Abstract
The human Fc receptor, FcγRIIA, is known to mediate phagocytosis and endocytosis, yet the greatest numbers of these receptors are expressed on the surface of non-phagocytic platelets, where they are involved in serotonin secretion. FcγRIIA harbors three tyrosine (Y) residues within its cytoplasmic domain. Y1 is upstream of both Y2 and Y3, which are contained within an immunoreceptor tyrosine-based activation motif (ITAM), required for many signaling events. We have demonstrated that the two ITAM tyrosines are required for phagocytic signaling and that mutation of a single ITAM tyrosine decreases but does not abolish phagocytic signaling. Furthermore, we have identified that the YMTL motif is required for endocytosis. These observations suggest that FcγRIIA utilizes different sequences for various signaling events. Therefore, we investigated the sequence requirements for another important FcγRIIA-mediated signaling event, serotonin secretion, using Rat Basophilic Leukemia (RBL-2H3) cells transfected with wildtype (WT) FcγRIIA or mutant FcγRIIA. Stimulation of cells expressing WT FcγRIIA induced release of serotonin at a level 7-fold greater than that in nonstimulated WT FcγRIIA-transfected cells or nontransfected RBL cells. Mutation of either ITAM tyrosine (Y2 or Y3) to phenylalanine was sufficient to abolish serotonin secretion. Further, while inhibition of Syk with piceatannol blocked phagocytosis as expected, it did not inhibit serotonin secretion. Additionally, inhibition of phosphoinositol-3-kinase (PI3K) with wortmannin only had a partial effect on serotonin signaling, despite the fact that the concentrations used completely abolished phagocytic signaling. These data suggest that the requirements for serotonin secretion differ from those for phagocytosis mediated by FcγRIIA.
Introduction
Receptors for immunoglobulin G (IgG), termed Fcγ receptors (FcγR), play important roles in immunologic responses. Among the FcγRs, FcγRIIA is expressed in humans but not in mice. It is the most widely distributed human FcγR and is expressed on macrophages/monocytes, neutrophils, dendritic cells and platelets.[1] Unlike most Fc receptors, FcγRIIA does not depend on an accessory subunit for signaling because it contains within its own cytoplasmic domain an immunoreceptor tyrosine-based activation motif (ITAM) required for many Ig gene family signaling events.[1] The ITAM typically contains two tyrosines (Y) in the following configuration: YXXL X(6–12) YXXL where X is any amino acid and L = leucine. The conserved cytoplasmic tyrosine residues of the ITAM are phosphorylated upon receptor crosslinking. As binding sites for the SH2 (Src homology-2) domains, the phosphotyrosines generated in the ITAM sequences are important for the interaction of Fcγ receptors with important signaling molecules such as the tyrosine kinase Syk, required for phagocytosis.
The cytoplasmic domain of FcγRIIA contains three tyrosine residues. The tyrosine at position 275 (Y1) is upstream of the ITAM sequence, and the tyrosines at positions 282 (Y2) and 298 (Y3) are within the ITAM sequence. Interestingly, the ITAM sequence in FcγRIIA is atypical in that there are 12 amino acids between the ITAM sequences, rather than the typical 6–8. Crosslinking FcγRIIA induces a host of signaling events including phagocytosis of IgG-opsonized particles,[2–6] endocytosis of IgG-containing immune complexes[1, 7–10] and serotonin and histamine release from platelets.[11–15] FcγRIIA has also been shown to participate in αIIbβ3 integrin signaling in platelets,[16] and may play a role in arterial vasoocclusive disease in type 2 diabetes.[17]
Transfection of FcγRIIA into normally non-phagocytic cells, such as fibroblasts and epithelial cells, endows these cells with the ability to ingest IgG coated particles.[18] We have demonstrated that an intact ITAM is required for full phagocytic activity in transfected COS-1 cells and further observed that mutation of a single ITAM tyrosine (Y2 or Y3) decreases but does not abolish phagocytic signaling if the upstream Y1 is available.[19] This observation has led to the thesis that the FcγRIIA non-ITAM tyrosine (Y1) can serve as a mechanism to partially rescue ITAM-dependant FcγRIIA signaling when one ITAM tyrosine is unavailable.[6]
Quantitatively, the majority of FcγRIIA in humans is found on platelets, owing to the vast numbers of these cells. In platelets, FcγRIIA mediates the release of serotonin, is involved in platelet activation and triggers endocytosis of IgG complexes.[10, 12, 13, 15] However, molecular signaling interactions are not easily manipulated in platelets and platelets are not readily transfectable. Thus, it is desirable to find a model system that can be used to study the molecular signaling interactions of serotonin secretion from platelets. Rat Basophilic Leukemia (RBL-2H3) cells, traditionally used as a model to study biochemical events in mast cell activation, can also serve as an attractive model for the study of platelet secretion. RBL cells are able to release serotonin upon receptor cross-linking and, like platelets, they lack other endogenous activating Fcγ receptors that could complicate experimental conditions.[11]
To study the cytoplasmic tail requirements for FcγRIIA-mediated serotonin secretion, we transfected RBL-2H3 cells with wild-type FcγRIIA or genetically engineered FcγRIIA with Tyrosine→Phenylalanine mutations both within and upstream of the ITAM domain (Y1F, Y2F, and Y3F). We compared the ITAM signaling requirements for serotonin secretion with those for FcγRIIA-mediated phagocytosis.
Unlike phagocytic signaling, serotonin secretion requires the presence of both ITAM tyrosines, i.e. mutation of either tyrosine completely abolishes secretion. Additionally, although mutation of Y1 alone slightly reduces phagocytosis in phagocytic signaling, the presence or absence of tyrosine at position Y1 has no impact on serotonin secretory function.[19] Given the differences between cytoplasmic tail requirements for phagocytosis and serotonin secretion in our model, we examined the requirements for several downstream signaling mediators. As expected, phagocytic signaling required both Syk and phosphoinositol-3-kinase (PI3K). However, while PI3K is required for serotonin secretion, inhibition of Syk does not reduce secretion. Differences exist in requirements for phagocytic and endocytic signaling.[9, 20] Our observations suggest that, similarly, alternate FcγRIIA signaling pathways exist for phagocytosis and serotonin secretion.
Materials and Methods
Cells and Reagents
RBL-2H3 cells were maintained in minimum essential medium containing 15% fetal calf serum (FCS; HyClone, UT) and 1% streptomycin (Gibco BRL, Grand Island, NY). Cells were grown in T-75 tissue culture flasks (Corning, Corning, NY) at 37°C under 5% CO2. Radiolabeled serotonin (5-HT) was purchased from Perkin Elmer (NET-498) and used within 6 months of purchase. Anti-FcγRIIA (IV.3) monoclonal antibody (mAb) was purified from hybridoma supernatants and digested into Fab fragments. Goat anti-mouse F(ab′)2 was purchased from Jackson Immunoresearch (West Grove, PA). The calcium ionophore A23187 was purchased from Sigma-Aldrich (St. Louis, MO).
Transfection and Selection
RBL-2H3 cells were stably transfected with WT FcγRIIA or mutants of FcγRIIA. Mutations were generated from the WT FcγRIIA ITAM-like sequence Y1QTANGGY2MTLNPRAPTDDDLNIY3LTL. Single and double mutations of tyrosine (Y) to phenylalanine (F) in the FcγRIIA cytoplasmic domain were generated by polymerase chain reaction. The FcγRIIA mutants are designated Y1F, Y2F, Y3F, Y1Y2F, Y1Y3F, Y2Y3F.
The cDNA sequences encoding FcγRIIA wild-type or tyrosine mutants were cloned into pcDNA3.1 and transfected into RBL-2H3 cells using Fugene-6 (Roche Applied Science, Indianapolis, IN) per the manufacturer’s instructions and selected using G-418 (Mediatech, Manassas, VA). Transfected cells were sorted for expression of FcγRIIA using fluorescence-activated cell sorting analysis with anti-FcγRII monoclonal antibody (IV.3) and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse secondary antibody (FACS Diva [B-D Biosciences]; Figure 1). These multi-clonal populations of each transfectant were assayed for serotonin secretion. Subsequently, single cell clones were generated by limited dilution. Single cell clones were then re-tested by flow cytometry for FcγRIIA expression, and serotonin secretion experiments were conducted on these single cell clones as described below.
Figure 1.
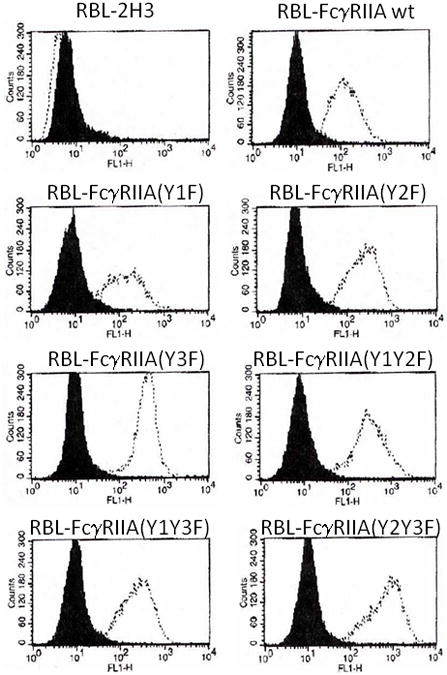
Cell surface expression of wildtype (WT) and mutant FcγRIIA in transfected RBL cells. In each panel, a dotted line indicates the histogram of cells stained with anti-FcγRIIA antibody, whereas the black shaded area indicates staining with isotype control.
Serotonin Release Assay
One day before assay, 2×104 RBL-2H3 cells from single cell clones were plated in triplicate in 96-well plates. Before receptor crosslinking, cells were preloaded with 2 μCi/ml 3H-serotonin at 37°C for 1 hour. Cells were washed, incubated with fresh medium for 1 hour and washed again. Washed cells were incubated on ice for 30 minutes in medium containing F(ab)′2 mAb IV.3 and then incubated an additional 30 minutes after addition of the secondary goat-anti-mouse antibody GAM F(ab)′ 2 (IV.3+GAM). Controls were cells incubated in 1) medium alone (med); 2) medium containing F(ab)′2 mAb IV.3 (IV.3); and 3) medium containing F(ab)′2 goat anti-mouse IgG (GAM). Alternatively, cells were treated with anti-DNP IgE (Sigma-Aldrich) and incubated on ice for 30 minutes followed by addition of DNP-BSA (Invitrogen, Carlsbad, CA) to stimulate serotonin release. Following stimulation, cells were placed at 37°C for 30 minutes to allow secretion to proceed. Secretion was terminated by addition of 100μl ice-cold medium and placement of cells on ice. After supernatants were removed from each well, cells were lysed by addition of phosphate-buffered saline (PBS) containing 1% sodium dodecyl sulfate (SDS). The 3H-serotonin in supernatants and lysates was determined by liquid scintillation counting. Serotonin release is calculated as the percent of the total serotonin available for secretion (serotonin release mediated by the calcium ionophore A23187). For inhibition assays, cells were preincubated with medium containing either 25 μg/ml piceatannol or 10 nM wortmannin (Sigma) for 15 minutes at 37°C. These specific Syk and PI3K inhibitors were chosen for consistency with previous observations.
Phagocytosis Assay
5×105 cells were plated per well in 6-well tissue culture dishes. The following day, the medium was replaced with fresh complete medium containing 1×108 IgG-opsonized sheep red blood cells (EA). After 30 minutes at 37°C, externally bound EA were lysed by exposure for 1 minute to cold hypotonic saline. Washed cells were fixed in Wright-Giemsa stain, and phagocytosis of EA was assessed in at least 300 cells by light microscopy. For inhibition studies, cells were preincubated for 15 minutes at 37°C with either 25 μg/ml piceatannol or 10 nM wortmannin.
Statistical Analysis
Statistics were performed using Students t-test.
Results
Expression of wildtype or mutant FcγRIIA is quantitatively equivalent in selected stably transfected RBL cell lines
To create a model system to investigate FcγRIIA tirggered serotonin secretion, wildtype FcγRIIA or mutants of FcγRIIA were stably transfected into RBL-2H3 cells. FACS analysis with anti-FcγRII monoclonal antibody (IV.3) and FITC-conjugated goat anti-mouse secondary antibody demonstrated that the selected cell lines transfected with FcγRIIA-wt or the various mutant FcγRIIA plasmids expressed quantitatively equivalent levels of FcγRII on the surface (Figure 1).
Cross-linking FcγRIIA induces serotonin secretion
When stimulated with FcγRII-specific mAb IV.3 F(ab)′2/GAM F(ab)′2 (IV.3+GAM), FcγRIIA-transfected RBL cells preloaded with 3H-serotonin released an average of 21% of total available radioactive serotonin (Figure 2A). This release represents an almost 7-fold increase over what is observed for RBL-2H3 cells into which FcγRIIA had not been transfected (less than 4%, Figure 2A). Less than 4% of total available serotonin is also released after mock stimulation of WT RBL-2H3 cells. This baseline release is considered due to general cell “leakiness”. Mock-stimulated FcγRIIA transfected RBL-2H3 cells also released baseline levels of serotonin (~3%), indicating that cell surface expression of FcγRIIA by itself does not increase release of serotonin (Figure 2A).
Figure 2.
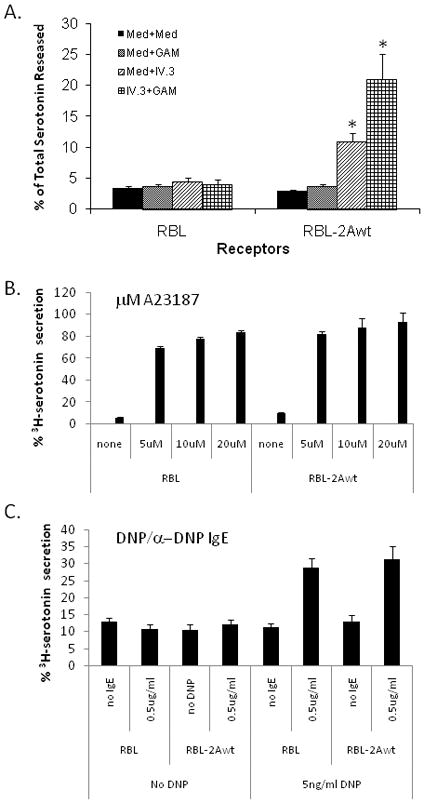
Serotonin release from RBL cells stably expressing wild-type (WT) FcγRIIA. Serotonin release was determined as described in Materials and Methods. Bars represent the percent of total available serotonin released and are the average of at least three separate experiments, each carried out in triplicate. Panel A demonstrates serotonin release from wild-type RBL-2H3 cells or FcγRIIA-expressing RBL-2H3 cells. MED indicates mock-stimulation with media alone; GAM indicates incubation with 2° antibody; IV.3 indicates incubation with primary antibody alone; and IV.3 + GAM indicate cross-linking with both primary and secondary antibodies. Panel B shows that stimulation of wild-type RBL-2H3 cells or FcγRIIA-expressing RBL-2H3 cells with calcium ionophore (A23187) or via the native IgE receptor with DNP/anti-DNP stimulation result in similar levels of serotonin release. Therefore, differences in serotonin release between clones are due to receptor ligation and signaling, and not artifacts of clone selection. (* p<0.05 compared to unstimulated and to untransfected RBL-2H3 cells)
In order to assure that differences in serotonin release were due to differences in receptor expression or signaling, clones of RBL-2H3 and FcγRIIA-expressing RBL-2H3 cells were stimulated with A23187, a potent stimulant that results in release of nearly 90% of total available serotonin. Release of serotonin after A23187 suggests that all clones have a similar amount of serotonin available for release (Figure 2B). Furthermore, each clone was exposed to anti-DNP IgE then stimulated with various concentrations of DNP to trigger serotonin secretion. As shown in Figure 2C, serotonin release via the rat IgE receptor resulted in similar levels in both wild-type RBL-2H3 cells and FcγRIIA-expressing RBL-2H3 cells suggesting that the transfection and selection process did not alter the ability of each to release serotonin.
Mutation of either ITAM tyrosine completely abolishes FcγRIIA-mediated serotonin secretion
We have previously shown that FcγRIIA-mediated phagocytosis is dependent on ITAM tyrosine residues (Y2 and Y3) and have demonstrated that the non-ITAM tyrosine (Y1) can partially rescue function in the absence of an intact ITAM domain.[19] Since the current model of phagocytic signaling is thought to involve phosphorylated ITAM tyrosines interacting with the SH2 domain of Syk as the initial downstream signaling event, we sought to determine whether serotonin secretion proceeds via the same pathway. To determine the relative importance of cytoplasmic domain tyrosines in signaling for serotonin secretion, we expressed FcγRIIA containing a single non-phosphorylatable tyrosine-to-phenylalanine mutation at positions Y1, Y2 or Y3 (Y1F, Y2F and Y3F), as well as pair-wise combinations of the above mutations (Y1Y2F, Y1Y3F, Y2Y3F).
Mutation of Y1 alone did not affect function (Figure 3A). However, mutation of either Y2 or Y3 to a non-phosphorable phenylalanine residue completely abrogated secretion, irrespective of the status of Y1 (Figure 3A). This is different from phagocytic signaling, where the availability of Y1 can rescue function. As expected, mutation of any two tyrosines likewise completely abolished secretion (Figure 3B).
Figure 3.
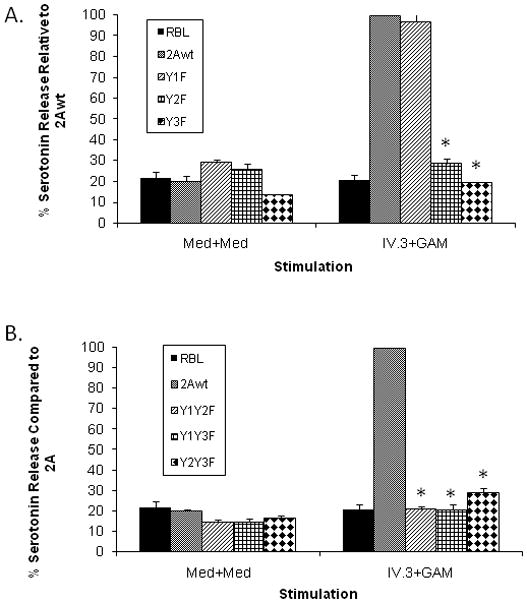
A. Effect of single tyrosine mutations on FcγRIIA mediated serotonin secretion. Serotonin release was determined as described in Materials and Methods. Bars represent the percent of total available serotonin released and are the average of at least three separate experiments, each carried out in triplicate. IV.3 + GAM indicates crosslinking with both primary and secondary antibodies; MED indicates mock-stimulation with media alone; GAM indicates incubation with 2° antibody; IV.3 indicates incubation with primary antibody alone. (* p<0.05 compared to wildtype FcγRIIA-mediated release)
B. Effect of double tyrosine mutations on FcγRIIA mediated serotonin secretion. Serotonin release was determined as described in Materials and Methods. Bars represent the percent of total available serotonin released and are the average of at least three separate experiments, each carried out in triplicate. IV.3 + GAM indicates crosslinking with both primary and secondary antibodies; MED indicates mock-stimulation with media alone; GAM indicates incubation with 2° antibody; IV.3 indicates incubation with primary antibody alone. (* p<0.05 compared to wildtype FcγRIIA-mediated release)
Effect of wortmannin and piceatannol on FcγRIIA mediated phagocytosis and serotonin release in RBL cells
According to the current understanding of FcγRIIA-mediated phagocytic signaling, the phosphorylated ITAM tyrosines recruit SH2 domains of additional enzymes and adapter proteins that participate in the signaling process.[1, 2] Given our findings that the ITAM and non-ITAM tyrosine requirements for serotonin secretion are different from those for phagocytosis, we next examined the requirements for two kinases identified in other FcγRIIA-mediated signaling cascades. Consistent with previous studies in other cell types, Figure 4A demonstrates that both Syk kinase and PI3K are required for phagocytosis in our model RBL cell system, and that at the concentrations used, inhibition of either kinase completely abolishes phagocytosis.[1, 2]
Figure 4.
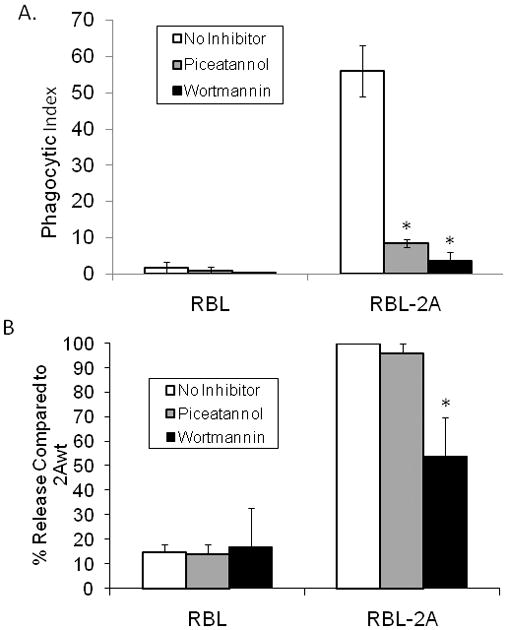
A. Effect of piceatannol and wortmannin on FcγRIIA mediated phagocytosis. Cells were pretreated with either piceatannol (25 μg/ml) or wortmannin (10nM) to specifically inhibit Syk kinase and PI3-K respectively, as described in Materials and Methods. Cells were then allowed to bind and internalize opsonized sheep erythrocytes (EA). External EA were then lysed and the RBL cells stained with Wright-Giemsa stain and viewed under brightfield microscopy. (* p<0.05 compared to wildtype FcγRIIA-mediated phagocytosis)
B. Effect of piceatannol and wortmannin on FcγRIIA mediated serotonin release. Cells were pretreated with either piceatannol (25 μg/ml) or wortmannin (10nM) to specifically inhibit Syk kinase and phosphoinositol-3-kinase (PI3K) respectively, as described in Materials and Methods. Serotonin secretion was then stimulated as described in Materials and Methods. (* p<0.05 compared to wildtype FcγRIIA-mediated release)
Our data also indicate that FcγRIIA-mediated serotonin secretion is at least partially dependant on PI3K. We observed that inhibition of PI3K by 10nM wortmannin (a relatively low concentration of inhibitor that completely inhibits phagocytosis) reduces FcγRIIA mediated serotonin secretion by nearly 50% (Figure 4B). However, inhibition of Syk with the Syk-selective inhibitor piceatannol did not inhibit serotonin release by cells expressing WT FcγRIIA, despite the fact that the concentration used (25 μg/ml) completely abolished phagocytosis. This concentration of piceatannol was also previously shown to abolish Syk functions in RBL cells, including serotonin secretion mediated by other receptors which signal via the gamma chain ITAM.[21, 22]
Discussion
FcγRIIA was previously shown to mediate phagocytosis, endocytosis, production of reactive oxygen metabolites, and release of vesicles containing proteases and other signaling molecules, e.g. serotonin, from leukocytes.[2–6] FcγRIIA is the only Fc receptor found on human platelets, where it plays a role in platelet activation, aggregation and serotonin secretion.[11–13, 15] We sought to study the cytoplasmic tail requirements of FcγRIIA for serotonin secretion. However, molecular signaling pathways are not easily manipulated in platelets, and platelets are not readily transfectable. Therefore, we created a model system for FcγRIIA-mediated serotonin secretion by stably expressing FcγRIIA in the rat basophilic cell line, RBL-2H3, which is known to have secretory potential. In addition, we established cell lines stably expressing FcγRIIA bearing tyrosine to phenylalanine mutations at the non-ITAM Y275 (Y1), and at the ITAM Y282 (Y2) and Y298 (Y3), as well as double mutants bearing each combination of the aforementioned mutations.
While there was a 7-fold increase in serotonin secretion for the FcγRIIA-expressing cell line, we observed that mutation of either ITAM tyrosine alone was sufficient to block serotonin secretion. While RBL-2H3 cells also express one other type of Fcγ receptor, FcγRIIB, this Fc receptor does not contain an ITAM domain, but rather has been found to inhibit Fc receptor function through its Immunoreceptor Tyrosine-based Inhibitory Motif (ITIM).[3] Of particular relevance is the observation that FcγRIIB does not mediate serotonin secretion in these cells.[11] Furthermore, the ITIM motif has been shown to negatively regulate FcγRIIA-mediated phagocytosis,[3] and PECAM-1 (which also contains an ITIM) has recently been found to negatively regulate FcγRIIA-mediated platelet aggregation[23]. It would therefore be interesting to determine if FcγRIIB, through its ITIM, similarly down-regulates FcγRIIA-mediated serotonin secretion in our model as well.
In light of the apparently differing structural requirements for FcγRIIA-mediated phagocytosis versus serotonin secretion, we next investigated the downstream signaling pathways involved in these signaling events. According to our current model of phagocytic signaling, once phosphorylated, the ITAM tyrosines recruit SH2 domains of additional enzymes and adapter proteins that participate in the signaling process.[1, 22] We therefore examined the requirements for two kinases identified in FcγRIIA phagocytic signaling cascades. Consistent with previous studies in other cell types, Figure 4A demonstrates that both Syk kinase and PI3K are required for phagocytosis in RBL cells.[1, 2] Our data also indicate that FcγRIIA mediated serotonin secretion is dependent on PI3K for full activity. We observed that inhibition of PI3K by the inhibitor wortmannin, at the low concentrations that are sufficient to abolish phagocytosis, also reduces FcγRIIA-mediated serotonin secretion by nearly 50% (Figure 4B).
Inhibition of Syk with the Syk-selective inhibitor piceatannol did not significantly inhibit serotonin release by cells expressing WT FcγRIIA, despite the fact that the concentration used (25 μg/ml) had been previously shown to reduce other Syk functions in RBL cells, including serotonin secretion mediated by other Fc receptor isotypes.[21, 24]
These data indicate that signaling for FcγRIIA-mediated serotonin release can bypass Syk kinase in RBL cells. In B cells, Syk kinase acts proximal to PI3K.[25] Likewise, in neutrophils stimulated through their IgG receptors, piceatannol treatment blocked the activation of PI3K, indicating that Syk acts proximal to PI3K.[25, 26] Our observation that FcγRIIA-mediated serotonin release is sensitive to PI3K inhibition but independent of Syk thus appears at odds with a current concept that Syk kinase, recruited early to the phosphorylated ITAM, must serve as an adapter to recruit PI3K for FcγR signaling. Rather, our data suggest that stimulation of FcγRIIA may directly engage PI3K and that this event is sufficient to initiate serotonin release. This sequence of events is consistent with other studies that indicate that PI3K can specifically bind to a phosphorylated ITAM without prior involvement of Syk kinase.[27]
On the basis of their discovery that PI3K can bind the phosphorylated ITAM independently of Syk, Cooney et al. proposed a model for phagocytic signaling whereby Syk and PI3K function in parallel.[27] It is highly possible that their discovery of PI3K’s direct recruitment to the phosphorylated ITAM of FcγRIIA has significant implications for secretion signaling. Our current studies likewise suggest a direct signaling role for PI3K. Since pharmacologic blockade of Syk does not reduce secretion, signaling via Syk appears less involved. While we cannot yet definitively state that there is a direct interaction between FcγRIIA and PI3K, our experiments clearly demonstrate that FcγRIIA-mediated signaling for secretion utilizes ITAM tyrosines and downstream signaling agents different from those required for phagocytosis.[3, 27]
These observations are consistent with evidence that the ITAM requirements for FcγRIIA triggered phagocytosis and endocytosis are very different. Specifically, mutations of ITAM tyrosines that completely block phagocytosis do not effect endocytosis. Furthermore, FcγRIIA triggered endocytosis has been linked to ITAM leucine residues.[20] Moreover, FcγRIIA mediated platelet activation has been reported to involve other accessory molecules such as Cbl.[15] Taken together, our observations suggest that separate and distinct signaling pathways are responsible for triggering phagocytosis, endocytosis and secretion. Further studies into the interaction of FcγRIIA with various signal and adapter molecules may shed light on the requirements for each of these processes.
Acknowledgments
This work was supported by grants from the National Institutes of Health, NHLBI (to ADS), an Arthritis Foundation Investigator Award (to RGW), and an American Academy of Allergy, Asthma and Immunology student research fellowship (to ABD).
References
- 1.Daeron M. Fc receptor biology. Annu Rev Immunol. 1997;15:203–34. doi: 10.1146/annurev.immunol.15.1.203. [DOI] [PubMed] [Google Scholar]
- 2.Indik ZK, Park JG, Hunter S, Schreiber AD. The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood. 1995 Dec 15;86:4389–99. [PubMed] [Google Scholar]
- 3.Hunter S, Indik ZK, Kim MK, Cauley MD, Park JG, Schreiber AD. Inhibition of Fcgamma receptor-mediated phagocytosis by a nonphagocytic Fcgamma receptor. Blood. 1998 Mar 1;91:1762–8. [PubMed] [Google Scholar]
- 4.Hunter S, Sato N, Kim MK, et al. Structural requirements of Syk kinase for Fcgamma receptor-mediated phagocytosis. Exp Hematol. 1999 May;27:875–84. doi: 10.1016/s0301-472x(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 5.Sato N, Kim MK, Schreiber AD. Enhancement of fcgamma receptor-mediated phagocytosis by transforming mutants of Cbl. J Immunol. 1999 Dec 1;163:6123–31. [PubMed] [Google Scholar]
- 6.Strzelecka A, Kwiatkowska K, Sobota A. Tyrosine phosphorylation and Fcgamma receptor-mediated phagocytosis. FEBS Lett. 1997 Jan 2;400:11–4. doi: 10.1016/s0014-5793(96)01359-2. [DOI] [PubMed] [Google Scholar]
- 7.Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. Embo J. 1994 Jul 1;13:2963–7. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matter K, Yamamoto EM, Mellman I. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol. 1994 Aug;126:991–1004. doi: 10.1083/jcb.126.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tse SM, Furuya W, Gold E, et al. Differential role of actin, clathrin, and dynamin in Fc gamma receptor-mediated endocytosis and phagocytosis. J Biol Chem. 2003 Jan 31;278:3331–8. doi: 10.1074/jbc.M207966200. [DOI] [PubMed] [Google Scholar]
- 10.Worth RG, Chien CD, Chien P, Reilly MP, McKenzie SE, Schreiber AD. Platelet FcgammaRIIA binds and internalizes IgG-containing complexes. Exp Hematol. 2006 Nov;34:1490–5. doi: 10.1016/j.exphem.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 11.Daeron M, Bonnerot C, Latour S, Fridman WH. Murine recombinant Fc gamma RIII, but not Fc gamma RII, trigger serotonin release in rat basophilic leukemia cells. J Immunol. 1992 Aug 15;149:1365–73. [PubMed] [Google Scholar]
- 12.Gratacap MP, Payrastre B, Viala C, Mauco G, Plantavid M, Chap H. Phosphatidylinositol 3,4,5-trisphosphate-dependent stimulation of phospholipase C-gamma2 is an early key event in FcgammaRIIA-mediated activation of human platelets. J Biol Chem. 1998 Sep 18;273:24314–21. doi: 10.1074/jbc.273.38.24314. [DOI] [PubMed] [Google Scholar]
- 13.Gratacap MP, Herault JP, Viala C, et al. FcgammaRIIA requires a Gi-dependent pathway for an efficient stimulation of phosphoinositide 3-kinase, calcium mobilization, and platelet aggregation. Blood. 2000 Nov 15;96:3439–46. [PubMed] [Google Scholar]
- 14.Lofgren R, Serrander L, Forsberg M, Wilsson A, Wasteson A, Stendahl O. CR3, FcgammaRIIA and FcgammaRIIIB induce activation of the respiratory burst in human neutrophils: the role of intracellular Ca(2+), phospholipase D and tyrosine phosphorylation. Biochim Biophys Acta. 1999 Oct 13;1452:46–59. doi: 10.1016/s0167-4889(99)00112-3. [DOI] [PubMed] [Google Scholar]
- 15.Saci A, Pain S, Rendu F, Bachelot-Loza C. Fc receptor-mediated platelet activation is dependent on phosphatidylinositol 3-kinase activation and involves p120(Cbl) J Biol Chem. 1999 Jan 22;274:1898–904. doi: 10.1074/jbc.274.4.1898. [DOI] [PubMed] [Google Scholar]
- 16.Boylan B, Gao C, Rathore V, Gill JC, Newman DK, Newman PJ. Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets. Blood. 2008 Oct 1;112:2780–6. doi: 10.1182/blood-2008-02-142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calverley DC, Baldermann LV, Heldt ML, Kinney GL, Hokanson JE. Increased platelet Fc receptor expression in diabetes is limited to those with type 2 disease and low LDL cholesterol levels. Atherosclerosis. 2006 Mar;185:173–6. doi: 10.1016/j.atherosclerosis.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Indik Z, Kelly C, Chien P, Levinson AI, Schreiber AD. Human Fc gamma RII, in the absence of other Fc gamma receptors, mediates a phagocytic signal. J Clin Invest. 1991 Nov;88:1766–71. doi: 10.1172/JCI115496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell MA, Huang MM, Chien P, Indik ZK, Pan XQ, Schreiber AD. Substitutions and deletions in the cytoplasmic domain of the phagocytic receptor Fc gamma RIIA: effect on receptor tyrosine phosphorylation and phagocytosis. Blood. 1994 Sep 15;84:1753–9. [PubMed] [Google Scholar]
- 20.Booth JW, Kim MK, Jankowski A, Schreiber AD, Grinstein S. Contrasting requirements for ubiquitylation during Fc receptor-mediated endocytosis and phagocytosis. EMBO J. 2002 Feb 1;21:251–8. doi: 10.1093/emboj/21.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeiffer JR, Oliver JM. Tyrosine kinase-dependent assembly of actin plaques linking Fc epsilon R1 cross-linking to increased cell substrate adhesion in RBL-2H3 tumor mast cells. J Immunol. 1994 Jan 1;152:270–9. [PubMed] [Google Scholar]
- 22.Indik ZK, Park JG, Hunter S, Mantaring M, Schreiber AD. Molecular dissection of Fc gamma receptor-mediated phagocytosis. Immunol Lett. 1995 Jan;44:133–8. doi: 10.1016/0165-2478(94)00204-5. [DOI] [PubMed] [Google Scholar]
- 23.Thai le M, Ashman LK, Harbour SN, Hogarth PM, Jackson DE. Physical proximity and functional interplay of PECAM-1 with the Fc receptor Fc gamma RIIa on the platelet plasma membrane. Blood. 2003 Nov 15;102:3637–45. doi: 10.1182/blood-2003-02-0496. [DOI] [PubMed] [Google Scholar]
- 24.Oliver JM, Burg DL, Wilson BS, McLaughlin JL, Geahlen RL. Inhibition of mast cell Fc epsilon R1-mediated signaling and effector function by the Syk-selective inhibitor, piceatannol. J Biol Chem. 1994 Nov 25;269:29697–703. [PubMed] [Google Scholar]
- 25.Beitz LO, Fruman DA, Kurosaki T, Cantley LC, Scharenberg AM. SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J Biol Chem. 1999 Nov 12;274:32662–6. doi: 10.1074/jbc.274.46.32662. [DOI] [PubMed] [Google Scholar]
- 26.Raeder EM, Mansfield PJ, Hinkovska-Galcheva V, Shayman JA, Boxer LA. Syk activation initiates downstream signaling events during human polymorphonuclear leukocyte phagocytosis. J Immunol. 1999 Dec 15;163:6785–93. [PubMed] [Google Scholar]
- 27.Cooney DS, Phee H, Jacob A, Coggeshall KM. Signal transduction by human-restricted Fc gamma RIIa involves three distinct cytoplasmic kinase families leading to phagocytosis. J Immunol. 2001 Jul 15;167:844–54. doi: 10.4049/jimmunol.167.2.844. [DOI] [PubMed] [Google Scholar]


