatively-activated representations.
Figure 1.
Mean (±SEM) percentage time in the food cup during the tone conditioned stimulus (CS) and pre-CS periods in the nonreinforced (labeled T1 and T2) and the reinforced (labeled R1-R6) test sessions of Experiment 1.
A group X block ANOVA of food cup responding during the tone in the nonreinforced test showed a significant effect of group, F(2, 27) = 3.36, p = 0.050. Planned contrasts showed that responding of Group PP was significantly higher than responding in each of the other groups, ps < 0.049, which did not differ, ps > 0.27. Pre-CS scores did not differ across groups, F < 1. (A food cup photocell in one chamber failed and so data from one rat in Group PP and one rat in Group UP were lost for this test only.)
A group X session blocks ANOVA of food cup responding during the tone CS in the reinforced test sessions showed significant effects of both group, F(2, 29) = 7.13, p = 0.003, and sessions, F(5, 145) = 19.11, p < 0.001, and no interaction, F < 1. Planned comparisons showed that Group PP displayed significantly more responding than either of the other two groups, ps < 0.004, which did not differ from each other, p = 0.567.
A comparable ANOVA of pre-CS responding also showed a significant effect of group, F(2, 29) = 3.70, p = 0.037; other Fs < 1. Post-hoc HSD comparisons among the groups revealed no significant differences (ps > 0.05), but planned comparisons, as performed with the during-CS data, showed that Group PU had higher pre-CS responding than each of the other two groups, ps < 0.038, which did not differ, p = 0.996. Importantly, the pattern of between-group differences in pre-CS responding was different from that observed in responding during the CSs. Thus those CS-dependent differences are not attributable to the pre-CS differences observed.
Discussion
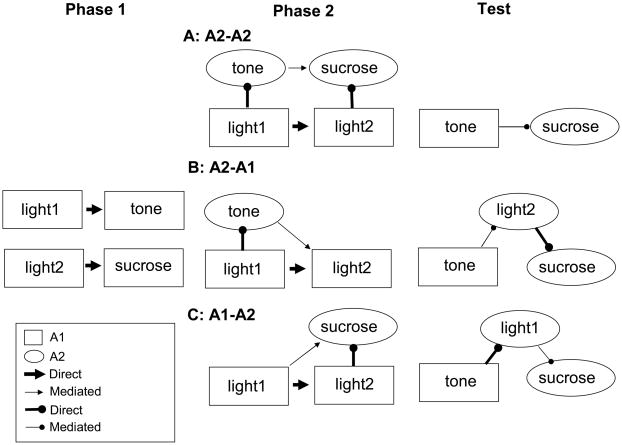
Rats in Group PP, which received pairings of two visual cues, one associated with a tone and the other associated with sucrose, displayed evidence for tone-sucrose learning. In both a simple assessment of responding to nonreinforced tones and in a reinforced savings test, these rats showed more conditioned responding than rats in two control conditions. Our interpretation of this result is that excitatory associations were acquired between two events when associatively-activated representations of them were paired (illustrated in Figure 2a). Thus, in this experiment A2-A2 learning was excitatory, consistent with Dwyer’s (2003) data, but contrasting with the inhibitory A2-A2 learning observed by Holland and Forbes (1982) and Holland and Ross (1981).
Figure 2.
Cartoon of “A2-A2” (Panel A), “A2-A1” (Panel B) and “A1-A2” (Panel C) accounts for the results of Experiment 1, which were evaluated in Experiment 2. See text for details.
However, these data may also be interpreted by assuming combinations of mediated learning and performance other than A2-A2 learning. For example, a combination of A2-A1 mediated learning in Phase 2 and representation-mediated performance in the test phase could also yield greater test responding in Group PP. By this account (Figure 2b), because light1 was paired with the tone in Phase 1, in Phase 2 light1 will activate a representation of the tone, which is then followed by presentation of light2. As a result of these Phase 2 pairings, the rats will form tone-light2 associations via excitatory A2-A1 learning (e.g., Holland, 1981). In test, the tone activates a representation of light2, which in turn activates a representation of sucrose (because of Phase 1 light2-sucrose) pairings. This light2-mediated activation of the sucrose representation produces food cup behavior. Similarly, the outcomes of Experiment 1 could result from the combination of bidirectional learning in Phase 1, A1-A2 learning in Phase 2, and representation-mediated performance in the test phase. By this account (Figure 2c), in Phase 2, light1 is associated with a representation of sucrose that is associatively-activated by light2. Then, in test the tone activates a representation of light1 (via its bidirectional associations with light1 established in Phase 1), which in turn activates a representation of sucrose. Experiment 2 evaluated the possible contributions of the A1-A2 or A2-A1 learning processes specified within these other accounts.
Experiment 2
To examine the contribution of A1-A2 or A2-A1 learning to the effects observed in Group PP of Experiment 1, in Experiment 2 we examined the effects of repeated exposure to either light1 or light2 prior to testing of responding to the tone. If responding to the tone occurred in test because of tone-sucrose associations established by A2-A2 learning in Phase 2 (Figure 2a), then that test responding should be independent of the status of the light1-tone or light2-food associations established in Phase 1. Extinction of these associations should however disrupt performance generated by the A1-A2 or A2-A1 accounts described earlier.
Three groups of rats received training identical to that of Group PP of Experiment 1, and a fourth group received control training identical to that of Group PU of Experiment 1. Then the rats in Group PP-L1 received repeated exposure to either light1 (Group PP-L1), light2 (Group PP-L2) or no cue (Groups PP-No and PU-No), before testing as in Experiment 1.
Comparison of the performances of Groups PP-No and PP-L2 would evaluate the possible contribution of A2-A1 tone-light2 learning in Phase 2 to test performance. If responding to the tone was enhanced in test because the tone activated a representation of light2, which then activated a representation of sucrose (Figure 2b), then extinction of light2’s ability to activate that sucrose representation in Group PP-L2 prior to the tone test should reduce responding to the tone. Likewise, comparison of the performances of Groups PP-No and PP-L1 would evaluate a possible role for A1-A2 light1-sucrose learning in Phase 2 in generating test performance. If responding to the tone was enhanced in test because the tone activated a representation of light1, which then activated a representation of food (Figure 2c), then extinction of L1’s ability to activate a sucrose representation in Group PP-L1 prior to the tone test should reduce responding to the tone.
Methods
Subjects and apparatus
The subjects were 32 male Sprague-Dawley rats similar to those that participated in Experiment 1. They were maintained as in Experiment 1. The apparatus was that used in Experiment 1.
Procedures
The procedures of Experiment 2 were identical to those of Experiment 1, except that (1) Group UP was omitted, (2) all rats received 5 64-min nonreinforced exposure sessions between Phase 2 and the test phase, and (3) Group PP was subdivided into three groups that received nonreinforced exposure to light1, light2, or the experimental context alone prior to testing responding to the tone. In each of the five nonreinforced exposure sessions, the rats in Group PP-L1 (n = 8) received 16 presentations of light1 (panel or house light, counterbalanced), the rats in Group PP-L2 (n = 8) received 16 presentations of light2, and the rats in Group PP-No (n = 8) and Group PU-No (n = 8) received no explicit event representations.
Results
Training phases
Phases 1 and 2 proceeded as in Experiment 1. Table 2 shows responding on the final session of each phase. In Phase 1, all rats acquired moderate levels of responding to light2, but after a period of generalization, reduced responding to light1. Furthermore, responding to the tone first increased and then decreased over training, probably reflecting carryover of generalized conditioned to light1, which preceded tone presentations in all groups. There was an insignificant trend for this responding to be higher in Group PP-No than in the other groups. In Phase 2, the discrimination between light1 and light2 was maintained in all groups. Finally, in the extinction phase, responding to light2 in Group PP-L2 extinguished rapidly, whereas the already low levels of responding to light1 were unchanged in Group PP-L1.
A group X cue X sessions ANOVA of phase 1 responding to the two visual cues showed significant main effects of cue, F(1, 28) = 129.19, p < 0.001, and sessions, F(9, 252) = 13.49, p < 0.001, and a significant interaction of those variables, F(9, 252) = 20.75, p < 0.001. However, there was no main effect of group, and no three-way interaction of group with cue and session, Fs < 1. Thus, discrimination learning was similar across groups. A group X session ANOVA of responding during the tone showed a significant effect of sessions, Fs(9, 252) = 11.00, p < 0.001, but insignificant group, F(3, 28) = 2.40, p = 0.089, and group X sessions interaction, F(27, 252) = 1.11, p = 0.33, effects. ANOVA of pre-CS responding showed an insignificant effect of group, F(3, 28) = 2.53, p = 0.078, but a significant effect of session, F(9, 252) = 2.16, p = 0.025, and a significant group X session interaction, F(27, 252) = 1.60, p = 0.034. This interaction was primarily due to maintained pre-CS responding in Group PP-No over sessions, while the other groups’ pre-CS responding declined.
A group X cue X sessions ANOVA of responding to the two visual stimuli in Phase 2 showed significant effects of cue, F(1, 28) = 99.55, p < 0.001, and session, F(1, 28) = 29.78, p < 0.001, and a significant cue X session interaction, F(1, 28) = 5.81, p = 0.023. There was no main effect of group, F < 1, and that variable did not interact significantly with any other variable, ps > 0.100. A group X cue X session ANOVA of pre-CS responding showed no significant effects or interactions, although the effect of Group approached significance, F(3, 28) = 2.34, p = 0.095, reflecting a slight nonsignficant superiority of Group PP-No. Finally, a group (group PP-L1 and group PP-L2 only) X sessions ANOVA of responding in Phase 3 extinction showed significant effects of group, F(1, 14) = 4.93, p = 0.043, and sessions, F(4, 56) = 5.20, p = 0.001, as well as a significant group X sessions interaction, F(4, 56) = 6.46, p < 0.001. The results of this ANOVA confirm the impression that responding to light2 extinguished over sessions (to 1.6 ± 1.1%) whereas responding to light1 was maintained at its already-low level (0.9 ± 0.5%).
Test
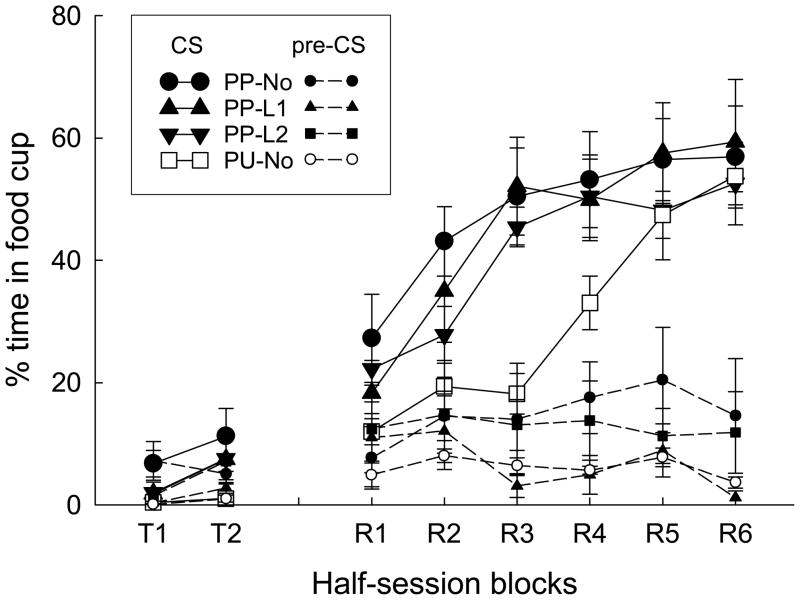
As in Experiment 1, rats that presumably received pairings of associatively-activated representations of tone and sucrose in Phase 2 (PP conditions) showed more responding than control (Group PU) rats in both a nonreinforced test and a reinforced savings test (Figure 3). Furthermore, repeated nonreinforced presentation of either light1 or light2 had no significant effects on test responding to the tone (Figure 3). Thus, there was no evidence for contributions of the A1-A2 or A2-A1 learning mechanisms described earlier (Figure 2).
Figure 3.
Mean (±SEM) percentage time in the food cup during the tone conditioned stimulus (CS) and pre-CS periods in the nonreinforced (labeled T1 and T2) and the reinforced (labeled R1-R6) test sessions of Experiment 2.
A group X block ANOVA of responding in the nonreinforced test session showed main effects of group, F(3, 28) = 3.46, p = 0.030, and block, F(1, 28) = 4.27, p = 0.048, and no interaction, F < 1. A planned contrast showed that the three PP-subgroups together displayed more responding than the PU-No (control) rats, F(1, 28) = 6.68, p = 0.015, although the only significant individual group comparison was that between groups PP-No and PU-No, p = 0.003; others ps > 0.102. Comparable ANOVA of pre-CS responding revealed no significant effects, ps > 0.160.
A group X blocks ANOVA of food cup responding showed significant effects of block, F(5, 140) = 40.78, p < 0.001, and a group X block interaction, F(15, 140) = 1.97, p = 0.022. Because food cup responding in the reinforced test sessions reached asymptote more quickly in this study than in Experiment 1, we also performed an ANOVA confined to the first 4 half-session blocks. This ANOVA showed significant effects of group, F(3, 28) = 4.70, p = 0.009, and blocks, F(3, 84) = 31.75, p < 0.001, and no interaction, F(9, 84) = 1.68, p = 0.107. Planned comparisons showed that responding in each PP subgroup differed significantly from responding in the control (PU) group, ps < 0.020; no other differences were significant, ps > 0.301. ANOVA of pre-CS responding showed no significant main effects or interactions, Fs < 1.
Discussion
The results of Experiment 2 replicated those of Experiment 1. Rats that received pairings of associates of a tone and sucrose displayed evidence of excitatory tone-sucrose learning. Furthermore, neither light1 (associated with the tone) nor light2 (associated with sucrose) presentations after light1-light2 pairings had any significant effect on responding to the tone in test. Thus, accounts of Experiment 1’s findings that involved A1-A2 or A2-A1 excitatory learning and mediation by representations of light1 or light2 at the time of test were not supported.
On the other hand, our failure to find effects of repeated light1 or light2 presentations does not unequivocally rule out such mechanisms. Although in many circumstances, post-training extinction of one element of an associated pair of stimuli reduces responding to the other (e.g., Rescorla, 1980a,b; Holland, 1985; Holland & Ross, 1981; Rizley & Rescorla, 1972), in other cases it does not (e.g. Holland & Rescorla, 1975; Rescorla, 1980a; Rizley & Rescorla, 1972). However, it is worth noting that failures to observe these extinction effects are typically attributed to the formation of S-R associations of one cue with the emotional response to the other cue, rather than of associations that enable the cue to activate a rich stimulus representation of the other cue. In that case, all of the various alternative mediated-learning accounts would be moot. Thus, we believe the post-training extinction manipulations were appropriate tests of the alternative accounts portrayed in Figure 2.
Experiment 3
In agreement with Dwyer’s (2003) results, in Experiments 1 and 2 we found evidence for excitatory learning between two associatively-activated event representations. As noted in the Introduction, using sensory preconditioning procedures, Wheeler et al. (2008) found both excitatory and inhibitory learning between two such representations, as a function of the temporal arrangement of the cues that activated them. Excitatory associations were formed between noise and tone CSs when an associate of the noise preceded an associate of the tone, but inhibitory noise-tone associations were formed when those associates were presented simultaneously or in the opposite (backward) order.
In Experiment 3, we used the procedures of Experiment 1 to examine the consequences of simultaneous and backward arrangements of light1 and light2 in Phase 2 for the formation of associations between tone and sucrose. If these arrangements yielded inhibitory A2-A2 learning, that is, inhibitory tone-sucrose associations, then responding to the tone in the reinforced tone-sucrose test sessions should be acquired more slowly after this training than after control training, in which light1 and light2 were unpaired in Phase 2. Thus, in Experiment 3, the reinforced test could be construed as a retardation test for inhibition rather than as a savings test for excitation, as in Experiments 1 and 2.
Methods
Subjects and apparatus
The subjects were 24 male Sprague-Dawley rats obtained and maintained as in Experiments 1 and 2. The apparatus was the same as that used in Experiments 1 and 2.
Procedures
The procedures and stimuli were identical to those of Experiment 1 with two exceptions. First, Group UP was omitted; rats that received the same training as rats in Group PU in Experiment 1 served as the sole control group (Group Unpaired, n = 8). Second, Group PP of Experiment 1 (which received light1→ light2 pairings in Phase 2) was replaced by two groups, Group Backward (n = 8), which received 8 light2→ light1 pairings in each Phase 2 session, and Group Simultaneous (n = 8), which received 8 presentations of a 10-s simultaneous light1+light2 compound (steady panel light and intermittent house light) in each Phase 2 session.
Results
Training phases
Phase 1 proceeded as in Experiments 1 and 2 (Table 1). In Phase 2, rats received light1 and light2 in the order light2→ light1, simultaneously, or on separate trials. Responding to the simultaneous compound was lower than responding to light2 in the other two groups, suggesting generalization decrement, but higher than responding to light1 alone in those groups, indicating that light2 at least in part maintained its identity when compounded with light1.
In Phase 2 (Table 2), responding to light2 was maintained in the two groups in which that element was presented individually (Backward and Unpaired), but was suppressed somewhat in Group Simultaneous, in which it was presented simultaneously with light1. A group X sessions ANOVA of responding to light2 or the light1+light2 compound showed a significant effect of group, F(2,21) = 9.33, p = 0.001. Post-hoc HSD tests showed that responding to the compound visual cue in Group Simultaneous was lower than responding to light2 in each of the other two groups, ps < 0.002. A comparable ANOVA for light1 or light1+light2 compound responding also showed a significant effect of group, F(2,21) = 7.87, p = 0.003. Responding to the compound in Group Simultaneous was higher than responding to light1 in Group Unpaired, p = 0.005, but similar to that elicited by light1 in Group Backward. It is likely that this level of responding to light1 in Group Backward was due to carryover of responding elicited by light2 (recall that in Phase 1 sucrose was delivered immediately after light2 presentation, during the time interval occupied by light1 in Phase 2). Pre-CS responding did not differ among the groups, F < 1.
Test
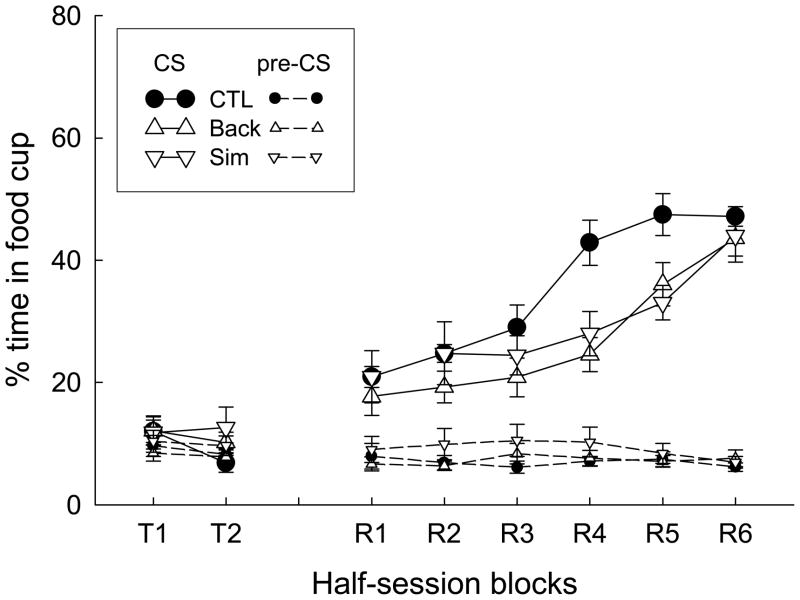
Essentially no responding over and above pre-CS responding was observed in the nonreinforced test session (Figure 4). In the reinforced test, the acquisition of responding to the tone was significantly slower in Groups Simultaneous and Backward than in Group Unpaired. This outcome is consistent with the assertion that simultaneous or backward pairings of two associatively-activated event representations produce inhibitory associations between the represented events.
Figure 4.
Mean (±SEM) percentage time in the food cup during the tone conditioned stimulus (CS) and pre-CS periods in the nonreinforced (labeled T1 and T2) and the reinforced (labeled R1-R6) test sessions of Experiment 3.
A group X block ANOVA of responding in the nonreinforced test session showed a main effect of block, F(1, 21) = 4.57, p = 0.044, but no effect of group, F<1. A group X block ANOVA of responding during the tone in the reinforced test showed significant effects of block, F(5, 105) = 93.21, p < 0.001, and a significant group X block interaction, F(10, 105) = 4.77, p < 0.001. An analysis of trends within that interaction showed significant differences between the quadratic trend in Group Unpaired with that in each of the other two groups, Fs (1, 21) > 8.80, ps < 0.008, and responding in Group Unpaired was higher than responding in each of the other two groups in blocks 4 and 5, ps < 0.02. Pre-CS responding did not differ between groups in either the nonreinforced or reinforced tests, Fs < 1.
Discussion
Unlike in Experiments 1 and 2, in which we found excitatory A2-A2 learning after forward pairings of CS and US surrogates, here we found evidence for inhibitory tone-sucrose learning with both simultaneous and backward pairings of surrogates of those events. In the backward condition, this outcome is consistent with the results of many experiments with directly-presented events; backward pairings of a CS and US typically produce inhibitory CS-US associations (e.g., Chang, Blaisdell, & Miller, 2003; Cole & Miller, 1999; Delamater, Lolordo, & Sosa, 2003; Heth, 1976; Williams & Overmier, 1988). Although simultaneous presentations of CSs and USs have often been found to yield excitatory learning (e.g., Heth, 1976), some studies have observed inhibitory learning (e.g., Heth, 1976; Moscovitch & LoLordo, 1968). Most important, our observation of inhibitory learning when two event representations are simultaneously activated into the A2 sate is consistent with the observations of Wheeler et al. (2008). Those authors, using sensory preconditioning procedures, also found inhibitory A2-A2 learning after simultaneous or backward pairings of surrogate events.
It might be argued that generalization between the two visual stimuli in Phase 1 may have provided the opportunity for inhibitory A1-A2 tone-sucrose learning in that phase. To the extent that light1 activated a representation of sucrose via generalization from light2, light1→ tone pairings in Phase 1 may have produced inhibition to the tone by contrast with reinforcement. Stimuli presented at the time an expected US is omitted are frequently found to acquire conditioned inhibition (e.g. LoLordo, 1969). However, in Experiment 3 all three groups had equal opportunity for such learning, but both Groups Simultaneous and Backward showed more evidence for inhibition than Group PU. Furthermore, it is notable that in Experiment 1, Group PU showed no evidence for slower acquisition of tone-sucrose associations than Group UP, which did not receive light1→ tone pairings. Thus it seems unlikely that inhibitory tone-sucrose associations were acquired as a result of Phase 1 training alone in any of these studies. Thus, the results of Experiment 3 support the view that inhibitory associations may be formed between two events when their associatively-activated representations are presented in appropriate temporal arrangements.
General Discussion
The results of these three experiments join a range of previous findings (e.g., Dwyer, 2003; Hall, 1996; Holland, 1990; Holland & Wheeler, 2007) that indicate that animals can learn about absent events, when previously-trained associates of those events serve as their surrogates. Two aspects of these results are more novel. First, associations were learned between two events when neither was actually present during the learning trials (A2-A2 learning). Tone-sucrose associations were formed when a visual cue that had been paired with the tone was paired with a visual cue that had been paired with sucrose. Second, the nature of the associations formed between these two absent events depended on the temporal relations arranged between their associates. Excitatory tone-sucrose associations were formed when the surrogate for tone preceded the surrogate for sucrose, but inhibitory associations were formed when those surrogates were presented simultaneously or in reverse (backward) order.
Our results are consistent with suggestions of Shevill and Hall (2004). In the context of a study of retrospective revaluation, those authors also proposed that simultaneous activation of two event representation into the A2 state may produce inhibitory learning whereas forward pairings might produce excitatory learning. However, as Shevill and Hall (2004) pointed out, their results admitted of a number of other interpretations, which did not invoke mediated learning.
The present results are more directly related to those of Wheeler et al. (2008), who found similar outcomes, using surrogate cues for neutral stimuli in sensory preconditioning procedures. Whereas in the present experiments, associations between a tone CS and a sucrose US were formed when associates of those events were paired, in Wheeler et al.’s (2008) studies, associations were formed between a noise and a tone when visual associates of those cues were paired. The presence of those noise-tone associations was revealed by subsequent tone-food conditioning, followed by tests of responding to the noise, as in sensory preconditioning experiments. Analogous to the results of the present experiments, Wheeler et al. (2008) found excitatory noise-tone associations when noise and tone surrogates were presented in a forward manner, but inhibitory noise-tone associations when they were presented simultaneously or in backward order. Wheeler et al. (2008) raised the possibility that their results may have depended on their use of sensory preconditioning procedures, in which both events represented in the A2 state were of relatively neutral value. Miller and Matute (1996) suggested that the results of so-called backward blocking experiments, in which the modification of associative strengths of absent elements is also often asserted to occur, may depend on the biological significance of the events involved. The present results show that Wheeler et al.’s (2008) findings are not limited to the formation of associations between the representations of neutral events.
It is valuable to contrast our observations of inhibitory A2-A2 learning (Experiment 3 and Wheeler et al., 2008, Experiment 1) with prior reports of inhibitory A2-A2 learning (Holland & Forbes, 1982; Holland & Ross, 1981). In those prior studies, the second event (the target of the inhibition) was retrieved into the A2 state by the A2-state representation of the first event. In Holland and Forbes’s (1982) experiments, inhibitory food-illness associations were thought to be formed after a tone retrieved a representation of food into the A2 state, which in turn retrieved a representation of illness into the A2 state. Similarly, Holland and Ross (1981) found mediated extinction of tone-food learning, indicative of the acquisition of inhibitory tone-food associations, after repeated presentation of a light that had previously been paired with the tone. Presentation of the light retrieved a representation of the tone into the A2 state, which in turn retrieved a representation of food into the A2 state, resulting in inhibitory A2-A2 tone-food learning.
In both Holland and Forbes’s (1982) and Holland and Ross’s (1981) experiments, the occurrence of excitatory (rather than inhibitory) A2-A2 learning seems particularly unlikely, because it would require that an analog of a straightforward extinction procedure (presenting food in the absence of illness or tone in the absence of food) generate excitatory learning. Thus, these observations of inhibitory A2-A2 learning could be easily reconciled with Dwyer’s (1983) observations of excitatory A2-A2 learning by adding a caveat to Dickinson and Burke’s (1996) account which would prevent the occurrence of “bootstrapped” excitatory learning. That is, a CS should not be able to condition itself by virtue of activating a representation of its reinforcer and then associating itself with that reinforcer representation (but see Rorbaugh & Riccio, 1970). Indeed, Holland (1983, 2006) found considerable evidence against such bootstrapping in the context of flavor-potentiated odor aversion learning. From this perspective, A2-A2 learning would be excitatory except when the second A2 event was retrieved by the first A2 event. However, our results (Experiment 3; Wheeler et al., 2008, Experiment 1) show that simply adding such a caveat is insufficient. The inhibitory A2-A2 learning that occurred in our studies involved retrieval of two events into the A2 state by the presentation of two separate associates, which were each in the A1 state (that is, physically present), as in Dwyer’s studies.
Taken together with Wheeler et al’s (2008) results, the present data discourage blanket assertions relating event activation status to the formation of excitatory or inhibitory associations (e.g. Dickinson & Burke, 1996; Holland, 1983). These two sets of studies show that associations formed between two absent events (A2-A2 learning) may be either excitatory or inhibitory, depending on the temporal relations between the associatively-activated event representations, just as is the case with real events. Similarly, although the presentation of a CS when a US is associatively-activated but physically absent (A1-A2 learning), typically results in the formation of inhibitory CS-US associations, there are some reports of excitatory CS-US learning under those circumstances. For example, after CS1-US pairings, CS2+CS1 presentations in the absence of the US typically produce inhibitory CS2-US associations. When excitatory learning is observed to CS2 in those circumstances, as is the case in second-order conditioning, that learning is typically attributed to within-compound S2-S1 learning or the formation of associations between S2 and the emotional conditioned response to S1, rather than to CS2-US learning (e.g., Rescorla, 1980). However, some investigators (Davey & McKenna, 1983; Ward-Robinson, 2004; Winterbauer & Balleine, 2005) reported evidence for excitatory CS2-US learning in those situations, as suggested by Konorski’s (1967) account for second-order conditioning. The identification of variables that affect the nature of learning involving associatively-activated event representations will be critical for the reformulation of models of associative learning to accommodate these variations in the contents of learning. For example, one recent model (Harris, 2006), allows for both excitatory and inhibitory learning between associatively-activated CSs and USs, depending on the level of activation achieved by the US representation, either within or outside a limited-capacity attention buffer. However, it remains to be seen whether quantitative implementations of that model would embrace the present and related data.
The question of whether directly- and associatively-activated event representations follow the same rules when they participate in associative learning is one aspect of the more general issue of how directly-activated and associatively-activated representations of the same event are related. Take the case of the sucrose solution used as the US in the present studies. Presentation of sucrose itself is likely to arouse rich A1-state processing, including, for example, its sensory aspects (such as taste, temperature, and texture), its hedonic aspects, and its response aspects, such as licking. What is the nature of A2 processing of sucrose produced by the presentation of a previously-established associate, such as light2 in the present study? On the one hand, the light might activate an extremely impoverished representation of sucrose that included only, for example, its response aspects, as in traditional views of stimulus-response learning. At the other extreme, one could imagine that the light activated an exact replica of the original processing of sucrose produced by presentation of sucrose itself. Such an associatively-activated sucrose representation might include a literal re-experience (Nyberg, Habib, McIntosh, & Tulving, 2000) or hallucination (Konorski, 1967) of sucrose presentation, with all its attending sensory, motivational and response properties.
It seems unlikely that directly- and associatively-activated event representations would be indistinguishable. But it has long been known that CSs can code detailed sensory aspects of their associated reinforcers (Dickinson & Balleine, 1995; Holland, 1990; Rescorla, 1980a), and there is increasing evidence that cues instigate activity in brain systems involved in processing the absent associates of those cues. For example, in human episodic memory, recall of sensory aspects of memory for an experience is often accompanied by brain activity in the appropriate sensory-specific cortex (e.g. Gottfried, Smith, Rugge, & Dolan, 2004; Prince, Daselaan, & Cabeza, 2005; Vaidya, Zhao, Desmond, & Gabrieli, 2002; Wheeler, Petersen, & Buckner, 2000). Similary, in settings more reminiscent of associative learning studies, after presentations of an auditory→ visual sequence, presentation of the auditory cue alone can be sufficient to produce activity in areas of visual cortex (McIntosh, Cabeza, & Lobaugh, 1998). In the context of Pavlovian conditioning of rats, Kerfoot, Agarwal, Lee, and Holland (2007) found that an auditory cue previously paired with the intraoral delivery of liquid produced activity in gustatory cortex as well as brain regions implicated in processing of attractive tastes when it was presented along with unflavored water (rather than sucrose) in testing. Furthermore, in Kerfoot et al.’s (2007) study, after tone-sucrose conditioning but prior to testing, the sucrose was devalued in some rats by pairing it with illness produced by injection of lithium chloride. In addition to activity in gustatory cortex, tone→ water presentations in those rats also produced activity in brain regions normally associated with processing of aversive tastes, as if the tone caused the rats to taste the sugar when presented with plain water.
The puzzle remaining is that the same conditions that provoke CRs and other aspects of processing of absent events also establish inhibition if the anticipated event is undelivered (or otherwise overexpected). What variables determine the extent to which a novel event that accompanies such an episode forms excitatory and/or inhibitory associations with that absent event? Event timing and salience may be critical. For example, the associative activation of an outcome representation is likely to be immediate, whereas the detection of the omission of that event is likely to be both delayed and uncertain. Holland (1984, 1988; Holland & Kenmuir, 2005) noted how both the temporal dynamics of the “moment of nonreinforcement” and the salience of the omitted event could determine the occurrence of excitatory or inhibitory learning in unblocking experiments, in which a novel stimulus signaled the omission of the second element of an expected serial food→ food reinforcer. In those experiments, rats acquired net inhibitory tendencies to the novel cue when the two food events originally occurred relatively contiguously and when the omitted event was highly salient, but excitatory tendencies when they had been more widely separated in time, or the second event was less salient. It remains to be seen whether variables such as these are systematically related to the establishment of excitatory or inhibitory associations among associatively-activated event representations.
Acknowledgments
We thank Vanessa McKenna and Heather El-Amamy for their technical assistance. This research was supported in part by Grant MH65879 from the National Institutes of Health to PCH, and by a Keck Foundation summer research fellowship to AS, from Allegheny College.
References
- Chang RC, Blaisdell AP, Miller RR. Backward conditioning: Mediation by the context. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:171–183. doi: 10.1037/0097-7403.29.3.171. [DOI] [PubMed] [Google Scholar]
- Cole RP, Miller RR. Conditioned excitation and conditioned inhibition acquired through backward conditioning. Learning and Motivation. 1999;30:129–156. [Google Scholar]
- Davey GCL, McKenna I. The effects of postconditioning revaluation of CS1 and UCS following Pavlovian second-order electrodermal conditioning in humans. Quarterly Journal of Experimental Psychology. 1983;35B:125–133. doi: 10.1080/14640748308400899. [DOI] [PubMed] [Google Scholar]
- Delamater AR, LoLordo VM, Sosa W. Outcome-specific conditioned inhibition in Pavlovian backward conditioning. Learning & Behavior. 2003;31:393–402. doi: 10.3758/bf03196000. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Balleine B. Motivational control of instrumental action. Current Directions in Psychological Science. 1995;4:162–167. [Google Scholar]
- Dickinson A, Burke J. Within-compound associations mediate the retrospective revaluation of causality judgements. Quarterly Journal of Experimental Psychology B. 1996;49:60–80. doi: 10.1080/713932614. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Retrospective revaluation or mediated conditioning? The effect of different reinforcers. Quarterly Journal of Experimental Psychology B. 1999;52:289–306. doi: 10.1080/027249999393013. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Formation of a novel preference and aversion by simultaneous activation of the representations of absent cues. Behavioural Processes. 2000;48:159–164. doi: 10.1016/s0376-6357(99)00080-7. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Mediated conditioning and retrospective revaluation with LiCl then flavour pairings. The Quarterly Journal of Experimental Psychology B. 2001;54:145–165. doi: 10.1080/713932750. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Learning about cues in their absence: Evidence from flavour preferences and aversions. The Quarterly Journal of Experimental Psychology B. 2003;56:56–67. doi: 10.1080/02724990244000160. [DOI] [PubMed] [Google Scholar]
- Dwyer DM, Mackintosh NJ, Boakes RA. Simultaneous activation of the representations of absent cues results in the formation of an excitatory association between them. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:163–171. [Google Scholar]
- Gottfried JA, Smith APR, Rugg MD, Dolan RJ. Remembrance of odors past: Human olfactory cortex in cross-modal recognition memory. Neuron. 2004;42:687–695. doi: 10.1016/s0896-6273(04)00270-3. [DOI] [PubMed] [Google Scholar]
- Hall G. Learning about associatively-activated stimulus representations: Implications for acquired equivalence and perceptual learning. Animal Learning & Behavior. 1996;24:233–255. [Google Scholar]
- Harris JA. Elemental representations of stimuli in associative learning. Psychological Review. 2006;113:584–605. doi: 10.1037/0033-295X.113.3.584. [DOI] [PubMed] [Google Scholar]
- Heth CD. Simultaneous and backward fear conditioning as a function of number of CS-UCS pairings. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:117–129. doi: 10.1037//0097-7403.2.2.117. [DOI] [PubMed] [Google Scholar]
- Holland PC. Acquisition of Representation-Mediated Conditioned Food Aversions. Learning and Motivation. 1981;12:1–18. doi: 10.1016/j.lmot.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Representation-mediated overshadowing and potentiation of conditioned aversions. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:1–13. [Google Scholar]
- Holland PC. Unblocking in Pavlovian appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:476–497. [PubMed] [Google Scholar]
- Holland PC. Element pretraining influences the content of appetitive serial compound conditioning in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:367–387. [PubMed] [Google Scholar]
- Holland PC. Event representation in Pavlovian conditioning: Image and action. Cognition. 1990;37:105–131. doi: 10.1016/0010-0277(90)90020-k. [DOI] [PubMed] [Google Scholar]
- Holland PC. Limitations on representation-mediated potentiation of flavour or odour aversions. Quarterly Journal of Experimental Psychology. 2006;59:233–250. doi: 10.1080/17470210500242904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Forbes DT. Representation-mediated extinction of conditioned flavor aversions. Learning and Motivation. 1982;13:454–471. [Google Scholar]
- Holland PC, Kenmuir C. Variations in unconditioned stimulus processing in unblocking. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:155–171. doi: 10.1037/0097-7403.31.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Rescorla RA. Second order conditioning with food unconditioned stimulus. Journal of Comparative and Physiological Psychology. 1975;88:459–467. doi: 10.1037/h0076219. [DOI] [PubMed] [Google Scholar]
- Holland PC, Ross RT. Within-compound associations in serial compound conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:228–241. [Google Scholar]
- Holland PC, Wheeler DS. Representation-mediated food aversions. In: Reilly S, Schachtman T, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford: Oxford University Press; 2008. in press. [Google Scholar]
- Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: a FOS and behavioral analysis. Learning and Memory. 2007;14:581–589. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain. Chicago: University of Chicago Press; 1967. [Google Scholar]
- LoLordo VM. Positive conditioned reinforcement from aversive situations. Psychological Bulletin. 1969;72:193–203. doi: 10.1037/h0027907. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Cabeza RE, Lobaugh NJ. Analysis of neural interactions explains the activation of occipital cortex by an auditory stimulus. Journal of Neurophysiology. 1998;80:2790–2796. doi: 10.1152/jn.1998.80.5.2790. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matute H. Biological significance in forward and backward blocking: Resolution of a discrepancy between animal conditioning and human causal judgment. Journal of Experimental Psychology: General. 1996;125:370–386. doi: 10.1037//0096-3445.125.4.370. [DOI] [PubMed] [Google Scholar]
- Moscovitch AB, LoLordo VM. Role of safety in the Pavlovian backward fear conditioning procedure. Journal of Comparative and Physiological Psychology. 1968;66:673–678. doi: 10.1037/h0026548. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Habib R, McIntosh AR, Tulving E. Reactivation of encoding-related brain activity during memory retrieval. Proceedings of the National Academy of Sciences USA. 2000;97:11120–11124. doi: 10.1073/pnas.97.20.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural Correlates of Relational Memory: Successful Encoding and Retrieval of Semantic and Perceptual Associations. Journal of Neuroscience. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Effect of US habituation following conditioning. Journal of Comparative and Physiological Psychology. 1973;82:137–143. doi: 10.1037/h0033815. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian Second order conditioning: Studies in associative learning. Hillsdale, N. J: Erlbaum; 1980. [Google Scholar]
- Rescorla RA. Simultaneous and successive associations in sensory preconditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1980;6:207–216. doi: 10.1037//0097-7403.6.3.207. [DOI] [PubMed] [Google Scholar]
- Rizley R, Rescorla RA. Associations in second order conditioning and sensory preconditioning. Journal of Comparative and Physiological Psychology. 1972;81:1–11. doi: 10.1037/h0033333. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh M, Riccio DC. Paradoxical enhancement of learned fear. Journal of Abnormal Psychology. 1970;75:210–216. doi: 10.1037/h0028974. [DOI] [PubMed] [Google Scholar]
- Shevill I, Hall G. Retrospective revaluation effects in the conditioned suppression procedure. Quarterly Journal of Experimental Psychology. 2004;57B:331–347. doi: 10.1080/02724990344000178. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Zhao M, Desmond JE, Gabrieli JD. Evidence for cortical encoding specificity in episodic memory: memory-induced re-activation of picture processing areas. Neuropsychologia. 2002;40:2136–2143. doi: 10.1016/s0028-3932(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Ward-Robinson J. An analysis of second-order autoshaping. Learning and Motivation. 2004;35:1–21. [Google Scholar]
- Wheeler DS, Sherwood A, Holland PC. Excitatory and inhibitory learning with absent stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 2008 doi: 10.1037/0097-7403.34.2.247. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory’s echo: vivid remembering reactivates sensory-specific cortex. Proceedings of the National Academy of Sciences USA. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA, Overmier JB. Backward inhibitory conditioning with signaled and unsignaled unconditioned stimuli: Distribution of trials across days and intertrial interval. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:26–35. [Google Scholar]
- Winterbauer NE, Balleine BW. Motivational control of second-order conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:334–340. doi: 10.1037/0097-7403.31.3.334. [DOI] [PubMed] [Google Scholar]