Abstract
A non-human primate model was used to evaluate its potential for identification of rotavirus viral protein 6 (VP6) CD4+ T cell epitopes. Four juvenile rhesus macaques were inoculated with a mixed inoculum (G1P[8] and G9P[8]) of human rotaviruses. Infection accompanied by G1P[8] shedding was achieved in the two macaques that had no rotavirus immunoglobulin A (IgA) in plasma. To measure the interferon gamma (IFN-γ) and tumor necrosis factor (TNF) anti-viral cytokines produced by peripheral CD4+ cells that recognize VP6 epitopes, whole blood cells from one infected macaque were stimulated in vitro with VP6 peptides. Stimulation with peptide pools derived from the simian rotavirus VP6161–395 region revealed reactivity of CD4+ T cells with the VP6281–331 domain. A VP6301–315 region was identified as the epitope responsible for IFN-γ production while a broader VP6293–327 domain was linked to TNF production. These results suggest that human rotavirus-infected macaques can be used for identification of additional epitopes and domains to address specific questions related to the development of pediatric vaccines.
Short Report
Rotaviruses are the most common etiological agents of severe diarrhea in infants and young children worldwide. Globally, each year ca. 600,000 deaths in children less than 5 years of age are linked to rotavirus infection (Parashar et al. 2003). Since only live attenuated rotavirus strains have been evaluated as vaccines in clinical trials, the testing of non-living alternative vaccines in non-human primate model is of immediate interest (Parez, 2007). Most rotavirus-induced antibody responses are directed against the VP6 protein that comprises the intermediate capsid layer of the virus particle (Franco et al. 1995; Banos et al. 1997; Choi et al. 2000). Although humoral immunity has been identified as an important component of protection against rotavirus infection in various animal models (Conner et al. 1991; O'Neal et al. 1997; Ruggeri et al. 1998; Yuan et al. 1998; Yuan and Saif, 2002; Jiang et al. 2002; Gonzalez et al. 2003), several studies have suggested that T cell-mediated immunity also plays a role (Banos et al. 1997; Jaimes et al. 2002, 2004, 2005; Rojas et al. 2003; McNeal et al. 2002, 2007). These studies carried out with rotavirus-infected humans and rodents suggest that both CD4+ and CD8+ T cells, particularly those that secrete IFN-γ, function as effectors of immune protection. Ultimate evidence demonstrating the indispensable role of T cell-mediated immunity to rotavirus was shown in BALB/c mice immunized intranasally with recombinant VP6 and a mucosal adjuvant where protection was shown to be dependent on CD4+ T cells rather than B cells (McNeal et al. 2002). In addition, the mechanisms of protection and the immune responses generated after live oral infection were found to be different from those induced by VP6 immunization (Van Cott et al. 2006). It is not clear, however, if these findings can be translated to genetically outbred primate populations such as humans.
Our studies with rotavirus-infected rhesus macaques corroborated that production of IFN-γ and other inflammatory cytokines by CD4+ and CD8+ T cells does occur in rotavirus-infected primates (Sestak et al. 2004). Based on findings with rotavirus-infected mice, there is some evidence to suggest that conserved protein sequences of rotavirus VP6 might cross-react with T cells obtained from different host species (Banos et al. 1997; Jaimes et al. 2005). If such VP6 epitopes can be identified also in primates, rotavirus vaccine development could shift from current “VP4/VP7 Live Attenuated Reassortant” approach toward better defined “synthetic peptide and/or DNA” approach. We reported that up to 86% nucleotide identity is identified between VP6 of simian and human rotaviruses (Zhao et al. 2005; McNeal et al. 2005). Challenge studies with human rotavirus-infected primates may thus provide new information in respect to identification of pediatric vaccine-relevant synthetic T cell epitopes. In this study, we attempted to identify rotavirus VP6-specific CD4+ T cell epitopes in macaques that were infected with a human rotavirus. Based on the assumption that rotavirus IgA is the major correlate of protection, four juvenile macaques (2 seronegative and 2 with marginal levels of plasma rotavirus IgA) were selected for experimental inoculation with wild type human rotaviruses each containing 3 × 104 focus forming units (f.f.u.). Two human stool suspensions containing rotaviruses, both identified as P[8] strains but belonging to either G1 or G9 as determined using RT-PCR genotyping and nucleotide sequencing methods (Das et al. 1994; Gentsch et al. 1992; Gouvea et al. 1990; Griffin et al. 2002) were used for inoculation. It was predicted that a viable strain (possibly reassortant) would emerge during replication in the infected animals and produce symptomatic infection. Following infection of one of the seronegative animals, whole blood cells were obtained and stimulated in vitro with synthetic 15-mer peptides overlapping by 11 amino acids, spanning the VP6161–395 region of simian TUCH rotavirus (McNeal et al. 2005) to identify epitopes that recognized CD4+ T cells.
Due to endemic nature of rotavirus in colony macaques, 203 macaques (100 juveniles of <3 years and 103 adults of 3–10 years of age, irrespective of sex) had to be pre-screened to identify individuals negative for rotavirus antibodies (McNeal et al. 2005). Negative cut-off ELISA values for IgG and IgA were determined at <50 and <20 Units/ml, respectively, in accordance with our previous studies (Sestak et al. 2004; McNeal et al. 2005; Zhao et al. 2005). All but 5 juvenile macaques (<3 years-old) tested positive for rotavirus antibodies (IgA, IgG, or both). Of these 5, 1 animal was unsuitable due to an unrelated study assignment. Thus, 4 macaques were challenged with rotavirus as previously described (Sestak et al. 2004).
Confirmatory testing of 4 selected macaques was performed three months later after initial prescreening—with post challenge day 0 (PCD 0) plasmas: No rotavirus antibodies were detected in plasma from macaques FC85 and GA02 while macaques FF64 and FN09 exhibited low but detectable levels of rotavirus IgA (Fig. 1A). By PCD 7, plasma rotavirus IgA levels had increased in 3 of the 4 animals (including both seronegative macaques) and continued to increase until PCD 14 (Fig. 1A). By PCD 21, IgA levels had begun to decline in both of the previously seronegative macaques (Fig. 1A).
Figure 1.
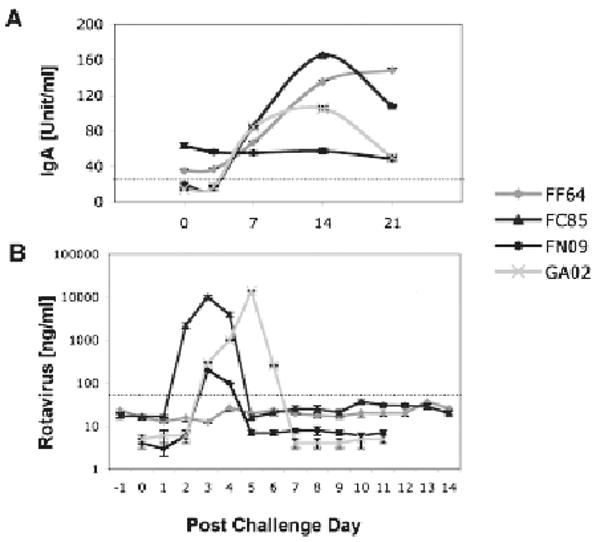
Rotavirus plasma IgA (A) and fecal shedding (B) are shown upon experimental challenge of 4 juvenile macaques with human rotavirus. Dotted lines represent cut-offs between negative and positive measurements—as previously established by average values corresponding to populations of control (negative and positive) macaques. Immunoassays used to measure the rotavirus plasma IgA and fecal shedding were described in detail elsewhere (Sestak et al. 2004; McNeal et al. 2005; Zhao et al. 2005).
Quantitative evaluation of rotavirus shedding in macaque stools (Fig. 1B) was consistent with our past results (Sestak et al. 2004; McNeal et al. 2005) and with the pattern of plasma rotavirus IgA in the 2 seronegative animals. By PCD 7, no shedding of rotavirus was detected in any of the 4 inoculated macaques. Only transient clinical symptoms of pasty diarrhea were recorded between PCD 3 and PCD 6 in the 2 previously seronegative macaques. Using RT-PCR genotyping and nucleotide sequencing methods, a single rotavirus P type i.e. P[8], and G1 but not G9 type was confirmed in stools collected from infected macaques (Das et al. 1994; Gentsch et al. 1992; Gouvea et al. 1990; Griffin et al. 2002).
The macaque with highest detected level of rotavirus fecal shedding (GA02) was selected for VP6 epitope mapping. This was done with the realization that rotavirus VP6 CD4+ T cell epitopes would differ between outbred primates, thus disallowing any generalization of the results. Because T cell immune responses to rotavirus were reported to peak in mice at PCD 7–14 (Jaimes et al. 2005), peripheral blood samples collected from macaque GA02 during this time period were used. In past studies with YM rotavirus-infected (Banos et al. 1997) or MBP∷VP6 immunized (McNeal et al. 2007) BALB/c mice, CD4+ T cell epitopes were identified in VP6289–302 and VP6242–259 regions, respectively. Another study with BALB/c mice identified CD8+ T cell epitopes linked to VP653–62 and VP6357–366 domains of murine EC rotavirus (Jaimes et al. 2005). Thus, our CD4+ T cell epitope mapping focused on the C-terminus VP6161–395 (60% of protein length).
Given the >90% homology between human and simian rotavirus VP6 genes (McNeal et al. 2005), we used human rotavirus to induce infection in macaques, and simian G3P[24] TUCH rotavirus for epitope mapping. A complete 1,194 bp sequence of TUCH rotavirus VP6 (GenBank, access code AY594670) was used as the template for peptide synthesis. Ninety-six individual peptides, each consisting of 15 aa residues overlapping by 11 aa, were produced to high (>80%) purity by Sigma-Genosys, St. Louis, MO, so that entire VP6 sequence was spanned (Maecker et al. 2001). One mg of each peptide was received from the manufacturer and stored in lyophilized form at −80 °C until its use. Prior to use, each peptide was reconstituted in 80% dimethyl sulfoxide (DMSO, Sigma), 20% deionized water at a concentration of 10 mg/ml and made into aliquots. Selected reconstituted peptides were combined to form the following 6 stimulation pools: VP6161–211, VP6201–251, VP6241–291, VP6281–331, VP6321–371, and VP6361–395 so that suspected CD4+ T cell epitope C-terminus regions were all included (Banos et al. 1997; Jaimes et al. 2005; McNeal et al. 2007). The final concentration of each peptide in each peptide pool was 1 mg/ml and standard stimulation protocol was followed (Maecker et al. 2001). Reconstituted peptides were stored at −80 °C until their use. Expression of IL-2, IFN-γ, and TNF (BD Pharmingen clones MQ1-17H12, 4S.B3 and MAB11, respectively) by blood CD3+CD4+ T lymphocytes (SP34-2 and L200 clones) upon stimulation with VP6 peptides was evaluated by five-color flow cytometry using the above antibodies (Sestak et al. 2004; Pahar et al. 2006).
While no production of 3 cytokines by CD3+CD4+ cells was observed at PCD 0, at PCD 7, stimulation of CD3+CD4+ cells (Fig. 2a) with peptide pools derived from the TUCH rotavirus VP6161–395 region revealed higher reactivity of the VP6281–331 domain than other VP6 regions, i.e. production of IL-2, TNF and IFN-γ was elevated above the base line level (p < 0.05) as determined by Mann Whitney Rank Test. Some increase of IFN-γ production was also observed after stimulation with the VP6241–291 peptide pool (Fig. 2b). The VP6281–331 peptide pool was split into individual overlapping 15-mers, and tested with blood sample collected at PCD 14 for production of IFN-γ (Fig. 2c). Stimulation of CD4+ T cells with the VP6301–315 peptide resulted in ∼5-fold increase in IFN-γ production above the base line (Fig. 2c). This was lower than expected based on response measured at PCD 7, suggesting a decline of IFN-γ production in GA02 by PCD 14. In addition to VP6301–315, three other peptides were identified in the VP6281–331 pool that elicited increased TNF production (p < 0.05), including VP6297–315 and VP6313–327 regions (Supplementary Fig. 1). In summary, VP6301–315 was identified as the epitope associated with IFN-γ while a broader VP6293–327 domain was linked with TNF production (Table 1). One out of the 2 incomplete (1 aa mismatch with TUCH peptides) murine CD4+ epitopes used in this study as controls also contained a portion of the sequence associated in this study with antiviral cytokine production (Table 1). The more dispersed location of TNF domain including its overlap with IFN-γ epitope could be explained by redundancy and pleiotropy of anti-viral cytokines and the fact that commercial anti-human TNF antibodies used in this study recognized several components of the TNF family, such as TNF-α, TNF-β, etc. (BD Pharmingen). Partial overlap and potential cross-reactivity between human, simian and mouse CD4+ T cell epitopes corroborates our hypothesis that rotavirus VP6 epitopes might be exploited for preparation of heterologous vaccines.
Figure 2.
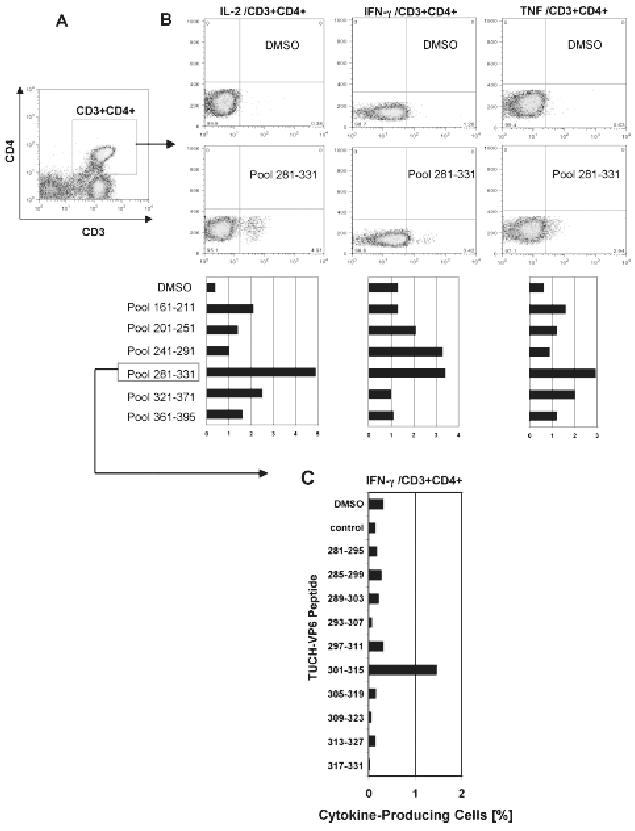
Rotavirus VP6 epitope mapping was performed with peripheral blood cells collected at peak of virus infection (PCD 7) from GA02 macaque. Stimulation was performed in vitro with overlapping VP6 15-mer peptide pools corresponding to the TUCH rotavirus (Maecker et al. 2001; Jaimes et al. 2005). Following staining with appropriate FACS antibodies, analysis of intracellular cytokine production was performed (Sestak et al. 2004; Pahar et al. 2006). Gating was done through populations of CD3+CD4+ T cells (A). The amino acid sequence ranges are shown for each pool (B). Highest production of IL-2, TNF and IFN-γ cells was found upon stimulation with the VP6281–331 pool although some cytokine production was also seen upon stimulation with other VP6 pools, primarily for IFN-γ after stimulation with the VP6241–291 peptide pool. DMSO values indicate baseline levels. Individual peptides from the VP6281–331 pool were used to stimulate peripheral blood cells collected at PCD 14 for their ability to induce production of IFN-γ (C). The highest IFN-γ production following stimulation with VP6301–315 peptide is shown (C).
Table 1.
TUCH rotavirus VP6 aa sequences that were associated with cytokine production.
| Position (TUCH) | Sequence | Suggested function |
|---|---|---|
| aa 241–255 | …..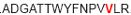 ….. ….. |
Mouse CD4+ T cell epitope* |
| aa 281–295 | …..IARNFDTIRLSFQLL….. | |
| aa 285–299 | …..FDTIRLSFQLLRPPN….. | |
| aa 289–303 | …..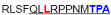 ….. ….. |
Mouse CD4+ T cell epitope* |
| aa 293–307 | …..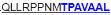 ….. ….. |
|
| aa 297–311 | ….. ….. ….. |
|
| aa 301–315 | ….. ….. ….. |
Macaque CD4+ T cell epitope |
| aa 305–319 | ….. ….. ….. |
|
| aa 309–323 | ….. ….. ….. |
|
| aa 313–327 | …..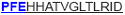 ….. ….. |
|
| aa 317–331 | …..HATVGLTLRIDSAVC….. | |
| aa 321–335 | …..GLTLRIDSAVCESVL….. | |
| aa 325–339 | …..RIDSAVCESVLADAN….. |
The IFN-γ-associated sequence (blue bold-underlined) corresponds to a TUCH rotavirus VP6301–315 while TNF-associated region (VP6293–327) is underlined, including the IFN-γ region. Partially modified sequences (1 aa mismatch in red) of BALB/c mouse VP6 epitopes that were used as controls (McNeal et al. 2007*, Banos et al. 1997†, and Jaimes et al. 2005†) are indicated.
In rhesus macaques, at least 33 MHC II (Mamu-DRB) configurations were so far identified in contrast to 5 human HLA-DRB regions (Doxiadis et al. 2001). Thus, it would be possible to pre-select haplotypes of candidate macaques against their MHC II background in follow up studies (Otting et al. 2000; Knapp et al. 1997). In order to link the epitopes identified above with particular MHC II haplotypes, future CD4+ T cell epitope mapping studies need to be performed with MHC II-uniform animals rather than with outbred individuals. Such rigorous studies with inclusion of intestinal biopsy cells derived from MHC pre-selected macaques should reveal additional rotavirus CD4+/CD8+/CD20+ epitopes.
Another objective of this study was to produce an infection of juvenile macaques with human rotavirus. Past attempts of other groups to accomplish such an objective yielded mixed results although some groups reported successful infection including diarrhea symptoms (Wyatt et al. 1976; Mitchell et al. 1977; Majer et al. 1978; Soike et al. 1980; Westerman et al. 2005, 2006). It is important to highlight that colostrum deprivation will not completely preclude passive transfer of maternal antibodies to the primate infant due to transplacental transfer. Our results suggest that macaques with even marginal levels of pre-challenge plasma rotavirus IgA may not establish infection characterized with high loads of virus in stools. Differences between the seronegative macaques (FC85 and GA02) and the two animals with low levels of pre-challenge IgA (FN09 and FF64) were characterized by a) reduced or no virus shedding in stools, b) no rise of post-challenge rotavirus IgA in FN09, and c) absence of clinical symptoms of disease in FN09 and FF64 macaques.
The typical symptoms of rotavirus-induced disease in children include dehydrating diarrhea, vomiting, and fever. To accomplish a manifestation of such severe illness in conventional primates is problematic due to several reasons. The first is related to the endemic nature of rotavirus in non-human primate colonies where the vast majority of animals possesses some level of immune protection. A second reason is the possibility of age-dependent susceptibility and the presence of unknown factor(s) of innate resistance in rhesus macaques. Based on our studies with rotavirus-challenged macaques, animals older than one year will not develop severe disease even when they are completely seronegative. The age of animals selected for this study ranged between 1.5–3 years. Although two seronegative animals developed pasty diarrhea, none of the four inoculated macaques showed watery diarrhea that can be sometimes observed in <1-year-old nursery macaques with natural rotavirus infection (Sestak et al. unpublished observations). Thus, candidate primates for future challenge studies should fulfill other criteria in addition to seronegativity: Such animals should be less than 1-year-old, fed with milk formula, and raised in a pathogen-free (BL2) environment. In this study, 203 candidate macaques including 100 juvenile animals <3 years-old were pre-screened in order to identify 5 suitable subjects from which 4 were assigned for the study. For this purpose, we took advantage of TNPRC research resource, i.e. a facility that houses the largest colony of non-human primates in the U.S.A. Giving the availability of such valuable resource, we hope to conduct further studies on the subject of rotavirus vaccine development and molecular pathogenesis.
Supplementary Material
Acknowledgments
The technical assistance of Julie Bruhn, Calvin Lanclos, Gloria Jackson, Monica M. McNeal (Cincinnati Children's Hospital), Drs. Erin Ribka, Marion Ratterree, and Pyone P. Aye is greatly appreciated. We thank Dr. Jon R. Gentsch laboratory (Centers for Disease Centrol and Prevention) for typing the rotavirus present in inoculum and stools of infected macaques. We also thank Dr. Richard Ward (Cincinnati Children's Hospital) for reviewing the manuscript. This work was supported by grant P51RR00164 from the National Institute of Health.
Footnotes
Copyright in this article, its metadata, and any supplementary data is held by its author or authors. It is published under the Creative Commons Attribution By licence. For further information go to: http://creativecommons.org/licenses/by/3.0/.
References
- Banos DM, Lopez S, Arias CF, Esquivel FR. Identification of a T-helper cell epitope on the rotavirus VP6 protein. J Virol. 1997;71:419–26. doi: 10.1128/jvi.71.1.419-426.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AH, Basu M, McNeal MM, Flint J, VanCott JL, Clements JD, Ward RL. Functional mapping of protective domains and epitopes in the rotavirus VP6 protein. J Virol. 2000;74:11574–80. doi: 10.1128/jvi.74.24.11574-11580.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner ME, Gilger MA, Estes MK, Graham DY. Serologic and mucosal immune response to rotavirus infection in the rabbit model. J Virol. 1991;65:2562–71. doi: 10.1128/jvi.65.5.2562-2571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das BK, Gentsch JR, Cicirello HG, Woods PA, Gupta A, Ramachandran M, Kumar R, Bahn MK, Glass RI. Characterization of rotavirus strains from newborns in New Delhi, India. J Clin Microbiol. 1994;32:1820–2. doi: 10.1128/jcm.32.7.1820-1822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxiadis GGM, Otting N, de Groot NG, Bontrop RE. Differential evolutionary MHC class II strategied in humans and rhesus macaques: relevance for biomedical studies. Immunol Rev. 2001;183:76–85. doi: 10.1034/j.1600-065x.2001.1830106.x. [DOI] [PubMed] [Google Scholar]
- Franco MA, Greenberg HB. Role of B-cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J Virol. 1995;69:7800–6. doi: 10.1128/jvi.69.12.7800-7806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch JR, Glass RI, Woods P, Gouvea V, Gorziglia M, Flores J, Das BK, Bhan MK. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–73. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AM, Jaimes MC, Cajiao I, Rojas OL, Cohen J, Pothier P, Kohli E, Butcher EC, Greenberg HB, et al. Rotavirus-specific B-cells induced by recent infection in adults and children predominantly express the intestinal homing receptor alpha4beta7. Virology. 2003;305:93–105. doi: 10.1006/viro.2002.1708. [DOI] [PubMed] [Google Scholar]
- Gouvea V, Glass RI, Woods P, Taniguichi K, Clark HF, Forrester B, Fang ZY. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–82. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DD, Nakagomi T, Hoshino Y, Nakagomi O, Kirkwood CD, Parashar UD, Glass RI, Gentsch JR. Characterization of nontypeable rotavirus strains from the United States: identification of a new rotavirus reassortant (P2A[6],G12) and rare P3[9] strains related to bovine rotaviruses. Virology. 2002;294:256–69. doi: 10.1006/viro.2001.1333. [DOI] [PubMed] [Google Scholar]
- Jaimes MC, Rojas OL, Gonzalez AM, Cajiao I, Charpilienne A, Pothier P, Kohli E, Greenberg HB, Franco MA, Angel J. Frequencies of virus-specific CD4(+) and CD8(+) T lymphocytes secreting gamma interferon after acute natural rotavirus infection in children and adults. J Virol. 2002;76:4741–9. doi: 10.1128/JVI.76.10.4741-4749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes MC, Rojas OL, Kunkel EJ, Lazarus NH, Soler D, Butcher EC, Bass D, Angel J, Franco MA, Greenberg HB. Maturation and trafficking markers on rotavirus-specific B. cells during acute infection and convalescence in children. J Virol. 2004;78:10967–76. doi: 10.1128/JVI.78.20.10967-10976.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaimes MC, Feng N, Greenberg HB. Characterization of homologous and heterologous rotavirus-specific T-cell responses in infant and adult mice. J Virol. 2005;79:4568–79. doi: 10.1128/JVI.79.8.4568-4579.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Gentsch JR, Glass RI. The role of serum antibodies in the protection against rotavirus disease: an overview. Clin Infect Dis. 2002;34:1351–61. doi: 10.1086/340103. [DOI] [PubMed] [Google Scholar]
- Knapp LA, Cadavid LF, Eberle ME, Knechtle SJ, Bontrop RE, Watkins DI. Identification of new mamu-DRB alleles using DGGE and direct sequencing. Immunogenetics. 1997;45:171–9. doi: 10.1007/s002510050186. [DOI] [PubMed] [Google Scholar]
- Maecker HT, Dunn HS, Suni MA, Khatamzas E, Pitcher CJ, Bunde T, Persaud N, Trigona W, Fu TM, et al. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Methods. 2001;255:27–40. doi: 10.1016/s0022-1759(01)00416-1. [DOI] [PubMed] [Google Scholar]
- Majer M, Behrens F, Weinmann E, Mauler R, Maas G, Baumeister HG, Luthardt T. Dairrhea in newborn cynomolgus monkeys infected with human rotavirus. Infection. 1978;6:71–2. doi: 10.1007/BF01642161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal MM, VanCott JL, Choi AH, Basu M, Flint JA, Stone SC, Clements JD, Ward RL. CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after intranasal immunization with a chimeric VP6 protein and the adjuvant LT(R192G) J Virol. 2002;76:560–8. doi: 10.1128/JVI.76.2.560-568.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal MM, Sestak K, Choi AH, Basu M, Cole MJ, Aye PP, Bohm RP, Ward RL. Development of a rotavirus-shedding model in rhesus macaques, using a homologous wild-type rotavirus of a new P genotype. J Virol. 2005;79:944–54. doi: 10.1128/JVI.79.2.944-954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal MM, Basu M, Bean JA, Clements JD, Choi AH, Ward RL. Identification of immunodominant CD4+ T cell epitope in the VP6 protein of rotavirus following immunization of BALB/c mice. Virology. 2007;363:410–8. doi: 10.1016/j.virol.2007.01.041. [DOI] [PubMed] [Google Scholar]
- Mitchell JD, Lambeth LA, Sosula L, Murphy A, Albrey M. Transmission of rotavirus gastroenteritis from children to a monkey. Gut. 1977;18:156–60. doi: 10.1136/gut.18.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal CM, Crawford SE, Estes MK, Conner ME. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707–17. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otting N, de Groot NG, Noort MC, Doxiadis GG, Bontrop RE. Allelic diversity of MHC-DRB alleles in rhesus macaques. Tissue Antigens. 2000;56:58–68. doi: 10.1034/j.1399-0039.2000.560108.x. [DOI] [PubMed] [Google Scholar]
- Pahar B, Cantu MA, Zhao W, Kuroda MJ, Veazey RS, Montefiori DC, Clements JD, Aye PP, Lackner AA, et al. Single epitope mucosal vaccine delivered via immuno-stimulating complexes induces low level of immunity against simian-HIV. Vaccine. 2006;24:6841–51. doi: 10.1016/j.vaccine.2006.06.050. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–72. doi: 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parez N. Rotavirus gastroenteritis: Why to back up the development of new vaccines? Comp Immunol Microbiol Infect Dis. 2007 doi: 10.1016/j.cimid.2007.07.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Rojas OL, Gonzalez AM, Gonzalez R, Perez-Schael I, Greenberg HB, Franco MA, Angel J. Human rotavirus specific T cells: quantification by ELISPOT and expression of homing receptors on CD4+ T cells. Virology. 2003;314:671–9. doi: 10.1016/s0042-6822(03)00507-5. [DOI] [PubMed] [Google Scholar]
- Ruggeri FM, Johansen K, Basile G, Kraehenbuhl JP, Svensson L. Antirotavirus Immunoglobulin A Neutralizes Virus In Vitro after Transcytosis through Epithelial Cells and Protects Infant Mice from Diarrhea. J Virol. 1998;72:2708–14. doi: 10.1128/jvi.72.4.2708-2714.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak K, McNeal MM, Choi A, Cole MJ, Ramesh G, Alvarez X, Aye PP, Bohm RP, Mohamadzadeh M, Ward RL. Defining T-Cell-Mediated Immune Responses in Rotavirus-Infected Juvenile Rhesus Macaques. J Virol. 2004;78:10258–64. doi: 10.1128/JVI.78.19.10258-10264.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soike KF, Gary GW, Gibson S. Susceptibility of nonhuman primate species to infection by simian rotavirus SA-11. Am J Vet Res. 1980;41:1098–103. [PubMed] [Google Scholar]
- VanCott JL, Prada AE, McNeal MM, Stone SC, Basu M, Huffer B, Jr, Smiley KL, Shao M, Bean JA, et al. Mice develop effective but delayed protective immune responses when immunized as neonates either intranasally with nonliving VP6/LT(R192G) or orally with live rhesus rotavirus vaccine candidates. J Virol. 2006;80:4949–61. doi: 10.1128/JVI.80.10.4949-4961.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman LE, Xu J, Jiang B, McClure HM, Glass RI. Experimental infection of pigtailed macaques with a simian rotavirus, YK-1. J Med Virol. 2005;75:616–25. doi: 10.1002/jmv.20308. [DOI] [PubMed] [Google Scholar]
- Westerman LE, Jiang B, McClure HM, Snipes-Magaldi LJ, Griffin DD, Shin G, Gentsch JR, Glass RI. Isolation and characterization of a new simian rotavirus, YK-1. Virol J. 2006;3:40. doi: 10.1186/1743-422X-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt RG, Sly DL, London WT, Palmer AE, Kalica AR, Van Kirk DH, Chanock RM, Kapikian AZ. Induction of diarrhea in colostrum-deprived newborn rhesus monkeys with the human reovirus-like agent of infantile gastroenteritis. Arch Virol. 1976;50:17–27. doi: 10.1007/BF01317997. [DOI] [PubMed] [Google Scholar]
- Yuan L, Kang SY, Ward LA, To TL, Saif LJ. Antibody-secreting cell responses and protective immunity assessed in gnotobiotic pigs inoculated orally or intramuscularly with inactivated human rotavirus. J Virol. 1998;72:330–8. doi: 10.1128/jvi.72.1.330-338.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. 2002;87:147–60. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Xia M, Bridges-Malveo T, Cantu M, McNeal MM, Choi AH, Ward RL, Sestak K. Evaluation of rotavirus dsRNA load in specimens and body fluids from experimentally infected juvenile macaques by real-time PCR. Virology. 2005;341:248–56. doi: 10.1016/j.virol.2005.06.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


