Abstract
Three lick suppression experiments with rats investigated backward blocking in first-order conditioning. As has been suggested in prior studies, the experiments demonstrated that backward blocking is difficult to obtain in conventional first-order conditioning situations. However, we demonstrate here that backward blocking is observed in first-order conditioning if the target cue’s behavioral control is weak at the time of elemental training of the blocking cue. The target cue’s behavioral control was weakened through forward blocking of the target cue by a third cue (Experiment 1), conducting compound and elemental training with backward temporal relationships to the US (Experiment 2), and extinguishing the target cue following compound training (Experiment 3). The results of these experiments suggest that weak control of behavior by the blocked cue at the time of elemental training of the blocking cue is a critical determinant of whether blocking can be observed. Prior failures to detect backward blocking in first-order conditioning are seemingly due to a difficulty in decreasing the response-eliciting potential of a cue by indirect means such as associative inflation of a competing cue.
Kamin (1968) reported that responding to a target conditioned stimulus (CS; X) that has been paired with an unconditioned stimulus (US) in compound with another CS (A) is weaker when A has been previously paired with the US than when prior training with A was omitted. This phenomenon, called blocking, has attracted considerable attention from researchers in the field of associative learning. The observation of blocking challenged the then prevailing view of associative learning as a simple bonding process between the representations of two stimuli which is determined only by the intensities of the stimuli and their spatiotemporal contiguity. Blocking, together with the other cue competition phenomena such as overshadowing (Pavlov, 1927), stimulated the development of more sophisticated theories (e.g., Rescorla & Wagner, 1972) and became a benchmark for evaluating contemporary models of associative learning.
A typical blocking experiment consists of two types of training trials: pairings of the blocking cue (A) with the US (i.e., A-US) and pairings of a compound of the target cue (X) and A with the US (i.e., AX-US). If all of the A-US pairings precede all of the AX-US pairings, then the procedure and resultant deficit in responding to X are called forward blocking. In contrast, if the AX-US pairings precede the A-US pairings, the procedure and impairment in responding to X (if any) are called backward blocking. The demonstration of forward blocking by Kamin (1968) was obtained in the conditioned suppression preparation with rats as subjects. Later, Dickinson, Shanks, and Evenden (1984) reported a similar blocking effect in human contingency judgment, which suggested that Pavlovian conditioning in nonhumans and human contingency learning share common underlying mechanisms. However, the commonality of mechanisms underlying nonhuman conditioning and human associative learning suggested by forward blocking was challenged by the apparent absence of backward blocking in nonhumans. Backward blocking was first demonstrated in a human contingency judgment situation by Shanks (1985) and has been repeatedly demonstrated in human learning situations (e.g., Beckers, De Houwer, Pineño, & Miller, 2005; Wasserman & Berglan, 1998). In contrast, evidence of backward blocking in Pavlovian conditioning (with nonhumans) is scanty. For example, Miller, Hallam, and Graham (1990) and Nakajima and Kawai (1997) both reported failures to obtain backward blocking in Pavlovian conditioning with nonhumans.
The absence of backward blocking in conditioning situations with nonhumans is compatible with some associative theories which view cue competition effects as a learning failure that occurs at the time of target training (e.g., Mackintosh, 1975; Pearce, 1987; Pearce & Hall, 1980; Rescorla & Wagner, 1972; Wagner, 1981). These theories readily explain forward blocking by assuming that prior A-US pairings result in acquisition of an A-US association, which interferes with acquisition of an X-US association at the time of compound training. However, these theories do not have any mechanism which enables subjects to change the associative status of the target cue retrospectively (i.e., retrospective revaluation). In the framework of these acquisition-focused theories, the strength of a cue-outcome association changes only when the cue itself is physically presented. Thus, A-US pairings should not impact the X-US association when they occur after the AX-US pairings (which is inconsistent with observations of backward blocking). One might suspect that the difficulty in obtaining backward blocking with nonhuman subjects relative to humans is due to a fundamental difference between conditioning in nonhumans and human contingency judgment. Pavlovian conditioning in nonhumans may be driven by relatively primitive associative processes (such as those hypothesized by simple associative theories), whereas human contingency judgment may be based on a more complex process (perhaps conscious inference) and consequently is susceptible to the retrospective revaluation of cues (e.g., backward blocking).
There are, however, other lines of evidence which suggest that Pavlovian conditioning with nonhumans and human contingency learning share the same basic underlying mechanisms. First, although backward blocking is difficult to obtain in conditioning with nonhuman subjects, another type of retrospective revaluation has been demonstrated repeatedly in both nonhumans (see below) and humans (e.g., Van Hamme & Wasserman). Specifically, when a competing cue undergoes associative deflation (e.g., extinction) after cue competition training has been completed, responding to a target cue often recovers despite the cue itself not being physically presented. Such recovery from cue competition has been reported in various cue competition phenomena such as overshadowing (Kaufman & Bolles, 1981; Matzel, Schachtman, & Miller, 1985) and forward blocking (Blaisdell, Gunther, & Miller, 1999). These studies cast doubt upon the view that nonhumans are incapable of retrospective revaluation. Retrospective revaluation caused by posttraining deflation of a companion stimulus is anticipated by some recently proposed learning theories (e.g., Denniston, Savastano, & Miller, 2001; Dickinson & Burke, 1996; Miller & Matzel, 1988; Van Hamme & Wasserman, 1994). These contemporary theories, at least with some parameters, also predict backward blocking. Thus, the absence of backward blocking is incompatible with predictions made by these recent theories.
A second line of evidence arguing for common underlying learning mechanisms is provided by some recent studies (e.g., Denniston, Miller, & Matute, 1996; Miller & Matute, 1996; Pineño, Urushihara, & Miller, 2005; Pineño, Urushihara, Stout, Fuss, & Miller, 2006) demonstrating that backward blocking can be obtained with nonhuman subjects when the two training phases were embedded in what is normally the first phase of a sensory preconditioning procedure (Brogden, 1939), a type of higher-order conditioning. In a sensory preconditioning situation, the target CS is first paired with an innocuous stimulus (a surrogate outcome, S) rather than with a US. Then, the surrogate outcome is paired with a conventional US, such as food or an electric shock in order to provide a motivational basis for responding, prior to testing of the target CS. Applied to backward blocking, the AX compound is first paired with S, and later A-S pairings are administered. Subsequent S-US pairings allow the X-S association to be evaluated. These studies demonstrated that backward blocking in nonhumans is indeed possible at least with this more complicated design.
An alternative explanation for the failure of backward blocking in nonhumans has been proposed by Miller and colleagues (Denniston et al., 1996; Miller & Matute, 1996). They argued that the difficulty in demonstrating backward blocking with nonhumans in a conventional conditioning situation results from a difficulty in decreasing the response potential of a CS, unless the cue itself is directly manipulated (e.g., extinction) or the US is devalued (e.g., Rescorla, 1973); it is quite difficult to decrease the cue’s response potential by indirect means such as manipulating the status of associates of the target cue. This view focuses on the protection of associative strength afforded by the target CS’s biological significance and explains why forward blocking and recovery from overshadowing but not backward blocking are commonplace in Pavlovian conditioning with nonhumans. A fundamental difference between forward and backward blocking is that, in a forward blocking experiment, the blocking cue is paired with the US and consequently becomes excitatory. Next, the target cue is paired with the US in compound with the previously conditioned blocking cue. Finally, responding to the target cue is tested. Thus, in a forward blocking experiment, the question is whether the target cue will come to elicit conditioned responding during the compound training phase. In contrast, in a backward blocking experiment, the target cue is paired with the US in compound with the blocking cue in the first phase. As a result, the target cue (as well as the blocking cue) should acquire appreciable response potential (assuming parameters are used that minimize overshadowing). Then, the blocking cue alone is further paired with the US, and finally responding to the target cue is assessed. Thus, in a backward blocking preparation, the situation is starkly different from that in a forward blocking preparation. The question in backward blocking is whether the target cue will lose its previously-acquired response potential when the elemental training of the blocking cue is later conducted. According to Miller and colleagues, once a CS obtains control of behavior (i.e., becomes biologically significant), it is difficult to decrease the response potential of the target CS by indirect means such as manipulating an associate of the target CS.
The fact that backward blocking in nonhumans is obtainable in a sensory preconditioning situation (e.g., Denniston et al., 1996; Miller & Matute, 1996; Pineño et al., 2005, 2006) is consistent with the explanation proposed by Miller and colleagues (Denniston et al.; Miller & Matute). An important feature of sensory preconditioning preparations is that little responding to any stimulus is expected based on target training with the surrogate outcome because it is conducted in the absence of a conventional US. Backward blocking is possible within a sensory preconditioning situation because the target cue is protected from acquiring potential to elicit responding throughout the two phases of backward blocking. Presumably backward blocking is relatively easy to obtain in human contingency learning for the same reason. In human contingency learning, motivationally salient stimuli such as electric shock are rarely used because of ethical considerations, and the motivational basis for responding is usually established through verbal instructions.
Recent studies provided support for the explanation proposed by Miller and colleagues concerning why backward blocking has proven elusive (Denniston et al., 1996; Miller & Matute, 1996). Second-order conditioning (Pavlov, 1927) is another well-known higher-order conditioning procedure that is identical to the sensory preconditioning except for the order of the two training phases. That is, in second-order conditioning, a cue (S) is first paired with the US and subsequently another cue (A) is paired with S. As a result, A, as well as the S, comes to elicit excitatory responding. A notable difference between second-order conditioning and sensory preconditioning is that in sensory preconditioning the target stimulus does not acquire the potential to elicit conditioned responding until pairings of the surrogate outcome and the US are administered, whereas in second-order conditioning, because S-US pairings are conducted first, the target cue comes to elicit conditioned responding immediately after A-S pairings. Thus, although the procedure for second-order conditioning closely resembles that of sensory preconditioning, based on the explanation for the difficulty of obtaining backward blocking proposed by Miller and his colleagues, there is no reason to expect backward blocking to occur in second-order conditioning situations. Consistent with this expectation, Denniston et al. (Experiment 2) reported that backward blocking was obtained in a sensory preconditioning situation, but not in a similar second-order conditioning situation.
Several recent studies further demonstrated that whether the target stimulus has any potential for eliciting vigorous responding (i.e., biological significance) is one critical determinant of whether cue competition will occur. Miller and Matute (1996, Experiment 3) found that when relatively loud (thus possessing inherent potential to elicit vigorous responding) auditory stimuli were used as CSs, forward blocking was diminished. Oberling, Bristol, Matute, and Miller (2000) also reported that other cue competition phenomena, including overshadowing (Pavlov, 1927), the relative stimulus validity effect (Wagner, Logan, Haberlandt, & Price, 1968), and the degraded contingency effect (Rescorla, 1968), are attenuated when relatively loud auditory stimuli were used as CSs. Denniston et al. (1996, Experiment 1) demonstrated that even in sensory preconditioning, backward blocking is weak if loud auditory stimuli are used as CSs. Furthermore, other studies revealed that the use of a target stimulus that had previously acquired a potential to elicit responding through pairings with a conventional US is partially protected from subsequent cue competition. For example, Oberling et al. (2000, Experiment 2B) demonstrated that overshadowing in conditioned suppression with a footshock US was attenuated when the target stimulus had been paired with an appetitive US (saccharin solution) in advance of the overshadowing treatment. This suggests that counterconditioning of the target cue interfered with overshadowing. Similarly, Blaisdell, Denniston, Savastano, and Miller (2000) reported that when the target cue was paired with a CS which was previously paired with an appetitive US (i.e., appetitive second-order conditioning) following overshadowing treatment with an aversive US, fear to the target cue became greater. That is, counterconditioning to the target cue attenuated previously established overshadowing. These studies suggest that the potential of a stimulus to elicit responding protects the stimulus from response decrements caused by cue competition, regardless of whether this potential is inherent or acquired and whether the motivational state underlying the response eliciting potential is consistent or inconsistent with the target behavior.
Collectively, the aforementioned studies suggest that whether or not the target cue comes to control behavior during training is one of the critical determinants of cue competition, and that backward blocking is obtainable in sensory preconditioning preparations because the target stimulus does not have the opportunity to control behavior until the backward blocking treatment has been completed. If the previous successes in obtaining backward blocking in nonhumans in sensory preconditioning resulted from the target cue not acquiring potential to elicit conditioned responding during blocking training, then backward blocking should be obtainable outside of sensory preconditioning preparations provided that the target cue does not otherwise control behavior during backward blocking treatment. That is, even though a target cue is directly paired with a conventional, motivational US (i.e., in first-order conditioning), backward blocking should be observed if the target cue is somehow prevented from having response-eliciting potential. In this report, we tested the possibility that backward blocking would occur in a first-order conditioning situation with nonhuman subjects using three different procedures which prevented the target cue from having control over behavior during blocking treatment. Thus, the two goals of this series were to demonstrate that backward conditioning can occur in nonhuman subjects outside of sensory preconditioning preparations and to assess why it is ordinarily difficult to see in first-order conditioning.
In addition, the experiments were expected to provide further knowledge about cue competition. As we have discussed above, several experiments have suggested that cue competition is affected by whether or not the target stimulus has control over behavior. However, the evidence is somewhat biased. On one hand, impairment of the effect of cue competition by endowing the stimulus in question with potential to elicit any responding has been demonstrated through various approaches such as using a high intensity target cue, using a counterconditioning technique, or using second-order conditioning in place of sensory preconditioning. On the other hand, enhancement of conventional cue competition or demonstrations of specific cue competition seen within retrospective revaluation such as backward blocking produced by attenuating the potential of the target to elicit responding has been, as far as we know, achieved only through the use of sensory preconditioning. This leaves some ambiguity about whether the difficulty in obtaining backward blocking in first-order conditioning really results from the target stimulus having response potential during training. For example, an alternative explanation might be possible that presentations of stimuli such as conventional USs during conditioning might distract nonhuman subjects and interfere with relatively higher-level processing such as evaluating cues retrospectively. To evaluate this possibility, the three experiments in this report were conducted using conventional USs (i.e., footshock) during conditioning, and used ploys other than sensory preconditioning to attenuate behavioral control by the target during backward blocking treatment.
Experiment 1
In Experiment 1, we used findings reported by Blaisdell et al. (1999, Experiment 3) to test the possibility that backward blocking could occur in first-order conditioning in nonhumans. They demonstrated that, after the two conventional phases of forward blocking, responding to a target cue recovered as a result of massive extinction of the forward blocking cue. Use of this recovery-from-forward blocking effect enabled us to keep the target cue of the present backward blocking experiment from acquiring potential to elicit conditioned responding during the initial phase of backward blocking training. Specifically, in the recovery-from-forward-blocking effect, elemental training of the forward-blocking cue is followed by compound pairings of the forward blocking cue and the target cue with the US. As a result, despite the target cue being paired with a US, the target cue should not come to elicit conditioned responding. Then, responding to the target cue emerges when massive extinction of the forward blocking cue is conducted. This is the point at which the target comes to elicit conditioned responding. In Experiment 1, we embedded a backward blocking design into this sequence.
The design of Experiment 1 is depicted in Table 1. There were five groups, Backward Blocking-Forward Block (BB-FwdBlock), Control-Forward Block (Ctrl-FwdBlock), Backward Blocking-No Forward Block (BB-No FwdBlock), Control-No Forward Block (Ctrl-No FwdBlock), and Forward Block-No Extinction (FwdBlock-No Ext). For all subjects, a potential forward-blocking stimulus (C) was paired with the US in Phase 1. Then, in Phase 2, subjects in the two groups of the FwdBlock condition (the BB-FwdBlock and Ctrl-FwdBlock groups) received pairings of a target cue (X) and a backward-blocking cue (A) with the US in compound with the forward-blocking cue (C). In these two groups, the target cue X and the backward blocking cue A were expected to be blocked by C (thus they should not have control over behavior at this point). In contrast, subjects in the two groups in the No FwdBlock condition (the BB-No FwdBlock and Ctrl-No FwdBlock groups) received pairings of the AX compound with the US without C in Phase 2. This corresponds to the compound training of a conventional backward-blocking procedure. Then, in Phase 3, A-US pairings were administered to the two backward-blocking groups (BB-FwdBlock and BB-No FwdBlock), whereas in the two control groups (Ctrl-FwdBlock and Ctrl-No FwdBlock), B-US pairings were administered with B being an irrelevant control stimulus; thus, these four groups constituted a 2 × 2 factorial design. Finally, massive extinction of the forward blocking stimulus (C) was conducted in Phase 4; in Groups BB-FwdBlock and Ctrl-FwdBlock, A and X were expected to be released from forward blocking by C. As C was irrelevant to the backward blocking procedure in the BB-No FwdBlock and Ctrl-No FwdBlock groups, it was anticipated that backward blocking would not be observed in these two groups. The question was whether backward blocking would be observed in Group BB-FwdBlock relative to Group Ctrl-FwdBlock, in which X was forward blocked by C and consequently prevented from evoking conditioned responding until after the backward-blocking procedure.
Table 1.
Design summary of Experiment 1
| Group | Phase 1 (Context 1) | Phase 2 (Context 1) | Phase 3 (Context 1) | Phase 4 (Context 2) | Test (Context 2) |
|---|---|---|---|---|---|
| BB-FwdBlock | 20 C-US | 4 CAX-US | 20 A-US | 800 C- | X? |
| Ctrl-FwdBlock | 20 C-US | 4 CAX-US | 20 B-US | 800 C- | X? |
| BB-No FwdBlock | 20 C-US | 4 AX-US | 20 A-US | 800 C- | X? |
| Ctrl-No FwdBlock | 20 C-US | 4 AX-US | 20 B-US | 800 C- | X? |
| FwdBlock–No Ext | 20 C-US | 4 CAX-US | 20 B-US | Context only | X? |
Note: BB = backward blocking; Ctrl = control condition for backward blocking; FwdBlock = AX compound training in compound with C in Phase 2; No FwdBlock = AX compound training without C in Phase 2; FwdBlock-No Ext = control group for forward blocking by C; X = click train; A and B = high-frequency tone or white noise, counterbalanced; US = footshock; “-“ = no US. Numbers refer to the number of each trial type.
There was one additional control group (the FwdBlock-No Ext group), which received the same manipulation as the Ctrl-FwdBlock group except for Phase 4 training. The FwdBlock-No Ext group received no extinction treatment of C; thus, the target cue X should have been forward blocked at the time of testing. This group was included in order to ascertain that the forward-blocking procedure by C and release from it worked with our parameters; this group was compared to the Ctrl-FwdBlock group. If forward blocking by C was effective and it was eliminated by extinction of C in Phase 4, responding to X in this group should be lower than in the Ctrl-FwdBlock group. Forward blocking parameters were largely borrowed from Blaisdell et al. (1999).
Method
Subjects
Thirty male (262–335 g) and 30 female (183–241 g), experimentally naïve, Sprague-Dawley descended rats bred in our colony served as subjects. The animals were randomly assigned to one of the five groups (ns=12), counterbalanced for sex. The animals were individually housed in wire-mesh cages in a vivarium maintained on a 16-hr light/8-hr dark cycle. Experimental manipulations were conducted approximately midway in the light portion of the cycle. A progressive water deprivation schedule was imposed over 4 days prior to the beginning of the experiment until water availability was limited to 30 min per day. All subjects were handled for 30 s three times per week from weaning until the initiation of the study.
Apparatus
The apparatus consisted of 12 chambers (counterbalanced within groups), each measuring 25 × 20 × 20 cm (l × w × h). All chambers had clear Plexiglas ceilings and side walls, and metal front and back walls. Chamber floors were 4-mm grids spaced 1.7 cm apart center-to-center, connected with NE-2 neon bulbs, which allowed 1.0-mA, 0.5-s constant-current footshock to be delivered by means of a high voltage AC circuit in series with a 1.0-MΩ resistor. This served as the US. A metal column measuring 8 × 12 × 20 cm (l × w × h) was attached to one of the sidewalls of the chamber. The column had an opening (4.5 × 4.0 × 4.5 cm) positioned 4.0 cm above the grid floor left-right centered. A water-filled lick tube could be placed in the column opening. When the lick tube was inserted, its tip extended 1 cm from the rear of the opening. Inside the opening, an infrared photobeam was projected horizontally, 1 cm in front of the lick tube. To drink from the lick tube, the subjects had to insert their head into the opening, thereby breaking the photobeam. The amount of time spent by subjects drinking was monitored by this device and served as the dependent variable. Each chamber was housed in a sound- and light-attenuating cubicle. A 60-W incandescent bulb was mounted on the back wall of each environmental chest. This bulb could be flashed (0.17 s on-0.17 s off) to serve as a visual stimulus. Three 45-Ω speakers mounted on three different interior walls of each environmental chest could deliver a complex tone (3000 and 3200 Hz) 6 dB above the ambient background (C scale, SPL), a 6-Hz click train 8 dB (C) above background, and a white noise 6 dB (C) above background which was 78 dB ambient noise produced primarily by a ventilation fan. Each chamber was dimly illuminated by a dim (#1820) houselight. The click train was used as the target CS X and the flashing light was used as CS C for all subjects. The complex tone and the white noise were used as CSs A and B, counterbalanced within groups. All CSs were presented for 10 s during treatment. On reinforced trials, the US overlapped with the last 0.5 s of the CSs.
From the 12 chambers noted above, two distinct contexts were created that differed in tactile and odor cues. Context 1 was identical to that described above except that the lick tube was removed. This context was used for forward blocking and backward blocking treatments (i.e., Phases 1, 2, and 3). Context 2 was created by covering the grid floor of the chamber with a Plexiglas plate and by presenting an odor stimulus, which was produced by placing two drops of 98% methyl salicylate (a mint odor) on the top of a wooden cube placed inside the environmental isolation chest but outside of the experimental chamber. Acclimation, Phase 4 training, reacclimation, and testing were conducted in Context 2. This was done to minimize possible differences between groups in the associative status of the test context, and was modeled after Blaisdell et al. (1999) who successfully obtained forward blocking in first-order conditioning. In order to make the contextual difference more conspicuous, the chamber used as Context 1 for each subject was different from that used as Context 2 for that subject. The change of the context was intended to minimize any associative summation of the test context with the test CS.
Procedure
Acclimation
On Day 1, all subjects were exposed to Context 2 for 60 min. Subjects established baseline licking behavior in this session.
Phase 1: Elemental training of the forward-blocking cue
On Days 2–6, subjects received in Context 1 four daily pairings of CS C and the US, 8, 23, 35, and 53 min from the initiation of each daily 60-min session.
Phase 2: Compound training
On Day 7, subjects received four daily compound conditioning trials in Context 1. Trials occurred 8, 20, 35, and 53 min from the initiation of the 60-min session. Subjects in the BB-FwdBlock, Ctrl-FwdBlock, and FwdBlock-No Ext groups received four pairings of a compound of CSs A, X, and C with the US. Subjects in the BB-No FwdBlock and Ctrl-No FwdBlock received four pairings of a compound of CSs A and X with the US.
Phase 3: Elemental training of the backward blocking cue
On Days 8–12, subjects in the BB-FwdBlock and BB-No FwdBlock groups received four daily pairings of CS A and the US, whereas subjects in the Ctrl-FwdBlock, Ctrl-No FwdBlock, and FwdBlock-No Ext groups received four daily pairings of a novel cue (B) and the US in Context 1. Subjects received conditioning trials 7, 25, 40, and 52 min into each daily 60-min session.
Phase 4: Extinction of the forward-blocking cue
On Days 13–20, subjects in the BB-FwdBlock, Ctrl-FwdBlock, BB-No FwdBlock, and Ctrl-No FwdBlock groups received 100 nonreinforced exposures of CS C in each daily 100-min session in Context 2, with the mean intertrial interval (ITI) of 50 s (range 25–75). Subjects in the FwdBlock-No Ext group received equivalent handling and exposure to Context 2 with no nominal stimulus presentation.
Reacclimation
On Days 21 and 22, all subjects received baseline recovery sessions in Context 2. During these sessions, subjects were left in Context 2 and allowed to access to the water-filled lick tube for 60 min without any nominal stimulus presentation.
Testing
On Day 23, testing of CS X was conducted in Context 2 for all subjects. In the testing session, subjects were allowed access to the water-filled lick tube and exposure of CS X started at the moment each subject completed their first 5 cumulative seconds of drinking. Times to complete 5 cumulative seconds in the absence (pre-CS time) and in the presence (CS time) of CS X were recorded. The subjects that required more than 60 s to complete their first cumulative 5 s of drinking on the test day were eliminated from the statistical analyses because of their unusually high fear of Context 2. All pre-CS and CS latencies were log (base 10) transformed in order to improve the within-group normality of the scores, and thereby better meet the requirements for the use of parametric statistical tests.
Results and Discussion
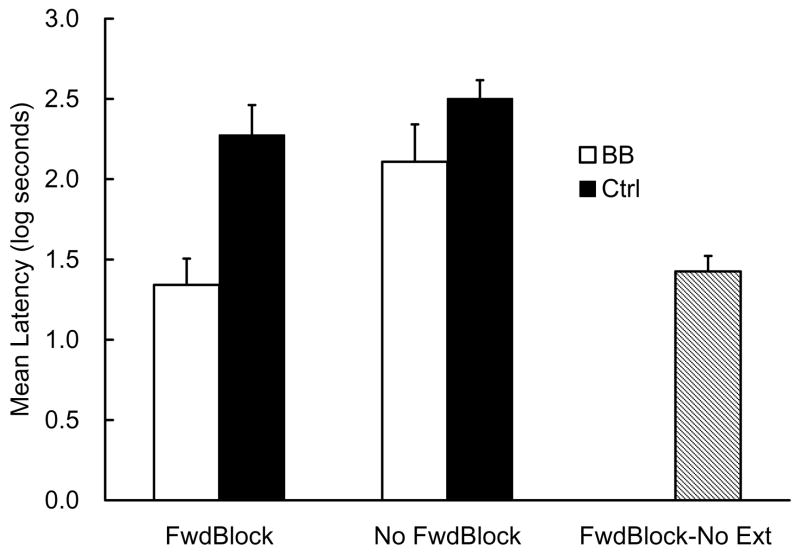
The data from one subject in the BB-No FwdBlock group were excluded from the following analysis because of the pre-CS lick criterion. The mean latencies to complete the first 5 cumulative seconds of drinking in the presence of CS X, which were measured on Day 23, are depicted in Figure 1. Both BB groups exhibited weaker suppression than their corresponding Ctrl groups, which is suggestive of backward blocking, whereas the difference was greater between the two groups in the FwdBlock condition than that between the groups in the No FwdBlock condition. The FwdBlock-No Ext group showed weaker suppression than the Ctrl-FwdBlock group, suggesting that considerable forward blocking was obtained by conducting compound training in the presence of CS C during Phase 2 and massive extinction of CS C in Phase 4 caused substantial recovery from forward blocking of CS X by CS C.
Figure 1.
Results of Experiment 1. Mean log latencies to complete the first 5 cumulative seconds of drinking in the presence of the target cue (X) are depicted. Error bars represent the standard errors of the mean. See Table 1 for the design.
The mean pre-CS scores (log transferred to better normalize the within group distributions) on Day 23 in the five groups were, 1.25, 1.21, 1.22, 1.09, and 1.29 log s for the BB-FwdBlock, Ctrl-FwdBlock, BB-No FwdBlock, Ctrl-No FwdBlock, and FwdBlock-No Ext groups, respectively. These pre-CS scores were analyzed with a one-way analysis of variance (ANOVA). The ANOVA yielded no main effect of group, F(4, 54) = 1.04, p >.39. Thus, there were no appreciable differences among groups in suppression to the CS to differences in fear of the test context. The CS data were analyzed with a similar one-way ANOVA, which detected an effect of group, F(4, 54) = 9.38, p <.01. Planned comparisons revealed the following four relationships. First, suppression in the Ctrl-FwdBlock group was greater than in the FwdBlock-No Ext group, F(1, 54) = 12.59, p <.01. Second, the BB-FwdBlock group showed weaker suppression than the Ctrl-FwdBlock group, F(1, 54) = 15.21, p <.01. Third, the BB-No FwdBlock group exhibited more suppression than the BB-FwdBlock group, F(1, 54) = 9.80, p <.01. Finally, as has been demonstrated previously in most studies of backward blocking with nonhumans, the difference in suppression between the BB-No FwdBlock and the Ctrl-No FwdBlock groups did not reach the conventional statistical level, F(1, 54) = 2.59, p >.11, meaning that statistically significant backward blocking did not occur in this condition.
The CS data were further analyzed with a 2 (cue contingency: BB vs. Ctrl) × 2 (blocking by CS C: FwdBlock vs. No FwdBlock) ANOVA excluding the FwdBlock-No Ext group. This ANOVA yielded main effects of cue contingency and blocking by CS C, F(1, 43) = 12.89, p <.01, and F(1, 43) = 7.20, p <.02, respectively. The interaction was not significant, F(1, 43) = 2.13, p >.15. Because of the absence of an interaction, we cannot readily conclude that backward blocking was significantly facilitated by the forward blocking manipulation. However, the planned comparisons reported above revealed that there was a significant difference between the BB-FwdBlock and Ctrl-FwdBlock, whereas there was no difference in suppression between the BB-No FwdBlock and the Ctrl-No FwdBlock groups as has been shown in most previous studies. Thus, we can minimally conclude that backward blocking is obtainable even in first-order conditioning situations when the target training was forward blocked by another cue, which prevented the target cue from acquiring potential to elicit conditioned responding until the backward-blocking training had been completed.
The present experiment suggests that the critical factor for backward blocking to occur is whether the target cue has the potential to elicit conditioned responding during backward blocking training, and not whether the outcome used during training is of high motivational basis, such as footshock. Thus, our tentative conclusion here is that the difficulty in obtaining backward blocking is not related to this property of the outcome. In the following experiments, we further tested the possibility of backward blocking in first-order conditioning by using other techniques to protect the target cue from having control over behavior during blocking treatment.
Experiment 2
Pavlov (1927) found that when a CS was paired with a US in a backward temporal relationship (i.e., US → CS), the CS comes to elicit little conditioned responding despite its temporal contiguity with the US. Since then, it has been repeatedly demonstrated that backward conditioning is far less effective in establishing conditioned responding to a CS than is forward conditioning. Although there have been some reports of excitatory conditioned responding as the result of backward conditioning (e.g., Keith-Lucas & Guttman, 1975; Mahoney & Ayres, 1976), these studies employed some unusual parameters such as very small numbers of conditioning trials. Moreover, conditioned responding was relatively weak in these cases. Thus, unlike forward conditioning, backward conditioning appears to endow the CS with little potential to control behavior.
There is, however, evidence that backward conditioning results in the formation of an excitatory association between the CS and the US despite the absence of excitatory conditioned responding to the CS itself. As the result of backward conditioning, the CS gains the potential to support excitatory second-order conditioning or sensory preconditioning to another CS (Barnet, Cole, & Miller, 1997; Barnet & Miller, 1996; Cole & Miller, 1999; Matzel, Held, & Miller, 1988). For example, Barnet et al. demonstrated that when a cue (CS1) is paired with the US in a backward temporal relationship (US → CS1), it elicits little excitatory conditioned responding. However, when CS1 is subsequently paired with another cue (CS2) such that CS2 temporally precedes CS1 (i.e., CS2 → CS1), CS2 comes to elicit strong excitatory second-order conditioned responding because it, unlike CS1, has a forward temporal relationship to the US when the representations of the different phases of treatment are superimposed. The implication here is that associations encode not only the identity of the associates, but the temporal relationship of the associates to one another. These studies suggested that, despite a CS acquiring little or no potential to elicit responding as a result of backward conditioning, an excitatory association between the CS and the US is formed which can be assessed through the potential of the CS to support excitatory second-order conditioning to another stimulus. Furthermore, recent studies revealed that associations resulting from backward conditioning, which are usually behaviorally silent (i.e., little potential to elicit behavior) but can be assessed through their potential to support excitatory higher-order conditioning, can be subjected to cue competition effects such as overshadowing (Blaisdell, Denniston, & Miller, 1997, Experiment 3; Burger, Mallemat, & Miller, 2000, Experiment 3).
In Experiment 2, we designed a backward blocking experiment that, in different groups, used forward and backward conditioning procedures, and tested the potential of the backward blocked cues to support excitatory second-order conditioning to another cue. As has been shown in previous studies and in Experiment 1, backward blocking is difficult to obtain with ordinary forward conditioning procedures. Thus, there was little reason to believe that backward blocking would be observed in forward conditioning groups by just introducing a second-order conditioning test. The central question was whether backward blocking would be observed in the backward conditioning groups, in which the target cue itself did not have potential to control vigorous behavior throughout training.
One might suggest that the use of higher-order testing makes the experiment redundant as there already have been demonstrations of backward blocking in sensory preconditioning, which is a form of excitatory higher-order conditioning (Denniston et al., 1996; Miller & Matute, 1996; Pineño et al., 2005, 2006). However, the current experiment clearly differed from these previous demonstrations of backward blocking. In the previous studies, backward blocking was demonstrated using intrinsically innocuous stimuli (e.g., audiovisual cues such as tone and light) as both outcomes and cues. The experimental and the control groups in those studies differed in the contingencies between neutral stimuli (including cues and the surrogate outcome) while the contingency between the surrogate outcome and the real US was identical among groups. That is, backward blocking of a CS-CS association was demonstrated. In contrast, in the present experiment, backward blocking was sought using an intrinsically significant stimulus (a footshock) as an outcome. The experimental and the control groups differed in contingencies between CSs and the conventional footshock US, but the contingency between the blocked CS and the CS which was tested (i.e., the second-order CS) was identical. That is, although second-order conditioning was used to assess the excitatory backward association, backward blocking of a CS-US association was what was critically examined in the present experiment.
The design of Experiment 2 is depicted in Table 2. The experiment included four groups of subjects, Backward Blocking-Backward Conditioning (BB-Backward), Control-Backward Conditioning (Ctrl-Backward), Backward Blocking-Forward Conditioning (BB-Forward), and Control-Forward Conditioning (Ctrl-Forward), which constituted a 2 (cue contingency; BB vs. Ctrl) × 2 (temporal relationship; Forward vs. Backward) factorial design. Subjects in all groups received compound training in Phase 1, which consisted of pairings of the compound AX with the US. Then, in Phase 2, subjects in the two groups in the backward blocking condition (i.e., the BB-Backward and BB-Forward groups) received further reinforced training with the backward blocking cue A, whereas subjects in the two groups in the control condition (i.e., the Ctrl-Backward and Ctrl-Forward groups) received the same number of reinforced trials with a control cue (B). Notably, for the two groups in the backward condition (i.e., the BB-Backward and Ctrl-Backward groups), all the pairings of CS (s) and the US were conducted in a backward temporal relationship (i.e., the US was followed by that of the CS), whereas for the two groups in the forward condition (i.e., the BB-Forward and Ctrl-Forward groups), all the pairings were in a forward temporal relationship (i.e., the CS presentation preceded the US presentation). Then, in Phase 3, all subjects received pairings of the second-order CS (Z) and the blocked CS X, with presentation of CS Z temporally preceding presentation of CS X. Finally, excitatory responding to CS Z and X was assessed for all groups. Backward conditioning parameters were largely borrowed from Burger et al. (2000, Experiment 3).
Table 2.
Design summary of Experiment 2
| Group | Phase 1 (Context 1) | Phase 2 (Context 1) | Phase 3 (Context 1) | Test Z (Context 2) | Test X (Context 2) |
|---|---|---|---|---|---|
| BB-Backward | 6 US→AX | 24 US→A | 4 Z→X | Z? | X? |
| Ctrl-Backward | 6 US→AX | 24 US→ B | 4 Z→X | Z? | X? |
| BB-Forward | 6 AX→US | 24 A→US | 4 Z→X | Z? | X? |
| Ctrl-Forward | 6 AX→US | 24 B→US | 4 Z→X | Z? | X? |
Note: BB = backward blocking; Ctrl = control condition for backward blocking; Backward = CS-US pairing is conducted in backward temporal relationship in Phases 1 and 2; Forward = CS-US pairing is conducted in forward temporal relationship in Phases 1 and 2; “→” = pairing of stimuli with 5 s (Phases 1 and 2) or 7 s (in Phase 3) temporal gap. X = flashing light; Z = click train; A and B = high-frequency tone or white noise, counterbalanced; US = footshock. Numbers refer to the number of each trial type.
Method
Subjects and apparatus
Twenty-four male (275–336 g) and 24 female (176–247 g), experimentally naïve, Sprague-Dawley descended rats bred in our colony served as subjects. The animals were randomly assigned to one of the four groups (ns=12), counterbalanced for sex. Housing, deprivation, and handling conditions were the same as in Experiment 1. The apparatus was the same as that described in Experiment 1 except the click train (6 Hz, 8 dB above background) was used as the second-order target CS Z and the flashing light (0.25 s on-0.25 s off) was used as first-order target (CS X). All CSs were presented for 5 s during conditioning. A 1.0-mA, 0.5-s footshock was used as the US. By changing tactile and odor cues in the same way as in Experiment 1, two distinct contexts (Contexts 1 and 2) were created. Context 1 was used for backward blocking and second-order conditioning treatments (i.e., Phases 1, 2, and 3). Context 2 was used for acclimation, reacclimation, and testing. As before, for each subject different chambers were used for Contexts 1 and 2, and the sipper tube was present only in Context 2.
Procedure
Acclimation
On Day 1, all subjects were exposed to Context 2 for 60 min. Subjects established baseline licking behavior during this session.
Phase 1: Compound training
On Days 2 and 3, subjects received three daily conditioning trials in Context 1 with US presentations occurring 15, 50, and 75 min into each daily 90-min session. Subjects in the BB-Backward and Ctrl-Backward groups received backward pairings of the compound stimulus and the US, in which the 5-s CS X-CS A compound stimulus was presented starting 5 s after the termination of the US. Subjects in the BB-Forward and Ctrl-Forward groups received forward pairings of the compound stimulus and the US, in which the 5-s CS X-CS A compound stimulus terminated 5 s before the US onset. Thus, in both conditions, there was a 5-s gap between the compound CS presentation and the US presentation.
Phase 2: Elemental training
On Days 4–7, subjects in the BB-Backward and BB-Forward groups received six daily pairings of CS A and the US, whereas subjects in the Ctrl-Backward and Ctrl-Forward groups received six daily pairings of CS B and the US. US presentations occurred 7, 25, 40, 52, 66, and 82 min into each daily 90-min session. For subjects in the BB-Backward and Ctrl-Backward groups, the 5-s presentations of CS A or CS B, respectively, were initiated 5 s after the termination of the US. For subjects in the BB-Forward and Ctrl-Forward groups the 5-s presentations of CS A or CS B, respectively, terminated 5 s before the US onset. Thus, in both conditions, there was 5-s gap between the CS and the US.
Phase 3: Second-order conditioning
On Day 8, all subjects received four Z-X pairings in a 60-min session in Context 1, with the pairings at 8, 23, 35, and 53 min into the session. In each trial, 5-s CS Z presentations terminated 7 s before the onset of 5-s CS X presentations.
Reacclimation and testing
On Days 9 and 10, all subjects received baseline recovery sessions in Context 2 in the same manner as in Experiment 1. On Day 11, CS Z was tested in Context 2 for all subjects in the same manner as in Experiment 1 except that CS Z was presented to all subjects for a full 15 min in order to minimize differences in the amount of extinction of CS Z, thereby minimizing any differential effects of testing with Z on subsequent responding to X. On Day 12, testing of CS X was conducted in the same manner. The subjects that had pre-CS times greater than 60 s on either test day were scheduled to be eliminated from the statistical analyses.
Results and Discussion
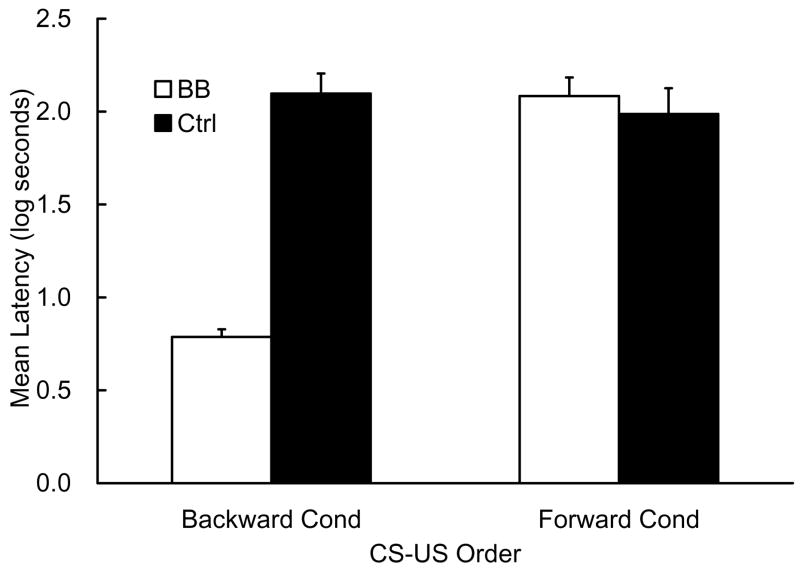
The data of one subject in the Ctrl-Forward group were excluded from the following analysis because of a failure to meet the pre-CS lick criterion. Mean latencies to complete the first 5 cumulative seconds of drinking in the presence of CS Z measured on Day 11 are depicted in Figure 2. As can be seen, there was a substantial difference in suppression between the BB-Backward and the Ctrl-Backward groups, which is suggestive of backward blocking when training was conducted with backward temporal relationships. In contrast, there was little difference between the Ctrl-Forward and the BB-Forward groups, which is suggestive of an absence of backward blocking when training was conducted in a forward temporal relationship (i.e., a conventional excitatory conditioning procedure). Although the order of CS-US presentation was reversed between the forward and the backward conditions, the suppression of the two groups in the Ctrl condition was almost the same, suggesting that the backward conditioned stimulus had the potential to support excitatory second-order conditioning to another stimulus (CS Z) comparable to that of the forward conditioned stimulus (see Barnet & Miller, 1996, for an explanation of this surprising result in terms of temporal coding). The following statistical analyses support these conclusions.
Figure 2.
Results of Experiment 2. Mean log latencies to complete the first 5 cumulative seconds of drinking in the presence of the second-order target cue (Z) are depicted. Error bars represent the standard errors of the mean. See Table 2 for the design.
The mean pre-CS scores (log transferred) in the four groups on Day 11 were 1.06, 1.15, 1.14, and 1.11 log s for the BB-Backward, Ctrl-Backward, BB-Forward, and Ctrl-Forward groups, respectively. These scores were analyzed with a 2 (cue contingency: BB vs. Ctrl) × 2 (CS-US order: Backward vs. Forward) ANOVA. The ANOVA detected neither a main effect nor an interaction, Fs < 1. The CS data were analyzed with a similar 2 × 2 ANOVA, which found a main effect of cue contingency, F(1, 43) = 32.42, p <.01, a main effect of CS-US order, F(1, 43) = 31.00, p <.01, and an interaction, F(1, 43) = 43.62, p <.01. Planned comparisons revealed that suppression of the BB-Backward group was weaker than that of the Ctrl-Backward group, F(1, 43) = 77.34, p <.01, whereas the suppression in the BB-Forward group did not differ significantly from that of the Ctrl-Forward group, F < 1.
The mean log latencies to complete the first 5 cumulative seconds of drinking in the presence of CS X, which were measured on Day 12, were 0.81, 0.84, 1.01, and 1.13 log s, for the BB-Backward, Ctrl-Backward, BB-Forward, and Ctrl-Forward groups, respectively. The suppressions in all groups were far lower than those to CS Z tested on Day 11 even in the Forward groups. A possible explanation for the low suppression to CS X is stimulus generalization decrement. Specifically, as the flashing light served as CS X in this experiment, the way subjects perceived CS X might have been different between the conditioning phases (when subjects were not likely to poke their head into metal opening because the lick tube was not available inside it) and the testing phase (when subjects were poking their heads into the sipper opening at least at the time of the onset of the CS X). When subjects poked their heads into the opening in order to access the lick tube, their eyes might have been partially covered. Consequently, greater generalization decrement might have occurred between conditioning and testing for CS X than for CS Z which was auditory. In any case, although the magnitude of responding across all groups seems relatively weaker, the suppression in the Forward condition was still greater than that in the Backward condition, which is congruent with our expectation. This conclusion was supported by the following statistical analyses. First of all, in order to make sure that there was no significant difference in fear to the test context, the pre-CS data were analyzed. The mean pre-CS scores (log transferred) in the four groups on Day 12 were, 1.17, 1.27, 1.25, and 1.28 log s for the BB-Backward, Ctrl-Backward, BB-Forward, and Ctrl-Forward groups, respectively. These baseline scores were analyzed with a 2 (cue contingency: BB vs. Ctrl) × 2 (CS-US order: Backward vs. Forward) ANOVA. This ANOVA did not yield any main effect or interaction, Fs < 1. A similar 2 × 2 ANOVA conducted on the CS data yielded a main effect for CS-US order, F(1, 43) = 11.07, p <.01. Neither the main effect of cue contingency nor the interaction proved significant, F(1, 43) = 1.02, p >.31 and F < 1, respectively. Thus, although the test data for CS X might have been somewhat weakened because of generalization decrement caused by physical modality of CS X, there were still detectable differences between groups in the potential of CS X to elicit conditioned suppression as a function of stimulus order during compound conditioning.
The results of Experiment 2 are consistent with the hypothesis that the potential of a cue to elicit responding at the time of elemental training is a critical determinant of backward blocking. That is, with a forward conditioning procedure, which generally imparts considerable potential to control behavior to a cue, backward blocking did not emerge as the results of either second-order testing or testing of the blocked cue itself. However, with a backward conditioning procedure, which generally imparts a target cue with little potential to elicit conditioned responding, backward blocking was evident in second-order testing. In other words, a decrement in the potential of the blocked cue (X) to support excitatory second-order conditioning of another cue (Z) was observed relative to both a comparable unblocked cue and a cue that was embedded in first-order conditioning. Together with the results of Experiment 1, our tentative conclusion is that backward blocking is possible even with an outcome of high motivational value as long as the target does not come to elicit conditioned responding during blocking training.
Experiment 3
In Experiment 3 we examined the possibility of obtaining backward blocking in a first-order conditioning situation by taking advantage of the phenomenon of renewal (Bouton & Bolles, 1979). Renewal refers to a recovery of responding to an extinguished CS that occurs when the physical context at the time of testing differs from that of extinction treatment. In the present experiment, we used the renewal effect to further test the possibility of obtaining backward blocking in first-order conditioning. The central feature of the renewal effect is that, when acquisition training with a CS is conducted in one context (Context 1) and then extinction treatment is administered in another context (Context 2), the CS still elicits conditioned responding when it is tested in Context 1 but not when tested in Context 2. The question examined in Experiment 3 was whether backward blocking is possible if elemental training of the blocking cue is conducted in Context 2 where the target cue has lost its potential to elicit conditioned responding as the result of previous extinction treatment within that context. Specifically, subjects in the group in question first received compound training of backward blocking in Context 1, and then experienced extinction of the target CS in Context 2. Then they received elemental training of the backward blocking cue in Context 2, where the target CS should have lost its potential to elicit conditioned responding. Finally, the target CS was presented in Context 1, where recovery of responding to the target cue should otherwise be expected due to renewal.
Notably, Experiment 3 was clearly different from the previous two experiments in one aspect. In Experiments 1 and 2, the target cue did not come to elicit excitatory responding until the backward blocking treatment was completed. In Experiment 3, the target cue presumably came to elicit excitatory responding as a result of compound reinforced training, and elemental training of the blocking cue was conducted while the target cue transiently (between compound training and testing) lost its control of behavior as a result of extinction treatment. Assuming that a target cue’s behavioral control works against its potential to be backward blocked, it is still unclear if backward blocking could be observed in this situation. That is, if the target cue’s behavioral control at the time of compound training is important, one might expect that backward blocking would fail even if the target cue’s behavioral control is attenuated between compound and elemental conditioning. In contrast, if the target cue’s behavioral control at the time of elemental training is important, one would expect to observe backward blocking when the target cue’s behavioral control is transiently reduced between compound and elemental training. Thus, the results of Experiment 3 were expected to provide further information concerning situations in which backward blocking can be obtained.
The design of Experiment 3 is depicted in Table 3. There were five groups, Backward Blocking-Extinction (BB-Extinction), Control-Extinction (Ctrl-Extinction), Overshadowing-Extinction (OV-Extinction), Backward Blocking-No Extinction (BB-No Extinction), and Control-No Extinction (Ctrl-No Extinction). Subjects in all groups first received compound training (i.e., AX-US pairings) in Context 1 in Phase 1. Then, in Phase 2, subjects in the Extinction condition (i.e., the BB-Extinction, Ctrl-Extinction, and OV-Extinction groups) received extinction treatment of the target cue X in Context 2. During Phase 2, subjects in the No Extinction condition (i.e., the BB-No Extinction and Ctrl-No Extinction groups) experienced the same exposure to Context 2 without any nominal stimulus exposure. Then in Phase 3, subjects in the BB condition (i.e., the BB-Extinction and BB-No Extinction groups) received elemental training of the blocking cue (A) in Context 2, whereas subjects in the Ctrl condition (i.e., the Ctrl-Extinction and Ctrl-No Extinction groups) received the same number of elemental training trials with an irrelevant stimulus (B) in Context 2. Subjects in the OV-Extinction group experienced the same amount of exposure to Context 2 without any nominal stimulus presentations. Suppression to the target cue X was then tested in Context 1 for all subjects.
Table 3.
Design summary of Experiment 3
| Group | Phase 1 (Context 1) | Phase 2 (Context 2) | Phase 3 (Context 2) | Test X (Context 1) |
|---|---|---|---|---|
| BB-Extinction | 4 AX-US | 108 X- | 24 A-US | X? |
| Ctrl-Extinction | 4 AX-US | 108 X- | 24 B-US | X? |
| BB-No Extinction | 4AX-US | - | 24 A-US | X? |
| Ctrl-No Extinction | 4 AX-US | - | 24 B-US | X? |
| OV-Extinction | 4 AX-US | 108 X- | - | X? |
Note: BB = backward blocking; Ctrl = control condition for backward blocking; OV = overshadowing control for backward blocking; Extinction = X is extinguished during Phase 2; No Extinction = no X exposure during Phase 2; US = footshock; “-“ = no presentation of the US. X = click train; A and B = high-frequency tone or white noise, counterbalanced. Numbers refer to the number of each trial type.
The design of Experiment 3 consisted of four groups composing a 2 (stimulus contingency; BB vs. Ctrl) × 2 (extinction; Extinction vs. No Extinction) factorial design plus an additional control group (OV-Extinction). The central question was whether backward blocking occurs in the Extinction and No Extinction conditions. According to the view that the potential of a stimulus to control behavior is a critical determinant of cue competition, backward blocking might result in the Extinction condition because the elemental training of the backward blocking CS is conducted when the target cue should have little potential to elicit conditioned responding as a consequence of extinction treatment in Phase 2. In contrast, backward blocking is not likely to result in the No Extinction condition because the target cue kept its potential to control behavior during the backward blocking treatment.
Method
Subjects and apparatus
Thirty male (300–397 g) and 30 female (201–243 g), experimentally naïve, Sprague-Dawley descended rats bred in our colony served as subjects. The animals were randomly assigned to one of the five groups (ns=12), counterbalanced for sex. Housing, deprivation, and handling conditions were the same as in Experiments 1 and 2. The apparatus was the same as in Experiments 1 and 2. The click train (6 Hz, 8 dB above background) was used as the target CS X. The complex tone (8 dB above background) and the white noise (8 dB above background) were used as CSs A and B, counterbalanced within groups. All CSs were presented for 10 s during conditioning. A 0.7-mA, 1.0-s footshock was used as the US. In Experiment 3, the flashing light was not used.
Contextual cues
From the 12 operant chambers, two distinct contexts were created that differed in visual and odor cues. Context 1 was identical to Context 1 in Experiments 1 and 2, which was used for acclimation, compound training (i.e., Phase 1), reacclimation, and testing. Context 2 was created by turning off the houselight, removing the lick tube, and presenting an odor stimulus (methyl salicylate). Extinction of the target cue and elemental training (i.e., Phases 2 and 3) were conducted in Context 2.
Procedure
Acclimation
On Day 1, all subjects were exposed to Context 1 for 30 min. Subjects established baseline licking behavior in Context 1 in this session. Within the same day, subjects were exposed to Context 2 for 30 min, approximately 5 hours after the acclimation to the Context 1. The presentation of two contexts within the same day was intended to facilitate discrimination between them.
Phase 1: Compound training
On Day 2, subjects received four conditioning trials in Context 1 during a 60-min session. Trials began 7, 25, 40, and 52 min into the session. In this session, 10-s presentations of the AX compound were immediately followed by the US presentation. During these sessions, the lick tube was not available in order to avoid licking being accidentally paired with footshock.
Phase 2: Extinction of the target cue
On Days 3–5, subjects in the BB-Extinction, Ctrl-Extinction, and OV-Extinction groups received 36 daily CS X-alone presentations, whereas subjects in the BB-No Extinction and Ctrl-No Extinction groups spent the same amount of time in Context 2 but without any nominal stimulus presentations. The X-alone trials were distributed pseudorandomly in each daily 24 min session with an average ITI (measured CS onset to CS onset) of 40 s.
Phase 3: Elemental training
On Days 5–7, the BB-Extinction and BB-No Extinction groups received eight daily pairings of CS A and the US in Context 2, whereas subjects in the Ctrl-Extinction and Ctrl-No Extinction groups received eight daily pairings of CS B and the US in Context 2. These trials occurred 8, 20, 35, 53, 65, 80, 98, and 113 min from the initiation of each daily 120-min session. On each trial, a 10-s presentation of A or B was immediately followed by the US. The OV-Extinction group did not receive any nominal stimuli but spent equivalent time in Context 2. Notably, the first session of this phase was conducted immediately after the last session of Phase 2; on Day 5, subjects received a 2 h and 16 min long session, in which the first 24 min included 36 X- trials (for the Extinction groups only) and the latter 1 h and 52 min included eight reinforced trials of CS A or CS B or no nominal stimulus (for all except the OV-Extinction group). The first reinforced trial occurred 24 min after the beginning of the session; that is, in the BB-Extinction and Ctrl-Extinction groups, the first reinforced trial of CS A or CS B occurred 40 s after the last extinction trial of the target cue. This was intended to minimize any possible spontaneous recovery (Pavlov, 1927) of responding to CS X at the time of the first reinforced trial. If we had inserted a day between termination of Phase 2 and initiation of Phase 3, CS X was likely to have reacquired potential to elicit conditioned responding to a degree at the time of the first trial of elemental training as a result of spontaneous recovery from extinction.
Reacclimation and testing
On Days 8 and 9, all subjects received baseline recovery sessions in Context 1 in the same manner as in Experiments 1 and 2. On Day 10, testing of CS X was conducted in Context 1 for all subjects in the same manner as in Experiment 1. The subjects that had pre-CS times greater than 60 s were scheduled to be eliminated from the statistical analyses.
Results and Discussion
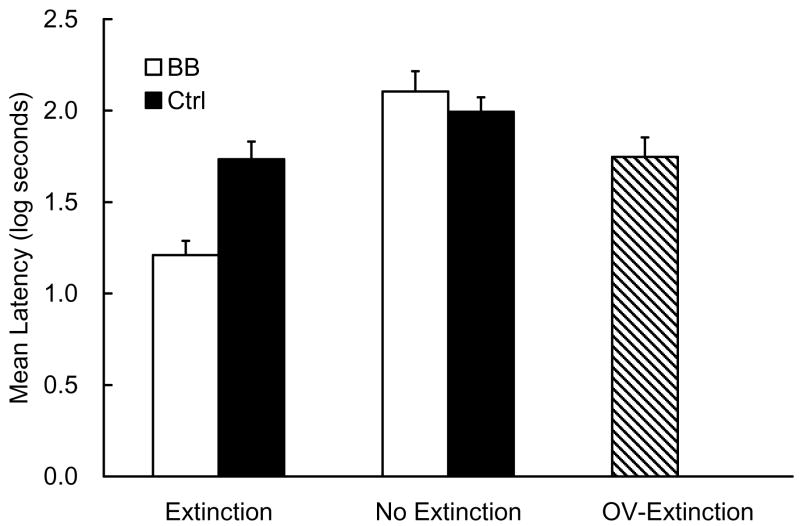
No animal met the pre-CS lick criterion for elimination. The mean log latencies to complete the first 5 cumulative seconds of drinking in the presence of CS X measured on Day 10 are depicted in Figure 3. There was little difference in suppression between Groups BB-No Extinction and Ctrl-No Extinction, suggesting a failure to obtain backward blocking in first-order conditioning. However, there was a considerable difference in suppression between Groups BB-Extinction and Ctrl-Extinction, which suggests backward blocking in this condition. In addition, there was little difference in suppression between the Ctrl-Extinction and OV-Extinction groups. The following statistical analyses support these impressions.
Figure 3.
Results of Experiment 3. Mean log latencies to complete the first 5 cumulative seconds of drinking in the presence of the target cue (X) are depicted. Error bars represent the standard errors of the mean. See Table 3 for the design.
The mean pre-CS scores (log transferred) in the five groups on Day 10 were 1.20, 1.10, 1.16, 1.19, and 1.19 log s for the BB-Extinction, Ctrl-Extinction, OV-Extinction, BB-No Extinction, and Ctrl-No Extinction groups, respectively. These scores were analyzed with a one-way ANOVA, which yielded no effect of group, F < 1. Thus, there were no appreciable differences among groups in fear of the context. The CS score was analyzed with a similar one-way ANOVA, which yielded an effect of group, F(4, 55) = 12.00, p <.01. Planned comparisons using the error term of the ANOVA revealed that suppression in the BB-Extinction group was weaker than in both the Ctrl-Extinction and the OV-Extinction groups, F(1, 55) = 13.83 and 14.52, ps <.01, respectively. An additional planned comparison revealed that there was no difference between the BB-No Extinction and Ctrl-No Extinction groups, F < 1. The data were also analyzed with 2 (cue contingency: BB vs. Ctrl) × 2 (extinction: Extinction vs. No Extinction) ANOVAs excluding the OV-Extinction group. The ANOVA conducted on pre-CS scores yielded no main effect nor interaction, Fs < 1. A similar ANOVA conducted on the CS score yielded a main effect of cue contingency, F(1, 44) = 4.59, p <.05, a main effect of extinction, F(1, 44) = 35.85, p <.01, and an interaction, F(1, 44) = 10.86, p <.01.
The data from the two groups in the No Extinction condition again suggest that obtaining backward blocking in ordinary first-order conditioning situations is difficult. In contrast, the data from the three groups in the Extinction condition clearly suggest that if the elemental training of the blocking cue is conducted while the target cue temporarily lost its potential to elicit conditioned responding as a result of extinction treatment, backward blocking is obtainable even in first-order conditioning. The results of the present experiment are congruent with the view that potential of the target stimulus to control behavior is a critical determinant of cue competition. That is, when a cue controls behavior, it is very difficult to decrease the cue’s response-eliciting potential by indirect means such as manipulation of a companion stimulus. This restriction makes it very difficult to demonstrate backward blocking in conventional first-order conditioning. The results of Experiment 3 also suggest that the status of the target cue at the time of elemental (and not compound) training of a blocking cue is especially important for backward blocking to occur. Although beyond the scope of current experiment, the findings in Experiment 3 lead to further questions. For example, what would happen if extinction was conducted after elemental training of the backward blocking cue (i.e., reversing the order of Phases 2 and 3 in this experiment)? Future research will be needed to address these questions.
The observed difference between the BB-Extinction and the Ctrl-Extinction groups, however, is not sufficient to conclude that backward blocking occurred. The difference between these two groups is also explicable by mechanisms other than backward blocking, such as differences in magnitude of reinstatement (e.g., Rescorla & Heth, 1975) or concurrent recovery (e.g., Weidemann & Kehoe, 2004). Reinstatement refers to a recovery of responding to a previously extinguished cue which occurs as the result of mere exposure to the US. The US presentation in the elemental training phase, especially on the first trial, might have been more surprising in the Ctrl-Extinction group than in the BB-Extinction group because CS A had been previously paired with the US (so it provided the subjects with information about the US occurrence), but CS B had not (so the US occurrence was highly surprising). This difference in surprise value of the US might have caused differential reinstatement between these two groups, resulting in greater reinstatement in the Ctrl-Extinction group than the BB-Extinction group. The difference in responding between the two groups is also explicable in terms of different magnitudes of concurrent recovery. Concurrent recovery refers to recovery of responding to a previously extinguished stimulus caused by reinforced training of an irrelevant stimulus after the extinction treatment. Weidemann and Kehoe reported that concurrent recovery is prevented (or at least attenuated) when the irrelevant stimulus is trained in advance of the extinction treatment. This finding suggests the possibility that weaker concurrent recovery observed in the BB-Extinction group than in the Ctrl-Extinction group caused the difference in suppression between the two groups. Specifically, in the BB-Extinction group, the stimulus reinforced elementally after extinction of the target cue was the blocking stimulus A, which had already been reinforced (during Phase 1 in compound with the target cue) before extinction of the target cue.
The two alternative explanations mentioned above are both based on greater recovery of behavioral control being caused by Phase 3 elemental training of CS B in the Ctrl-Extinction group rather than weaker behavioral control caused by elemental training of CS A in the BB-Extinction group. We can evaluate this possibility by comparing the BB-Extinction and Ctrl-Extinction groups with the third control group, OV-Extinction, which received no additional training of any stimulus during Phase 3. If the difference in conditioned suppression between the BB-Extinction and the Ctrl-Extinction groups resulted from different levels of reinstatement or concurrent recovery, suppression in the two groups should have been greater than that in the OV-Extinction group, in which neither reinstatement nor concurrent recovery should have occurred. However, as can be seen, this was not the case.
A previous study by Bonardi, Honey, and Hall (1990) reported that the effect of forward blocking is decreased when the elemental training of a blocking cue is conducted in a context other than that in which the compound training and the testing are conducted. As the elemental training of the blocking cue in the present experiment was conducted in a context different from that used in compound training, the same problem should have occurred to some extent here. In order to minimize this effect, relatively many elemental training trials were conducted in Phase 3. Perhaps due to this, substantial backward blocking was observed in the Extinction condition in the current experiment. Thus, although the magnitude of backward blocking might have been weakened by our having changed the physical context, our conclusion does not change. Genuine backward blocking was demonstrated in first-order conditioning as a result of our embedding of backward blocking treatment in a renewal procedure.
One alternative explanation for the present data, however, is that different treatment in Phase 3 experienced by subjects in the three extinction groups resulted in changes in subjective similarity between two contexts. Specifically, subjects in the BB-Extinction group uniquely received the same stimulus (A) in both Contexts 1 and 2; hence, the two contexts may have been perceived as more similar in this group than in the other two extinction groups (i.e., Ctrl-Extinction and OV-Extinction). The greater subjective similarity between contexts could have resulted in less renewal in this group, which would resemble a backward blocking effect. Such an explanation of the weak responding in the BB-Extinction group is possible, but it is not entirely consistent with the full results observed in Experiment 3. If one supposes that presentation of A in both contexts enhances similarity between two contexts, not only A but also the US should have had this effect. Moreover, it is reasonable to assume that the effect caused by the US, a highly significant stimulus for subjects, would be much stronger than that caused by A. Because the US was experienced in both Contexts 1 and 2 by the subjects in the BB-Extinction and Ctrl-Extinction groups but only in Context 1 by the subjects in the OV-Extinction group, not only the BB-Extinction but also the Ctrl-Extinction group should have experienced an increase in the subjective similarity of the two contexts compared to the OV-Extinction group. In addition, subjects in the Ctrl-Extinction group experienced presentations of B instead of A in Phase 3, which eventually became fearful through training in Phase 3 as A did in Phase 1. This should also have increased subjective similarity between the two contexts. Thus, according to this account, not only responding to X by the BB-Extinction group but also that of the Ctrl-Extinction group should be weaker than that by the OV-Extinction group, which is not consistent with the data, in that suppression in the OV-Extinction and Ctrl-Extinction groups were almost identical. In order to explain the results of Experiment 3 in terms of subjective similarity between contexts caused by presentation of a specific stimulus in both contexts, one has to explain why such effect was caused by A but not by the US. Moreover, this account fails to speak to the conceptually similar findings of Experiments 1 and 2, in which the target cue’s behavioral control worked against backward blocking.
Importantly, the results of the present experiment not only demonstrated that backward blocking is possible using a renewal technique but also suggest that renewal can be overridden by backward blocking treatment. That is, reinforcement of the target cue’s companion stimulus (in the case of the present experiment, CS A) after extinction of the target cue prevented response recovery from extinction caused by our changing the physical context, despite training of the companion cue itself producing little decrement in responding to the unextinguished target cue. However, as the focus of the present experiment was not on the impairment of ABA renewal, the control conditions included in this experiment were not sufficient to draw clear conclusions concerning this issue.
General Discussion
There has traditionally been great difficulty in obtaining backward blocking in conditioning situations with nonhumans (e.g., Miller et al., 1990; Nakajima & Kawai, 1997), which contrasts with the relative ease of obtaining the same effect in human contingency learning (e.g., Beckers et al., 2005; Shanks, 1985; Wasserman & Berglan, 1998). This discrepancy contrasts sharply with the fact that forward blocking is similarly obtainable both in human contingency learning (e.g., Dickinson et al., 1984) and Pavlovian conditioning with nonhumans (e.g., Kamin, 1968). Several researchers (Denniston et al., 1996; Miller & Matute, 1996) interpreted the difficulty in obtaining backward blocking in nonhumans as arising from the target cue having potential to control behavior at the time the blocking cue is elementally trained. That is, in Pavlovian conditioning with nonhumans, a stimulus of inherent motivational value is used as an outcome (i.e., a US), whereas in human learning, the use of such motivational or emotional stimuli is usually avoided. In a typical backward blocking experiment, the target cue comes to elicit conditioned responding in the first phase of training, which might prevent subsequent elemental training of the blocking cue from decreasing the response potential of the target cue. In contrast, in a typical forward blocking experiment, elemental training of the blocking cue is expected to prevent the target cue from gaining the potential to evoke responding during the subsequent compound training phase. Thus, there is no point at which the target cue comes to control behavior. According to this view, backward blocking is much more difficult to obtain than forward blocking because a backward blocked cue’s behavioral control is strong before blocking treatment, which causes the cue to be less sensitive to impairment of response potential as the result of cue competition (e.g., Blaisdell et al., 2000; Denniston et al., 1996; Oberling et al., 2000). Alternatively, one could argue on the basis of previous studies that backward blocking (but not forward blocking) relies on higher-order reasoning, which may be lacking in nonhumans.
The three experiments in this report were conducted in order to determine whether backward blocking in first-order conditioning with nonhumans is possible if the target cue does not control behavior at the time of elemental training of the blocking cue. The results are generally compatible with the prediction concerning backward blocking derived from the view that the potential of a stimulus to elicit conditioned responding during blocking treatment is a critical determinant of cue competition. That is, even though the target cue was directly paired with a stimulus of high motivational value (i.e., a footshock), backward blocking was clearly observed when the target cue did not control behavior at the time of elemental training. This was confirmed when the target cue’s response potential during elemental training was low due to forward blocking of the target cue by another cue (Experiment 1), pairing the target cue with the US in a backward temporal relationship (Experiment 2), and extinction of the target cue between compound training and elemental training (Experiment 3). Notably, the three experiments not only demonstrated backward blocking in first-order conditioning but also replicated the difficulty in obtaining backward blocking in conventional first-order conditioning situations which has been reported in several prior studies (e.g., Miller et al., 1990; Nakajima & Kawai, 1997). The two groups in the No FwdBlock condition in Experiment 1, the two groups in the Forward condition in Experiment 2, and the two groups in the No Extinction condition in Experiment 3, did not differ in suppression to the target cue. Together with the successful observations of backward blocking in the experimental conditions (i.e., when the target cue was forward blocked, embedded in backward conditioning, or extinguished) of the three experiments, these failures strongly support the role of the potential of stimulus to control behavior (i.e., biological significance) in determining whether cue competition will occur. As predicted by many contemporary learning theories (e.g., Denniston et al., 2001; Dickinson & Burke, 1996; Van Hamme & Wasserman, 1994), backward blocking should be readily obtainable unless the experimental design encourages behavioral control of the target cue prior to elemental conditioning.
There are some recent studies that included backward blocking treatment in first-order conditioning situations with nonhuman animals, which are worthy of attention. Balleine, Espinet, and Gonzalez (2005) demonstrated that perceptual learning between competing cues enhanced retrospective revaluation. Specifically, they found that retrospective revaluation, in the form of weaker responding to the target cue (X) as the result of backward-blocking treatment (i.e., AX+ followed by A+) than that resulting from recovery-from-overshadowing treatment (AX+ followed by the A−), was observed only when alternating preexposures of the two cues, A and X, were massively administered to enhance discrimination between the two cues through perceptual learning. When no preexposure was administered or when the same number of preexposure trials were conducted in blocked rather than alternating order (which would be expected to result in less perceptual learning), a tendency opposite to backward blocking was observed (i.e., stronger responding to the target as a result of backward-blocking treatment than that resulting from recovery-from-overshadowing treatment). They interpreted their data as contradicting the claim made by Miller and his colleagues (e.g., Denniston et al., 1996; Miller & Matute, 1996) that the potential of the target cue to elicit vigorous responding is a critical determinant of retrospective revaluation, based on the fact that clear evidence of retrospective revaluation was obtained in first-order conditioning situation.
The claim by Balleine et al. (2005) concerning the view proposed by Miller and his colleagues (Denniston et al., 1996; Miller & Matute, 1996), however, is somewhat missing the point. According to the view of Miller and his colleagues, not all variations of retrospective revaluation are supposed to be difficult to obtain in first-order conditioning; backward blocking should be hard to obtain, whereas recovery from overshadowing should not be. Thus, their demonstration of retrospective revaluation in first-order conditioning does not challenge the view of Miller et al. Because they compared the backward-blocking condition directly with a recovery-from-overshadowing condition, it is not possible to determine whether the difference observed in their experiment was due to backward blocking or recovery from overshadowing, or both. According to the account focused on the potential of the target cue to control behavior, it is predicted that the difference observed in such a retrospective revaluation design in first-order conditioning should result mostly from the recovery of overshadowing, which is supported by a recent study (Liljeholm & Balleine, 2006).
As the focus of the present series of experiments was selectively on backward blocking rather than on retrospective revaluation in general, comparing a backward-blocking condition with a recovery-from-overshadowing control condition, as Balleine et al. (2005) did, was not appropriate because this would open the possibility that differences in recovery from overshadowing could have contributed to our results. Thus, we adopted a control condition in which subjects received pairings of a neutral cue (B) and the US in Phase 2 (i.e., AX+ followed by B+). Using this control condition also equated the number of USs received in the experimental and control conditions and thus facilitates rejection of some nonassociative accounts, such as habituation to the US during Phase 2, of any apparent backward blocking that is observed (see Wheeler & Miller, 2007, for evidence that the forward blocking equivalent of the recovery from overshadowing control condition is less informative than control conditions that equate the number of US presentations).
It should be noted that some recent studies cast doubt upon the appropriateness of the use of aforementioned control group in both forward and backward blocking experiments. Using honey bees as subjects, Blaser, Couvillon, and Bitterman (2006) reported a nonassociative phenomenon resembling blocking, which they called pseudoblocking. Their experimental group received the two conventional phases of blocking treatment but without the target cue (X) present. That is, the subjects received A+ and A+ in two successive phases. In contrast, a control group received A+ in one phase and B+ in another phase; again X was absent. Upon testing on X, Blaser et al. observed weaker responding to X by the experimental group than by the control group. One explanation for the pseudoblocking effect is that more stimulus generalization to X from A and B occurred in the control group than from A alone in the experimental group. Their findings suggested that any apparent blocking effect obtained using the AX+→B+ control group discussed above, including our current backward blocking experiments, might be explicable in terms of greater stimulus generalization in control conditions.
Taking several facts into consideration, however, it seems to be difficult to explain all of the present results in terms of pseudoblocking. Notably, Guez and Miller (2008) recently tried to replicate the pseudoblocking effect in our laboratory using a similar conditioned suppression preparation with rats and found that the effect was obtainable only when an additional discrete cue was presented throughout two phases of pseudoblocking and testing phase. According to their interpretation, the pseudoblocking effect observed in Blaser et al. (2006) arose because a feeder used to present sucrose US, which was regarded as a training context by Blaser et al., served as another punctuate cue rather than a training context. Guez and Miller demonstrated that blocking or control training without the target cue (in these conditions, responding to the target cue should be supported only by stimulus generalization from other cues) did not result in appreciable responding to the target cue. Taylor, Joseph, Balsam, and Bitterman (2008, Experiment 3) also found similar results in an appetitive conditioning situation with rats. These results are understandable considering that highly discriminable stimuli were carefully selected with the intent of minimizing such generalization between stimuli. Despite of some differences in duration and intensity, the set of stimuli used in the present experiments was similar to those used by Guez and Miller. Thus, it does not seem to be plausible to suppose that any apparent blocking effect was generated by different levels of stimulus generalization between cues. Furthermore, the results of Experiment 3 were not compatible with the explanation in terms of stimulus generalization. According to the pseudoblocking explanation, the suppression in the OV-Extinction group should have been weaker than in the BB-Extinction and Ctrl-Extinction groups, which was not the case.
The three experiments in this report were designed based on the view that for blocking to occur the target cue must not have the potential to elicit behavior during elemental training of the backward blocking cue. In each of the three experiments, a different manipulation was used to prevent the target from controlling behavior at the time of elemental training of the blocking cue and backward blocking was observed, thereby providing support for this view. However, we must note that at least some parts of results are explicable through other processes. For example, in Experiment 1, the recovery-from-forward-blocking technique was used to prevent the target cue from possessing a potential to elicit conditioned responding during training in the BB-FwdBlock and Ctrl-FwdBlock groups. At the same time, this resulted in generally weaker responding in the FwdBlock groups than in the No FwdBlock groups. An alternative explanation is that generally weak responding in the FwdBlock condition enabled backward blocking. Specifically, backward blocking in first-order conditioning might be more readily obtained when the expected responding is relatively weak; that is, the difference in responding between the backward blocking and control conditions might be detectable only when responding is moderate. The same line of explanation seems to be applicable to the results of Experiment 3; responding in the three groups in the Extinction condition was generally weaker than in the No Extinction condition. However, such explanation is implausible for the results of Experiment 2, in which responding in the control groups did not differ between the backward and forward conditions. Furthermore, such an interpretation is not compatible with previous findings that backward blocking is possible in sensory preconditioning but not in second-order conditioning (Denniston et al., 1996) or that counterconditioning can decrease the effect of cue competition (Blaisdell et al., 2000; Oberling et al., 2000). Thus, it is unlikely that the difficulty in obtaining backward blocking was due exclusively to the magnitude of responding.
An implication from the results of present three experiments, together with previous studies, is that the relative ease of obtaining backward blocking in human learning situation might result from the use of an innocuous outcome. Notably, in a human electrodermal conditioning situation with visual CSs and an electric shock US, Mitchell and Lovibond (2002, Experiment 2) investigated the effect on backward blocking of providing participants with verbal instructions concerning the additivity of the effect of CSs presented in compound. A reliable backward blocking effect was observed when the instructions about additivity were given to the participants before conditioning, whereas backward blocking was not seen when the instructions were not provided. Superficially, their results appear inconsistent with our assertion that the potential of a stimulus to control behavior is a critical determinant for cue competition because, unlike other examples of backward blocking in humans, an electric shock was used as the US. However, in their experiment, the intensity of the electric shock was set so that it was “definitely uncomfortable, but not painful” for each participant. Thus, the intensity of their electric shock was possibly not strong enough to endow the cue with potential to elicit appreciable emotional responding, which we propose is necessary to prevent backward blocking from occurring. However, it is also possible that, unlike nonhumans, human subjects might be able to show backward blocking regardless of whether stimuli of high motivational value are used. Whether common mechanisms in humans and nonhumans determine when cue competition will and will not be observed is a question for future research.
The present experiments, as well as previous studies, have suggested that the potential of a stimulus to control vigorous behavior is one of the important determinants for cue competition. However, the question of why this property of a stimulus is an important factor for cue competition still remains unanswered. Although we do not have sufficient evidence to definitively answer this question, one might speculate that retaining information that supports immediate responding to a stimulus has fostered survival for organisms in their evolutionary past. That is, failing to escape from predators or missing opportunities to obtain food in order to conserve energy is far more deleterious for animals than is expending energies in escaping unnecessarily from safe situations or searching for food on barren feeding grounds (see Miller & Matute, 1996, for elaboration). The constraints for cue competition might be an evolved conservative strategy to retain biologically important information in such circumstances.
Acknowledgments
This research was conducted while Kouji Urushihara was visiting SUNY-Binghamton as a research fellow of the Japan Society for the Promotion of Science at Osaka Kyoiku University. The work was supported by NIMH Grant 33881 to Ralph R. Miller, by Grant-in-Aid for JSPS Fellows to Kouji Urushihara, and by Grant-in-Aid for Special Purpose from Ministry of Education, Culture, Sports, Science, and Technology of Japan to Kouji Urushihara (19539004). The authors would like to thank Eric Curtis, Sean Gannon, Ryan Green, Mario Laborda, Bridget McConnell, Lisa Ng, Heather Sissons, Gonzalo P. Urcelay, and James Witnauer for their comments on an earlier version of this manuscript. We also thank Danielle Beaumont and Wan Yui See for their assistance in running the experiments.
References
- Balleine BW, Espinet A, Gonzalez F. Perceptual learning enhances retrospective revaluation of conditioned flavor preference in rats. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:341–350. doi: 10.1037/0097-7403.31.3.341. [DOI] [PubMed] [Google Scholar]
- Barnet RC, Cole RP, Miller RR. Temporal integration in second-order conditioning and sensory preconditioning. Animal Learning & Behavior. 1997;25:221–233. [Google Scholar]
- Barnet RC, Miller RR. Second-order conditioning mediated by a backward conditioned inhibitor. Journal of Experimental Psychology: Animal Behavior Processes. 1996;22:279–296. doi: 10.1037//0097-7403.22.3.279. [DOI] [PubMed] [Google Scholar]
- Beckers T, De Houwer J, Pineno O, Miller RR. Outcome additivity and outcome maximality influence cue competition in human causal learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31:238–249. doi: 10.1037/0278-7393.31.2.238. [DOI] [PubMed] [Google Scholar]
- Blaisdell AP, Denniston JC, Miller RR. Temporal encoding as a determinant of overshadowing. Journal of Experimental Psychology: Animal Behavior Processes. 1997;24:72–83. doi: 10.1037//0097-7403.24.1.72. [DOI] [PubMed] [Google Scholar]
- Blaisdell AP, Denniston JC, Savastano HI, Miller RR. Counterconditioning of an overshadowed cue attenuate overshadowing. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26:74–86. doi: 10.1037//0097-7403.26.1.74. [DOI] [PubMed] [Google Scholar]
- Blaisdell AP, Gunther LM, Miller RR. Recovery from blocking through deflation of the block stimulus. Animal Learning & Behavior. 1999;27:63–76. [Google Scholar]
- Blaser RE, Couvillon PA, Bitterman ME. Blocking and pseudoblocking: New control experiments with honeybees. Quarterly Journal of Experimental Psychology. 2006;59:68–76. doi: 10.1080/17470210500242938. [DOI] [PubMed] [Google Scholar]
- Bonardi C, Honey RC, Hall G. Contextual specificity of conditioning in flavor-aversion learning: Extinction and blocking tests. Animal Learning & Behavior. 1990;18:229–237. [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learning and Motivation. 1979;10:445–466. [Google Scholar]
- Brogden WJ. Sensory pre-conditioning. Journal of Experimental Psychology. 1939;25:323–332. doi: 10.1037/h0058465. [DOI] [PubMed] [Google Scholar]
- Burger DC, Mallemat H, Miller RR. Overshadowing of subsequent events and recovery thereafter. Quarterly Journal of Experimental Psychology. 2000;53B:149–171. doi: 10.1080/713932724. [DOI] [PubMed] [Google Scholar]
- Cole RP, Miller RR. Conditioned excitation and conditioned inhibition acquired through backward conditioning. Learning and Motivation. 1999;30:129–156. [Google Scholar]
- Denniston JC, Miller RR, Matute H. Biological significance as a determinant of cue competition. Psychological Science. 1996;7:325–331. [Google Scholar]
- Denniston JC, Savastano HI, Miller RR. The extended comparator hypothesis: Learning by contiguity, responding by relative strength. In: Mowrer R, Klein S, editors. Handbook of contemporary learning theories. Hillsdale, NJ: Erlbaum; 2001. pp. 65–117. [Google Scholar]
- Dickinson A, Burke J. Within-compound associations mediate the retrospective revaluation of causality judgments. Quarterly Journal of Experimental Psychology. 1996;49B:60–80. doi: 10.1080/713932614. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Shanks D, Evenden J. Judgement of act-outcome contingency: The role of selective attribution. Quarterly Journal of Experimental Psychology. 1984;36A:29–50. [Google Scholar]
- Guez D, Miller RR. Blocking and pseudoblocking: The reply of Rattus norvegicus to Apis mellifera. Quarterly Journal of Experimental Psychology. 2008;61:1186–1198. doi: 10.1080/17470210701480238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin L. “Attention-like” processes in classical conditioning. In: Jones MR, editor. Miami symposium on the prediction of behavior: Aversive stimulation. Coral Gables, FL: University of Miami Press; 1968. pp. 9–31. [Google Scholar]
- Kaufman MA, Bolles RC. A nonassociative aspect of overshadowing. Bulletin of the Psychonomic Society. 1981;18:318–320. [Google Scholar]
- Keith-Lucas T, Guttman N. Robust single-trial delayed backward conditioning. Journal of Comparative and Physiological Psychology. 1975;88:468–476. [Google Scholar]
- Liljeholm M, Balleine BW. Stimulus salience and retrospective revaluation. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:481–487. doi: 10.1037/0097-7403.32.4.481. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcements. Psychological Review. 1975;82:276–298. [Google Scholar]
- Mahoney WJ, Ayres JJB. One-trial simultaneous and backward fear conditioning as reflected in conditioned suppression of licking in rats. Animal Learning & Behavior. 1976;4:357–362. [Google Scholar]
- Matzel LD, Held FP, Miller RR. Information and expression of simultaneous and backward associations: Implications for contiguity theory. Learning and Motivation. 1988;19:317–344. [Google Scholar]
- Matzel LD, Schachtman TR, Miller RR. Recovery of overshadowed association achieved by extinction of the overshadowing stimulus. Learning and Motivation. 1985;16:398–412. [Google Scholar]
- Miller RR, Hallam SC, Grahame NJ. Inflation of comparator stimuli following CS training. Animal Learning & Behavior. 1990;18:434–443. [Google Scholar]
- Miller RR, Matute H. Biological significance in forward and backward blocking: Resolution of a discrepancy between animal conditioning and human causal judgment. Journal of Experimental Psychology: General. 1996;125:370–386. doi: 10.1037//0096-3445.125.4.370. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. Orlando, FL: Academic Press; 1988. pp. 51–92. [Google Scholar]
- Mitchell CJ, Lovibond PF. Backward and Forward blocking in human electrodermal conditioning: Blocking requires an assumption of outcome additivity. Quarterly Journal of Experimental Psychology. 2002;55B:311–329. doi: 10.1080/02724990244000025. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Kawai N. Failure of retrospective inference in rats’ taste aversion. Japanese Psychological Research. 1997;39:87–97. [Google Scholar]
- Oberling P, Bristol AS, Matute H, Miller RR. Biological significance attenuates overshadowing, relative validity, and degraded contingency effects. Animal Learning & Behavior. 2000;28:172–186. [Google Scholar]
- Pavlov IP. Conditioned reflexes: an investigation of the physiological activities of the cerebral cortex. Oxford, UK: Oxford University Press; 1927. [Google Scholar]
- Pearce JM. A model for stimulus generalization in Pavlovian conditioning. Psychological Review. 1987;94:61–73. [PubMed] [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Pineño O, Urushihara K, Miller RR. Spontaneous recovery from forward and backward blocking. Journal of Experimental Psychology: Animal Behavior Processes. 2005;31:172–183. doi: 10.1037/0097-7403.31.2.172. [DOI] [PubMed] [Google Scholar]
- Pineño O, Urushihara K, Stout SC, Fuss J, Miller RR. When more is less: Extending training of the blocking association following compound training attenuates the blocking effect. Learning & Behavior. 2006;34:21–36. doi: 10.3758/bf03192868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Effect of US habituation following conditioning. Journal of Comparative and Physiological Psychology. 1973;82:137–143. doi: 10.1037/h0033815. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1:88–96. [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black A, Prokasy WF, editors. Classical conditioning II. New York: Appleton-Century-Crofts; 1972. [Google Scholar]
- Shanks DR. Forward and backward blocking in human contingency judgments. Quarterly Journal of Experimental Psychology. 1985;37B:1–21. [Google Scholar]
- Taylor KM, Joseph VT, Balsam PD, Bitterman ME. Target-absent controls in blocking experiments with rats. Learning & Behavior. 2008;36:145–148. doi: 10.3758/lb.36.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hamme LJ, Wasserman EA. Cue competition in causality judgment: The role of nonpresentation of compound stimulus elements. Learning and Motivation. 1994;25:127–151. [Google Scholar]
- Wagner AR. SOP: a model of autonomic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Lawrence Erlbaum Associates; 1981. pp. 1–47. [Google Scholar]
- Wagner AR, Logan FA, Haberlandt K, Price T. Stimulus selection in animal discrimination learning. Journal of Experimental Psychology. 1968;76:171–180. doi: 10.1037/h0025414. [DOI] [PubMed] [Google Scholar]
- Wasserman EA, Berglan LR. Backward blocking and recovery from overshadowing in human causal judgment: The role of within-compound associations. Quarterly Journal of Experimental Psychology. 1998;51B:121–138. doi: 10.1080/713932675. [DOI] [PubMed] [Google Scholar]
- Weidemann G, Kehoe EJ. Recovery of rabbit’s conditioned nictitating membrane response without direct reinforcement after extinction. Learning & Behavior. 2004;32:409–426. doi: 10.3758/bf03196038. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Miller RR. Contrasting reduced overshadowing and blocking. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33:349–359. doi: 10.1037/0097-7403.33.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]