Abstract
NF-κB comprises a family of transcription factors that are critically involved in various inflammatory processes. In this paper, the role of NF-κB in inflammation and atherosclerosis and the regulation of the NF-κB signaling pathway are summarized. The structure, function, and regulation of the NF-κB inhibitors, IκBα and IκBβ, are reviewed. The regulation of NF-κB activity by glucocorticoid receptor (GR) signaling and IκBα sumoylation is also discussed. This paper focuses on the recently reported regulatory function that adipocyte enhancer-binding protein 1 (AEBP1) exerts on NF-κB transcriptional activity in macrophages, in which AEBP1 manifests itself as a potent modulator of NF-κB via physical interaction with IκBα and a critical mediator of inflammation. Finally, we summarize the regulatory roles that recently identified IκBα-interacting proteins play in NF-κB signaling. Based on its proinflammatory roles in macrophages, AEBP1 is anticipated to serve as a therapeutic target towards the treatment of various inflammatory conditions and disorders.
1. NF-κB Signaling Pathway
The ability to sense external stimuli that could be lethal to cells coupled with the potential to respond to such cytotoxic signals by switching on defensive genes to sustain cell growth and survival is a remarkable facet of nuclear factor kappa B (NF-κB). Since NF-κB is ubiquitously expressed in almost all types of cells and is a transcription factor that is sequestered in an inactive state in the cytosol but can become activated by a wide range of diverse internal and external stimuli, NF-κB has long been considered an ideal safeguard to defend the cell against countless stimuli and maintain its homeostasis [1]. Moreover, NF-κB is a unique transcription factor in that its function is not solely dependent on its expression when needed. Rather, NF-κB is constitutively expressed in the cell, but it does not become active until it is called upon for action, in which it will be ready and its mission can be accomplished in a timely regulated fashion.
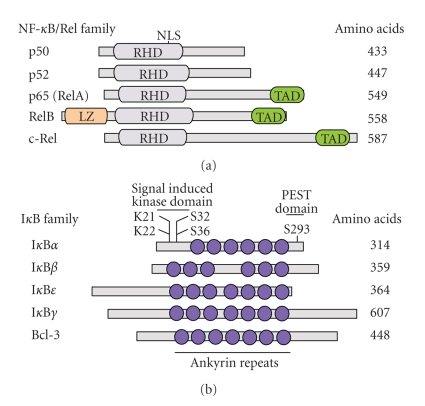
NF-κB comprises a family of ubiquitously expressed, eukaryotic transcription factors that participate in the regulation of multiple immediate genes that are expressed at the onset of many vital biological processes such as cell growth, immunoregulation, apoptosis, and inflammation [2, 3]. Modulation of NF-κB activity can lead to many abnormal cellular processes and diseases including asthma, arthritis, atherosclerosis, obesity, and various types of cancers [2–7]. NF-κB exists in cells as a heterodimer of members of the Rel family of proteins, including p50, p52, p65 (RelA), RelB, and c-Rel, which share a high degree of structural similarity (Figure 1).
Figure 1.
Structural Organization of NF-κB/Rel and IκB Proteins. (a) A schematic representation of some members of the Rel family of proteins. Members of this family contain a unique, highly conserved Rel homology domain (RHD) towards the N-terminus, and this domain carries a nuclear localization signal (NLS). Most members of the Rel family contain a C-terminally located transactivation domain (TAD) that is important for optimal transcriptional activity. RelB is a structurally unique member of the NF-κB protein family in that it contains a leucine zipper-like (LZ) region at its N-terminus. (b) A schematic representation of some members of the IκB family of proteins, which are uniquely characterized by the presence of 30-33-amino acid ankyrin (ANK) repeats. At least for IκBα and IκBβ the most well-characterized members of the IκB family, there are two conserved serine residues at the N-terminus preceding the first ANK repeat. Phosphorylation of these two serine residues is known to be crucial for signaling IκB proteins for ubiquitination and proteolytic degradation. At the C-terminus of IκB proteins, there is a region rich with proline, glutamate, serine, and threonine residues, and hence, it is named the PEST domain. The number of amino acid residues within the indicated proteins in mouse is shown.
2. Roles of NF-κB in Inflammation and Atherosclerosis
One of the major functions of NF-κB is its key involvement in inducing an effective immune/inflammatory response against viral and bacterial infections. The importance of NF-κB role in initiating a potent inflammatory response cannot be better signified than recognizing that the κB consensus sequence is found in the promoter/enhancer regions of more than 50 diverse genes whose expression is known to be crucial in driving an inflammatory response [8–10]. Inducible genes that are known to be transactivated by NF-κB include, but are not limited to, IL-1β, IL-6, IL-8, TNFα, IFNγ, MCP-1, iNOS, COX-2, intracellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) [2, 3, 10–19]. These molecules play critical roles in key biological events involving cell recruitment, attachment, differentiation, proliferation, and activation constituting an active inflammatory response. NF-κB is also known to cooperate with other active transcription factors such as activator protein-1 (AP-1) in upregulating the expression of some MMPs [20, 21], which play destructive roles in atherosclerotic lesions rendering them unstable and prone to rupture.
Genetic knockout models have provided lucid evidence that NF-κB proteins are absolutely essential for the development of a normal, effective immune system, since NF-κB genetic ablation, in general, renders mice immunocompromised and prone to pathogenic infections. Specifically, p50−/− mice develop normally but are defective in immunoglobulin production, and thus, humoral immune responses [22]. Likewise, p52−/− mice develop normally but their B-cell follicles and germinal centers do not develop normally, and the mice are unable to launch an adequate humoral response against T-cell-dependent antigens [23, 24]. Although ablation of p65 causes embryonic lethality due to liver apoptosis [25], ablation of TNFα or TNFR rescues p65−/− from the lethal phenotype [26, 27]. However, p65−/−/TNFα −/− mice are highly susceptible to bacterial infections and unable to provoke an innate immune response. In addition, T and B lymphocytes of c-Rel−/− mice are unresponsive to various mitogenic stimuli, and the mice are unable to generate a humoral immune response [28]. Lastly, RelB−/− mice are severely defective in generating adaptive immune responses [29]. Thus, it is evident that NF-κB proteins are indispensable in generating effective inflammatory, innate, and adaptive immune responses against viral and bacterial pathogens.
The first experimental evidence of NF-κB role in atherosclerosis, a progressive inflammatory disease, came from a study demonstrating that active NF-κB can be detected in aortae with evident atherosclerotic lesions but not in normal, nonlesional aortae [30]. In fact, a strong signal of active NF-κB can be detected in endothelial cells, macrophages, and to a lesser extent, T lymphocytes within atherosclerotic lesions [30, 31]. Interestingly, oxLDL is potentially capable of activating NF-κB in endothelial cells and macrophages in culture systems as well as in atherosclerotic lesions [32–35]. In the context of atherosclerosis, NF-κB activation is believed to promote the expression of various factors that mediate various processes such as proliferation, chemotaxis, adhesion, inflammation, and thrombosis, key events in atherogenesis [36]. Wolfrum and colleagues have shown that mice which overexpress TNF-inducible protein A20, a cytosolic zinc finger protein that inhibits NF-κB activity by blocking IκB degradation, display significantly smaller atherosclerotic lesions compared to control mice [37]. A recent study has clearly demonstrated that endothelium-specific inhibition of NF-κB activity is accompanied by significant reduction in atherosclerotic lesion formation in apolipoprotein E null (ApoE−/−) mice [38]. In fact, inhibition of NF-κB leads to abrogated macrophage recruitment to the atherosclerotic lesions and reduced expression of cytokines and chemokines in the aortae of ApoE−/− mice [38]. Indeed, a large number of naturally occurring products have been shown to attenuate the pathogenesis of atherosclerosis by virtue of their ability to interfere with NF-κB signaling [39–43]. Furthermore, several studies have demonstrated a positive correlation between NF-κB activity and incidence of myocardial infarction [44–51]. Due to its critical role in atherosclerosis and myocardial infarction, NF-κB is proposed to be a promising therapeutic target for reducing, if not eliminating, the risks of atherosclerosis and its complications.
3. Structure of NF-κB/Rel Proteins
Although several homodimers and heterodimers are formed by various members of the NF-κB protein family, NF-κB is a term that is often used to describe the p50/p65 heterodimer, which was the first NF-κB dimer to be described [18, 52]. Indeed, p50 and p65 are the first members of the NF-κB gene family to be cloned and characterized [53–56]. As shown in Figure 1, members of the NF-κB/Rel protein family contain a highly conserved, N-terminal 300-amino acid region known as the rel homology domain (RHD), which mediates dimerization, interaction with IκB proteins, nuclear translocation due to the presence of a nuclear localization signal (NLS) within RHD, as well as binding to specific sites within the promoters of target genes [10]. Although the majority of NF-κB dimers are capable of transactivating target genes, in vivo data demonstrated that some dimers such as p50/p50 and p52/p52 homodimers can be inactive or repressive [57–59]. The fact that p50 and p52 lack a C-terminal region that is conserved in the majority of other NF-κB proteins suggests that this region confers on NF-κB proteins a transcriptional potential, and hence, it is called the transactivation domain (TAD) [10]. Mutations of important residues within TAD render activating NF-κB dimers transcriptionally inactive [60]. RelB is a structurally unique member of the NF-κB protein family in that it contains a leucine zipper-like (LZ) region at its N-terminus, which is required for its full transcriptional activity [61].
4. Regulation and Activity of NF-κB/Rel Proteins
Under basal conditions, most NF-κB subunits are sequestered in the cytosol, where they are constitutively bound by members of the NF-κB inhibitor family of proteins, mainly IκBα and IκBβ [54, 62]. However, diverse stimuli including inflammatory cytokines, mitogens, lipopolysaccharides, UV light, as well as bacterial and viral pathogens can transduce a signal that ultimately results in NF-κB liberation from its inhibitors, allowing NF-κB dimers to translocate to the nucleus and become transcriptionally active [9, 63, 64]. Except for RelB, all other NF-κB proteins contain a protein kinase A (PKA) phosphorylation site 20–30 amino acids N-terminal of the NLS within RHD, and phosphorylation of this site (S337 in p50 and S276 in p65) seems to be essential for nuclear translocation [65, 66]. Moreover, phosphorylation of the PKA phosphorylation site is important in protecting the transcriptional and DNA-binding activities of active NF-κB dimers [67–70]. Studies have shown that S276 in p65 is a major phosphorylation site that is subject to compartment-specific and stimulus-specific phosphorylation by PKAc in the cytoplasm [70] and the mitogen- and stress-activated protein kinase-1 (MSK1) in the nucleus [71]. S276 phosphorylation is required for optimal NF-κB activity in different cell types [71–73]. Other important phosphorylation sites have been demonstrated to be critical for optimal NF-κB transactivation potential. Such sites include protein kinase C zeta-(PKCζ-) phosphorylated S311 [74], casein kinase II (CKII)-phosphorylated S529 [75], IKKα / β-phosphorylated S536 [76–78]. S536 in p65 has also been shown to be subject to phosphorylation by other kinases such as AKT/protein kinase B (PKB) [79, 80], ribosomal S6 kinase 1 (RSK1) [81], TRAF family member-associated (TANK)-binding kinase 1 (TBK1) [82, 83], and IKKε [83] under certain circumstances.
Dimerization of NF-κB proteins is a prerequisite for NF-κB to become transcriptionally active, and it is mediated by specific motifs within RHDs of both members of NF-κB dimers [9]. Studies have shown that the dimerization motifs are located at the C-terminus of RHD, and mutation of critical residues within such motifs interferes with dimerization [84–86]. Site-directed mutagenesis experiments also revealed the importance of certain residues within the dimerization motifs in determining partner specificity [85–87]. Once in the nucleus, active NF-κB dimers can bind to specific DNA-binding sites, known as κB binding sites, within the regulatory regions of their target genes, leading to gene transactivation [10, 88]. The κB site has a conserved consensus sequence of 10 nucleotides (GGGRNNYYCC where N is any base, R is a purine, and Y is a pyrimidine), and slight variations of the κB nucleotide sequence confer preference to different dimer combinations of NF-κB subunits [9, 88]. The N-terminus of RHD is known to be essential for DNA-binding activity of NF-κB proteins [84, 89]. Although point mutations of specific residues within this region do not interfere with dimerization, they completely abrogate the DNA-binding activity of NF-κB dimers [84, 90].
5. Inhibitors of NF-κB
Work from Baltimore's laboratory provided initial characterization of NF-κB coordinate regulation via physical interaction with its inhibitors, members of the IκB family of proteins [8, 91]. The observation that nuclear NF-κB exists in an IκB-unbound state indicated that IκB proteins can sequester NF-κB in an inactive state in the cytosol. Initial characterization of IκB proteins that associate with NF-κB led to the identification of 37-kDa and 43-kDa proteins, which are now known as IκBα and IκBβ, respectively [92]. IκBα and IκBβ are the most well-characterized members of the mammalian IκB family of proteins, which contains a number of structurally related proteins besides IκBα and IκBβ, including IκBγ 1, IκBγ 2, IκBδ, IκBε, IκBR, IκBL, p100, p105, and Bcl-3 [9, 93]. Recently, a new member of the IκB protein family was identified and named IκBζ [94]. Except for Bcl-3 and IκBζ, which are constitutively localized in the nucleus [94, 95], all other IκB proteins are localized in the cytosol [93]. Nuclear localization of Bcl-3 and IκBζ indicates that these proteins do not regulate NF-κB translocation into the nucleus, but rather, they seem to be involved in regulating NF-κB transcriptional and DNA-binding activities [94, 96–98].
6. Structure of IκBα and IκBβ
Structural organization of IκB proteins started to be uncovered upon molecular cloning and characterization of the IκBα gene (also known as MAD-3) in the early 1990s [99, 100]. Now, it is clear that all IκB proteins known to date possess three to seven centrally located, 30–33 amino acid repeated sequences known as ankyrin (ANK) repeats (also known as notch-related motifs, cell cycle repeats, and cdc10/SW16 repeats) (Figure 1) [9, 93, 101]. These repeats were initially identified in the SW16 protein expressed by Saccharomyces cerevisiae [102]. Although the exact amino acid sequences of ANK repeats found in different IκB proteins can be distinct, ANK repeats have a consensus amino acid sequence (XGXTPLHLAARXGHVEVVKLLLDXGADVNAXTK, where X can be any amino acid) [103, 104]. Even within the same IκB protein, ANK repeats can be quite distinct, and this is thought to be an important determinant in the specificity and selectivity of the protein-protein interaction between IκB and NF-κB proteins [105]. The presence of ANK repeats in IκB proteins renders them capable of physically interacting with regions within the RHD of target NF-κB dimers [106–108]. Additionally, IκBα, IκBβ, and IκBε have N-terminal signal-receiving domain (SRD) containing two highly conserved serine residues, which are known to be important phosphorylation sites involved in the regulation of IκB function [9, 93]. IκBα, IκBβ, IκBγ 1, IκBγ 2, IκBδ, IκBR, IκBL, p100, and p105 contain a region at the C-terminus that is rich in proline, glutamate, aspartate, serine, and threonine residues, and hence, it is called the PEST domain [9, 93]. The PEST domain plays an important role in the inhibition of NF-κB DNA-binding activity [109], as well as in IκB protein stability/turnover [52, 110–113]. Although deletion of the N-terminus and/or the C-terminus does not affect IκBα ability to interact with NF-κB dimers, point mutations of certain residues within the N-terminus of IκBα render it resistant to signal-induced phosphorylation and degradation [114–117], while deletion of the C-terminus of IκBα interferes with its ability to dissociate NF-κB from its DNA binding sites [107, 109, 118]. Finally, two nuclear export signal (NES) sequences have been identified in the N-terminus [119] and C-terminus of IκBα [120]. Actually, the more conserved N-terminal NES was shown to be necessary and sufficient for IκBα nuclear export [119]. Efficient nuclear translocation and cytosolic relocalization (i.e., nuclear export) of IκBα is ensured by the presence of NLS and NES, respectively.
7. Function of IκBα and IκBβ
Since IκBα and IκBβ are the best studied members of the IκB protein family, special emphasis will be allotted to these two molecules throughout this paper. Members of the IκB protein family are constitutively and ubiquitously expressed proteins that localize in the cytosol, except for Bcl-3 and IκBζ which are primarily present in the nucleus [94, 95]. The main function of IκB proteins is to inhibit NF-κB activity when it is not required, and this happens via protein-protein interaction that takes place between IκB proteins and NF-κB dimers in the cytosol. IκBα and IκBβ interact via their ANK repeats with the RHD of NF-κB dimerized proteins in such a way that masks the positively charged regions of the NLSs within the RHDs of NF-κB dimers [121, 122]. As a result, NF-κB dimers are prevented from translocating to the nucleus, and thus, they are kept in an inactive, IκB-bound state in the cytosol [123–125]. Although IκB-NF-κB interaction is mediated by ANK repeats of IκB proteins, not all ANK repeats are involved in this interaction [107, 118, 126]. In an extensive site-directed mutagenesis study performed to assess the significance of every ANK repeat within IκBα [107], a number of interesting findings were revealed. First, the C-terminus of IκBα is required for the protein to be functional, and thus, the ANK repeats are not sufficient on their own to exert an inhibitory action towards NF-κB. Second, lack of the third ANK repeat does not impede IκBα inhibitory function, suggesting that this ANK repeat is dispensable for IκBα inhibitory function. Third, the only mutant forms of IκBα that are unable to inhibit NF-κB activity are those that were incapable of interacting with NF-κB. Another study suggests that the first ANK repeat of IκBα is mostly responsible for its inhibitory activity, and substituting the first ANK repeat in IκBβ with that of IκBα significantly enhances the former's inhibitory activity [127]. It is evident that the ANK repeats and the C-terminal region (i.e., PEST domain) of IκBα form a tertiary structure that is capable of interacting with NF-κB proteins, and that such interaction confers NF-κB transcriptional inactivity [107].
It is known that NF-κB is itself an upregulator of IκBα and IκBβ, in which NF-κB activation via various and distinct stimuli is usually followed by rapid induction of IκBα and IκBβ expression [19, 52, 128] due to the presence of a κB DNA-binding site within the IκB promoter [129–131]. This negative feedback regulatory loop sets a molecular switch that ensures rapid, controlled, and transient activation of target genes by NF-κB. Induced expression of IκBα allows translocation of nascently synthesized IκBα into the nucleus, where it binds to active NF-κB dimers that are bound to κB sites within the promoters of their target genes. Interaction between nuclear IκBα and active NF-κB dimers leads to dissociation between NF-κB dimers and DNA, and it forces a conformational change in IκBα that exposes the nuclear export signal (NES), eventually leading to resequesterization of IκBα and NF-κB dimers in the cytosol [92, 120, 132]. This highly complex, tightly regulated reciprocal regulatory process involving NF-κB and IκB proteins confers the NF-κB signaling pathway a central regulatory function in many key biological events that requires transient, short-term NF-κB activity.
8. Regulation of IκBα and IκBβ
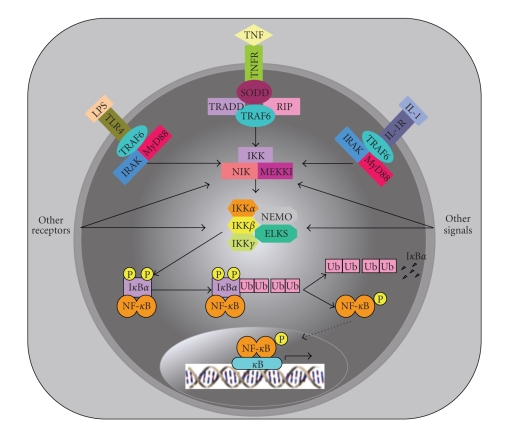
There are at least two well-characterized signaling pathways leading to NF-κB activation, classical and alternative, and both rely on the catalytic activity of known IκB kinases (IKKs) (Figure 2). The classical NF-κB signaling pathway is typically triggered by a vast number of proinflammatory cytokines (e.g., IL-1β and TNFα), viruses, and bacteria, and hence, it leads to a coordinate inflammatory/immune response culminating in the expression of multiple cytokines, chemokines, adhesion molecules, and proinflammatory proteolytic enzymes [133, 134]. On the other hand, the alternative NF-κB signaling pathway is normally triggered by non-proinflammatory cytokines (e.g., lymphotoxin β (LTβ), B-cell activating factor (BAFF), and CD40 ligand (CD40L)) as well as some viruses (e.g., human T-cell leukemia virus (HTLV) and Epstein-Barr virus (EBV)) [133, 134]. The alternative NF-κB signaling pathway is triggered to induce the expression of genes whose products play fundamental roles in the development and maintenance of secondary lymphoid organs [133]. Unlike the classical pathway, the alternative pathway is NEMO independent in that it does not require the IκB kinase (IKK) complex, which contains the scaffold protein NF-κB essential modulator (NEMO), IKKα, IKKβ, IKKγ, and other adaptor proteins [133–140]. Instead, the alternative pathway relies on the activity of the NF-κB inducing kinase (NIK) that transactivates IKKα-IKKα homodimers, which upon activation transduce a signal that culminates in profound NF-κB activation [134]. In the next sections, special attention will be paid to the classical NF-κB signaling pathway.
Figure 2.
NF-κB Signaling Pathway. A cartoon representing the cascade of biochemical events that are initiated by various stimuli, eventually leading to NF-κB nuclear translocation and transcriptional activation. Recruitment of different adaptor molecules to different receptor complexes coupled with activation of different downstream kinases is shown. There are mainly two signaling pathways leading to NF-κB activation, classical (also known as canonical) and alternative. The formation and activation of the IKK complex, which consists of catalytically active kinases (e.g., IKKα, IKKβ, and IKKγ) and noncatalytic regulatory proteins (e.g., NEMO and ELKS), is a universal event in both signaling pathways. In the classical signaling pathway, ligand binding to a cell surface receptor leads to the recruitment of adaptor proteins (e.g., TRAF6) to the receptor, leading to the recruitment of IKK complex and subsequent phosphorylation and degradation of the IκB proteins. Unlike the classical signaling pathway, the alternative signaling pathway, which is normally triggered by non-proinflammatory cytokines (e.g., LTβ, BAFF, and CD40L) as well as some viruses (e.g., HTLV and EBV), does not allow the recruitment of NEMO. Instead, ligand binding to a cell surface receptor leads to the recruitment of NIK, which in turn phosphorylates and activates IKKα dimers. Typically, the classical signaling pathway leads to the activation of NF-κB dimers consisting of RelA, c-Rel, RelB, and p50, while the alternative signaling pathway leads to the activation of NF-κB dimers consisting primarily of RelB and p52.
9. Basal Turnover/Degradation of IκBα and IκBβ
Besides signal-induced proteolytic degradation of IκBα and IκBβ, these proteins have been shown to be susceptible to degradation under basal, unstimulatory conditions. In fact, IκBα and IκBβ have been shown to be constitutively phosphorylated in absence of stimuli [67, 141], and specific serine/threonine residues (S283, S289, S293, and T291) within the PEST domain of IκBα have been shown to be the target of constitutive phosphorylation by CKII [111, 113, 117, 142]. Phosphorylation of the PEST domain renders IκBα susceptible to degradation, indicating that the PEST domain is essential for controlling IκBα intrinsic protein stability [111–113]. Likewise, it was shown that the PEST domain of IκBβ is required for its degradation [143]. Unlike signal-induced degradation of IκBα, which required ubiquitination, basal degradation of IκBα seems to be ubiquitination independent, in which degradation of unubiquitinated IκBα is evident in unstimulated cells in vitro [144]. This data is supported by the observation that a mutant form of IκBα carrying lysine-to-arginine substitutions at the two ubiquitination sites (K21 and K22) is as prone to basal degradation as the wild type form (WT) of IκBα [145]. In other words, ubiquitination of IκBα is a signal-induced event and is not required for basal degradation of IκBα. However, the ubiquitin-independent IκBα degradation pathway is proteasome dependent, since proteasome inhibitors block basal, as well as signal-induced, degradation of IκBα [144].
Until the emergence of a paper published by Phillips and Ghosh in 1997 [146], the 26S proteasome-mediated proteolysis pathway was the only known cellular process responsible for basal and signal-induced degradation of IκBα and IκBβ. However, the use of selective proteasome inhibitors revealed the existence of a novel proteolysis pathway that leads to IκBα and IκBβ degradation in an ubiquitin-independent, proteasome-independent manner in immature B cells [146, 147]. Indeed, such a novel pathway was subsequently shown to be dependent on the presence of free calcium, most likely imported from outside the cell [145]. Further examination of this pathway revealed that phosphorylation of the PEST domain of IκBα allows it to bind to the calmodulin-like domain (CaMLD) of the large subunit of the calcium-dependent thiol protease complex, calpain [148, 149]. Interaction between IκBα and calpain is followed by N-terminal cleavage and further proteolysis of IκBα [148, 149]. These studies suggest that IκBα and IκBβ can also be regulated by protease machineries other than the intrinsic, well-known 26S proteasome complex.
10. Signal-Induced IκBα and IκBβPhosphorylation by the IKK Complex
In order for NF-κB to become activated, IκBα/β must become phosphorylated at specific serine residues at the N-terminus, followed by ubiquitination (not for IκBβ) and proteolytic degradation of phosphorylated IκBα and IκBβ in the cytosol. Phosphorylation and subsequent proteolytic degradation of IκBα and IκBβ liberate NF-κB dimers, which become phosphorylated, translocate to the nucleus, and bind to specific κB binding sites within the promoter/enhancer regions of their target genes, leading to their transactivation. Binding of TNFα to its receptor (TNFR1) is known to trigger NF-κB activation through the classical pathway, leading to TNF-induced cell death [150]. Under basal conditions, constitutive activation of the TNF-induced cell death pathway is prevented by the blocking potential of a protein called the silencer of death domains (SODDs), which binds to TNFR1 and prevents downstream signal transduction [150]. Upon TNFα-TNFR1 binding, SODD dissociates from TNFR1 and this allows recruitment of adaptor molecules TNF receptor-associated death domain (TRADD), receptor interacting protein (RIP), and TNFR associated factor 2 (TRAF2), which bind to TNFR1 as a complex through TRADD. Sequential recruitment of NIK and the IKK complex to the TRADD complex bound to TNFR1 is mediated by TRAF2 [151, 152]. Stimulation signals triggered by LPS, IL-1β, and TNFα also lead to the recruitment and activation of MEKK1 [153]. The recruited IKK complex also contains an IκB kinase regulatory subunit called ELKS (glutamic acid, leucine, lysine, and serine-rich protein), which allows IκBα recruitment and interaction with the IKK complex at the membrane [154]. Although NEMO, IKKα, IKKβ, IKKγ, and ELKS are the main components of the cytoplasmic serine-protein-kinase multi-subunit IKK complex, other proteins are identified as essential elements of the complex [135–140, 155–157]. NIK and MEKK1 are upstream upregulators of the IKK complex, in which they phosphorylate and transactivate IKKα and IKKβ within the complex [155, 158].
Membrane recruited IKK complex with catalytically active IKKα and IKKβ is responsible for phosphorylating two N-terminally located conserved serine residues in IκBα and IκBβ (Ser32 and Ser36 in IκBα; Ser19 and Ser23 in IκBβ) [137, 159]. Interestingly, cell lines that lack NEMO display severe defects in NF-κB activation and they are unresponsive to a wide range of potent stimuli [139], indicating that catalytically active IKKα and IKKβ are insufficient in phosphorylating IκBα and IκBβ in the absence of complex formation. Although some studies have initially suggested a major role of IKKα in IκBα and IκBβ phosphorylation [135, 137, 156, 160, 161], a study demonstrated that mutation of two serine residues within the activation loop of IKKβ, but not IKKα, renders the IKK complex catalytically inactive [162], indicating that IKKβ is the predominant kinase component of the IKK complex. This observation is supported by an experiment demonstrating that IKKα −/− cells display normal IKK activity towards IκBα and IκBβ upon LPS, IL-1β, and TNFα treatment [163]. Moreover, IKKβ −/− mice resemble p65−/− mice in that they suffer from embryonic lethality due to severe liver apoptosis [164]. The apoptotic phenotype of IKKβ −/− mice combined with the observation that TNFα deficiency eliminate embryonic lethality of p65−/− mice [165] and that NF-κB mediates TNFα-induced apoptosis [166] strongly suggest that IKKβ catalytic activity is absolutely required for NF-κB activation. Direct experimental evidence indicates that IKKβ −/− embryonic stem cells and fibroblasts display defective IKK activity towards IκBα, and no NF-κB activity [164]. These findings indicate that IKKα cannot compensate for IKKβ loss, and that IKKβ is solely responsible for phosphorylating IκBα and IκBβ in vivo. Interestingly, phosphorylation of S32/S36 and S19/S23 in IκBα and IκBβ, respectively, does not force IκBα and IκBβ dissociation from their NF-κB dimer partners in the cytosol [116, 167], but it renders them susceptible to ubiquitination and subsequent proteolytic degradation [101, 167–171].
11. Signal-Induced Degradation of IκBα and IκBβ
Proteolysis, or proteolytic degradation, is a highly regulated cellular multistep process that involves enzymes called proteases that are capable of hydrolyzing peptide bonds within polypeptides that are usually ubiquitinated, ultimately leading to protein degradation (Figure 3). Protein ubiquitination and subsequent degradation were thought to be molecular mechanisms undertaken by cells to eradicate misfolded or defective proteins [172, 173]. Nevertheless, it is well recognized that protein degradation is a process that is not directed only against imperfect proteins, but also against some fully functional proteins as a means to regulate and control various key biological processes [10]. Cyclins, proteins involved in the regulation of the cell cycle, are a prime example of functional proteins that are regulated by ubiquitination-dependent proteolytic degradation pathways [174, 175].
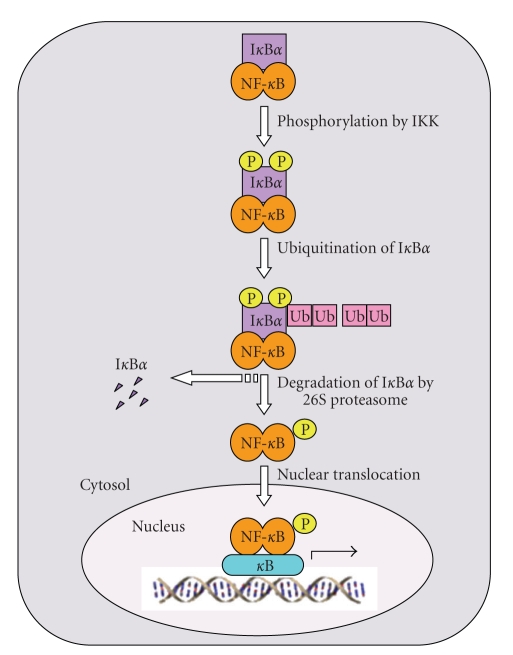
Figure 3.
Regulation of IκBα and IκBβ. A cartoon representing the most critical intracellular events leading to NF-κB transcriptional activation. Interaction between diverse ligands and their receptors eventually leads to activation of the IKK complex, which allows IκBα and IκBβ phosphorylation in the cytosol. This phosphorylation step is followed by IκBα, but not IκBβ, ubiquitination, and subsequently, IκBα and IκBβ are subjected to proteolytic degradation. Once IκBα and IκBβ molecules are degraded, NF-κB dimers are liberated, and they translocate to the nucleus subsequent to unmasking of their NLS. Once in the nucleus, NF-κB dimers bind to κB sites within the promoter/enhancer regions of their target genes, driving gene transactivation.
It is evident that signal-induced phosphorylation of IκBα and IκBβ must be followed by their degradation for NF-κB transactivation potential to be manifested [167, 176–182]. The most compelling evidence indicating the necessity of IκBα and IκBβ degradation for NF-κB activation came from a study showing that treatment of stimulated cells with protease inhibitors does not eliminate phosphorylation of IκBα and IκBβ, but protects NF-κB-IκBα and NF-κB-IκBβ cytosolic complexes, and thus, prevents NF-κB activation [143, 167, 178, 181, 182]. Under stimulatory conditions, proteolytic degradation of IκBα and IκBβ occurs via an ubiquitination- and proteasome-dependent mechanism [169, 170].
The 26S proteasome is composed of a core protease, known as the 20S proteasome, and the 19S regulatory complex (RC), which is composed of at least 18 different subunits in two subcomplexes known as the lid and the base [183]. The involvement of the 26S proteasome in signal-induced NF-κB activation was originally signified by studies demonstrating that treatment with selective inhibitors of the 26S proteasome blocks NF-κB activation [178, 184]. Subsequent to phosphorylation of the two serine residues within the signal-induced kinase domain of IκBα, multiple 76-amino acid ubiquitin polypeptides covalently attach to the N-terminus of phosphorylated IκBα, rendering IκB proteins susceptible to 26S proteasome-dependent degradation [169, 170, 185, 186]. For IκBα, ubiquitination primarily takes place on two adjacent lysine residues (K21 and K22) in the N-terminus of the protein, and mutation of these two lysine residues prevents IκBα ubiquitination and subsequent proteolytic degradation [187, 188]. Indeed, conservative substitution of K21 and/or K22 by arginine precludes not only ubiquitination, but also signal-induced degradation of IκBα, ultimately preventing NF-κB activation [187, 188]. During initial characterization of IκBβ regulation, it was shown that treatment of cells with protease inhibitors prevents IκBβ degradation [143], suggesting that IκBβ may be under control of the ubiquitin-proteasome machinery in a phosphorylation-dependent fashion, as in IκBα. Indeed, site-directed mutagenesis of S19 and/or S23 renders IκBβ somewhat resistant to degradation [143]. Strikingly, however, alanine substitution of K9 has no effect on IκBβ degradation [143], indicating that ubiquitination is not a prerequisite for IκBβ degradation. So, although phosphorylation of the two, N-terminal conserved serine residues is required for inducing IκBα and IκBβ degradation, ubiquitination of the N-terminal lysine residues is required for proteasome-dependent degradation of IκBα, but not IκBβ. Interestingly, although the PEST domain of IκBα and IκBβ is not required for S32/S36 and S19/S23 phosphorylation, respectively, its deletion eliminates signal-induced degradation of IκBα [115, 117, 189–191] and IκBβ [192–194]. In sum, for signal-induced NF-κB transactivation activity to manifest, at least six main biochemical events must precede: (1) phosphorylation of IκBα and IκBβ by the IKK complex, (2) ubiquitination of phosphorylated IκBα, (3) proteasome-mediated degradation of IκBα and IκBβ, (4) phosphorylation of NF-κB dimer, (5) nuclear translocation of NF-κB dimer, and (6) NF-κB dimer-κB DNA interaction (Figure 3).
12. Regulation of NF-κB Activity via IκBα Sumoylation
Signal-induced IκBα phosphorylation and ubiquitination, followed by its proteolytic degradation, are not the only posttranslational modifications that target IκBα and regulate NF-κB activity in cells. Sumoylation is defined as process by which a small ubiquitin-like modifier (SUMO) (~20 kDa) is covalently attached to lysine residues on target proteins [195–197]. Similar to ubiquitination, the process of sumoylation involves three enzymatic events that proceed sequentially, ultimately culminating in SUMO conjugation to the protein substrate by forming an isopeptide bond between SUMO and the ε-amino group of a lysine side chain [198]. In 1998, Desterro and colleagues have reported for the first time the existence of a modified, slower migrating form of IκBα [199]. This modified, slower migrating protein has been identified as an SUMO-1-modified IκBα in several mammalian cells including human embryonic kidney HEK 293 cells, monkey COS-7, human T leukemic Jurkat cells, and HeLa cells [199]. Intriguingly, only a small fraction of total IκBα protein was found to be modified by SUMO-1, and the degree of sumoylation varied depending on the cell type with 50% being the maximum proportion of sumoylated IκBα of the total IκBα pool [199]. Notably, nuclear localization of IκBα was deemed necessary for its sumoylation [200]. Significantly, the sumoylated form of IκBα was further shown to be highly resistant to signal-induced ubiquitination and subsequent proteasome-mediated degradation compared to unmodified IκBα [199]. Desterro and colleagues went on to show that overexpression of SUMO-1 inhibits signal-induced activation of NF-κB-dependent transcription. In a later study, Guo and colleagues have reported the identification and cloning of SUMO-4, which was proposed to conjugate with IκBα leading to NF-κB downregulation [201]. Very recently, SUMO-4-mediated downregulation of NF-κB was shown to be dependent on modification of IκBα by SUMO-4 [202]. Intriguingly, a κB binding motif was also identified in SUMO-4 promoter, and mutagenesis of this motif interfered with NF-κB-dependant transactivation of its target genes, suggesting a feedback loop mechanism by which SUMO-4 regulates NF-κB activity [202].
Unlike ubiquitination, which requires phosphorylation at S32 and S36, phosphorylation at these sites interferes with IκBα sumoylation [199], likely due to a conformational change that hinders SUMO-1 conjugation. Using site-directed mutagenesis, it was also shown that SUMO-1 conjugation requires K21 and K22 at the N-terminus of IκBα; with K21 being the primary site for sumoylation [199]. Strikingly, K21 and K22 are the target residues for ubiquitination, providing a plausible explanation for the prolonged stability of sumoylated IκBα compared to unmodified IκBα. This observation also suggests that SUMO-1 and ubiquitin molecules compete for these residues to regulate IκBα function, and thus, NF-κB activity. Moreover, many hydrolases that can potentially cleave the bond between SUMO-1 and its targeted lysine residue on the substrate, hence known as desumoylating enzymes, have been identified [203–206]. Hence, controlling the balance between sumoylation and desumoylation of IκBα may serve as a mechanism underlying the regulation of NF-κB activity. Sumoylation of IκBα may be a physiologically significant anti-inflammatory mechanism undertaken by cells to suppress lethal inflammatory responses via converting IκBα proteins from their degradation-susceptible, unmodified form into a degradation-resistant, sumoylated one. Interestingly, in an in vitro study, it was demonstrated that epithelial cells exposed to increasing periods of hypoxia; a condition that triggers a wide range of inflammatory events, responded by increasing the proportion of SUMO-1-bound IκBα and cAMP-response element-binding protein (CREB) [207]; a transcription factor that induced the expression of various proinflammatory cytokines. Consistently, induced hypoxia led to a significant increase in transcriptional expression of SUMO-1 [207]. Very recently, it was demonstrated that adenosine signaling mediates SUMO-1 modification of IκBα during hypoxia [208]. Several studies have demonstrated a tight link between SUMO-4 polymorphism and susceptibility to type-I diabetes [201, 209, 210]. However, this correlation between SUMO-4 polymorphism and susceptibility to type-I diabetes seems to be more prevailing among Asian populations compared to Caucasians. Wang and colleagues have recently reviewed the correlation between SUMO-4 polymorphism and type-I diabetes, and they provided some insights to explain the discrepancy noted among different populations and the mechanisms through which SUMO4 contributes to the pathogenesis of type-I diabetes [211]. SUMO-4 polymorphism seems to have no correlation with susceptibility of other inflammatory conditions including Grave's disease [212], rheumatoid arthritis [213–215], and systemic lupus erythematosus [216]. Finally, it is conceivable that modulation of IκBα sumoylation may be utilized as a mechanism to aggravate or alleviate the symptoms of various NF-κB-driven inflammatory conditions.
It is important to mention that other proteins involved in NF-κB signaling pathways are also subject to sumoylation. For example, sumoylation on K277 and K309 residues of NEMO by SUMO-1 conjugation has been shown to mediate NF-κB activation by genotoxic stress [217]. In fact, regulation of NF-κB activity by NEMO sumoylation occurs under a variety of other stress conditions including oxidative stress, ethanol exposure, heat shock, and electric shock [218]. Details regarding the mechanisms involved in NEMO sumoylation were revealed by a recent study demonstrating that NEMO sumoylation is mediated by protein inhibitor of activated STATy (PIASy), which seems to preferentially stimulate site-selective modification of NEMO by SUMO-1, but not SUMO-2 or SUMO-3, in vitro [219].
13. Cross-Talk between Glucocorticoid Receptor (GR) and NF-κB Signaling
Although signal-induced posttranslational modification of IκBs by phosphorylation, ubiquitination, sumoylation, and proteolytic degradation serves as the central molecular mechanism by which NF-κB signaling is regulated, several IκB-independent mechanisms have been proposed as effective, alternative cellular events that are crucial in the regulation of NF-κB activity. Such IκB-independent mechanisms seem to be critical in the alternative, noncanonical NF-κB signaling pathway and they occur via post-translational modification of various proteins, other than IκBs, that are critically involved in NF-κB signaling. Like IκBs, some members of the Rel family of proteins can be subject to signal-induced post-translational modifications that ultimately lead to modulation of NF-κB activity [134, 220, 221]. Signal-induced phosphorylation of RelA, which was first documented about fifteen years ago [67, 141], is by far the most extensively studied post-translational modification in the regulation of NF-κB signaling (for review, refer to [134, 220, 221]). Similarly, post-translational modifications of RelB [222, 223], c-Rel [224–229], and p50 [67, 230] have also been documented as IκB-independent regulatory mechanisms involved in NF-κB signaling.
Moreover, other IκB-independent mechanisms involving a complex interplay between NF-κB and other NF-κB-unrelated proteins have been recently revealed. The glucocorticoid receptor (GR) is a prime example of such regulatory proteins that are critically implicated in the control of NF-κB signaling pathways. GR is a hormone-dependent transcription factor belonging to the nuclear receptor superfamily, and it is critically involved in mediating the immunosuppressive functions of glucocorticoids by repressing the expression of major cytokines. Although the exact molecular mechanisms underlying the repression function of GR on NF-κB activity are not fully understood, experimental evidence suggests that it is primarily the ligand-induced physical interaction between GR and DNA-bound NF-κB subunits (RelA and p50) that ultimately inhibits NF-κB activity [231–236]. The regulation of NF-κB activity via the interaction between GR and NF-κB subunits seems to be independent on GR DNA binding and homodimerization [236–238]. Although there is a consensus among researchers that the GR-mediated regulation of NF-κB signaling takes place in the nucleus, it was proposed that ligand-induced activation of GR by glucocorticoids may regulate NF-κB signaling in the cytoplasm by increasing IκBα protein level in HeLa cells, monocytic cells, and T cells [239, 240]. However, induction of IκBα seems to be cell type-specific mechanism underlying GR-mediated repression of NF-κB activity since glucocorticoid treatment caused inhibition of NF-κB activity without any detectable change in IκBα expression in endothelial cells [241] or epithelial cells [242, 243]. In an in vitro study involving human pulmonary epithelial A549 cells, monkey COS-1 cells, and human breast cancer T47D cells, it was shown that GR-mediated inhibition of NF-κB activity occurs via a dual mechanism involving both protein-protein interaction between GR and NF-κB subunits and induction of IκBα expression; with the former mechanism being predominant in NF-κB regulation [244]. Moreover, it seems that GR-mediated repression of NF-κB activity via IκBα induction is not only cell type-specific, but it is also dependent on the type of ligand, the NF-κB target gene, the presence of certain cofactors, the status of chromatin, and probably other conditions [221, 242, 245].
Noteworthy, other mechanisms underlying GR-mediated repression of NF-κB activity have been proposed. For example, GR activation was shown to be associated with suppression of histone acetylase (HAT) activity via inhibited recruitment of large coactivator complexes containing HAT regulatory proteins such as CREB-binding protein (CBP) and p300 [246]. Moreover, GR activation was shown to cause induced expression of histone deacetylase 2 (HDAC2) accompanied by recruitment of GR to NF-κB target genes leading to their transrepression [246]. It seems that the concentration of glucocorticoids is one determining factor in controlling the balance between GR-mediated suppression of HAT activity and induction of HDAC activity leading to modulated NF-κB activity [246]. Interestingly, GR itself is subject to deacetylation by HDAC2, which leads to GR nuclear translocation and physical interaction with NF-κB subunits [247]. It is also documented that induced histone methylation, rather than suppressed histone acetylation, may serve as a mechanism that underlies GR-mediated repression of NF-κB functions [248, 249]. Additionally, experimental evidence revealed that attenuation of GR-mediated transrepression of its target genes is accompanied by recruitment of potent corepressors such as nuclear receptor corepressor (NCoR) and silencing mediator of retinoid and thyroid hormone receptor (SMRT) [250–253]. Furthermore, it was proposed that GR interferes with NF-κB-dependent serine phosphorylation of the C-terminal domain of RNA polymerase II leading to suppressed expression of NF-κB target genes [235]. These findings suggest that GR activation may repress NF-κB activity without influencing NF-κB DNA binding potential. Consistent with this proposal, treatment of asthmatic patients with inhaled glucocorticoids suppresses inflammation via inhibition of NF-κB-mediated expression of inflammatory mediators with no detectable reduction in NF-κB DNA binding ability [254]. Together, these findings strongly suggest that GR can modulate NF-κB activity in the nucleus by regulating several key events including NF-κB DNA binding, HAT and/or HDAC expression, coactivator(s) and/or corepressor(s) recruitment, as well as RNA polymerase II-induced transactivation of NF-κB target genes. Finally, glucocorticoids may serve as inhibitors of NF-κB activity via their ability to modulate the activity of proteins involved in the mitogen-activated protein kinase (MAPK) pathways including c-jun N-terminal kinase (JNK), p38, and MAP kinase phosphatase-1 (MKP-1), all of which can cross-talk and modulate NF-κB activity, and thus, inflammatory responses [255–263].
14. AEBP1 Is a Multifunctional Protein
Adipocyte enhancer-binding protein-1 (AEBP1) is a ubiquitously expressed protein whose expression seems to be the highest in adipose tissue, liver, lung, spleen, and brain [264]. Recently, AEBP1 was shown to be abundantly expressed in primary macrophages as well as macrophage cell lines [265–267]. AEBP1 shares a remarkable amino acid sequence homology with two members of the regulatory carboxypeptidase family of enzymes, CPX1 and CPX2, all of which contain N-terminal discoidin-like domain (DLD) and homologous central carboxypeptidase (CP) domain [268]. Unlike CPX1 and CPX2, which are catalytically inactive [269, 270], AEBP1 functions as an active carboxypeptidase capable of catalyzing hydrolysis of arginine and lysine in hippuryl-arg and hippuryl-lys synthetic carboxypeptidase B (CPB) substrates, respectively [271, 272]. Studies from Ro's laboratory have demonstrated that deletion of residues 429 to 460 in the CP domain, which encompasses the active site, renders AEBP1 catalytically inactive [271]. Moreover, the carboxypeptidase activity of AEBP1 has been shown to be responsive to carboxypeptidase activators and inhibitors, and that DNA binding enhances AEBP1 hydrolytic activity [272], indicating that AEBP1 functions as an active carboxypeptidase.
AEBP1 is highly expressed in preadipocytes, and its expression persists during the first stages of adipogenesis [271, 273]. However, AEBP1 levels drop dramatically as preadipocytes differentiate into mature adipocytes, and AEBP1 expression is completely abolished in terminally differentiated, nonproliferative adipocytes [264, 271, 273]. Because of the altered expression pattern of AEBP1 during adipogenesis, AEBP1 was suspected to play a negative regulatory role in adipose P2 (aP2) expression in preadipocytes. Indeed, in vitro studies have demonstrated that AEBP1 specifically binds adipocyte enhancer-1 (AE-1) DNA sequence [271], and transcriptionally represses aP2 in 3T3-L1 preadipocytes and other cell lines [271, 273, 274]. Transcriptional repression of aP2 by AEBP1 is physiologically significant since targeted over-expression of AEBP1 in adipose tissue causes diet-induced obesity in mice [275]. AEBP1 seems to induce massive obesity in mice with targeted, tissue-specific overexpression of AEBP1 (AEBP1TG mice) by inducing adipocyte proliferation in vivo, leading to adipocyte hyperplasia in white adipose tissue [275]. In contrast, AEBP1-deficient mice (AEBP1−/− mice) display 25% reduction in total body weight due to significantly reduced fat pads caused by enhanced apoptosis and impaired survival signal [276]. Indeed, AEBP1TG preadipocytes display augmented proliferation [273, 275], while AEBP1−/− preadipocytes exhibit a defective proliferative potential in vitro [276].
MAPK pathways are a network of serine/threonine kinases and dual-specificity kinases, whose function is implicated in various key biological processes in the cell including proliferation, inflammation, and tumorigenesis [277–280]. Kinases involved in MAPK pathways include JNK1/2, extracellular signal-regulated kinases 1 and 2 (ERK1/2), and other MAP kinases (e.g., MEK, MEKK, and MEKKK). In vitro and in vivo experimental studies revealed that AEBP1 physically interacts with ERK1/2 via its DLD [273]. This protein-protein interaction is critical for MAPK activity since it leads to protection of ERK1/2 from dephosphorylation by its specific phosphatase (MKP-3), leading to sustained activation of ERK1/2 [273]. AEBP1 inhibits differentiation of preadipocytes into mature adipocytes, thus impeding adipogenesis, by means of enhancing ERK1/2 activity in preadipocytes [273].
Recently, AEBP1 was shown to be a critical regulator of macrophage cholesterol homeostasis, foam cell formation, and macrophage inflammatory responsiveness [265]. In fact, AEBP1 was shown to manifest its proinflammatory effects by promoting NF-κB activity via impeding the inhibitory function of IκBα in macrophages, an event that seems to be dependent on AEBP1-IκBα physical interaction [266]. Most recently, experimental evidence indicates that AEBP1 mediates LPS-induced foam cell formation by virtue of its ability to directly suppress peroxisome proliferator-activated receptor γ 1 (PPARγ1) and liver X receptor α (LXRα) activity in macrophages, suggesting that AEBP1 may play a critical regulatory role in bacterial infection-induced atherosclerosis [267].
15. DLD Mediates AEBP1-IκBα Interaction
The N-terminus of AEBP1 contains DLD that is remarkably homologous to discoidin, a lectin expressed in the slime mold Dictyostelium discoideum [281], and hence the name. In Dictyostelium discoideum, discoidin has been shown to be crucial for proper cell aggregation and migration [282] as well as protein-protein interaction [283, 284]. Indeed, DLD of AEBP1 was found to be required for protein-protein interaction between AEBP1 and MAPK [273]. Similarly, it was shown that AEBP1 is capable of physically interacting with IκBα by means of its DLD, whose deletion eliminated AEBP1-IκBα interaction [266]. It is worth mentioning that despite the structural similarities between IκBα and IκBβ, co-immunoprecipitation experiments suggest that AEBP1 is capable of interacting with IκBα, but not IκBβ, in macrophages [266]. Analysis of IκBα-IκBβ amino acid sequence alignment reveals that there are three main structural differences between IκBα and IκBβ (Figure 4). First, the first 12 amino acid residues in IκBα are absent in IκBβ. Second, there is a 41-amino acid stretch located between the third and forth ANK repeat of IκBβ that is not present in IκBα. Third, there is an 18-amino acid stretch at the C-terminus of IκBβ that is absent in IκBα. Based on sequence analysis, it is conceivable that either the presence of the first 12 amino acid residues in IκBα is required for interaction with AEBP1 or that the presence of the extra-amino acid stretches in IκBβ allows the formation of a tertiary structure that does not permit protein-protein interaction with AEBP1. It is also conceivable that the extra-amino acid stretches in IκBβ somehow mask the region, or domain, that is necessary for protein-protein interaction with AEBP1. We are currently attempting to map the exact region of IκBα that mediates protein-protein interaction with AEBP1, which will shed more light on the differential ability of AEBP1 to regulate IκBα and IκBβ functions. Further investigation of the AEBP1-interacting region of IκBα will shed more light on how AEBP1 is capable of differentially regulating IκBα and IκBβ functions in vivo. Although modulation of IκBα expression has been previously shown to be a mechanism explaining altered NF-κB activity [285–287], the data we have recently demonstrated is the first of its kind to propose a molecular mechanism behind modulated NF-κB activity by which IκBα protein stability is altered via protein-protein interaction and is independent of alterations in IKK complex kinetic activity [266].
Figure 4.
Amino Acid Sequence Comparison between IκBα and IκBβ. (a) Alignment of amino acid sequences of mouse IκBα and IκBβ is shown. The six highly conserved ANK repeats in both proteins are highlighted in grey, yellow, green, red, pink, and blue, respectively. (b) A cartoon illustrating a comparison of the slightly different structural domain organizations of mouse IκBα and IκBβ proteins. The signal-induced kinase domain, six ANK repeats, and PEST domain of each protein are shown.
Since about 50% of AEBP1 protein population exists in the nucleus [266, 288], and since newly synthesized IκBα is known to translocate to the nucleus to bind DNA-bound NF-κB dimers and resequester them into the cytosol [64], it is possible that AEBP1 and IκBα interact in the nucleus. This is an interesting possibility since AEBP1-IκBα interaction in the nucleus can interfere with the ability of nuclear IκBα to bind to its target, DNA-bound NF-κB dimers, leading to sustained NF-κB-driven transactivation of target genes (e.g., proinflammatory genes). Thus, by virtue of its cytosolic/nuclear localization and its ability to bind IκBα, it is reasonable to propose that AEBP1 can impede IκBα inhibitory functions towards NF-κB both at the cytosolic and nuclear levels.
Noteworthy, DLD has been identified in several extracellular and intracellular proteins including discoidin domain receptor tyrosine kinase (DDR) [283], the blood coagulation cofactors V and VIII [289], milk-fat globule proteins [290], muskelin [284], retinoschisin [291], and developmental endothelial locus-1 (Del-1) [292]. It would be of great interest to assess whether such DLD-containing proteins have the potential to physically interact with IκBα, as does AEBP1. Such assessment coupled with careful analysis of the amino acid variations among the DLD sequences of these proteins will assist in mapping the exact amino acid stretch within DLD that mediates interaction with IκBα.
16. DLD Mediates AEBP1 Protein-Protein Interaction with Other Proteins
DLD has been suggested to mediate cell-cell adhesion [293], protein self-association [284], and protein-protein interaction [283, 284]. In fact, protein-protein interaction between AEBP1 and MAPK in the cytosol, which prolongs MAPK activation by protecting it from dephosphorylation by its specific phosphatase (MKP-3, also known as PYST1), has been shown to be mediated by DLD of AEBP1 [273]. Similarly, AEBP1 was shown to be capable of physically interacting with cytosolic IκBα via its DLD, whose deletion eliminates AEBP1-IκBα protein-protein interaction [266]. So, these findings further support a role of DLD in protein-protein interaction in mammalian systems. Furthermore, these findings underscore the importance of DLD in mediating very critical functions undertaken by AEBP1 to control key biological processes in the cell. Intriguingly, we propose that the presence of DLD creates a molecular competition between MAPK and IκBα in the cytosol to bind to AEBP1. This proposal is interesting given that sustained MAPK activation and IκBα proteolytic degradation followed by NF-κB activation culminate in diverse biological outcomes in different cell types. Although the molecular mechanisms that signal AEBP1 to interact predominantly with MAPK or IκBα are unknown, it is conceivable that AEBP1 can be utilized by the cell as an on/off switch to promote or inhibit MAPK and NF-κB activities via balancing AEBP1 protein-protein interaction with MAPK and IκBα.
17. AEBP1 and NF-κB: A Positive Relationship
Since its initial identification by Sen and Baltimore about two decades ago [294], NF-κB has been the focus of many researchers in an attempt to understand the various molecular mechanisms involved in inflammatory diseases and cancer. Modulation of NF-κB activity can result in many abnormal cellular processes and diseases including asthma, arthritis, atherosclerosis, obesity, and various types of cancers [2–7]. Recently, we have provided experimental evidence establishing a positive relationship between AEBP1 expression and NF-κB activity in macrophages [266]. Nuclear p65 protein level was shown to be barely detectable in AEBP1−/− macrophages, compared to AEBP1+/+ counterparts [266]. Consistently, electrophoretic mobility gel shift assay clearly illustrates that ablation of AEBP1 expression in macrophages correlates with inhibited NF-κB DNA-binding activity [266]. This positive relationship seems to be a consequence of a negative relationship between AEBP1 expression and IκBα protein stability in macrophages. Interestingly, AEBP1 was shown to promote IκBα phosphorylation followed by its proteolytic degradation, liberating the NF-κB subunits, which translocate into the nucleus and become transcriptionally active [266]. Furthermore, this negative regulation imposed by AEBP1 on IκBα function in the cytosol seems to be mediated by protein-protein interaction that requires DLD of AEBP1, as confirmed by co-immunoprecipitation analysis [266]. Consistent with the proposal that AEBP1-IκBα protein-protein interaction, which is mediated by DLD, provokes destabilization of IκBα shortening its half-life, the N-terminus deletion (ΔN) and carboxypeptidase (CP) mutant forms of AEBP1, which are devoid of DLD, have no influence on IκBα protein stability, unlike AEBP1 derivatives retaining DLD [266]. Importantly, in contrast to the WT form of AEBP1, ΔN and CP mutant forms possess marginal or no upregulatory function towards NF-κB activity [266], confirming that AEBP1-IκBα interaction is a key biological event that is crucial for AEBP1-mediated IκBα-induced degradation and subsequent NF-κB up-regulation in macrophages.
It is known that alteration of IKKβ kinetic activity ultimately leads to modulation of IκBα phosphorylation and proteolytic degradation [164]. Given that AEBP1 regulates IκBα phosphorylation status and its proteolytic degradation, one may speculate that AEBP1 is capable of modulating IκBα function in macrophages by means of altering the kinetic potential of IKKβ. However, this possibility was ruled out by in vitro kinase assays demonstrating that IKKβ kinetic activity against a bacterially expressed GST-IκBα (aa 1-54) fusion protein is comparable in AEBP1+/+ and AEBP1−/− macrophages under both basal and LPS-stimulatory conditions [266]. In addition, it was shown that AEBP1 is not a component of the IKK complex nor it influences the composition of the IKK complex in macrophages [266].
In a recent report, we have hypothesized that the positive regulatory role that AEBP1 imposes on NF-κB activity may not be macrophage specific [266]. In fact, abrogation of NF-κB activity has been shown to cause embryonic lethality due to liver apoptosis [25, 26, 164]. If AEBP1-mediated positive regulation of NF-κB is a universal process that takes place in cells and tissues other than macrophages (e.g., liver), one would expect that AEBP1−/− embryos may suffer from liver apoptosis that is life-threatening. Although NF-κB activity has not been evaluated in AEBP1−/− hepatocytes, it is fascinating that AEBP1−/− mice suffer from about 50% embryonic lethality [276]. Hence, severe diminishment of NF-κB activity, which can potentially lead to liver apoptosis, that is caused by AEBP1 deficiency may serve as a molecular mechanism underlying embryonic lethality in AEBP1−/− mice. Interestingly, our recent findings reveal that the levels of the apoptotic markers p-STAT3 and cleaved caspase-3 are significantly higher in the livers of AEBP1−/− mice compared to control mice (unpublished), suggesting that AEBP1 plays an antiapoptotic role in vivo. Consistent with its ability to promote NF-κB activity in various cell types, preliminary findings suggest that AEBP1 also promotes NF-κB activity in mammary gland tissue, in which NF-κB activity is significantly enhanced and diminished in the mammary gland tissues obtained from mice that over-express and lack AEBP1, respectively (unpublished). In sum, AEBP1-mediated promotion of NF-κB activity seems to be a regulatory event that occurs in various cells and tissues.
18. Differential Regulation of IκBα and IκBβ: A Possible Role for AEBP1?
Despite their structural homology, individual IκB proteins have distinctive structural features and they exhibit differential ability and preference to associate with and inhibit various combinations of NF-κB dimers in the cytosol. For example, both IκBα and IκBβ preferentially interact with and inhibit the activity of NF-κB dimers containing p50, p65, and c-Rel [54, 123, 295], IκBγ prefers p50 homodimers and p50/p65 heterodimers [296], IκBε prefers p65 and c-Rel homo- and heterodimers [297], and Bcl-3 prefers p50 and p52 homodimers [95, 298]; whereas p100 and p105 seem to bind to almost all possible dimer combinations of NF-κB proteins [89, 299]. It is also known that different NF-κB dimers display differential intrinsic preference with regard to DNA binding specificity [88, 300], and this DNA binding specificity confers distinct NF-κB dimers a differential transactivation potential towards a diverse set of genes [301].
Additionally, IκBα and IκBβ are known to be differentially regulated in various cell types and under several stimulatory conditions [143, 295]. Although both IκBα and IκBβ become ubiquitinated upon phosphorylation, the two lysine residues (Lys21 and Lys22) at the N-terminus of IκBα are required for ubiquitination; whereas the lysine residue (Lys9) at the N-terminus of IκBβ is not required for ubiquitination [143]. In addition, several studies have demonstrated that while IκBα is subject to rapid degradation upon cell stimulation by various stimuli including LPS, IL-1β, TNFα, and PMA in most cell types, IκBβ degradation cannot be induced except by very few potent stimuli such as LPS and IL-1β in certain cell types and it tends to be a relatively slow process [147, 295]. However, other studies have shown that S19/S23-phosphorylated IκBβ is subject to degradation induced by PMA or TNFα treatment [143, 302]. The slower kinetics associated with IκBβ degradation has been suggested to be probably due to the slower rate of IκBβ phosphorylation by the IKK complex, which seems to favor IκBα as a more efficient substrate [192]. So, depending on the potency of signals, IκBβ may or may not become subject to phosphorylation and subsequent degradation [10]. The differential ability of IKKβ to phosphorylate IκBα and IκBβ has been suggested as a mechanism to explain the differential proteolytic degradation kinetics of IκBα and IκBα [159]. Here, it can be argued that AEBP1, by virtue of its differential ability to interact with IκBα, but not IκBβ, may play a determining role in making IκBα more susceptible than IκBβ to signal-induced phosphorylation and subsequent degradation. Hence, AEBP1 physical interaction with IκBα, but not IκBβ, has been proposed to serve as a mechanism that may elucidate the differential regulatory functions exhibited by these two molecules in vitro and in vivo [266].
Since the PEST domain plays a critical role in IκB protein turnover/stability [52, 110, 143], it is arguable that the function of this domain is differentially regulated in IκBα and IκBβ, thus leading to their differential regulation. However, studies have shown that deletion or mutations within the PEST domain confer resistance to signal-induced degradation for both IκBα [110, 115, 117, 189–191] and IκBβ [143, 192–194]. In light of these results, understanding the role of PEST domain does not seem to help in explaining the differential regulation imposed on IκBα and IκBβ. In addition, while the two N-terminal lysine residues (K21 and K22) of IκBα are known to be ubiquitination sites that are required for signal-induced degradation of IκBα [187, 188], the only N-terminal lysine residue (K9) in IκBβ does not seem to be an exclusive ubiquitination site, and its mutation has no effect on signal-induced degradation of IκBβ [143]. Moreover, it was shown that IκBβ is phosphorylated on Ser19 and Ser23 in unstimulated cells; whereas Ser32 and Ser36 phosphorylation in IκBα is only signal induced [143].
In sum, the differential specificity of IκB/NF-κB interaction combined with the differential transactivation potential of different NF-κB dimers may explain how differential regulation of distinct IκB proteins can lead to differential regulation of NF-κB dimer activity, and thus, differential expression control of discrete genes. However, due to the remarkable similarities between IκBα and IκBβ in terms of their structure and NF-κB dimer specificity, understanding the molecular mechanisms behind the differential regulatory functions undertaken by these two molecules in different cell types and under different conditions has proven to be a tremendous challenge, and so far, a crystal-clear explanation of such differential regulation of these two molecules is still lacking.
19. AEBP1-IκBα Interaction Leads to IκBαDegradation: Unknown Mechanism
To date, two pathways have been suggested as molecular mechanisms responsible for IκBα proteolytic degradation. First, upon stimulation, IκBα is thought to be degraded via a classical, signal-induced proteasome-dependent pathway that involves the 26S proteasome [170]. Second, in vitro studies using immature B cells have demonstrated that IκBα can be subject to constitutive proteasome-independent, Ca++-dependent degradation under basal conditions [145]. It was also shown that constitutive phosphorylation of serine/threonine residues within the C-terminal PEST domain of IκBα by CKII is required for IκBα turnover [111–113]. Also, accumulation of free IκBα in the cytosol triggers its rapid degradation through a phosphorylation, ubiquitination-independent proteasome-dependent pathway [144]. The exact molecular mechanism(s) underlying the regulatory role of AEBP1 towards IκBα activity is not yet identified. However, we have questioned the molecular mechanisms by which AEBP1-IκBα interaction leads to IκBα phosphorylation and subsequent proteolytic degradation, and three speculative points regarding such molecular mechanisms were offered [266]. First, AEBP1-IκBα interaction could cause a conformational change in the latter rendering it more susceptible to Ser32/Ser36 phosphorylation and degradation via the ubiquitination-dependent proteasome-dependent pathway. Second, IκBα-bound AEBP1 could serve as a recruiting scaffold protein that facilitates recruitment of the constitutive proteasome-independent Ca++-dependent proteolytic or ubiquitination-independent proteasome-dependent machineries. Third, it is possible that AEBP1-bound IκBα is more prone to constitutive phosphorylation on serine/threonine residues within the PEST domain, inducing its proteasome-dependent proteolytic degradation. Here, we speculate that AEBP1 may also serve as a “bridge” that brings IκBα in proximity to IKKα / β in the cytosol, forcing IκBα phosphorylation and subsequent proteolytic degradation. In addition, it is possible that AEBP1 somehow enhances the catalytic activity of an “unknown” kinase that can potentially phosphorylate S32 and S36 in IκBα. Moreover, one may speculate that AEBP1 interferes with an “unknown” phosphatase that exercises its catalytic activity on S32/S36-phosphorylated IκBα in the cytosol. Finally, AEBP1 interaction with IκBα may protect the latter from sumoylation, favoring its ubiquitination and subsequent proteolytic degradation. Examination of these possibilities may shed light on the exact molecular mechanism undertaken by AEBP1 to hamper IκBα inhibitory function towards NF-κB.
20. AEBP1-Mediated NF-κB Upregulation Is Independent of PPARγ1 and LXRαModulation
Experimental evidence suggesting that PPARγ 1 and LXRα play anti-inflammatory roles is overwhelming. PPARγ and LXR ligands suppress inflammation by interfering with the NF-κB, AP-1, and STAT signaling pathways [303–312]. PPARγ 1 and LXRα repression by AEBP1 serves as a mechanism that satisfactorily explains the proinflammatory properties exhibited by AEBP1 in macrophages. Since AEBP1 represses PPARγ1 and LXRα transcriptional activity in macrophages [265, 267], active PPARγ 1 and LXRα interfere with NF-κB activity [303, 304, 307, 313], and AEBP1 enhances NF-κB activity, it is reasonable to suggest that PPARγ 1 and LXRα transcriptional repression by AEBP1 may contribute to AEBP1-mediated NF-κB up-regulation in macrophages. However, this effect may be negligible since deletion of DLD, which does not influence the ability of AEBP1 to repress PPARγ1 or LXRα [265], completely eliminates the ability of AEBP1 to up-regulate NF-κB activity [266]. In agreement, deletion of the C-terminus of AEBP1, which completely eliminates the ability of AEBP1 to repress PPARγ1 or LXRα [265], does not interfere with the ability of AEBP1 to up-regulate NF-κB activity [266]. Additionally, Glass and colleagues have shown that neither treatment of RAW 264.7 macrophages with PPARγ ligand nor PPARγ 1 overexpression in absence of its ligand had any anti-inflammatory effects [304]. Rather, PPARγ-mediated anti-inflammatory effects are only observed when PPARγ 1 is over-expressed and ligand activated [143]. Similarly, LXRα-mediated anti-inflammatory effects can only be observed in the presence of LXR ligands and under LPS-stimulatory conditions [307]. However, AEBP1 was shown to enhance NF-κB activity in macrophages expressing endogenous PPARγ 1 and LXRα in the absence of PPARγ or LXR ligands under both basal and LPS-stimulatory conditions [266]. Collectively, we conclude that co-ordinate AEBP1-mediated IκBα proteolytic degradation and subsequent NF-κB up-regulation is independent of AEBP1-mediated PPARγ 1 and LXRα repression in macrophages.
21. Potential Role of AEBP1 in Septic Shock Syndrome
Septic shock syndrome is a very serious medical condition that can lead to failure of many body organs, eventually causing death. Septic shock is caused by an exaggerated immune response against the LPS component of various Gram-negative bacteria via TLR signaling [320, 321]. Very recently, LPS was shown to significantly induce AEBP1 expression in macrophages, and that LPS-induced down-regulation of the pivotal anti-inflammatory mediators PPARγ1 and LXRα is largely mediated by AEBP1 [267]. Given the role that AEBP1 plays in LPS signaling in macrophages, and given AEBP1 role in inducing macrophage proinflammatory responsiveness [265] via promoting NF-κB activity in macrophages [266], it is conceivable that AEBP1−/− mice may be resistant to LPS-induced septic shock and Gram-negative bacterial infection-induced atherosclerosis. In contrast, AEBP1TG mice with targeted overexpression of AEBP1 in adipose tissue and macrophages [275] are expected to be more susceptible to LPS-induced septic shock and Gram-negative bacterial infection-induced atherosclerosis compared to their WT counterparts. It would be very intriguing to investigate the susceptibility of AEBP1−/− and AEBP1TG mice to develop septic shock syndrome upon administration of pathogenic Gram-negative bacteria such as C. pneumonia.
22. Other IκBα-Interacting Proteins and Modulation of NF-κB Activity
Recently, very few studies have proposed that protein-protein interactions involving IκBα may serve as a molecular mechanism explaining the potential of some proteins to modulate NF-κB activity in vitro and in vivo. Besides NF-κB subunits, only a handful of proteins have been shown to physically interact with IκBα using yeast two-hybrid system or co-immunoprecipitation experiments. The X protein of hepatitis B virus (HBV) has been shown to physically interact with IκBα, but not IκBβ, and this interaction leads to sustained NF-κB activation following TNFα treatment [314]. Mutagenesis analysis revealed that the 249-253 amino acid sequence towards the C-terminus of IκBα is critical for protein-protein interaction between X protein and IκBα [314]. Human β-arrestin2, a cytosolic protein that is expressed predominantly in the spleen and neuronal tissue, has been shown to directly interact with IκBα leading to inhibited phosphorylation and degradation of IκBα, ultimately leading to NF-κB down-regulation [315]. The first 60 amino acids within the N-terminus of β-arrestin2 comprise the IκBα interacting region, and the C-terminal domain (40 amino acids) of IκBα contributes to the β-arrestin2 binding [315]. Using GST fusion protein pull-down assays, the poxvirus and zinc finger (POZ) domain at the N-terminus of FBI-1 (factor that binds to the inducer of short transcripts of human immunodeficiency virus-1), a ubiquitously expressed nuclear protein, has been shown to mediate protein-protein interaction between FBI-1 and IκBα [316]. Additionally, ChlaDub1 is a deubiquitinating protease from Chlamydia trachomatis that has been shown to physically interact with IκBα, leading to impaired ubiquitination and proteolytic degradation of IκBα and blocked NF-κB activation in transfected HeLa and HEK293N cells [317]. Similarly, human G protein-coupled receptor kinase 5 (GRK5), a protein that is highly expressed in the heart, placenta, lung and skeletal muscle, has been shown to interact with IκBα in BAEC and HEK293 cells [318]. GRK5-IκBα interaction has been shown to enhance nuclear accumulation of IκBα, ultimately causing diminished NF-κB-driven transcription and NF-κB DNA binding [318]. The regulator of gene protein signaling homology domain of GRK5 (RH) and the N-terminal domain of IκBα have been identified as the regions involved in GRK5-IκBα interaction [318]. Finally, a yeast two-hybrid screen of a human library and co-immunoprecipitation experiments have recently revealed that N-terminal protease (Npro) of classical swine fever virus (CSFV), a protein that is localized in the cytosolic and nuclear compartments, physically interacts with IκBα leading to transient nuclear accumulation of pIκBα in the infected porcine kidney cell line PK-15 [319]. Yet, NF-κB activation does not seem to be significantly affected in PK-15 cells that were stably transfected with Npro [319]. It seems that the C-terminal (aa 213-317) region of IκBα is essential for Npro-IκBα interaction [319].
To date, however, AEBP1 is the only protein that was shown to be an interacting partner of IκBα in macrophages, and AEBP1-IκBα interaction seems to be physiologically significant with regard to NF-κB transactivation and macrophage inflammatory responsiveness [266]. In an attempt to identify a consensus sequence that may be responsible of mediating protein-protein interaction between the IκBα-interacting proteins mentioned above and IκBα, the amino acid sequences of the IκBα-interacting regions within these proteins were compared. Amino acid sequence analysis revealed that there is no obvious consensus sequence or considerable similarities within the identified IκBα-interacting regions of AEBP1, FBI-1, β-arrestin2, and GRK5. Since the exact region of IκBα that mediates physical interaction between IκBα and these proteins is either different or unknown, it is difficult to search for other potential, yet unknown, IκBα-interacting protein based on amino acid sequence similarities. The so-far identified IκBα-interacting proteins and the domains/sequences involved in such interactions are outlined in Table 1.
Table 1.
IκBα-Interacting Proteins and Impact on NF-κB Activity.
| IκBα-Interacting Protein | Species | IκBα-Interacting Region | Interaction Region of IκBα | Impact on NF-κB Activity | Reference |
|---|---|---|---|---|---|
| X protein | Hepatitis B Virus | Unknown | C-terminus (aa 249-253) | ↑ | [314] |
| β-arrestin2 | Homo sapiens | N-terminus (aa 1-60) | C-terminus (aa 276-317) | ↓ | [315] |
| FBI-1 | Homo sapiens | POZ domain (aa 24-131) | Unknown | ↑ | [316] |
| AEBP1 | Mus musculus | DLD (aa 1-166) | Unknown | ↑ | [266] |
| ChlaDub1 | Chlamydia trachomatis | Unknown | Unknown | ↓ | [317] |
| GRK5 | Homo sapiens | RH domain (aa 50–176) | N-terminus (aa 1–58) | ↓ | [318] |
| Npro | Classical Swine Fever Virus | Unknown | C-terminus (aa 213–317) | ↔ | [319] |
23. Conclusion
NF-κB signaling is critically involved in various biological processes that are crucial for cell growth and survival. The role that NF-κB plays in inflammatory reactions cannot be underestimated. By virtue of its ability to act as a direct interacting partner of IκBα via its DLD, AEBP1 exerts a potent upregulatory function toward NF-κB activity in macrophages. Hence, AEBP1 manifests itself as a critical modulator of inflammatory responses. Finally, we anticipate that AEBP1 may serve as a likely molecular target towards the development of novel therapeutic strategies for the prevention or treatment of various inflammatory disorders such as atherosclerosis and septic shock syndrome.
Abbreviations
- AE-1:
Adipocyte enhancer-1
- AEBP1:
Adipocyte enhancer-binding protein-1
- ANK:
Ankyrin
- AP-1:
Activator protein-1
- aP2:
Adipose P2
- ApoE:
Apolipoprotein E
- cAMP:
Cyclic adenosine monophosphate
- CBP:
CREB-binding protein
- CKII:
Casein kinase II
- COX-2:
Cyclooxygenase 2
- CP:
Carboxypeptidase
- CPB:
Carboxypeptidase B
- DLD:
Discoidin-like domain
- ELKS:
Glutamic acid, leucine, lysine, and serine-rich protein
- ERK:
Extracellular signal-regulated kinase
- FBI-1:
Factor that binds to the inducer of short transcripts of HIV-1
- GR:
Glucocorticoid receptor
- GRK:
G protein-coupled receptor kinase 5
- GST:
Glutathione S transferase
- HAT:
Histone acetylase
- HDAC:
Histone deacetylase
- ICAM-1:
Intracellular adhesion molecule-1
- IL:
Interleukin
- IFNγ:
Interferon γ
- IκB:
Inhibitor of NF-κB
- IKK:
IκB kinase
- iNOS:
Inducible nitric oxide synthase
- IRAK:
Interleukin 1 receptor-associated kinase
- JNK:
C-jun N-terminal kinase
- LPS:
Lipopolysaccharide
- LXRα:
Liver X receptor α
- MAPK:
Mitogen-activated protein kinase
- MCP-1:
Monocyte chemoattractant protein-1
- MEKK:
MEK kinase (also designated as MAPKKK)
- MKP:
MAP kinase phosphatase
- MMP:
Matrix metalloproteinase
- NCoR:
Nuclear receptor co-repressor
- NEMO:
NF-κB essential modulator
- NES:
Nuclear export signal
- NF-κB:
Nuclear factor kappa B
- NIK:
NF-κB inducing kinase
- NLS:
Nuclear localization signal
- Npro:
N-terminal protease
- oxLDL:
Oxidized low density lipoprotein
- PEST:
Proline-glutamate/aspartate-serine-threonine
- PIASy:
Protein inhibitor of activated STATy
- PKA:
Protein kinase A
- PKB/AKT:
Protein kinase B
- PKC:
Protein kinase C
- PMA:
Phorbol-12-myristate-13-acetate
- POZ:
Poxvirus and zinc finger domain
- PPARγ1:
Peroxisome proliferator-activated receptor γ 1
- RHD:
Rel homology domain
- RIP:
Receptor interacting protein
- SMRT:
Silencing mediator of retinoid and thyroid hormone receptor
- STAT:
Signal transducer and activator of transcription
- SUMO:
Small ubiquitin-like modifier
- TAD:
Transactivation domain
- TNFα:
Tumor necrosis factor α
- TRADD:
TNF receptor-associated death domain
- TRAF:
TNFR associated factor
- VCAM-1:
Vascular cell adhesion molecule-1
- WT:
Wild type
References
- 1.Baeuerle PA. Pro-inflammatory signaling: last pieces in the NF-κB puzzle? Current Biology. 1998;8(1):R19–R22. doi: 10.1016/s0960-9822(98)70010-7. [DOI] [PubMed] [Google Scholar]
- 2.Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. Journal of Clinical Investigation. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. Journal of Clinical Investigation. 2001;107(2):135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoelson SE, Lee J, Yuan M. Inflammation and the IKKβ/IκB/NF-κB axis in obesity- and diet-induced insulin resistance. The International Journal of Obesity. 2003;27(supplement 3):S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 5.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118(6):671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-κB: its role in health and disease. The Journal of Molecular Medicine. 2004;82(7):434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- 7.Monaco C, Paleolog E. Nuclear factor κB: a potential therapeutic target in atherosclerosis and thrombosis. Cardiovascular Research. 2004;61(4):671–682. doi: 10.1016/j.cardiores.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 8.Baeuerle PA, Baltimore D. IκB: a specific inhibitor of the NF-κB transcription factor. Science. 1988;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 9.May MJ, Ghosh S. Rel/NF-κB and IκB proteins: an overview. Seminars in Cancer Biology. 1997;8(2):63–73. doi: 10.1006/scbi.1997.0057. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh S, May MJ, Kopp EB. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annual Review of Immunology. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(8):2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barath P, Fishbein MC, Cao J, Berenson J, Helfant RH, Forrester JS. Tumor necrosis factor gene expression in human vascular intimal smooth muscle cells detected by in situ hybridization. American Journal of Pathology. 1990;137(3):503–509. [PMC free article] [PubMed] [Google Scholar]
- 13.Brand K, Fowler BJ, Edgington TS, Mackman N. Tissue factor mRNA in THP-1 monocytic cells is regulated at both transcriptional and posttranscriptional levels in response to lipopolysaccharide. Molecular and Cellular Biology. 1991;11(9):4732–4738. doi: 10.1128/mcb.11.9.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cybulsky MI, Gimbrone MA., Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 15.Mackman N, Brand K, Edgington TS. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor κB binding sites. Journal of Experimental Medicine. 1991;174(6):1517–1526. doi: 10.1084/jem.174.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 17.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annual Review of Immunology. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 18.Kopp EB, Ghosh S. NF-κB and Rel proteins in innate immunity. Advances in Immunology. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin AS., Jr. The NF-κB and IκB proteins: new discoveries and insights. Annual Review of Immunology. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 20.Yokoo T, Kitamura M. Dual regulation of IL-1β-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-κB and AP-1. American Journal of Physiology. 1996;270:F123–F130. doi: 10.1152/ajprenal.1996.270.1.F123. [DOI] [PubMed] [Google Scholar]
- 21.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor κB: differential regulation of collagenase 1 and collagenase 3. Arthritis and Rheumatism. 2000;43(4):801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80(2):321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 23.Caamaño JH, Rizzo CA, Durham SK, et al. Nuclear factor (NF)-κB2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. Journal of Experimental Medicine. 1998;187(2):185–196. doi: 10.1084/jem.187.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franzoso G, Carlson L, Poljak L, et al. Mice deficient in nuclear factor (NF)-κB/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. Journal of Experimental Medicine. 1998;187(2):147–159. doi: 10.1084/jem.187.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376(6536):167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 26.Doi TS, Takahashi T, Taguchi O, Azuma T, Obata Y. NF-κB RelA-deficient lymphocytes: normal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. Journal of Experimental Medicine. 1997;185(5):953–961. doi: 10.1084/jem.185.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alcamo E, Mizgerd JP, Horwitz BH, et al. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-κB in leukocyte recruitment. Journal of Immunology. 2001;167(3):1592–1600. doi: 10.4049/jimmunol.167.3.1592. [DOI] [PubMed] [Google Scholar]
- 28.Kontgen F, Grumont RJ, Strasser A, et al. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes & Development. 1995;9(16):1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 29.Weih F, Carrasco D, Durham SK, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family. Cell. 1995;80(2):331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 30.Brand K, Page S, Rogler G, et al. Activated transcription factor nuclear factor-κB is present in the atherosclerotic lesion. Journal of Clinical Investigation. 1996;97(7):1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaltschmidt C, Kaltschmidt B, Lannes-Vieira J, et al. Transcription factor NF-κB is activated in microglia during experimental autoimmune encephalomyelitis. Journal of Neuroimmunology. 1994;55(1):99–106. doi: 10.1016/0165-5728(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 32.Parhami F, Fang ZT, Fogelman AM, Andalibi A, Territo MC, Berliner JA. Minimally modified low density lipoprotein-induced inflammatory responses in endothelial cells are mediated by cyclic adenosine monophosphate. Journal of Clinical Investigation. 1993;92(1):471–478. doi: 10.1172/JCI116590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng H-B, Rajavashisth TB, Libby P, Liao JK. Nitric oxide inhibits macrophage-colony stimulating factor gene transcription in vascular endothelial cells. Journal of Biological Chemistry. 1995;270(28):17050–17055. doi: 10.1074/jbc.270.28.17050. [DOI] [PubMed] [Google Scholar]
- 34.Rajavashisth TB, Yamada H, Mishra NK. Transcriptional activation of the macrophage-colony stimulating factor gene by minimally modified LDL: involvement of nuclear factor-κB. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15(10):1591–1598. doi: 10.1161/01.atv.15.10.1591. [DOI] [PubMed] [Google Scholar]
- 35.Brand K, Eisele T, Kreusel U, et al. Dysregulation of monocytic nuclear factor-κB by oxidized low-density lipoprotein. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17(10):1901–1909. doi: 10.1161/01.atv.17.10.1901. [DOI] [PubMed] [Google Scholar]
- 36.Brand K, Page S, Walli AK, Neumeier D, Baeuerle PA. Role of nuclear factor-κB in atherogenesis. Experimental Physiology. 1997;82(2):297–304. doi: 10.1113/expphysiol.1997.sp004025. [DOI] [PubMed] [Google Scholar]
- 37.Wolfrum S, Teupser D, Tan M, Chen KY, Breslow JL. The protective effect of A20 on atherosclerosis in apolipoprotein E-deficient mice is associated with reduced expression of NF-κB target genes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(47):18601–18606. doi: 10.1073/pnas.0709011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gareus R, Kotsaki E, Xanthoulea S, et al. Endothelial cell-specific NF-κB inhibition protects mice from atherosclerosis. Cell Metabolism. 2008;8(5):372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Kim KM, Choi JY, Yoo S-E, et al. HMCO5, herbal extract, inhibits NF-κB expression in lipopolysaccharide treated macrophages and reduces atherosclerotic lesions in cholesterol fed mice. Journal of Ethnopharmacology. 2007;114(3):316–324. doi: 10.1016/j.jep.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Geng C, Jiang L, et al. The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. European Journal of Nutrition. 2008;47(5):235–243. doi: 10.1007/s00394-008-0717-8. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Zhang R, Xu Y, Zhou H, Wang B, Li S. Genistein inhibits the development of atherosclerosis via inhibiting NF-κB and VCAM-1 expression in LDLR knockout mice. Canadian Journal of Physiology and Pharmacology. 2008;86(11):777–784. doi: 10.1139/Y08-085. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Yan F, Liu Y, et al. Aqueous extract of rhubarb stabilizes vulnerable atherosclerotic plaques due to depression of inflammation and lipid accumulation. Phytotherapy Research. 2008;22(7):935–942. doi: 10.1002/ptr.2429. [DOI] [PubMed] [Google Scholar]
- 43.Li H, Dai M, Jia W. Paeonol attenuates high-fat-diet-induced atherosclerosis in rabbits by anti-inflammatory activity. Planta Medica. 2009;75(1):7–11. doi: 10.1055/s-0028-1088332. [DOI] [PubMed] [Google Scholar]
- 44.Thourani VH, Brar SS, Kennedy TP, et al. Nonanticoagulant heparin inhibits NF-κB activation and attenuates myocardial reperfusion injury. American Journal of Physiology. 2000;278(6):H2084–H2093. doi: 10.1152/ajpheart.2000.278.6.H2084. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki H, Ray PS, Zhu L, Galang N, Maulik N. Oxidative stress due to hypoxia/reoxygenation induces angiogenic factor VEGF in adult rat myocardium: possible role of NFκB. Toxicology. 2000;155(1–3):27–35. doi: 10.1016/s0300-483x(00)00274-2. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki H, Ray PS, Zhu L, Otani H, Asahara T, Maulik N. Hypoxia/reoxygenation promotes myocardial angiogenesis via an NFκB-dependent mechanism in a rat model of chronic myocardial infarction. Journal of Molecular and Cellular Cardiology. 2001;33(2):283–294. doi: 10.1006/jmcc.2000.1299. [DOI] [PubMed] [Google Scholar]
- 47.Yoshiyama M, Omura T, Takeuchi K, et al. Angiotensin blockade inhibits increased JNKs, AP-1 and NFκB DNA-binding activities in myocardial infarcted rats. Journal of Molecular and Cellular Cardiology. 2001;33(4):799–810. doi: 10.1006/jmcc.2001.1351. [DOI] [PubMed] [Google Scholar]
- 48.Izumi T, Saito Y, Kishimoto I, et al. Blockade of the natriuretic peptide receptor guanylyl cyclase-A inhibits NF-κB activation and alleviates myocardial ischemia/reperfusion injury. Journal of Clinical Investigation. 2001;108(2):203–213. doi: 10.1172/JCI12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimizu M, Tamamori-Adachi M, Arai H, Tabuchi N, Tanaka H, Sunamori M. Lipopolysaccharide pretreatment attenuates myocardial infarct size: a possible mechanism involving heat shock protein 70-inhibitory κBα complex and attenuation of nuclear factor κB. Journal of Thoracic and Cardiovascular Surgery. 2002;124(5):933–941. doi: 10.1067/mtc.2002.122305. [DOI] [PubMed] [Google Scholar]
- 50.Thiemermann C. Inhibition of the activation of nuclear factor κB to reduce myocardial reperfusion injury and infarct size. Cardiovascular Research. 2004;63(1):8–10. doi: 10.1016/j.cardiores.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 51.Lu L, Chen SS, Zhang JQ, Ramires FJ, Sun Y. Activation of nuclear factor-κB and its proinflammatory mediator cascade in the infarcted rat heart. Biochemical and Biophysical Research Communications. 2004;321(4):879–885. doi: 10.1016/j.bbrc.2004.07.048. [DOI] [PubMed] [Google Scholar]
- 52.Verma IM, Stevenson JK, Schwarz EM, van Antwerp D, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes & Development. 1995;9(22):2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 53.Kawakami K, Scheidereit C, Roeder RG. Identification and purification of a human immunoglobulin-enhancer-binding protein (NF-κB) that activates transcription from a human immunodeficiency virus type 1 promoter in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(13):4700–4704. doi: 10.1073/pnas.85.13.4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baeuerle PA, Baltimore D. A 65-κD subunit of active NF-κB is required for inhibition of NF-κB by IκB. Genes & Development. 1989;3(11):1689–1698. doi: 10.1101/gad.3.11.1689. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh S, Gifford AM, Riviere LR, Tempst P, Nolan GP, Baltimore D. Cloning of the p50 DNA binding subunit of NF-κB: homology to Rel and dorsal. Cell. 1990;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- 56.Zabel U, Schreck R, Baeuerle PA. DNA binding of purified transcription factor NF-κB. Affinity, specificity, Zn2+ dependence, and differential half-site recognition. Journal of Biological Chemistry. 1991;266(1):252–260. [PubMed] [Google Scholar]
- 57.Kang S-M, Tran A-C, Grilli M, Lenardo MJ. NF-κB subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992;256(5062):1452–1455. doi: 10.1126/science.1604322. [DOI] [PubMed] [Google Scholar]
- 58.Plaksin D, Baeuerle PA, Eisenbach L. KBF1 (p50 NF-κB homodimer) acts as a repressor of H-2Kb gene expression in metastatic tumor cells. Journal of Experimental Medicine. 1993;177(6):1651–1662. doi: 10.1084/jem.177.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown AM, Linhoff MW, Stein B, et al. Function of NF-κB/Rel binding sites in the major histocompatibility complex class II invariant chain promoter is dependent on cell-specific binding of different NF-κB/Rel subunits. Molecular and Cellular Biology. 1994;14(5):2926–2935. doi: 10.1128/mcb.14.5.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blair WS, Bogerd HP, Madore SJ, Cullen BR. Mutational analysis of the transcription activation domain of RelA: identification of a highly synergistic minimal acidic activation module. Molecular and Cellular Biology. 1994;14(11):7226–7234. doi: 10.1128/mcb.14.11.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dobrzanski P, Ryseck RP, Bravo R. Both N- and C-terminal domains of RelB are required for full transactivation: role of the N-terminal leucine zipper-like motif. Molecular and Cellular Biology. 1993;13(3):1572–1582. doi: 10.1128/mcb.13.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghosh S, Baltimore D. Activation in vitro of NF-κB by phosphorylation of its inhibitor IκB. Nature. 1990;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- 63.Israel A. The IKK complex: an integrator of all signals that activate NF-κB? Trends in Cell Biology. 2000;10(4):129–133. doi: 10.1016/s0962-8924(00)01729-3. [DOI] [PubMed] [Google Scholar]
- 64.Hayden MS, Ghosh S. Signaling to NF-κB. Genes & Development. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 65.Mosialos G, Hamer P, Capobianco AJ, Laursen RA, Gilmore TD. A protein kinase-A recognition sequence is structurally linked to transformation by p59(v-rel) and cytoplasmic retention of p68(c-rel) Molecular and Cellular Biology. 1991;11(12):5867–5877. doi: 10.1128/mcb.11.12.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Norris JL, Manley JL. Selective nuclear transport of the Drosophila morphogen dorsal can be established by a signaling pathway involving the transmembrane protein Toll and protein kinase A. Genes & Development. 1992;6(9):1654–1667. doi: 10.1101/gad.6.9.1654. [DOI] [PubMed] [Google Scholar]
- 67.Naumann M, Scheidereit C. Activation of NF-κB in vivo is regulated by multiple phosphorylations. EMBO Journal. 1994;13(19):4597–4607. doi: 10.1002/j.1460-2075.1994.tb06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li CCH, Dai RM, Chen E, Longo DL. Phosphorylation of NF-κB1-p50 is involved in NF-κB activation and stable DNA binding. Journal of Biological Chemistry. 1994;269(48):30089–30092. [PubMed] [Google Scholar]
- 69.Li CCH, Korner M, Ferris DK, Chen E, Dai RM, Longo DL. NF-κB/Rel family members are physically associated phosphoproteins. Biochemical Journal. 1994;303(2):499–506. doi: 10.1042/bj3030499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-κB is regulated by the IκB- associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89(3):413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 71.Vermeulen L, De Wilde G, van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-κB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO Journal. 2003;22:1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Molecular Cell. 1998;1(5):661–671. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 73.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-κB determines its association with CBP/p300 or HDAC-1. Molecular Cell. 2002;9(3):625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 74.Duran A, Diaz-Meco MT, Moscat J. Essential role of RelA Ser311 phosphorylation by ζPKC in NF-κB transcriptional activation. EMBO Journal. 2003;22(15):3910–3918. doi: 10.1093/emboj/cdg370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang D, Westerheide SD, Hanson JL, Baldwin AS., Jr. Tumor necrosis factor α-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. Journal of Biological Chemistry. 2000;275(42):32592–32597. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 76.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. Journal of Biological Chemistry. 1999;274(43):30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 77.Jiang X, Takahashi N, Matsui N, Tetsuka T, Okamoto T. The NF-κB activation in lymphotoxin β receptor signaling depends on the phosphorylation of p65 at serine 536. Journal of Biological Chemistry. 2003;278(2):919–926. doi: 10.1074/jbc.M208696200. [DOI] [PubMed] [Google Scholar]
- 78.O’Mahony AM, Montano M, van Beneden K, Chen L-F, Greene WC. Human T-cell lymphotropic virus type 1 tax induction of biologically active NF-κB requires IκB kinase-1-mediated phosphorylation of RelA/p65. Journal of Biological Chemistry. 2004;279(18):18137–18145. doi: 10.1074/jbc.M401397200. [DOI] [PubMed] [Google Scholar]
- 79.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr. Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-κB through utilization of the IκB kinase and activation of the mitogen-activated protein kinase p38. Journal of Biological Chemistry. 2001;276(22):18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 80.Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin- 1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Molecular and Cellular Biology. 1999;19(7):4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bohuslav J, Chen L-F, Kwon H, Mu Y, Greene WC. p53 induces NF-κB activation by an IκB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. Journal of Biological Chemistry. 2004;279(25):26115–26125. doi: 10.1074/jbc.M313509200. [DOI] [PubMed] [Google Scholar]
- 82.Fujita F, Taniguchi Y, Kato T, et al. Identification of NAP1, a regulatory subunit of IκB kinase-related kinases that potentiates NF-κB signaling. Molecular and Cellular Biology. 2003;23(21):7780–7793. doi: 10.1128/MCB.23.21.7780-7793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buss H, Dorrie A, Schmitz ML, Hoffmann E, Resch K, Kracht M. Constitutive and interleukin-1-inducible phosphorylation of p65 NF-κB at serine 536 is mediated by multiple protein kinases including IκB kinase (IKK)-α, IKKβ, IKKε, TRAF family member-associated (TANK)-binding kinase 1 (TBK1), and an unknown kinase and couples p65 to TATA-binding protein-associated factor II31-mediated interleukin-8 transcription. Journal of Biological Chemistry. 2004;279(53):55633–55643. doi: 10.1074/jbc.M409825200. [DOI] [PubMed] [Google Scholar]
- 84.Logeat F, Israel N, Ten R, et al. Inhibition of transcription factors belonging to the rel/NF-κB family by a transdominant negative mutant. EMBO Journal. 1991;10(7):1827–1832. doi: 10.1002/j.1460-2075.1991.tb07708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruben SM, Narayanan R, Klement JF, Chen C-H, Rosen CA. Functional characterization of the NF-κB p65 transcriptional activator and an alternatively spliced derivative. Molecular and Cellular Biology. 1992;12(2):444–454. doi: 10.1128/mcb.12.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ganchi PA, Sun S-C, Greene WC, Ballard DW. A novel NF-κB complex containing p65 homodimers: implications for transcriptional control at the level of subunit dimerization. Molecular and Cellular Biology. 1993;13(12):7826–7835. doi: 10.1128/mcb.13.12.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ryseck R-P, Novotny J, Bravo R. Characterization of elements determining the dimerization properties of RelB and p50. Molecular and Cellular Biology. 1995;15(6):3100–3109. doi: 10.1128/mcb.15.6.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kunsch C, Ruben SM, Rosen CA. Selection of optimal κB/Rel DNA-binding motifs: interaction of both subunits of NF-κB with DNA is required for transcriptional activation. Molecular and Cellular Biology. 1992;12(10):4412–4421. doi: 10.1128/mcb.12.10.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rice NR, MacKichan ML, Israel A. The precursor of NF-κB p50 has IκB-like functions. Cell. 1992;71(2):243–253. doi: 10.1016/0092-8674(92)90353-e. [DOI] [PubMed] [Google Scholar]
- 90.Bressler P, Brown K, Timmer W, Bours V, Siebenlist U, Fauci AS. Mutational analysis of the p50 subunit of NF-κB and inhibition of NF-κB activity by trans-dominant p50 mutants. Journal of Virology. 1993;67(1):288–293. doi: 10.1128/jvi.67.1.288-293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baeuerle PA, Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of the NF-κB transcription factor. Cell. 1988;53(2):211–217. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]
- 92.Zabel U, Baeuerle PA. Purified human IκB can rapidly dissociate the complex of the NF-κB transcription factor with its cognate DNA. Cell. 1990;61(2):255–265. doi: 10.1016/0092-8674(90)90806-p. [DOI] [PubMed] [Google Scholar]
- 93.Whiteside ST, Israel A. IκB proteins: structure, function and regulation. Seminars in Cancer Biology. 1997;8(2):75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- 94.Totzke G, Essmann F, Pohlmann S, Lindenblatt C, Janicke RU, Schulze-Osthoff K. A novel member of the IκB family, human IκBζ, inhibits transactivation of p65 and its DNA binding. Journal of Biological Chemistry. 2006;281(18):12645–12654. doi: 10.1074/jbc.M511956200. [DOI] [PubMed] [Google Scholar]
- 95.Zhang Q, Didonato JA, Karin M, Mckeithan TW. BCL3 encodes a nuclear protein which can alter the subcellular location of NF-κB proteins. Molecular and Cellular Biology. 1994;14(6):3915–3926. doi: 10.1128/mcb.14.6.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Franzoso G, Bours V, Park S, Tomita-Yamaguchi M, Kelly K, Siebenlist U. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-κB-mediated inhibition. Nature. 1992;359(6393):339–342. doi: 10.1038/359339a0. [DOI] [PubMed] [Google Scholar]
- 97.Franzoso G, Bours V, Azarenko V, et al. The oncoprotein Bcl-3 can facilitate NF-κB-mediated transactivation by removing inhibiting p50 homodimers from select κB sites. EMBO Journal. 1993;12(10):3893–3901. doi: 10.1002/j.1460-2075.1993.tb06067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Inoue J, Takahara T, Akizawa T, Hino O. Bcl-3, a member of the IκB proteins, has distinct specificity towards the Rel family of proteins. Oncogene. 1993;8(8):2067–2073. [PubMed] [Google Scholar]
- 99.Haskill S, Beg AA, Tompkins SM, et al. Characterization of an immediate-early gene induced in adherent monocytes that encodes IκB-like activity. Cell. 1991;65(7):1281–1289. doi: 10.1016/0092-8674(91)90022-q. [DOI] [PubMed] [Google Scholar]
- 100.Davis N, Ghosh S, Simmons DL, et al. Rel-associated pp40: an inhibitor of the Rel family of transcription factors. Science. 1991;253(5025):1268–1271. doi: 10.1126/science.1891714. [DOI] [PubMed] [Google Scholar]
- 101.Beg AA, Baldwin AS., Jr. The IκB proteins: multifunctional regulators of Rel/NF-κB transcription factors. Genes & Development. 1993;7(11):2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- 102.Breeden L, Nasmyth K. Similarity between cell-cycle genes of budding yeast and fission yeast and the Notch gene of Drosophila. Nature. 1987;329(6140):651–654. doi: 10.1038/329651a0. [DOI] [PubMed] [Google Scholar]
- 103.Lux SE, John KM, Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature. 1990;344(6261):36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- 104.Michaely P, Bennett V. The ANK repeat: a ubiquitous motif involved in macromolecular recognition. Trends in Cell Biology. 1992;2(5):127–129. doi: 10.1016/0962-8924(92)90084-z. [DOI] [PubMed] [Google Scholar]
- 105.Davis JQ, Bennett V. The anion exchanger and Na+K+-ATPase interact with distinct sites on ankyrin in in vitro assays. Journal of Biological Chemistry. 1990;265(28):17252–17256. [PubMed] [Google Scholar]
- 106.Hatada EN, Nieters A, Wulczyn FG, et al. The ankyrin repeat domains of the NF-κB precursor p105 and the protooncogene Bcl-3 act as specific inhibitors of NF-κB DNA binding. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(6):2489–2493. doi: 10.1073/pnas.89.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Inoue J-I, Kerr LD, Rashid D, Davis N, Bose HR, Jr., Verma IM. Direct association of pp40/IκBβ with rel/NF-κB transcription factors: role of ankyrin repeats in the inhibition of DNA binding activity. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(10):4333–4337. doi: 10.1073/pnas.89.10.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ganchi PA, Sun S-C, Greene WC, Ballard DW. IκB/MAD-3 masks the nuclear localization signal of NF-κB p65 and requires the transactivation domain to inhibit NF-κB p65 DNA binding. Molecular Biology of the Cell. 1992;3(12):1339–1352. doi: 10.1091/mbc.3.12.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ernst MK, Dunn LL, Rice NR. The PEST-like sequence of IκBα is responsible for inhibition of DNA binding but not for cytoplasmic retention of c-Rel or RelA homodimers. Molecular and Cellular Biology. 1995;15(2):872–882. doi: 10.1128/mcb.15.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beauparlant P, Lin R, Hiscott J. The role of the C-terminal domain of IκBα in protein degradation and stabilization. Journal of Biological Chemistry. 1996;271(18):10690–10696. doi: 10.1074/jbc.271.18.10690. [DOI] [PubMed] [Google Scholar]
- 111.McElhinny JA, Trushin SA, Bren GD, Chester N, Paya CV. Casein kinase II phosphorylates IκBα at S-283, S-289, S-293, and T-291 and is required for its degradation. Molecular and Cellular Biology. 1996;16(3):899–906. doi: 10.1128/mcb.16.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin R, Beauparlant P, Makris C, Meloche S, Hiscott J. Phosphorylation of IκBα in the C-terminal PEST domain by casein kinase II affects intrinsic protein stability. Molecular and Cellular Biology. 1996;16(4):1401–1409. doi: 10.1128/mcb.16.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schwarz EM, van Antwerp D, Verma IM. Constitutive phosphorylation of IκBα by casein kinase II occurs preferentially at serine 293: requirement for degradation of free IκBα . Molecular and Cellular Biology. 1996;16(7):3554–3559. doi: 10.1128/mcb.16.7.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brockman JA, Scherer DC, McKinsey TA, et al. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Molecular and Cellular Biology. 1995;15(5):2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267(5203):1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 116.Traenckner EB-M, Pahl HL, Henkel T, Schmidt KN, Wilk S, Baeuerle PA. Phosphorylation of human IκB-α on serines 32 and 36 controls IκB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO Journal. 1995;14(12):2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Whiteside ST, Ernst MK, LeBail O, Laurent-Winter C, Rice N, Israel A. N- and C-terminal sequences control degradation of MAD3/IκBα in response to inducers of NF-κB activity. Molecular and Cellular Biology. 1995;15(10):5339–5345. doi: 10.1128/mcb.15.10.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leveillard T, Verma IM. Diverse molecular mechanisms of inhibition of NF-κB/DNA binding complexes by IκB proteins. Gene Expression. 1993;3(2):135–150. [PMC free article] [PubMed] [Google Scholar]
- 119.Johnson C, van Antwerp D, Hope TJ. An N-terminal nuclear export signal is required for the nucleocytoplasmic shuttling of IκBα . EMBO Journal. 1999;18(23):6682–6693. doi: 10.1093/emboj/18.23.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arenzana-Seisdedos F, Turpin P, Rodriguez M, et al. Nuclear localization of IκBα promotes active transport of NF-κB from the nucleus to the cytoplasm. Journal of Cell Science. 1997;110(3):369–378. doi: 10.1242/jcs.110.3.369. [DOI] [PubMed] [Google Scholar]
- 121.Ghosh G, van Duyne G, Ghosh S, Sigler PB. Structure of NF-κB p50 homodimer bound to a κB site. Nature. 1995;373(6512):303–310. doi: 10.1038/373303a0. [DOI] [PubMed] [Google Scholar]
- 122.Muller CW, Rey FA, Sodeoka M, Verdine GL, Harrison SC. Structure of the NF-κB p50 homodimer bound to DNA. Nature. 1995;373(6512):311–317. doi: 10.1038/373311a0. [DOI] [PubMed] [Google Scholar]
- 123.Beg AA, Ruben SM, Scheinman RI, Haskill S, Rosen CA, Baldwin AS., Jr. IκB interacts with the nuclear localization sequences of the subunits of NF-κB: a mechanism for cytoplasmic retention. Genes & Development. 1992;6(10):1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- 124.Henkel T, Zabel U, van Zee K, Muller JM, Fanning E, Baeuerle PA. Intramolecular masking of the nuclear location signal and dimerization domain in the precursor for the p50 NF-κB subunit. Cell. 1992;68(6):1121–1133. doi: 10.1016/0092-8674(92)90083-o. [DOI] [PubMed] [Google Scholar]
- 125.Zabel U, Henkel T, Silva MS, Baeuerle PA. Nuclear uptake control of NF-κB by MAD-3, an IκB protein present in the nucleus. EMBO Journal. 1993;12(1):201–211. doi: 10.1002/j.1460-2075.1993.tb05646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dobrzanski P, Ryseck R-P, Bravo R. Differential interactions of Rel-NF-κB complexes with IκBα determine pools of constitutive and inducible NF-κB activity. EMBO Journal. 1994;13(19):4608–4616. doi: 10.1002/j.1460-2075.1994.tb06782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Simeonidis S, Stauber D, Chen G, Hendrickson WA, Thanos D. Mechanisms by which IκB proteins control NF-κB activity. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(1):49–54. doi: 10.1073/pnas.96.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Suyang H, Phillips R, Douglas I, Ghosh S. Role of unphosphorylated, newly synthesized IκBβ in persistent activation of NF-κB. Molecular and Cellular Biology. 1996;16(10):5444–5449. doi: 10.1128/mcb.16.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun S-C, Ganchi PA, Ballard DW, Greene WC. NF-κB controls expression of inhibitor IκBα: evidence for an inducible autoregulatory pathway. Science. 1993;259(5103):1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 130.Cheng Q, Cant CA, Moll T, et al. NF-κB subunit-specific regulation of the IκBα promoter. Journal of Biological Chemistry. 1994;269(18):13551–13557. [PubMed] [Google Scholar]
- 131.Chiao PJ, Miyamoto S, Verma IM. Autoregulation of IκBα activity. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(1):28–32. doi: 10.1073/pnas.91.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Arenzana-Seisdedos F, Thompson J, Rodriguez MS, Bachelerie F, Thomas D, Hay RT. Inducible nuclear expression of newly synthesized IκBα negatively regulates DNA-binding and transcriptional activities of NF-κB. Molecular and Cellular Biology. 1995;15(5):2689–2696. doi: 10.1128/mcb.15.5.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Luo J-L, Kamata H, Karin M. IKK/NF-κB signaling: balancing life and death—a new approach to cancer therapy. Journal of Clinical Investigation. 2005;115(10):2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Viatour P, Merville M-P, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends in Biochemical Sciences. 2005;30(1):43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 135.Mercurio F, Zhu H, Murray BW, et al. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278(5339):860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 136.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science. 1997;278(5339):866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 137.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell. 1997;91(2):243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 138.Rothwarf DM, Zandi E, Natoli G, Karin M. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature. 1998;395(6699):297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 139.Yamaoka S, Courtois G, Bessia C, et al. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell. 1998;93(7):1231–1240. doi: 10.1016/s0092-8674(00)81466-x. [DOI] [PubMed] [Google Scholar]
- 140.Mercurio F, Murray BW, Shevchenko A, et al. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Molecular and Cellular Biology. 1999;19(2):1526–1538. doi: 10.1128/mcb.19.2.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mellits KH, Hay RT, Goodbourn S. Proteolytic degradation of MAD3 (IκBα) and enhanced processing of the NF-κB precursor p105 are obligatory steps in the activation of NF-κB. Nucleic Acids Research. 1993;21(22):5059–5066. doi: 10.1093/nar/21.22.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barroga CF, Stevenson JK, Schwarz EM, Verma IM. Constitutive phosphorylation of IκBα by casein kinase II. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(17):7637–7641. doi: 10.1073/pnas.92.17.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weil R, Laurent-Winter C, Israel A. Regulation of IκBβ degradation: similarities to and differences from IκBα . Journal of Biological Chemistry. 1997;272(15):9942–9949. doi: 10.1074/jbc.272.15.9942. [DOI] [PubMed] [Google Scholar]
- 144.Krappmann D, Wulczyn FG, Scheidereit C. Different mechanisms control signal-induced degradation and basal turnover of the NF-κB inhibitor IκBα in vivo. EMBO Journal. 1996;15(23):6716–6726. [PMC free article] [PubMed] [Google Scholar]
- 145.Miyamoto S, Seufzer BJ, Shumway SD. Novel IκBα proteolytic pathway in WEHI231 immature B cells. Molecular and Cellular Biology. 1998;18(1):19–29. doi: 10.1128/mcb.18.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Phillips RJ, Ghosh S. Regulation of IκBβ in WEHI 231 mature B cells. Molecular and Cellular Biology. 1997;17(8):4390–4396. doi: 10.1128/mcb.17.8.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shumway SD, Miyamoto S. A mechanistic insight into a proteasome-independent constitutive inhibitor κBα (IκBα) degradation and nuclear factor κB (NF-κB) activation pathway in WEHI-231 B-cells. Biochemical Journal. 2004;380(1):173–180. doi: 10.1042/BJ20031796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shumway SD, Maki M, Miyamoto S. The PEST domain of IκBα is necessary and sufficient for in vitro degradation by μ-calpain. Journal of Biological Chemistry. 1999;274(43):30874–39881. doi: 10.1074/jbc.274.43.30874. [DOI] [PubMed] [Google Scholar]
- 149.Shumway SD, Berchtold CM, Gould MN, Miyamoto S. Evidence for unique calmodulin-dependent nuclear factor-κB regulation in WEHI-231 B cells. Molecular Pharmacology. 2002;61(1):177–185. doi: 10.1124/mol.61.1.177. [DOI] [PubMed] [Google Scholar]
- 150.Jiang Y, Woronicz JD, Liu W, Goeddel DV. Prevention of constitutive TNF receptor 1 signaling by silencer of death domains. Science. 1999;283(5401):543–546. doi: 10.1126/science.283.5401.543. [DOI] [PubMed] [Google Scholar]
- 151.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J-I, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398(6724):252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 152.Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. International Immunopharmacology. 2001;1(4):625–635. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 153.Nemoto S, DiDonato JA, Lin A. Coordinate regulation of IκB kinases by mitogen-activated protein kinase kinase kinase 1 and NF-κB-inducing kinase. Molecular and Cellular Biology. 1998;18(12):7336–7343. doi: 10.1128/mcb.18.12.7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ducut Sigala JL, Bottero V, Young DB, Shevchenko A, Mercurio F, Verma IM. Activation of transcription factor NF-κB requiries ELKS, an IκB kinase regulatory subunit. Science. 2004;304(5679):1963–1967. doi: 10.1126/science.1098387. [DOI] [PubMed] [Google Scholar]
- 155.Regnier CH, Song HY, Gao X, Goeddel DV, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90(2):373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 156.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388(6642):548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 157.Zandi E, Karin M. Bridging the gap: composition, regulation, and physiological function of the IκB kinase complex. Molecular and Cellular Biology. 1999;19(7):4547–4551. doi: 10.1128/mcb.19.7.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nakano H, Shindo M, Sakon S, et al. Differential regulation of IκB kinase α and β by two upstream kinases, NF-κB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(7):3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu C, Ghosh S. Differential phosphorylation of the signal-responsive domain of IκBα and IκBβ by IκB kinases. Journal of Biological Chemistry. 2003;278(34):31980–31987. doi: 10.1074/jbc.M304278200. [DOI] [PubMed] [Google Scholar]
- 160.Zandi E, Chen Y, Karin M. Direct phosphorylation of IκB by IKKα and IKKβ: discrimination between free and NF-κB-bound substrate. Science. 1998;281(5381):1360–1363. doi: 10.1126/science.281.5381.1360. [DOI] [PubMed] [Google Scholar]
- 161.Ling L, Cao Z, Goeddel DV. Nf-κB-inducing kinase activates IKK-α by phosphorylation of Ser-176. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(7):3792–3797. doi: 10.1073/pnas.95.7.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Delhase M, Hayakawa M, Chen Y, Karin M. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science. 1999;284(5412):309–313. doi: 10.1126/science.284.5412.309. [DOI] [PubMed] [Google Scholar]
- 163.Hu Y, Baud V, Delhase M, et al. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science. 1999;284(5412):316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 164.Li Z-W, Chu W, Hu Y, et al. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. Journal of Experimental Medicine. 1999;189(11):1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Doi TS, Marino MW, Takahashi T, et al. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(6):2994–2999. doi: 10.1073/pnas.96.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274(5288):782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 167.DiDonato JA, Mercurio F, Karin M. Phosphorylation of IκBα precedes but is not sufficient for its dissociation from NF-κB. Molecular and Cellular Biology. 1995;15(3):1302–1311. doi: 10.1128/mcb.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Traenckner EB-M, Baeuerle PA. Appearance of apparently ubiquitin-conjugated IκB-α during its phosphorylation-induced degradation in intact cells. Journal of Cell Science. 1995;19:79–84. doi: 10.1242/jcs.1995.supplement_19.11. [DOI] [PubMed] [Google Scholar]
- 169.Chen Z, Hagler J, Palombella VJ, et al. Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin-proteasome pathway. Genes & Development. 1995;9(13):1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 170.Alkalay I, Yaron A, Hatzubai A, Orian A, Ciechanover A, Ben-Neriah Y. Stimulation-dependent IκBα phosphorylation marks the NF-κb inhibitor for degradation via the ubiquitin-proteasome pathway. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(23):10599–10603. doi: 10.1073/pnas.92.23.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li C-CH, Dai R-M, Longo DL. Inactivation of NF-κB inhibitor IκBα: ubiquitin-dependent proteolysis and its degradation product. Biochemical and Biophysical Research Communications. 1995;215(1):292–301. doi: 10.1006/bbrc.1995.2465. [DOI] [PubMed] [Google Scholar]
- 172.Ciechanover A. The ubiquitin-proteasome proteolytic pathway. Cell. 1994;79(1):13–21. doi: 10.1016/0092-8674(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 173.Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Current Opinion in Cell Biology. 1995;7(2):215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 174.Murray A. Cyclin ubiquitination: the destructive end of mitosis. Cell. 1995;81(2):149–152. doi: 10.1016/0092-8674(95)90322-4. [DOI] [PubMed] [Google Scholar]
- 175.Wilkinson KD. Ubiquitin-dependent signaling: the role of ubiquitination in the response of cells to their environment. Journal of Nutrition. 1999;129(11):1933–1936. doi: 10.1093/jn/129.11.1933. [DOI] [PubMed] [Google Scholar]
- 176.Henkel T, Machleidt T, Alkalay I, Kronke M, Ben-Neriah Y, Baeuerle PA. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature. 1993;365(6442):182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 177.Sun S-C, Elwood J, Beraud C, Greene WC. Human T-cell leukemia virus type I Tax activation of NF-κB/Rel involves phosphorylation and degradation of IκBα and RelA (p65)-mediated induction of the c-rel gene. Molecular and Cellular Biology. 1994;14(11):7377–7384. doi: 10.1128/mcb.14.11.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Traenckner EB-M, Wilk S, Baeuerle PA. A proteasome inhibitor prevents activation of NF-κB and stabilizes a newly phosphorylated form of IκB-α that is still bound to NF-κB. EMBO Journal. 1994;13(22):5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Finco TS, Beg AA, Baldwin AS., Jr. Inducible phosphorylation of IκBα is not sufficient for its dissociation from NF-κB and is inhibited by protease inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(25):11884–11888. doi: 10.1073/pnas.91.25.11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Miyamoto S, Maki M, Schmitt MJ, Hatanaka M, Verma IM. Tumor necrosis factor α-induced phosphorylation of IκBα is a signal for its degradation but not dissociation from NF-κB. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(26):12740–12744. doi: 10.1073/pnas.91.26.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lin Y-C, Brown K, Siebenlist U. Activation of NF-κB requires proteolysis of the inhibitor IκB-α: signal-induced phosphorylation of IκB-α alone does not release active NF- κB. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(2):552–556. doi: 10.1073/pnas.92.2.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Alkalay I, Yaron A, Hatzubai A, et al. In vivo stimulation of IκB phosphorylation is not sufficient to activate NF-κB. Molecular and Cellular Biology. 1995;15(3):1294–1301. doi: 10.1128/mcb.15.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hartmann-Petersen R, Gordon C. Proteins interacting with the 26S proteasome. Cellular and Molecular Life Sciences. 2004;61(13):1589–1595. doi: 10.1007/s00018-004-4132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78(5):773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 185.Rodriguez MS, Wright J, Thompson J, et al. Identification of lysine residues required for signal-induced ubiquitination and degradation of IκB-α in vivo. Oncogene. 1996;12(11):2425–2435. [PubMed] [Google Scholar]
- 186.Griscavage JM, Wilk S, Ignarro LJ. Inhibitors of the proteasome pathway interfere with induction of nitric oxide synthase in macrophages by blocking activation of transcription factor NF-κB. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(8):3308–3312. doi: 10.1073/pnas.93.8.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Scherer DC, Brockman JA, Chen Z, Maniatis T, Ballard DW. Signal-induced degradation of IκBα requires site-specific ubiquitination. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(24):11259–11263. doi: 10.1073/pnas.92.24.11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Baldi L, Brown K, Franzoso G, Siebenlist U. Critical role for lysines 21 and 22 in signal-induced, ubiquitin-mediated proteolysis of IκB-α . Journal of Biological Chemistry. 1996;271(1):376–379. doi: 10.1074/jbc.271.1.376. [DOI] [PubMed] [Google Scholar]
- 189.Rodriguez MS, Michalopoulos I, Arenzana-Seisdedos F, Hay RT. Inducible degradation of IκBα in vitro and in vivo requires the acidic C-terminal domain of the protein. Molecular and Cellular Biology. 1995;15(5):2413–2419. doi: 10.1128/mcb.15.5.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Aoki T, Sano Y, Yamamoto T, Inoue J-I. The ankyrin repeats but not the PEST-like sequences are required for signal-dependent degradation of IκBα . Oncogene. 1996;12(5):1159–1164. [PubMed] [Google Scholar]
- 191.Sun S-C, Elwood J, Greene WC. Both amino- and carboxyl-terminal sequences within IκBα regulate its inducible degradation. Molecular and Cellular Biology. 1996;16(3):1058–1065. doi: 10.1128/mcb.16.3.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.DiDonato J, Mercurio F, Rosette C, et al. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Molecular and Cellular Biology. 1996;16(4):1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Harhaj EW, Maggirwar SB, Good L, Sun S-C. CD28 mediates a potent costimulatory signal for rapid degradation of IκBβ which is associated with accelerated activation of various NF-κB/Rel heterodimers. Molecular and Cellular Biology. 1996;16(12):6736–6743. doi: 10.1128/mcb.16.12.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.McKinsey TA, Brockman JA, Scherer DC, Al-Murrani SW, Green PL, Ballard DW. Inactivation of IκBβ by the tax protein of human T-cell leukemia virus type 1: a potential mechanism for constitutive induction of NF-κB. Molecular and Cellular Biology. 1996;16(5):2083–2090. doi: 10.1128/mcb.16.5.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Dohmen RJ. SUMO protein modification. Biochimica et Biophysica Acta. 2004;1695(1–3):113–131. doi: 10.1016/j.bbamcr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 196.Hay RT. SUMO: a history of modification. Molecular Cell. 2005;18(1):1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 197.Mabb AM, Miyamoto S. SUMO and NF-κB ties. Cellular and Molecular Life Sciences. 2007;64(15):1979–1996. doi: 10.1007/s00018-007-7005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liu B, Shuai K. Regulation of the sumoylation system in gene expression. Current Opinion in Cell Biology. 2008;20(3):288–293. doi: 10.1016/j.ceb.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Desterro JMP, Rodriguez MS, Hay RT. SUMO-1 modification of IκBα inhibits NF-κB activation. Molecular Cell. 1998;2(2):233–239. doi: 10.1016/s1097-2765(00)80133-1. [DOI] [PubMed] [Google Scholar]
- 200.Rodriguez MS, Dargemont C, Hay RT. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. Journal of Biological Chemistry. 2001;276(16):12654–12659. doi: 10.1074/jbc.M009476200. [DOI] [PubMed] [Google Scholar]
- 201.Guo D, Li M, Zhang Y, et al. A functional variant of SUMO4, a new IκBα modifier, is associated with type diabetes. Nature Genetics. 2004;36(8):837–841. doi: 10.1038/ng1391. [DOI] [PubMed] [Google Scholar]
- 202.Wang C-Y, Yang P, Li M, Gong F. Characterization of a negative feedback network between SUMO4 expression and NFκB transcriptional activity. Biochemical and Biophysical Research Communications. 2009;381(4):477–481. doi: 10.1016/j.bbrc.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 203.Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. Journal of Cell Biology. 1996;135(6):1457–1470. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88(1):97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- 205.Müller S, Matunis MJ, Dejean A. Conjugation with the ubiquitin-related modifier SUMO-1 regulates the partitioning of PML within the nucleus. EMBO Journal. 1998;17(1):61–70. doi: 10.1093/emboj/17.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kim JH, Baek SH. Emerging roles of desumoylating enzymes. Biochimica et Biophysica Acta. 2009;1792(3):155–162. doi: 10.1016/j.bbadis.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 207.Comerford KM, Leonard MO, Karhausen J, Carey R, Colgan SP, Taylor CT. Small ubiquitin-related modifier-1 modification mediates resolution of CREB-dependent responses to hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(3):986–991. doi: 10.1073/pnas.0337412100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Liu Q, Li J, Khoury J, Colgan SP, Ibla JC. Adenosine signaling mediates SUMO-1 modification of IκBα during hypoxia and reoxygenation. Journal of Biological Chemistry. 2009;284(20):13686–13695. doi: 10.1074/jbc.M809275200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Noso S, Ikegami H, Fujisawa T, et al. Genetic heterogeneity in association of the SUMO4 M55V variant with susceptibility to type 1 diabetes. Diabetes. 2005;54(12):3582–3586. doi: 10.2337/diabetes.54.12.3582. [DOI] [PubMed] [Google Scholar]
- 210.Tsurumaru M, Kawasaki E, Ida H, et al. Evidence for the role of small ubiquitin-like modifier 4 as a general autoimmunity locus in the Japanese population. Journal of Clinical Endocrinology and Metabolism. 2006;91(8):3138–3143. doi: 10.1210/jc.2006-0206. [DOI] [PubMed] [Google Scholar]
- 211.Wang C-Y, She J-X. SUMO4 and its role in type 1 diabetes pathogenesis. Diabetes/Metabolism Research and Reviews. 2008;24(2):93–102. doi: 10.1002/dmrr.797. [DOI] [PubMed] [Google Scholar]
- 212.Jennings CE, Owen CJ, Wilson V, Pearce SHS. No association of the codon 55 methionine to valine polymorphism in the SUMO4 gene with Graves’ disease. Clinical Endocrinology. 2005;62(3):362–365. doi: 10.1111/j.1365-2265.2005.02224.x. [DOI] [PubMed] [Google Scholar]
- 213.Gibbons LJ, Thomson W, Zeggini E, et al. The type 1 diabetes susceptibility gene SUMO4 at IDDM5 is not associated with susceptibility to rheumatoid arthritis or juvenile idiopathic arthritis. Rheumatology. 2005;44(11):1390–1393. doi: 10.1093/rheumatology/kei041. [DOI] [PubMed] [Google Scholar]
- 214.Costas J, Perez-Pampin E, Ferreiros-Vidal I, et al. SUMO4 and MAP3K7IP2 single nucleotide polymorphisms and susceptibility to rheumatoid arthritis. Journal of Rheumatology. 2006;33(6):1048–1051. [PubMed] [Google Scholar]
- 215.Orozco G, Sánchez E, González-Gay MA, et al. SLC22A4, RUNX1, and SUMO4 polymorphisms are not associated with rheumatoid arthritis: a case-control study in a Spanish population. Journal of Rheumatology. 2006;33(7):1235–1239. [PubMed] [Google Scholar]
- 216.Orozco G, Sánchez E, Gómez LM, et al. Study of the role of functional variants of SLC22A4, RUNX1 and SUMO4 in systemic lupus erythematosus. Annals of the Rheumatic Diseases. 2006;65(6):791–795. doi: 10.1136/ard.2005.044891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Huang TT, Wuerzberger-Davis SM, Wu Z-H, Miyamoto S. Sequential modification of NEMO/IKKγ by SUMO-1 and ubiquitin mediates NF-κB activation by genotoxic stress. Cell. 2003;115(5):565–576. doi: 10.1016/s0092-8674(03)00895-x. [DOI] [PubMed] [Google Scholar]
- 218.Wuerzberger-Davis SM, Nakamura Y, Seufzer BJ, Miyamoto S. NF-κB activation by combinations of NEMO SUMOylation and ATM activation stresses in the absence of DNA damage. Oncogene. 2007;26(5):641–651. doi: 10.1038/sj.onc.1209815. [DOI] [PubMed] [Google Scholar]
- 219.Mabb AM, Wuerzberger-Davis SM, Miyamoto S. PIASy mediates NEMO sumoylation and NF-κB activation in response to genotoxic stress. Nature Cell Biology. 2006;8(9):986–993. doi: 10.1038/ncb1458. [DOI] [PubMed] [Google Scholar]
- 220.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor κB pathway. Oncogene. 2006;25(51):6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 221.Neumann M, Naumann M. Beyond IκBs: alternative regulation of NF-κB activity. FASEB Journal. 2007;21(11):2642–2654. doi: 10.1096/fj.06-7615rev. [DOI] [PubMed] [Google Scholar]
- 222.Marienfeld R, Berberich-Siebelt F, Berberich I, Denk A, Serfling E, Neumann M. Signal-specific and phosphorylation-dependent RelB degradation: a potential mechanism of NF-κB control. Oncogene. 2001;20(56):8142–8147. doi: 10.1038/sj.onc.1204884. [DOI] [PubMed] [Google Scholar]
- 223.Maier HJ, Marienfeld R, Wirth T, Baumann B. Critical role of RelB serine 368 for dimerization and p100 stabilization. Journal of Biological Chemistry. 2003;278(40):39242–39250. doi: 10.1074/jbc.M301521200. [DOI] [PubMed] [Google Scholar]
- 224.Druker BJ, Neumann M, Okuda K, Franza BR, Jr., Griffin JD. rel Is rapidly tyrosine-phosphorylated following granulocyte-colony stimulating factor treatment of human neutrophils. Journal of Biological Chemistry. 1994;269(7):5387–5390. [PubMed] [Google Scholar]
- 225.Fognani C, Rondi R, Romano A, Blasi F. cRel-TD kinase: a serine/threonine kinase binding in vivo and in vitro c-Rel and phosphorylating its transactivation domain. Oncogene. 2000;19(18):2224–2232. doi: 10.1038/sj.onc.1203543. [DOI] [PubMed] [Google Scholar]
- 226.Martin AG, Fresno M. Tumor necrosis factor-α activation of NF-κB requires the phosphorylation of Ser-471 in the transactivation domain of c-Rel. Journal of Biological Chemistry. 2000;275(32):24383–24391. doi: 10.1074/jbc.M909396199. [DOI] [PubMed] [Google Scholar]
- 227.Yu S-H, Chiang W-C, Shih H-M, Wu K-J. Stimulation of c-Rel transcriptional activity by PKA catalytic subunit β . The Journal of Molecular Medicine. 2004;82(9):621–628. doi: 10.1007/s00109-004-0559-7. [DOI] [PubMed] [Google Scholar]
- 228.Harris J, Olière S, Sharma S, et al. Nuclear accumulation of cRel following C-terminal phosphorylation by TBK1/IKKε . The Journal of Immunology. 2006;177(4):2527–2535. doi: 10.4049/jimmunol.177.4.2527. [DOI] [PubMed] [Google Scholar]
- 229.Sánchez-Valdepeñas C, Martín AG, Ramakrishnan P, Wallach D, Fresno M. NF-κB-inducing kinase is involved in the activation of the CD28 responsive element through phosphorylation of c-Rel and regulation of its transactivating activity. The Journal of Immunology. 2006;176(8):4666–4674. doi: 10.4049/jimmunol.176.8.4666. [DOI] [PubMed] [Google Scholar]
- 230.Guan H, Hou S, Ricciardi RP. DNA binding of repressor nuclear factor-κB p50/p50 depends on phosphorylation of Ser337 by the protein kinase A catalytic subunit. Journal of Biological Chemistry. 2005;280(11):9957–9962. doi: 10.1074/jbc.M412180200. [DOI] [PubMed] [Google Scholar]
- 231.Ray A, Prefontaine KE. Physical association and functional antagonism between the p65 subunit of transcription factor NF-κB and the glucocorticoid receptor. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(2):752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Caldenhoven E, Liden J, Wissink S, et al. Negative cross-talk between RelA and the glucocorticoid receptor: a possible mechanism for the antiinflammatory action of glucocorticoids. Molecular Endocrinology. 1995;9(4):401–412. doi: 10.1210/mend.9.4.7659084. [DOI] [PubMed] [Google Scholar]
- 233.Scheinman RI, Gualberto A, Jewell CM, Cidlowski JA, Baldwin AS., Jr. Characterization of mechanisms involved in transrepression of NF-κB by activated glucocorticoid receptors. Molecular and Cellular Biology. 1995;15(2):943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Liden J, Rafter I, Truss M, Gustafsson J-A, Okret S. Glucocorticoid effects on NF-κB binding in the transcription of the ICAM-1 gene. Biochemical and Biophysical Research Communications. 2000;273(3):1008–1014. doi: 10.1006/bbrc.2000.3079. [DOI] [PubMed] [Google Scholar]
- 235.Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFκB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes & Development. 2000;14(18):2314–2329. doi: 10.1101/gad.827900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Martens C, Bilodeau S, Maira M, Gauthier Y, Drouin J. Protein-protein interactions and transcriptional antagonism between the subfamily of NGFI-B/Nur77 orphan nuclear receptors and glucocorticoid receptor. Molecular Endocrinology. 2005;19(4):885–897. doi: 10.1210/me.2004-0333. [DOI] [PubMed] [Google Scholar]
- 237.Reichardt HM, Kaestner KH, Tuckermann J, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93(4):531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 238.Reichardt HM, Tuckermann JP, Göttlicher M, et al. Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO Journal. 2001;20(24):7168–7173. doi: 10.1093/emboj/20.24.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270(5234):286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 240.Scheinman RI, Cogswell PC, Lofquist AK, Baldwin AS., Jr. Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science. 1995;270(5234):283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 241.Brostjan C, Anrather J, Csizmadia V, et al. Glucocorticoid-mediated repression of NFκB activity in endothelial cells does not involve induction of IκBα synthesis. Journal of Biological Chemistry. 1996;271(32):19612–19616. doi: 10.1074/jbc.271.32.19612. [DOI] [PubMed] [Google Scholar]
- 242.Heck S, Bender K, Kullmann M, Göttlicher M, Herrlich P, Cato ACB. IκBα-independent downregulation of NF-κB activity by glucocorticoid receptor. EMBO Journal. 1997;16(15):4698–4707. doi: 10.1093/emboj/16.15.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Newton R, Hart LA, Stevens DA, et al. Effect of dexamethasone on interleukin-1β-(IL-1β)-induced nuclear factor-κB (NF-κB) and κB-dependent transcription in epithelial cells. The European Journal of Biochemistry. 1998;254(1):81–89. doi: 10.1046/j.1432-1327.1998.2540081.x. [DOI] [PubMed] [Google Scholar]
- 244.Wissink S, van Heerde EC, van der Burg B, van der Saag PT. A dual mechanism mediates repression of NF-κB activity by glucocorticoids. Molecular Endocrinology. 1998;12(3):355–363. doi: 10.1210/mend.12.3.0081. [DOI] [PubMed] [Google Scholar]
- 245.de Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-κB or activator protein-1: molecular mechanisms for gene repression. Endocrine Reviews. 2003;24(4):488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 246.Ito K, Barnes PJ, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1β-induced histone H4 acetylation on lysines 8 and 12. Molecular and Cellular Biology. 2000;20(18):6891–6903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ito K, Yamamura S, Essilfie-Quaye S, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-κB suppression. The Journal of Experimental Medicine. 2006;203(1):7–13. doi: 10.1084/jem.20050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kagoshima M, Wilcke T, Ito K, et al. Glucocorticoid-mediated transrepression is regulated by histone acetylation and DNA methylation. The European Journal of Pharmacology. 2001;429(1–3):327–334. doi: 10.1016/s0014-2999(01)01332-2. [DOI] [PubMed] [Google Scholar]
- 249.Saccani S, Pantano S, Natoli G. p38-dependent marking of inflammatory genes for increased NF-κB recruitment. Nature Immunology. 2002;3(1):69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 250.Schulz M, Eggert M, Baniahmad A, Dostert A, Heinzel T, Renkawitz R. RU486-induced glucocorticoid receptor agonism is controlled by the receptor N terminus and by corepressor binding. Journal of Biological Chemistry. 2002;277(29):26238–26243. doi: 10.1074/jbc.M203268200. [DOI] [PubMed] [Google Scholar]
- 251.Wang Q, Blackford JA, Jr., Song L-N, Huang Y, Cho S, Simons SS., Jr. Equilibrium interactions of corepressors and coactivators with agonist and antagonist complexes of glucocorticoid receptors. Molecular Endocrinology. 2004;18(6):1376–1395. doi: 10.1210/me.2003-0421. [DOI] [PubMed] [Google Scholar]
- 252.Garside H, Stevens A, Farrow S, et al. Glucocorticoid ligands specify different interactions with NF-κB by allosteric effects on the glucocorticoid receptor DNA binding domain. Journal of Biological Chemistry. 2004;279(48):50050–50059. doi: 10.1074/jbc.M407309200. [DOI] [PubMed] [Google Scholar]
- 253.Ronacher K, Hadley K, Avenant C, et al. Ligand-selective transactivation and transrepression via the glucocorticoid receptor: role of cofactor interaction. Molecular and Cellular Endocrinology. 2009;299(2):219–231. doi: 10.1016/j.mce.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 254.Hart L, Sam L, Adcock I, Barnes PJ, Chung KF. Effects of inhaled corticosteroid therapy on expression and DNA-binding activity of nuclear factor κB in asthma. The American Journal of Respiratory and Critical Care Medicine. 2000;161(1):224–231. doi: 10.1164/ajrccm.161.1.9809019. [DOI] [PubMed] [Google Scholar]
- 255.Kassel O, Sancono A, Kraötzschmar J, Kreft B, Stassen M, Cato ACB. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO Journal. 2001;20(24):7108–7116. doi: 10.1093/emboj/20.24.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Lasa M, Brook M, Saklatvala J, Clark AR. Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Molecular and Cellular Biology. 2001;21(3):771–780. doi: 10.1128/MCB.21.3.771-780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Molecular and Cellular Biology. 2002;22(22):7802–7811. doi: 10.1128/MCB.22.22.7802-7811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Engelbrecht Y, de Wet H, Horsch K, Langeveldt CR, Hough FS, Hulley PA. Glucocorticoids induce rapid up-regulation of mitogen-activated protein kinase phosphatase-1 and dephosphorylation of extracellular signal-regulated kinase and impair proliferation in human and mouse osteoblast cell lines. Endocrinology. 2003;144(2):412–422. doi: 10.1210/en.2002-220769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wu W, Chaudhuri S, Brickley DR, Pang D, Karrison T, Conzen SD. Microarray analysis reveals glucocorticoid-regulated survival genes that are associated with inhibition of apoptosis in breast epithelial cells. Cancer Research. 2004;64(5):1757–1764. doi: 10.1158/0008-5472.can-03-2546. [DOI] [PubMed] [Google Scholar]
- 260.Fürst R, Schroeder T, Eilken HM, et al. MAPK phosphatase-1 represents a novel anti-inflammatory target of glucocorticoids in the human endothelium. FASEB Journal. 2007;21(1):74–80. doi: 10.1096/fj.06-6752com. [DOI] [PubMed] [Google Scholar]
- 261.Cho IJ, Kim SG. A novel mitogen-activated protein kinase phosphatase-1 and glucocorticoid receptor (GR) interacting protein-1-dependent combinatorial mechanism of gene transrepression by GR. Molecular Endocrinology. 2009;23(1):86–99. doi: 10.1210/me.2008-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Bladh L-G, Johansson-Haque K, Rafter I, Nilsson S, Okret S. Inhibition of extracellular signal-regulated kinase (ERK) signaling participates in repression of nuclear factor (NF)-κB activity by glucocorticoids. Biochimica et Biophysica Acta. 2009;1793(3):439–446. doi: 10.1016/j.bbamcr.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 263.King EM, Holden NS, Gong W, Rider CF, Newton R. Inhibition of NF-κB-dependent transcription by MKP-1: transcriptional repression by glucocorticoids occuring via p38 MAPK. Journal of Biological Chemistry. 2009;284(39):26803–26815. doi: 10.1074/jbc.M109.028381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Ro H-S, Kim S-W, Wu D, Webber C, Nicholson TE. Gene structure and expression of the mouse adipocyte enhancer-binding protein. Gene. 2001;280(1-2):123–133. doi: 10.1016/s0378-1119(01)00771-5. [DOI] [PubMed] [Google Scholar]
- 265.Majdalawieh A, Zhang L, Fuki IV, Rader DJ, Ro H-S. Adipocyte enhancer-binding protein 1 is a potential novel atherogenic factor involved in macrophage cholesterol homeostasis and inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2346–2351. doi: 10.1073/pnas.0508139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Majdalawieh A, Zhang L, Ro H-S. Adipocyte enhancer-binding protein-1 promotes macrophage inflammatory responsiveness by up-regulating NF-κB via IκBα negative regulation. Molecular Biology of the Cell. 2007;18(3):930–942. doi: 10.1091/mbc.E06-03-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Majdalawieh A, Ro H-S. LPS-induced suppression of macrophage cholesterol efflux is mediated by adipocyte enhancer-binding protein 1. International Journal of Biochemistry and Cell Biology. 2009;41(7):1518–1525. doi: 10.1016/j.biocel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 268.Reznik SE, Fricker LD. Carboxypeptidases from A to Z: implications in embryonic development and Wnt binding. Cellular and Molecular Life Sciences. 2001;58(12-13):1790–1804. doi: 10.1007/PL00000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Xin X, Day R, Dong W, Lei Y, Fricker LD. Identification of mouse CPX-2, a novel member of the metallocarboxypeptidase gene family: cDNA cloning, mRNA distribution, and protein expression and characterization. DNA and Cell Biology. 1998;17(10):897–909. doi: 10.1089/dna.1998.17.897. [DOI] [PubMed] [Google Scholar]
- 270.Lei Y, Xin X, Morgan D, Pintar JE, Fricker LD. Identification of mouse CPX-1, a novel member of the metallocarboxypeptidase gene family with highest similarity to CPX-2. DNA and Cell Biology. 1999;18(2):175–185. doi: 10.1089/104454999315565. [DOI] [PubMed] [Google Scholar]
- 271.He G-P, Muise A, Li AW, Ro H-S. A eukaryotic transcriptional repressor with carboxypeptidase activity. Nature. 1995;378(6552):92–96. doi: 10.1038/378092a0. [DOI] [PubMed] [Google Scholar]
- 272.Muise AM, Ro H-S. Enzymic characterization of a novel member of the regulatory B-like carboxypeptidase with transcriptional repression function: stimulation of enzymic activity by its target DNA. Biochemical Journal. 1999;343(2):341–345. [PMC free article] [PubMed] [Google Scholar]
- 273.Kim S-W, Muise AM, Lyons PJ, Ro H-S. Regulation of adipogenesis by a transcriptional repressor that modulates MAPK activation. Journal of Biological Chemistry. 2001;276(13):10199–10206. doi: 10.1074/jbc.M010640200. [DOI] [PubMed] [Google Scholar]
- 274.Lyons PJ, Muise AM, Ro H-S. MAPK modulates the DNA binding of adipocyte enhancer-binding protein 1. Biochemistry. 2005;44(3):926–931. doi: 10.1021/bi0480178. [DOI] [PubMed] [Google Scholar]
- 275.Zhang L, Reidy SP, Nicholson TE, et al. The role of AEBP1 in sex-specific diet-induced obesity. Molecular Medicine. 2005;11(1–12):39–47. doi: 10.2119/2005-00021.Ro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ro H-S, Zhang L, Majdalawieh A, et al. Adipocyte enhancer-binding protein 1 modulates adiposity and energy homeostasis. Obesity. 2007;15(2):288–302. doi: 10.1038/oby.2007.569. [DOI] [PubMed] [Google Scholar]
- 277.Mansour SJ, Matten WT, Hermann AS, et al. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265(5174):966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 278.Wilkinson MG, Millar JBA. Control of the eukaryotic cell cycle by MAP kinase signaling pathways. FASEB Journal. 2000;14(14):2147–2157. doi: 10.1096/fj.00-0102rev. [DOI] [PubMed] [Google Scholar]
- 279.Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101(12):4667–4679. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 280.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiology and Molecular Biology Reviews. 2004;68(2):320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Rosen SD, Kafka JA, Simpson DL, Barondes SH. Developmentally regulated, carbohydrate binding protein in Dictyostelium discoideum. Proceedings of the National Academy of Sciences of the United States of America. 1973;70(9):2554–2557. doi: 10.1073/pnas.70.9.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Springer WR, Cooper DNW, Barondes SH. Discoidin I is implicated in cell-substratum attachment and ordered cell migration of Dictyostelium discoideum and resembles fibronectin. Cell. 1984;39(3):557–564. doi: 10.1016/0092-8674(84)90462-8. [DOI] [PubMed] [Google Scholar]
- 283.Johnson JD, Edman JC, Rutter WJ. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(12):5677–5681. doi: 10.1073/pnas.90.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Prag S, Collett GDM, Adams JC. Molecular analysis of muskelin identifies a conserved discoidin-like domain that contributes to protein self-association. Biochemical Journal. 2004;381(2):547–559. doi: 10.1042/BJ20040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Delerive P, Gervois P, Fruchart J-C, Staels B. Induction of IκBα expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-α activators. Journal of Biological Chemistry. 2000;275(47):36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 286.Azuma M, Motegi K, Aota K, Yamashita T, Yoshida H, Sato M. TGF-β1 inhibits NF-κB activity through induction of IκB-α expression in human salivary gland cells: a possible mechanism of growth suppression by TGF-β1. Experimental Cell Research. 1999;250(1):213–222. doi: 10.1006/excr.1999.4503. [DOI] [PubMed] [Google Scholar]
- 287.Saile B, Matthes N, El Armouche H, Neubauer K, Ramadori G. The bcl, NF-κB and p53/p21WAF1 systems are involved in spontaneous apoptosis and in the anti-apoptotic effect of TGFb or TNFα on activated hepatic stellate cells. The European Journal of Cell Biology. 2001;80(8):554–561. doi: 10.1078/0171-9335-00182. [DOI] [PubMed] [Google Scholar]
- 288.Park J-G, Muise A, He G-P, Kim S-W, Ro H-S. Transcriptional regulation by the γ5 subunit of a heterotrimeric G protein during adipogenesis. EMBO Journal. 1999;18(14):4004–4012. doi: 10.1093/emboj/18.14.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kane WH, Davie EW. Cloning of a cDNA coding for human factor V, a blood coagulation factor homologous to factor VIII and ceruloplasmin. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(18):6800–6804. doi: 10.1073/pnas.83.18.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Stubbs JD, Lekutis C, Singer KL, et al. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(21):8417–8421. doi: 10.1073/pnas.87.21.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Vijayasarathy C, Takada Y, Zeng Y, Bush RA, Sieving PA. Retinoschisin is a peripheral membrane protein with affinity for anionic phospholipids and affected by divalent cations. Investigative Ophthalmology and Visual Science. 2007;48(3):991–1000. doi: 10.1167/iovs.06-0915. [DOI] [PubMed] [Google Scholar]
- 292.Arakawa A, Matsuo-Takasaki M, Takai A, et al. The secreted EGF-Discoidin factor xDel1 is essential for dorsal development of the Xenopus embryo. Developmental Biology. 2007;306(1):160–169. doi: 10.1016/j.ydbio.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 293.Baumgartner S, Hofmann K, Chiquet-Ehrismann R, Bucher P. The discoidin domain family revisited: new members from prokaryotes and a homology-based fold prediction. Protein Science. 1998;7(7):1626–1631. doi: 10.1002/pro.5560070717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- 295.Thompson JE, Phillips RJ, Erdjument-Bromage H, Tempst P, Ghosh S. IκB-β regulates the persistent response in a biphasic activation of NF-κB. Cell. 1995;80(4):573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 296.Inoue J-I, Kerr LD, Kakizuka A, Verma IM. IκBγ, a 70 kd protein identical to the C-terminal half of p110 NF-κB: a new member of the IκB family. Cell. 1992;68(6):1109–1120. doi: 10.1016/0092-8674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- 297.Whiteside ST, Epinat J-C, Rice NR, Israeöl A. IκBε, a novel member of the IκB family, controls RelA and cRel NF-κB activity. EMBO Journal. 1997;16(6):1413–1426. doi: 10.1093/emboj/16.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Nolan GP, Fujita T, Bhatia K, et al. The bcl-3 proto-oncogene encodes a nuclear IκB-like molecule that preferentially interacts with NF-κB p50 and p52 in a phosphorylation- dependent manner. Molecular and Cellular Biology. 1993;13(6):3557–3566. doi: 10.1128/mcb.13.6.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Mercurio F, DiDonato JA, Rosette C, Karin M. p105 and p98 precursor proteins play an active role in NF-κB-mediated signal transduction. Genes & Development. 1993;7(4):705–718. doi: 10.1101/gad.7.4.705. [DOI] [PubMed] [Google Scholar]
- 300.Fujita T, Nolan GP, Ghosh S, Baltimore D. Independent modes of transcriptional activation by the p50 and p65 subunits of NF-κB. Genes & Development. 1992;6(5):775–787. doi: 10.1101/gad.6.5.775. [DOI] [PubMed] [Google Scholar]
- 301.Lin R, Gewert D, Hiscott J. Differential transcriptional activation in vitro by NF-κB/Rel proteins. Journal of Biological Chemistry. 1995;270(7):3123–3131. doi: 10.1074/jbc.270.7.3123. [DOI] [PubMed] [Google Scholar]
- 302.Pomerantz JL, Denny EM, Baltimore D. CARD11 mediates factor-specific activation of NF-κB by the T cell receptor complex. EMBO Journal. 2002;21(19):5184–5194. doi: 10.1093/emboj/cdf505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Chinetti G, Griglio S, Antonucci M, et al. Activation of proliferator-activated receptors α and γ induces apoptosis of human monocyte-derived macrophages. Journal of Biological Chemistry. 1998;273(40):25573–25580. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 304.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 305.Zhou Y-C, Waxman DJ. Cross-talk between Janus kinase-signal transducer and activator of transcription (JAK-STAT) and peroxisome proliferator-activated receptor-α (PPARα) signaling pathways: growth hormone inhibition of PPARα transcriptional activity mediated by STAT5b. Journal of Biological Chemistry. 1999;274(5):2672–2681. doi: 10.1074/jbc.274.5.2672. [DOI] [PubMed] [Google Scholar]
- 306.Straus DS, Pascual G, Li M, et al. 15-deoxy-Δ12,14-prostaglandin J2 inhibits multiple steps in the NF-κB signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(9):4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nature Medicine. 2003;9(2):213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 308.Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. Journal of Biological Chemistry. 2003;278(12):10443–10449. doi: 10.1074/jbc.M213071200. [DOI] [PubMed] [Google Scholar]
- 309.Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARγ and PPARδ negatively regulate specific subsets of lipopolysaccharide and IFNγ target genes in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(11):6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Chang L, Zhang Z, Li W, Dai J, Guan Y, Wang X. Liver-X-receptor activator prevents homocysteine-induced production of IgG antibodies from murine B lymphocytes via the ROS-NF-κB pathway. Biochemical and Biophysical Research Communications. 2007;357(3):772–778. doi: 10.1016/j.bbrc.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 311.Bonfield TL, Thomassen MJ, Farver CF, et al. Peroxisome proliferator-activated receptor-γ regulates the expression of alveolar macrophage macrophage colony-stimulating factor. The Journal of Immunology. 2008;181(1):235–242. doi: 10.4049/jimmunol.181.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Park SJ, Lee KS, Kim SR, et al. Peroxisome proliferator-activated receptor gamma agonist down-regulates IL-17 expression in a murine model of allergic airway inflammation. The Journal of Immunology. 2009;183(5):3259–3267. doi: 10.4049/jimmunol.0900231. [DOI] [PubMed] [Google Scholar]
- 313.Chung SW, Kang BY, Kim SH, et al. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-γ and nuclear factor-κB. Journal of Biological Chemistry. 2000;275(42):32681–32687. doi: 10.1074/jbc.M002577200. [DOI] [PubMed] [Google Scholar]
- 314.Weil R, Sirma H, Giannini C, et al. Direct association and nuclear import of the hepatitis B virus X protein with the NF-κB inhibitor IκBα . Molecular and Cellular Biology. 1999;19(9):6345–6354. doi: 10.1128/mcb.19.9.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Gao H, Sun Y, Wu Y, et al. Identification of β-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-κB pathways. Molecular Cell. 2004;14(3):303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 316.Lee D-K, Kang J-E, Park H-J, et al. FBI-1 enhances transcription of the nuclear factor-κB (NF-κB)-responsive E-selectin gene by nuclear localization of the p65 subunit of NF-κB. Journal of Biological Chemistry. 2005;280(30):27783–27791. doi: 10.1074/jbc.M504909200. [DOI] [PubMed] [Google Scholar]
- 317.Le Negrate G, Krieg A, Faustin B, et al. ChlaDub1 of Chlamydia trachomatis suppresses NF-κB activation and inhibits IκBα ubiquitination and degradation. Cellular Microbiology. 2008;10(9):1879–1892. doi: 10.1111/j.1462-5822.2008.01178.x. [DOI] [PubMed] [Google Scholar]
- 318.Sorriento D, Ciccarelli M, Santulli G, et al. The G-protein-coupled receptor kinase 5 inhibits NFκB transcriptional activity by inducing nuclear accumulation of IκBα . Proceedings of the National Academy of Sciences of the United States of America. 2008;105(46):17818–17823. doi: 10.1073/pnas.0804446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Doceul V, Charleston B, Crooke H, Reid E, Powell PP, Seago J. The Npro product of classical swine fever virus interacts with IκBα, the NF-κB inhibitor. The Journal of General Virology. 2008;89(8):1881–1889. doi: 10.1099/vir.0.83643-0. [DOI] [PubMed] [Google Scholar]
- 320.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Current Opinion in Immunology. 1999;11(1):13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 321.Zhang G, Ghosh S. Molecular mechanisms of NF-κB activation induced by bacterial lipopolysaccharide through Toll-like receptors. The Journal of Endotoxin Research. 2000;6(6):453–457. doi: 10.1179/096805100101532414. [DOI] [PubMed] [Google Scholar]