Abstract
Background
Necrotizing enterocolitis (NEC) is the leading surgical cause of death in premature infants. We have accumulated evidence supporting a role for heparin-binding EGF-like growth factor (HB-EGF) in protection of the intestines from NEC. The aim of the current study was to evaluate the effect of loss-of-function of endogenous HB-EGF on susceptibility to NEC.
Methods
Neonatal HB-EGF(-/-) knockout (KO) mice and their HB-EGF(+/+) wild type (WT) counterparts were exposed to experimental NEC. An additional group of HB-EGF KO pups were also exposed to NEC, but had HB-EGF added to their formula. To examine gut barrier function, HB-EGF KO and WT pups received intragastric fluorescein isothiocyanate-labeled dextran (FITC-dextran) under basal and stressed conditions, and serum FITC-dextran levels measured.
Results
WT mice had an incidence of NEC of 53% whereas HB-EGF KO mice had a significantly increased incidence of NEC of 80% (p=0.04). Importantly, administration of exogenous HB-EGF to HB-EGF KO pups significantly reduced the incidence of NEC to 45% (p=0.04). HB-EGF KO mice had significantly increased intestinal permeability compared to WT mice under basal and stressed conditions.
Conclusions
Our results provide evidence that loss of the HB-EGF gene increases susceptibility to NEC, and that administration of exogenous HB-EGF reverses this susceptibility.
Keywords: HB-EGF, NEC, intestine, intestinal permeability
Introduction
Necrotizing enterocolitis (NEC) is the most common acquired intra abdominal emergency in infants and the most common surgical emergency in the neonatal intensive care unit.[1,2] Although several lines of evidence suggest that neonatal risk factors, including prematurity, asphyxia, intestinal ischemia, and formula feeding, all contribute to the occurrence of NEC, the pathogenesis of this disease remains unclear.[3] With aggressive management leading to the salvage of premature infants from the pulmonary standpoint, the incidence of NEC is increasing, and is expected to replace pulmonary insufficiency as the leading cause of death in premature infants.
Heparin-binding EGF-like growth factor (HB-EGF) was initially identified in the conditioned medium of cultured human macrophages [4] and later found to be a member of the EGF family of growth factors.[5] HB-EGF exerts its mitogenic effects by binding to and activation of EGF receptor subtypes ErbB-1 and ErbB-4.[6] In addition, HB-EGF exerts chemotactic effects when binding to the HB-EGF specific receptor N-arginine dibasic convertase (NrdC).[7] The ability of HB-EGF to evoke a mitogenic response in a variety of cell types, and its expression in a large number of tissues, suggests multiple potential roles for HB-EGF in vivo. Importantly, endogenous HB-EGF is protective in various pathologic conditions, and plays a pivotal role in mediating the earliest cellular responses to proliferative stimuli and cellular injury.
Using a model of intestinal ischemia/reperfusion (I/R) injury based on superior mesenteric artery occlusion (SMAO), we have shown that HB-EGF KO mice have increased intestinal histologic injury, decreased intestinal restitution, and decreased survival.[8] Using a model of hemorrhagic shock and resuscitation (HS/R), we have shown that HB-EGF KO mice have increased intestinal epithelial cell apoptosis and increased gut barrier failure.[9] These findings support our premise that HB-EGF is an important intestinal cytoprotective agent. It is particularly interesting that despite the redundancy among epidermal growth factor (EGF) family members, a family now known to contain ten related members, deletion of the HB-EGF gene alone leads to profound susceptibility to intestinal injury that is not compensated for by other family members.
The intestinal mucosa is composed of a layer of proliferative epithelial cells that maintain a barrier between the interior and the exterior milieu, representing the first line of defense against the invasion of noxious substances into the sub-epithelial tissue.[10] The inherent capacity of the intestinal epithelium to regenerate leads to intestinal repair and healing even after extensive mucosal destruction. However, the loss of surface epithelium and villous structure during intestinal injury undermines gut barrier function and allows bacterial translocation and absorption of toxins, antigens, proteases and other molecules. Impaired gut barrier function leads to local infection followed by distant organ pathology.
Our previous studies have shown that administration of exogenous HB-EGF decreases the incidence and severity of NEC in a neonatal rat model,[11] with simultaneous preservation of gut barrier integrity.[12] Although we have previously shown that HB-EGF KO mice have increased susceptibility to intestinal injury after intestinal I/R and after HS/R, these studies utilized adult animals. The goals of the current study were to demonstrate the role of endogenous HB-EGF gene expression in susceptibility to intestinal injury and in preservation of gut barrier function in a newborn mouse model of experimental NEC.
Materials and Methods
Neonatal mouse model of experimental necrotizing enterocolitis
The experimental protocol was performed according to the guidelines for the ethical treatment of experimental animals and approved by our Institutional Animal Care and Use Committee (#02205AR). HB-EGF KO mice on a C57BL/6J × 129 background and HB-EGF WT C57BL/6J × 129 mice were kindly provided by Dr. David Lee (Chapel Hill, NC).[13] In HB-EGF KO mice, HB-EGF exons 1 and 2 were replaced with PGK-Neo, thus deleting the signal peptide and propeptide domains. The desired targeting events were verified by Southern blots of genomic DNA and exon-specific polymerase chain reaction, with Northern blots confirming the absence of the respective transcripts.[13]
NEC was induced using a modification of the model initially described by Barlow et al.[14], and modified for mice by Jilling et al.[15] Pregnant time-dated mice were delivered by C-section under inhaled 2% Isoflurane (Butler Animal Health, Dublin, OH) anesthesia on day 18.5 of gestation. Newborn mouse pups were placed in an incubator (37°C) and fed via gastric gavage with formula containing 15 g Similac 60/40 (Ross Pediatrics, Columbus, OH) in 75 mL Esbilac (Pet-Ag, New Hampshire, IL), providing 836.8 kJ/kg per day. Feeds were started at 0.03 mL every 3h beginning 2h after birth and advanced as tolerated up to a maximum of 0.05 mL per feeding by the fourth day of life. Animals were stressed by exposure to hypoxia (100% nitrogen for 1 min) followed by hypothermia (4°C for 10 min) once a day beginning immediately after birth until the end of the experiment. Exposure of pups to hypoxia, hypothermia and hypertonic feeds will subsequently be referred to as exposure to “stress”.
To investigate the effects of HB-EGF loss-of-function on susceptibility to NEC, HB-EGF WT (n=19) and HB-EGF KO (n=31) pups were exposed to experimental NEC. An additional group of HB-EGF KO pups (n=33) were exposed to experimental NEC as described, but received HB-EGF (800 μg/kg/dose) added to each feed. The HB-EGF used was Good Manufacturing Practice (GMP) grade human mature HB-EGF produced in Pichia pastoris yeast (Trillium Therapeutics, Inc., Toronto, Canada). The dose of HB-EGF chosen was based upon previous studies from our laboratory which investigated doses of HB-EGF ranging from 25-800 μg/kg/dose that were added to the feeds of rat pups exposed to experimental NEC. Increasing doses of HB-EGF led to decreased histologic injury and improved survival in a dose-dependent fashion, with the best results found at 800 μg/kg/dose.[11] In all experiments, pups were euthanized upon development of clinical signs of NEC (abdominal distention, bloody bowel movements, respiratory distress, lethargy). Remaining animals were sacrificed 96 h after birth.
Mucosal Permeability
To investigate mucosal permeability, we used fluorescein isothiocyanate (FITC)-labeled dextran molecules (molecular weight, 73 kDa) (Sigma-Aldrich Inc, St Louis, MO) as a probe. Previous studies by others have shown that use of 73-kDa dextran molecules results in a reliable assessment of mucosal perturbations 4 h after enteral administration.[16] In this experiment, FITC-labeled dextran molecules (750 mg/kg) were administered via orogastric tube to rat pups. After 4 h, blood was collected and plasma FITC-dextran levels were measured using spectrophotofluorometry (Molecular Devices, SpectraMax M2, Sunnyvale,Ca). The amount of dextran in the plasma was calculated based on standard dilution curves of known dextran concentrations. Mouse pups were divided into 4 groups as follows: WT mice that received intragastric FITC-dextran immediately after birth with no exposure to stress (n=15); 2) HB-EGF KO mice that received intragastric FITC-dextran immediately after birth with no exposure to stress (n=17); 3) WT mice that received intragastric FITC dextran after 24 h of stress (n=13); and 4) HB-EGF KO mice that received intragastric FITC dextran after 24 h of stress (n=10).
Histologic Injury Score
Upon sacrifice, the gastrointestinal tract was carefully removed and visually evaluated for signs of NEC (areas of bowel necrosis, intestinal hemorrhage, perforation). Three pieces of duodenum, jejunum, ileum, and colon from every animal were fixed in 10% formalin for 24 h, paraffin-embedded, sectioned at 5 μm thickness, and stained with hematoxylin and eosin for histological evaluation of the presence and/or degree of NEC using the NEC histologic injury scoring system described by Caplan et al.[17] Histological changes were graded as follows: grade 0, no damage; grade 1, epithelial cell lifting or separation; grade 2, sloughing of epithelial cells to the mid villus level; grade 3, necrosis of the entire villus; and grade 4, transmural necrosis (Figure 1). Tissues were graded blindly by two independent observers. Tissues with histological scores of 2 or higher were considered positive for NEC.
Figure 1.
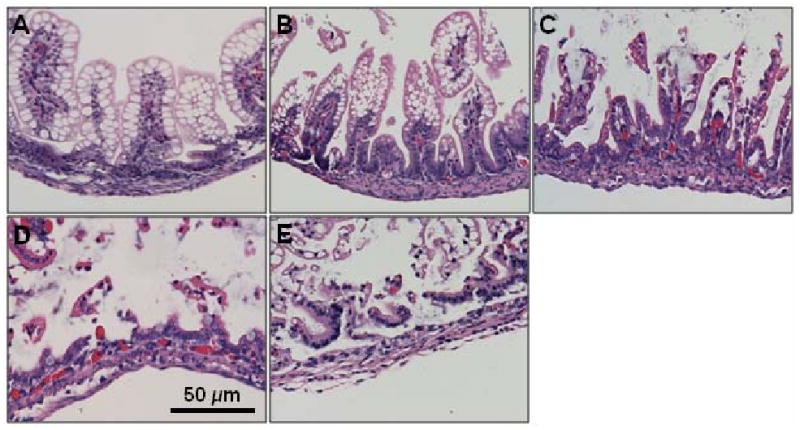
Histologic injury score in mouse pups subjected to experimental NEC. Shown are representative H&E stained sections showing: A) normal intestine; B) Grade 1, epithelial cell lifting or separation; C) Grade 2, sloughing of epithelial cells to the mid villous level; D) Grade 3, necrosis of the entire villus; and E) Grade 4, transmural necrosis. Magnification × 20.
Statistical Analyses
The Chi-square test was used for comparing the incidence of NEC between groups. Serum concentrations of FITC-dextran were compared using the Student's t test. p-values less then 0.05 were considered statistically significant. All statistical analyses were performed using SAS software (Version 9.1, SAS Institute, Cary, NC).
Results
Histologic Injury
Histologic analyses revealed that WT mouse pups had an incidence of NEC of 53%, with grade 2 injury seen in 100% of the animals that developed NEC (Figure 2). HB-EGF KO mice had a significantly increased incidence of NEC of 80% (p=0.04), with histopathologic changes ranging from moderate, mid-level villous necrosis (grade 2) to severe necrosis of the entire villous (grade 3). Of the 80% of pups that developed NEC, 48% had grade 2 injury and 32% had grade 3 injury. HB-EGF KO pups exposed to stress but with HB-EGF (800 μg/kg/dose) added to the feeds showed a significant decrease in the incidence of NEC to 45% compared to stressed pups that were not treated with HB-EGF (p=0.004). In addition to a decreased incidence of NEC, supplementation of HB-EGF to the formula of HB-EGF KO pups resulted in decreased severity of NEC. Of the 45% of HB-EGF-treated pups that developed NEC, 44% had grade 2 injury and only 3% had grade 3 injury.
Figure 2.
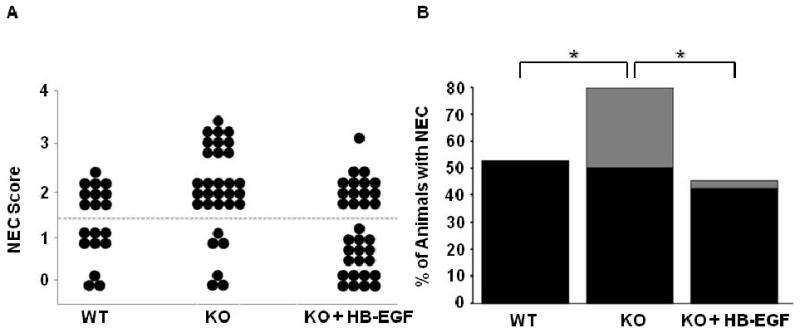
Incidence and severity of NEC in HB-EGF WT and KO mice. A) NEC score. Each dot represents a single rat pup exposed to stress, and the NEC score for each pup is shown. HB-EGF KO pups that received HB-EGF received 800 μg/kg/dose added to each feed. B) Incidence and severity of NEC. The percent of animals with NEC is shown. Black bars, grade 2 injury; grey bars, grade 3 injury; *p<0.05
Gut Barrier Function
We next examined intestinal permeability to determine gut barrier function in WT and HB-EGF KO pups exposed to experimental NEC. Under basal, non-stressed conditions immediately after birth, HB-EGF KO pups had significantly increased serum FITC-dextran levels compared to WT pups (179.73 ± 58.43 μg/ml vs. 47.79 ±14.39 μg/ml; p=0.04) (Figure 3). After 24 h of exposure to stress, WT mice had increased serum FITC-dextran levels compared to WT mice under basal conditions (119.86 ± 36.39 μg/ml vs. 47.79 ± 14.39 μg/ml; p=0.00003). On the other hand, HB-EGF KO pups exposed to stress for 24 h had a much smaller increase in serum FITC-dextran levels compared to KO mice under basal conditions (190.70 ± 61.54 μg/ml vs. 179.73 ± 58.43 μg/ml), but still had much higher serum FITC-dextran levels compared to WT mice exposed to stress for 24 h (190.70 ± 61.54 μg/ml vs. 119.86 ± 36.39 μg/ml; p=0.3). The FITC-dextran serum levels in WT animals after birth are low, indicating intact intestinal barrier function, but as the animals are exposed to stress for 24 h there is an increase in serum FITC-dextran levels indicating damage to the mucosal barrier. HB-EGF KO mice have increased FITC-dextran serum levels immediately after birth and maintain high serum levels at the 24 hour time point as well, suggesting a baseline deficit in gut barrier function that may explain, in part, their increased susceptibility to NEC.
Figure 3.
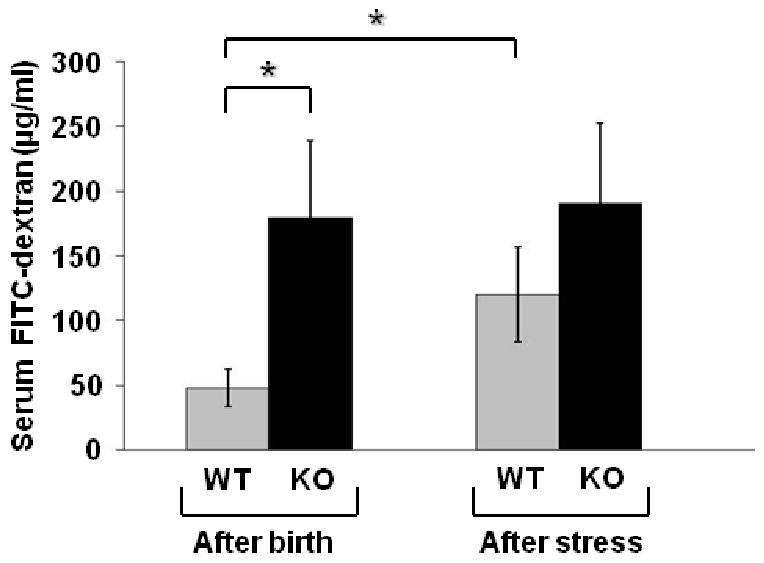
Gut barrier function in HB-EGF WT and KO mice. Gut barrier function was determined by measuring serum FITC-dextran (μg/ml) levels 4 h after gastric administration of FITC-dextran. *p<0.05
Discussion
Previous studies from our laboratory as well as others have shown that expression of endogenous HB-EGF is significantly increased in response to tissue damage,[18,19] hypoxia [20] and oxidative stress,[21] and during wound healing and regeneration.[22] This pattern of expression is consistent with a pivotal role for HB-EGF in tissue regeneration and repair. In addition, we have shown that administration of exogenous HB-EGF under experimental conditions protects the intestines from diverse injuries including intestinal I/R,[23] HS/R [24] and NEC.[11,25] Furthermore, we have previously shown that adult HB-EGF KO mice have increased intestinal injury upon exposure to intestinal I/R [8] or HS/R.[9] The current study now demonstrates that newborn HB-EGF KO mice have increased susceptibility to experimental NEC, and shows that they have increased intestinal permeability under both basal and stressed conditions. Despite this increased intestinal permeability under basal conditions, HB-EGF KO mice do not develop intestinal disease in the absence of exposure to stress. It is possible that under basal conditions, the other functional EGF family members are capable of maintaining normal intestinal physiology in HB-EGF KO mice. However, upon exposure to stress, other EGF family members may no longer be able to compensate for lack of HB-EGF, resulting in increased intestinal injury in the KO mice.
We have previously examined HB-EGF mRNA expression in intestine resected from patients with NEC, comparing intestinal samples from areas afflicted with acute NEC to the more normal intestine at the resection margins. We found that NEC-afflicted intestine had significantly lower HB-EGF mRNA levels compared to intestine at the resection margins.[26] These findings suggest that decreased endogenous HB-EGF may predispose the intestines to the development of NEC. These findings are similar to those reported here, inasmuch as lack of HB-EGF expression in the intestine increases the susceptibility of that intestine to NEC. An important finding of the current study is that the effects of lack of endogenous HB-EGF on the intestine can be compensated for by administration of exogenous enteral HB-EGF. These findings support the concept of administration of HB-EGF to patients with or at risk of developing NEC in order to prevent the progression of or development of the disease.
Studies in critically ill adults have shown that impairment of mucosal barrier function with overgrowth of pathogenic bacteria in the gastrointestinal tract enhances translocation of bacteria and endotoxin, resulting in a septic inflammatory response and multiorgan failure.[27,28] Piena-Spoel et al. [29] evaluated changes in intestinal permeability in 13 children with NEC compared to 10 control patients undergoing surgery by measuring lactulose to rhamnose ratios in urine samples. They found that lactulose to rhamnose ratios in NEC patients were increased for prolonged periods of time, with high peaks seen in patients with sepsis, indicative of gut barrier failure. Control patients had increased intestinal permeability only in the first days after surgery, which normalized rapidly afterwards. Beach et al. [30] observed increased intestinal permeability during the first week of life in neonates of gestational age 31-36 weeks, while Weaver et al. [31] showed that premature newborns born prior to 34 weeks gestation exhibited higher intestinal permeability than more mature newborns. The impaired gut barrier function of premature babies under basal conditions may be similar to the impaired intestinal permeability we report here in newborn HB-EGF KO mice under basal conditions. When HB-EGF expression is decreased or absent, as in the intestine of neonates afflicted with NEC or in HB-EGF KO mice, gut barrier function is impaired, which may contribute to bacterial translocation leading to a systemic inflammatory response.
Global deletion of the HB-EGF gene results in abnormal embryonic cardiac cushion development resulting in defective valvulogenesis, with stenosis of the semilunar and atrioventricular valves leading to cardiac hypertrophy.[13] Chalothorn et al. used 4 month old HB-EGF KO mice to study the effect of HB-EGF on angiogenesis in a hindlimb ischemia model.[32] As part of those studies, the authors demonstrated that HB-EGF KO mice had cardiac hypertrophy without evidence of heart failure, with normal hemodynamic parameters. Furthermore, 6 month old HB-EGF KO mice only had a 25% reduction in left ventricular fractional shortening.[13] This is less than the mildest level of human heart failure which is characterized by an ejection fraction that is reduced by at least 40%.[13] In addition, the fact that our studies were performed in newborn HB-EGF KO mice makes it even less likely that cardiac hypertrophy or hemodynamic disturbances affected our results. Furthermore, the fact that administration of enteral HB-EGF to HB-EGF KO pups subjected to stress increased their resistance to NEC further argues against cardiac status affecting the results, since administration of short-term HB-EGF in this fashion would certainly not affect the cardiac function of these mice.
Jackson et al. have also reported that HB-EGF KO mice have defects in the lungs, including fewer alveoli, abnormally thickened mesenchymal tissue and a high fraction of immature, glycogen-containing cells.[13]. Despite these findings, HB-EGF KO mouse pups did not display any clinical evidence of pulmonary distress in our studies. Furthermore, in parallel studies of HB-EGF KO compared to WT adult mice, we noted no differences in oxygen saturation of these mice either prior to or after surgical manipulation (unpublished observations).
The results of the current study, demonstrating increased intestinal injury and increased intestinal permeability in HB-EGF KO mice exposed to experimental NEC support the contention that HB-EGF expression is important in protection of the intestines from NEC. The fact that administration of exogenous HB-EGF to HB-EGF KO mice protects the intestines from experimental NEC supports the clinical administration of HB-EGF to patients with or at risk of developing NEC in an effort to treat or prevent the disease.
Acknowledgments
This work was funded by NIH R01 DK074611 (GEB). The authors thank Dr. David Lee (Chapel Hill, NC) for supplying HB-EGF KO and WT mice, and Gregory Young, M.S. (The Ohio State University Center for Biostatistics) for assistance with statistical analyses.
Footnotes
Financial Disclosures: GEB has a Consulting Agreement and Sponsored Research Agreement with Trillium Therapeutics, Inc., however, the experiments performed in this paper were not funded under this research agreement.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kliegman RM, Fanaroff AA. Necrotizing enterocolitis. N Engl J Med. 1984;310:1093–1103. doi: 10.1056/NEJM198404263101707. [DOI] [PubMed] [Google Scholar]
- 2.Kafetzis DA, Skevaki C, Costalos C. Neonatal necrotizing enterocolitis: an overview. Current opinion in infectious diseases. 2003;16:349–355. doi: 10.1097/00001432-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Henry MC, Moss RL. Current issues in the management of necrotizing enterocolitis. Seminars in perinatology. 2004;28:221–233. doi: 10.1053/j.semperi.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Besner G, Higashiyama S, Klagsbrun M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990;1:811–819. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Higashiyama S, Abraham JA, Miller J, et al. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 6.Junttila TT, Sundvall M, Maatta JA, et al. Erbb4 and its isoforms: selective regulation of growth factor responses by naturally occurring receptor variants. Trends in cardiovascular medicine. 2000;10:304–310. doi: 10.1016/s1050-1738(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 7.Nishi E, Prat A, Hospital V, et al. N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration. The EMBO journal. 2001;20:3342–3350. doi: 10.1093/emboj/20.13.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Assal ON, Paddock H, Marquez A, et al. Heparin-binding epidermal growth factor-like growth factor gene disruption is associated with delayed intestinal restitution, impaired angiogenesis, and poor survival after intestinal ischemia in mice. J Pediatr Surg. 2008;43:1182–1190. doi: 10.1016/j.jpedsurg.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Radulescu A, Besner GE. Heparin-Binding EGF-Like Growth Factor Is Essential for Preservation of Gut Barrier Function after Hemorrhagic Shock and Resuscitation in Mice. Surgery. doi: 10.1016/j.surg.2009.02.020. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potten CS. Epithelial cell growth and differentiation. II. Intestinal apoptosis. Am J Physiol. 1997;273:253–257. doi: 10.1152/ajpgi.1997.273.2.G253. [DOI] [PubMed] [Google Scholar]
- 11.Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2006;41:144–149. doi: 10.1016/j.jpedsurg.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2006;41:742–747. doi: 10.1016/j.jpedsurg.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Jackson LF, Qiu TH, Sunnarborg SW, et al. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. The EMBO journal. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlow B, Santulli TV, Heird WC, et al. An experimental study of acute neonatal enterocolitis--the importance of breast milk. J Pediatr Surg. 1974;9:587–595. doi: 10.1016/0022-3468(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 15.Jilling T, Simon D, Lu J, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177:3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caplan MS, Miller-Catchpole R, Kaup S, et al. Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 17.Caplan MS, Hedlund E, Adler L, et al. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol. 1994;14:1017–1028. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 18.Cribbs RK, Harding PA, Luquette MH, et al. Endogenous production of heparin-binding EGF-like growth factor during murine partial-thickness burn wound healing. J Burn Care Rehabil. 2002;23:116–125. doi: 10.1097/00004630-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Xia G, Rachfal AW, Martin AE, et al. Upregulation of endogenous heparin-binding EGF-like growth factor (HB-EGF) expression after intestinal ischemia/reperfusion injury. J Invest Surg. 2003;16:57–63. [PubMed] [Google Scholar]
- 20.Jin K, Mao XO, Sun Y, et al. Heparin-binding epidermal growth factor-like growth factor: hypoxia-inducible expression in vitro and stimulation of neurogenesis in vitro and in vivo. J Neurosci. 2002;22:5365–5373. doi: 10.1523/JNEUROSCI.22-13-05365.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank GD, Mifune M, Inagami T, et al. Distinct mechanisms of receptor and nonreceptor tyrosine kinase activation by reactive oxygen species in vascular smooth muscle cells: role of metalloprotease and protein kinase C-delta. Mol Cell Biol. 2003;23:1581–1589. doi: 10.1128/MCB.23.5.1581-1589.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy DW, Downing MT, Brigstock DR, et al. Production of heparin-binding epidermal growth factor-like growth factor (HB-EGF) at sites of thermal injury in pediatric patients. J Invest Dermatol. 1996;106:49–56. doi: 10.1111/1523-1747.ep12327214. [DOI] [PubMed] [Google Scholar]
- 23.El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609–625. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 24.El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142:234–242. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Radulescu A, Zorko NA, Yu X, et al. Preclinical neonatal rat studies of heparin-binding EGF-like growth factor in protection of the intestines from necrotizing enterocolitis. Pediatr Res. 2009;65:437–442. doi: 10.1203/PDR.0b013e3181994fa0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng J, El-Assal ON, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:167–174. doi: 10.1053/j.sempedsurg.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Deitch EA. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch Surg. 1990;125:403–404. doi: 10.1001/archsurg.1990.01410150125024. [DOI] [PubMed] [Google Scholar]
- 28.Hadfield RJ, Sinclair DG, Houldsworth PE, et al. Effects of enteral and parenteral nutrition on gut mucosal permeability in the critically ill. American journal of respiratory and critical care medicine. 1995;152:1545–1548. doi: 10.1164/ajrccm.152.5.7582291. [DOI] [PubMed] [Google Scholar]
- 29.Piena-Spoel M, Albers MJ, Ten Kate J, et al. Intestinal permeability in newborns with necrotizing enterocolitis and controls: Does the sugar absorption test provide guidelines for the time to (re-)introduce enteral nutrition? J Pediatr Surg. 2001;36:587–592. doi: 10.1053/jpsu.2001.22288. [DOI] [PubMed] [Google Scholar]
- 30.Beach RC, Menzies IS, Clayden GS, et al. Gastrointestinal permeability changes in the preterm neonate. Archives of disease in childhood. 1982;57:141–145. doi: 10.1136/adc.57.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver LT, Laker MF, Nelson R. Intestinal permeability in the newborn. Archives of disease in childhood. 1984;59:236–241. doi: 10.1136/adc.59.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalothorn D, Moore SM, Zhang H, et al. Heparin-binding epidermal growth factor-like growth factor, collateral vessel development, and angiogenesis in skeletal muscle ischemia. Arterioscler Thromb Vasc Biol. 2005;25:1884–1890. doi: 10.1161/01.ATV.0000175761.59602.16. [DOI] [PubMed] [Google Scholar]


