Abstract
Objective
We compared longitudinal changes in the hippocampal structure in subjects with very mild dementia of the Alzheimer type (DAT) treated with donepezil, untreated very mild DAT subjects and nondemented controls.
Design
MPRAGE sequences were collected approximately two years apart on two 1.5T Siemens Vision systems, yielding two cohorts. Large-deformation high-dimensional brain mapping was used to compute deformation of hippocampal subfields.
Subjects
Subjects came from two sources: 18 untreated DAT subjects and 26 controls were drawn from a previous longitudinal study; 18 treated DAT subjects were studied prospectively, and 44 controls were drawn from a longitudinal study from the same time period.
Interventions
Patients attending a dementia clinic at Washington University School of Medicine were prescribed Donepezil by their physician.
Results
There was no significant cohort effect at baseline; therefore the two groups of control subjects were combined. The potential confounding effect of cohort/scanner was dealt with by including it as a covariate in statistical tests. There was no significant group effect in the rate-of-change of hippocampal volume or subfields deformation. Further exploration showed that compared with the untreated DAT subjects, the treated DAT subjects did not differ in the rate of change in any of the hippocampal measures. They also did not differ from the controls, while the untreated DAT subjects differed from the controls in the rates-of-change of hippocampal volume, CA1 and subiculum subfield deformations.
Conclusions
Treatment with donepezil did not alter the progression of hippocampal deformation in DAT subjects in this study. Small sample size may have contributed to this outcome.
Keywords: donepezil, Alzheimer’s disease, hippocampus, magnetic resonance imaging
INTRODUCTION
The effects of acetylcholinesterase (AChE) inhibitors on the symptoms of dementia of the Alzheimer type (DAT) can be highly variable1. Although AChE inhibitors demonstrate modest overall benefits compared with placebo for stabilizing or slowing decline in mild to moderate Alzheimer’s disease2, summary estimates show small effect sizes3. It is not currently possible to identify those who will respond to treatment prior to treatment4. Cognitive improvements associated with AChE inhibitor treatment are often only temporary and when declines in cognition resume, they proceed similarly in patients that have received treatment and placebo2–4. Moreover, there is no obvious neural mechanism by which AChE inhibitors might exert neuroprotective effects5, 6. Neuromorphometric measures are increasingly being studied as potential biomarkers for improved antemortem diagnosis of DAT and detection of disease modification following treatment7, 8. Using large-deformation high-dimensional brain mapping (HDBM-LD), we reported previously that reduced hippocampal volumes and inward deformations of the CA1 and subiculum subfields were present in very mild DAT subjects9, 10 and were correlated with a poorer response to donepezil treatment11. However, while volumetric MRI of whole brain or medial temporal lobe structures is now being considered as possible outcome measures in therapeutic trials for patients with DAT and mild cognitive impairment (MCI)12, 13, few studies have assessed the effects of treatment with AChE inhibitors on changes in brain structure, such as hippocampal degeneration, associated with the disease pathophysiology.
In this study, we used HDBM-LD to compare changes in hippocampal volume and surfaces proximal to the CA1 and subiculum subfields between healthy older adults and two groups of DAT subjects: One group treated with donepezil in an open-label study11, and a second group from a study prior to the availability of donepezil and which was therefore untreated. Although a prospective randomized study of this kind is desirable, it would be unethical to withhold treatment with an approved treatment for DAT patients for a period of time sufficient to assess the effects of treatment on brain structure. We tested the hypothesis that treatment with donepezil would alter the rate of hippocampal volume loss and surface deformation in patients with very mild DAT.
METHODS
Subject
Subjects in this study were drawn from two sources. The first source was a previously published prospective longitudinal study in 18 subjects with very mild DAT and 26 nondemented subjects9. These DAT subjects had been enrolled in the study prior to the widespread use of donepezil and were therefore untreated.
The second source of subjects included 18 donepezil-treated patients with very mild DAT who were participating in a longitudinal study of the treatment of mild DAT with donepezil11. The treating clinicians for these subjects were dementia specialists at the Memory Diagnostic Center at Washington University School of Medicine (WUSM). These patients received baseline clinical assessments within 30 days of treatment initiation, and every three months thereafter, for a maximum of two years. Forty four age-matched, community-dwelling, nondemented elderly subjects were selected as a control group from an ongoing longitudinal study of memory and aging at the WUSM Alzheimer Disease Research Center (ADRC) from the same time period.
Exclusion criteria for all subjects included genetic mutations linked to familial forms of dementia, or clinical diagnoses of other neuropsychiatric disorders (e.g., Lewy body disease, vascular dementia, depression) that could have confounded the diagnosis of DAT.
Clinical Assessment
The subjects were assessed at the time of magnetic resonance (MR) scanning (within a month). The Clinical Dementia Rating scale (CDR)14 was given to assess the presence, and if present, the severity of dementia symptoms. The CDR rates the presence or absence of cognitive impairment. A CDR score of 0 indicates no dementia and scores of 0.5, 1, 2, and 3 indicate very mild, mild, moderate and severe dementia, respectively. CDR assessments have been shown to have high inter-rater reliability when used at our ADRC15 and in multicenter studies of DAT16. Only CDR 0.5 subjects with DAT and CDR 0 subjects were included in this study.
ApoE allele status was known in 100 of the 106 subjects. Fifty nine of these 100 subjects had no ApoE4 alleles, 34 had a single ApoE4 allele, and 7 subjects had two ApoE4 alleles. In the treated DAT subject group, 3 had no ApoE4 alleles, 7 had a single ApoE4 allele and 2 had two ApoE4 alleles.
Cognitive Assessment
The subjects’ cognitive function performance was assessed independently and blinded of the clinical assessment in a comprehensive neuropsychological battery that included measures of episodic memory, semantic memory, speeded psychomotor performance, visuospatial ability, and attention17. This battery was used previously to describe the pattern of cognitive deficits in 407 individuals with very mild and mild DAT (CDR 0.5 and 1)18, and three factors (Temporal Factor, Parietal Factor, Frontal Factor) relating to the frequency of β-amyloid plaques in the temporal, parietal and frontal lobes were revealed. A general factor score was also computed19. In the present study, these four factor scores were calculated for each of the subjects using unit weightings of z-scores based on means and standard deviations derived from the prior study18. Mini mental state examination (MMSE) was not available for the untreated DAT subjects at the time of study. We therefore examined the Short Blessed Test20 and WMS logical memory21 scores as an additional comparison of the treated and untreated DAT subject characteristics.
Imaging and Brain Mapping
The subjects from the first source were scanned approximately two years apart on a 1.5-Tesla Vision system (Siemens Medical Systems), using a standard head coil and a MPRAGE sequence (TR=9.7ms, TE=4.0ms, flip angle=8°, 1×1×1 mm3 voxel resolution, scan time=11.0min) to collect one 3D T1-weighted volume.
The subjects from the second source were scanned approximately 1.5 years apart on a different 1.5T Siemens Vision system, using a standard head coil and identical MPRAGE sequence as above with slightly different protocols (TR=9.7ms, TE=4.0ms, flip angle=10°, 1×1×1.25mm3 voxel resolution, scan time=6.5 min) to collect multiple (2–4) 3D T1-weighted image volumes. The scans for each subject were aligned with the first scan and averaged to create a low-noise image volume22.
Landmark-based, large-deformation high-dimensional diffeomorphic mapping (HDBM-LD) was used to generate the surfaces of the hippocampus in baseline scans10, 23. For longitudinal mapping, baseline and follow-up scans were first registered using a nine-parameter affine transformation (rigid-body rotation and translation plus cardinal axis stretch)24 to adjust for changes in head position and scanner-drift25. Next, the landmarks that were placed in the baseline scans were transferred to the follow-up scans by applying the above affine transformations to the baseline landmarks. Then, the above HDBM-LD procedure was applied to all of the follow-up scans. (See Wang el al9, App. A, for rationale for this approach.)
Measurement of Changes in Hippocampal Structure
Left and right hippocampal volumes in each subject were calculated as the volumes enclosed by the hippocampal surfaces. An average hippocampal surface constructed from 86 healthy subjects10 was used as a reference surface, from which perpendicular deformation of each subject’s hippocampal surface was calculated at each surface point. Hippocampal surface zones corresponding to underlying subfields (i.e., CA1, subiculum and remainder) were obtained using methods previously described10, 26. For each subject, deformations were averaged within each surface zone to represent surface deformations for CA1, subiculum and remainder subfields10.
Statistical Analysis
Rates of change (per year) of the clinical, cognitive and hippocampal measures for each subject were calculated by dividing the difference at the two assessments by the interval. Group differences in the rates of change of hippocampal volume and subfield deformation were examined with general linear models using SAS 9.127, where left and right sides were treated as repeated measures. Appropriate post-hoc contrasts were used to examine pair-wise group differences in the rates of change for treated CDR 0.5 vs. CDR 0, untreated CDR 0.5 vs. CDR 0 and particularly treated vs. untreated comparisons. The analyses were also performed with gender as an additional covariate since the CDR 0 subjects consisted of mostly females (M/F=26/44) and while the CDR 0.5 subjects was male predominant (M/F=21/15) (chi-square=4.4, df=2, p=0.11). Similar analyses were performed on the rates of change of psychometric scores (without hemisphere).
RESULTS
The groups of 18 untreated CDR 0.5 subjects (mean±SD age=73.7±4.4 years, M/F=11/7) and 26 CDR 0 subjects (mean age=73.0±7.0 years, M/F=12/14) from the first source of subjects had similar ages and gender distributions. The groups of 18 treated CDR 0.5 subjects (mean age=74.0±5.2 years, M/F=10/8) and 44 CDR 0 subjects (mean age=74.7±5.4 years, M/F=14/30) from the second source had similar ages, but somewhat different gender distributions. The two groups of nondemented subjects did not differ in age (t=−0.68, df=68, p=0.50) or gender distribution (chi-square=1.4, df=1, p=0.23).
The two groups of nondemented subjects did not differ in baseline hippocampal volume (left: t=−0.81, p=0.42; right: t=−0.26, p=0.80; for all t-tests in this section, df=68), CA1 (left: t=−0.78, p=0.44; right: t=−41, p=0.68), or subiculum (left: t=−0.84, p=0.40; right: t=−1.4, p=0.17) subfield deformation, however they differed in the right remainder subfield deformation (t=−4.4, p<.0001), but not the left (t=−0.57, p=0.59). The two groups of nondemented subjects differed in the rates of change of left hippocampal volume (t=2.24, p=0.028), and left and right CA1 subfield deformation (left: t=2.28, p=0.026; right: t=2.32, p=0.023). The two groups of nondemented subjects were combined for use as a single comparison group, and cohort source was used as a covariate in all subsequent statistical analyses. We present subject sample characteristics and hippocampal measures for the combined control cohorts and the two DAT groups in Table 1 and Table 2, and separately for the two control cohorts in Table 3. We observe that for both baselin) measures and their rates of change, the variances renamed about the same whether the two cohorts of control subjects were combined or not.
Table 1.
Subject Age and Mean (SD) Psychometric Measurements at Each Visit.
| Group (M/F) | Visit 1 | Visit 2 | |
|---|---|---|---|
| Control (26/44) | Age | 73.7 (5.9) | 75.7 (6.0) |
| MMSE | 29 (1) | 29 (1) | |
| Short Blessed Test | 0.80 (1.22) | 0.81 (1.75) | |
| WMS Logical Memory | 10.0 (3.1) | 10.5 (2.7) | |
| General Factor | 0.62 (0.85) | 0.59 (0.90) | |
| Temporal Factor | 1.38 (0.62) | 1.47 (0.62) | |
| Parietal Factor | 0.97 (0.45) | 0.95 (0.46) | |
| Frontal Factor | 0.77 (0.65) | 0.70 (0.62) | |
| Treated (10/8) | Age | 74.7 (5.4) | 76.3 (5.4) |
| MMSE | 25 (4) | 25 (4) | |
| Short Blessed Test | 6.7 (5.7) | 12.3 (9.7) | |
| WMS Logical Memory | 4.7 (2.8) | 4.5 (2.6) | |
| General Factor | −1.86 (1.36) | −2.28 (1.48) | |
| Temporal Factor | 0.05 (0.88) | 0.22 (0.84) | |
| Parietal Factor | 0.24 (0.66) | −0.10 (0.87) | |
| Frontal Factor | −0.30 (0.37) | −0.43 (0.63) | |
| Untreated (11/7) | Age | 73.7 (4.4) | 75.7 (4.2) |
| MMSE | N/A | N/A | |
| Short Blessed Test | 4.6 (4.5) | 7.1 (8.4) | |
| WMS Logical Memory | 5.6 (3.2) | 5.1 (3.5) | |
| General Factor | −1.06 (1.05) | −1.37 (1.40) | |
| Temporal Factor | −0.01 (0.69) | −0.07 (0.76) | |
| Parietal Factor | 0.40 (0.52) | 0.26 (0.81) | |
| Frontal Factor | 0.11 (0.58) | −0.07 (0.59) |
Table 2. Statistical Test Results of Psychometric Measures.
Results of general linear models where cohort and gender were used as covariates. Significant or near-significant effects after Bonferroni corrections are shown in bold. For the comparison of rates of change, using baseline measures as an additional covariate is also reported. SBT: Short Blessed Test. WLM: WMS Logical Memory. The untreated and treated DAT subjects differed from the nondemented healthy subjects in the rates of change of the general and parietal factors. However, the untreated and treated DAT subjects did not differ from each other in the rates of change in any of the factors.
| Comparison | Factor Measure | Group Effect: F (df1, df2), p | Contrast: p | ||
|---|---|---|---|---|---|
| untreated vs. control | treated vs. control | treated vs. untreated | |||
| Baseline | WLM | 31 (2,99) <.0001 | <.0001 | <.0001 | 0.61 |
| SBT | 29 (2,99) <.0001 | <.0001 | <.0001 | 0.15 | |
| General | 46 (2,89) <.0001 | <.0001 | <.0001 | 0.42 | |
| Frontal | 19 (2,89) <.0001 | <.0001 | <.0001 | 0.77 | |
| Parietal | 15 (2,87) <.0001 | 0.0001 | 0.0002 | 0.58 | |
| Temporal | 37 (2,88) <.0001 | <.0001 | <.0001 | 0.36 | |
| Rates of Change | WLM | 2.4 (2,100) 0.096 | 0.12 | 0.12 | 0.95 |
| SBT | 12 (2,100) <.0001 | 0.11 | <0.0001 | 0.049 | |
| General | 6.5 (2,84) 0.0023 | 0.015 | 0.0098 | 0.67 | |
| Frontal | 0.03 (2,84) 0.97 | 0.98 | 0.81 | 0.84 | |
| Parietal | 4.1 (2,82) 0.021 | 0.34 | 0.0088 | 0.12 | |
| Temporal | 1.2 (2,81) 0.31 | 0.14 | 0.75 | 0.24 | |
| Rates of Change (with baseline as covariate) | WLM | 10.7 (2,98) <.0001 | 0.0007 | 0.0003 | 0.90 |
| SBT | 9.6 (2,98) 0.0002 | 0.070 | <.0001 | 0.050 | |
| General | 2.3 (2,83) 0.11 | 0.092 | 0.073 | 0.71 | |
| Frontal | 0.35 (2,83) 0.71 | 0.45 | 0.64 | 0.88 | |
| Parietal | 3.6 (2,81) 0.031 | 0.32 | 0.011 | 0.11 | |
| Temporal | 1.4 (2,80) 0.24 | 0.10 | 0.92 | 0.26 | |
Table 3. Mean (SD) Hippocampal Measurements for the two Control Cohorts.
For surface deformations, negative values for these measures represented inward variation of the surface while positive values for these measures represent outward variation of the surface. Rates of change (per year) of the hippocampal volume and subfield deformation were calculated by dividing the raw difference at the two time points by the inter-scan time interval. The two cohorts differed in the right remainder subfield deformation (t=−4.4, p<.0001) at visit 1, and differed in the rates of change of left hippocampal volume (t=2.24, p=0.028), left and right CA1 subfield deformation (left: t=2.28, p=0.026; right: t=2.32, p=0.023).
| Control Cohort | Left | Right | |||||
|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Rate of Change | Visit 1 | Visit 2 | Rate of Change | ||
| Volume (mm3) | Source 1 | 2081 (355) | 2094 (369) | 7.9 (92) | 2600 (481) | 2594 (518) | −1.1 (73) |
| Source 2 | 2153 (357) | 2105 (357) | −35 (67) | 2627 (364) | 2576 (390) | −31 (82) | |
| CA1 (mm) | Source 1 | −0.15 (0.39) | −0.13 (0.38) | 0.011 (0.092) | −0.037 (0.48) | −0.011 (0.51) | 0.014 (0.054) |
| Source 2 | −0.065 (0.43) | −0.12 (0.45) | −0.052 (0.12) | 0.005 (0.37) | −0.031 (0.39) | −0.028 (0.084) | |
| Subiculum (mm) | Source 1 | −0.036 (0.26) | −0.029 (0.28) | 0.004 (0.055) | −0.050 (0.27) | −0.052 (0.27) | −0.0002 (0.029) |
| Source 2 | 0.013 (0.22) | −0.0041 (0.22) | −0.009 (0.025) | 0.022 (0.17) | 0.0042 (0.20) | −0.009 (0.049) | |
| Remainder (mm) | Source 1 | −0.075 (0.24) | −0.060 (0.32) | 0.009 (0.086) | −0.041 (0.22) | −0.066 (0.23) | −0.014 (0.051) |
| Source 2 | −0.041 (0.24) | −0.27 (0.25) | −0.016 (0.052) | 0.19 (0.21) | 0.12 (0.26) | −0.047 (0.13) | |
There was a group difference in the between-scan time interval (F=4.5, df=2,103, p=0.014), with the treated CDR 0.5 subject having a shorter average interval (1.55±0.42 years) compared to the untreated subjects (1.96±0.37 years, LS Means contrast p=0.055) as well as the combined group of nondemented subjects (2.05±0.71 years, contrast p=0.0035). Therefore, rates of change of the hippocampal volume and subfield deformation were used in statistical analyses as opposed to using the hippocampal volume and subfield deformations at each time point in a repeated-measures type of analysis of variance.
Statistical comparison results for the hippocampal and psychometric measures are shown in Table 4 and Table 5, with gender and cohort used as covariates. Effect sizes (Cohen’s d) are also provided for the hippocampal measures (unadjusted for the covariates). At baseline, the main group effect was significant for hippocampal volume, left and right CA1 and left and right subiculum subfield deformations. Contrasts showed that while both untreated and treated groups differed from the nondemented subjects, the treated and untreated DAT groups did not differ from each other in any of these measures (as expected at baseline).
Table 4. Mean (SD) Hippocampal Measurements at Each Visit.
For surface deformations, negative values for these measures represented inward variation of the surface while positive values for these measures represent outward variation of the surface. Individual rates of change (per year) of the hippocampal volume and subfield deformation were calculated by dividing the raw difference at the two time points by the inter-scan time interval.
| Group | Left | Right | |||||
|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 2 | Rate of Change | Visit 1 | Visit 2 | Rate of Change | ||
| Volume (mm3) | Control | 2126 (355) | 2101 (359) | −19 (80) | 2617 (409) | 2583 (439) | −20 (80) |
| Treated | 1996 (387) | 1938 (371) | −50 (70) | 2328 (539) | 2262 (542) | −63 (114) | |
| Untreated | 1714 (224) | 1659 (210) | −32 (30) | 2184 (370) | 2102 (366) | −49 (54) | |
| CA1 (mm) | Control | −0.095 (0.42) | −0.12 (0.42) | −0.028 (0.11) | −0.011 (0.41) | −0.023 (0.44) | −0.012 (0.076) |
| Treated | −0.14 (0.51) | −0.22 (0.51) | −0.067 (0.083) | −0.22 (0.60) | −0.29 (0.62) | −0.057 (0.087) | |
| Untreated | −0.60 (0.29) | −0.66 (0.27) | −0.038 (0.029) | −0.35 (0.43) | −0.42 (0.43) | −0.036 (0.040) | |
| Subiculum (mm) | Control | −0.0053 (0.23) | −0.013 (0.24) | −0.004 (0.039) | −0.0044 (0.21) | −0.017 (0.23) | −0.005 (0.042) |
| Treated | −0.10 (0.23) | −0.13 (0.23) | −0.020 (0.041) | −0.18 (0.23) | −0.20 (0.23) | −0.021 (0.067) | |
| Untreated | −0.24 (0.18) | −0.28 (0.18) | −0.025 (0.024) | −0.24 (0.19) | −0.28 (0.21) | −0.025 (0.041) | |
| Remainder (mm) | Control | −0.053 (0.24) | −0.060 (0.27) | −0.007 (0.067) | 0.10 (0.24) | 0.054 (0.26) | −0.035 (0.105) |
| Treated | −0.22 (0.28) | −0.27 (0.31) | −0.028 (0.038) | 0.0343 (0.24) | −0.041 (0.270 | −0.044 (0.045) | |
| Untreated | −0.055 (0.19) | −0.064 (0.20) | −0.007 (0.02) | −0.17 (0.25) | −0.25 (0.25) | −0.082 (0.191) | |
Table 5. Statistical Test Results of Hippocampal Measures.
Results of general linear models where left and right sides were treated as repeated measures, and cohort and gender were used as covariates. Significant or near-significant effects are shown in bold. For the comparison of rates of change, using baseline measures as an additional covariate did not change the outcome. For the main effects, Bonferroni corrections (4) were used to determine significance of main effects but not for contrasts. The main effect of cohort was significant for baseline measures of remainder subfield deformations and rates of change of hippocampal volume and CA1 subfield deformation. The effect of hemisphere was significant only for the remainder subfield deformation (F=8.4, df=1,101, p=0.0045). Effect sizes were calculated as Cohen’s d (unadjusted for covariates), and negative value indicates smaller or more inward than the reference group.
| Comparison | Measure | Group Effect: F(df1, df2), p | Contrast: P (Effect Size) | ||
|---|---|---|---|---|---|
| untreated vs. control | treated vs. control | treated vs. untreated | |||
| Baseline | Volume |
11 (2,101) <.0001 |
0.0002 (−1.2) | 0.0071 (−0.55) | 0.32 (0.59) |
| CA1 |
7.5 (2,101) 0.0009 |
0.0008 (−1.1) | 0.071 (−0.31) | 0.19 (0.68) | |
| Subiculum |
11 (2,101) <.0001 |
0.0009 (−1.1) | 0.0015 (−0.66) | 0.76 (0.50) | |
| Remainder | 4.1 (2,101) 0.0195 | 0.44 (−0.69) | 0.0067 (−0.59) | 0.19 (0.09) | |
| Rates of Change (slope) | Volume | 2.7 (2,101) 0.072 | 0.046 (−0.30) | 0.24 (−0.48) | 0.50 (−0.23) |
| CA1 | 2.48 (2,101) 0.089 | 0.048 (−0.20) | 0.32 (−0.46) | 0.43 (−0.41) | |
| Subiculum | 3.42 (2,101) 0.037 | 0.020 (−0.60) | 0.25 (−0.39) | 0.33 (0.11) | |
| Remainder | 1.08 (2,101) 0.34 | 0.32 (−0.07) | 0.28 (−0.43) | 0.99 (−0.42) | |
| Rates of Change (with baseline as covariate) | Volume | 1.9 (2,99) 0.25 | 0.062 | 0.46 | 0.34 |
| CA1 | 1.9 (2,99) 0.16 | 0.057 | 0.71 | 0.22 | |
| Subiculum | 2.2 (2,99) 0.11 | 0.040 | 0.57 | 0.23 | |
| Remainder | 1.12 (2,99) 0.33 | 0.30 | 0.27 | 0.99 | |
Longitudinally, there was no overall main group effect after Bonferroni corrections in the rates of change for any of the hippocampal measures. Exploratory examination of the contrasts showed that the untreated CDR 0.5 subjects differed from the nondemented healthy subjects in the rates of change of hippocampal volume, CA1 and subiculum subfield deformations. The treated CDR 0.5 subjects did not differ from the nondemented healthy subjects or the untreated CDR 0.5 subjects. Using each measure’s baseline values as an additional covariate did not alter the results.
All neuropsychological battery factor scores and the Short Blessed Test and WMS logical memory scores showed main effects of group at baseline. Contrasts showed that while untreated and treated CDR 0.5 subjects differed from the nondemented healthy subjects, the two groups of CDR 0.5 subjects did not differ from each other in any of these scores at baseline. While the untreated and treated CDR 0.5 subjects differed from the nondemented healthy subjects in the rates of change of the general and parietal factors, the untreated and treated CDR 0.5 subjects did not differ from each other in the rates of change in any of the scores.
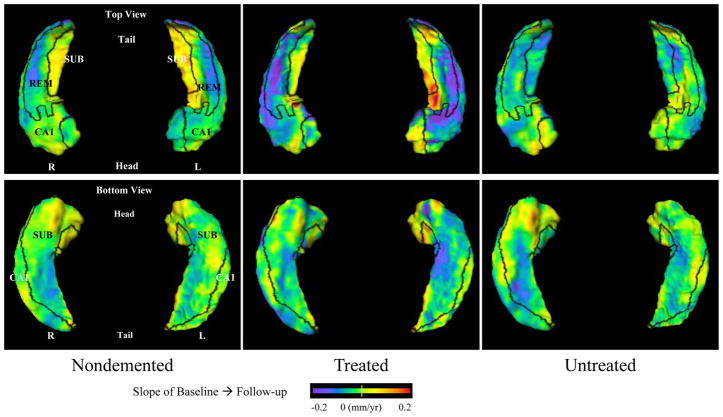
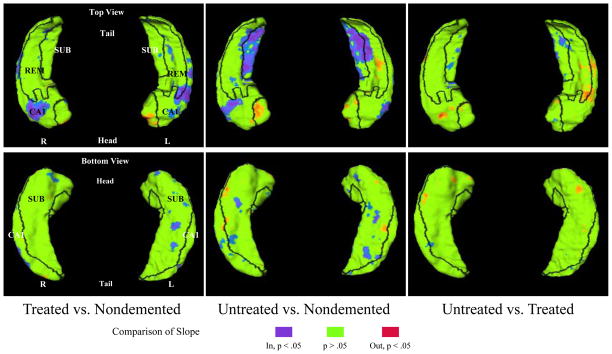
Patterns of rates of change on the hippocampal surfaces in CDR 0.5 subjects treated with donepezil, untreated and nondemented subjects are visualized in Figure 1. Comparisons of rates of change among CDR 0.5 subjects treated with donepezil, untreated and nondemented subjects are visualized in Figure 2. We observed that compared with controls, while the two CDR 0.5 groups showed rates of change in similar regions of the CA1 subfield, the untreated CDR 0.5 subjects showed additional difference in the subiculum that the treated CDR 0.5 subjects did not, however these differences were not significant between these two groups of CDR 0.5 subjects.
Figure 1. Visualization of the pattern of longitudinal change on the hippocampal surfaces in nondemented subjects and subjects with very mild DAT, either treated with donepezil or untreated.
Top panels show the left and right hippocampi viewed from the top (dorsal surface), while bottom panels shows the left and right hippocampi viewed from the bottom (ventral surface). Baseline-to-follow-up changes for the nondemented subject, untreated subjects and treated subjects are shown in the left, middle and right columns, respectively. Boundaries between the three zones of the hippocampal surface (i.e., lateral, superior and inferior-medial) are drawn in black and all three zones are labeled (CA1 = CA1, SUB = Subiculum, REM = Remainder, which is comprised of CA2–4 plus the dendate gyrus). Rates of change (slopes) of inward variation of the hippocampal surface is represented by cooler flame colors (i.e., blue to purple), while slopes of outward variation is represented by warmer colors (i.e., orange to red).
Figure 2. Comparison of the patterns of longitudinal change on the hippocampal surfaces in subjects among very mild DAT treated with donepezil, untreated DAT subjects and nondemented subjects.
Top panels show the left and right hippocampi viewed from the top (dorsal surface), while bottom panels shows the left and right hippocampi viewed from the bottom (ventral surface). Comparisons (difference in the mean, unadjusted for covariates) between treated and nondemented subjects are shown in the left column. Comparisons between untreated and nondemented subjects are shown in the middle column. Comparisons between treated and untreated subjects are shown in the right column. Boundaries between the three zones of the hippocampal surface (i.e., lateral, superior and inferior-medial) are drawn in black and all three zones are labeled (CA1 = CA1, SUB = Subiculum, REM = Remainder). Rates of change (slopes) of hippocampal surface variation are compared. Surface locations showing significant differences (p<0.05, uncorrected for multiple comparisons) are visualized with blue to purple colors (for inward) and orange to red colors (for outward).
Since the presence of one or ApoE4 alleles have been shown previously to predict a poorer response to AChE inhibitors5, 6, we examined the rates of change in the psychometric and hippocampal measures separately for the 3 treated DAT subjects with no ApoE4 allele and 9 treated DAT subjects with 1 or 2 ApoE4 alleles. Due to the small sample size, Wilcoxon exact tests were used. The comparisons showed that while the rates of decline for MMSE (p=0.036), WMS logical memory scores (p=0.032) and inward deformation in the left subiculum (p=0.018) were significantly more severe in subjects with 1 or more ApoE4 alleles as compared with the 3 subjects without any ApoE4 allele, none of the other psychometric or hippocampal measures showed any significant difference in the rates of change.
DISCUSSION
Progressive hippocampal volume loss, assessed using MR imaging, is a characteristic neuroanatomical feature of Alzheimer disease (AD). However, it is unknown whether treatment with medications marketed for the treatment of AD, such as donepezil, can alter hippocampal volume loss. Volumetric MRI of whole brain or medial temporal lobe structures is now being considered as a possible outcome measure in therapeutic trials for patients with AD and MCI12, 13. In patients with moderate stages of amnestic MCI, cognitive testing may provide more predictive accuracy for disease progression than measures of ventricular volume expansion or whole brain, entorhinal cortex or hippocampal volume loss28. In this study, we compared rates of change in hippocampal volume as well as CA1 and subiculum subfield deformation in donepezil-treated and untreated very mild DAT subjects, and with nondemented comparison subjects. The main finding of this study was that the donepezil-treated and untreated DAT subjects did not differ in their rates of changes of these hippocampal measures. This finding suggests that whatever symptomatic benefits might have been experienced by treatment with donepezil in our subjects was not accompanied by a parallel effect on hippocampal structure. Because treatment (or no treatment) with donepezil was naturalistic in this study and not systematically evaluated using structured instruments, the degree of symptom amelioration experienced by the donepezil-treated patients is not known.
Our findings disagree with some previous reports29, 30. In a study of hippocampal volume loss over 1-year intervals in 54 DAT patients treated with donepezil compared with historical controls, the mean annual rate of hippocampal atrophy in the donepezil-treated group (3.82%) was significantly lower than that in the historical comparison group (5.04%)29. Another report described a significant effect of donepezil treatment in 67 DAT patients in a prospective, double-blind, placebo-controlled 6-month trial30. In our current study, the assessment of changes in hippocampal volumes over time were not hypothesized but rather were assessed in an exploratory fashion. The reasons for the discrepancy of our findings with these previous findings may be the small samples in our study, which would have precluded detecting a volume difference in slopes (expressed as percent change per year) of less than 1.6% (the slope difference between the treated and untreated subjects in the current study was 0.64% on the left and 0.46% on the right).
Of note, we found that the pattern of longitudinal change for the treated subjects is more similar with the pattern for the nondemented subjects than with the untreated subjects (Figure 1). Heterogeneity in the clinical populations included in the three studies may also be a likely explanation. Also, different methods were used to assess time-dependent changes in hippocampal structure. The methods used in the present study were developed for the specific purpose of assessing time-dependent changes in both the volume and surface variation of the hippocampus, and were shown to be capable of detecting small degrees of volume loss and surface deformation10, 31.
Our study has several limitations. Although a prospective randomized, placebo-controlled study is desirable, it is unattainable today because of ethical considerations. The donepezil-treated DAT subjects were prospectively recruited into this longitudinal study of hippocampal structure along with age-matched controls, while the untreated DAT subjects were drawn from a previously published longitudinal study of the natural course of AD. The potential confounding effect of these two naturalistic cohorts was dealt with by including it as a covariate in all statistical tests and examining the cohort effect in these tests. We also observed that for both baseline measures and their rates of change, the variances remained about the same whether the two cohorts of control subjects were combined or not. Nonetheless, clinical differences that may have influenced the capacity of the patients to respond to treatment cannot be completely excluded as confounds. Also, the numbers of subjects included in the treated and untreated DAT groups were relatively small. Larger numbers of subjects may have allowed us to detect more subtle effects of drug treatment on the measures of hippocampal structure selected as outcome measures for the study (trend observed in Figure 2). However, the measures we selected for study were adequate to detect disease progression in untreated DAT subjects9. Finally, we cannot exclude the possibility that measures of neuroanatomical progression other than the selected measures of hippocampal structure may have been able to detect an effect of treatment. While the amelioration of dementia symptoms in DAT patients is a critical measure of the utility of any treatment for AD, it is also important to assess the effects of treatment on some measure that reflects the underlying disease process. This will become especially important as putative “disease-altering” treatments are developed and tested in clinical trials. Changes in neuroanatomical structures are likely to be important indicators in such studies.
Acknowledgments
This research was supported in part by NIH grants R01-MH60883, P01-AG03991, P50-AG05681. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The principal investigator (JGC) takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Cummings JL. Alzheimer’s disease. N Engl J Med. 2004 Jul 1;351(1):56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- 2.Hansen RA, Gartlehner G, Webb AP, Morgan LC, Moore CG, Jonas DE. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Clin Interv Aging. 2008;3(2):211–225. [PMC free article] [PubMed] [Google Scholar]
- 3.Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008 Mar 4;148(5):379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 4.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farlow MR, Lahiri DK, Poirier J, Davignon J, Schneider L, Hui SL. Treatment outcome of tacrine therapy depends on apolipoprotein genotype and gender of the subjects with Alzheimer’s disease. Neurology Mar. 1998;50(3):669–677. doi: 10.1212/wnl.50.3.669. [DOI] [PubMed] [Google Scholar]
- 6.Poirier J, Delisle MC, Quirion R, et al. Apolipoprotein E4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12260–12264. doi: 10.1073/pnas.92.26.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR, Jr, Petersen RC, Xu Y, et al. Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology. 1998;51(4):993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mungas D, Reed BR, Jagust WJ, et al. Volumetric MRI predicts rate of cognitive decline related to AD and cerebrovascular disease.[see comment] Neurology. 2002;59(6):867–873. doi: 10.1212/wnl.59.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Swank JS, Glick IE, et al. Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. Neuroimage. 2003;20(2):667–682. doi: 10.1016/S1053-8119(03)00361-6. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Miller JP, Gado MH, et al. Abnormalities of hippocampal surface structure in very mild dementia of the Alzheimer type. Neuroimage Mar. 2006;30(1):52–60. doi: 10.1016/j.neuroimage.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csernansky JG, Wang L, Miller JP, Galvin JE, Morris JC. Neuroanatomical predictors of response to donepezil therapy in patients with dementia. Arch Neurol Nov. 2005;62(11):1718–1722. doi: 10.1001/archneur.62.11.1718. [DOI] [PubMed] [Google Scholar]
- 12.Jack CR, Jr, Petersen RC, Grundman M, et al. Longitudinal MRI findings from the vitamin E and donepezil treatment study for MCI. Neurobiol Aging Sep. 2008;29(9):1285–1295. doi: 10.1016/j.neurobiolaging.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modrego PJ. The effect of drugs for Alzheimer disease assessed by means of neuroradiological techniques. Curr Med Chem. 2006;13(28):3417–3424. doi: 10.2174/092986706779010289. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 15.Berg L, McKeel DW, Jr, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch Neurol. 1998;55(3):326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 16.Morris JC, Ernesto C, Schafer K, et al. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology. 1997;48(6):1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- 17.Storandt M, Grant EA, Miller JP, Morris JC. Rates of progression in mild cognitive impairment and early Alzheimer’s disease. Neurology. 2002 Oct 8;59(7):1034–1041. doi: 10.1212/wnl.59.7.1034. [DOI] [PubMed] [Google Scholar]
- 18.Kanne SM, Balota DA, Storandt M, McKeel DW, Jr, Morris JC. Relating anatomy to function in Alzheimer’s disease: neuropsychological profiles predict regional neuropathology 5 years later. Neurology. 1998;50(4):979–985. doi: 10.1212/wnl.50.4.979. [DOI] [PubMed] [Google Scholar]
- 19.Rubin EH, Storandt M, Miller JP, et al. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Arch Neurol Mar. 1998;55(3):395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- 20.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry Jun. 1983;140(6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D. Wechsler Memory Scale. 3. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 22.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage Oct. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Csernansky JG, Wang L, Joshi SC, Ratnanather JT, Miller MI. Computational anatomy and neuropsychiatric disease: probabilistic assessment of variation and statistical inference of group difference, hemispheric asymmetry, and time-dependent change. Neuroimage. 2004;23 (Suppl 1):S56–68. doi: 10.1016/j.neuroimage.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Freeborough PA, Woods RP, Fox NC. Accurate registration of serial 3D MR brain images and its application to visualizing change in neurodegenerative disorders. J Comput Assist Tomogr. 1996;20(6):1012–1022. doi: 10.1097/00004728-199611000-00030. [DOI] [PubMed] [Google Scholar]
- 25.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005 Aug 24;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csernansky JG, Wang L, Miller JP, et al. Preclinical Detection of Alzheimer’s Disease: Hippocampal Shape and Volume Predict Dementia Onset in the Elderly. Neuroimage. 2005 doi: 10.1016/j.neuroimage.2004.12.036. (accepted) [DOI] [PubMed] [Google Scholar]
- 27.SAS System for Windows, V9.1.3. Cary, North Carolina: SAS Institute Inc; 2004. [computer program]. Version. [Google Scholar]
- 28.Fleisher AS, Sun S, Taylor C, et al. Volumetric MRI vs clinical predictors of Alzheimer disease in mild cognitive impairment. Neurology. 2008 Jan 15;70(3):191–199. doi: 10.1212/01.wnl.0000287091.57376.65. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E. Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? Am J Psychiatry Apr. 2005;162(4):676–682. doi: 10.1176/appi.ajp.162.4.676. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan KR, Charles HC, Doraiswamy PM, et al. Randomized, placebo-controlled trial of the effects of donepezil on neuronal markers and hippocampal volumes in Alzheimer’s disease. Am J Psychiatry Nov. 2003;160(11):2003–2011. doi: 10.1176/appi.ajp.160.11.2003. [DOI] [PubMed] [Google Scholar]
- 31.Csernansky JG, Wang L, Swank J, et al. Preclinical detection of Alzheimer’s disease: hippocampal shape and volume predict dementia onset in the elderly. Neuroimage. 2005 Apr 15;25(3):783–792. doi: 10.1016/j.neuroimage.2004.12.036. [DOI] [PubMed] [Google Scholar]