Abstract
At both empirical and theoretical levels, multiple functional roles of contextual information upon memory performance have been proposed without a clear dissociation of these roles. Some theories have assumed that contexts are functionally similar to cues, whereas other views emphasize the retrieval facilitating properties of contextual information. In Experiment 1, we observed that one critical parameter, the spacing of trials, could determine whether the context would function as a cue or as a retrieval cue for memories trained in different phases. Experiments 2 and 3 doubly dissociated these functions by selectively disrupting one role but not the other, and vice versa. Overall, these observations identify one determinant of different functions of contextual information and pose a major challenge to theories of learning that assume exclusively one or the other function of the context. Moreover, these data emphasize the importance of parametric variations on behavioral control, which has critical implications for studies designed to understand the role of the hippocampus in processing of contextual attributes.
Keywords: Pavlovian fear conditioning, contextual information, occasion setter, double dissociation, trial spacing
Two roles of the context in Pavlovian fear conditioning
By definition, learning theorists have always been interested in the determinants of behavioral control by a cue. While early work only focused on discrete cues (e.g., a tone or a flashing light), research conducted in the middle of the last century identified an important role for contextual information upon behavioral control (for a review, see Balsam & Tomie, 1985). By contextual information, we mean the environment in which the learning event takes place. For example, consider a Pavlovian fear conditioning experiment with rats as subjects. In such a situation, the physical chamber where punctate conditioned stimuli (CSs) and unconditioned stimuli (USs) are presented is said to be the context of learning, but contexts can also be determined by internal states (e.g., a drug state; Overton, 1985) or any other protracted attribute that is present on a given learning experience (e.g., an enduring odor). The perceived importance of the context in learning and memory phenomena grew in the middle of last century. This is evident from a simple literature search in Psych Info with “context” and “memory” as criteria present in the title. Starting with 1 article in 1953–7, the number has grown each five years to 123 in 2003–07. Clearly, attention to contextual attributes has grown impressively in the last 50 years.
Interest in the context grew on the basis of empirical findings that pointed to a role of the context as an aid for memory retrieval. That is, it became clear that some associations between punctate events are better retrieved with the aid of contextual stimuli. A well known example of this is state dependent retrieval, which is the decrement in memory performance that results from altering attributes of the internal context. In a classic example, human subjects were trained one day on memory tasks either sober or under the influence of alcohol. On the next day, they were tested either in the same or opposite state. Memory performance was better when the internal states were similar during both phases of the experiments (Goodwin, Powell, Bremer, Hoine, & Stern, 1969; also see Overton, 1964, for a demonstration with nonhuman animals). Similar effects with changes in physical contexts (e.g., outside a swimming pool or under water; Godden & Baddeley, 1975) have also been reported in humans. In these two cases, the memory seems to be available, but subjects appear to rely upon contextual cues to facilitate the retrieval of the target information. Numerous other contextual effects on retrieval using nonhuman animals such as rats were also documented (e.g., Klein, 1972). Common to all these observations is the idea that the context facilitates the retrieval of memories.
Theories of memory performance did not take long to account for this facilitative role of the context upon memory retrieval. For example, the encoding specificity principle advanced by Tulving and Thomson (1973) stated that contextual attributes are encoded as part of the memory representation, and that on a subsequent memory recall test these contextual attributes are critical for memory retrieval. Similarly, Spear articulated a position that strongly relies upon contextual attributes for successful memory retrieval (Spear, 1973; 1978). Recently, Bouton (1993) has extended this analysis by proposing that the dependence of memory retrieval upon contextual attributes is larger when a stimulus has more than one relationship with an outcome (e.g., when acquisition is followed by extinction so presumably the animal possesses both CS-US and CS-noUS associations). Moreover, he also extended the above-mentioned analysis to temporal attributes of the context, which expands in explanatory power by providing a heuristic explanation of long-term retention changes in memory performance such as recency-to-primacy shifts. Overall, these theoretical views all agree that the context is encoded as part of an association and plays a major role in memory retrieval. Thus, at least under certain circumstances, memory retrieval is dependent on the context, that is, the context has a facilitative role upon memory performance.
Although very few researchers would dispute the notion that the context plays a facilitative role in the retrieval of memories, the history of the field of associative learning and memory shows that this is not the only role that contextual stimuli can play. Contemporary with the observation of context-dependent retrieval, Rescorla (1968) conducted a study with rats in which he varied the contingency between a cue and the presentation of the outcome (shock). More specifically, he varied both the probability that the target CS was followed by shock and the probability that the shock was preceded by the CS. Rescorla observed that behavioral control decreased as the probability of the US in the presence of the CS was decreased, and as the probability of the US in the absence of the CS was increased. These findings are often cited because they pose a problem for simple contiguity theory in that they suggest that informational variables (i.e., contingency or relative predictiveness of the CS in the presence of the context) have a strong impact upon behavioral control by the CS. For purposes of the present discussion, it is important to note that Rescorla’s findings, together with Kamin’s observation of blocking (1968), gave rise to a new family of models that we will refer to as total-error correction models. These models, in order to account for Rescorla’s above-mentioned data, assumed that the context plays the role of another cue presented during a conditioning trial. That is, in order to explain the decremental effect of adding unsignaled USs to a given training regimen, these models posit that the context gains associative strength and this context-US association then competes with acquisition of the CS-US association (e.g., Mackintosh, 1975; Pearce & Hall, 1980; Rescorla & Wagner, 1972; Wagner, 1981). Comparator models make a similar assumption (Denniston, Savastano, & Miller, 2001; Gibbon & Balsam, 1981; Miller & Matzel, 1988; Stout & Miller, 2007), but instead of positing that the context prevents the formation of a CS-US association, these models assume that context-outcome associations interfere with performance governed by the CS at the time of testing. The idea that the context plays a role similar to that of punctate cues has received strong support in the associative literature. It explains trial massing effects (e.g., Barela, 1999), degraded contingency effects (Durlach, 1983; Urcelay & Miller, 2006), and US pre-exposure effects (Baker, Mercier, Gabel, & Baker, 1981), among other relevant phenomena. What is important for the present discussion is that the same (i.e., contextual) stimuli that have been proposed to facilitate the retrieval of the target memory are also assumed to compete with acquisition or expression of the target memory.
The central question that the present research was designed to address was: Why does the context sometimes facilitate the retrieval of memories and sometimes compete with memories for behavioral control? This question is important because, in the last 50 years, the notion of the context as a determinant of behavioral control has gained attention not only in the field of associative learning but also in the field of neuroscience (see Holland & Bouton, 1999); yet, we do not know why the context sometimes competes and sometimes facilitates retrieval of target memories. Of note, the idea that there is more than one potential role for contextual information is not new. In the book “Context and Learning”, edited more than 20 years ago (Balsam & Tomie, 1985), Balsam (Chapter 1) described several functions of the context, among which some are facilitative in nature (retrieval, occasion setting, response selection, summation) and others which are competitive in nature (competition, comparison, stimulus generalization). In the same volume, Miller and Schachtman (1985; Chapter 7) described the summation hypothesis of the role of the context (which assumes that the context plays a role similar to a punctate cue) and the hypothesis of contextual potentiation of retrieval of nominal CS-US associations. Although many researchers have described diverse functions of the context (e.g., Holland & Bouton, 1999), we will use these two (i.e., competitive and facilitative) as broad functions which may later be decomposed into multiple roles. But there is limited value in further describing different roles given that we have not yet been able to identify the variables that determine which of these two basic functions will be evidenced. The research presented here is an attempt to dissociate these two fundamental roles of the context.
In an effort to better understand the factors that determine the roles of the context, Balaz, Capra, Hartl, and Miller (1981) observed that a context could facilitate behavioral control by a cue that received training in that context (Experiment 1), while at the same time noting that such a context would not increase responding to another cue trained in a different context, thus precluding that the effect observed was due to context-US associations summating with target CS-US associations. In other words, they observed that the context could facilitate retrieval of a target CS-US association without direct context-US associations being responsible for such facilitated retrieval. Additionally, in Experiment 2 they increased the shock intensity in an attempt to enhance context-US associations and saw that responding to a target cue trained in a different context was also related to the strength of context-US associations after these parametric variations. However, posttraining exposure to the context alone (i.e., context extinction) or pretraining exposure to the context alone (i.e., latent inhibition to the context; Balaz, Capra, Kasprow, & Miller, 1982) decreased the potential of the context to summate with CS-US associations, while leaving intact the potential of the context to facilitate retrieval of the CS-US association acquired in that context. Taken together, these experiments suggest that the context may have more than one role: summation with the target CS and retrieval facilitation of a CS independent of summation.
One approach to appreciate the dual nature of contextual function is to look at a paradox in the Pavlovian conditioning literature resulting from two seemingly similar treatments. One such treatment is US preexposure prior to CS-US pairings (Baker et al., 1981). Consistent with the assumption that the context plays the role of a cue and enters into associations with the US, US preexposure ordinarily retards acquisition of behavioral control by a target cue. Moreover, consistent with an explanation of this effect in terms of blocking of the target CS by the context, signaling the US alone exposures with an irrelevant CS alleviates the US preexposure effect, as if the signal overshadowed the context, thus preventing the context from blocking behavioral control by the target cue (Baker et al., 1981). Similar effects have been observed by signaling the added US exposures of degraded contingency treatments in which the added US exposures are delivered interspersed among the CS-US training trials (Durlach, 1983; Witnauer & Miller, 2007). In contrast to preexposure, the second treatment is proactive interference. Interference effects first gained attention in the human verbal learning literature (Underwood, 1969) and only recently have received attention from a Pavlovian conditioning perspective. In proactive interference, a CS1-US association trained during a first phase interferes with the expression of a second CS2-US association trained in a second phase (e.g., Amundson, Escobar, & Miller, 2003; Escobar, Matute, & Miller, 2001). Because this is an example of interference between two cues that are never trained together, one would want to make sure that it is the CS1-US association (and not mere exposure to the CS1 or the US) which causes proactive interference. Hence, one obvious control condition for proactive interference is a group that experiences unpaired CS1 and US exposures in the first phase. This unpaired control results in higher responding to CS2 than the group that received CS1-US pairings during the first phase. These two effects (US preexposure and proactive interference) both depend on the status of the context; both effects wane if the two phases occur in different contexts. The context dependence of the US preexposure has been observed in an eyelid conditioning preparation with rabbits (Hinson, 1982), in an autoshaping preparation with pigeons (Tomie, 1976) and in fear conditioning with rats (Matzel, Brown & Miller, 1987; Matzel, Schachtman & Miller, 1988;; Randich & Ross, 1984). The contextual dependence of proactive interference has recently been reported in fear conditioning with rats by Amundson and Miller (2008; also see Escobar, Matute & Miller, 2001, for a similar observation in retroactive interference with both changes in physical contexts and manipulations involving priming stimuli). Critically, in the US preexposure effect the context seems to function as a competing cue, whereas in proactive interference it links the two memories so that the CS1-US association from Phase 1 can proactively interfere with the CS2-US association of Phase 2. We discuss this in more detail later. The paradox is that, in the US preexposure effect, signaling the USs (that were not paired with the target cue) with an unrelated cue increases responding to the target, whereas in proactive interference signaling these USs decreases responding to the target.
We undertook a literature survey aimed at understanding the parametric differences that result in this seeming paradox. One feature of note is that the common control condition for proactive interference is a group that receives unpaired CS1 and US presentations during Phase 1 and, after CS2-US pairings in Phase 2, exhibits high levels of responding to CS2. However, these unpaired presentations in Phase 1 do not result in a large US preexposure effect presumably because the US presentations are widely distributed which allows extinction of the context to occur between US presentations. Consistent with this assertion, we systematically found across different experiments that to obtain a reliable proactive interference effect the optimal trial spacing is distributed, whereas to obtain a US preexposure effect massed trial spacing is optimal. Figure 1 illustrates how the effect of signaling the US is differentially affected by whether the trials are distributed or massed. This may explain why in some cases a US preexposure effect is observed (i.e., weak responding to the CS) and in other cases no response diminution is seen, as in the control condition for proactive interference (i.e., strong responding to the CS). For example, Amundson et al. (2003) observed proactive interference with 5-s CSs, and a mean intertrial interval (ITI) of 40 min, whereas Urushihara and Miller (2006) observed a reliable US preexposure effect with 30-s CSs and a mean ITI of 9 min. Using a metric that captures the relationship between total CS exposure versus context exposure (C/T, Gibbon & Balsam, 1981), the former example results in a C/T of 480 (i.e., widely spaced), whereas the later results in a C/T of 18 (i.e., relatively massed). Based on this logic, we hypothesized that varying only the spacing of trials during Phase 1 training would make the context either a cue that could block behavioral control by the target trained in Phase 2 or a memory facilitator that could retrieve the Phase 1 (CS1-US) memory and allow it to interfere with the expression of Phase 2 (CS2-US) memory. More precisely, massed US alone treatment in Phase 1 should result in reliable context conditioning, which should block behavioral control by the target during Phase 2. Context conditioning should be abolished by signaling the US exposures during Phase 1. Conversely, spaced Phase 1 training should make the context a memory facilitator that retrieves the Phase 1 [CS1-US] training and allows it to proactively interfere with that of Phase 2 [CS2-US]. Under these circumstances, unpaired presentations of the CS and US during Phase 1 should results in a weak US preexposure effect, presumably due to extinction of the context-US association during the long ITIs in which the context alone is presented. These predictions were assessed in Experiment 1.
Figure 1.
A paradoxical interaction that occurs depending on the spacing of trials during Phase 1 and the effect of signaling the USs in Phase 1. With massed trials, US alone exposures decrease behavioral control by the cue trained in Phase 2 (i.e., the US preexposure effect). Such a decrement is alleviated by signaling those US exposures. With spaced trials the opposite is true. No decrement is observed after US alone exposures, and signaling those trials decreases responding to the target (i.e., proactive interference).
Experiment 1
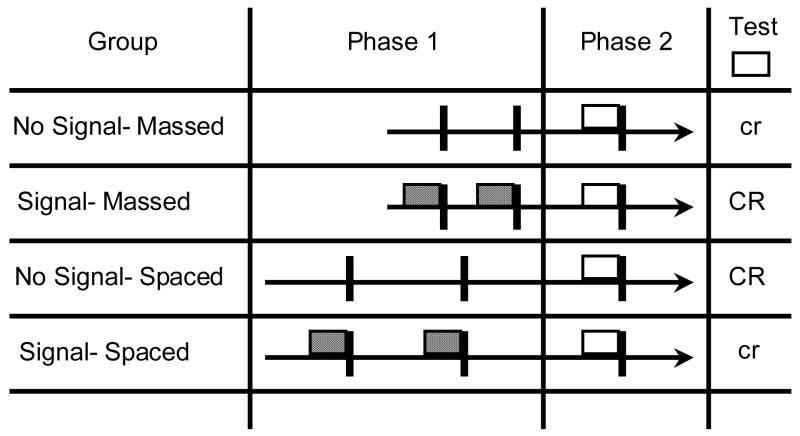
In Experiment 1 we used a 2 × 2 factorial between-subjects design with US signal (Signal vs. NoSignal) and trial spacing (Massed vs. Spaced) as factors. Two of the groups (Condition NoSignal) experienced 12 outcome alone exposures (+) in Phase 1, and the remaining two groups experienced 12 outcome presentations signaled by an irrelevant cue A (A+; Condition Signal). Moreover, these groups were orthogonally given massed (10-min sessions) or spaced (120-min sessions) Phase 1 training. In Phase 2, all groups received two pairings of the target CS (X) with the US. We anticipated that spaced treatment in Phase 1 would discourage direct conditioning of the context and favor the context becoming a memory facilitator; thus signaled outcome (A+) presentations would decrease responding to the target cue X (i.e., proactive interference). In contrast, massed Phase 1 treatment should favor the context becoming a cue that competes with the target in Phase 2 (US preexposure). Consequently, signaling the outcome alone exposures should increase responding to the target presumably because the signal overshadows the context, leaving it unable to compete with the target. Table 1 shows the design and predictions of Experiment 1.
Table 1.
Design of Experiment 1
| Group | Phase 1 | Phase 2 | Exp Test X |
|---|---|---|---|
| NoSignal-Massed | 12 + | 2 X+ | cr |
| Signal-Massed | 12 A+ | 2 X+ | CR |
| NoSignal-Spaced | 12 + | 2 X+ | CR |
| Signal-Spaced | 12 A+ | 2 X+ | cr |
Note: + = footshock. Numbers next to the stimuli indicate number of trials. Stimulus X was a 20-s white noise and A was a 20-s clicker. Phases 1 and 2 were conducted in one context (Training) and the rest of the treatments in a different context (Test). Group labels refer to the presence of A during Phase 1 and the spacing of trials. Expectations (Exp) are based on previous experiments investigating the proactive interference and US preexposure effects. CR = strong conditioned response; cr = weak conditioned response.
Method
Subjects
The subjects were 24 female and 24 male Sprague-Dawley descended, experimentally naïve, young adult rats, bred in our colony. Body weight range for the females was 179–248 g and for the males was 286–371 g. Subjects were individually housed and maintained on a 16-hr light/8-hr dark cycle with experimental sessions occurring roughly midway through the light portion. All subjects were handled for 30 s three times per week from weaning until the initiation of the study. Subjects had free access to food in the home cage. One week prior to initiation of the experiment, water availability was progressively reduced to 20 min per day, provided approximately two hours after any scheduled treatment.
Apparatus and stimuli
Two contexts were used in this experiment (Chamber V and Chamber R). Chamber V was 27-cm long, 29.5-cm high, 21.5-cm wide at the top, and 5.5-cm wide at the bottom. The ceiling was clear Plexiglas, the front and back walls were black Plexiglas, and the side walls were stainless steel. The floor was comprised of two 27-cm long plates, 2-cm wide, with a 1.5-cm gap between the two plates. A 0.6-mA, 0.5-s constant-current footshock, produced by a high voltage AC circuit in series with a 1.0-MΩ resistor could be delivered through the metal walls and floor of the chamber. Each of six copies of Chamber V was housed in a separate sound- and light-attenuating environmental isolation chest. Each chamber was illuminated by a 7-W (nominal at 120 VAC, but driven at 60 VAC) incandescent light bulb, which was mounted on the inside wall of its environmental enclosure, approximately 30-cm from the center of the experimental chamber. Light entered the chamber primarily by reflection from the ceiling of the environmental chest.
Chamber R was rectangular, measuring 24.0 × 9.0 × 12.5-cm (l × w × h). The walls and ceiling of Chamber R were clear Plexiglas, and the floor comprised of stainless steel rods measuring 0.5-cm diameter, spaced 1.5-cm apart (center to center). The rods were connected by NE-2 bulbs, which allowed for the delivery of 0.6-mA, 0.5-s constant-current footshock. Each of six copies of Chamber R was housed in separate light- and sound-attenuating environmental isolation chambers. Each chamber was dimly illuminated by a 2-W (nominal at 120 VAC, but driven at 60 VAC) incandescent house light mounted on an inside wall of the environmental chest located approximately 30-cm from the animal enclosure. The light intensities inside the two types of chambers were approximately equal due to the difference in opaqueness of the walls of Chambers V and R.
Each chamber could be equipped with a water-filled lick tube that extended 1-cm into a 5 cm deep cylindrical niche, which was 4.5-cm in diameter, left-right centered on a narrow wall, with its bottom 1.75-cm above the floor of the apparatus. There was a photobeam 1-cm in front of the lick tube that was broken whenever the subject licked the tube. Two 45-Ω speakers on the inside walls of the isolation chests could deliver either a white noise 6 dB (C-scale) above background (76 dB, produced mainly by a ventilation fan) or a 6-Hz click train 6 dB above background. The 0.6-mA footshock served as the US. In this experiment, the white noise and click CSs were 20-s in duration, and the footshock US was 0.5 s in duration. For all animals, the click served as CS A, the white noise served as CS X.
Procedure
Subjects were randomly assigned to one of four groups counterbalanced for sex (ns= 12). Phases 1 and 2 were conducted in one [training] context (V or R) and all other treatments in the remaining [test] context in a counterbalanced manner within groups. This was done to minimize differential fear of the test context summating with fear to the CS.
Acclimation
Before the beginning of training, one day of preexposure to the test context was conducted. Subjects were exposed to the experimental chamber during a 45-min session with the water lick tubes available.
Phase 1
Prior to Phase 1, the lick tubes were removed from the training chambers. On Days 2–4, subjects in Condition Spaced received 4 daily US alone (condition NoSignal) or 4 daily A-US trials (condition Signal) in the training context within a 120-min session. The mean ITI was 24 min. Two different schedules were used on alternate days. In schedule 1, US presentations occurred at 10, 30, 80, and 115 min into the 120-min session. In schedule 2, these trials occurred 5, 54, 89, and 110 into the 120-min session. Subjects in the Massed condition received a similar treatment but within a 10 min session. The mean ITI was 2 min. For these subjects, signaled or unsignaled trials (US presentations) on schedule 1 occurred at 3, 4.5, 6, and 9 min. into the session. Trials in schedule 2 occurred at 3, 6, 7.5, and 9 min into the session. Schedule 1 was used on Days 2 and 4 and schedule 2 was used on Day 3.
Phase 2
On Day 5, all subjects experienced 2 X-US pairings within a 120-min session in the training context. Stimulus onset occurred at 30 and 90 min into the session. Critically, the 120-min of context exposure during Phase 2 should not have appreciably influenced behavior in our preparation because preliminary studies indicated that at least 480 min of context exposure are needed to influence responding to CS X in the absence of Phase 1 treatment.
Reacclimation
On Days 6 and 7, the lick tubes were reinserted and subjects were allowed to drink during daily 45-min sessions in the test context. This treatment was intended to restabilize baseline levels of drinking. These sessions did not include any nominal stimulus presentations.
Test
On Day 8 with the lick tubes inserted in the test context, all subjects were tested for suppression to X during a 16-min session. This was accomplished by presenting CS X immediately on completion of the first 5 cumulative seconds of licking (as measured by the total amount of time the infrared photobeam was disrupted). Thus, all subjects were drinking at the time of CS onset. Time to complete this initial 5 cumulative seconds of licking (pre-CS scores) and time to complete 5 additional seconds after the onset of the test CS (CS score) were recorded. A 15-min ceiling was imposed on CS scores.
Data Analysis
Following the convention of our laboratory, subjects that took more than 60 s to complete their first 5 cumulative seconds of licking (i.e., prior to CS onset) on the test day, thereby exhibiting an unusual reluctance to drink in the test context, were eliminated from all analyses. In Experiment 1, no rat met this criterion. Suppression data were transformed to log (base 10) scores to better approximate a normal distribution of scores within groups, thereby facilitating the use of parametric statistics. An alpha level of.05 was adopted for all statistical tests. The transformed data were analyzed with a factorial analysis of variance (ANOVA). When appropriate, we report effect size calculated using the algorithm provided byMyers and Well (2003, p. 210).
Results and Discussion
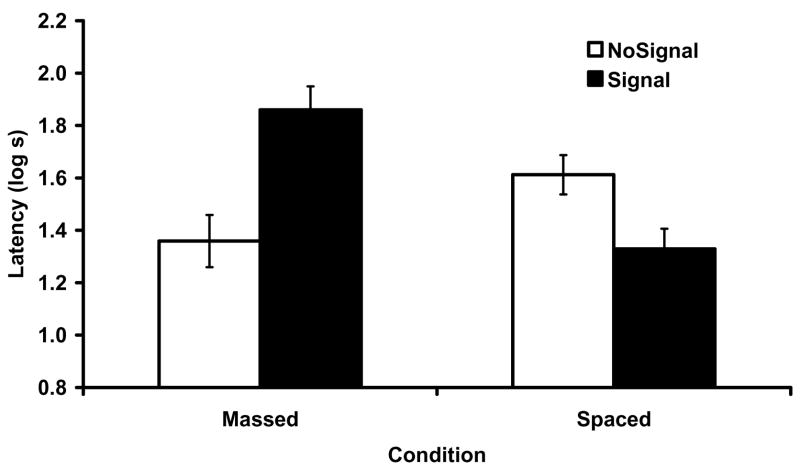
Figure 2 shows the results of the test of the target CS X. As can be seen, subjects that experienced the US alone in short sessions during Phase 1 (i.e., unsignaled massed trials) showed weak conditioned suppression to X, reflecting a US preexposure effect that resulted either from blocked acquisition during Phase 2 (Rescorla & Wagner, 1972) or impaired retrieval of the target X-US association at test (Stout & Miller, 2007). This decrement was alleviated if the Phase 1 US presentations were signaled by a nontarget cue. As predicted, the opposite pattern was observed when Phase 1 of training was conducted with spaced trials. With these parameters, US alone exposures had less of an effect on responding to X, but signaling those (otherwise ineffective) US exposures with the nontarget A resulted in proactive interference to the target. Thus, the effect of the signal A upon behavioral control by the target was reversed as a function of trial spacing in Phase 1. These observations were supported by the following statistical analyses.
Figure 2.
Mean suppression ratios to test presentations of X in Experiment 1. Error brackets depict the standard error of the mean. A US preexposure was obtained with massed Phase 1 trials, and this was alleviated by signaling those trials. The opposite happened with spaced trials. Less decrement is observed with spaced US preexposure (relative to the massed condition) and a proactive interference emerges after signaling the US alone exposures.
A 2 (Spacing: Massed vs. Spaced) × 2 (Signal: Signal vs. NoSignal) ANOVA conducted on the transformed latencies to begin drinking (i.e., prior to CS onset) in the testing context did not reveal any main effects or interactions, thus suggesting similar baselines across all groups (smallest p =.19). A similar ANOVA conducted on the transformed latencies to resume drinking in the presence of the target CS did not reveal any main effects but did detect a crossover interaction, F(1, 44) = 20.58, p <.01, MSE = 0.09, Cohen’s f = 0.63. The crossover interaction suggests opposite behavioral effects of signaling the US depending on the spacing of the Phase 1 trials. This interaction was decomposed with planned comparisons using the overall error term from the omnibus ANOVA. These comparisons revealed that with massed Phase 1 trials, signaling the Phase 1 US exposures recovered the otherwise low responding to the target X that resulted from unsignaled US preexposure. In other words, Group Signal-Massed responded more than Group NoSignal-Massed, F(1, 44) = 16.81, p <.01, Cohen’s f = 0.57. Moreover, a planned comparison revealed more responding in Group NoSignal-Spaced than in Group NoSignal-Massed, which suggests an attenuation of the US preexposure effect when Phase 1 training was spaced, F(1, 44) = 4.28, p <.05, Cohen’s f = 0.26. This result is entirely consistent with an analysis of the context in terms of its cue properties. Thus, we observed a reduction in the US preexposure effect when we signaled the USs or when we widely spaced the Phase 1 trials. Consistent with an analysis of the context in terms of memory facilitating properties, Group Signal-Spaced responded less than Group NoSignal-Spaced, F(1, 44) = 5.36, p <.05, Cohen’s f = 0.30, which suggests that signaling the otherwise ineffective Phase 1 US exposures decreased responding to the target, presumably because the context retrieved the representation of the A-US memory which proactively interfered with acquisition or expression of the target association.
The present pattern of results leads to several important conclusions. First, the signaling of Phase 1 US exposures can have opposite effects based on the spacing of the US presentations. Second, because both of these decremental phenomena (the US preexposure effect and proactive interference) appear to be context dependent, the context is apparently playing different roles in each effect. In the US preexposure effect the context is acting as a cue, and therefore US preexposure in a different context should attenuate the effect (Randich & Ross, 1984). In the case of proactive interference, the commonality of the context between Phases 1 and 2 allows the representation of Phase 1 association (A-US) to proactively interfere with responding to the target X based on the X-US association acquired in Phase 2. Proactive interference has been shown to wane if the context is changed between Phases 1 and 2 (Amundson & Miller, 2008; Experiment 2). Thus, both effects are context dependent because both are attenuated if the context between Phases 1 and 2 is changed. However, the large difference in context exposure that each effect necessitates (US preexposure is better observed with massed Phase 1 trials and proactive interference is better observed with spaced Phase 1 trials) suggests that the roles of the contexts are functionally dissimilar. One approach to testing this proposition is to double dissociate them. This was the purpose of Experiments 2 and 3.
Experiment 2
In Experiment 2, we wanted to assess if the two hypothesized functions for the context in the US preexposure effect (context as a cue) and proactive interference effect (context as a facilitator of interference) could be dissociated. By dissociation here we mean: Is there one manipulation of the context that will attenuate one effect without similarly altering the other effect, and another manipulation that will attenuate the other effect without similarly altering the first? Therefore, in Experiment 2 we chose a manipulation that we anticipated would affect the properties of the context as a cue, but not as a memory facilitator.
Latent inhibition (Lubow & Moore, 1959), the retarded emergence of behavioral control observed when the target cue has undergone nonreinforced preexposure, is a phenomenon observed across many species and preparations. There are several explanations of the latent inhibition phenomenon, some of them attentional in nature (e.g., Lubow, 1989) and some of them associative (e.g., Miller & Matzel, 1988). Regardless of both the theoretical interpretation of the phenomena and the fact that this effect has been investigated primarily with punctate cues, there are some observations that suggest that contexts can also undergo latent inhibition with sufficient preexposure (e.g., Cole, Van Tilburg, Burch-Vernon, & Riccio, 1996). Moreover, above we cited evidence reported by Balaz et al. (1981) suggesting that with select parameters of US intensity, the context can become a cue which elicits responding that summates with responding to any nominal CS (as opposed to simply facilitating retrieval of a nominal CS-US association). Balaz et al. (1982) also observed that context preexposure (i.e., latent inhibition) decreased the role of these context-US associations. Finally, Hinson (1982; Exp 3) observed that context preexposure alleviated the US-preexposure effect using rabbits in an eyelid conditioning preparation. Thus, evidence suggests that contexts can undergo latent inhibition and that this treatment can decrease the potential of the context to act as nominal cue. Critical for Experiment 2 are the observations by Oberling, Gunther, and Miller (1999), in which they saw that massive preexposure to punctate stimuli would not affect the potential of the stimulus to act as an occasion setter1. Thus, in Experiment 2 we tested the hypothesis that, if the context is acting as a cue in the US preexposure effect, then massive preexposure to the context should attenuate the US preexposure effect. Conversely, if the context plays the role of a memory facilitator in the proactive interference effect, then massive preexposure to the context should not have a strong impact on proactive interference.
In Experiment 2, we used a 2 × 2 factorial design in which two groups of subjects received proactive interference treatment (Phase 1 containing signaled USs with long ITIs) and two groups of subjects received US preexposure treatment (Phase 1 containing unsignaled USs with short ITIs). One group from each of these two conditions received massive (10 hours) preexposure to the context. We expected that the latent inhibition treatment of the context would only affect the role of the context as a cue and therefore selectively decrease the US preexposure effect but not the proactive interference effect. In proactive interference, the context presumably plays a role akin to a memory facilitator that mediates interference. Consequently, based on the data of Oberling and colleagues (1999), we did not expect that latent inhibition would affect proactive interference. The experimental design with these predictions is summarized in Table 2.
Table 2.
Design of Experiment 2
| Group | Latent Inhibition | Phase 1 | Phase 2 | Exp Test X |
|---|---|---|---|---|
| Control-PI | Handling | 12 A+ (S) | 2 X+ | cr |
| LI-PI | 600 min | 12 A+ (S) | 2 X+ | cr |
| Control-US | Handling | 12 + (M) | 2 X+ | cr |
| LI-US | 600 min | 12 + (M) | 2 X+ | CR |
Note: + = footshock. Numbers next to the stimuli indicate number of trials. Group labels refer to context preexposure (LI vs. Control) and Phase 2 treatment (PI [proactive interference] vs. US [US preexposure]). Phases 1, 2, and 3 were conducted in one context (Training) and all other treatments in the remaining context (Test). S = Spaced, M = Massed. Expectations (Exp) = expectations about conditioned responding to the target X. CR = strong conditioned response; cr = weak conditioned response.
Method
Subjects
The subjects were 24 female (187 – 260 g) and 24 male (280 – 362 g), experimentally naïve, Sprague-Dawley descended rats obtained from our own breeding colony. They were randomly assigned to one of four groups (ns = 12), counterbalanced within groups for sex. Subjects were individually housed, maintained, and water deprived in a similar manner to Experiment 1.
Apparatus and stimuli
The apparatus and stimuli were the same as those used in Experiment 1.
Procedure
Subjects were randomly assigned to one of four groups based on the Phase 2 treatment (Proactive interference [PI] or US preexposure [US]) and on the experimental manipulation of the training context in Phase 1 (Latent inhibition [LI] vs. Control). Phases 1, 2, and 3 were conducted in one [training] context (V or R chamber) and all other treatments in the remaining [test] context in a counterbalanced manner within groups.
Acclimation
Before the beginning of training, a one-day, 45-min, pre-exposure session to the test context was conducted as in Experiment 1.
Latent Inhibition
Prior to Phase 1 the lick tubes were removed from the training chambers. On Days 2–5 subjects in Condition LI experienced massive daily exposure to the training context (150 min) in order to decrease the potential of the context to become conditioned with the US, and subjects in the Condition Control were given equivalent handling each day. Specifically, subjects in this condition were loaded into the cart used to carry the animals from the colony to the experimental room, brought to the door of the experimental room, taken out of the cart, immediately placed back into the cart, and brought back to their cages in the colony. Thus, Condition LI received a total of 10 hours of exposure to the training context, and subjects in Condition Control got no exposure at all. For subjects in the LI condition, the sound attenuating enclosures were opened every 50 min to ensure that the subjects were awake.
Phase 1
On Days 6–8, subjects in Condition Proactive Interference (PI) received four daily A-US pairings in the training context within a 120-min session. Trials occurred 10, 30, 80, and 115 min into the session. Subjects in the US Preexposure condition (US) received four unsignaled trials (US presentations) that occurred 3, 4.5, 6, and 9 min into a 10-min session.
Phase 2
On Day 9 all subjects experienced two X-US pairings within a 120-min session in the training context. Stimulus onset occurred 30 and 90 min into the session.
Reacclimation
On Days 10 and 11, the lick tubes were reinserted and subjects were allowed to drink during daily 45-min sessions in the test context as in Experiment 1.
Test
On Day 12 with the lick tubes inserted in the test context, all subjects were tested for suppression to X as was done in Experiment 1. One rat in Group Control-PI group was eliminated from all analyses because of a pre-CS score greater than 60 s.
Results and Discussion
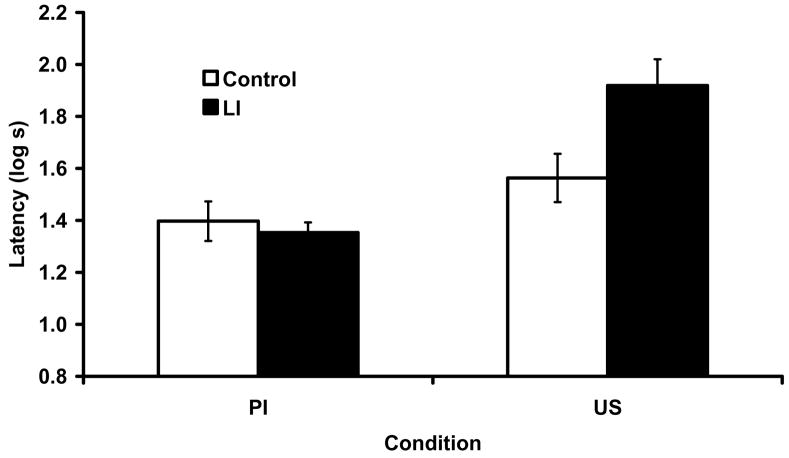
The suppression data from Experiment 2 are shown in Figure 3. The groups in the Control condition that received proactive interference and US preexposure showed weak responding to the target. Importantly, massive latent inhibition of the training context selectively decreased the US preexposure effect without affecting the proactive interference effect. Thus, this experiment complements the findings of Experiment 1 by showing that we were able to manipulate the role of the context as a competitor cue (thereby alleviating the US preexposure effect), but not as memory facilitator. These observations were supported by the following statistical analysis.
Figure 3.
Mean suppression ratios to test presentations of X in Experiment 2. Error brackets depict the standard error of the mean. Prior exposure to the context alone alleviated the US preexposure effect, but had no effect on the proactive interference effect.
A 2 × 2 factorial ANOVA with context preexposure (Latent Inhibition vs. Control) and treatment (Proactive Interference vs. US Preexposure) as main factors was conducted on the transformed latencies to start drinking water in the test context. This analysis did not reveal any main effects or an interaction (smallest p =.20). A similar ANOVA conducted on the transformed latencies to resume drinking in the presence of the target CS revealed a marginal main effect of context preexposure, F(1, 44) = 3.63, p =.06, MSE = 0.08, Cohen’s f = 0.24, a main effect of Phase 2 treatment, F(1, 43) = 20.09, p <.01, MSE = 0.08, Cohen’s f = 0.63, and an interaction, F(1, 43) = 5.98, p <.01, MSE = 0.08, Cohen’s f = 0.32. The detection of both main effects together with the interaction suggests that the interaction is driven by a difference between groups at one level of one of the independent variables but not the other. To confirm this pattern, we conducted planned comparisons which revealed that context latent inhibition treatment alleviated the US-preexposure effect in that Group LI-US suppressed more than Control-US, F(1, 43) = 9.69, p <.01, MSE = 0.08, Cohen’s f = 0.42, but had no effect on proactive interference. Groups LI-PI and Control-PI did not differ F(1, 43) = 0.14, p =.70, MSE = 0.08. Moreover, to determine if latent inhibition selectively reduced the US-Preexposure effect but not Proactive Interference, we compared the two groups that received the Latent Inhibition treatment. This comparison revealed that Group LI-US suppressed more than Group LI-PI, F(1, 43) = 24.55, p <.01, MSE = 0.08, Cohen’s f = 0.70. Thus, as expected, the two roles of the context are differentially affected by the latent inhibition manipulation.
The results of Experiment 2 suggest that the observations of Experiment 1, in which we saw that the addition of a signal for the US and an increase in trial spacing in Phase 1 had opposite effects upon expression of the X-US association learned from Phase 2, are mediated by contextual attributes that play fundamentally different roles upon the expression of the X-US association. By massively preexposing subjects to the training context, we were able to reduce the US preexposure effect without any appreciable change in proactive interference. The aim of Experiment 3 was to further dissociate these two separate roles by conducting a manipulation that would selectively decrease the proactive interference effect without significantly changing the US preexposure effect.
Experiment 3
Experiment 3 was designed to further dissociate the two roles of the context that we observed in Experiment 1. Our working hypothesis was that in the US preexposure effect, the context plays the role of a cue like any other punctate stimulus, thereby blocking behavior controlled by the X-US association. Conversely, we hypothesized that in the proactive interference effect the context allows the Phase 1 association (A-US) to proactively interfere with expression of X-US association learned during Phase 2. Thus, the context bridges the two memories and allows one memory to (proactively) interfere with expression of the other memory. As we mentioned in the introduction, Bouton (1993) has proposed that contexts may be analyzed in terms of both physical and temporal attributes. In Bouton’s analysis, as time passes after a learning experience, the context provided by both internal and external states changes so the passage of time on itself represents a context change (Bouton, in press). A prediction that can be derived from this approach is that changes in temporal and physical attributes of the context should alleviate interference. This is supported by a recent experiment in which Amundson and Miller (2008) observed that proactive interference depended critically on the two conflicting memories sharing some attributes. For example, when they conducted Phase 1 training in a different physical context, the proactive interference effect was alleviated. Similarly, Escobar et al. (2001; Experiment 1b) observed that retroactive interference (a decrement similar to the proactive interference observed here, with the only difference being that the phases of the interfering and target memory are reversed) was alleviated when a 14-day retention interval was interposed between training the target and interfering memories. Based on this interpretation of the role of context and the data collected by Escobar et al. (2001) and Amundson and Miller (2008), we anticipated that a retention interval between Phases 1 and 2 should alleviate proactive interference. On the contrary, there are several reports on the durability of fear memories. For example, recent data by Wiltgen and Silva (2007) suggest that fear memories of context conditioning (where the context plays a role similar to that of a cue) last for at least 36 days. Moreover, Matzel and colleagues (Matzel, Brown, & Miller, 1987) observed a reliable US preexposure effect even when they interposed an 8-day retention interval between Phases 1 and 2 and a second 8-day interval between Phase 2 and test. Thus, we expected that fear memories of the context (which presumably underlie the US preexposure effect) would not be alleviated by the retention interval.
In Experiment 3 we used a 2 × 2 factorial design in which subjects were randomly assigned to one of four groups based on the treatment they received (Proactive Interference [PI] or US preexposure [US]) and the delay between Phases 1 and 2 (1 day [NoDelay] vs. 15 day [Delay]). Groups PI-NoDelay and US-NoDelay constituted the Control condition, as they received treatments similar to Groups Control-PI and Control-US in Experiment 2. Groups PI-Delay and US-Delay received similar treatments, but a 15-day retention interval was imposed between Phases 1 and 2. Based on our analysis of the context in terms of two different roles, we anticipated that the retention interval would selectively disrupt the proactive interference effect (Group PI-Delay) without a significant change in the US-preexposure effect (Group US-Delay). The design of Experiment 3 is depicted in Table 3.
Table 3.
Design of Experiment 3
| Group | Phase 1 | Retention Interval | Phase 2 | Exp Test X |
|---|---|---|---|---|
| PI-NoDelay | 12 A+ (S) | 1 Day | 2 X+ | cr |
| PI-Delay | 12 A+ (S) | 15 Days | 2 X+ | CR |
| US-NoDelay | 12 + (M) | 1 Day | 2 X+ | cr |
| US-Delay | 12 + (M) | 15 Days | 2 X+ | cr |
Note: + = footshock. Numbers next to the stimuli indicate number of trials. Group labels refer to Phase 1 treatment (PI [proactive interference] vs. US [US preexposure]) and retention interval between Phases 1 and 2 (NoDelay [1 Day] vs. Delay [15 Days]). Phases 1, and 2 were conducted in one context (Training) and all other treatments in the remaining context (Test) with the exception of Phase 2, the retention interval which subjects spent in their home cages. S = Spaced, M = Massed. Expectations (Exp) = expectations about conditioned responding to the target X. CR = strong conditioned response; cr = weak conditioned response.
Method
Subjects and apparatus
Subjects were 24 female and 24 male Sprague-Dawley descended, experimentally naïve, young adult rats, bred in our colony. Body weight range for females was 186–246 g and for males was 263–351 g. The apparatus and stimuli were identical to those used in Experiments 1 and 2.
Procedure
Subjects were randomly assigned to one of four groups (ns = 12) based on Phase 1 treatment (proactive interference [A-US] or outcome preexposure [US alone]) and on the retention interval between Phases 1 and 2 (NoDelay vs. Delay). Phases 1 and 2 were conducted in one [training] context (V or R) and all other treatments in the remaining [test] context in a counterbalanced manner within groups. This was done to minimize differential fear of the test context summating with fear to the CS.
Acclimation
Before the beginning of training, a one-day pre-exposure to the test context was conducted as in Experiments 1 and 2.
Phase 1
Prior to Phase 1 the lick tubes were removed from the training chambers. On Days 2–4 (Condition Delay) or 16–18 (Condition NoDelay) subjects received US preexposure and proactive interference treatments identical to those administered in Experiments 1 and 2.
Retention interval
On Days 5 through 18, subjects in the Delay condition (and Days 2–15 for subjects in the NoDelay condition), animals remained in their home cages and were handled for 30 s three times a week. They were also given access to water for 30 min each day.
Phase 2
On Day 19 all subjects experienced two X-US pairings within a 120-min session in the training context. Stimulus onset occurred at 30 and 90 min into the session.
Reacclimation
On Days 20–23, the lick tubes were reinserted, and subjects were allowed to drink during daily 45-min sessions in the test context.
Test
On Day 24, with the lick tubes reinserted in the test context, all subjects were tested for suppression to X as in the previous experiments.
Results and Discussion
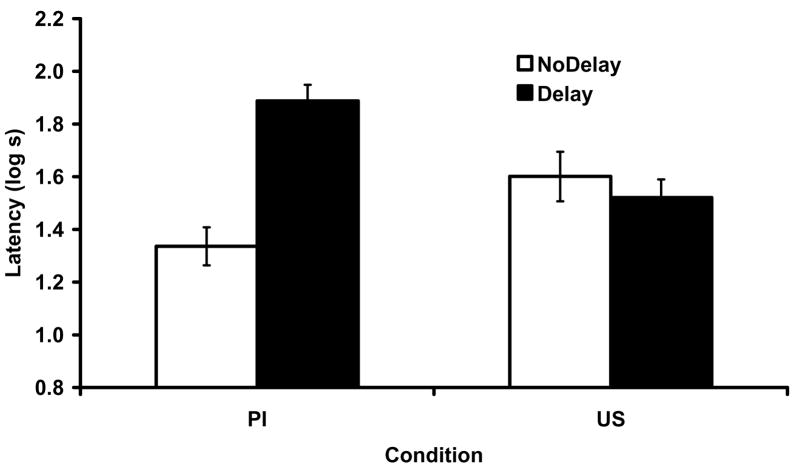
The results of Experiment 3 are depicted in Figure 4. Conditioned suppression was low in the control groups. Importantly, we observed attenuation of proactive interference with a 15-day retention interval between training of the interfering memory and the target X-US association. No attenuation of the US preexposure was observed. Consistent with our expectations, the retention interval imposed between Phases 1 and 2 selectively affected the proactive interference effect, but had no effect on the groups that received US preexposure. These impressions were supported by the following statistical analyses.
Figure 4.
Mean suppression ratios to test presentations of X in Experiment 3. Error brackets depict the standard error of the mean. A retention interval between Phases 1 and 2 alleviated the proactive interference effect, but had no appreciable effect on the US preexposure effect.
A 2 × 2 factorial ANOVA with treatment (Proactive Interference vs. US Preexposure) and retention interval (NoDelay vs. Delay) conducted on the transformed latencies to start drinking did not reveal any differences between groups, indicating no baseline differences, smallest p =.32. A similar ANOVA conducted on the transformed latencies revealed a main effect of retention interval, F(1, 44) = 9.71, p <.01, MSE = 0.07, Cohen’s f = 0.42, and an interaction, F(1, 44) = 17.43, p <.01, MSE = 0.07, Cohen’s f = 0.58. The interaction suggests that the effect of the retention interval was different for the two treatments. To assess this interpretation, we conducted a planned comparison between Groups Proactive Interference-Control and Proactive Interference-Delay, which revealed attenuated proactive interference with the imposition of the delay, F(1, 44) = 26.59, p <.01, MSE = 0.07, Cohen’s f = 0.73. However, a similar comparison between the two Groups that received US-preexposure treatment revealed no differences, F(1, 44) = 0.56, p =.45, MSE = 0.07. Moreover, Group Interference-Delay responded more than Group US Preexposure-Delay F(1, 44) = 11.79, p <.01, MSE = 0.07, Cohen’s f = 0.47, consistent with the expectation that the delay selectively affected proactive interference. Thus, this experiment complements Experiment 2; conjointly they double dissociate the two roles of the context. Whereas in Experiment 2 context preexposure affected the role of the context as a cue but not as a memory facilitator, in Experiment 3 a retention interval affected the role of the context as a memory facilitator but not the role of the context as a cue.
General Discussion
Although different roles of contextual information have been described and its importance highlighted on several occasions (Balsam, 1985; Bouton, in press; Fanselow, 2007; Miller & Schachtman, 1985), the exact determinants of these roles have not been elucidated. The present experiments were designed to address this theoretical and empirical discrepancy in the learning and memory literature regarding different roles of contextual information. We identified a paradoxical interaction between memories and contexts (see Figure 1) that results from seemingly similar training procedures but have opposite effects upon the target memory (X→US). Based on a literature review, we identified a parameter, the spacing of trials, that we hypothesized might determine whether the context plays the role of a cue or the role of a memory facilitator. That is, in Experiment 1, we observed that varying the spacing of trials during Phase 1 determined whether the context played the role of another cue (and hence, competed with the target memory) or facilitate the retrieval of a competing memory (that favored the observation of proactive interference). This resulted in an interaction between the spacing of trials and signaling of US presentations during Phase 1. These pairings increased responding to the target when training was conducted with massed trials, but they decreased responding to the target when training was conducted with spaced trials. Importantly, we identified one determinant (i.e., the spacing of trials) of these two opposite outcomes. We further hypothesized that if the observations of Experiment 1 resulted from the context playing two different functions, it should be possible to dissociate them by selectively affecting one function but not the other, and vice versa. Consistent with this hypothesis, in Experiments 2 and 3 we doubly-dissociated these two functions by alleviating the US-preexposure effect through context preexposure (Experiment 2) and the proactive interference effect by imposing a retention interval between Phases 1 and 2 (Experiment 3).
As we noted in the Introduction, learning theories generally have assumed one of two types of roles for contextual information. Some theories have assumed that, due to the establishment of context-US associations, the context is functionally similar to a punctate cue and therefore competes for behavioral control with any punctuate cue trained in that context (Denniston et al., 2001; Gibbon & Balsam, 1981; Mackintosh, 1975; Miller & Matzel, 1988; Pearce, 1987; 1994; 2002; Pearce & Hall, 1980; Rescorla & Wagner, 1972; Stout & Miller, 2007; Wagner, 1981). These theories have successfully accounted for degraded contingency treatments (Rescorla, 1968) and for trial massing effects, among other phenomena. Contrary to the assumption that the context competes with the target memory, there are theories that assume that the context facilitates memory retrieval, so that in the absence of contextual cues retrieval failure is observed. For example, Spear (1978) proposed that the context is encoded as a memory attribute that facilitates retrieval of the target memory at the time of testing. Moreover, he claimed that forgetting is largely due to the absence of retrieval cues at the time of testing. Bouton (1993) has made a similar assumption to account for various forms of interference including extinction treatments. As applied to proactive interference, the context facilitates the retrieval of Phase 1 learning, which allows this memory to proactively interfere with the Phase 2 memory. In order to account for various forms of interference between cues or outcomes trained together (stimulus competition phenomena) and cues or outcomes trained apart (interference effects), Miller and Escobar (2002) recently proposed a dual-process model that assumed that for cues and outcomes trained together, a comparator mechanism active at the time of testing produces competition between two sources of information as a net result, whereas for cues or outcomes trained apart, a priming mechanism operates for memory retrieval by which activation of one association of a cue [or outcome] inhibited activation of another association to that cue [or outcome]. However, they did not identify any critical parameter for these two different roles of contextual information. The present research integrates these two processes and importantly identifies one variable that determines whether the context will act as a competitor cue or as a priming stimulus.
Throughout this paper, we have referred to the functions of the context seen in proactive interference as memory facilitating or priming. Bouton and Swartzentruber (1986) refered to these functions as occasion setting. Although we did not conduct any specific test of occasion setting properties, this analogy is based on the fact that memory facilitating contexts and occasion setters both control behavior to a target independently of its own associations with the US. Further evidence supporting this analogy between the memory facilitating function of contexts and occasion setters was reported by Swartzentruber and Bouton (1988). They observed transfer of positive occasion setting properties of a context to a second stimulus that was also trained as an occasion set CS. In other words, like occasion setters, contexts can control the retrieval of CS-US associations while not eliciting any conditioned responses on their own, and this control may transfer to another CS. Moreover, the present discussion of the context having two different roles in memory performance is not restricted to the Pavlovian conditioning literature. In the instrumental conditioning literature, there are examples that also suggest that the context plays two different roles, although these roles have not been dissociated as we did in Experiments 2 and 3. For example, Pearce and Hall (1979) administered to rats variable interval (VI) 60-s training with immediate reinforcement and subsequently they extinguished the training context. Consistent with the view that the context where instrumental behavior was acquired facilitated (or invigorated) instrumental behavior, post-training extinction of the training context diminished instrumental behavior in a subsequent test. They concluded that the context invigorates instrumental behavior, which is consistent with the role of the context as amemory facilitator assumed in the present research. In contrast, Reed and Reilly (1990) also administered VI 60-s instrumental training but, instead of presenting the reinforcer immediately, they used a delayed reward procedure in which the reinforcer was presented 5 s after lever pressing. Critically, when they extinguished the context after instrumental training, they observed an increase in instrumental behavior rather than a decrease as observed by Pearce and Hall (1979). In other words, with highly similar parameters of training and context extinction, they saw opposite effects after extinguishing the context. The critical difference between these studies was the delay in reinforcement that was introduced by Reed and Reilly (1990), which presumably allowed the context to play a role more akin to any other cue and compete for behavioral control with the Response→Outcome association. Similarly, Balsam (1985) described an autoshaping experiment using ring doves in which he measured key pecking (reflecting associative strength between the key and the US) and also general activity, which had previously been shown to reflect the associative value of the context. The experiment was run for 28 sessions, during each of which animals experienced 20 trials. Critically, the correlation between general activity (reflecting context-US strength) and keypeck rate (reflecting CS-US strength) was negative during the first half of the experiment but progressively switched to positive. That is, early in training the higher the context-US association (general activity), the less key pecking was observed. However, during the second half of training, the value of the context and the key pecking rate were positively correlated. It seems from this description that early in training the context competed for behavioral control with the target cue, but it later summated with behavior controlled by the cue. Taken together with the examples with rats cited above, these results point at two different roles of the context in instrumental behavior. In the two examples provided above (Pearce & Hall, 1979; Reed & Reilly, 1990), the trace interval between response emission and reinforcer presentation seems to be the critical variable, whereas in the example with the birds (Balsam, 1985), the amount of training seems to be the critical variable that determines whether the context plays one role or the other.
In the present experiments, the critical variable that determined whether the context played the role of another cue or the role of a memory facilitator was the spacing of trials (i.e., the amount of context exposure) during Phase 1. That is, short ITIs made the context act like a cue and long ITIs made the context act like a memory facilitator. Thus, the total exposure to the context, apparently independent of the amount of exposure to the punctate cues, was the critical determinant of the putative role that the context played. However, there is no reason for assuming that signal value as a cue and occasion setting are mutually exclusive (Holland, 1992). We will further argue here that there is no fundamental difference between punctate stimuli (i.e., cues) and contexts regarding these different functions based on duration of exposure. That is, under certain circumstances, punctate cues (such as a light or a tone) can have properties similar to contexts and vice versa. This is evident in experiments assessing the role of so-called local context in Pavlovian fear conditioning (Barnet, Grahame, & Miller, 1993a; 1993b; 1995; Yin, Barnet, & Miller, 1994). In these experiments, the local context was generated by a punctate cue (a bright light) that was turned on soon prior to the onset of a target cue and off soon after termination of the target cue. Irrespective of session duration, in these experiments phenomena similar to the present effects of the context were achieved by the presentation of the bright light. For example, in one experiment (Yin, Barnet, & Miller, 1994; Experiment 1), a trial massing effect was obtained irrespective of the spacing of trials and with a similar session length for all groups. What Yin and colleagues manipulated was the local context surrounding each of five reinforced trials that were presented in a single session. Some groups received five massed trials (the ITI from US termination to CS onset was 45 s), whereas other groups received five spaced trials (the ITI was 900 s). Orthogonally, the bright light was turned on for almost all of the session or only near each trial (on 22.5 s before the target CS onset and off 22.5 s after the target CS terminated). If the trial spacing effect depended on the absolute temporal distance between trials, then all massed groups should have displayed less conditioned responding at test. However, if the trial spacing depended on the local context (here we assume that the context plays the role of a cue), then less conditioned responding should have been observed when the local context was briefly presented around a trial, which is what Yin and colleagues observed. Moreover, consistent with the notion that the context was playing the role of a cue, posttraining extinction of the light (i.e., the local context) produced a recovery from the trial massing effect. In all groups the bright light acted as a context. However, when the bright light was brief, this context acted as a cue and competed for behavioral control with the target, whereas when the light was long, this context did not compete for behavioral control with the target cue. In other words, when presented briefly the bright light prevented the expression of behavioral control by the target cue, but when the bright light was prolonged, it had a negligible effect upon behavioral control by the target, which is similar to what we observed in the present experiments but with the context (as defined by the environment where training occurred) replacing the light.
Recent work by Waddell, Morris, and Bouton (2006) also suggests that punctate cues can act as contexts under circumstances of prolonged duration of exposure. Instead of using a bright light, they trained rats in a bar-press suppression preparation with a clicker that was 60 s or 600 s in duration. Prior to training, they conducted lesions of the bed nucleus of the stria terminalis (BNST), which presumably mediates freezing to contextual but not punctate cues (Sullivan et al., 2004). Consistent with this hypothesis, in their Experiment 1 they observed that the lesions disrupted conditioning to the long CS but not to the short CS. Moreover, in their Experiment 3, similar lesions abolished reinstatement after fear extinction, an effect that depends critically upon context-US associations. Thus, behavioral (Barnet et al., 1993a; 1993b; 1995; Yin et al., 1994) and neurobiological evidence (Waddell et al., 2006), using punctate stimuli of different sensory modalities, suggest that, based on the duration of exposure, these cues can act as contexts in a manner functionally similar to the effects obtained in the present experiments. Overall, the results reviewed above suggest that punctate stimuli of short duration function as cues, but with long durations they can function as contexts. Thus, at a psychological level, there seems to be no fundamental distinction between environments (i.e., the chamber) and punctate stimuli (e.g., a tone) in the way they function. What seems to be critical is the amount of exposure to these sources of stimulation. These conjectures seem obvious even to the naïve reader, but what the research presented here does is to provide experimental evidence of such functional equivalence, at least with the case being made for the environments.
The results observed in the present experiments also have implications for research aimed at understanding the role of the hippocampus in the processing of contextual memories (Maren, 2008). Although it has been widely held that the hippocampus processes contextual memories and the amygdala processes target cues (Maren, 2001), this view has been questioned in light of recent data. For example, some hippocampal lesion studies have failed to observe a decrement in context fear learning (Wiltgen Sanders, Anagnostaras, Sage, & Fanselow, 2006), and recent studies have been designed to determine whether the dorsal or ventral hippocampus (Moser & Moser, 1998) processes contextual fear memories. The data to date have been inconclusive. For example, some studies have observed decreased contextual fear after lesions (or inactivation) of the dorsal hippocampus (Bast, Zhang, & Feldon, 2003; Chowdhury, Quinn, & Fanselow, 2005; Misane et al., 2005; Phillips & LeDoux, 1992; 1994), but other studies have failed to observe such effects (Maren, Aharonov, & Fanselow, 1997; Maren & Holt, 2004; Quinn, Wied, Ma, Tinsley, & Fanselow, 2008, Exp 2). The proposed reasons for these discrepancies have been diverse, in general pointing to procedural differences that allow for speculation concerning the causes of the different results. Based on the previously mentioned relationship between cue exposure and total context exposure (C/T; Gibbon & Balsam, 1981), we suggest that the dorsal hippocampus is involved in contextual fear but only when training is conducted with relatively massed trials (i.e., small C/T ratios) which, according to the results presented here, should make the context function as a cue. That is, in the studies that found an effect, the C/T ratio is consistently below seven, whereas in those studies in which lesions or inactivation of the dorsal hippocampus failed to have an effect on contextual fear, the C/T ratio is seven or larger. Thus, an analysis in terms of C/T allows predictions concerning the parameters with which dorsal hippocampal lesions will impede context learning. In relation to the experiments presented here, it is likely that the dorsal hippocampus has an effect on context fear when the context is more akin a punctate cue (i.e., a low C/T ratio) than when the spacing of trials is larger. With widely spaced trials, the dorsal hippocampus is likely to disrupt the involvement of the context as an occasion setter, which is consistent with recent observations of decreased renewal after dorsal hippocampal inactivation or lesions (Corcoran & Maren, 2004; Ji & Maren, 2005). Thus, the present analysis and the functional dissociation that we propose encourage predictions about the involvement of the dorsal hippocampus upon contextual fear memories (i.e., context as a cue) and context-dependent fear memories (i.e., context as a memory facilitator or occasion setter).
In summary, the research presented here isolates one parameter (the spacing of trials) that determines whether the context will play the role of a cue or a memory facilitator. We further observed that these two roles are dissociable, in that different manipulations can selectively decrease one role or the other. It should be noted that these two roles are not mutually exclusive, as amount of exposure is a variable in which spaced and massed are two extremes. Likely intermediate points in this continuum would result in the context playing a mixture of both roles. What this research suggests is that the two roles can be dissociated, at least at a behavioral level.
Acknowledgments
This research was supported by National Institute of Mental Health Grant 33881. The authors thank Joseph Alesandro, Eric Curtis, Mario Laborda, Bridget McConnell, Gonzalo Miguez, Cody Polack, Sarah Sterling, Yumu Tanaka, and James Witnauer for their comments on an earlier version of this manuscript. We would also like to thank James Esposito for assistance in conducting these experiments.
Footnotes
Bouton and Swartzentruber (1986) applied the term occasion setting to the functions of the context that control responding to a target stimulus independently of its direct associations with the US. In the General Discussion, we will discuss these properties and the relationship between the memory facilitating effects of contexts and traditional occasion setters as traditionally defined by Holland (1992).
References
- Amundson JC, Miller RR. Associative interference in Pavlovian conditioning: A function of similarity between the interfering and target associative structures. Quarterly Journal of Experimental Psychology. 2008;61:1340–1355. doi: 10.1080/17470210701560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundson JC, Escobar M, Miller RR. Proactive interference in first-order Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavioral Processes. 2003;29:311–322. doi: 10.1037/0097-7403.29.4.311. [DOI] [PubMed] [Google Scholar]
- Baker AG, Mercier P, Gabel J, Baker PA. Contextual conditioning and the US preexposure effect in conditioned fear. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:109–128. doi: 10.1037//0097-7403.7.2.109. [DOI] [PubMed] [Google Scholar]
- Balaz MA, Capra S, Hartl P, Miller RR. Contextual potentiation of acquired behavior after devaluing direct context-US associations. Learning and Motivation. 1981;12:383–397. [Google Scholar]
- Balaz MA, Capra S, Kasprow WJ, Miller RR. Latent inhibition of the conditioning context: Further evidence of contextual potentiation of retrieval in the absence of context-US associations. Animal Learning & Behavior. 1982;10:242–248. [Google Scholar]
- Balsam PD. The functions of context in learning and performance. In: Balsam PD, Tomie A, editors. Context and learning. Hillsdale, NJ: Erlbaum; 1985. pp. 1–21. [Google Scholar]
- Balsam PD, Tomie A, editors. Context and learning. Hillsdale, NJ: Erlbaum; 1985. [Google Scholar]
- Barela PB. Theoretical mechanisms underlying the trial-spacing effect in pavlovian fear conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1999;25:177–193. doi: 10.1037//0097-7403.25.2.177. [DOI] [PubMed] [Google Scholar]
- Barnet RC, Grahame NJ, Miller RR. Local context and the comparator hypothesis. Animal Learning & Behavior. 1993a;21:1–13. [Google Scholar]
- Barnet RC, Grahame NJ, Miller RR. Local time horizons in Pavlovian learning. Journal of Experimental Psychology: Animal Behavior Processes. 1993b;19:215–230. [PubMed] [Google Scholar]
- Barnet RC, Grahame NJ, Miller RR. Trial spacing effects in Pavlovian conditioning: A role for local context. Animal Learning & Behavior. 1995;23:340–348. [Google Scholar]
- Bast T, Zhang W, Feldon J. Dorsal hippocampus and classical fear conditioning to tone and context in rats: effects of local NMDA-receptor blockade and stimulation. Hippocampus. 2003;13:657–675. doi: 10.1002/hipo.10115. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of pavlovian learning. Psychological Bulletin. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. The multiple forms of “context” in associative learning. In: Feldman Barrett L, Mesquita B, Smith E, editors. The mind in context. New York: Guilford; in press. [Google Scholar]
- Bouton ME, Swartzentruber D. Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian discrimination. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:333–350. [Google Scholar]
- Chowdhury N, Quinn J, Fanselow M. Dorsal hippocampus involvement in trace fear conditioning with long, but not short, trace intervals in mice. Behavioral Neuroscience. 2005;119:1396–1402. doi: 10.1037/0735-7044.119.5.1396. [DOI] [PubMed] [Google Scholar]
- Cole K, VanTilburg D, Burch-Vernon A, Riccio D. The importance of context in the US preexposure effect in CTA: Novel versus latently inhibited contextual stimuli. Learning and Motivation. 1996;27:362–374. doi: 10.1006/lmot.1996.0021. [DOI] [PubMed] [Google Scholar]
- Corcoran K, Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learning & Memory. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denniston JC, Savastano HI, Miller RR. The extended comparator hypothesis: Learning by contiguity, responding by relative strength. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Hillsdale, NJ: Erlbaum; 2001. pp. 65–117. [Google Scholar]
- Durlach PJ. Effect of signaling intertrial USs in autoshaping. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:374–389. [PubMed] [Google Scholar]
- Escobar M, Matute H, Miller RR. Cues trained apart compete for behavioral control in rats: Convergence with the associative interference literature. Journal of Experimental Psychology: General. 2001;130:97–115. doi: 10.1037/0096-3445.130.1.97. [DOI] [PubMed] [Google Scholar]
- Escobar M, Matute H, Miller RR. Cues trained apart compete for behavioral control in rats: Convergence with the associative interference literature. Journal of Experimental Psychology: General. 2001;130:97–115. doi: 10.1037/0096-3445.130.1.97. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Context: What’s so special about it? In: Roediger HL, Dudai Y, Fitzpatrick SM, editors. Science of memory: Concepts. Oxford, UK: Oxford University Press; 2007. pp. 101–105. [Google Scholar]
- Gibbon J, Balsam P. Spreading association in time. In: Locurto CM, Terrace HS, Gibbon J, editors. Autoshaping and conditioning theory. New York: Academic Press; 1981. pp. 219–253. [Google Scholar]
- Godden D, Baddeley A. Context-dependent memory in two natural environments: On land and underwater. British Journal of Psychology. 1975;66:325–331. [Google Scholar]
- Goodwin DW, Powell B, Bremer D, Hoine H, Stern J. Alcohol and recall: State-dependent effects in man. Science. 1969;163:1358–1360. doi: 10.1126/science.163.3873.1358. [DOI] [PubMed] [Google Scholar]
- Hinson RE. Effects of UCS preexposure on excitatory and inhibitory rabbit eyelid conditioning: An associative effect of conditioned contextual stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 1982;8:49–61. [PubMed] [Google Scholar]
- Holland PC. Occasion setting in Pavlovian conditioning. In: Medin DL, editor. The Psychology of learning and motivation. Vol. 28. San Diego, CA: Academic Press; 1992. pp. 69–125. [Google Scholar]
- Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Current Opinion in Neurobiology. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learning & Memory. 2005;12:270–276. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin LJ. Attention-like processes in classical conditioning. In: Jones MR, editor. Miami symposium on the prediction of behavior: Aversive stimulation. Miami, FL: University of Miami Press; 1968. pp. 9–31. [Google Scholar]
- Klein SB. Adrenal-pituitary influence in reactivation of avoidance-learning memory in the rat after intermediate intervals. Journal of Comparative and Physiological Psychology. 1972;79:341–359. doi: 10.1037/h0032809. [DOI] [PubMed] [Google Scholar]
- Lubow RE. Latent inhibition and conditioned attention theory. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- Lubow RE, Moore AU. Latent inhibition: The effect of nonreinforced preexposure to the conditioned stimulus. Journal of Comparative and Physiological Psychology. 1959;52:415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Mackintosh NJ. A theory of attention: Variations in the associability of stimuli with reinforcement. Psychological Review. 1975;82:276–298. [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: Cautions and caveats. European Journal of Neuroscience. 2008;28:1661–1666. doi: 10.1111/j.1460-9568.2008.06485.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow M. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioural Brain Research. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W. Hippocampus and Pavlovian fear conditioning in rats: muscimol infusions into the ventral, but not dorsal, hippocampus impair the acquisition of conditional freezing to an auditory conditional stimulus. Behavioral Neuroscience. 2004;118:97–110. doi: 10.1037/0735-7044.118.1.97. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Brown A, Miller RR. Associative effects of US preexposure: Modulation of conditioned responding by an excitatory training context. Journal of Experimental Psychology: Animal Behavior Processes. 1987;13:65–72. [PubMed] [Google Scholar]
- Matzel LD, Schachtman TR, Miller RR. Learned irrelevance exceeds the sum of CS-preexposure and US-preexposure deficits. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:311–319. [PubMed] [Google Scholar]
- Miller RR, Escobar M. Associative interference between cues and between outcomes presented together and presented apart: An integration. Behavioural Processes. 2002;57:163–185. doi: 10.1016/s0376-6357(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. Orlando, FL: Academic Press; 1988. pp. 51–92. [Google Scholar]
- Miller RR, Schachtman TR. The several roles of context at the time of retrieval. In: Balsam PD, Tomie A, editors. Context and learning. Hillsdale, NJ: Erlbaum; 1985. pp. 167–194. [Google Scholar]
- Misane I, Tovote P, Meyer M, Spiess J, Ögren S, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Moser M, Moser E. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Myers JL, Well AD. Research design and statistical analysis. 2. Mahwah, NJ: Erlbaum; 2003. [Google Scholar]
- Oberling P, Gunther L, Miller RR. Latent inhibition and learned irrelevance of occasion setting. Learning and Motivation. 1999;30:157–182. [Google Scholar]
- Overton DA. State dependent or “dissociated” learning produced with pentobarbital. Journal of Physiological and Comparative Psychology. 1964;57:3–12. doi: 10.1037/h0048023. [DOI] [PubMed] [Google Scholar]
- Overton DA. Contextual stimulus effects of drugs and internal states. In: Balsam PD, Tomie A, editors. Context and learning. Hillsdale, NJ: Erlbaum; 1985. pp. 357–384. [Google Scholar]
- Pearce JM. A model for stimulus generalization in Pavlovian conditioning. Psychological Review. 1987;94:61–73. [PubMed] [Google Scholar]
- Pearce JM. Similarity and discrimination: A selective review and a connectionist model. Psychological Review. 1994;101:587–607. doi: 10.1037/0033-295x.101.4.587. [DOI] [PubMed] [Google Scholar]
- Pearce JM. Evaluation and development of a connectionist theory of configural learning. Animal Learning & Behavior. 2002;30:73–95. doi: 10.3758/bf03192911. [DOI] [PubMed] [Google Scholar]
- Pearce JM, Hall G. The influence of context-reinforcer associations on instrumental performance. Animal Learning & Behavior. 1979;7:504–508. [Google Scholar]
- Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychological Review. 1980;87:532–552. [PubMed] [Google Scholar]
- Phillips R, LeDoux J. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Phillips R, LeDoux J. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learning & Memory. 1994;1:34–44. [PubMed] [Google Scholar]
- Quinn J, Wied H, Ma Q, Tinsley M, Fanselow M. Dorsal hippocampus involvement in delay fear conditioning depends upon the strength of the tone-footshock association. Hippocampus. 2008;18:640–654. doi: 10.1002/hipo.20424. [DOI] [PubMed] [Google Scholar]
- Randich A, Ross R. Mechanisms of blocking by contextual stimuli. Learning and Motivation. 1984;15:106–117. [Google Scholar]
- Reed P, Reilly S. Context extinction following conditioning with delayed reward enhances subsequent instrumental responding. Journal of Experimental Psychology: Animal Behavior Processes. 1990;16:48–55. [PubMed] [Google Scholar]
- Rescorla RA. Probability of shock in the presence and absence of CS in fear conditioning. Journal of Comparative and Physiological Psychology. 1968;66:1–5. doi: 10.1037/h0025984. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current research and theory. New York: Appleton Century Crofts; 1972. pp. 64–99. [Google Scholar]
- Spear NE. Retrieval of memories in animals. Psychological Review. 1973;80:163–194. [Google Scholar]
- Spear NE. The processing of memories: Forgetting and retention. Hillsdale, NJ: Erlbaum; 1978. [Google Scholar]
- Stout SC, Miller RR. Sometimes-competing retrieval (SOCR): A formalization of the comparator hypothesis. Psychological Review. 2007;114:759–783. doi: 10.1037/0033-295X.114.3.759. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Aspergis J, Bush DE, Johnson LR, Hou M, LeDoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128:7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Swartzentruber D, Bouton ME. Transfer of positive contextual control across different conditioned stimuli. Bulletin of the Psychonomic Society. 1988;26:569–572. [Google Scholar]
- Tomie A. Interference with autoshaping by prior context conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1976;2:323–334. [Google Scholar]
- Tulving E, Thomson DM. Encoding specificity and retrieval processes in episodic memory. Psychological Review. 1973;80:352–73. [Google Scholar]
- Underwood BJ. Attributes of memory. Psychological Review. 1969;76:559–573. [Google Scholar]
- Urcelay GP, Miller RR. Counteraction between overshadowing and degraded contingency treatments: Support for the extended comparator hypothesis. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:21–32. doi: 10.1037/0097-7403.32.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushihara K, Miller RR. Overshadowing and the outcome-alone exposure effect counteract each other. Journal of Experimental Psychology: Animal Behavior Processes. 2006;32:253–270. doi: 10.1037/0097-7403.32.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Waddell J, Morris R, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: Aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behavioral Neuroscience. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. Journal of Neuroscience. 2006;26:5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen B, Silva A. Memory for context become less specific with time. Learning & Memory. 2007;14:313–317. doi: 10.1101/lm.430907. [DOI] [PubMed] [Google Scholar]
- Witnauer JE, Miller RR. Degraded contingency revisited: Posttraining extinction of a cover stimulus attenuates a target cue’s behavioral control. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33:440–450. doi: 10.1037/0097-7403.33.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Barnet RC, Miller RR. Trial spacing and trial distribution effects in Pavlovian conditioning: Contributions of a comparator mechanism. Journal of Experimental Psychology: Animal Behavior Processes. 1994;20:123–134. doi: 10.1037//0097-7403.20.2.123. [DOI] [PubMed] [Google Scholar]