Mast cells can promote inflammation and other tissue changes in IgE-associated allergic disorders, as well as in certain innate and acquired immune responses that are thought to be independent of IgE. However, mast cells can also have anti-inflammatory and immunosuppressive functions. Here, we review the evidence that mast cells can have negative, as well as positive, immunomodulatory roles in vivo, and propose that mast cells may both enhance and later suppress certain features of an immune response.
Introduction
Mast cells can function as effector cells during innate1-3 and acquired1,4-6 immune responses. Such ‘effector functions’ of mast cells include killing pathogens2,3,7, degrading potentially toxic endogenous peptides8-10 or components of venoms9,11, and regulating the numbers, viability, distribution, phenotype or ’non-immune’ functions of structural cells, such as fibroblasts and vascular endothelial cells. Mast cells can exert their effector functions through the direct or indirect actions of a wide spectrum of mast-cell-derived products, and such effects can be observed in both innate1-3,12 and acquired1,5,6,12,13 immune responses (BOX 1).
Mast cells also can influence many aspects of the biology of immune cells, including granulocytes, monocytes/macrophages, dendritic cells (DCs), T cells, B cells, natural killer (NK) and NKT cells. Here, the effects of mast cells on the recruitment, survival, development, phenotype or function of immune cells are referred to as ’immunomodulatory functions’ (BOX 1).
Through effector and/or immunomodulatory functions, mast cells can promote the initiation and increase the magnitude of inflammation, tissue remodelling and, in some cases, tissue injury associated with the innate or adaptive immune responses to pathogens, as well as during allergic or autoimmune disorders1-3,5-7,13. Given the many mechanisms by which mast cells can mediate these effects, they are often thought of as cells whose primary role is to ‘turn on an immune response’. However, as described below, mast cells also have functions that can reduce inflammation, tissue remodelling and tissue injury. Accordingly, a new picture of the function of mast cells is emerging — these cells have the potential to turn immune responses off, as well as to turn them on. Unlike the specific regulatory T-cell subset14, there is no evidence to date that suggests the existence of a specific developmentally and phenotypically distinct subset of immunoregulatory mast cells with specific suppressive functions. Accordingly, we refer to the anti-inflammatory or immunosuppressive functions of mast cells as ‘negative immunomodulatory’ functions, and to those functions that enhance the initiation, magnitude or duration of immune responses as ‘positive immunomodulatory’ functions (BOX 1).
In this Perspective article, we highlight some basic aspects of mast-cell biology and briefly discuss some of the functions, products and surface receptors expressed by mast cells that contribute to the immunomodulatory function of these cells (reviewed in REFS1,3,5,6,13). We describe mouse models used to analyse mast-cell function in vivo and to identify immunomodulatory roles for mast cells during specific immune responses.
Based on this evidence, we present three hypotheses: that the potential to perform negative, as well as positive, immunomodulatory functions is a basic property of the mast-cell lineage; that mast cells can both enhance and later help to limit certain innate and acquired immune responses; and that the extent to which mast cells actually perform such positive or negative immunomodulatory functions during specific immune responses in vivo is highly dependent on the individual biological setting.
The basic biology of mast cells
Origin and tissue distribution
Mast cells are derived from haematopoietic stem cells, but do not ordinarily circulate in a mature form; instead, differentiation and maturation occurs locally, following migration of their precursors to the vascularised tissues or serosal cavities in which mast cells will ultimately reside15-17 (Figure 1). In vertebrates, mast cells are widely distributed throughout the vascularised tissues, particularly near surfaces that are exposed to the environment, including the skin, airways and gastrointestinal tract, where pathogens, allergens and other environmental agents are frequently encountered15-17. Thus, mast cells are well-positioned to be, along with DCs, one of the first cells of the immune system to interact with environmental antigens and allergens, invading pathogens or environmentally derived toxins.
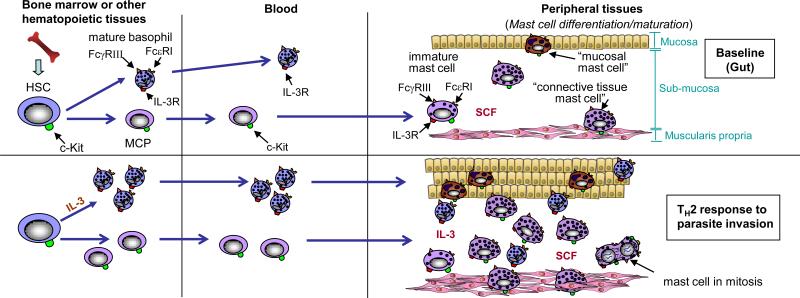
Figure 1. Mast cell development and tissue distribution during examples of immune responses.
Tissue mast cells are derived from haematopoietic stem cells (HSCs), which ultimately give rise to mast-cell progenitors (MCPs). MCPs circulate in the blood and enter the tissues where they undergo differentiation and maturation to become mature mast cells. Stem-cell factor (SCF) ordinarily is required to maintain mast-cell survival, but the phenotype of mature mast cells can vary depending on the growth factor milieu (for example the presence or absence of additional cytokines with effects on mast-cell proliferation or phenotype, such as interleukin-3 (IL-3), IL-4, IL-9 and TGF-β1 and other microenvironmental factors. For example, mucosal mast cells are found in the mucosa of the gut, whereas connective tissue mast cells, that exhibit certain phenotypic characteristics that differ from those of mucosal mast cells, reside in the submucosa and muscularis propria. The numbers of these mast-cell populations can increase dramatically during a T helper 2 (TH2) type response to parasitic infection of the gut, which may reflect a combination of increased recruitment, survival and/or differentiation and maturation of MCPs, as well as proliferation of mast cells resident at that site.
Mast cells are long-lived cells that, similar to monocytes and macrophages, can re-enter the cell cycle and proliferate following appropriate stimulation. Increased recruitment and/or retention, as well as local maturation of mast-cell progenitors, can also contribute to the expansion of mast-cell populations in the tissue15-18 (Figure 1). In addition, expansion of mast-cell numbers, as well as local changes in their tissue distribution and/or phenotypic characteristics, can occur during T helper 2 (TH2)-cell responses, persistent inflammation and/or tissue remodelling15-18 (Figure 1). Such TH2-cell responses are often associated with an increase in the number of circulating basophils, which are haematopoietic cells developmentally distinct from mast cells but that can secrete various mediators, including histamine, that are also produced by mast cells.
Both the regulation of mast-cell survival and proliferation and the modulation of important phenotypic characteristics of this lineage — including its susceptibility to activation by various stimuli during innate or acquired immune responses, the ability of the cell to store and/or produce various secreted products, and the magnitude and nature of its secretory responses to specific stimuli of activation — can be finely controlled or ‘tuned’1. The main survival and developmental factor for mast cells is stem-cell factor (SCF; also known as Kit ligand), but many growth factors, cytokines and chemokines can influence mast-cell numbers and phenotype, including interleukin-3 (IL-3), which is especially important in mice, TH2-associated cytokines, such as IL-4 and IL-9, and TGF-β115-19.
Activation and secretion of mast-cell factors
Various stimuli, in addition to IgE and specific antigen, can activate mast cells to release a diverse array of biologically active products, many of which can potentially mediate pro-inflammatory, anti-inflammatory and/or immunosuppressive functions (summarized in REFS1,2,6,13). Furthermore, mast cells can participate in multiple cycles of activation for mediator release and can be differentially activated to release distinct patterns of mediators or cytokines, depending on the type and strength of the activating stimuli1,6,13,18,20,21. The strength and nature of the responsiveness of mast cells to various activating stimuli may be influenced by genetic or microenvironmental factors that affect the expression pattern or functional properties of the surface receptors or signalling molecules that contribute to such responses1,4,20.
Accordingly, it can be problematic to generalize findings from studies on a single mast-cell population, such as phenotypically ‘immature’ mast cells derived in vitro from mouse haematopoietic cells, to other mast-cell populations, such as mature mast cells in vivo. Also, although co-incubation of mast cells with other cell types under defined conditions in vitro is possible (and this may provide very valuable information on the nature and potential importance of such interactions) it may be exceedingly difficult (probably impossible) to fully recapitulate in vitro the conditions experienced by mast cells in vivo. For this reason, we think that to understand most completely the functions of mast cells during health and disease, these cells should be studied in vivo.
Mouse models of mast-cell function
Mast-cell knock-in mice
Although mice that specifically lack only mast cells have not been reported,c-kit mutant mice, which are deficient in mast cells but have several other phenotypic abnormalities, can be used to analyse the in vivo functions of mast cells1,3,15,22. The most commonly used animals for such studies are the WBB6F1-KitW/W-v mice and the more recently characterized C57BL/6-KitW-sh/W-sh mice1,3,5,13,22-24.KitW is a point mutation that produces a truncated c-Kit, which lacks its transmembrane domain and is therefore not expressed on the cell surface; KitW-v is a (Thr660Met) mutation at the c-kit tyrosine kinase domain that substantially reduces the kinase activity of the receptor; and KitW-sh is an inversion mutation of the transcriptional regulatory elements upstream of the c-kit transcription start site on mouse chromosome 5 (reviewed in REFS1,5,13).
Adult WBB6F1-KitW/W-v and C57BL/6-KitW-sh/W-sh mice are profoundly deficient in mast cells and melanocytes1,15,22. WBB6F1-KitW/W-v mice exhibit several other phenotypic abnormalities, such as macrocytic anaemia, a reduction in the number of bone-marrow and blood neutrophils, sterility, and a marked reduction in number of interstitial cells of Cajal, which are found in the gastrointestinal tract1,15,22,24,25. By contrast, C57BL/6-KitW-sh/W-sh mice are neither anaemic nor sterile, and appear to have normal numbers of bone-marrow and blood neutrophils22,24. Because the c-kit-related phenotypic abnormalities that affect lineages other than mast cells are generally milder in C57BL/6-KitW-sh/W-sh mice than in WBB6F1-KitW/W-v mice, and because C57BL/6-KitW-sh/W-sh mice are fertile and are on the C57BL/6 background, they are becoming increasingly popular for studies to elucidate the roles of mast cells in vivo.
Differences in the biological responses in c-kit mutant mice compared with wild-type mice may be due to any one of the abnormalities that result from the c-kit mutations in these animals and may not be due to the loss of mast cells. However, the lack of mast cells in c-kit mutant mice can be selectively repaired by the adoptive transfer of genetically compatible, in-vitro-derived wild-type or mutant mast cells1,5,15,22. Such in vitro-derived mast cells, for example bone-marrow-derived cultured mast cells, can be administrated intravenously, intraperitoneally or intradermally, or directly injected into the anterior wall of the stomach of c-kit mutant mice, to create so-called ‘mast-cell knock-in mice’. These mast-cell knock-in mice can then be used to assess the extent to which differences in the expression of biological responses observed in c-kit mutant mice, compared to those in wild-type mice, are due to the lack of mast cells in the in c-kit mutant mice.
Other approaches
It is possible to investigate the role of specific mast-cell-associated mediators in vivo by testing animals in which that mediator has been knocked out. If that mediator is selectively expressed by mast cells, and if its deletion does not significantly influence the expression of other mast-cell products, then it is possible to draw conclusions about the role of that mast-cell product in vivo. For example, mice that lack mouse mast-cell protease-1 (mMCP-1)26-28, mMCP-429,30, mMCP-631-33, or mouse mast cell-carboxypeptidase A (mMC-CPA)34, or that have a mutated mMC-CPA that essentially lacks enzymatic activity11, have been used to analyze whether the absence of these proteases (or their enzymatic activity) influences other aspects of the mast-cell phenotype, such as content of other stored mediators, as well as to define the functions of such mast-cell-associated proteases in vivo.
Other promising genetic approaches to investigate the specific functions of mast cells and their products are currently in development, such as ‘mast-cell-specific Cre’ mice that can be crossed with other strains in which the genes of interest are ‘floxed’35. When a gene sequence is flanked by loxP sites (floxed), Cre recombinase, which recognizes the loxP consensus sequence, can excise that specific segment from the gene sequence. Such approaches will be useful to analyze to what extent mast cells represent important sources of products with potential immunomodulatory effects that also can be derived from other cell types, such as histamine, leukotrienes, prostaglandins, cytokines and chemokines.
Pharmacological approaches or those based on the use of antibodies to deplete mast cells or to neutralize their products may also provide useful information, but are limited by the specificity of the drug or antibodies chosen. For example, anti-histamines block histamine whether it has been secreted from mast cells or non-mast cells and antibodies that neutralize SCF36 or block c-Kit37,38 can result in the depletion of mast cells in vivo, but may also influence other cell types that express c-Kit.
Drugs (or antibodies) that only interfere with mast-cell activation would be highly desirable for experimental studies and, possibly, for evaluation as therapeutic agents. One drug, disodium cromoglycate, is widely characterized as a ‘mast-cell stabilizer’ (that is, an agent that blocks the release of mast-cell mediators following appropriate activation of the cell) and has been used to suppress mouse mast-cell function in vivo39,40. However, the drug's molecular targets are not restricted to mast cells41 and the drug also influences the function of granulocytes and B cells42. Given the current limitations of the use of pharmacological or antibody-based approaches to eliminate mast cells or specifically to block their functional activation, we think that genetic approaches (including mast-cell knock-in mice, the use of mice deficient in specific mast-cell-associated mediators, and, if fully validated, approaches that genetically delete specific mediators selectively in mast cells) are the most definitive way to identify and characterize mast-cell functions in vivo.
Mast-cell immunomodulatory functions
Immunomodulatory activity of mast cells in vitro
Mast cells express MHC class I and II molecules, and have been reported to process and present antigen in vitro (reviewed in REFS13,43-45). Mast cells also can enhance antigen presentation in vitro by internalizing antigen bound to high-affinity Fc receptor for IgE (FcεRI)-associated IgE; this mechanism is independent of mast-cell MHC class II expression, but requires mast-cell apoptosis and phagocytosis by other antigen-presenting cells, which then present the antigen45. However, to date there is no evidence from in vivo studies suggesting that mast cells present antigen during naturally occurring or experimental immune responses.
The expression of various co-stimulatory molecules by mast cells is further evidence that these cells can have immunomodulatory roles. Co-stimulatory molecules expressed by mouse and/or human mast cells include members of the B7 family, members of tumour-necrosis factor (TNF)–TNF receptor families, CD28 and CD40 ligand (CD40L)6,13,46,47. Moreover, mast cells can exhibit costimulatory function in vitro. For example, engagement of the co-stimulatory ligand OX40L expressed by human46 or mouse47 mast cells and OX40 expressed by T cells is required for optimal mast-cell-dependent enhancement of T-cell proliferation46,47 or cytokine production47.
Mast cells represent potential sources of many mediators that can enhance or suppress the development, survival, proliferation, migration, maturation, or function of immune cells (reviewed in REFS1-3,5-8,13,16-18,21,30,31,43,44,46-56). Some individual mediators, such as histamine, can have both positive and negative immunomodulatory effects. Histamine, which can be produced by mast cells and other immune cells, can promote TH1-cell activation through H1 receptors but conversely can suppress both TH1 and TH2 cell activation through H2 receptors50. Mast cells can also produce cytokines that can influence the polarization and function of T-cell subsets1,5,6,13,43. However, there is only one study showing that such an effect of a mast-cell derived cytokine is important in vivo (see below)54. Similarly, several mast cell products, including IL-4, IL-5, IL-6, IL-13, CD40L and rat mast-cell protease I, can influence B-cell development and function, including IgE production6,13. However, the in vivo relevance of these observations remains to be determined.
Positive immunomodulatory functions in vivo
Some of the positive immunomodulatory functions of mast cells that have been proposed based on in vitro studies have been confirmed in vivo using mast cell knock-in mice1,54,57-70 or in mice lacking specific mast-cell-associated proteases26-28,32-34 or lacking specific protease enzymatic activity11 (Table 1 ). In many of these studies, the end points assessed included the recruitment of particular immune cells, such as granulocytes28,32,33,57-61,63,64,67-70, DCs62,64,65,71 or various subpopulations of lymphocytes64,67,70,72. Many of these studies also demonstrated that the lack of mast cells54,57-64,67,69,70 or a specific mast-cell product28,32,33,54,60,63,67,68,70 also reduced the pathology associated with the immune response or impaired its effectiveness in promoting host resistance to infection.
Table 1.
Mast-cell immunomodulatory functions demonstrated in vivo*
| Immunomodulatory functions | Mast cell mediators involved (if identified) | Comments | References† |
|---|---|---|---|
| Positive: | |||
| Promote recruitment of cells of innate immunity | TNF, CXCL2, leukotrienes, mMCP1, mMCP2, mMCP6. | In some studies (REFS60,63,67,68,70) the TNF was shown to be of mast cell origin by analyzing mice containing mast cells that could or could not make TNF; in the other studies, the TNF was not formally shown to be of mast cell origin. | 28,32,33,57-61,64,69,76 |
| Promote lymphocyte recruitment | TNF | 62,64,67,68,70,72,92 | |
| Promote DC migration | Histamine, TNF | 62,64,65,71,92 | |
| Promote pathology in EAE via enhancement of TH1-cell response | IL-4 | 54 | |
| Enhance sensitization in CHS¶ | Mediator unknown | Some in vivo studies strongly suggest that mast cells promote sensitization due to an effect, induced by the binding of antigen-non-specific IgE antibodies to mast-cell FcεRI, on mast-cell phenotype and/or function. | 88 |
| Promote a model of antibody-mediated arthritis | IL-1 | 66 | |
| Negative: | |||
| Suppress adaptive immune responses | Histamine, IL-10 | 23,49,82,93 | |
| Promote peripheral tolerance to skin allografts | IL-10? | 83 | |
| Suppress innate responses (to chronic UVB irradiation) | IL-10 | 23 |
Representative functions demonstrated in experiments performed in vivo using either mouse mast-cell protease-deficient mice or mast-cell-engrafted c-kit mutant mice (mast-cell knock-in mice). In some models using mast-cell knock-in mice, the key mast-cell mediators with immunomodulatory functions in that model have not yet been defined. Not included herein are many examples of studies using ‘mast-cell knock-in mice’ that have demonstrated pro-inflammatory effects of mast cells (for example promotion of leukocyte recruitment), but in which the key mast-cell-associated mediators have not yet been defined in vivo.
In some of the referenced studies, the mast-cell mediators that contribute to the function listed have not yet been identified.
Not yet demonstrated using mast-cell-knock-in mice.
CHS, contact hypersensitivity; CXCL2, CXC-chemokine ligand 2; DC, dendritic cell; EAE, experimental autoimmune encephalomyelitis; FcεRI, high-affinity Fc receptor for IgE; IL, interleukin; mMCP1, mouse mast-cell protease-1; TH1, T helper 1; TNF, tumour-necrosis factor.
Mast cells have been shown to be crucial for host survival in models of bacterial infection59,73-76 and, in some of these models, there was evidence that TNF also promoted survival59,73,74. However, the extent to which such TNF was produced by mast cells was not specifically analyzed in these studies. In addition, there is evidence that mast cells may be able to promote host survival in the cecal ligation and puncture (CLP) model of bacterial infection in mice that lack TNF74, suggesting that mast cells can enhance survival in this setting independently of TNF. As well as in bacterial infections, mast cells can promote host resistance to certain parasite infections. However, the mechanisms involved have not been fully defined and may be complex, involving both local and systemic mast-cell-dependent effects (reviewed in REFS1,64,77).
Several lines of evidence indicate that mast cell-derived proteases can promote host defence in certain models of innate or acquired immunity. Following intraperitoneal injection of the bacteria Klebsiella pneumoniae, mMCP-6-deficient mice exhibited both reduced neutrophil recruitment into the peritoneal cavity and significantly increased mortality32. A deficiency in mMCP-6 (or in IgE) was also associated with markedly reduced recruitment of eosinophils to the sites of larvae deposition in skeletal muscle during the chronic phase of Trichinella spiralis infection, but was not associated with a detectable abnormality in the intestinal expulsion of the parasite33. Recent evidence suggests that mMCP-2 can contribute to neutrophil recruitment and host survival during CLP, but that, in wild-type mice, mast-cell production of intra-cellular IL-15 ordinarily limits the mast cell's ability to produce this protease in that setting76.
In a myelin oligodendrocyte glycoprotein (MOG)-induced model of experimental autoimmune encephalomyelitis (EAE), mast cells can increase the incidence and severity of the disorder6,78. Remarkably, it appears that mast cells do not have to be within the CNS to exert at least some of their important effects in MOG-induced EAE. Therefore, although systemic engraftment of KitW/W-v mice with in vitro-derived bone-marrow-derived cultured mast cells does not result in the appearance of mast cells in the CNS of these mice, they exhibit a CNS disease severity that is similar to that in wild-type mice. One of the ways the extra-CNS activity of mast cells can influence autoimmune responses to MOG is through the production of IL-4 in the lymph nodes, which enhances the development of encephalogenic TH1 cells54.
Mast-cell-derived TNF contributes to airway hyperreactivity and inflammation in models of allergic airway inflammation67,70,79, probably in part due to its ability to promote T-cell recruitment67,70 and to enhance local levels of IL-4, IL-5, IL-13 and IL-17 (Ref.67). Mast cells also can contribute substantially to the disease pathology in models of autoantibody-induced destructive arthritis80, bullous pemphigoid (an autoimmune disease of the skin)81 and in a model of TH17-cell-dependent, neutrophil-associated lung inflammation68.
In summary, mast cells can exert positive immunoregulatory functions in vivo that can either enhance host defense or promote disease, that reflect actions of the mast cell's stored mediators and/or cytokines, and that can be mediated by functions of mast cells that reside either at the site of the immune response or within lymph nodes.
Negative immunomodulatory functions in vivo
It has been reported that mast cells also have immunomodulatory functions that significantly reduce the magnitude or duration of the response. Hart et al.49 showed that the ability of ultraviolet B (UVB) irradiation of the skin to induce systemic immunosuppression of contact hypersensitivity (CHS) responses to the hapten 2,4,6-trinitrochlorobenzene was markedly reduced in (C57BL/6 × DBA/2)F1-KitW-f/W-f mice but was restored following mast-cell knock-in. Several lines of evidence suggested that histamine was a major mediator of this UVB-induced, mast cell-dependent effect. Although histamine can be made by basophils and other immune cells, as well as by mast cells, mast cells represent a major source of histamine in the normal skin of mice. Mast cells were probably also responsible for the finding that UVB irradiation suppressed delayed-type hypersensitivity (DTH) responses to allogeneic spleen cells in KitW-f/+ mice (which contain dermal mast cells) but not in KitW-f/W-f mice (which do not contain dermal mast cells)49.
Mast cells can also mediate negative immunomodulatory functions in vivo by producing IL-10. Mast cells and mast-cell derived IL-10 markedly limited the magnitude, and promoted the resolution, of both innate responses to chronic low-dose UVB-irradiation and of CHS responses induced in response to the hapten 2,4-dinitro-1-fluorobenzene (DNFB) or to urushiol, which is the hapten-containing sap of poison ivy or poison oak23. Mast-cell derived IL-10 was shown to limit multiple aspects of these responses, including the numbers of granulocytes, macrophages and T cells at the reaction sites, as well as the local tissue swelling, epidermal hyperplasia and, importantly, full thickness epidermal necrosis and ulceration. Although the mechanisms by which mast-cell-derived IL-10 (and other mast-cell mediators that are relevant in this setting) function to limit the tissue changes in these models remain to be fully defined, mast cells and mast-cell-derived IL-10 may influence these responses through a complex combination of direct and indirect effector and immunoregulatory functions (Figure 2).
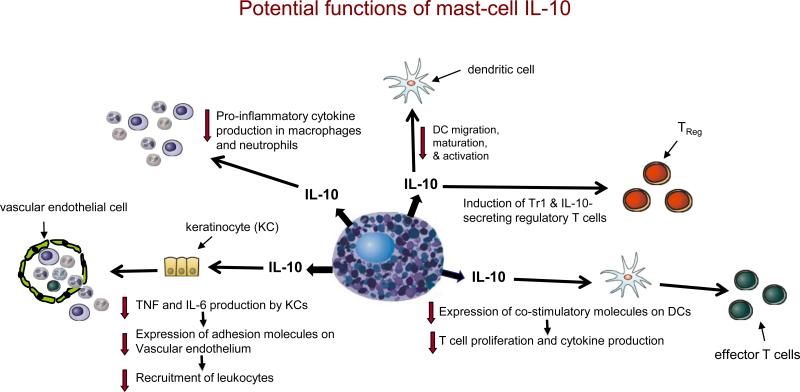
Figure 2. Potential functions of mast-cell-derived IL-10.
If produced in appropriate settings and amounts, mast-cell-derived interleukin-10 (IL-10) has the potential to promote the development of IL-10-secreting regulatory T cells (e.g., Tr1 cells and CD4+CD25+FOXP3+ T cells) and reduce DC migration, maturation and activation. It can also enhance the ability of DCs to reduce T-cell proliferation and cytokine production through the downregulation of costimulatory molecule expression by the DC. By directly inhibiting tumour-necrosis factor (TNF) and IL-6 production by keratinocytes, IL-10 can indirectly reduce the expression of adhesion molecules on vascular endothelial cells and thereby diminish the recruitment of circulating effector cells. IL-10 can directly inhibit the production of prostanoids by neutrophils and pro-inflammatory cytokines by macrophages. Although many of the specific functions indicated are based on evidence from in vitro studies of IL-10, mast-cell-derived IL-10 has been shown to mediate negative immunomodulatory functions in vivo.
Mast cells also contribute to two additional models of immunosuppression82,83. Depinay et al.82 reported that the bites of Anopheles mosquitoes can impair the development of antigen-specific T-cell responses in a model of DTH to ovalbumin in mice, but only if mast cells are present in the bitten skin. Lu et al.83 showed that mast cells were essential for the optimal induction of tolerance to skin allografts, which requires the participation of CD4+CD25+FOXP3+ regulatory T cells. Regulatory T cells are a source of IL-9, and IL-9 can mediate the suppression of alloreactive CD8+ T cells and act as a mast-cell survival and/or growth factor (reviewed in Ref.83). Local production of IL-9 (by regulatory T cells and/or other sources) may contribute to the development, and perhaps influence the function, of mast cells within the tolerant allografts.
How mast cells mediate negative immunomodulatory functions following Anopheles mosquito bites, or in peripheral tolerance to skin allografts, remains to be fully elucidated. However, IL-10 was implicated as a mechanism of immunosuppression in each of these studies, acting either in draining lymph nodes82 or at sites of skin allografts83. In addition, even though both Tr1 cells and CD4+CD25+FoxP3+ regulatory T cells can develop in vivo in the absence of IL-10 (Ref.84), it will be of interest to assess whether mast cells and mast-cell-derived IL-10 have important effects on regulatory T-cell numbers, phenotype and/or function in models of CHS or chronic UVB irradiation, or in other settings in which regulatory T cells are thought to have important roles84-86.
In summary, through the production of IL-10 and probably other products mast cells can exert negative immunoregulatory functions in vivo that can substantially limit the magnitude and/or duration of both certain acquired immune responses and the innate response to chronic irradiation with UVB light.
Conclusions
Here we show that mast cells can have both negative, as well as positive immunomodulatory functions, in addition to their well-established roles as effector cells. Understanding how individual positive or negative immunomodulatory functions can be induced or suppressed in various mast-cell populations will remain a topic of considerable interest. However, identifying and characterizing specific immunomodulatory roles of mast cells during particular immune responses in vivo may be quite challenging. For example, it already is clear that mast cells can have either positive or negative immunomodulatory functions in what would appear to be very similar settings, such as in different mouse models of CHS that employ different types and/or concentrations of haptens and/or different vehicles for administering these haptens5,13,60,87,88 (Figure 3). One may even speculate that in immune responses in which mast cells first promote the sensitization phase of the response, mast cells could then help to initiate the local inflammation that occurs when the host is subsequently exposed to specific antigen, and finally help to limit the extent of, and/or resolve, the ensuing inflammation and associated tissue pathology.
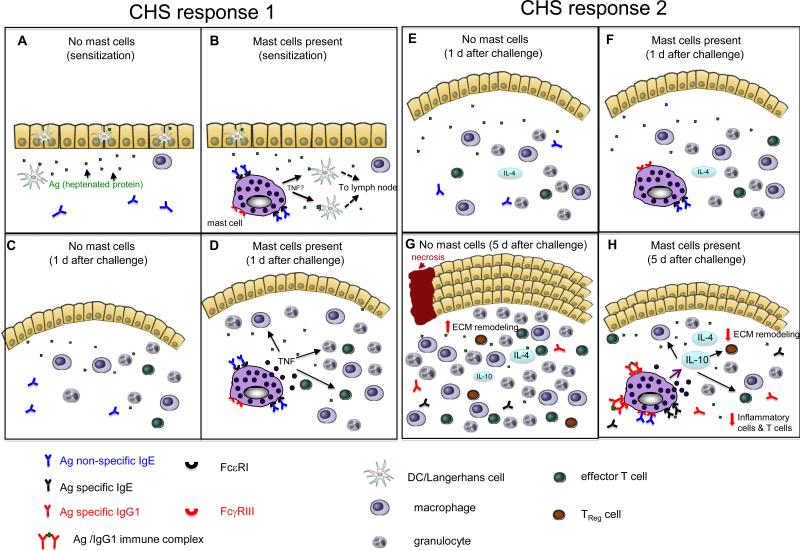
Figure 3. Hypothetical model of how mast cells might promote or limit features of different contact hypersensitivity responses.
(a-d) CHS response 1 is elicited by sensitizing mice epicutaneously on the abdomen with 2% oxazolone in 100% ethanol and challenging them on the ear 5 d later with 1% oxazolone in 100% ethanol. In comparison to reactions elicited in the absence of mast cells (a, c), mast cells promote migration of dendritic cells (DCs) from the site of cutaneous sensitization (b) and, 1 day after epicutaneous challenge (that is day 6 after sensitization), mast cells promote features of the effector phase of the response, such as swelling of the dermis (indicated in pink in the figure) and leukocyte recruitment, by directly or indirectly undergoing antigen-dependent activation and releasing mediators (including tumour-necrosis factor (TNF)) (d). In CHS response 1, mast cells have a net pro-inflammatory effect, by enhancing both the sensitization and the effector phases of the response. The ability of mast cells to enhance sensitization requires that the mast cells have antigen non-specific IgE bound to their FcεRI. The mechanism of mast-cell activation during the effector phase of the response is not clear. (e-h) CHS response 2 is elicited by sensitizing mice epicutaneously on the abdomen with DNFB (0.5% vol/vol) in 100% acetone and challenging them on the ear 5 d later with DNFB (0.2% vol/vol) in 100% acetone. (e,g) One day after epicutaneous challenge with a hapten (that is day 6 after sensitization), the ear swelling responses are similar in the presence (f) or absence (e) of mast cells. Therefore, mast-cell activation probably does not have a crucial role early after challenge. However, five days after epicutaneous challenge with hapten (g,h), when the mice have elevated circulating levels of antigen-specific IgG1 antibodies, mast-cell-derived interleukin-10 (IL-10) contributes to the ability of mast cells to limit the number of innate inflammatory cells and T cells, and tissue pathology, at the site of hapten challenge (h). Based on in vitro studies, we speculate that increased local expression of certain cytokines (such as IL-4) at the site of hapten challenge can increase mast-cell surface expression of low-affinity Fc receptor for IgG (FcγRIII), and perhaps have other effects on mast-cell phenotype and/or function. Such mast cells can then secrete higher levels of TNF and IL-10 following stimulation through their FcγRIII by immune complexes of specific antigen and IgG1 antibodies. In the absence of mast cells (and mast-cell-derived IL-10), the pathology associated with these contact hypersensitivity responses is substantially exacerbated and there is increased inflammation (including higher numbers of CD8+, CD4+, and CD4+CD25+ T cells), more marked thickening of the epidermis, more substantial increases in extracellular matrix (ECM) and other components of the dermis, as well as areas of full thickness epidermal necrosis and ulceration (g). In CHS response 2, mast cells do not appear to enhance sensitization (not shown) but have a net anti-inflammatory effect on the effector phase of the response.
In support of this hypothesis, both in vitro and in vivo data strongly suggest that one of the mechanisms that can promote mast-cell-dependent IL-10 production that in turn limits certain CHS responses is the activation of mast cells by immune complexes of specific antigen and IgG123. These antigen-specific IgG1 antibodies develop, probably by mast-cell-independent mechanisms, in response to the initial exposure to hapten during the sensitization phase of CHS23. Thus, in this model, the development of an aspect of the humoral component of the immune response to hapten challenge (the antigen-specific IgG1) results in the generation of a signal (the antigen-IgG1 immune complexes) that promotes an anti-inflammatory phenotype in the mast cells that are resident at the site of the local reaction (Figure 3h). The notion that mast cells might first promote the sensitization and/or elicitation phases of an immune response, and then help to limit or resolve the local tissue changes induced by antigen challenge, is consistent with the hypothesis that a key function of mast cells is to promote homeostasis — even in instances when mast cells also have a major role in perturbing homeostasis in order to promote host defence8-11.
It will be of interest to understand how, and under which circumstances, immunomodulatory functions of mast cells can influence the development, magnitude or kinetics of beneficial, or harmful, innate or acquired immune responses. The extent to which immunomodulatory effects of mast cells might contribute to some of the host–environment interactions that are thought to influence the development of the immune system (such as proposed in the hygiene hypothesis (reviewed in Ref.89)) should also be explored.
Finally, it will be important to assess whether positive or negative immunoregulatory functions of mast cells can be therapeutically manipulated. To give just one example, IL-10 is thought to contribute importantly to the effectiveness of antigen-specific immunotherapy (SIT) for allergic diseases, and successful SIT is associated with the development of strong antigen-specific IgG1 and IgG4 responses90. Aggregation of human IgG1 or IgG4 antibodies bound to surface FcγRI can activate mediator secretion in human mast cells that have been manipulated in vitro to upregulate FcγRI expression91. While it has not yet been reported whether IgG-dependent activation can induce secretion of IL-10 by human mast cells, it is tempting to speculate that during SIT in humans, as in CHS to urushiol or DNFB in mice, the phenotype and function of populations of mast cells is shifted from ‘net pro-inflammatory’ to ‘net anti-inflammatory’, and that this change in mast-cell phenotype reflects, at least in part, the ability of immune complexes of specific antigen and IgG to stimulate mast-cell IL-10 production. However, it remains to be seen whether this (or other) potential mast-cell-dependent immunoregulatory mechanisms actually contribute to the success of standard SIT, or might be harnessed in other settings to enhance immune responses that promote health or to suppress those that result in disease.
Box 1. Effector and immunomodulatory functions of mast cells: definitions and examples.
Effector functions
These functions include the physiological or pathological function of mast cells or the direct regulation of ‘non-immune’ cells, such as vascular endothelial cells, epithelial cells, fibroblasts, nerve cells, and muscle cells.
Examples
Promote clearance of pathogens by phagocytosis and/or secretion of antimicrobial peptides
Degrade potentially toxic endogenous peptides and components of venoms
Increase vascular permeability (for example, by histamine)
Stimulate bronchial smooth muscle-cell contraction (for example by leukotriene C4 (LTC4))
Promote fibroblast collagen synthesis (for example, by tryptase)
Immunomodulatory functions
These are effects on other immune cells (such as dendritic cells (DCs), T cells, B cells, monocytes/macrophages and granulocytes) and effects on structural cells (such as vascular endothelial cells, epithelial cells, smooth muscle cells) that alter their ability to influence immune cells.
Examples of positive immunomodulatory functions
Promote the migration, maturation, differentiation and function of immune cells via secretion of factors such as tumour-necrosis factor (TNF), chemokines, histamine, LTB4 and proteases.
Present antigen to T cells (via MHC class I or II molecules) or enhance antigen presentation by capturing IgE-bound-antigen via FcεRI and then undergoing apoptosis
Promote B cell IgE production (through IL-4, IL-13 and CD40L)
Promote expression of TSLP on epithelial cells (for example, by TNF, IL-4 and IL-13)
Promote recruitment of immune cells by production of TNF and other mediators that up-regulate adhesion molecule expression on vascular endothelial cells
Promote TH2 responses via effects of prostaglandin D2 on DC maturation
Promote airway smooth muscle production of chemokines & cytokines (via TNF, IL-4 and IL-13)
Examples of negative immunomodulatory functions
Suppress sensitization for contact hypersensitivity (via UVB-induced production of histamine)
Suppress cytokine production by T cells and monocytes (via IL-10)
Suppress production of pro-inflammatory cytokines and chemokines by keratinocytes (via IL-10)
Enhance ability of DCs to reduce T cell proliferation and cytokine production (via IL-10)
Supplementary Material
Acknowledgements
Supported by United States Public Health Service NIH grants to S.J.G. and an Australian National Health and Medical Research Council CJ Martin Fellowship to M.A.G. The authors regret that space limitations prevented the specific citation of the work of many authors who have contributed to this field. We thank Mark Larché for sharing his ideas about the possible role of mast cells in SIT.
Footnotes
Competing interest statement
The authors have no competing financial interests.
References
- 1.Galli SJ, et al. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 2.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol. Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- 3.Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007;19:31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 5.Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ. Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses. Curr Opin Immunol. 2006;18:751–760. doi: 10.1016/j.coi.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: the role of mast cells in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–739. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- 7.Malaviya R, Abraham SN. Mast cell modulation of immune responses to bacteria. Immunol. Rev. 2001;179:16–24. doi: 10.1034/j.1600-065x.2001.790102.x. [DOI] [PubMed] [Google Scholar]
- 8.Maurer M, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432:512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 9.Metz M, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 10.Piliponsky AM, et al. Mast cell degradation of neurotensin contributes to survival in a mouse model of sepsis. Nat Med. 2008;14:392–398. doi: 10.1038/nm1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med. 2007;204:2629–2639. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev. 2007;217:168–185. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 13.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 14.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 15.Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu. Rev. Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- 16.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol. Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat. Rev. Immunol. 2002;2:773–786. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 18.Ryan JJ, et al. Mast cell homeostasis: a fundamental aspect of allergic disease. Crit Rev Immunol. 2007;27:15–32. doi: 10.1615/critrevimmunol.v27.i1.20. [DOI] [PubMed] [Google Scholar]
- 19.Miller HR, Wright SH, Knight PA, Thornton EM. A novel function for transforming growth factor-beta1: upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific beta-chymase, mouse mast cell protease-1. Blood. 1999;93:3473–3486. [PubMed] [Google Scholar]
- 20.Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- 22.Grimbaldeston MA, et al. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 24.Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in Kit(W-sh) mice does not impair antibody-mediated arthritis. J Exp Med. 2007;204:2797–2802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chervenick PA, Boggs DR. Decreased neutrophils and megakaryocytes in anemic mice of genotype W/Wv. J Cell Physiol. 1969;73:25–30. doi: 10.1002/jcp.1040730104. [DOI] [PubMed] [Google Scholar]
- 26.Wastling JM, et al. Histochemical and ultrastructural modification of mucosal mast cell granules in parasitized mice lacking the beta-chymase, mouse mast cell protease-1. Am J Pathol. 1998;153:491–504. doi: 10.1016/s0002-9440(10)65592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 2000;192:1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence CE, Paterson YY, Wright SH, Knight PA, Miller HR. Mouse mast cell protease-1 is required for the enteropathy induced by gastrointestinal helminth infection in the mouse. Gastroenterology. 2004;127:155–165. doi: 10.1053/j.gastro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. J Exp Med. 2003;198:423–431. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 31.McNeil HP, Adachi R, Stevens RL. Mast cell-restricted tryptases: structure and function in inflammation and pathogen defense. J Biol Chem. 2007;282:20785–20789. doi: 10.1074/jbc.R700017200. [DOI] [PubMed] [Google Scholar]
- 32.Thakurdas SM, et al. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 33.Shin K, et al. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008;180:4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feyerabend TB, et al. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol Cell Biol. 2005;25:6199–6210. doi: 10.1128/MCB.25.14.6199-6210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scholten J, et al. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res. 2008;17:307–315. doi: 10.1007/s11248-007-9153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newlands GF, Miller HR, MacKellar A, Galli SJ. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N brasiliensis infection. Blood. 1995;86:1968–1976. [PubMed] [Google Scholar]
- 37.Brandt EB, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J. Clin. Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gekara NO, Weiss S. Mast cells initiate early anti-Listeria host defences. Cell Microbiol. 2008;10:225–236. doi: 10.1111/j.1462-5822.2007.01033.x. [DOI] [PubMed] [Google Scholar]
- 39.Soucek L, et al. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 40.Sun J, et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–3368. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98:1806–1818. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norris AA. Pharmacology of sodium cromoglycate. Clin Exp Allergy. 1996;26(Suppl 4):5–7. doi: 10.1111/j.1365-2222.1996.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 43.Mekori YA, Metcalfe DD. Mast cell-T cell interactions. J. Allergy Clin. Immunol. 1999;104:517–523. doi: 10.1016/s0091-6749(99)70316-7. [DOI] [PubMed] [Google Scholar]
- 44.Skokos D, et al. Mast cell-dependent B and T lymphocyte activation is mediated by the secretion of immunologically active exosomes. J. Immunol. 2001;166:868–876. doi: 10.4049/jimmunol.166.2.868. [DOI] [PubMed] [Google Scholar]
- 45.Kambayashi T, et al. Indirect involvement of allergen-captured mast cells in antigen presentation. Blood. 2008;111:1489–1496. doi: 10.1182/blood-2007-07-102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kashiwakura J, Yokoi H, Saito H, Okayama Y. T cell proliferation by direct cross-talk between OX40 ligand on human mast cells and OX40 on human T cells: comparison of gene expression profiles between human tonsillar and lung-cultured mast cells. J. Immunol. 2004;173:5247–5257. doi: 10.4049/jimmunol.173.8.5247. [DOI] [PubMed] [Google Scholar]
- 47.Nakae S, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176:2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 48.Frandji P, et al. Antigen-dependent stimulation by bone marrow-derived mast cells of MHC class II-restricted T cell hybridoma. J. Immunol. 1993;151:6318–6328. [PubMed] [Google Scholar]
- 49.Hart PH, et al. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J. Exp. Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jutel M, et al. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- 51.Nakae S, et al. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci U S A. 2005;102:6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xue L, et al. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175:6531–6536. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- 53.Theiner G, Gessner A, Lutz MB. The mast cell mediator PG D2 suppresses IL-12 release by dendritic cells leading to Th2 polarized immune responses in vivo. Immunobiology. 2006;211:463–472. doi: 10.1016/j.imbio.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 54.Gregory GD, Raju SS, Winandy S, Brown MA. Mast cell IL-4 expression is regulated by Ikaros and influences encephalitogenic Th1 responses in EAE. J Clin Invest. 2006;116:1327–1336. doi: 10.1172/JCI27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caughey GH. Mast cell tryptases and chymases in inflammation and host defense. Immunol Rev. 2007;217:141–154. doi: 10.1111/j.1600-065X.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevens RL, Adachi R. Protease-proteoglycan complexes of mouse and human mast cells and importance of their beta-tryptase-heparin complexes in inflammation and innate immunity. Immunol Rev. 2007;217:155–167. doi: 10.1111/j.1600-065X.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 57.Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991;87:446–453. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Ramos BF, Jakschik BA. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science. 1992;258:1957–1959. doi: 10.1126/science.1470922. [DOI] [PubMed] [Google Scholar]
- 59.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 60.Biedermann T, et al. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J Exp Med. 2000;192:1441–1452. doi: 10.1084/jem.192.10.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malaviya R, Abraham SN. Role of mast cell leukotrienes in neutrophil recruitment and bacterial clearance in infectious peritonitis. J Leukoc Biol. 2000;67:841–846. doi: 10.1002/jlb.67.6.841. [DOI] [PubMed] [Google Scholar]
- 62.Demeure CE, et al. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. J Immunol. 2005;174:3932–3940. doi: 10.4049/jimmunol.174.7.3932. [DOI] [PubMed] [Google Scholar]
- 63.Kakurai M, et al. Mast cell-derived tumor necrosis factor can promote nerve fiber elongation in the skin during contact hypersensitivity in mice. Am J Pathol. 2006;169:1713–1721. doi: 10.2353/ajpath.2006.060602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maurer M, et al. Skin mast cells control T cell-dependent host defense in Leishmania major infections. Faseb J. 2006;20:2460–2467. doi: 10.1096/fj.06-5860com. [DOI] [PubMed] [Google Scholar]
- 65.Suto H, et al. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 66.Nigrovic PA, et al. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc Natl Acad Sci U S A. 2007;104:2325–2330. doi: 10.1073/pnas.0610852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakae S, et al. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120:48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 68.Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109:3640–3648. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siebenhaar F, et al. Control of Pseudomonas aeruginosa skin infections in mice is mast cell-dependent. Am J Pathol. 2007;170:1910–1916. doi: 10.2353/ajpath.2007.060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reuter S, et al. Mast cell-derived tumour necrosis factor is essential for allergic airway disease. Eur Respir J. 2008;31:773–782. doi: 10.1183/09031936.00058907. [DOI] [PubMed] [Google Scholar]
- 71.Jawdat DM, Albert EJ, Rowden G, Haidl ID, Marshall JS. IgE-mediated mast cell activation induces Langerhans cell migration in vivo. J. Immunol. 2004;173:5275–5282. doi: 10.4049/jimmunol.173.8.5275. [DOI] [PubMed] [Google Scholar]
- 72.McLachlan JB, et al. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat. Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 73.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 74.Maurer M, et al. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J. Exp. Med. 1998;188:2343–2348. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Supajatura V, et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orinska Z, et al. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat Med. 2007;13:927–934. doi: 10.1038/nm1615. [DOI] [PubMed] [Google Scholar]
- 77.Matsuda H, et al. Necessity of IgE antibodies and mast cells for manifestation of resistance against larval Haemaphysalis longicornis ticks in mice. J. Immunol. 1990;144:259–262. [PubMed] [Google Scholar]
- 78.Robbie-Ryan M, Tanzola MB, Secor VH, Brown MA. Cutting edge: both activating and inhibitory Fc receptors expressed on mast cells regulate experimental allergic encephalomyelitis disease severity. J. Immunol. 2003;170:1630–1634. doi: 10.4049/jimmunol.170.4.1630. [DOI] [PubMed] [Google Scholar]
- 79.Kim YS, et al. Mast cells play a key role in the development of late airway hyperresponsiveness through TNF-alpha in a murine model of asthma. Eur J Immunol. 2007;37:1107–1115. doi: 10.1002/eji.200636612. [DOI] [PubMed] [Google Scholar]
- 80.Lee DM, et al. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 81.Chen R, et al. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J. Clin. Invest. 2001;108:1151–1158. doi: 10.1172/JCI11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Depinay N, Hacini F, Beghdadi W, Peronet R, Mecheri S. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. J Immunol. 2006;176:4141–4146. doi: 10.4049/jimmunol.176.7.4141. [DOI] [PubMed] [Google Scholar]
- 83.Lu LF, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 84.Maynard CL, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 85.Beissert S, Schwarz A, Schwarz T. Regulatory T cells. J Invest Dermatol. 2006;126:15–24. doi: 10.1038/sj.jid.5700004. [DOI] [PubMed] [Google Scholar]
- 86.O'Garra A, Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 87.Askenase PW, et al. Defective elicitation of delayed-type hypersensitivity in W/Wv and SI/SId mast cell-deficient mice. J Immunol. 1983;131:2687–2694. [PubMed] [Google Scholar]
- 88.Bryce PJ, et al. Immune sensitization in the skin is enhanced by antigen-independent effects of IgE. Immunity. 2004;20:381–392. doi: 10.1016/s1074-7613(04)00080-9. [DOI] [PubMed] [Google Scholar]
- 89.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 90.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 91.Woolhiser MR, Okayama Y, Gilfillan AM, Metcalfe DD. IgG-dependent activation of human mast cells following up-regulation of FcgammaRI by IFN-gamma. Eur J Immunol. 2001;31:3298–3307. doi: 10.1002/1521-4141(200111)31:11<3298::aid-immu3298>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 92.Jawdat DM, Rowden G, Marshall JS. Mast cells have a pivotal role in TNF-independent lymph node hypertrophy and the mobilization of Langerhans cells in response to bacterial peptidoglycan. J Immunol. 2006;177:1755–1762. doi: 10.4049/jimmunol.177.3.1755. [DOI] [PubMed] [Google Scholar]
- 93.Hochegger K, et al. Role of mast cells in experimental anti-glomerular basement membrane glomerulonephritis. Eur J Immunol. 2005;35:3074–3082. doi: 10.1002/eji.200526250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.