Abstract
Rationale
Calcium channel blockers (CCBs) exert their antihypertensive effect by reducing cardiac afterload but not preload, suggesting that Ca2+ influx through L-type Ca2+ channels (LTCC) mediates arterial but not venous tone.
Objective
The object of this study was to resolve the mechanism of venous resistance to CCBs.
Methods and Results
We compared the sensitivity of depolarization (KCl)-induced constriction of rat small mesenteric arteries (MA) and veins (MV) to the dilator effect of CCBs. Initial findings confirmed that nifedipine progressively dilated depolarization-induced constrictions in MA but not MV. However, Western blots showed a similar expression of the α1C pore-forming subunit of the LTCC in both vessels. Patch-clamp studies revealed a similar density of whole-cell Ca2+ channel current between single smooth muscle cells (SMCs) of MA and MV. Based on these findings, we hypothesized that LTCCs are expressed but “silenced” by intracellular Ca2+ in venous SMCs. After depletion of intracellular Ca2+ stores by the SERCA pump inhibitor thapsigargin, depolarization-induced constrictions in MV were blocked 80% by nifedipine suggesting restoration of Ca2+ influx through LTCCs. Similarly, KCl-induced constrictions were sensitive to block by nifedipine after depletion of intracellular Ca2+ stores by caffeine, ryanodine, or 2-aminoethoxydiphenyl borate. Cell-attached patch recordings of unitary LTCC currents confirmed rare channel openings during depolarization of venous compared to arterial SMCs, but chelating intracellular Ca2+ significantly increased the open-state probability of venous LTCCs.
Conclusions
We report that intracellular Ca2+ inactivates LTCCs in venous SMCs to confer venous resistance to CCB-induced dilation, a fundamental drug property that was previously unexplained.
Keywords: arteries, veins, L-type Ca2+ channels
INTRODUCTION
The organic calcium channel blocking drugs (CCBs) have been used for three decades as a mainstay of vasodilator therapy to treat hypertension, coronary vasospasm and other diseases of abnormal arterial tone.1 The three structural families of CCBs share a common mechanism of action to dilate small arteries and arterioles; they bind to the pore-forming α1C subunit of the L-type Ca2+ channel (LTCC) in arterial smooth muscle cells (SMCs) to reduce open-state probability.2 However, the usefulness of the CCBs is limited by their inability to dilate the venous circulation. Ankle edema, a common side effect of CCB therapy, is thought to result partly from CCB-induced dilation of arterioles in the absence of venous dilation; the resulting increase in capillary pressure promotes fluid exudation.3–6 Additionally, the antihypertensive effect of the CCBs mediated by arterial dilation may be buffered by persistent venoconstriction that sustains preload to maintain blood pressure elevation.7–10
Although it is well recognized clinically that the vasodilator effect of CCBs is limited to the arterial circulation, the mechanism that confers venous resistance to this class of drugs in vivo is unknown. One possibility is that the LTCCs expressed in arteries and veins are fundamentally different in their biophysical or pharmacological properties. In this regard, only several studies have characterized the biophysical properties and drug profiles of the LTCCs in patch-clamped venous SMCs or even documented their contribution to the contraction of isolated veins. Surprisingly, these studies suggest that similar to arteries, LTCCs in the SMCs of large veins retain sensitivity to the CCBs. For example, depolarization-induced constrictions of porcine femoral and saphenous veins are sensitive to calcium channel blockade.11 Additionally, voltage-elicited Ca2+ currents in patch-clamped SMCs from canine saphenous vein are blocked by nanomolar concentrations of CCBs. The biophysical properties of these channels appear indistinguishable from their arterial counterparts.12 Finally, the LTCCs in rabbit portal vein myocytes also display a customary profile of voltage-dependent opening, channel kinetics and sensitivity to blockade by nisoldipine, verapamil and diltiazem.13 Collectively, these findings suggest that the SMCs of conduit veins express prototypic LTCCs, although patch-clamp studies in SMCs isolated from veins or venules that regulate changes in venous capacitance apparently are unavailable.
In contrast, small veins and venules from diverse origins fail to dilate to the CCBs, suggesting a lack of functional LTCCs in these venous SMCs in situ. In striated muscle of spontaneously hypertensive rats, the LTCC blockers verapamil, nifedipine and felodipine selectively dilate arterioles but not venules.14 Similarly nifedipine only dilates the arterioles but not venules in the hamster cheek pouch, although the venules are sensitive to other dilator stimuli.15 Finally, nifedipine and benipidine dilate the arterioles but not venules of the rat mesenteric circulation.16,17 Thus, the insensitivity of the venous circulation to CCB-induced dilation, that is clinically recognized also, is observed experimentally in small veins.
Using the second order branches of small mesenteric arteries (MA) and mesenteric veins (MV) of the rat as a model, the present study was designed to resolve the mechanisms that confer venous insensitivity to CCB–induced dilation. We used depolarizing concentrations of high KCl to directly activate voltage-gated LTCCs to induce constriction and thereby circumvent complex receptor-mediated signaling pathways that may differ between arterial and venous SMCs. Complementary vascular reactivity and Ca2+ imaging studies were performed in pressurized MA and MV. Additionally, Westerm blot and patch-clamp studies compared the expression, properties, and CCB sensitivity of LTCCs between arterial and venous preparations.
METHODS
An expanded Methods section is available in the Online Data Supplement at http://circres.ahajournals.org.
Diameter and Em recording in pressurized vessels
Procedures using animals were performed in accordance with the Guide for the Care and Use of Laboratory Animals at the University of Arkansas for Medical Sciences. Second order mesenteric arteries (MA) and mesenteric veins (MV) were dissected from 10-week old male Sprague Dawley rats (Harlan), and cannulated with tapered glass micropipettes. The inflow pipette was connected to a reservoir to control intraluminal pressure.18 Arteries and veins were pressurized at their native pressures of 80 mmHg and 6 mmHg, respectively. Internal diameters were recorded on-line using an upright microscope/Spot RT camera with images captured by Metavue acquisition software (Metamorph). Intracellular membrane potential (Em) was recorded using glass microelectrodes.19
Ca2+ imaging in Fluo-4 loaded vessels
Second order MA and MV were cleaned of adhered fat and connective tissue and loaded with the Ca2+ indicator fluo-4 (2 µmol/L) plus pluronic acid (0.01%) in PSS at room temperature for 1.5 hours in the dark. Images were captured using a 10× S Fluor objective (NA = 0.5) on a Nikon TE2000-U microscope with a QImaging Retiga 2000R camera and iVision software (version 4.0.14, Biovision Inc.) that also was used for analysis. For fluorescence quantitation, a region of interest in the lumen of the vessel was chosen, avoiding the vessel walls and sympathetic nerves.
Detection of the α1C subunit
Whole-cell protein homogenates were prepared from MA and MV pooled from 2 rats. The expression level of the pore-forming α1C subunit of the LTCC was assessed by Western blotting using a polyclonal antibody (Alomone Labs, Jerusalem, Israel).20
Recording of Ca2+ channel currents
SMCs were enzymatically isolated from rat MA and MV.19–21 Whole-cell Ca2+ channel currents were recorded using standard pulse protocols and solutions.22 Single LTCC current openings in freshly isolated MA and MV SMCs were obtained in the cell-attached mode.
RESULTS
Depolarization-induced constriction in MV is resistant to nifedipine
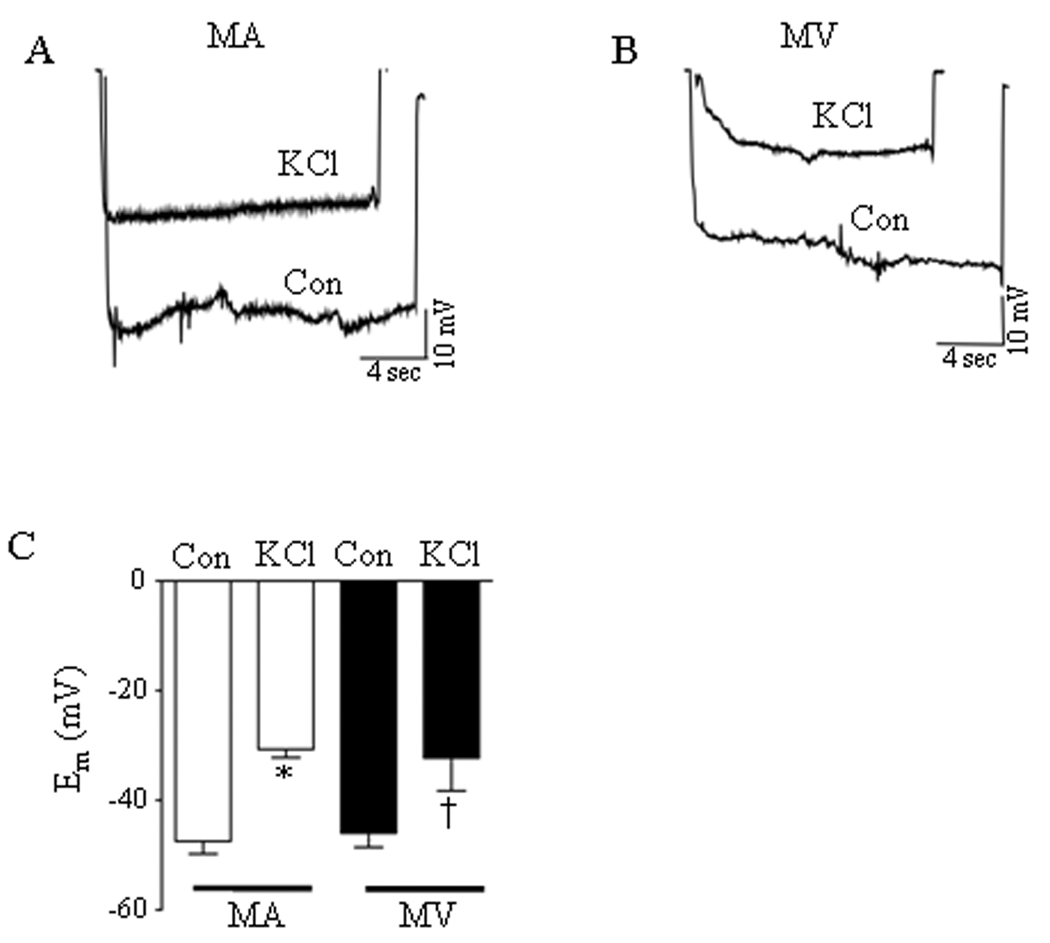
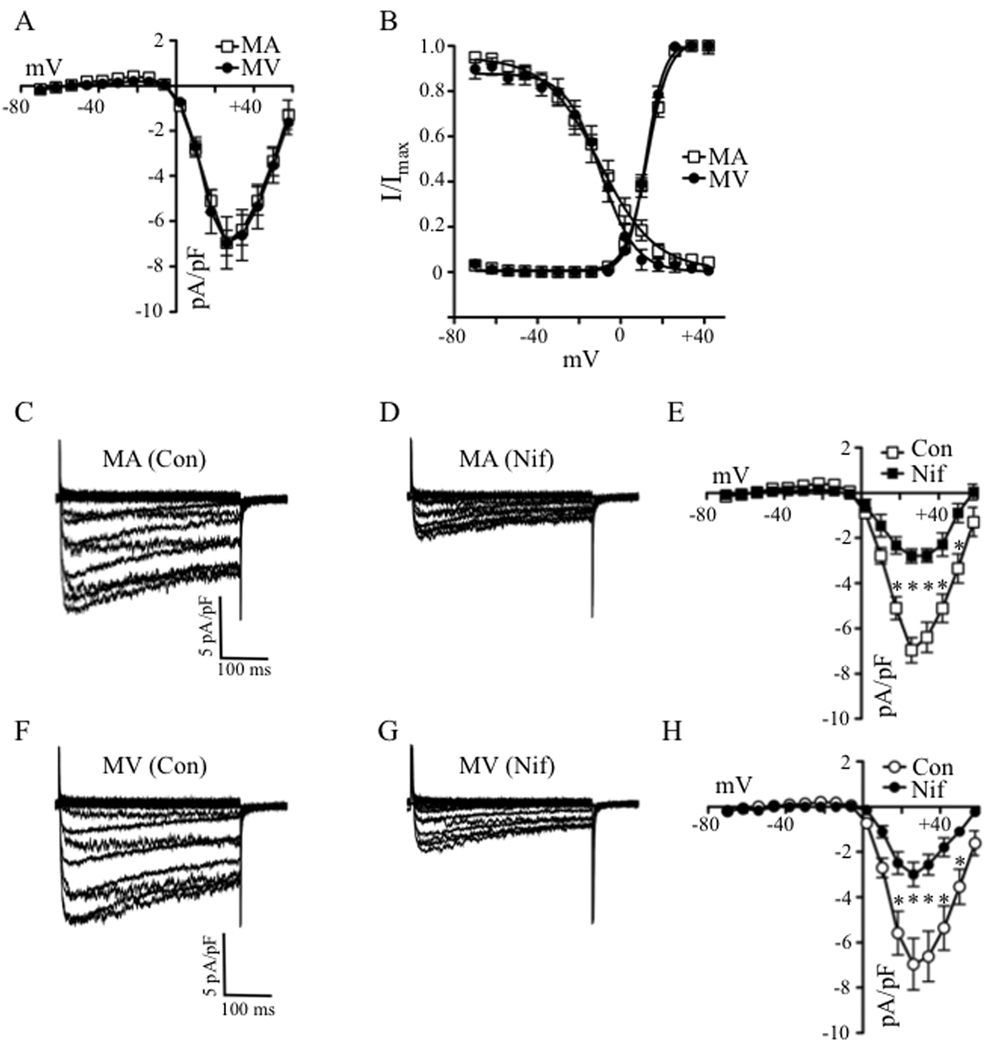
Direct depolarization of isolated veins by high concentrations of KCl has been used to directly evaluate the contribution of LTCC-mediated Ca2+ influx to venoconstriction.11 We used glass microelectrodes to compare the depolarizing responses of 2nd order branches of the rat MA and MV to high (60 mmol/L) KCl to ensure an equal voltage stimulus for LTCC opening in both preparations. Figures 1A and 1B show that KCl depolarization robustly reduced the membrane potential (Em) of MA and MV. The average resting Em in MA and MV were −47 ± 2 mV and −46 ± 3 mV, respectively (n = 6). Figure 1C demonstrates that 60 mmol/L KCl was equally effective in depolarizing the MA (Δ16 ± 2 mV) and MV (Δ14 ± 2 mV). The same depolarizing stimulus also similarly reduced the diameter of the MA and MV by 70 ± 6 % and 68 ± 6 %, respectively (Fig. 2A).
Figure 1.
Depolarization of rat mesenteric arteries (MA) and veins (MV) by 60 mmol/L KCl. (A, B) Membrane potential tracings in isolated, pressurized mesenteric arteries (MA) and mesenteric veins (MV), respectively, under control (Con) drug-free conditions and after incubation with KCl. (C) Resting membrane potential and amplitude of KCl-induced depolarization was not significantly different between MA and MV (n = 4–6). * = significant difference between Con and KCl (MA), † = significant difference between Con and KCl (MV) (p<0.05).
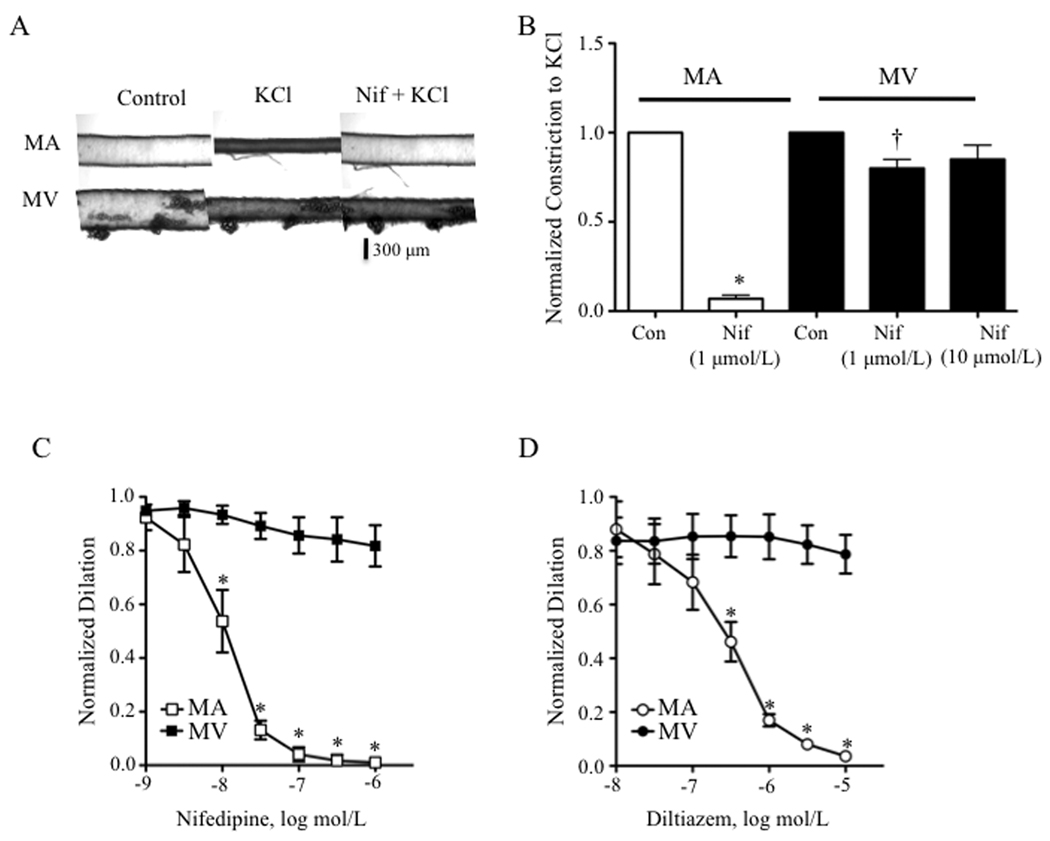
Figure 2.
KCl -induced constrictions in the mesenteric vein (MV) but not artery (MA) are resistant to the LTCC blockers, nifedipine and diltiazem. (A) Nifedipine (Nif) fully blunts depolarization-induced constriction in MA whereas the constriction in MV was only mildly attenuated. Panels (left to right): resting diameters (Control, Con), KCl (60 mmol/L)-induced constriction, and KCl–induced constriction in the presence of 1 µmol/L Nif. (B) Averaged values of 5 experiments as in Fig. 2A. * = significant difference between Control and KCl in MA, † = significant difference between Control and KCl in MV (p<0.05). (C) Nifedipine (1 nmol/L – 1 µmol/L) and (D) diltiazem (10 nmol/L – 10 µmol/L) concentration-dependently dilates KCl (60 mmol/L)-induced constrictions of MA but not MV (n = 4 – 5). * = significant dilation of MA (p<0.05).
In spite of these similarities, whereas pre-incubation of MA with nifedipine (Nif, 1 µmol/L) for 10 min inhibited KCl constriction by 93%, similar constrictions of the MV were resistant to 1 µmol/L or 10 µmol/L Nif, showing only a 20% and 15% loss of constriction, respectively (Figs. 2A and 2B). Neuronally-released norepinephrine did not contribute to KCl constriction of small MV, since the α-adrenergic receptor antagonist, phentolamine (1 µmol/L), did not blunt this response (Online Figure I). Finally, Nif and a structurally unrelated CCB, diltiazem, failed to reverse established KCl constrictions of rat MV whereas both drugs concentration-dependently reversed arterial constriction (Figs. 2C and 2D).
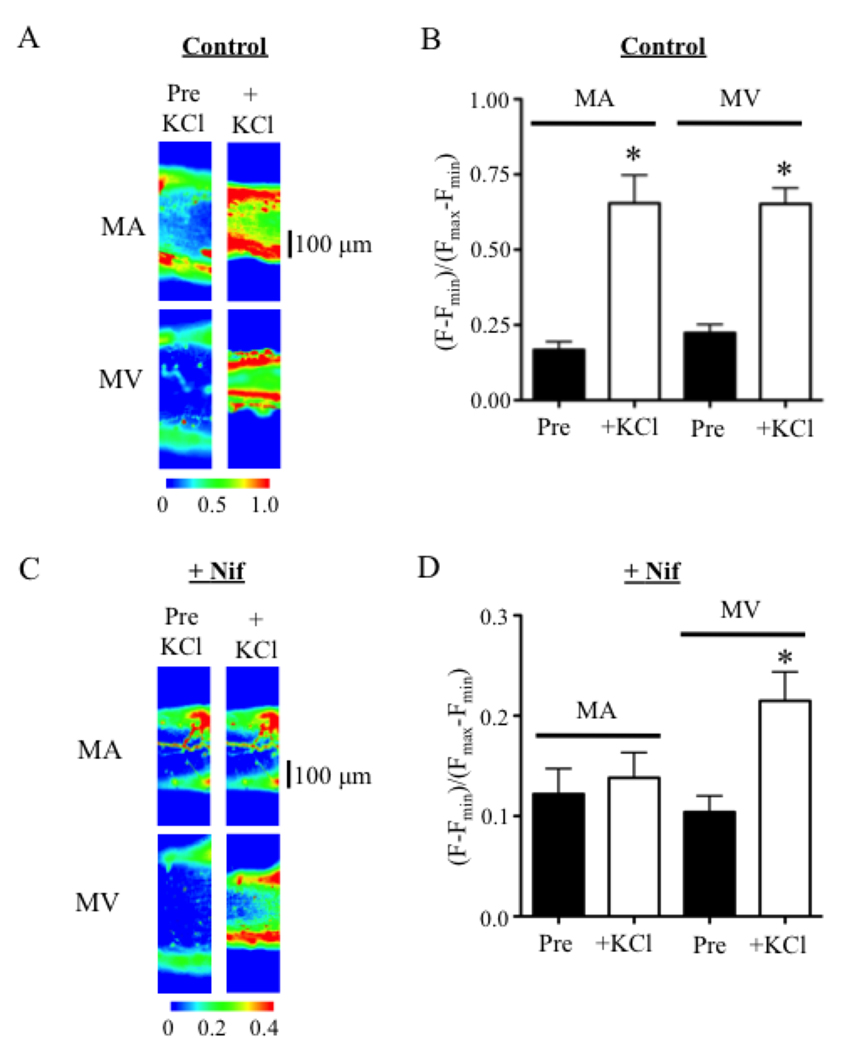
Studies using fluo-4 loaded MA and MV verified that LTCCs contribute less to depolarization-induced Ca2+ mobilization in venous compared to arterial SMCs. In drug-free solution, KCl induced a 3.9-fold and 2.9-fold rise in cytosolic free Ca2+ ([Ca]i) that did not differ significantly between MA and MV, respectively (Figs. 3A and 3B). After a 10-minute incubation with 10 µmol/L Nif, KCl failed to increase [Ca]i in MA (Figs. 3C and 3D). However, KCl still induced a 2.0-fold rise in [Ca]i in MV implying that the venous SMCs mobilized Ca2+ in the absence of functional LTCCs (Figs. 3C and 3D).
Figure 3.
Nifedipine (Nif) blocks KCl-induced elevations of [Ca]i in pressurized MA but not MV. (A, B) KCl (60 mmol/L) stimulates a similar rise of [Ca]i in pressurized MA and MV loaded with fluo-4 (2 µmol/L) (n = 5–7). (C, D) Nif (10 µmol/L, +Nif) prevents KCl-induced increases in [Ca]i in MA, while [Ca]i still rises 2.0-fold in MV. (n = 5) * = significant difference from pre-KCl levels (Pre) compared to the maximum response after KCl (+KCl) in the same preparation (p<0.05).
Similar expression of α1C in arterial and venous SMCs
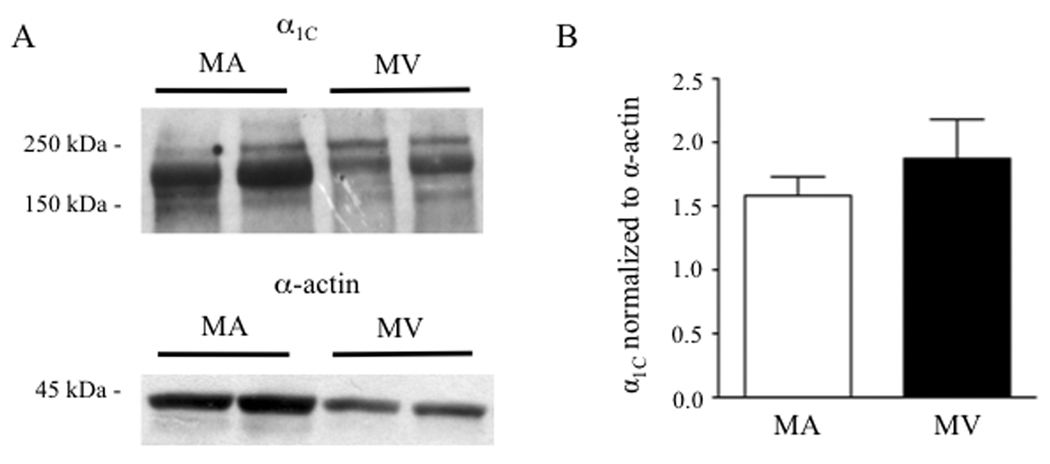
Considering the resistance of KCl constrictions in MV to CCBs and the diminished contribution of venous LTCCs to [Ca]i elevation during depolarization, we explored if MV express fewer LTCC α1C pore-forming subunits compared to MA. Western blots using protein lysates from MA and MV showed that the doublet immunoreactive band corresponding to the long and short forms of α1C was 48% less in MV (Fig. 4A, top blot, n = 4). However, after normalization of α1C to the reduced smooth muscle α-actin signal in venous lysates (Fig. 4A, lower blot, n = 4) to account for the lower SMC content in MV,23 densitometric analysis suggested that the MA and MV express similar levels of the α1C protein (Fig. 4B).
Figure 4.
(A) Western blot analysis of the α1C pore-forming subunit of the LTCC (top panel) and smooth muscle α-actin (lower panel) in protein lysates from rat mesenteric artery (MA) and vein (MV). Forty (40) µg of MA or MV lysate (pooled from 2 rats) was added to each lane. (B) After normalization to smooth muscle-specific α-actin, α1C expression was not significantly different between MA and MV (n = 4).
LTCCs in patch-clamped arterial and venous SMCs have similar properties
Subsequently, we used whole-cell patch-clamp methods to determine if the α1C immunoreactive signal detected in venous lysates corresponded to functional LTCCs. Inward currents of similar amplitudes were observed in venous and arterial SMCs (Figs. 5A, 5C and 5F). Peak Ba2+ current elicited at +18 mV was similar between MA and MV SMCs (−6.4 ± 0.7 pA/pF and −6.6 ± 1.1 pA/pF, respectively) (Fig. 5A; n = 14, 7). Membrane capacitance also was similar (MA: 14.8 ± 0.9 pF; MV: 15.7 ± 1.3 pF). Standard pulse protocols were used to obtain the half-maximal activation and inactivation voltages (Fig. 5B).23 These analyses provided similar values between arterial and venous SMCs for half-maximal (V1/2) current activation (MA: 12.2 ± 1.1 mV; MV: 12.0 ± 0.6 mV) and inactivation (MA: −9.3±3.3 mV; MV: −8.6 ± 2.7 mV), revealing no voltage-dependent abnormalities of the venous channels. Interestingly, exposure of the patch-clamped SMCs to Nif at a concentration (100 nmol/L) that failed to dilate the MV but fully dilated the MA (Fig. 2C), was equally effective in blocking inward current in both cell types (Figs. 5C–D, 5F–G). Peak Ba2+ current in arterial SMCs was blocked 56% by Nif compared to 55% inhibition in venous SMCs (Figs. 5E and 5H). Thus, although KCl constrictions in isolated, pressurized MV were insensitive to CCBs, the venous SMCs subjected to whole-cell patch-clamp conditions expressed prototypic nifedipine-sensitive LTCCs with properties indistinguishable from the arterial channels.
Figure 5.
Similar properties and densities of whole-cell LTCC currents are observed in SMCs of rat MA and MV. (A) Current-voltage (I–V) relationships for Ca2+ channel currents in MA and MV reveal similar current densities (n = 14, 7). (B) Voltage-dependent activation and inactivation were not significantly different between SMCs of MA (n = 9, 14) and MV (n = 5, 9). (C–E) Arterial SMCs treated with nifedipine (Nif, 100 nmol/L) showed a 56% reduction in LTCC channel current density (n = 9–14). (F–H) Venous SMCs showed a similar level of nifedipine–induced block of 55% (n = 4–7). * = significant difference from control (Con) in the same preparation (p<0.05).
Depleting intracellular Ca2+ restores venous SMC sensitivity to CCBs
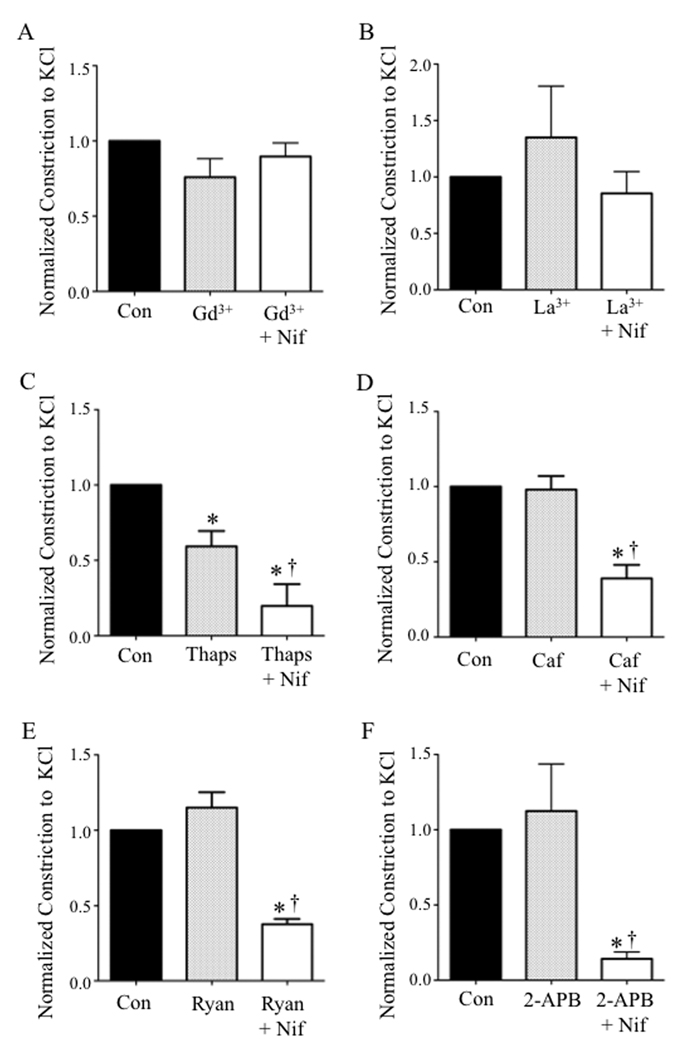
The divergent observations that LTCCs in MV did not contribute to KCl constriction (Fig. 2) whereas depolarization elicited whole-cell LTCC currents in dialyzed SMCs of MV (Fig. 5), led us to consider the possibility that venous LTCCs are inactivated by cytosolic factors in situ, conferring resistance to CCB-induced dilation. In this regard, a rise in [Ca]i during cell excitation is recognized as a negative feedback mechanism that inactivates LTCCs in a variety of cell types.24–26 Initially we determined if Ca2+ influx through store-operated channels (SOCs) inactivated LTCCs during depolarization of MV. Pressurized MV were pre-incubated with gadolinium (Gd3+) or lanthanum (La3+) to non-selectively block SOCs. We observed that neither Gd3+ (100 µmol/L) nor La3+ (100 µmol/L) reduced the amplitude of KCl constrictions in MV or conferred venous sensitivity to nifedipine (Figs. 6A and 6B).
Figure 6.
Pharmacological depletion of intracellular Ca2+ stores restores nifedipine (Nif) sensitivity to depolarization-induced contractions in rat MV. Blocking store-operated Ca2+ release with Gd3+ (A, 100 µmol/L) or La3+ (B, 100 µmol/L) did not reduce venous KCl-induced constriction or restore sensitivity to Nif in veins. (n = 4–5) (C) KCl-induced constrictions after Thaps were blocked 80% by Nif (10 µmol/L). (n = 6) (D) Store depletion by caffeine (Caf, 10 mmol/L) also resulted in sensitivity to block by Nif. (E) Using ryanodine (Ryan, 10 nmol/L) to deplete intracellular Ca2+ stores also resulted in MV sensitivity to nifedipine, (n = 5–6), as did incubating with 2-APB (100 µmol/L) (F) (n = 6). * = significant difference from Control (Con), p<0.05; † = significant difference between Thaps, Caf, Ryan, or 2-APB in the presence and absence of Nif.
Since the sarcoplasmic reticulum is in close proximity to the plasma membrane in some venous SMCs,27 we next explored if depleting intracellular Ca2+ stores using the SERCA inhibitor thapsigargin (Thaps, 1 µmol/L)28 could restore depolarization-induced Ca2+ influx through LTCCs and thereby confer sensitivity to CCBs. Since Thaps alone robustly constricted MV (data not shown), vessels were kept in nominally Ca2+-free PSS during exposure to Thaps and then extracellular Ca2+ (1.5 mmol/L) was restored concurrently with depolarizing KCl. Depletion of intracellular Ca2+ stores with Thaps reduced KCl constriction in MV by 41%. However, after Thaps treatment, Nif blocked KCl constrictions in rat MV by 80% (n = 6) (Fig. 6C). We also pre-incubated the MVs in 10 mmol/L caffeine (Caf) to deplete intracellular Ca2+ stores by increasing ryanodine receptor activity.28 Caf did not alter KCl constrictions, but similar to Thaps, in the presence of Caf, KCl constrictions of MV were blunted 61% by Nif in the presence of Caf (Fig. 6D). Ryanodine (Ryan), which opens ryanodine receptors to a full conductance state also depletes intracellular Ca2+ stores28. Ryanodine (10 nmol/L) also did not affect KCl constrictions in MV, but in its presence, Nif blunted KCl constrictions by 62% (Fig. 6E). Finally, we used 2-Aminoethoxydiphenyl borate (2-APB), a non-selective IP3 receptor blocker and SOC channel blocker28, to deplete intracellular Ca2+ stores. We observed that whereas 2-APB (100 µmol/L) did not alter KCl constrictions in MV, Nif reduced KCl constrictions by 86% in the presence of 2-APB (Fig. 6F). Thus, depletion of intracellular Ca2+ stores in venous SMCs using four different pharmacological agents apparently restored depolarization-elicited Ca2+ influx through LTCCs in MV.
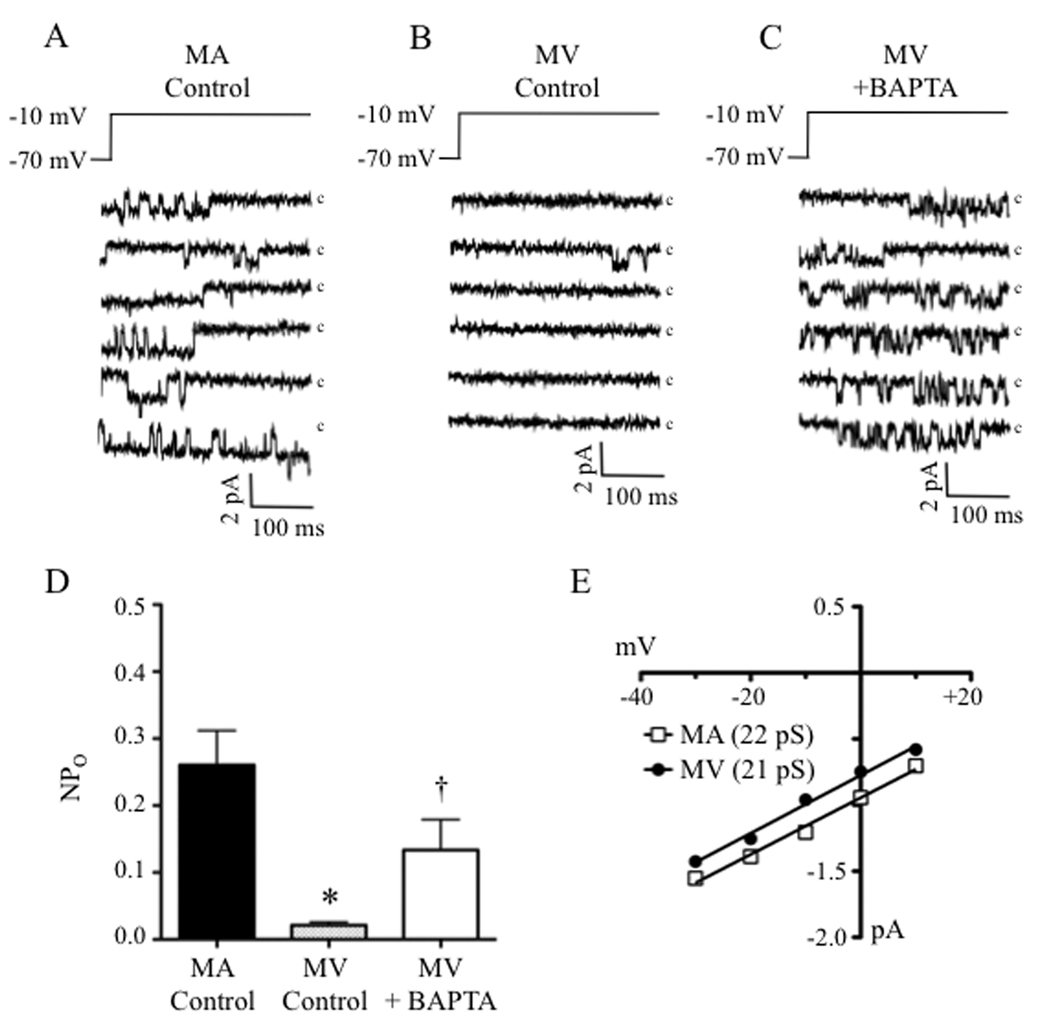
As a final proof-of-principle that the lack of effect of CCBs in MV relates to a “silencing” of LTCCs in venous SMCs by intracellular Ca2+ stores, we compared the open-state probability (NPo) of LTCCs in arterial and venous SMCs using the cell-attached patch configuration that minimally disturbs the intracellular milieu. Reducing the patch potential (PP) from −70 mV to −10 mV resulted in dense and often stacked single-channel currents in cell-attached patches of SMCs of MA (Fig. 7A) but only rare unitary currents in patches of MV (Fig. 7B). The corresponding NPo values at a PP of −10 mV were 0.26±0.05 (MA, 10 patches) and 0.02 ± 0.01 (MV, 7 patches) (Fig. 7D). However, incubating venous SMCs in the chelator BAPTA-AM (100 µmol/L) to deplete intracellular Ca2+ increased the NPo of venous LTCCs by 6.7–fold (NPo = 0.13 ± 0.05, 6 patches) (Fig. 7C and 7D). The single-channel conductance was not significantly different between SMCs from MA (22 pS) and MV (21 pS), showing values similar to those reported by others using 50 to 90 mmol/L Ba2+ as the charge carrier29,30 (Fig. 7E).
Figure 7.
Ca2+ inactivation may silence LTCCs during depolarization of venous smooth muscle (A) Tracings of single LTCC currents in freshly isolated SMCs of rat MA revealed frequent openings in the cell-attached mode. (B) Under the same conditions, venous SMCs showed only rare openings, but activity was greatly increased after chelating intracellular Ca2+ with BAPTA-AM (C, 100 µmol/L). (c = closed state). (D) The open-state probability (NPO) was significantly higher in control MA compared to control MV and chelating intracellular Ca2+ with BAPTA-AM significantly increased MV NPO (n = 6–10). (E) The single channel conductance was not significantly different between MA and MV (n = 30–40 openings from 3 different cell isolations). * = significant difference from MA Control (p<0.05) (n = 30–40 openings from 3 different cell isolations). † = significantly different from MV Control (p<0.05).
DISCUSSION
The organic CCBs have been used for several decades to treat hypertension and vasospasm with particular emphasis on the dihydropyridine class that includes nifedipine as a selective vasodilator. It is widely recognized that the CCBs exert their antihypertensive effect by reducing the open-state probability of LTCCs in arterial SMCs to lower afterload, but that they have little suppressant effect on the tone of the venous circulation.9, 31–33 To our knowledge, the present study provides the first mechanistic explanation for the resistance of veins to CCB therapies, a characteristic that may limit their antihypertensive action and contribute to adverse effects.3–6 Based on our findings, we propose that the SMCs of small MV express prototypic LTCCs that retain the same sensitivity to CCB–induced blockade of constriction as arterial SMCs. However, during vascular activation, the LTCCs in the SMCs of small veins are inactivated by Ca2+ from intracellular stores. Thus, small veins express phenotypically normal but “silent” LTCCs whose function is only revealed after Ca2+ store depletion disinhibits the channels to enable Ca2+ influx.
In this regard, it is well recognized that Ca2+ influx through LTCCs primarily mediates arterial but not venous tone in vivo9, 31–33, although there is experimental evidence that some larger veins retain sensitivity to CCBs.11 The mechanism that confers the resistance of small veins to CCB–induced dilation has been elusive, and it may not be fair to extend our observations to all venous beds, as there are special venous beds, such as the portal veins and pulmonary veins that are exposed to high pO2 and high pressure, respectively, that may respond differently to CCBs because of their unique environments. Our studies used cannulated second order rat MA and MV, which were pressurized at their high and low in situ intraluminal pressures, respectively, as an experimental model to identify these mechanisms. We first considered the possibility that a more negative resting Em below the voltage required for LTCC activation and/or an inability of KCl to depolarize the venous SMCs may explain the apparent lack of depolarization-induced Ca2+ influx through LTCC in MV. The factors that regulate resting Em in veins are poorly understood and the in situ Em in rat MV is reportedly more negative than in the MA34 although the in vitro values of resting Em may not differ.35 Indeed, we observed similar levels of resting Em between pressurized rat MA and MV and the addition of 60 mmol/L KCl induced a comparable depolarization. Similarly, the lack of LTCC–dependent constriction in MV could not be attributed to fewer channels, since MA and MV expressed similar levels of pore-forming α1C subunits and venous SMCs exhibited whole-cell Ca2+ channel currents indistinguishable in properties and densities from arterial SMCs. Thus, the functional LTCCs in SMCs of MV appear to be prototypic channels. However, the paucity of unitary LTCC currents in cell–attached patches of venous SMCs indicated that when the cytosol is minimally disturbed in veins, the LTCCs are either not activated by depolarization or are inactivated by a cytosolic influence that is retained in the cell-attached configuration but lost during cell dialysis in whole-cell recording. Our findings that the Ca2+ chelator BAPTA-AM increased unitary LTCC currents in venous SMCs paired with the demonstration that pharmacological depletion of intracellular Ca2+ stores confers sensitivity of venous constriction to CCBs provide strong evidence that intracellular Ca2+ stores inactivate venous LTCCs during SMC depolarization. However, we cannot rule out the possibility that LTCC-mediated Ca2+ entry is partly buffered by SR uptake that acts as a sink for Ca2+ entering the cell as postulated by the superficial buffer barrier hypothesis.36
Our proposal that the silencing of LTCCs in venous SMCs is mediated by Ca2+-inactivation concurs with the recognition of this process as an important biophysical property of LTCCs that acts as a negative feedback mechanism to limit voltage-gated Ca2+ entry during cell excitation.24–26 The process of Ca2+-dependent inactivation requires the Ca2+ binding protein, calmodulin, which binds to the intracellular C-terminus of α1C to cause a conformational change that enables channel inactivation.37,38 In the heart, Ca2+ influx through LTCCs opens ryanodine receptors in the sarcoplasmic reticulum to trigger Ca2+-induced Ca2+ release and also mediates Ca2+-dependent inactivation of LTCCs to limit action potential duration.39 The process of Ca2+ inactivation of LTCCs also may assume a physiological role in vascular SMCs, although there are only a few findings in this regard.40–42 However, we report here that intracellular Ca2+ stores in venous SMCs appear to nearly eliminate a contribution of Ca2+ influx through LTCCs to constriction, thereby acting as a powerful physiological switch that determines the source of activator Ca2+ in this cell type. Additionally, this process also may account for the resistance of venous SMCs to the dilator effect of CCBs, since the inactivation of LTCCs negates a contribution of voltage-gated Ca2+ influx to venous contraction. Thus, a physiological event formerly assigned only a modulatory role in SMCs may have a major impact on the fundamental Ca2+ handling mechanisms that underlie venous contraction and also determine its pharmacological sensitivity to vasodilator drugs.
Our findings also suggest that venous SMCs rely on other mechanisms for increasing cytosolic Ca2+, which must differ from the LTCCs that predominate in arterial SMCs. Store-operated and receptor-operated Ca2+ channels are present on the plasma membrane of SMCs and additional voltage-gated Ca2+ channel types (i.e. T-type channels) have been proposed.43–45 Ryanodine receptors and IP3 receptors located in the membrane of the sarcoplasmic reticulum also have been described.27 We observed that the rat MV still can contract robustly to KCl-induced depolarization even in the presence of high concentrations of nifedipine or diltiazem, suggesting that depolarization triggers the release of intracellular Ca2+ in venous SMCs independently of LTCCs activity under our conditions. Indeed, the SR is present in close proximity to the plasma membrane in the SMCs of many blood vessel types including the small pulmonary arteries, aorta, and small mesenteric arteries and veins,45 suggesting coupling of intracellular Ca2+ stores and plasma membrane ion channels. This interaction has been demonstrated in human cerebral arteries, in which Ca2+ release from ryanodine receptors (i.e. Ca2+ sparks) activates the plasma membrane high-conductance, Ca2+-activated K+ channel.46 Thus, we speculate that there may be a difference in sarcoplasmic reticulum-plasma membrane association of ion channels in veins compared to arteries, such that intracellular Ca2+ stores in veins can interact with and silence plasma membrane LTCCs.
Limitations
There are several key limitations in our study that should be acknowledged. First, the in vivo environment of arteries and veins differs in many respects including oxygen tension, pH, and intraluminal pressure and these factors may alter vascular reactivity. We accounted for the different intraluminal pressures in our experiment by pressurizing arteries and veins near their physiological pressures. However, we exposed both MA and MV to physiological solutions bubbled with a 95% O2 – 5% CO2 gas mixture to attain a pH value of 7.4. Thus, differences in pO2, CO2 and extracellular pH did not account for venous resistance to CCBs in our study, but conceivably could modulate sensitivity in vivo. Second, CCBs including Nif can influence vascular tone aside from their pharmacological block of LTCC that mediates the main dilator effect. The CCBs may induce nitric oxide (NO) release from the endothelium47 and scavenge superoxide48. Since the endothelium was not removed from the rat small MA and MV in our study, it is possible that endothelial-derived NO complemented the dilator effects of Nif attributed to pharmacological block of LTCCs in SMCs. Third, to our knowledge, the types of ion channels and their regulation in venous SMCs have not been rigorously investigated. Thus, it is possible that the populations of ion channels expressed in arteries and veins are different. Although highly speculative, the lack of LTCC activity in veins also could relate to uncharacterized interactions with other cytosolic or membrane proteins. For example, in mouse motor nerve terminals, normally silent LTCCs are revealed by blockade of Ca2+-activated K+ channels.49
Perspectives
Our study has highlighted important differences in mechanisms of vasoconstriction between small MA and MV, and the findings suggest that venous depolarization-induced constriction can occur independently of Ca2+ influx via LTCCs. Our findings suggest that although prototypic LTCCs are expressed in the plasma membrane of venous SMCs, these channels are “silenced” in situ by intracellular Ca2+ stores. To our knowledge, this is the first report providing an explanation for why CCBs are selective arterial dilators in humans and animals in vivo, whereas veins fail to respond to these important vasodilator agents. Conversely, defining the unique mechanisms of venoconstriction may present novel targets for new blood pressure lowering drugs that selectively target the venous circulation.
Novelty and Significance
What is known?
Calcium channel blockers block Ca2+ entry through voltage-gated L-type Ca2+ channels and are widely prescribed as antihypertensive medications to lower high blood pressure.
These drugs block Ca2+ influx into arteries but not veins to increase vessel diameter and lower blood pressure, but the reason why veins fail to dilate to calcium channel blockers is unclear.
What new information does this article contribute?
The present study shows that Ca2+ channel blockers prevent the constriction of small arteries but not veins from the rat mesenteric circulation; the mesenteric circulation is a key vascular bed that controls blood pressure in animals and humans.
Next, we show that L-type Ca2+ channels are present in similar numbers in mesenteric arteries and veins, but the Ca2+ channels in veins are not functional.
Finally, we provide evidence that in veins, Ca2+ from intracellular stores “silences” L-type Ca2+ channels.
Summary of Novelty and Significance
The reason why Ca2+ channel blocking drugs lower blood pressure by dilating arteries but not veins is unknown. Our goal was to determine why veins fail to dilate in response to this class of drugs. Using the rat mesenteric circulation as a model, we observed that although L-type Ca2+ channels exist in both arteries and veins, Ca2+ channel blockers prevent arteries but not veins from constricting. However, depleting intracellular Ca2+ stores in the muscle cells of veins restores sensitivity to Ca2+ channel blockers. These findings suggest that intracellular Ca2+ stores “silence” L-type Ca2+ channels in veins, thereby negating their contribution to constriction. Thus, we provide evidence for a novel mechanism of “Ca2+ channel silencing” that confers venous insensitivity to Ca2+ channel blockers. Our data suggest that Ca2+ from intracellular stores may mediate venoconstriction, and these Ca2+ mobilizing pathways may represent targets for the development of new venodilator therapies. In the clinical setting, the selective dilation of the venous circulation reduces blood return to the heart to lower its workload, which is beneficial in some cardiovascular diseases such as heart failure.
Supplementary Material
Acknowledgments
Sources of Funding: This study was supported by NIH grants R01 HL064806-09 (to N.J.R.) and F32 HL095284-01 (to K.T.).
Non-standard Abbreviations and Acronyms
- 2-APB
2-aminoethoxydiphenyl borate
- Caf
caffeine
- CCB
calcium channel blocker
- LTCC
L-type Ca2+ channel
- MA
mesenteric arteries
- MV
mesenteric veins
- Nif
nifedipine
- PP
patch potential
- PSS
physiological salt solution
- Ryan
ryanodine
- SMC
smooth muscle cell
- Thaps
thapsigargin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Subject codes: [136] Calcium cycling/excitation-contraction coupling, [97] Other vascular biology
Disclosures: None
References
- 1.Stern S, Bayes de Luna A. Coronary artery spasm: a 2009 update. Circulation. 2009;119:2531–2534. doi: 10.1161/CIRCULATIONAHA.108.843474. [DOI] [PubMed] [Google Scholar]
- 2.Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 3.Major TC, Dhamija S, Black N, Liachenko S, Morenko B, Sobocinski G, Okerberg C, Tinholt P, Madore S, Kowala MC. The T- and L-type calcium channel blocker (CCB) mibefradil attenuates leg edema induced by the L-type CCB nifedipine in the spontaneously hypertensive rat: a novel differentiating assay. J Pharmacol Exp Ther. 2008;325:723–731. doi: 10.1124/jpet.107.133892. [DOI] [PubMed] [Google Scholar]
- 4.Noll G, Lüscher TF. Comparative pharmacological properties among calcium channel blockers: T-channel versus L-channel blockade. Cardiology. 1998;89:10–15. doi: 10.1159/000047274. [DOI] [PubMed] [Google Scholar]
- 5.Opie LH. Calcium channel antagonists. Part IV: Side effects and contraindications drug interactions and combinations. Cardiovasc Drugs Ther. 1988;2:177–189. doi: 10.1007/BF00051233. [DOI] [PubMed] [Google Scholar]
- 6.Weir MR. Incidence of pedal edema formation with dihydropyridine calcium channel blockers: issues and practical significance. J Clin Hypertens (Greenwich) 2003;5:330–335. doi: 10.1111/j.1524-6175.2003.02216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson RJ, Galligan JJ, Fink GD. Effect of an ET(B)-selective and a mixed ET(A/B) endothelin receptor antagonist on venomotor tone in deoxycorticosterone-salt hypertension. J Hypertens. 2001;19:431–440. doi: 10.1097/00004872-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Palacios B, Lim SL, Pang CC. Subtypes of endothelin receptors that mediate venous effects of endothelin-1 in anaesthetized rats. Br J Pharmacol. 1997;122:993–998. doi: 10.1038/sj.bjp.0701474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricksten SE, Yao T, Thoren P. Peripheral and central vascular compliance in conscious normotensive and spontaneously hypertensive rats. Acta Physiol Scand. 1981;112:169–177. doi: 10.1111/j.1748-1716.1981.tb06801.x. [DOI] [PubMed] [Google Scholar]
- 10.Safar ME, London GM, Weiss YA, Milliez PL. Altered blood volume regulation in sustained hypertension: A hemodynamic study. Kidney Internat. 1975;8:42–47. doi: 10.1038/ki.1975.74. [DOI] [PubMed] [Google Scholar]
- 11.Magnon M, Gallix P, Cavero I. Intervessel (arteries and veins) and heart/vessel selectivities of therapeutically used calcium entry blockers: variable, vessel-dependent indexes. J Pharmacol Exp Ther. 1995;275:1157–1166. [PubMed] [Google Scholar]
- 12.Yatani A, Seidel CL, Allen J, Brown AM. Whole-cell and single-channel calcium currents of isolated smooth muscle cells from saphenous vein. Circ Res. 1987;60:523–533. doi: 10.1161/01.res.60.4.523. [DOI] [PubMed] [Google Scholar]
- 13.Cox RH, Katzka D, Morad M. Characteristics of calcium currents in rabbit portal vein myocytes. Am J Physiol. 1992;263:H453–H463. doi: 10.1152/ajpheart.1992.263.2.H453. [DOI] [PubMed] [Google Scholar]
- 14.Messing M, Van Essen H, Smith TL, Smits JF, Struyker-Boudier HA. Microvascular actions of calcium channel antagonists. Eur J Pharmacol. 1991;198:189–195. doi: 10.1016/0014-2999(91)90620-6. [DOI] [PubMed] [Google Scholar]
- 15.Kushiro T, Watanabe N, Takahashi A, Koike M, Saito F, Otsuka Y, Kanmatsuse K. Different effects of L-type and L+N-type calcium channel blockers on hamster cheek pouch venules. J Cardiovasc Pharmacol. 2004;44(6):672–675. doi: 10.1097/00005344-200412000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama K, Horikawa N, Ogawa T, Kohno F, Ishii K, Kubo K, Imabeppu S. Arteriolar and venular vasodilating properties of benidipine hydrochloride, a 1,4-dihydropyridine Ca2+ antagonist with long-lasting action, assessed in rat mesenteric microcirculation. J Cardiovasc Pharmacol. 1999;33:540–548. doi: 10.1097/00005344-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Araki H, Itoh M, Nishi K. Effects of nipradilol on the microvascular tone of rat mesentery: comparison with other beta-blockers and vasodilators. Arch Int Pharmacodyn Ther. 1992;318:47–54. [PubMed] [Google Scholar]
- 18.Pesic A, Madden JA, Pesic M, Rusch NJ. High blood pressure upregulates arterial L-type Ca2+ channels: is membrane depolarization the signal? Circ Res. 2004;94:e97–e104. doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- 19.Albarwani S, Nemetz LT, Madden JA, Tobin AA, England SK, Pratt PF, Rusch NJ. Voltage-gated K+ channels in rat small cerebral arteries: molecular identity of the functional channels. J Physiol. 2003;551:751–763. doi: 10.1113/jphysiol.2003.040014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pratt PF, Bonnet S, Ludwig LM, Bonnet P, Rusch NJ. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension. 2002;40:214–219. doi: 10.1161/01.hyp.0000025877.23309.36. [DOI] [PubMed] [Google Scholar]
- 21.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation. 1997;4:35–50. doi: 10.3109/10739689709148316. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Rusch NJ, Striessnig J, Sarna SK. Down-regulation of L-type calcium channels in inflamed circular smooth muscle cells of the canine colon. Gastroenterology. 2001;120:480–489. doi: 10.1053/gast.2001.21167. [DOI] [PubMed] [Google Scholar]
- 23.Rondelli CM, Szasz IT, Kayal A, Thakali K, Watson RE, Rovner AS, Eddinger TJ, Fink GD, Watts SW. Preferential myosin heavy chain isoform B Expression may contribute to the faster velocity of contraction in veins versus arteries. J Vasc Res. 2007;44:264–272. doi: 10.1159/000100991. [DOI] [PubMed] [Google Scholar]
- 24.Kass RS, Sanguinetti MC. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984;84:705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romanin C, Karlsson JO, Schindler H. Activity of cardiac L-type Ca2+ channels is sensitive to cytoplasmic calcium. Pflugers Arch. 1992;421:516–518. doi: 10.1007/BF00370266. [DOI] [PubMed] [Google Scholar]
- 26.Haack JA, Rosenberg RL. Calcium-dependent inactivation of L-type calcium channels in planar lipid bilayers. Biophys J. 1994;66:1051–1060. doi: 10.1016/S0006-3495(94)80886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devine CE, Somlyo AV, Somlyo AP. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972;52:690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laporte R, Hui A, Laher I. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol Rev. 2004;56:439–513. doi: 10.1124/pr.56.4.1. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Sperelakis N. Tyrosine kinases modulate the activity of single L-type calcium channels in vascular smooth muscle cells from rat portal vein. Can J Physiol Pharmacol. 1997;75:1063–1068. [PubMed] [Google Scholar]
- 30.Ohya Y, Tsuchihashi T, Kagiyama S, Abe I, Fujishima M. Single L-type calcium channels in smooth muscle cells from resistance arteries of spontaneously hypertensive rats. Hypertension. 1998;31:1125–1129. doi: 10.1161/01.hyp.31.5.1125. [DOI] [PubMed] [Google Scholar]
- 31.Bertolissi M, De Monte A, Giordano F. Comparison of intravenous nifedipine and sodium nitroprusside for treatment of acute hypertension after cardiac surgery. Minerva Anestesiol. 1998;64:321–328. [PubMed] [Google Scholar]
- 32.Kelbaek H, Aldershvile J, Skagen K, Hildebrandt P, Nielsen SL. Pre- and afterload reduction in chronic mitral regurgitation: a double-blind randomized placebo-controlled trial of the acute and 2 weeks effect of nifedipine or isosorbide dinitrate treatment on left ventricular function and the severity of mitral regurgitation. Br J Clin Pharmacol. 1996;41:493–497. doi: 10.1046/j.1365-2125.1996.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klugmann S, Salvi A, Camerini F. Haemodynamic effects of nifedipine in heart failure. Br Heart J. 1980;43:440–446. doi: 10.1136/hrt.43.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harder DR, Contney SJ, Willems WJ, Stekiel WJ. Norepinephrine effect on in situ venous membrane potential in spontaneously hypertensive rats. Am J Physiol. 1981;240:H837–H842. doi: 10.1152/ajpheart.1981.240.6.H837. [DOI] [PubMed] [Google Scholar]
- 35.Stekiel WJ, Contney SJ, Lombard JH. Small vessel membrane potential, sympathetic input, and electrogenic pump rate in SHR. Am J Physiol. 1986;250:C547–C556. doi: 10.1152/ajpcell.1986.250.4.C547. [DOI] [PubMed] [Google Scholar]
- 36.Chen Q, Cannell M, van Breemen C. The superficial buffer barrier in vascular smooth muscle. Can J Physiol Pharmacol. 1992;70:509–514. doi: 10.1139/y92-066. [DOI] [PubMed] [Google Scholar]
- 37.Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron. 2001;31:973–985. doi: 10.1016/s0896-6273(01)00438-x. [DOI] [PubMed] [Google Scholar]
- 38.Pitt GS, Zühlke RD, Hudmon A, Schulman H, Reuter H, Tsien RW. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J Biol Chem. 2001;276:30794–30802. doi: 10.1074/jbc.M104959200. [DOI] [PubMed] [Google Scholar]
- 39.Takamatsu H, Nagao T, Ichijo H, Adachi-Akahane S. L-type Ca2+ channels serve as a sensor of the SR Ca2+ for tuning the efficacy of Ca2+-induced Ca2+ release in rat ventricular myocytes. J Physiol. 2003;552:415–424. doi: 10.1113/jphysiol.2003.050823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heaps CL, Bowles DK, Sturek M, Laughlin MH, Parker JL. Enhanced L-type Ca2+ channel current density in coronary smooth muscle of exercise-trained pigs is compensated to limit myoplasmic free Ca2+ accumulation. J Physiol. 2000;528:435–445. doi: 10.1111/j.1469-7793.2000.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganitkevich VYa, Shuba MF, Smirnov SV. Inactivation of calcium channels in single vascular and visceral smooth muscle cells of the guinea-pig. Gen Physiol Biophys. 1991;10:137–161. [PubMed] [Google Scholar]
- 42.Petkov GV, Fusi F, Saponara S, Gagov HS, Sgaragli GP, Boev KK. Characterization of voltage-gated calcium currents in freshly isolated smooth muscle cells from rat tail main artery. Acta Physiol Scand. 2001;173:257–265. doi: 10.1046/j.1365-201X.2001.00907.x. [DOI] [PubMed] [Google Scholar]
- 43.Chalmers S, Olson ML, MacMillan D, Rainbow RD, McCarron JG. Ion channels in smooth muscle: regulation by the sarcoplasmic reticulum and mitochondria. Cell Calcium. 2007;42(4–5):447–466. doi: 10.1016/j.ceca.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005;83(3):215–242. doi: 10.1139/y05-016. [DOI] [PubMed] [Google Scholar]
- 45.Ureña J, del Valle-Rodríguez A, López-Barneo J. Metabotropic Ca2+ channel-induced calcium release in vascular smooth muscle. Cell Calcium. 2007;42(4–5):513–520. doi: 10.1016/j.ceca.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Wellman GC, Nathan DJ, Saundry CM, Perez G, Bonev AD, Penar PL, Tranmer BI, Nelson MT. Ca2+ sparks and their function in human cerebral arteries. Stroke. 2002;33:802–808. doi: 10.1161/hs0302.104089. [DOI] [PubMed] [Google Scholar]
- 47.Berkels R, Egink G, Marsen TA, Bartels H, Roesen R, Klaus W. Nifedipine increases endothelial nitric oxide bioavailability by antioxidative mechanisms. Hypertension. 2001;37:240–245. doi: 10.1161/01.hyp.37.2.240. [DOI] [PubMed] [Google Scholar]
- 48.Brovkovych VV, Kalinowski L, Muller-Peddinghaus R, Malinski T. Synergistic antihypertensive effects of nifedipine on endothelium: Concurrent release of NO and scavenging of superoxide. Hypertension. 2001;37:34–39. doi: 10.1161/01.hyp.37.1.34. [DOI] [PubMed] [Google Scholar]
- 49.Flink MT, Atchison WD. Iberiotoxin-induced block of Ca2+-activated K+ channels induces dihydropyridine sensitivity of ACh release from mammalian motor nerve terminals. J Pharmacol Exp Ther. 2003;305:646–652. doi: 10.1124/jpet.102.046102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.