Abstract
Objective
To describe the relationships of lung function and emphysema, measured with spirometry and computed tomography (CT) scan, to early AMD in a multi-ethnic sample of whites, blacks, Hispanics, and Chinese.
Methods and Design
3,399 persons aged 45–84 years residing in six United States communities participating in a period cross-sectional study. AMD was measured from digital retinal photographs at the second Multi-Ethnic Study of Atherosclerosis (MESA) examination. Forced expiratory volume in one second (FEV1) and FEV1/forced vital capacity (FVC) ratio were measured at the third or fourth MESA examination. Percent emphysema was measured from cardiac CT scans at baseline. Apical and basilar lung segments were defined as the cephalad or caudal regions of the lung on the cardiac CT scan. Logistic regression models were used to examine the relationship of lung function and structure to AMD controlling for age, gender, and other factors.
Results
The prevalence of early AMD was 3.7%. Early AMD was not associated with FEV1 (odds ratio [OR], 95% confidence interval [CI], and P value, 0.82, 0.58–1.15, P=0.25), FEV1/ FVC ratio (0.92, 0.76–1.12, 0.43), percent emphysema (1.13, 0.91–1.40, 0.26) and apical-basilar difference in percent emphysema (1.14, 0.95–1.37, 0.17). Associations were stronger in smokers. Apical-basilar difference in percent emphysema was significantly associated with early AMD among ever smokers (1.28, 1.02–1.60, 0.03). Associations were not modified by race/ethnicity.
Conclusions
Lung function and emphysema on CT scan were not cross-sectionally associated with AMD; this might be explained by the relatively low smoking exposure in this cohort.
Keywords: Age-related macular degeneration, inflammation, endothelial dysfunction, epidemiology
Age-related macular degeneration (AMD) is an important cause of visual loss in Americans 65 years of age or older.1 Its pathogenesis remains poorly understood. Aside from age, smoking, and genetic factors, few risk factors have been found to be consistently associated with this condition in epidemiological studies.1-3 Two population-based studies have reported poorer respiratory function and a history of physician diagnosis of emphysema to be associated with the risk of AMD.4-6 In the Framingham Eye Study, decreased forced vital capacity (FVC) and a history of pulmonary symptoms were associated with AMD.4 In the Beaver Dam Eye Study, a self-reported history of emphysema at baseline was associated with the 15-year incidence of retinal pigment epithelium (RPE) depigmentation and exudative AMD (odds ratio [OR] 3.0, 95% confidence interval [CI], 1.0–8.4, P=0.04).6 These associations were independent of smoking and other factors.
These findings were hypothesized to be related to systemic inflammation and/or decreased oxygenation associated with chronic obstructive pulmonary disease (COPD). However, two prior case-control studies failed to find such relationships,7,8 possibly due to problems with the ascertainment of COPD. Given the growing recognition of the role of inflammation and genes involved in inflammatory regulation in the development and progression of AMD and COPD, we examine the associations of functional measures of obstructive lung disease on spirometry and anatomical measures of percent emphysema on computed tomography (CT) scans with signs of AMD in the Multi-Ethnic Study of Atherosclerosis (MESA).
METHODS
Study Sample
The MESA is a prospective cohort study of men and women aged 45–85 years without a history of clinical cardiovascular disease (CVD) living in six United States communities.9 The study objectives of the MESA were to identify risk factors for subclinical CVD, progression of subclinical CVD, and transition from subclinical to clinical CVD. Selection of the study population has been reported in detail elsewhere.9 At the first examination, carried out from July 17, 2000, to August 29, 2002, there were 6,814 participants: 1,086 from Baltimore, MD, 1,164 from Chicago, IL, 1,077 from Forsyth County, NC, 1,319 from Los Angeles County, CA, 1,102 from New York, NY, and 1,066 from St. Paul, MN. Tenets of the Declaration of Helsinki were followed, and institutional review board approval was granted at each study site. Written informed consent was obtained from each participant.
Retinal Photography and Measurement of AMD
Fundus photography using a 45° 6.3 mega pixel digital non-mydriatic camera was performed at the second examination immediately following the baseline examination from September 9, 2002, to February 7, 2004, at each site using a standardized protocol.10,11 Two photographic fields were taken of each eye; the first centered on the optic disc (Early Treatment Diabetic Retinopathy Study [ETDRS] Field 1) and the second centered on the fovea (ETDRS Field 2).12 Images were obtained from 6,176 participants.
Fundus Image Grading
Capture and grading of digital images and quality control have been described in detail elsewhere.13,14 Each image was graded twice (a preliminary and a detail grade) on-line using a modification of the Wisconsin Age-related Maculopathy Grading scheme.14 For the purposes of this report, there were 5,887 (98.9%) persons photographed with at least one eye that could be evaluated for AMD (right eye [211], left eye [200], and both eyes [5,476]). Of these, 3399 had lung data and are included in the analyses (Figure 1). There were no statistically significant differences in gradability for AMD among the four racial/ethnic groups in the study (data not shown).
Figure 1.
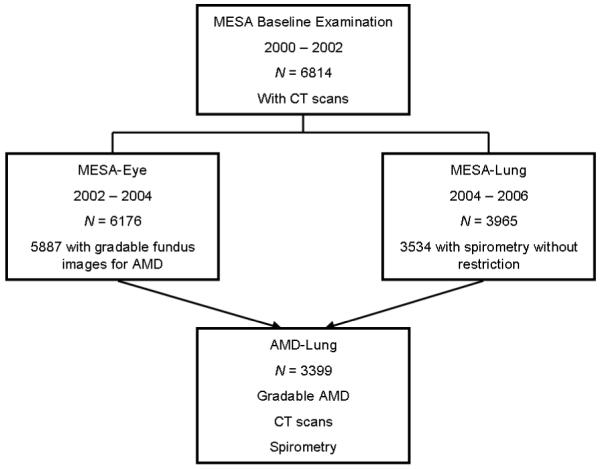
Participants in the Multi-Ethnic Study of Atherosclerosis (MESA) Study with Age-Related Macular Degeneration (AMD), CT Scans, and Spirometry.
Definitions of Variables
The AMD features evaluated were drusen size, type and area, increased retinal pigment, retinal pigment epithelial (RPE) depigmentation, pure geographic atrophy, and signs of exudative macular degeneration (i.e., subretinal hemorrhage, subretinal fibrous scar, RPE detachment, and/or serous detachment of the sensory retina or laser or photodynamic treatment for neovascular AMD). Soft distinct drusen were defined by size (between 63 and 300 μm in diameter) and appearance (sharp margins and a round nodular appearance with a uniform density [color] from center to periphery). Soft indistinct drusen were the same size as the soft distinct but have indistinct margins and a softer, less solid appearance. Increased retinal pigment appeared as a deposition of granules or clumps of grey or black pigment in or beneath the retina. RPE depigmentation was characterized by faint grayish-yellow or pinkish-yellow areas of varying density and configuration without sharply defined borders. Early AMD was defined by either the presence of any soft drusen (distinct or indistinct) and pigmentary abnormalities (either increased retinal pigment or RPE depigmentation) or the presence of a large soft drusen ≥ 125 μm in diameter with a large drusen area (> 500 μm in diameter circle) or large ( ≥ 125 μm in diameter) soft indistinct drusen in the absence of signs of late AMD. Late AMD was defined by the presence of any of the following: geographic atrophy or pigment epithelial detachment, subretinal hemorrhage or visible subretinal new vessels, subretinal fibrous scar or laser treatment scar for AMD.
When two eyes of a participant were discrepant for the severity of a lesion, the grade assigned for the participant was that of the more severely involved eye. For example, in assigning the prevalence of soft drusen, if soft drusen were present in one eye but not in the other eye, the participant was considered to have soft drusen. When drusen or signs of AMD could not be graded in an eye, the participant was assigned a score equivalent to that in the other eye.
Spirometry
Functional measures of obstructive lung disease were quantified by spirometry conducted in the third and fourth MESA examinations as part of the MESA-Lung Study in accordance with the American Thoracic Society/European Respiratory Society guidelines.15 The MESA-Lung Study enrolled 3,965 MESA participants of 4,484 selected who were sampled randomly among those who consented to genetic analyses, underwent baseline measures of endothelial function, and attended an examination during the MESA-Lung recruitment period in 2004–2006 (99%, 89%, and 91% of the MESA cohort, respectively). Asians were over-sampled. All participants performed at least three acceptable maneuvers on a dryrolling-sealed spirometer with software that performed automated quality checks in real time (Occupational Marketing, Inc., Houston, TX). All spirometry exams were reviewed by one investigator and each test was graded for quality.16 The intra-class correlation coefficients (ICC) of both the forced expiratory volume in one second (FEV1) and FVC on random 10% replicate testing was 0.99. The FEV1 and FEV1/ FVC ratio were treated as the primary measures of airflow obstruction. For the current analysis related to obstructive lung disease, we excluded 322 participants with a restrictive pattern of spirometry, defined as a FVC less than the lower limit of normal17 with a FEV1/FVC ratio above 0.70.
Percent of Emphysema-like Lung
Quantitative anatomical measures of emphysema were performed on the lung fields of cardiac CT scans, which captured approximately 70% of the lung volume from the carina to the lung bases. Cardiac CT scans were performed at full inspiration on multi-detector and electron-beam CT scanners in the first MESA examination following a standardized protocol.18 Two scans were performed on each participant; since these measures are affected by level of inspiration, the scan with the higher air volume was used for analyses except in cases of discordant scan quality, in which case the higher quality scan was used.19
Image attenuation was assessed using modified Pulmonary Analysis Software Suite20-23 at a single reading center by trained readers without knowledge of other participant information. Attenuation of air was measured outside the chest on a random sample of scans to confirm scanner calibration at −1,000 Hounsfield Units (HU). Percent of emphysema-like lung (also known as percent low attenuation area and hereafter referred to as percent emphysema) was defined as the percentage of the total voxels (pixels x slice thickness) in the lung which fell below −910 HU. This threshold was chosen based upon pathology comparisons24 and the generally mild degree of emphysema in the sample. The ICC of the 100% replicate scanning was 0.94.
Percent emphysema measures from the carina to lung base are highly correlated (r=0.99) with full-lung measures on the same full-lung scans in smokers (Reddy, S, Barr RG, Berbaum K, et al. Quantitative evaluation of emphysema presence and distribution via coronary calcium CT compared with full lung study. Abstract presented at: The international conference of the Radiological Society of North America, November 27-December 2, 2005; Chicago). Emphysema measures from cardiac CT scans correlated with those from full-lung scans from the same MESA participants (e.g., 0.93 on MD-CT scanner).19
The apical and basilar lung segments were defined as the cephalad or caudal regions of the lung divided along the z-axis scan coverage of the cardiac CT scan.
Assessment of Other Risk Factors
Participants underwent an interview and assessment of cardiovascular risk factors during the course of the study.9,25 Other variables or confounders were based on data collected at the first or fourth examination. Resting blood pressure was measured three times with participants in the seated position (Dinamap model Pro 100 automated oscillometric sphygmomanometer; Critikon, Tampa, FL). The average of the last two measurements was used. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or current use of anti-hypertensive medications. Height and weight were measured with participants wearing light clothing and no shoes and the body mass index (BMI) was calculated as kg/m2.
A detailed questionnaire was used to obtain information about past medical history (e.g., hypertension, diabetes, asthma, arthritis, emphysema), cigarette smoking and alcohol consumption (defined as current, and past/never), and medication use. Cotinine was measured by immunoassay (Immulite 2000 Nicotine Metabolite Assay; Diagnostic Products Corp., Los Angeles, CA).
Statistical Analysis
Logistic regression was used to estimate the OR and its 95% CI for early and specific AMD lesions associated with different lung function measurements. Each lung function measure was entered into a separate model. Odds ratios for continuous variables are presented per standard deviation increase. Interactions between race/ethnicity, sex, and smoking status and AMD were tested by including an interaction term in the logistic regression models. In the CT analysis, we adjusted for covariates measured at baseline, while for the spirometry measures, we adjusted for covariates at the time of that examination. Two logistic regressions are presented for each risk factor. Using measures collected at baseline, Model 1 for the CT measures included age, sex, racial/ethnic group, height, body mass index, and study site. Model 2 for the CT measures included Model 1 and additional adjustment for smoking status, pack-years smoked, serum cotinine level, and CT protocol. Using measures collected at spirometry examination, Model 1 for the spirometry measures included age, sex, racial/ethnic group, height, and study site. Model 2 for the spirometry measures included Model 1 and additional adjustment for body mass index, smoking status, pack-years smoked, and asthma prior to the age of 45 years. Version 9.1 of SAS (Cary, NC) was used for all analyses.
RESULTS
Selected characteristics including risk factors and potential confounders for and AMD/lung disease relationship for the full cohort and each of the four racial/ethnic groups among participants with available data (n=3,399 at baseline) are shown in Table 1. There were 1,196 (35.2%) whites, 558 (16.4%) Chinese, 896 (26.4%) blacks, and 749 (22.0%) Hispanics in the cohort. Fifty-three percent of the cohort never smoked, 35% smoked in the past, and 12% smoked currently. Among ever smokers the median number of pack years was 17.8.
Table 1.
Characteristics of the Multi-Ethnic Study of Atherosclerosis (MESA) Cohort in Persons with the Age-Related Macular Degeneration Gradings at Second Examination and Lung Data at Baseline or Fourth Examination
| Total Cohort | Whites | Chinese | Blacks | Hispanics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic* | N | Mean/% | SD | N | Mean/% | SD | N | Mean/% | SD | N | Mean/% | SD | N | Mean/% | SD | P-value |
| Baseline MESA Examination | ||||||||||||||||
| Age, years | 3399 | 61.2 | 9.9 | 1196 | 61.6 | 9.9 | 558 | 61.4 | 10.1 | 896 | 61.3 | 9.6 | 749 | 60.1 | 9.9 | 0.011 |
| Percent male | 3399 | 49.2 | 1196 | 50.3 | 558 | 52.3 | 896 | 47.2 | 749 | 47.5 | 0.168 | |||||
| Percent current smoker | 3395 | 11.9 | 1195 | 9.6 | 558 | 5.6 | 893 | 18.1 | 749 | 13.0 | <.0001 | |||||
| Percent past smoker | 3395 | 35.4 | 1195 | 45.2 | 558 | 20.4 | 893 | 34.9 | 749 | 31.4 | <.0001 | |||||
| Pack years smoked† | 1279 | 15.0 | 613 | 17.8 | 140 | 13.1 | 388 | 16.4 | 312 | 10.0 | ||||||
| Body mass index (kg/m2) | 3399 | 27.9 | 5.2 | 1196 | 27.7 | 5.0 | 558 | 24.0 | 3.1 | 896 | 29.7 | 5.6 | 749 | 29.2 | 4.8 | <.0001 |
| Symptoms of asthma before age of 45 years |
3399 | 7.8 | 1196 | 8.9 | 558 | 3.9 | 896 | 8.9 | 749 | 7.5 | 0.001 | |||||
| Hypertension by JNC VI (1997) criteria |
3399 | 41.8 | 1196 | 36.9 | 558 | 35.7 | 896 | 55.6 | 749 | 37.7 | <.0001 | |||||
| Percent completed high school | 3395 | 83.7 | 1195 | 96.6 | 558 | 79.93 | 893 | 90.1 | 749 | 58.3 | <.0001 | |||||
| Percent emphysema-like lung | 3398 | 17.1 | 12.1 | 1196 | 21.5 | 13.0 | 558 | 14.1 | 10.1 | 896 | 16.6 | 11.8 | 748 | 13.1 | 9.9 | <.0001 |
| Apical-basilar difference in per- cent emphysema-like lung |
3391 | 1.02 | 6.79 | 1191 | 0.56 | 7.32 | 558 | 3.2 | 5.85 | 895 | 0.83 | 7.23 | 747 | 0.30 | 5.57 | <.0001 |
| Fourth MESA Examination | ||||||||||||||||
| Height (cm) | 3399 | 165.9 | 10.1 | 1196 | 168.6 | 9.7 | 558 | 161.7 | 8.8 | 896 | 168.2 | 9.9 | 749 | 161.7 | 9.3 | <.0001 |
| Symptoms of chronic bronchitis | 3383 | 7.8 | 1194 | 10.0 | 556 | 3.4 | 887 | 7.9 | 746 | 7.6 | <.0001 | |||||
| Pack years smoked† | 1584 | 17.8 | 708 | 20.3 | 167 | 17.4 | 522 | 19.1 | 375 | 10.9 | ||||||
| Forced expiratory volume at one minute, % predicted | 3298 | 95.92 | 17.32 | 1155 | 93.58 | 16.51 | 552 | 101.00 | 16.73 | 856 | 95.17 | 18.94 | 735 | 96.64 | 16.2 | <.0001 |
| FEV1/FVC ratio | 3282 | 0.747 | 0.086 | 1148 | 0.730 | 0.085 | 550 | 0.752 | 0.777 | 851 | 0.750 | 0.094 | 733 | 0.766 | 0.079 | <.0001 |
| Forced vital capacity, % predicted | 3284 | 97.89 | 14.70 | 1148 | 96.34 | 13.28 | 550 | 101.05 | 14.76 | 853 | 98.28 | 16.65 | 733 | 97.50 | 9.97 | <.0001 |
Abbreviations: FEV1/FVC = Forced expiratory volume in one second/forced vital capacity; JNC=Joint National Committee; SD=standard deviation.
Sample size vary due to some being measured in whole cohort at baseline and some at third or fourth examination in persons with gradable fundus photographs for any of the age-related macular degeneration outcomes at second examination.
Median value among ever smokers
There were significant differences in the frequency and distribution of most characteristics among the racial/ethnic groups. For example, blacks were more likely to be current cigarette smokers while Chinese were least likely to have a history of asthma before 45 years of age and to have the lowest BMI. Whites had the lowest FEV1/FVC ratio indicative of obstructive lung disease.
Early AMD was present in 3.7%, late AMD in 0.4%, large drusen in 9%, soft drusen in 17%, increased pigment in 2%, RPE depigmentation in 0.8%, exudative AMD in 0.3%, and geographic atrophy in 0.2% of the cohort. Because of the small number of persons with signs of late AMD, associations of pulmonary factors with this endpoint are not examined.
Associations
Table 2 shows no statistically significant associations of functional measures of airflow obstruction and anatomic measures of emphysema with early AMD for the whole cohort while controlling for age, sex, race/ethnicity, height, and study site. There were also no associations of airflow obstruction and percent emphysema with size, type, and area of drusen and pigmentary abnormalities in the whole cohort (data not shown). However, all associations were in the hypothesized directions (Table 2).
Table 2.
Relation of Various Pulmonary Characteristics of the Multi-Ethnic Study of Atherosclerosis Cohort to Early Age-Related Macular Degeneration
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Percent emphysema-like lung, per 12.11 units | 1.10 | (0.89 – 1.36) | 0.38 | 1.13 | (0.91 – 1.40) | 0.26 |
| Apical-basilar difference in % emphysema-like lung, per 6.79 units | 1.13 | (0.94 – 1.35) | 0.15 | 1.14 | (0.95 – 1.37) | 0.17 |
| Forced expiratory volume at 1 minute per 734 units | 0.84 | (0.61 – 1.14) | 0.27 | 0.82 | (0.58 – 1.15) | 0.25 |
| Forced vital capacity per 950 units | 0.88 | (0.60 – 1.29) | 0.52 | 0.84 | (0.57 – 1.25) | 0.39 |
| FEV1/FVC ratio per 0.086 | 0.91 | (0.76 – 1.09) | 0.30 | 0.92 | (0.76 – 1.12) | 0.43 |
| Symptoms of chronic bronchitis | 1.11 | (0.56 – 2.18) | 0.77 | 1.08 | (0.54 – 2.14) | 0.83 |
Abbreviations: CI = confidence interval; FEV1/FVC = Forced expiratory volume in one second /forced vital capacity; OR = odds ratio.
Using measures collected at baseline, Model 1 for the CT measures included age, sex, racial/ethnic group, height, body mass index, and study site. Model 2 for the CT measures included Model 1 and additional adjustment for smoking status, pack-years smoked, serum cotinine level and CT protocol.
Using measures collected at spirometry examination, Model 1 for the spirometry measures included age, sex, racial/ethnic group, height, and study site. Model 2 for the spirometry measures included Model 1 and additional adjustment for body mass index, smoking status, pack-years smoked, and asthma prior to the age of 45 years.
These associations were modified by smoking status (Table 3). Apical-basilar differences in percent emphysema was associated with early AMD in ever smokers (OR 1.28, 95% CI, 1.02–1.60, P=0.03) but not in never smokers (OR 0.93, 95% CI, 0.69–1.26, P=0.66; P-interaction = 0.11). The relation of apical-basilar difference in percent emphysema with early AMD remained statistically significant (P=.04) while further controlling for pack years smoked in ever smokers.
Table 3.
Relation of Various Pulmonary Characteristics of Multi-Ethnic Study of Atherosclerosis Cohort to Early Age-Related Macular Degeneration by Smoking Status Controlling for Age, Sex, Race, and Study Site.
| Never Smoked | Ever Smoked | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Percent emphysema-like lung, per 12.11 units | 0.96 | (0.68 – 1.35) | 0.80 | 1.21 | (0.93 – 1.59) | 0.16 |
| Apical-basilar difference in % emphysema-like lung, per 6.79 units | 0.93 | (0.69 – 1.26) | 0.66 | 1.28 | (1.02 – 1.60) | 0.03 |
| Forced expiratory volume at 1 minute per 734 units | 1.00 | (0.54 – 1.84) | 0.99 | 0.80 | (0.55 – 1.16) | 0.24 |
| Forced vital capacity per 950 units | 0.82 | (0.42 – 1.61) | 0.57 | 0.94 | (0.59 – 1.40) | 0.79 |
| FEV1/FVC ratio per 0.086 | 1.04 | (0.74 – 1.46) | 0.81 | 0.87 | (0.70 – 1.08) | 0.21 |
| Symptoms of chronic bronchitis | 1.19 | (0.35 – 4.03) | 0.78 | 1.05 | (0.46 – 2.39) | 0.95 |
Abbreviations: CI = confidence interval; FEV1/FVC = Forced expiratory volume in one second /forced vital capacity; OR = odds ratio.
There were no statistically significant interactions of airflow obstruction and percent emphysema with AMD endpoints by age, sex, and race (data not shown).
DISCUSSION
MESA provided a unique opportunity to examine the relationship of anatomical and functional measures of lung disease to AMD in a large, multi-ethnic cohort free from clinical CVD at the baseline examination.9 After controlling for age, sex, race, height, and study site in multivariate models, we found no statistically significant associations to early AMD in the whole cohort. However, one association was modified by smoking history and apical-basilar emphysema was associated with early AMD in ever smokers.
Findings in the MESA Study contrast with findings from the two earlier population-based studies.4-6 As noted above, in the Framingham Eye Study, decreased FVC and a history of lung infection were associated with AMD and in the Beaver Dam Eye Study, a self-reported history of emphysema at baseline was associated with the 15-year incidence of RPE depigmentation (OR 2.5, 95% CI, 1.3–4.8, P=0.006) and exudative AMD (OR 3.0, 95% CI, 1.0–8.4, P=0.04).4,6 The associations in the Beaver Dam Eye Study remained after controlling for smoking and other factors. Additionally, in the Beaver Dam Eye Study, a history of respiratory symptoms (cough/phlegm/wheezing), first obtained at the 10-year followup, was associated with the 5-year incidence of exudative AMD (OR 3.6, 95% CI, 1.4–9.3, P=0.01) and progression of AMD (OR 2.9, 95% CI, 1.4–6.0, P=0.004), independent of a history of smoking. While controlling for age, smoking status, and a history of emphysema, peak expiratory flow rate was inversely associated with the 5-year incidence of two signs of early AMD, large drusen (OR 4th versus 1st quartile 0.4, 95% CI, 0.2–0.7, P=0.001) and soft drusen (OR 0.5, 95% CI, 0.3–0.9, P=0.01), and progression of AMD (OR 0.5, 95% CI, 0.2–1.0, P=0.04). These prior cohort studies suggest that airflow obstruction and COPD may be a risk factor for AMD. We did not, in general, confirm these findings cross-sectionally in MESA, using lung function and novel measures of percent emphysema on CT scan. Since the association of airflow obstruction and emphysema appear to be modified by smoking history, this difference may be due to underlying differences in the smoking history of the three cohorts. The MESA cohort had no clinical cardiovascular disease at baseline and participants in the current sample had to survive to participate in the third-fourth MESA examination. The current sample is thus drawn from a ‘healthy’ population, and the smoking exposure is less than other cohorts previously studied.
Consistent with this notion, arguably the most sensitive of these available measures for smoking-related damage to the lung, apical-basilar difference in emphysema, was associated with early AMD in ever smokers. Classically, smoking preferentially damages the apices of the lung and this difference in degree of emphysema is detectable on CT scan. Interestingly, many prior studies of inflammatory and genetic risk in COPD have found stronger associations for apical-basilar difference in emphysema than for diffuse emphysema.26
Although our study has many strengths (e.g., objective grading of AMD, spirometry and a validated measure of percent emphysema which is novel in a population-based multi-ethnic cohort), caution must be exercised when interpreting the findings for several reasons. We examined many variables. The significance of the one association of lung density and early AMD found in persons with a history of smoking may have been due to the large number of analyses and may have led to chance findings or type I error. In addition, the infrequency of late AMD (0.5%) limited us from analysis of lung factors with this endpoint. Thus, we could not examine the association of emphysema with exudative AMD as reported in the Beaver Dam Eye Study. The period cross-sectional nature of the study design (a single eye examination two years after or before the risk factors were measured) limits our ability to detect associations with factors which may manifest subsequently as pathologic changes over some different interval of time. Another possible cause of concern is the possibility of misclassification of AMD status because of the use of minified 45° nonstereoscopic fundus images in the analyses compared with 30° stereoscopic images. However, we have no reason to believe that this will result in systemic biases in evaluating associations. Furthermore, the emphysema measures were drawn from cardiac rather than full-lung scans, although we have previously validated these measures against full-lung scans.19 Finally, we cannot determine whether there were biases caused by nonparticipation or selective mortality that affected the relationships.
In summary, these data from the MESA show no statistically significant associations between pulmonary function or emphysema and early AMD signs in the whole cohort. In a secondary but potentially important analysis, apical-basilar difference in percent emphysema was associated with early AMD among ever smokers. While our data suggest that these factors are not related to early AMD in a general predominantly non-smoking cohort without clinical signs of cardiovascular disease, the reader should be cautious regarding the implications of applying our findings to the general population which has a higher proportion of smokers and persons with respiratory and cardiovascular disease. Longitudinal data in samples of larger size with greater frequency of smoking and late AMD will be important in further investigating the possibility of a causal relationship between lung structure and function and AMD.
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Support was provided by NIH Intramural Research program award Z01000403 from the National Eye Institute (Cotch MF). Additional support was provided by NIH grants HL69979-03 (Klein R and Wong TY) and HL077612 (Barr RG).
Footnotes
None of the authors have any financial/conflicting interests to disclose.
Reference List
- 1.Klein R. Epidemiology. In: Berger JW, Fine SL, Maguire MG, editors. Age-related macular degeneration. Mosby; St. Louis, MO: 1999. pp. 31–55. [Google Scholar]
- 2.Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108(4):697–704. doi: 10.1016/s0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Klein BE, Tomany SC, Moss SE. Ten-year incidence of age-related maculopathy and smoking and drinking: the Beaver Dam Eye Study. Am J Epidemiol. 2002;156(7):589–598. doi: 10.1093/aje/kwf092. [DOI] [PubMed] [Google Scholar]
- 4.Kahn HA, Leibowitz HM, Ganley JP, et al. The Framingham Eye Study. II. Association of ophthalmic pathology with single variables previously measured in the Framingham Heart Study. Am J Epidemiol. 1977;106(1):33–41. doi: 10.1093/oxfordjournals.aje.a112429. [DOI] [PubMed] [Google Scholar]
- 5.Klein R, Klein BE, Tomany SC, Cruickshanks KJ. Association of emphysema, gout, and inflammatory markers with long-term incidence of age-related maculopathy. Arch Ophthalmol. 2003;121(5):674–678. doi: 10.1001/archopht.121.5.674. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Knudtson MD, Klein BE. Pulmonary disease and age-related macular degeneration: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126(6):840–846. doi: 10.1001/archopht.126.6.840. [DOI] [PubMed] [Google Scholar]
- 7.Hyman LG, Lilienfeld AM, Ferris FL, III, Fine SL. Senile macular degeneration: a case-control study. Am J Epidemiol. 1983;118(2):213–227. doi: 10.1093/oxfordjournals.aje.a113629. [DOI] [PubMed] [Google Scholar]
- 8.Delaney WV, Jr., Oates RP. Senile macular degeneration: a preliminary study. Ann Ophthalmol. 1982;14(1):21–24. [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Klein R, Islam FM, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141(3):446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis. Ophthalmology. 2006;113(3):373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs--an extension of the modified Airlie House classification. Ophthalmology. 1991;98(5 Suppl):786–806. ETDRS report number 10. [PubMed] [Google Scholar]
- 13.Klein R, Meuer SM, Moss SE, Klein BE, Neider MW, Reinke J. Detection of age-related macular degeneration using a nonmydriatic digital camera and a standard film fundus camera. Arch Ophthalmol. 2004;122(11):1642–1646. doi: 10.1001/archopht.122.11.1642. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98(7):1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of ATS-Recommended Spirometry Reference Values in a Multiethnic Sample of Adults: The MESA-Lung Study. Chest. 2009 Sep 9; doi: 10.1378/chest.09-0919. Epub ahead of print, doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 18.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and validity of lung density measures from cardiac CT Scans--The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16(6):689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo J, Reinhardt JM, Kitaoka H, et al. Integrated system for CT-based assessment of parenchymal lung disease; Proceedings of the IEEE International Symposium on Biomedical Imaging; Washington, DC. 2002 Jul 7-10; 2002. pp. 871–4. 2002. [Google Scholar]
- 21.Tschirren J, McLennan G, Palagyi K, Hoffman EA, Sonka M. Matching and anatomical labeling of human airway tree. IEEE Trans Med Imaging. 2005;24(12):1540–1547. doi: 10.1109/TMI.2005.857653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu S, Hoffman EA, Reinhardt JM. Automatic lung segmentation for accurate quantitation of volumetric X-ray CT images. IEEE Trans Med Imaging. 2001;20(6):490–498. doi: 10.1109/42.929615. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Hoffman EA, Reinhardt JM. Atlas-driven lung lobe segmentation in volumetric X-ray CT images. IEEE Trans Med Imaging. 2006;25(1):1–16. doi: 10.1109/TMI.2005.859209. [DOI] [PubMed] [Google Scholar]
- 24.Coxson HO, Mayo JR, Behzad H, et al. Measurement of lung expansion with computed tomography and comparison with quantitative histology. J Appl Physiol. 1995;79(5):1525–1530. doi: 10.1152/jappl.1995.79.5.1525. [DOI] [PubMed] [Google Scholar]
- 25.MESA Coordinating Center . Multi-Ethnic Study of Atherosclerosis Field Center Manual of Operations. University of Washington; Seattle, WA: Jan 5, 2007. [Google Scholar]
- 26.Hoffman EA, Simon BA, McLennan GA. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:A519–34. doi: 10.1513/pats.200603-086MS. [DOI] [PMC free article] [PubMed] [Google Scholar]


