Cyanide ion, a versatile diatomic ligand, has been extensively investigated as both a classic inhibitor and as a ligand for exploring properties of hemes and hemoproteins. Unlike CO and O2 which bind only to iron(II) species, CN− can bind to both iron(II) and -(III) hemo-proteins. Stable low-spin (LS) iron(III) proteins can be straightforwardly prepared.[1–3] In contrast, (cyano)iron(II) hemoproteins are usually indirectly formed by reduction of (cyano)iron(III) proteins. Cyanide bound, iron(II) forms of myoglobin,[4] hemoglobin,[5] horseradish peroxidase[6] and a number of cytochrome oxidase derivatives[7] are known. Many, but not all, of the iron(II) species, have lower binding constants than the iron(III) analogues. The equilibrium constant for cyanide binding for iron(III) hemoproteins is often ≥105 M−1 compared to ≤102 M−1 for iron(II) species.[3]
Since the first reported isolation of a (cyano)heme was reported by us in 1980,[8] a number of electronic and geometric structure issues have been brought forward.[9] All of the known species are LS iron(III) derivatives, either bis(cyano) [FeIII(Por)(CN)2]− or mixed-ligand [FeIII(Por)(CN)(L)] complexes.[9] However, there are currently no (cyano)iron(II) porphyrinate derivatives reported, presumably because of its known lower stability/affinity compared to iron(III). It might be thought that (cyano)iron(II) species would be preferred since a filled d6 shell should strongly π-bond to the π-accepting cyanide ligand.
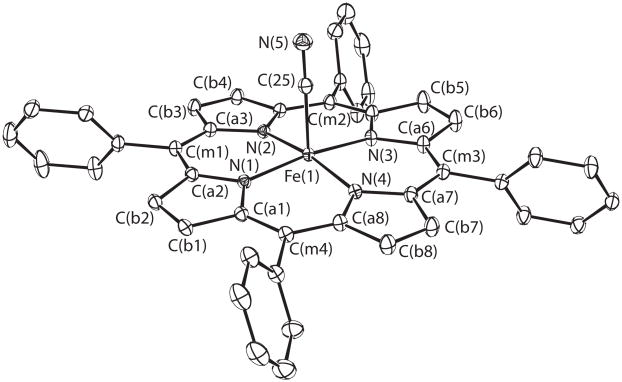
We now report the first (cyano)iron(II) porphyrinate species, five-coordinate [K(222)][Fe(TPP)(CN)] (Figure 1). The average equatorial Fe–Np bond distance (1.986 (7) Å) and the axial Fe–C distance (1.8783 (10) Å) are consistent with a LS state.[10] However, T-dependent Mössbauer spectra reveal a more complicated picture of the iron spin state. A single quadrupole doublet is observed, whose value decreases from 1.827 mm/s at 25 K to 0.85 mm/s at 300 K; the isomer shift varies between 0.37 to 0.47 mm/s. The most probable explanation is that a thermally induced spin crossover is occurring, whose interconversion is rapid on the Mössbauer time scale (< 10−8 s).[11]a This interpretation has been confirmed by both DFT calculations and magnetic susceptibility measurements.
Figure 1.
100 K ORTEP diagram of [Fe(TPP)(CN)]−. Thermal ellipsoids are contoured at the 50% probability level. Hydrogens omitted for clarity.
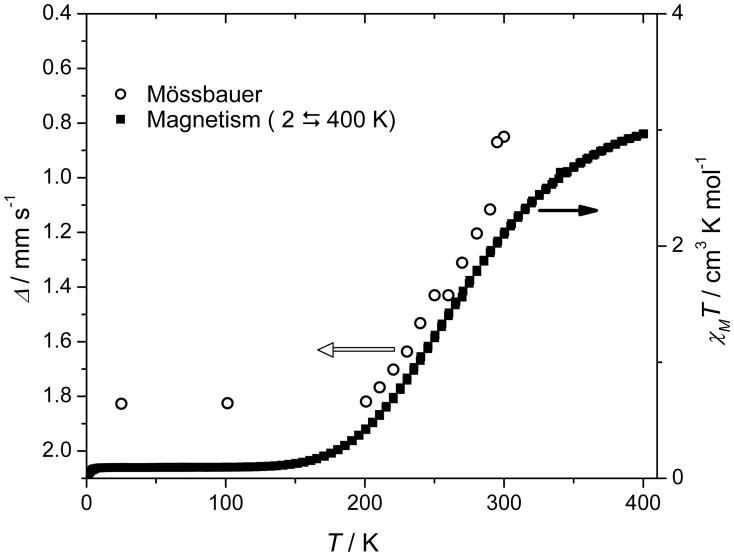
The magnetic susceptibility of [K(222)][Fe(TPP)(CN)] was investigated over the temperature range of 2–400 K. Figure 2 shows the product of the molar susceptibility (χm) (corrected for paramagnetism (TIP)) and temperature (T) in an external magnetic field of 2 T, which provides direct evidence for an S = 0 (LS) ↔ S = 2 (HS) spin crossover. AT 400 K, the value of χmT (2.96 cm3 K mol−1) is close to that expected for the HS state, but the lack of significant plateau suggests that the transition is not quite complete at this temperature. The spin-state transition occurs over a large temperature range (~175–400 K) and is reversible; both ascending and descending temperature measurements are shown in Figure 2 and no hysteresis was observed. The transition temperature T1/2 (defined as temperature at which complexes shows a population of 50% in the HS state) of this gradually proceeding spin transition is about 265 K. Figure 2 also plots the observed time-averaged quadrupole splitting value against temperature; the strong correlation between the quadrupole splitting and the susceptibility is clear.
Figure 2.
χmT versus T plot for [K(222)][Fe(TPP)(CN)] at 2T applied field. The Mössbauer quadrupole splitting values are also presented for comparison.
To gain a better understanding of the thermodynamics regarding the spin-states, density functional theory was employed (see Supporting Information).[12] At low temperature only the low-spin S = 0 state was thermodynamically accessible. With increasing temperature the S = 2 state became significant, and a spin-crossover event is predicted to occur near 325 K (Figure S1) in good agreement with the value of 265 K from experiment. The intermediate-spin S = 1 state was disfavored over the entire temperature range explored.
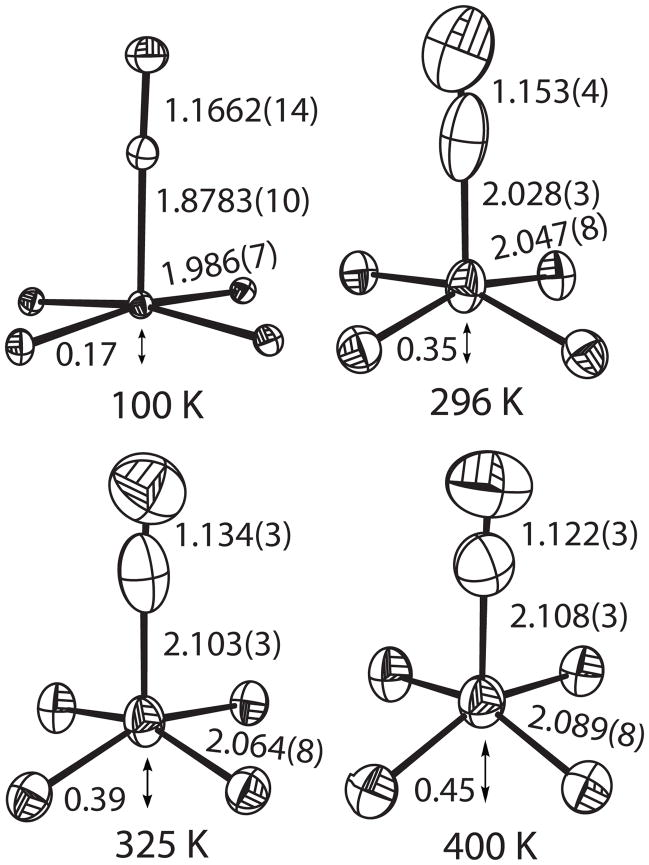
We have also investigated the T-dependent structures of the iron complex, since changes in metal–donor atom distances, along with changes in magnetic properties, are the two hallmarks of spin-state transitions. Structures have been determined at 100 K (two crystals) and 296, 325 and 400 K.[13] A change from a LS to a HS state in the five-coordinate complex is expected to lead to increases in the axial Fe–C distance, the equatorial Fe–Np bond distances, and the displacement of the iron atom from the mean porphinato plane. The results are summarized in the ORTEP drawings given in Figure 3, for simplicity only the cyanide group and FeN4 porphyrin core are shown. The Fe–C distance elongates by 0.23 Å (Figure 3), which is amongst the largest changes in bond lengths that have been observed for iron(II) spin crossover compounds.[11b]b This is in part because the axial and equatorial bond distance increases must be asymmetric owing to the macrocyclic constraints of the porphyrin ring; note that Fe–Np has increased by 0.103 Å over the same temperature range. The 100 K Fe–Np average bond length of 1.986 (7) Å is that for a pure LS state whereas the 400 K value of 2.089 (8) Å is slightly less than expected for anionic HS iron(II) complex, consistent with the idea that the spin state transition is not quite complete. Also completely consonant with expectation are the increases in the displacement of the iron from the mean plane of the four nitrogen plane.
Figure 3.
Four ORTEP diagrams of [K(222)][Fe(TPP)(CN)] displaying the cyanide groups and the core atoms of porphyrin (Fe and four pyrrole N atoms). Values of axial ligand and average equatorial bond distances are given as well as the iron displacement from the mean N4 plane. Thermal ellipsoids are contoured at the 50% probability level.
The anisotropic thermal parameters also show evidence of the spin crossover. As expected, the magnitude of all atomic anisotropic displacement parameters increase upon increasing temperature. However, the cyanide carbon atom shows different behavior over the temperature range. The thermal parameters at 100 and 400 K are close to isotropic, consistent with a single carbon atom site, whereas at intermediate temperatures with substantial populations of two spin states and differing carbon sites, the thermal parameters are much more prolate with elongation along the Fe–C bond direction. Importantly, the C–N bond distance in all structures remains nearly constant, as expected if only CN− atoms occupy two sites.
Additional evidence for the spin crossover comes from T-dependent infrared spectra, which has the advantage of a shorter time scale (10−13 s) and thus can detect both spin isomers. Measurements at 296 K, as either Nujol mulls or KBr pellets, show two distinct ν(C–N) frequencies at 2070 and 2105 cm−1, with the first being the stronger. (S.I.) On cooling, the 2105 cm−1 peak gradually decreases while the 2070 cm−1 peak increases. At 150 to 160 K, the stretch at 2105 cm−1 disappears and thus corresponds to the HS stretch. A similar pattern of T-dependent azide stretches was observed in a 5/2, 3/2 spin crossover complex.[14]
In coordination chemistry, cyanide and CO have been deeply entrenched as strong field ligands.[15, 16] Recently, Miller et al. showed that [(NEt4)3][Cr(II)(CN)5][17] is a distorted trigonal bipyramidal complex that was not low spin. Two different theoretical calculations[18] have suggested that the HS state results from the buildup of electrostatic (ligand–ligand) repulsions and not the ligand field of cyanide per se; the cyanide ligand is behaving as a strong field ligand in this Cr complex. However, [K(222)][Fe(TPP)(CN)] represents a case where the CN− should unequivocally lead to LS species. That it does not, strongly demonstrates the weaker field nature of cyanide, even in a case where π-back bonding should be maximized.
In summary, the synthesis and characterization of the first cyanoiron(II) porphyrinate, [K(222)][Fe(TPP)(CN)], is presented. It forms a LS to HS crossover complex; coordination of a single axial cyanide ligand does not generate a sufficiently strong ligand field to ensure a low-spin complex under all conditions.[19] This is in distinct contrast to the five-coordinate CO complex, that is low spin under all known conditions.
Supplementary Material
Footnotes
We thank the National Institutes of Health for support of this research under Grant GM-38401 to WRS and the NSF for X-ray instrumentation (Grant CHE-0443233).
References
- 1.Antonini E, Brunori M. Hemoglobin and Myoglobin in Their Reactions with Ligands. North-Holland; Amsterdam: 1971. [Google Scholar]
- 2.Keilin D, Hartree EF. Biochem J. 1951;49:88. doi: 10.1042/bj0490088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milani M, Ouellet Y, Ouellet H, Guertin M, Boffi A, Antonini G, Bocedi A, Mattu M, Bolognesi M, Ascenzi P. Biochemistry. 2004;43:5213. doi: 10.1021/bi049870+. [DOI] [PubMed] [Google Scholar]
- 4.Reddy KS, Yonetani T, Tsuneshige A, Chance B, Kushkuley B, Stavrov SS, Vanderkooi JM. Biochemistry. 1996;35:5562. doi: 10.1021/bi952596m. [DOI] [PubMed] [Google Scholar]
- 5.a) Boffi A, Ilari A, Spagnuolo C, Chiancone E. Biochemistry. 1996;35:8068. doi: 10.1021/bi9601971. [DOI] [PubMed] [Google Scholar]; b) Booffi A, Chiancone E, Peterson ES, Wang J, Rousseau DL, Friedman JM. Biochemistry. 1997;36:4510. doi: 10.1021/bi961889s. [DOI] [PubMed] [Google Scholar]
- 6.a) Rakshit G, Spiro TG. Biochemistry. 1974;13:5317. doi: 10.1021/bi00723a010. [DOI] [PubMed] [Google Scholar]; b) Teraoka J, Kitagawa T. Biochem Biophys Res Common. 1980;93:694. doi: 10.1016/0006-291x(80)91133-x. [DOI] [PubMed] [Google Scholar]; c) Yoshikawa S, O’Keeffe DH, Caughey WS. J Biol Chem. 1985;260:3518. [PubMed] [Google Scholar]; d) Meunier B, Rodiguez-Lopez JN, Smith AT, Thorneley RNF, Rich PR. Biochemistry. 1995;34:14687. doi: 10.1021/bi00045a009. [DOI] [PubMed] [Google Scholar]
- 7.a) Caughey WS, Dong A, Sampath V, Yoshikawa S, Zhao XJ. J Bioenerg Biomembr. 1993;25:81. doi: 10.1007/BF00762850. [DOI] [PubMed] [Google Scholar]; b) Yoshikawa S, Mochizuki M, Zhao XJ, Caughey WS. J Biol Chem. 1995;270:4270. doi: 10.1074/jbc.270.9.4270. [DOI] [PubMed] [Google Scholar]; c) Kim Y, Babcock GT, Surerus KK, Fee JA, Dyer RB, Woodruff WH, Oertling WA. Biospectroscopy. 1998;4:1. doi: 10.1002/(sici)1520-6343(1998)4:1<1::aid-bspy1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]; d) Jafferji A, Allen JWA, Ferguson SJ, Fülöp V. J Biol Chem. 2000;275:25089. doi: 10.1074/jbc.M001377200. [DOI] [PubMed] [Google Scholar]; e) Mitchell R, Moddy AJ, Rich PR. Biochemistry. 1995;34:7576. doi: 10.1021/bi00023a003. [DOI] [PubMed] [Google Scholar]
- 8.Scheidt WR, Haller KJ, Hatano K. J Am Chem Soc. 1980;102:3017. [Google Scholar]
- 9.a) Li J, Noll BC, Schulz CE, Scheidt WR. Inorg Chem. 2007;46:2286. doi: 10.1021/ic061463u. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ikeue T, Ohgo Y, Saitoh T, Nakamura M, Fujii H, Yokoyama M. J Am Chem Soc. 2000;122:4068. [Google Scholar]; c) Ikezaki A, Nakamura M. Inorg Chem. 2002;41:2761. doi: 10.1021/ic0108383. [DOI] [PubMed] [Google Scholar]
- 10.Scheidt WR, Reed CA. Chem Rev. 1981;81:543. [Google Scholar]
- 11.a) Gütlich P, Goodwin HA. Top Curr Chem. 2004;233:1. [Google Scholar]; b) Gütlich P, van Koningsbruggen PJ, Renz F. Struct Bonding. 2004;107:27. [Google Scholar]
- 12.See supporting information for computational details.
- 13.CCDC 699087-699090 ([K(222)][Fe(TPP)(CN)] at 100, 296, 325 and 400 K) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 14.Neya S, Takahashi A, Ode H, Hoshino T, Hata M, Ikezaki A, Ohgo Y, Takahashi M, Hiramatsu H, Kitagawa T, Furutani Y, Kandori H, Funasaki N, Nakamura M. Eur J Inorg Chem. 2007:3188. [Google Scholar]
- 15.Alexander JJ, Gray HB. J Am Chem Soc. 1968;90:4260. [Google Scholar]
- 16.Cotton FA, Murillo CA, Bochmann M. Advanced Inorganic Chemistry. Wiley; New York: 1999. [Google Scholar]
- 17.Nelson KJ, Giles ID, Shum WW, Arif AM, Miller JS. Angew Chem. 2005;117:3189. doi: 10.1002/anie.200462763. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:3129. [Google Scholar]
- 18.a) Deeth RJ. Eur J Inorg Chem. 2006:2551. [Google Scholar]; b) Lord RL, Baik MH. Inorg Chem. 2008;47:4413. doi: 10.1021/ic8000653. [DOI] [PubMed] [Google Scholar]
- 19.We have also synthesized and characterized of six-coordinate cyanoferroporphyrinates, all are LS.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.