1. Introduction
1.1. History
Formaldehyde was described in the year 1855 by the Russian scientist Alexander Michailowitsch Butlerow. The technical synthesis by dehydration of methanol was achieved in 1867 by the German chemist August Wilhelm von Hofmann. The versatility that makes it suitable for use in various industrial applications was soon discovered, and the compound was one of the first to be indexed by Chemical Abstracts Service (CAS). In 1944, Walker published the first edition of his classic work Formaldehyde.(1) Between 1900 and 1930, formaldehyde-based resins became important adhesives for wood and wood composites. The first commercial particle board was produced during World War II in Bremen, Germany. Since 1950, particle board has become an attractive alternative to solid wood for the manufacturing of furniture. Particle board and other wood-based panels were subsequently also used for the construction of housing. Adverse health effects from exposure to formaldehyde in prefabricated houses, especially irritation of the eyes and upper airways, were first reported in the mid-1960s. Formaldehyde emissions from particle boards bonded with urea formaldehyde resin were soon identified as the cause of the complaints. As a consequence, a guideline value of 0.1 ppm was proposed in 1977 by the former German Federal Agency of Health to limit human exposure in dwellings. Criteria for the limitation and regulation of formaldehyde emissions from wood-based materials were established in 1981 in Germany and Denmark. The first regulations followed in the United States in 1985 or thereabouts. In Germany and the United States, large-scale test chambers were used for the evaluation of emissions. Although the chamber method is very reliable, it is also time-consuming and expensive. This meant there was a strong demand for simple laboratory test methods.(2)
1.2. Formaldehyde as a Priority Indoor Pollutant
Discussion about formaldehyde as a possible carcinogen started in 1980 when the carcinogenicity of formaldehyde in rats and mice after long-term inhalation exposure was reported.3,4 These publications and the results of studies of human exposure assessment for formaldehyde triggered an avalanche of scientific work as well as stories in the yellow press. Although electronic databases and powerful search engines are now available, it is still difficult to survey all papers in the technical and medical literature. Notwithstanding this, formaldehyde is definitely the most common and the best-known indoor air pollutant.
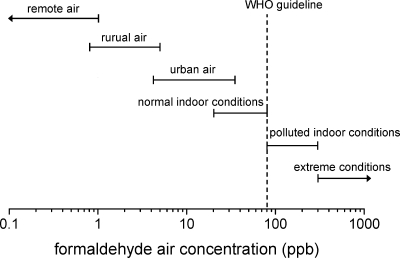
Over the years, the release of formaldehyde from building products has been decreasing. On the other hand, formaldehyde concentrations in ambient air are increasing continuously, especially in the urban environment. For this reason, formaldehyde slipped out of the primary focus of indoor research in the 1990s, although special formaldehyde-related events occasionally come to the attention of the general public. Well-known examples are reports about increasedformaldehyde emission from furniture coatings in Germany (1992) and high formaldehyde concentrations in mobile homes in the United States (2006). However, in 2004, formaldehyde discussions were generally taken up again when formaldehyde was considered as carcinogenic for humans. As a consequence, various authorities and institutions have proposed new indoor air guidelines, giving values that are nearly ubiquitous. Although a prioritized ranking of chemicals and exposures that cause concern is difficult and uncertain, the Scientific Committee on Health and Environmental Risks (SCHER)(5) states that formaldehyde (like carbon monoxide, nitrogen dioxide, benzene, naphthalene, environmental tobacco smoke (ETS), radon, lead, and organophosphate pesticides) is a compound of concern in the indoor environment.
1.3. Review of Literature
In this article, the current status of indoor-related formaldehyde research is summarized. This review is based on a literature search carried out using the “Web of Science” (ISI). The keywords “formaldehyde” and “indoor” gave 1240 hits for the period from 1990 to 2008. The results were cross-checked by searching Elsevier’s “ScienceDirect” (1850 hits), “Blackwell Synergy” (174 hits for the Indoor Air journal alone), the American Chemical Society, PubMed, SpringerLink, and Informaworld. Other references known to the authors such as standards (DIN, VDI, CEN, ISO, ASHRAE) and conference proceedings were also included.
2. General Description
2.1. Physical and Chemical Properties
Formaldehyde is produced on a large scale by the oxidation of methane or methanol in the presence of a catalyst.(6) At room temperature, it is a colorless gas that is flammable and highly reactive. The compound is soluble in water, ethanol, diethyl ether, and acetone. In aqueous solution, methylene glycol [CH2(OH)2] and polymethylene glycols [H(CH2O)nOH] are formed.(2) Formaldehyde is commonly purchased as a 37% solution in water, known as formalin, with 10% methanol as a stabilizer. The annual production of 37% formaldehyde is about 20 million tons worldwide.(7) In a recent review article, Tang et al.(8) estimate a global output of 32 million tons of formaldehyde in 2006, with the highest producers being China (34%), the United States (14%), and Germany (8%). More than 65% of the total formaldehyde is used to synthesize resins. The name paraformaldehyde describes a polymeric structure with 8−100 formaldehyde units per molecule.(1) The cyclic trimer of formaldehyde C3H6O3 is called 1,3,5-trioxane. Formaldehyde has a dipolar resonance structure (see Table 1), which makes the molecule a typical electrophile. According to Roffael(2) and Walker,(1) the most important reactions apart from polymerization are as follows:
Table 1. Physical and Chemical Properties of Formaldehyde.
| parameter | ref | |
| structure | 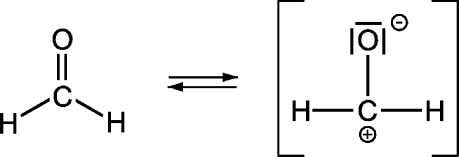 |
|
| synonyms | methanal, methyl aldehyde, methyl oxide | |
| CAS registry no. | 50-00-0 | |
| molecular formula | HCHO, CH2O | |
| SMILES | C=O | |
| molecular wt | 30.03 g mol−1 | (422) |
| melting pointa | −92 °C | (422) |
| boiling point | −21 °C | (422) |
| dipole moment | 2.33 D | (422) |
| solubility | soluble in water, ethanol, ether, acetone | |
| Henry’s law constant | 2.5 × 103 M atm−1 (25 °C) | (9) |
| log(Kow)b | −0.83 | |
| kOH•c | 9.37 × 10−12 cm3 molecule−1 s−1 (298 K) | (11) |
| kO3c | 2.09 × 10−24 cm3 molecule−1 s−1 (298 K) | (15) |
| kNO3c | 5.80 × 10−16 cm3 molecule−1 s−1 (298 K) | (12) |
| conversion factor | 0.1 ppm = 124.8 μg m−3 (293 K, 1013 mbar) | |
| 1 μg m−3 = 0.815 ppb (293 K, 1013 mbar) |
In some publications, a boiling point of −118 °C is given.
Calculated with SPARC (http://ibmlc2.chem.uga.edu/sparc/).
See also NIST Kinetics Database (http://kinetics.nist.gov).
• Reaction with ammonia to form hexamethylene tetramine
• Cannizzaro reaction
• Aldol reaction
• Tischenko reaction
The Henry’s law constant is 2.5 × 103 M atm−1 at 298 K (6.3 × 103 M atm−1 if diol formation is taken into account).(9) The calculated octanol/water partition coefficient is log(Kow) = −0.83. The World Health Organization (WHO) has published a value of log(Kow) = −1.(10) The reaction rate constant with the OH-radical is kOH = 9.3 × 10−12 cm3 molecule−1 s−1 at 298 K.(11) Assuming an atmospheric OH concentration of 106 molecules cm−3, this gives an HCHO lifetime against the OH reaction of 31 h. In the gas phase, formaldehyde shows a structured absorption spectrum between 260 and 360 nm.(12) The lifetimes against the photolytic processes HCHO → H2 + CO and HCHO → H + HCO, calculated for the latitude of 50°, are 6.9 and 2.1 h, respectively.(13) Atkinson(14) has calculated formaldehyde lifetimes in the atmosphere with respect to photolysis (τ = 4 h), reaction with the OH radical (τ = 1.2 days), reaction with the NO3 radical (τ = 80 days), and reaction with O3 (τ > 4.5 years). The gas-phase reaction of ozone with formaldehyde has been studied by Braslavsky and Heicklen.(15)
2.2. Toxicology
The high solubility of formaldehyde in water causes rapid absorption in the respiratory and gastrointestinal tract. Here, it can be oxidized to form formate and exhaled as carbon dioxide or incorporated in biological matrices. The biological half-life is extremely short at about 1 min.(16) As an electrophile, formaldehyde can react with nucleophilic biogenic compounds in the body.(17) Formaldehyde itself is produced in small amounts from methanol via the enzyme alcohol dehydrogenase (ADH),18,19 which is a human metabolite and can be measured in urine.20,21 According to a report published by “Health Canada”, which is based on human clinical studies and animal experiments, the primary effects of acute exposure to formaldehyde are irritation of the mucosa of the upper respiratory tract and the eyes.(22) The RD50 values (exposure concentration producing a 50% respiratory rate decrease as an indication of respiratory tract irritation) of male mice are 3.1−5.3 ppm for an exposure time of 5−10 min.(23) The lowest observable adverse effect levels (LOAEL) for human sensory irritation range from 0.4 ppm (rhinitis) to 3 ppm (eye, nasal, and throat irritation).(23) A recent study of formaldehyde and sensory irritation in humans showed that eye irritation is the most sensitive parameter. A no observed effect level (NOEL) of 0.5 ppm was derived in the case of constant exposure.(24)
Different threshold values are available for the odor perception of formaldehyde. Devos et al.(25) have calculated a standardized human olfactory threshold of 0.87 ppm (1.07 mg m−3). The WHO has estimated absolute odor thresholds (defined as the concentration at which 50% of the panel detects the odor) between 0.06 and 0.22 mg m−3.(10) In the INDEX report17,26 very low odor thresholds of 0.03 and 0.035 mg m−3 are specified, which refer to an updated WHO report(27) and an unavailable paper from 1917 cited in Devos et al.,(25) respectively.
In 2004, the International Agency for Research on Cancer (IARC) has classified formaldehyde as carcinogenic for humans (Group 1).(28) This evaluation is based on information regarding the relationship between nasopharyngeal cancer and leukemia related to the exposure to formaldehyde. In the European Union, formaldehyde is classified under Category 3 as a suspected carcinogen (Directive 2001/58/CEE). Since 1991, the U.S. EPA has regarded formaldehyde as a probable human carcinogen (B1) (http://cfpub.epa.gov/ncea/iris).
2.3. Application of Formaldehyde
Formaldehyde is a chemical feedstock for numerous industrial processes. It is also used as a preservative, disinfectant, and biocide. As far as the indoor environment is concerned, its use as a component of thermosetting adhesives is of particular significance. The reactions described below are described in detail by different authors.1,2,29−31
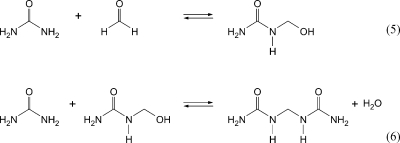
Urea-formaldehyde (UF) adhesives (so-called aminoplasts) are still the most commonly used products in the manufacturing of wood-based materials and furniture due to their rapid curing, their compatibility with additives, and their low price. In the first step, mono-, di-, and trimethylolurea are formed from formaldehyde and urea in a Mannich reaction. This is followed by condensation reactions to build up the polymer (see eqs 5 and 6). UF adhesives have poor water resistance: the presence of water results in a hydrolysis of the C−N bond and, as a consequence, the release of formaldehyde.
 |
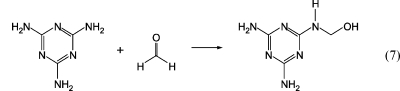
Melamine−urea−formaldehyde (MUF) adhesives are similar to UF adhesives. They are produced by mixing portions of UF and melamine−formaldehyde (MF) or by cocondensation of all monomers in one batch. Equation 7 shows the first step of the melamine−formaldehyde reaction.
 |
Phenol−formaldehyde (PF) adhesives (so-called phenoplasts) are made by electrophilic substitution to methylol phenol in the first step, as shown in eq 8. In alkaline solution, the reaction results in highly viscous resins of low molecular weight, called resols. A novolac with a high degree of cross-linking is formed in acidic solution. PF adhesives are very stable and water-resistant and have a high adherence to wood. In the past, plastics made of PF resins were also known as Bakelite and were, among other things, used as casings for telephones, radios, etc.
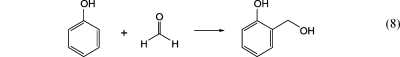 |
Melamine−urea−phenol−formaldehyde (MUPF) adhesives are used for the production of moisture-proofed wood-based products and for construction materials. Like MUF adhesives, they are produced by the addition of small amounts of phenol.
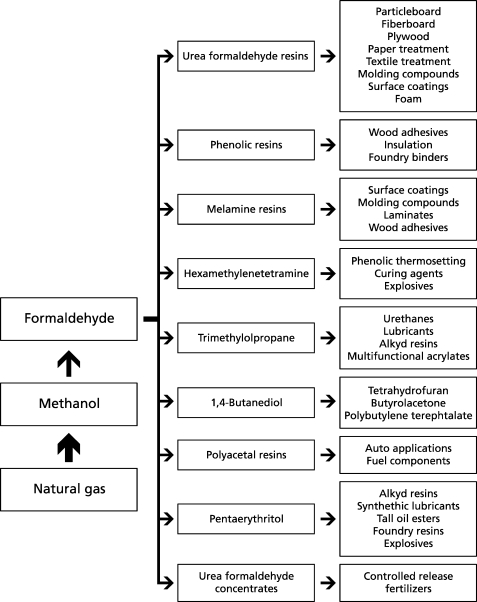
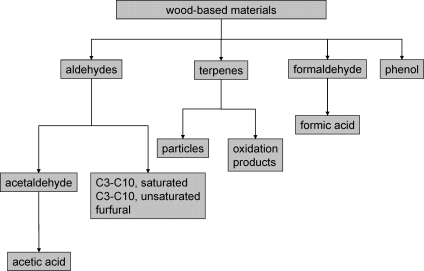
Figure 1 provides an overview of the industrial utilization of formaldehyde. Indoor-related applications of formaldehyde in the past and present have been summarized by a number of authors.32,33 A brief overview is given below:
Figure 1.
Survey of industrial applications for formaldehyde and formaldehyde products.
• Wood-based products (particle board, oriented-strand board (OSB), high-density fiber board (HDF), medium-density fiber board (MDF), plywood)
• Cork products (flooring materials)
• Insulation materials made of UF foam, mineral wool, or glass wool
• Paper products
• Coating materials, paints, and lacquers containing formaldehyde as preservative
• Textiles
• Cleaning and caring products
• Disinfectants and preservatives
• Photoprocessing chemicals
• Cosmetics.
3. Sources of Formaldehyde
3.1. Outdoor Sources
3.1.1. Formaldehyde as a Natural Compound
A number of natural and anthropogenic outdoor sources are known for formaldehyde.(34) Like other VOCs, it is a biogenic compound and part of plant physiological and plant/atmosphere exchange processes.(35) In 1927, Freudenberg and Harder identified formaldehyde as a decomposition product of lignin.(36) Müller et al.(37) found formaldehyde within and above a coniferous forest in Germany. Trapp et al.(38) mention formaldehyde as a degradation product of isoprene in a eucalyptus forest in Portugal. Carter and Atkinson(39) proposed a scheme for the formation of formaldehyde from isoprene via reaction with OH and NO. Kesselmeier et al.(40) have measured several parts per billion (ppb) of formaldehyde in a remote forest site in central Amazonia. Smidt et al.(41) have been able to detect low formaldehyde concentrations of 0.24−0.52 ppb in forests in the Austrian Alps (920 m) and 0.16−0.30 ppb at a mountaintop site (1758 m). Long-term measurements at rural European monitoring sites were carried out by Solberg et al.(42) Meyer and Boehme(43) have shown that formaldehyde is released from solid wood. Seco et al.(44) have reviewed VOC emission and uptake by plants. They point out that formaldehyde seems to be a product of methanol oxidation, but the exact origin within plants remains unclear. Other possible mechanisms, such as 5,10-methylene-tetrahydrofolate dissociation, glyoxylate decarboxylation, or oxidative demethylation reactions, have been proposed by Hanson and Roje.(45) Formaldehyde is also produced in the marine environment.(46)
3.1.2. Atmospheric Reactions
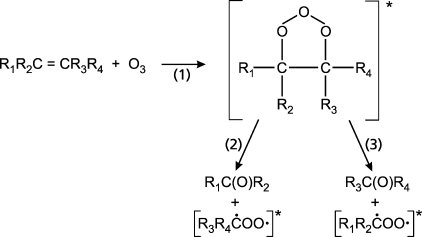
Thousands of organic compounds are released into the atmosphere from biogenic sources. According to Atkinson and Arey,(47) these organic compounds include isoprene, monoterpenes, sesquiterpenes, and a number of oxygenated compounds. In the troposphere, they react with hydroxyl (OH) radicals, nitrate (NO3) radicals, and ozone (O3), and they play an important role in the chemistry of the lower troposphere. The gas-phase reaction of ozone with unsaturated hydrocarbons is known to produce aldehydes, ketones, and acids as main components. As shown in Figure 2, an ozonide is formed from the reaction of the ozone with the double bond. The two decomposition pathways of the ozonide are of equal importance for alkenes of the structure RCH=CH2, R1CH=CHR, or R1R2C=CR3R4, but for alkenes with the structure R1R2C=CH2 or R1R2C=CHR3, the ozonide decomposes preferentially via pathway 3 while forming formaldehyde or R3CHO.(48) Grosjean and Grosjean(49) have identified formaldehyde in a number of alkene−ozone reactions. Grosjean et al.(50) have studied atmospheric oxidation reactions of biogenic hydrocarbons in a test chamber and have measured formaldehyde concentrations up to 26 ppb at 22 °C with excess cyclohexane to scavenge OH from the reaction of ozone (0.07−0.1 ppm) with β-pinene (1.0 ppm), d-limonene (1.2 ppm), and trans-caryophyllene (0.2−0.5 ppm), respectively. Formaldehyde formation from ozonolysis of carvone, carveol, geraniol, and citral has been reported by Nunes et al.(51) Griesbaum et al.(52) have identified formaldehyde by means of NMR spectroscopy as a byproduct of the gas-phase ozonolysis of terpenes. Relatively high formaldehyde outdoor concentrations can be found in the urban air of heavily polluted megacities. Here, HCHO is directly released into the atmosphere or produced by photochemical gas-phase reaction of hydroxyl radicals with so-called nonmethane hydrocarbons (NMHC). During one ozone episode in the city of Beijing, Duan et al.(53) measured a concentration of 36 μg m−3 formaldehyde in urban air. The rate constant for the reaction of the hydroxyl radical with methane is low (kOH(CH4) = 6.3 × 10−15 cm3 molecule−1 s−1). This means that the formation of formaldehyde from methane is only important in remote areas.
Figure 2.
Formation of carbonyl compounds from alkene−ozone reactions.12,421
3.1.3. Outdoor Combustion
The combustion of wood is also a natural source of formaldehyde.(54) Hedberg et al.(55) have studied birch combustion and report formaldehyde emission rates of 180−710 mg/kg wood. This is in accordance with data by Schauer et al.(56) for oak (759 mg/kg), pine (1165 mg/kg), and eucalyptus (599 mg/kg). Enhanced formaldehyde concentrations can be found under the influence of wildfire activity.(57) Reisen and Brown(58) have measured levels up to 0.57 ppm for the personal exposure of Australian firefighters. Formaldehyde is a known component of automobile exhaust gas.(59) Public interest in biodiesel fuel has recently stimulated fresh discussion of that topic. Machado Correra and Arbilla(60) and also Guarieiro et al.(61) have shown that carbonyl emissions are dependent on the biodiesel content and that the biodiesel ester molecules are probably the source of these carbonyls. However, Peng et al.(62) arrive at a different conclusion and attribute lower formaldehyde emissions to more complete combustion and increases in engine performance.
3.1.4. Formaldehyde Release into the Atmosphere
The WHO(27) pointed out that industrial formaldehyde releases can occur at any stage of the production, use, storage, transportation, or disposal of products with residual formaldehyde. Emissions have been detected from chemical manufacturing plants, pulp and paper mills, forestry product plants, tire and rubber plants, coal processing plants, textile mills, automotive manufacturing plants, and the metal products industry. Hauptmann et al.(7) have evaluated data from different references. On the basis of data from Canada, they provide the following estimated breakdown of emissions into outdoor air: traffic (70%), aircraft (11%), shipping (7%), the formaldehyde processing industry (10%), and power plants and waste incineration (<1%). It is difficult to determine the global emission of formaldehyde. The WHO report(27) mentions total releases of 8960 t/a from U.S. industries into the environment for the year 1992. The nationwide emission estimate for the United States of 4500 t/a made by Nazaroff and Alvarez-Cohen(63) is based on a U.S. EPA report from 1993.
3.2. Indoor Sources
Generally speaking, exposure to formaldehyde is higher indoors than outdoors. This is mainly due to the stronger sources and low air exchange rates in the indoor environment.(64) A special situation arises for workplaces, which are not, however, treated in detail in this review. A general overview of formaldehyde sources, which might contribute to increased indoor concentrations, is shown in Figure 3. More specific information on different sources is provided in Table 2.
Figure 3.
Possible indoor- and outdoor-related formaldehyde sources.
Table 2. Potential Formaldehyde Sources in the Indoor Environment as Determined in Different Studies.
| source | comments | ref |
|---|---|---|
| Wood and Wood-Based Products | ||
| solid wood | oak, Douglas fir, beech, spruce, pine | (43) |
| particle board | effect of hot-pressing | (412) |
| particle board | recycled wood-waste sprayed with PMDI/PF | (423) |
| particle board, MDF | comparison of standard methods | (133) |
| particle board | effect of aging | (424) |
| particle board | effect of humidity and temperature | (425) |
| particle board | (168) | |
| oriented-strand board | comparison of analytical techniques | (246) |
| wood-based composites | laminate, engineered flooring, MDF, particle board | (247,426) |
| wood based panels | effect of loading and ventilation | (427) |
| wood panels | interlaboratory comparison | (178) |
| particle board, plywood | with carpet and insulation | (428,429) |
| pressed wood products | (179,430) | |
| wood-based flooring materials | effect of ozone, infrared, sunlight, UV-A, UV-B | (256) |
| Insulation Materials | ||
| mineral wool | (249) | |
| mineral wool | interlaboratory comparison | (431) |
| Flooring Materials | ||
| carpet | interaction of ozone | (78,85) |
| laminate | effect of temperature | (248) |
| Cork products | natural cork and cork tiles | (415) |
| building finishing materials | effect of temperature | (432) |
| Coating Materials | ||
| latex paint | (74,75) | |
| latex paint | presence of ozone | (88) |
| water-based paint | emission of biocides | (76) |
| natural paint | presence of ozone | (408) |
| photocatalytic paint | effect of irradiation | (93−95) |
| Combustion | ||
| wood burning | wood-heated homes in Quebec, Canada | (433) |
| wood burning | wood-heated homes in Sweden | (434) |
| cooking stoves | (435) | |
| cooking | residential cooking activities in a test house | (97) |
| burning of incense | measured in temples | (436) |
| cigarette smoking | (98,100,261) | |
| mosquito coils and candles | (437) | |
| Miscellaneous | ||
| personal computers | (438) | |
| laser printers, photocopiers | (439−441) | |
| miscellaneous building materials | pine wood, gypsum board, wallpaper, carpet, PVC, linoleum, paint, and presence of ozone | (71) |
| furniture and home equipment | parquet, sofa, table, chair, carpet, book shelves | (244) |
| miscellaneous building materials | plywood, particle board, hard board, carpet, barrier materials | (243) |
| miscellaneous building materials | test house study | (442) |
| textiles, permanent-press fabrics | effect of aging, temperature, humidity | (73,443,444) |
| wall coverings | paper, acrylic, PVC | (70) |
| cleaning products, air fresheners | (445) | |
| car air freshener | presence of ozone | (84) |
| chemical products | formaldehyde and formaldehyde releasers | (446) |
| cleaning activities | (447) | |
| household products | presence of ozone | (82,448) |
| consumer products | 55 materials studied | (73) |
| miscellaneous materials | carpet, wall, floor, cooking oil, and presence of ozone | (72) |
| miscellaneous materials | wood-based products, carpet, textiles, heaters, burners, cigarettes | (263) |
| miscellaneous polymeric materials | PVC, carpet, SBR, wall coverings, rubber foam backing | (449) |
| VOC mixtures | presence of ozone | (255,450,451) |
| portable air cleaners | with and without air freshners | (258) |
| miscellaneous materials | ozone reactions during disinfection | (86,87) |
| miscellaneous materials | aircraft cabin materials and clothing fabrics | (91) |
| newspaper/books/journals | (452) | |
| preservative | anatomical dissection course | (288,328) |
| human metabolites | breath air | (103−106) |
3.2.1. Wood-Based Materials
In the past, there was a link between the two items “formaldehyde emission” and “wood-based products”. This negative image resulted from the high formaldehyde release from UF-bonded particle board under living conditions in the 1960s and 1970s. Dwellings in which particle board was used extensively, such as prefabricated houses and mobile homes, were particularly concerned, and many occupants complained about bad odors and adverse health effects. Subsequent emissions of formaldehyde are due to the presence of small amounts of free formaldehyde in the resin and to the reversibility of the urea−formaldehyde reaction (see eqs 5 and 6). In Germany, the first publication dealing with this topic appeared in 1962.(65) Formaldehyde emission from UF-bonded particle board may continue for months or even years,(66) but the emission potential decreases with increasing age. Since 1970 formaldehyde emission rates from particle board and other wood-based materials have decreased as a consequence of governmental and voluntary guidelines and regulations. Wood-based products bonded with PF adhesives show comparatively low formaldehyde emission potentials because the cross-linking is more stable. Furthermore, environmentally friendly adhesives using natural tannin have been developed to reduce the dependence on formaldehyde-based adhesives.67−69
3.2.2. Flooring Materials
Most laminates used in furniture production are impregnated with modified aminoplastic resins and finished with lacquer. The emission of formaldehyde from veneered and laminated wood-based products is mainly caused by adhesives and glueing. However, low-emitting resins and new manufacturing techniques have distinctly improved such products. Paper is known to be the main source for formaldehyde emission from wall coverings.(70) In the case of flooring materials such as carpet, parquet, laminate, PVC, and linoleum, the emission of formaldehyde is of no or only minor importance in the absence of ozone.71,72
3.2.3. Insulation Materials
Mineral wool is preferred for insulation purposes in walls or floorings. This product is made from molten glass, stone, or slag that is spun into fibers. Inorganic rock or slag is the main component (typically 97%) of stone wool. The remaining 3% organic content is generally a thermosetting resin binder and oil. Glass wool (GW) is made from sand or recycled glass, limestone, and soda ash and usually contains 95%−96% inorganic material. Urea-modified phenol−formaldehyde resins are used as binders, producing low emissions of formaldehyde during use. Higher emissions are known from insulation materials made of UF foam, but this form of insulation is of little importance today.
3.2.4. Coatings
As far as liquid coating materials are concerned, acid-curing lacquers made of modified urea− and melamine−formaldehyde resins were the strongest formaldehyde source.(73) Mainly for that reason they have almost completely disappeared from the market in central Europe. Nevertheless, acid-curing wood finishes are still applied in Scandinavia, Eastern Europe, and Asia (http://www.kompass.com). High emissions of formaldehyde from latex paint have been reported by Chang et al.74,75 Formaldehyde and formaldehyde-releasing compounds such as dimethylol glycol and dimethylol urea were routinely used as biocides in water-based paints and fungicidal products but have now been widely replaced by other compounds such as isothiazolinones.(76) The release of formaldehyde from catalytic paints will be treated in the next section.
3.2.5. Indoor Chemistry: General Aspects
Indoor chemistry is a special but sometimes important source of formaldehyde. Wolkoff et al.(77) stated that reactions between unsaturated VOCs and ozone can form irritants that may be responsible for many reported symptoms. One of the first indoor-related papers on this topic was published in 1992 by Weschler et al.,(78) who reported the emission of formaldehyde and other aldehydes from carpeting in the presence of ozone, while concentrations of unsaturated compounds such as 4-phenylcyclohexene (PCH), 4-vinylcyclohexene (VCH), and styrene decreased. In the indoor environment, we have a situation in which ozone concentrations are lower, as compared with the outdoor environment,(79) while concentrations of unsaturated compounds such as terpenes are in contrast distinctly higher. Many terpenoids present in indoor air, such as limonene (kO3 = 200 × 10−18 cm3 molecule−1 s−1), myrcene (kO3 = 470 × 10−18 cm3 molecule−1 s−1), and terpinolene (kO3 = 1880 × 10−18 cm3 molecule−1 s−1), exhibit high gas-phase reaction constants with ozone.80,81 Terpene/ozone reaction rates in indoor and outdoor air can therefore be of the same order of magnitude. It has been shown recently that many household products contain terpenes and can rapidly react with ozone under indoor-related conditions.82−84 Other building products have also been studied for the emission of so-called secondary products as the result of indoor-related chemical reactions.71,72,85−88 Furthermore, formaldehyde has been detected as a reaction product of ozone-initiated chemistry in aircraft cabins89,90 and as a byproduct of surface reactions with aircraft cabin materials.(91) Wisthaler et al.(90) and later Petrick and Dubowski(92) have identified oxidation reactions of squalene, which is a major component of the skin, as a directly human related formaldehyde source. Short-term formaldehyde emissions under test chamber conditions have been found with indoor wall paints equipped with modified TiO2 to serve as a catalyst under daylight or artificial light to reduce indoor air pollutants.93−95
3.2.6. Indoor Combustion: General Aspects
Thermal treatment and combustion are known to be strong sources of formaldehyde indoors. Balakrishnan et al. pointed out that about half of the world’s population, especially the developing countries, relies on traditional fuels such as biomass as the primary source of domestic energy.(96) Besides CO, NOx, SO2, polycyclic aromatic hydrocarbons (PAH), and particulates, formaldehyde is one of the main components emitted from biomass fuel smoke. Residential cooking activities were also identified as formaldehyde sources.(97) In developed countries, cigarette smoke is the primary combustion source indoors.98−101
3.2.7. Other Indoor-Related Sources
In his recent review on changes in indoor pollutants since the 1950s, Weschler(102) notes that “easy care” and “permanent press” fabrics, especially T-shirts, pants, and shirts, were introduced in the 1960s. These fabrics had been treated with formaldehyde resins and had significant formaldehyde emissions close to the breathing zone. These resins have since been improved, and such fabrics emit less formaldehyde today. There are many more potential sources of formaldehyde in the indoor environment, such as electronic equipment, paper, fabric dyes, inks, cosmetics, objects for anatomy dissection, etc., but it is not possible to examine them in detail here (see Table 2).
Last but not least, it should be mentioned that traces of formaldehyde have also been discovered as a product of human metabolic reactions. Lindinger et al.(103) have shown that formaldehyde is a component of exhaled human breath. Wehinger et al.(104) have identified increased levels of formaldehyde in exhaled breath samples from primary lung cancer patients. Moser et al.(105) report formaldehyde concentrations in the deep lung portion of human breath up to 72.7 ppbv with a median of 4.3 ppbv. Kushch et al.(106) have examined trace compounds in the exhaled breath of 81 smokers, 210 nonsmokers, and 79 ex-smokers. For formaldehyde, median values of about 10 ppb were measured and no statistically significant difference between smokers and nonsmokers was observed. However, the experimental technique of proton-transfer-reaction mass spectrometry (PTR-MS), being used in all cited studies for the measurement of formaldehyde,103−106 has been criticized by several authors(485) (see the next section).
4. Sampling and Analysis of Formaldehyde
4.1. Analytical Methods
4.1.1. In-Situ Methods
For the analysis of formaldehyde in the outdoor environment, spectroscopic techniques are convenient. Vairavamurthy et al.(107) have reviewed four in situ monitoring techniques: (a) differential optical absorption spectroscopy (DOAS); (b) Fourier transform infrared absorption (FTIR); (c) laser-induced fluorescence spectroscopy (LIFS); and (d) tunable diode laser spectroscopy (TDLS). Finlayson-Pitts and Pitts(12) have compared formaldehyde detection limits for FTIR, TDLS, and matrix isolation IR (see Table 3). Vairavamurthy et al. also pointed out that in situ techniques usually require long optical paths, which makes these methods unsuitable for routine applications. Infrared diode laser spectroscopy was nevertheless used by Hanoune et al. for formaldehyde measurements at ppb levels in libraries.108,109 Photoacoustic spectroscopy (PAS) has been used occasionally for the determination of formaldehyde in indoor air.110−112 However, this method is susceptible to interference and suffers from high detection limits. Cihelka et al.(113) used diode lasers in combination with FTIR and photacoustics and achieved detection limits of <100 ppb. Proton-transfer-reaction mass spectrometry (PTR-MS) is based on chemical ionization using H3O+ as the primary reactant ion.(486) The method has been successfully applied in monitoring formaldehyde in outdoor and indoor air,(103) although de Gouw and Warneke state that formaldehyde is a difficult compound to detect by PTR-MS.(114) According to Wisthaler et al.,(142) the PTR-MS method is less sensitive to formaldehyde than other carbonyl compounds due to the loss of protonated formaldehyde resulting from the reaction with water.(115) Kushch(106) suggested that m/z 31 ions (protonated formaldehyde) should be corrected for the isotope effects of NO. Fragments of the reaction products of ethanol and O2+ or methanol and O2+ may also be observed. Thekedar et al. have excluded m/z 31 from breath gas analysis for the reasons that the sensitivity depends strongly on humidity and that formaldehyde is present in room air samples in much higher concentration than in the exhaled breath.(116) As a potential alternative to PTR-MS, Spanel and Smith have applied selected ion flow tube mass spectrometry (SIFT-MS) for the detection of formaldehyde in breath gas.(484) Hak et al.(117) have made an intercomparison study of four different in situ techniques for ambient formaldehyde measurement in urban air. Formaldehyde concentrations obtained using continuously measuring DOAS, FTIR, and Hantzsch instruments agreed within 11%, while two-hour integrated samples obtained by DNPH (see below) presented concentrations up to 25% lower. In addition, the authors provide a detailed review of previous formaldehyde intercomparison studies.
Table 3. Overview of Sampling Methods and Analytical Techniques for the Determination of Formaldehyde in Air.
| method | comments | ref |
|---|---|---|
| In Situ | ||
| FTIR | LODa = 6 ppb at L = 1 km (ν̃b = 2779, 2781.5 cm−1) | (12) |
| TDLS | LODa = 0.05 ppb at L = 150 m (ν̃b = 2781 cm−1) | (12) |
| matrix isolation IR | LODa = 0.03 ppb | (12) |
| DOAS | (107,453) | |
| PTR-MS | relatively low sensitivity for formaldehyde | (103) |
| SIFT-MS | relatively low sensitivity for formaldehyde | (484) |
| photoacoustics | LODa = 60 ppb (λc = 3.6 μm), interferences with other pollutants | (110) |
| Derivatization | ||
| acetylacetone (acac) | UV/vis and fluorimetry (Nash reagent) | (132,138,139) |
| DNPH | HPLC-UV/vis (also can be used for higher aldehydes) | (146,148) |
| MBTH | LODa = 0.08 ppb, near real-time measurement | (112,124,125) |
| dimedone | UV/vis and fluorimetry | (454,455) |
| pararosaniline | UV/vis | (2,107,120,121) |
| chromotropic acid | UV/vis | (2,107,120) |
| AHMT | UV/vis | (2,107,120,126) |
| PFBOAd | headspace GC/MS | (456) |
| kinetic fluorimetry | formaldehyde as a catalyst for rhodamine B oxidation | (457) |
| Sensors | ||
| microgas sensor | LODa = 0.06 ppm | (156) |
| microgas sensor | MWCNT or palladium-doped (LODa = 0.03 ppm) | (158,159) |
| cataluminescence | online monitoring using gas sensor | (157) |
| biosensor | LODa = 50 ppb, online detection using dehydrogenase reaction | (162) |
| Comparison of Methods | ||
| derivatization | acac vs pararosaniline and chromotropic acid methods | (118) |
| derivatization | DNPH vs acac | (155) |
| derivatization + in situ | DNPH vs TDLS | (108) |
| in situ | DOAS, FTIR, acac, DNPH | (117) |
| derivatization + in situ | acac, DNPH, DOAS, PTR-MS | (142) |
LOD = limit of detection.
Wavenumber.
Center wavelength of pho-acoustic instrument.
o-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine.
4.1.2. Derivatization Methods
For indoor applications, batch-sampling methods are more convenient. Here, formaldehyde is trapped in an absorber or on impregnated filters and cartridges. The compound is then derivatized and the resulting chromophore can be analyzed by chromatography and/or spectroscopy. However, a common problem is their nonspecificity for formaldehyde if no chromatographic separation is applied. Most reagents also react with other carbonyl compounds, and these byproduct could interfere with the target analyte.(118) Different methods for the analysis of aldehydes have been reviewed and discussed by several authors.2,107,119 Brief descriptions will be given of most of the derivatization methods (see refs (2,107,120) for chemical reaction schemes), but the chromotropic acid, the acetylacetone, and the DNPH methods will be considered in more detail. Other techniques are summarized in Table 3. Titrimetric methods are not discussed in this review, and the reader is referred to the book by Roffael(2) and the review by Marutzky.(31)
Pararosaniline Method.(121) A magenta dye is formed from formaldehyde and pararosaniline in the presence of sodium sulfite. Under acidic conditions, this intermediate reacts with SO2 to form the chromophore. Its strong absorbance at 570 nm is used in UV/vis detection. Other aldehydes, such as acetaldehyde, acrolein, and propanal, interfere, but at pH ≤ 1.0, the reaction is specific for formaldehyde. In the presence of atmospheric SO2, a toxic Hg(II) reagent is required in order to eliminate the sulfite formed from SO2. Although the sensitivity of the modified pararosaniline method is limited and it is susceptible to interference, it has in the past been one of the most widely used techniques for the determination of formaldehyde.
The MBTH method122−124 (3-methyl-2-benzothiazolinonehydrazone) is a nonselective colorimetric method for the determination of aliphatic aldehydes of low molecular weight. MBTH reacts on aldehydes to give an azide. In parallel with this, a reactive cation is formed by oxidation of MBTH with Fe(III). In a further step, a blue ionic dye is formed. Absorbance is monitored at 628 nm. This reaction is less sensitive than that of the pararosaniline method. Other aldehydes undergo an analogous reaction, but the yield is generally lower. The MBTH method quantifies total aldehydes in ambient air in terms of their formaldehyde equivalents. Strong reducing agents can interfere with the determination of aldehydes. An online detection system has been described by Toda et al.(125)
AHMT (4-amino-3-hydrazino-5-mercapto-4H-1,2,4-triazole) reacts with aldehydes in strong alkaline media to form a colorless intermediate product. This product is oxidized by atmospheric oxygen to give a magenta-colored dye, detectable by colorimetry at a wavelength of 550 nm. The sensitivity of the AHMT reagent varies, depending on the type of reacting aldehyde. On the basis of the AHMT reaction, Kawamura et al.(126) have developed a sensor to measure formaldehyde concentrations in the range of 0.04−1 ppm with a sampling time of 3 min. The authors explain that their device has been developed for prevention and control of the “Sick Building Syndrome” (SBS). This statement has to be questioned, since SBS is a very complex phenomenon (see section ).
Chromotropic Acid Method.(127) In the presence of concentrated sulfuric acid, chromotropic acid (1,8-dihydroxynaphthalene-3,6-disulfonic acid) reacts with formaldehyde to give a red-violet hydroxydiphenylmethane derivative (see eq 9). In the second step of the reaction, a violet quinoid oxidation product is formed with atmospheric oxygen. The concentrated sulfuric acid is a catalyst for dehydration and oxidation. The absorption maximum at 580 nm is used for UV/vis detection. The reaction is specific for formaldehyde when the pH value is <1.0. Interference can be caused by phenols, some other organic substances, and strong oxidizers. One of its main disadvantages is the low stability of chromotropic acid in solution. Nevertheless, the method has been standardized in the USA by NIOSH(128) and is used for the determination of formaldehyde in large-scale chambers,(129) in small-scale chambers,(130) and in the desiccator method.(131)
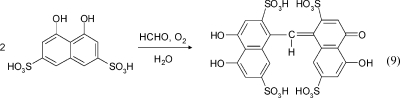 |
The acetylacetone (acac) method as described by Nash(132) is a widely applied(133) standard procedure and recommended in Europe134−136 and Japan(137) for the determination of formaldehyde emissions from wood-based materials. The reaction, which is based on the Hantzsch synthesis, involves the cyclization of 2,4-pentanedione (acac), ammonium acetate, and formaldehyde to form the dihydropyridine 3,5-diacetyl-1,4-dihydrolutidine (DDL) (see eq 10). Quantification can be performed by UV/vis spectroscopy at 412 nm (ε(H2O) = 7850 L mol−1 cm−1). The molecule also exhibits fluorescence (ϕ(H2O) = 0.005), thus offering the possibility of very selective fluorimetric determination at 510 nm,138,139 since other carbonyl compounds do not form strongly fluorescent Hantzsch products.107,140 Sampling is carried out by passing air through an absorber where formaldehyde is quantitatively trapped in distilled water. After addition of 2,4-pentanedione and ammonium acetate, the reaction to form DDL is completed within 10 min at 40 °C. The long sampling time of 40 min could be a drawback of the conventional acac method in the case of dynamic processes where the formaldehyde concentration rapidly changes over time. Portable instruments are now available which enable reliable in situ measurement of formaldehyde on a time scale of seconds.141−143 Acetoacetanilide has been introduced as an alternative to acac for the derivatization of formaldehyde based on the Hantzsch reaction.(144) The authors state that the reaction with acetoacetanilide can be carried out at room temperature.
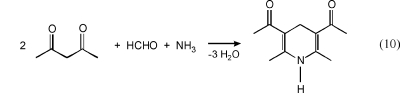 |
The DNPH method145,146 is frequently used for the simultaneous analysis of formaldehyde, other aldehydes, and ketones. In acidic solution, hydrazones are formed from 2,4-dinitrophenylhydrazine (DNPH) by nucleophilic addition to the carbonyl group, followed by elimination of water (see eq 11). In sampling, air is pulled through cartridges typically containing silica gel and coated with an acid solution of DNPH (today XAD-2 is only very rarely used as adsorbent(147)). To prevent water from condensing on the surface, the cartridge is sometimes covered with C18-alkyles.(148) After sampling, the cartridge is then eluted with acetonitrile. This eluate is used directly for HPLC analysis. Chromatographic separation of the hydrazones is achieved by means of a C18 column and water/acetonitrile solvent combinations with binary or ternary gradients. UV spectroscopy is used for detection, with the absorption maxima of different hydrazones ranging from 340 to 427 nm.(146) This method is described in U.S. EPA Method TO-11A,(149) ASTM D 5197(150) and is accepted as an international standard by ISO.(151) It is also applied in several standards for the determination of the emission of formaldehyde from building products.152,153
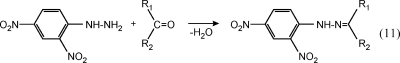 |
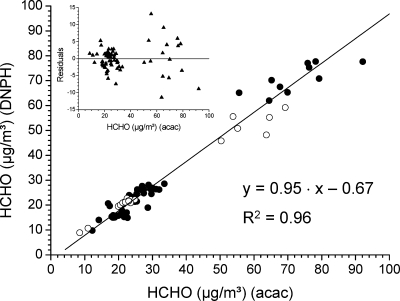
The data presented in Figure 4 are obtained from a recent study.(154) They show a very good agreement between the acac and DNPH methods, and both analytical techniques are equivalent as regards the determination of formaldehyde in indoor air. The statistical deviations between the acac and DNPH methods increase with higher formaldehyde concentrations. This is obvious from the plot of residuals (calculated value against measured value) (inset in Figure 4). A similar observation of increasing standard deviations was made by Hanoune et al.(108) when comparing the DNPH method with infrared diode laser spectroscopy. A fair correlation between the acac and DNPH methods was also observed by Trapp and De Serves(155) at low atmospheric concentrations. On the other hand, Wisthaler et al.(142) found that DNPH-HPLC data severely underestimated formaldehyde levels in the atmosphere simulation chamber (SAPHIR). This was explained by a suppressed hydrazine-to-hydrazone conversion at low humidities. The acac method with derivatization to DDL in aqueous solution followed by fluorimetric determination is probably the most reliable method of measuring formaldehyde in indoor air although the fluorescence response is dependent on temperature and DDL decomposes on exposure to heat or light.(139)
Figure 4.
Linear relationship between formaldehyde concentrations in a test house measured by the acac and the DNPH methods for 78 data points. The inset shows the unweighted residuals plotted (reprinted from ref (154) with permission from Elsevier).
4.1.3. Sensors
There is an increasing demand for fast and simple formaldehyde indoor monitoring methods, and this has stimulated research activities in the field of sensor technology. However, the sensors available still suffer from comparatively high detection limits, which makes the technique mainly suitable for workplace environments. Lv et al.(156) have developed a microgas sensor based on a microhot plate, which can detect a concentration of 0.06 ppm of indoor formaldehyde. Zhou et al.(157) describe a cataluminescence-based gas sensor using nanosized V2Ti4O13 as a probe for online determination of formaldehyde in air which has a detection limit of 0.06 mg m−3. A semiconductor gas sensor of tin oxide doped with hydroxyl-functionalized multiwall carbon nanotubes (MWCNTs) has been designed and tested by Wang et al.(158) The same authors describe a formaldehyde gas microsensor of palladium-doped tin dioxide on a silicon substrate.(159) Both sensors are able to detect formaldehyde concentrations of 0.03 ppm. Other common types of sensors are based on enzyme reactions. In the presence of oxidized nicotinamide adenine dinucleotide (NAD+) as a catalyst, formaldehyde is oxidized to form formic acid, which is detected by electrochemical or photochemical techniques.160−163 The conductometric biosensor developed by Vianello et al.(162) provides an indoor-related detection limit of 50 ppb. Seo et al. describe the biosensing of formaldehyde on the basis of fungal growth.(164)
4.1.4. Future Trends in Formaldehyde Analysis
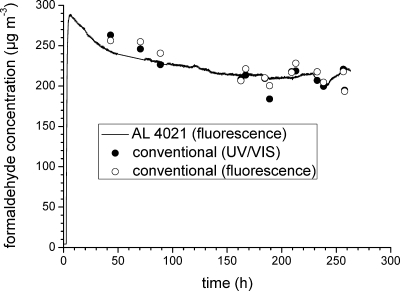
In the indoor environment, formaldehyde concentrations of interest range between 1 and 1000 ppb. Sampling or rather measuring devices should be transportable, robust, and easy to calibrate. Most of the spectroscopic techniques, such as DOAS, FTIR, and TDLS, do not fulfill these requirements, but they are useful for determining sub-ppb levels in rural or remote areas. Derivatization methods are state-of-the-art for the indoor analysis of formaldehyde, with the most important being the chromotropic acid, DNPH, and acetylacetone methods. All three have the drawback of long sampling times, typically 0.5−2 h. The standardized DNPH method(151) is also used for higher aldehydes. Nevertheless, the acetylacetone method is easier to use, specific, and highly sensitive for formaldehyde when combined with fluorescence spectroscopy. This makes the method very attractive for use in online measuring devices. The development of such techniques is urgently needed for the study of dynamic processes and is definitely among current and future trends. The formaldehyde online monitor AL-4021 (see http://www.aero-laser.de) is based on the acac method, is commercially available, and has been tested by Hak et al.(117) for measurement of formaldehyde in urban air. In our institute, AL-4021 has been successfully applied for the time-resolved determination of formaldehyde in test chambers. In Figure 5, the time vs formaldehyde concentration curve of an MDF board in a 1 m3 chamber (T = 23 °C, r.h. = 45%, n = 1.0 h−1, L = 1.0 m2 m−3) is shown. The solid line has been recorded by use of AL-4021 with a time resolution of 1 s. The data represented by solid and open circles have been measured with the conventional acac method by UV/vis and fluorescence detection, respectively.
Figure 5.
Formaldehyde emission from a MDF board in a 1 m3 chamber at T = 23 °C, r.h. = 45%, n = 1 h−1, and L = 1 m2 m−3. Solid line, online detection using an AL-4021 formaldehyde analyzer; solid circles (●), conventional acac method with UV/vis detection; open circles (○), conventional acac method with fluorescence detection.
Modern nanotechnology also opens up possibilities for new measurement techniques, especially sensors. At the present time, detection limits are inadequate, but it is foreseeable that the next generation of sensors may be suitable for many applications in active and passive formaldehyde measurement. A comprehensive review covering many aspects of carbonyl sampling and analysis in indoor air has recently been published by Barro et al.(165) In the present publication, the reader is referred to Table 3, where analytical procedures are summarized.
4.2. Emission Testing Using Chambers and Cells
4.2.1. Chambers
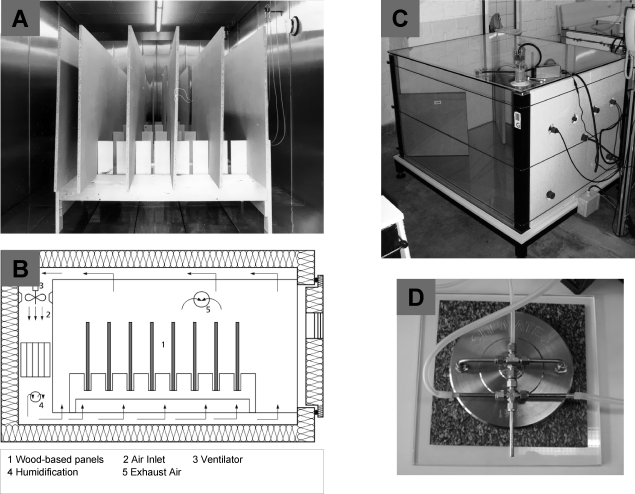
The evaluation of the formaldehyde emission potential of individual products and materials under indoor-related conditions and over defined time scales requires the use of climate-controlled emission testing systems, so-called emission test chambers, and cells, the size of which can vary between a few cubic centimeters, and several cubic meters, depending on the application. The first room-sized test chambers were developed in the mid-1970s when building authority regulations were being introduced concerning formaldehyde emissions from wood particle boards (see Figure 6A and B). Large chambers can be regarded as the “standard meter” in formaldehyde testing, as only with this type are real-life-related scenarios possible. The formaldehyde test methods used in North America, Europe, and China are derived from large-chamber studies. Nowadays, a common size for an emission test chamber is 1 m3 and the interior is usually made of glass(166) (see Figure 6C) or stainless steel.(167) Chambers smaller than this are used only occasionally for the testing of formaldehyde. However, Crump et al.(168) have demonstrated a good comparability between a 1 m3 chamber, a 4.5 L chamber, and a 2.4 L chamber for measuring formaldehyde emission from wood-based particle and fiber boards. In the United States the so-called Dynamic Micro Chamber (DMC) is used.(169) The DMC is an apparatus for measuring formaldehyde emission from composite wood products bonded with urea−formaldehyde adhesives and employs a combination of a small sample chamber and an electrochemical sensor. So far, the DMC method has not been developed into an international standard. Large and small chambers have only recently gained new importance due to the California Air Resources Board (CARB) (see http://www.arb.ca.gov) approving regulations to reduce the emission of formaldehyde from wood-based panels,(170) in accordance with ASTM E 1333 (large chamber) and ASTM D 6007 (small chamber).
Figure 6.
Different devices for measuring formaldehyde emission from building products under indoor-related conditions. (A) 48 m3 stainless steel chamber with particle board; (B) diagram of the 48 m3 stainless steel chamber; (C) 1 m3 glass chamber with particle board; (D) field and laboratory emission cell (FLEC) with floor covering.
During an emission investigation, the product or material is tested with regard to temperature (T), relative humidity (r.h.), air exchange rate (n), air velocity, and product loading factor (L = ratio of the surface of the product to be investigated to the volume of the emission test chamber) under standardized conditions in the testing device. This can be sealed to exclude gas from the outside atmosphere. This procedure is suitable for measuring the formaldehyde concentration in air and the product specific emission rate (SER). The latter can be related to sample length (μg m−1 h−1), volume (μg m−3 h−1), area (μg m−2 h−1), or unit (μg unit−1 h−1). Note that different standards use different methods for sample preparation, measurement, and expressing the results. In most cases, the result is presented as an area-specific emission rate129,153 or as a chamber concentration in the steady state(134) (see section ). The chamber test is a conventional process in which conditions are selected in such a way that they reflect those to be found in realistic indoor rooms. In interpreting the results of test chamber/cell investigations, it must, under certain circumstances, be accepted as a limiting factor that not all realistic conditions to be found in an indoor room can be simulated. Groah et al.(171) have compared different protocols and analytical methods for the large-chamber testing of formaldehyde in Europe and in North America. The European protocol produced values that were 20% lower than the North American protocol, but strong linear relationships between the tests could be observed.
4.2.2. Field and Laboratory Emission Cell (FLEC)
It is also desirable to have a measuring system that can be used to carry out emission testing and quality assurance on location. The relevant principle of a transportable emission testing cell for mobile application was implemented in Scandinavia for the first time in 1991 with the so-called field and laboratory emission cell (FLEC).172,173 The FLEC as an example of a frequently used type of cell is shown in Figure 6D. Air flow conditions in the FLEC have been described by several authors.174,175 The device now makes it possible to carry out nondestructive emission testing on surfaces within the framework of field investigations. The FLEC has been tested for formaldehyde applications,(176) but a standardized method for measuring formaldehyde emissions has not yet been established. The general requirements relating to the design and properties of an emission cell are described in ISO standard 16000-10.(177)
4.2.3. Determination of Emission Rates and Steady-State Concentrations
Air measurement in a chamber or cell initially produces the concentration C(t) at the time t of the measurement. For better comparability of the measured data, the specific emission rate (SER) independent of air exchange and loading is to be preferred. In some publications, the specific emission rate (area or unit) is called the emission factor (EF).
The time-dependent determination of the emission potential is carried out on the basis of the balance equation (eq 12), where C(t) = chamber concentration (in μg m−3 or mg m−3), n = air exchange (in h−1), and L = loading (in m2 m−3).
For a decaying concentration−time function, SER(t) is obtained from eq 12 by transition to the difference quotient according to eq 13.
where
This means that if n + 1 experimental data are available for concentration, it will be possible to obtain n − 1 emission rate values by this method. In the steady-state (dC/dt = 0), eq 12 progresses to eq 15, which is frequently used for the determination of emission rates.
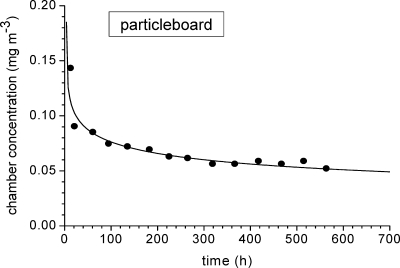
In the case of wood-based materials, the time vs concentration curve often decreases continuously and the power function 16, as proposed by Colombo et al.,(178) can be applied for the interpolation of the data. An example is provided in Figure 7 for the least-squares fit to eq 16 of the formaldehyde concentration data from a 48 m3 chamber test at T = 23 °C, r.h. = 45%, n = 1 h−1, and L = 1 m2 m−3 (see figure captions for fit parameters).
Figure 7.
Formaldehyde emission from particle board in a large chamber (48 m3) at T = 23 °C, r.h. = 45%, n = 1 h−1, and L = 1 m2 m−3. The interpolation curve was obtained from nonlinear regression analysis using eq 16 with A = 1.18, B = 4.76, and D = 0.29.
C(t) is the chamber concentration in milligrams per cubic meter, t is the time in hours, and A, B, and D are fit parameters. The steady-state concentration is determined from eq 16 on the basis of a procedure given in European standard EN 717-1. It must always be kept in mind that this function goes to infinity when approaching zero and does not take peak values into account.
4.3. Material Testing
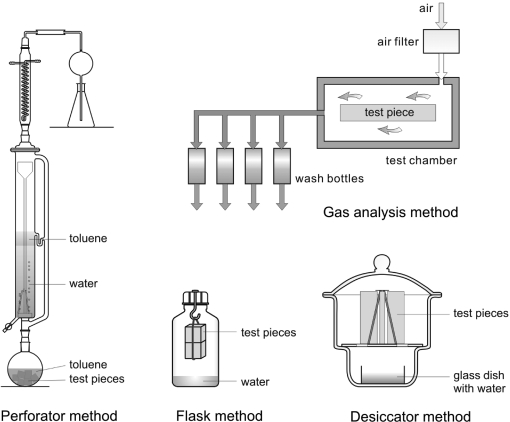
Formaldehyde testing in chambers and cells is usually time-consuming and calls for sophisticated equipment. For pretesting and production control, the so-called derived methods have been developed. These methods are based on determining the formaldehyde content or formaldehyde release under special testing conditions. Several derived methods are available for wood-based panels in particular (see Table 4 and Figure 8). Critical reviews of the methods are provided by Roffael,(2) Marutzky,(31) Marutzky and Margosian,(179) and Risholm-Sundmann et al.(133) The first correlations between chamber testing and derived methods were given by Mehlhorn.(180)
Table 4. Standard Methods for Formaldehyde Emission Testing.
| method | standard | volume | ref |
|---|---|---|---|
| chamber method | EN 717-1 | >12 m3, 1 m3, 0.225 m3 | (134) |
| JIS A 1901 | 20 L to 1 m3 | (152) | |
| JIS A 1911 | >1 m3 to 80 m3 | (153) | |
| ASTM E 1333 | >22 m3 | (458) | |
| ASTM D 6007 | 1 m3 | (130) | |
| desiccator method | ASTM D 5582 | ≈ 10.5 La | (131) |
| JIS A 1460 | 9−11 L | (137) | |
| JASb | 9−11 L | (459)c | |
| gas analysis method | EN 717-2 | 4 L chamber | (135) |
| flask method | EN 717-3 | 500 mL flask | (136) |
| perforator method | EN 120 | (460) |
Inside diameter is 250 mm.
JAS no. depends on product.
Refers to JAS 233 for plywood.
Figure 8.
Standardized laboratory methods (perforator, gas analysis, flask, desiccator) for the determination of formaldehyde release from wood-based materials.
4.3.1. Perforator Method
The formaldehyde content of wood-based panels is determined by the perforator method. The content principally correlates with the emission value, especially for wood composites of similar structure and density. The method was developed in the late 1960s by the former European Particleboard Federation (FESYP). Since 1984, it has become established as European standard EN 120. It is a procedure for extracting small samples of wood-based panels by means of boiling toluene and is suitable for unlaminated and uncoated wood-based panels. The extracted formaldehyde is sampled through perforation in water and is measured in the aqueous solution by a suitable analytical procedure. The original method using iodine proved to be too unspecific and was later on replaced by the specific acetylacetone method. The perforator value depends on the moisture content of the tested samples. Correction factors, based on a reference moisture content, are used to compensate for this influence.(181) The test procedure needs comparably simple equipment and has a short total running time of 3 h. For these reasons, it is widely used for production control in the wood-based panel industry, especially in Europe and China.
4.3.2. Flask Method
Another simple test for wood-based panels is the flask test. It was developed by Roffael in 1975.(182) The test is based on storing one to three board pieces with a total mass close to 20 g in a closed polyethylene bottle with a volume of 400 cm3. The pieces are stored over 50 mL of distilled water for a defined period of time—usually 24 h—at a constant temperature of 40 °C. The formaldehyde released is absorbed by the water. The formaldehyde content of the aqueous solution is determined photometrically at 412 nm by the acetylacetone method and referred to the dry weight of the tested pieces. A slightly modified version of the method was later standardized as EN 717-3. Disadvantages of the method are the small quantity of material which can be tested and the unrealistic ratio of open edges to surfaces of the tested specimens. In spite of these limitations, the method is most suitable for production control of panels with a similar structure. Variations of the methods have been developed with larger bottles and modified testing times.
4.3.3. Desiccator Methods
The so-called desiccator methods131,137 are based on the same principles as the flask method. Pieces of wood-based panel of known surface area are positioned over water for 24 h at a constant temperature. Instead of a small plastic bottle, a glass desiccator with a volume of 9−13 L is used, thus permitting larger quantities of test material. Analysis of formaldehyde is usually carried out by either the acetylacetone method(137) or the chromotropic acid method.(131) A number of variations of the desiccator method exist (see Table 4). In the meantime, a standard harmonized between the wood-based panel industries of Australia, Japan, and New Zealand has been accepted by the International Standardization Organization as ISO/CD 12460-4.
4.3.4. Gas Analysis
An eminently suitable derived formaldehyde test is the gas analysis method, which determines the accelerated formaldehyde release at an elevated temperature of 60 °C. It can be used for all types of panels, including coated boards. This test is also used for testing formaldehyde-emitting impregnated papers, laminates, and insulation foams. The method requires a specimen of 400 mm × 50 mm × thickness. The sample is placed in a test tube at a controlled temperature of 60 °C. A gas stream of 1 L per minute is passed through the tube. The emitted formaldehyde is absorbed by gas-washing bottles and measured photometrically. The bottles are changed once per hour over a total testing time of 4 h. Usually the values measured are averaged, ignoring the first hour. The result is expressed in mg h−1 m−2. This procedure is standardized as EN 717-2.(135)
4.4. Air Sampling Strategies
Appropriate sampling strategies for the measurement of formaldehyde in indoor air are discussed by Gavin et al.(183) and in the ISO 16000-2 standard.(184) Discontinuous methods for measuring room-air components can be subdivided into short-term and long-term types. While active sampling is suitable not only for short but also—provided the air flow rate has been reduced correspondingly—for longer measurement intervals, passive sampling is used mostly for long-term measurement. In discontinuous methods, the measured value is first determined by subsequent analysis in the laboratory. In the case of active sampling, air is passed through a sampling device using a pump and the air volume is accurately determined. A passive sampler is a device which is capable of taking air samples at a rate controlled by diffusion through a static layer or permeation through a membrane.(185) The flow of pollutants into the passive sampler is proportional to the difference of concentrations in the ambient air (Cair) and on the surface of the passive sampler (CA), as shown in eq 17.
Di is the diffusion coefficient for compound i, mi is the collected mass of compound i, A is the surface area of the sampler, l is the length of diffusion, and t is the collection time. In the case of active sampling with short measurement time intervals (10 min to approximately 4 h), international guidelines have been drawn up for interior air measurement.(151) Formaldehyde is a highly volatile compound, which means that the indoor concentration will generally depend on the source strength and on the air exchange rate. Strong sinks such as gypsum board may influence the concentration by adsorption and desorption effects.(186) Due to its high solubility, water and other polar liquids act as permanent sinks for formaldehyde. For a constant emission source and an air exchange rate n, the time required to reach a desired percentage of the steady-state concentration C∞ is given by eq 18.
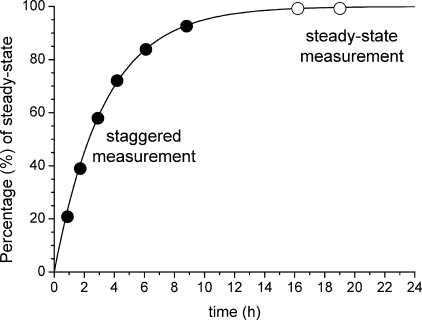
The solid curve in Figure 9 models the increase in formaldehyde concentration in a room after ventilation for an air exchange rate of n = 0.3 h−1 in the absence of sinks. Two sampling strategies can be applied: the solid circles in Figure 9 stand for a 1 h staggered strategy, in which sampling starts immediately after the windows are closed. The steady-state value must be calculated from eq 18. The open circles in Figure 9 represent possible sampling times at the steady-state level. This method depends on a good guess at the air exchange rate.
Figure 9.
Modeled increase of the formaldehyde concentration after ventilation for a continuous source and an air exchange rate of n = 0.3 h−1 in the absence of sinks (see eq 18). The solid circles (●) represent a staggered measuring strategy; the open circles (○) represent a steady-state measuring strategy (note: the circles represent possible sampling times, not data from measurements).
Passive sampling, whose theoretical fundamentals were described in detail and summarized by Crump,(185) is enjoying increasing popularity in indoor air testing, since it can be employed without causing any nuisance to room users. Different types of formaldehyde passive samplers have been employed.187−193 However, it must be borne in mind that passive collectors are usually left in a room for days or even weeks at a time without continuous monitoring by the analyst, and this means that the possibility of tampering cannot be excluded. One of the advantages of passive sampling is that person-related exposure can be determined in a simple manner by having the passive collector worn by an individual for a specific period.194,195 However, the result of passive sampling will depend on temperature, since the accumulation of molecules is driven by diffusion, which is a function of the gas kinetic properties. Another critical parameter is the flow rate, and low air velocities will cause undervalued concentrations. A standard procedure for passive sampling of formaldehyde in indoor air has been described by ISO.(196)
5. Fomaldehyde Indoor Guidelines
5.1. Guidelines by Category
Several safety and occupational health authorities worldwide have laid down permissible exposure levels of formaldehyde by inhalation. Most levels are based on results of epidemiological and toxicological test outcomes obtained from both human and animal data for a certain exposure time or are based on health hazard assessments in the relevant toxicological literature. Limit values are basically separated into two main categories: workplace environments in which occupational exposure occurs and nonoccupational (i.e., residential) environments. Such occupational threshold limit values (TLV) are often categorized as time-weighted average (TWA), short-term exposure limit (STEL), and ceiling (C) values, with the last defining the exposure limit, which should not be exceeded at any time.
The Occupational Safety and Health Administration (OSHA)(197) has set the STEL for formaldehyde at 2 ppm in 15 min and the permissible exposure limit time-weighted average (PEL-TWA) at 0.75 ppm. The TLV-C proposed by the American Conference of Governmental Industrial Hygienists (ACGIH)(198) is 0.3 ppm. The National Institute for Occupational Safety and Health (NIOSH)(199) has set a more stringent STEL of 0.1 ppm and a recommended exposure limit for occupational exposure of 0.016 ppm. Other occupational formaldehyde guideline values may be found in the papers by Duhayon et al.(200) and Paustenbach et al.(201) In general, occupational limit values are higher than indoor guideline values on account of two important factors. The basic difference between these guideline values depends on the vulnerability of the people staying in these environments. One of the factors is that nonoccupational indoor environments cover the general population, including infants, children, the elderly, pregnant women, and people allergic to formaldehyde. The other factor is that the general population is often exposed to lower formaldehyde levels over long time periods (i.e., during their lifetimes), while workers are assumed to be exposed to formaldehyde for about 8 h in a working day and 5 days a week. In a recent review, Zhang et al.(202) discussed and compared occupational and indoor formaldehyde guideline values.
As can be seen from Table 5, indoor guideline values can be roughly categorized into two groups based on exposure durations. The short-time exposure levels are used for preventing acute health effects on individuals while long-term exposure levels are used for preventing the chronic health effects of formaldehyde. The most common short-term exposure limit is 100 μg m−3 as a 0.5 h average value aimed at preventing significant sensory irritation in the general population and is recommended by the WHO.
Table 5. International Guideline Values and Recommendations for Formaldehyde in Indoor Air.
| country | year issued | value | comments | |
|---|---|---|---|---|
| Australia | 1982(226) | 0.1 ppm | 120 μg m−3 | short-duration |
| 2006(227) | 0.08 ppm | 100 μg m−3 | ||
| Canada | 1987(220) | 0.1 ppm | 120 μg m−3 | action level |
| 1987 | 0.05 ppm | 60 μg m−3 | target level | |
| 2005(22) | 0.1 ppm | 123 μg m−3 | 1 h | |
| 2005 | 0.04 ppm | 50 μg m−3 | 8 h | |
| China | 2003(225) | 0.08 ppm | 100 μg m−3 | 1 h average |
| Denmark | 1990(207) | 0.15 mg m−3 | ||
| Finland | 2001(209) | 30 μg m−3 | S1 | |
| 50 μg m−3 |
S2 | |||
| 100 μg m−3 |
S3 | |||
| France | 2008(213) | 50 μg m−3 | 2 h (proposed) | |
|
10 μg m−3 |
long-term exposure (proposed) | |||
| Germany | 1977(216) | 0.1 ppm | ||
| Singapore | 1996(224) | 0.1 ppm | 120 μg m−3 | 8 h |
| Hong Kong | 1999 | 0.025 ppm | 30 μg m−3 | level 1 (8 h) |
| 0.081 ppm |
100 μg m−3 | level 2 (8 h) | ||
| 0.3 ppm |
370 μg m−3 | level 3 (8 h) | ||
| 2003(221) | 0.025 ppm | 30 μg m−3 | excellent | |
| 0.081 ppm |
100 μg m−3 | good | ||
| Japan | 1997(223) | 0.08 ppm | 100 μg m−3 | 0.5 h |
| Korea | 2004(222) | 0.1 ppm | 120 μg m−3 | 8 h |
| Norway | 1990(210) | 0.05 ppm | 60 μg m−3 | 24 h average |
| 1999(211) | 0.05 ppm | 100 μg m−3 | 30 min average | |
| Sweden | 2000 | 0.08 ppm | 100 μg m−3 | adopted from WHO |
| Poland | 1996(215) | 0.04 ppm | 50 μg m−3 | category A: 24 h |
| 0.08 ppm |
100 μg m−3 | category B: 8−10 h | ||
| U.K. | 2004(208) | 100 μg m−3 | 0.5 h | |
| USA (California) | 1991(217) | 0.1 ppm | 120 μg m−3 | action level |
| 0.05 ppm |
60 μg m−3 | target level (ALARA)a | ||
| 1999(203) | 0.076 ppm | 94 μg m−3 | 1 h (acute REL)b | |
| 2004(219) | 0.027 ppm | 33 μg m−3 | 8 h (interim REL) | |
| 2005(218) | 0.002 ppm | 3 μg m−3 | annual average (chronic REL) | |
| WHO | 1987(228) | 0.08 ppm | 100 μg m−3 | 0.5 h average |
ALARA = as low as reasonably achievable.
REL = reference exposure limit.
Long-term exposure values in indoor guidelines are often based on 8 or 24 h time durations. These time-weighted average (TWA) values were set to protect the public in indoor environments from the chronic effects of formaldehyde and are considered to offer adequate protection to individuals exposed to formaldehyde continuously over their lifetimes. Chronic noncancer health effects have been assessed based on a threshold concentration or dose which is below a level at which no adverse health effects would occur. Reference Exposure Levels (RELs), as estimated by the OEHHA,(203) are designed to protect the most sensitive individuals in the population and include margins of safety.
Some organizations try to encourage the use of low-emitting products for reducing particular indoor air pollutants, mainly formaldehyde. An example of this is the U.S. Green Building Council (USGBC),(204) which published the Leadership in Energy and Environmental Design (LEED) Green Building Rating System, which is based on voluntarily participation and aims at facilitating high-performance buildings. Similarly, the “Standard for the Design of High-Performance, Green Buildings Except Low-Rise Residential Buildings” set by ASHRAE(205) only accepts urea-formaldehyde being used on the exterior envelope material of the buildings. European labeling systems are surveyed in a report published by the European Commission.(206)
5.2. Guidelines by Regions
Table 5 shows the current formaldehyde indoor guideline values set in different countries by different organizations. Most of the cited documents are published as official government publications and are available on the Internet. An overview of guideline values (12 countries in the year 1990) is given in a report published by the European Commission.(207)
5.2.1. Europe
In the U.K., the Committee on the Medical Effects of Air Pollutants (COMEAP)(208) recommended a limit value of 100 μg m−3 (0.5 h) for indoor formaldehyde in 2004. Finland(209) has set up a different system. The indoor climate is classified as S1 (individual indoor climate), S2 (good indoor climate), and S3 (satisfactory indoor climate), in which formaldehyde target values were set as 30 μg m−3, 50 μg m−3, and 100 μg m−3, respectively. An indoor formaldehyde level was specified by the Norwegian Health Directorate (NHD)(210) in 1990 in the Guidelines for Indoor Air Quality, in which a 24-h average indoor formaldehyde level was set at 60 μg m−3. Stranger et al.(211) cite a guideline value of 100 μg m−3 (0.5 h exposure), applicable in Norway since 1999. The Danish guideline value of 0.15 mg m−3 has not been revised since 1990.(207) Sweden has adopted the WHO-guideline value, but a further reduction to 60 μg m−3 is currently under discussion.(212) In France, the French Agency for Environmental and Occupational Health Safety (AFSSET) has proposed guideline values of 10 μg m−3 and 50 μg m−3 for long-term exposure and short-term exposure (2 h), respectively.213,214 The Polish Ministry of Health and Social Welfare(215) issued a decree to reduce the pollutants emitted by building materials and furnishings in inhabited enclosed areas. The maximum allowable concentrations for formaldehyde, categorized as Category A (up to 24 h exposure per day) and Category B (8−10 h exposure per day), are 50 μg m−3 and 100 μg m−3, respectively. Germany established an indoor guideline value of 0.1 ppm in 1977. The Federal Institute for Risk Assessment (BfR) and the Federal Environment Agency stated in 2006 that a revision of this guideline value is not required.(216)
5.2.2. USA/Canada
In 1991 the California Environmental Protection Agency set indoor formaldehyde levels at 0.10 ppm as an action level and at 0.05 ppm as a target value.(217) These values were recently lowered by the Office of Environmental Health Hazard Assessment (OEHHA). Formaldehyde levels for acute exposure, 8-h exposure, and chronic exposure were set at 0.076 ppm (94 μg m−3), 0.027 ppm (33 μg m−3), and 0.002 ppm (3 μg m−3), respectively.203,218,219 Health Canada22,220 and the Federal Provincial Advisory Committee on Occupational and Environmental Health (CEOH) laid down indoor air quality guidelines in 1987 and revised them in 1989. In these guidelines, formaldehyde target and action levels were set at 0.05 ppm (60 μg m−3) and 0.1 ppm (120 μg m−3), respectively. Following reclassification of formaldehyde as a carcinogen by the IARC and on the basis of the results of epidemiological and toxicological studies, Health Canada set new limits in 2006. These new guidelines, the Proposed Residential Indoor Air Quality Guidelines, specified a short-term (1 h) exposure limit of 0.1 ppm (123 μg m−3) and a long-term (8 h) exposure limit of 0.04 ppm (50 μg m−3).
5.2.3. Asia
The Indoor Air Quality Management Group in Hong Kong published indoor formaldehyde guidelines in 1999 entitled “Guidance Notes for the Management of Indoor Air Quality in Office and Public Places”. In these guidelines, the indoor air quality (8 h average) in offices and public environments was classified into three categories: Level 1 represents very good indoor air quality, Level 2 represents the recommended indoor air quality standards for the general public, and Level 3 represents the indoor air quality required as protection for workers. The indoor formaldehyde concentrations corresponding to these three levels were 30 μg m−3, 100 μg m−3, and 370 μg m−3. The guidelines were modified in 2003 in the form of an indoor air quality certification scheme. Two indoor air quality levels (8 h average) were defined as benchmarks, namely “excellent class” (<30 μg m−3) and “good class” (<100 μg m−3).(221)
In Korea, the indoor formaldehyde was set at 0.1 ppm (8 h) according to the Air Quality Standard in Office and Indoor Air Quality Management Act in 2004.(222) The Ministry of Health and Welfare (MHW) in Japan laid down an indoor air guideline value of 0.08 ppm (0.5 h) in June 1997.(223) Guidelines for good indoor air quality in office premises were promulgated by the Singapore Ministry of the Environment in 1996.(224) According to these guidelines, the maximum formaldehyde concentration limit for acceptable indoor air quality is 0.1 ppm (8 h). In China, indoor air quality became a governmental concern about a decade ago, and a guideline value termed the “Indoor Air Quality Standard” of 100 μg m−3 (1 h average) was issued in 2002.(225)
5.2.4. Australia
The National Health and Medical Research Council (NHMRC) recommended an indoor formaldehyde level of 130 μg m−3 (0.1 ppm) in 1982 due to concerns regarding the urea−formaldehyde foam insulation (UFFI) used in buildings.(226) Recently, the Australian Government has specified indoor formaldehyde guideline values for various indoor environments in the National Industrial Chemicals Notification and Assessment Scheme (NICNAS), where the recommended indoor air guidance value of formaldehyde for a short duration was set at 0.08 ppm.(227)
In 1987 the World Health Organization (WHO) recommended a concentration < 0.08 ppm (100 μg m−3) for short-term exposure (0.5 h) to prevent nose and throat irritation.(228) Mendell(229) has reviewed chemical emissions as risk factors for respiratory and allergic effects in children. This author criticizes the wide range of existing formaldehyde guideline values and suggests a need for research to quantify risk/response relationships as guidance for the necessary preventive action.
6. Formaldehyde Emission Data
Comparison of product emission rates is difficult because different methods and units are used. The test chamber is still the standard device for emission testing, and it is convenient to express the emission rate directly in (mg m−2 h−1) or as a chamber concentration (ppm, ppb, mg m−3) in the case of the steady state. Moreover, different test protocols use different L and n. For this reason, the emission rates of different building products are cited in Table 6 as they appear in the references together with the information provided about chamber conditions.
Table 6. Compilation of Indoor-Related Formaldehyde Emission Studiesa.
| material | T (°C) | r.h. (%) | L (m2 m−3) | n (h−1) | HCHO emission | comment | yearref |
|---|---|---|---|---|---|---|---|
| chipboard | 22 | 35 | 1.7 | 0.5 | 0.70 ppm | 1975(230) | |
| particle board | 22 | 30 | 1.6 | 0.2 | 0.40 ppm | 1980(234) | |
| 27 | 60 | 1.6 | 0.2 | 1.70 ppm | |
||
| 27 | 30 | 1.6 | 0.5 | 0.80 ppm | |
||
| 22 | 60 | 1.6 | 0.5 | 0.90 ppm | |
||
| particle board | 25 | 50 | 0.131−0.426 | 0.25−0.50 | 0.095−0.29 ppm | 10 samples | 1987(233) |
| OSB | 23 | 45 | 1.0 | 1.0 | 0.01−0.09 ppm | 31 samples (median: 0.04 ppm) | 2008(246) |
| solid wood | 23 | 45 | 1.0 | 1.0 | 2−9 ppb | beech, Douglas fir, oak, spruce, pine | 1996(43) |
| UF wood products (bare) | 22 | 50 | 0.46 | 1.0 | 8.6−1580 μg m2 h−1 | 19 samples (median: 164 μg m2 h−1) | 1999(73) |
| UF wood products (bare) | 27 | 50 | 0.46 | 0.3 | 6.8−1170 μg m2 h−1 | 19 samples (median: 158 μg m2 h−1) | |
| PF wood products (bare) | 22 | 50 | 0.46 | 1.0 | 4.1−9.2 μg m2 h−1 | 4 samples | |
| PF wood products (bare) | 27 | 50 | 0.46 | 0.3 | 9.5−13 μg m2 h−1 | 4 samples | |
| UF wood products (coated) | 22 | 50 | 0.46 | 1.0 | <2.7−460 μg m2 h−1 | 14 samples (median: 8.6 μg m2 h−1) | |
| UF wood products (coated) | 27 | 50 | 0.46 | 0.3 | 4.6−1300 μg m2 h−1 | 14 samples (median: 15 μg m2 h−1) | |
| decorative laminates | 22 | 50 | 1.83 | 1.0 | 4.0−51 μg m2 h−1 | 3 samples | |
| cabinetry materials | 23 | 50 | 1.9 | 5.7 | 8−470 μg m2 h−1 | particle board, hard board, plywood | 2002(243) |
| particle board (18 mm) | 25 | 50 | 2.16 | 0.5 | 0.45 mg m2 h−1 | 7 days after sample installation | 2006(247) |
| MDF (18 mm) | 25 | 50 | 2.16 | 0.5 | 0.33 mg m2 h−1 | 7 days after sample installation | |
| laminate | 25 | 50 | 2.16 | 0.5 | 0.03 mg m2 h−1 | 7 days after sample installation | |
| engineered flooring | 25 | 50 | 2.16 | 0.5 | 0.04 mg m2 h−1 | 7 days after sample installation | |
| floor covering: natural wood | 23.2 | n.d. | n.d. | n.d | 288 μg m2 h−1 | 1 h exposure to 750 ppb ozone | 2009(256) |
| floor covering: wood-based | 23.2 | n.d. | n.d. | n.d. | 31 μg m2 h−1 | 1 h exposure to 750 ppb ozone | |
| floor covering: PVCb | n.d. | 50 | 505 | 514−686 | <5−18 μg m2 h−1 | new building (mean: 9 μg m2 h−1) | 2007(250) |
| floor covering: parquetb | n.d. | 50 | 505 | 514−686 | <5−10 μg m2 h−1 | new building (mean: 7 μg m2 h−1) | |
| ceilingb | n.d. | 50 | 505 | 514−686 | 5−96 μg m2 h−1 | new building (mean: 42 μg m2 h−1) | |
| wallsb | n.d. | 50 | 505 | 514−686 | <5−11 μg m2 h−1 | new building (mean: 7 μg m2 h−1) | |
| mineral wool | 23 | 45 | 1.0 | 1.0 | 0.02−0.10 ppm | 5 samples (4 samples <0.05 ppm) | 1993(249) |
| fiberglass products | 23 | 50 | 0.87−1.04 | 1.0 | 16−32 μg m2 h−1 | 3 samples | 1999(73) |
| wall coverings | 23 | 45 | 1.0 | 1.0 | 0.05−0.035 ppm | 10 samples (median: 0.015 ppm) | 1993(70) |
| carpet | 23 | 45 | 0.4 | 0.5 | 8−15 μg m−3 | 4 samples, 24 h values | 2008(251) |
| latex paint | n.d. | 18−58 | flow chamber (2.5 L/min) | 0.13−6.29 μg h−1 | 11 samples exposed to ozone | 1995(88) | |
| paints | 22 | 50 | 1.04 | 1.0 | 326−663 μg m2 h−1 | 3 products, initial emission | 1999(73) |
| paints | 22 | 50 | 1.04 | 1.0 | 8.1−9.8 μg m2 h−1 | 3 products, final emission | |
| carpet | 23 | 50 | n.d. | 7 | 4−30 μg m2 h−1 | 4 samples exposed to ozone | 2006(85) |
| household products | 21−23 | 36−55 | n.d. | 0.95−1.08 | 8.2−23.7 ppb | 3 products exposed to ozone | 2006(83) |
| textiles | 22 | 50 | 7.05 | 1.0 | 107 μg m2 h−1 | permanent-press T-shirts (unwashed) | 1999(73) |
| 42 μg m2 h−1 | permanent-press T-shirts (washed) | ||||||
| temporary housing units | 22−25 | 49−58 | n.d. | 0.15−0.39 | 164−266 μg m2 h−1 | morning (unoccupied) | 2008(307) |
| 26−30 | 46−49 | 257−347 μg m2 h−1 | afternoon (unoccupied) | ||||
| photocopy machines | 26−31 | 30−35 | 1c | 2.0 | <500−2600 μg h−1 | 4 dry-process photocopy machines | 1996(441) |
| building products | 23 | 30−50 | 8.2−14.7 | 12 | 5.5−40.6 μg m2 h−1 | 14 products exposed to ozone | 2007(71) |
| burning of incense | 23 | 50 | 0.5 | ca. 20−300 μg m−3 | 3 sticks in 18.26 m3 chamber | 2004(260) | |
| |
ca. 300−1700 μg g−1 | 10 products tested | |||||
| wood burning | 180−710 mg kg−1 | 4 samples (birch) tested in wood stove | 2002(55) | ||||
| wood burning | 599−1165 mg kg−1 | oak, pine, eucalyptus | 2001(56) | ||||
| cigarette smoking | 20−25 | 45−55 | 0.022 | 234.1 μg m−3 | average of 8 commercial cigarettes | 2004(98) | |
| experimental cigarettes | 30−57 μg cigarette−1 | 13 cigarettes with added saccharides | 2006(261) | ||||
| formaldehyde in breath | 1.2−72.7 ppb | median: 4.3 ppb (deep lung portion) | 2005(105) | ||||
The emission values are presented as they appear in the references.
Measured on site by use of the FLEC.
Units per volume.
6.1. Emission Modeling
One of the classic papers on the indoor air pollution caused by formaldehyde emission from chipboard was published in 1975 by Andersen et al.(230) The authors studied the influence of climatic parameters on steady-state concentration in climate chambers. A hyperbolic decrease in formaldehyde concentration occurred as air exchange rates increased, while the equilibrium concentration was directly proportional to temperature and humidity. On the basis of these findings, Andersen et al. have formulated a relationship (eq 19) between the steady-state chamber concentration Css and the climatic parameters.
T is the temperature in °C, H is the humidity in g kg−1, L is the loading rate in m2 m−3, and n is the air exchange in h−1 while a, b, c, S, and R are constants depending on the type and surface coating of the chipboard. An updated model was developed later by Mølhave et al.(231) Myers and Nagaoka(232) and also Lehmann(233) have applied the Hoetjer equation (eq 20) to describe the effect of ventilation and loading rates on emissions from particle board and MDF in large test chambers.
Ceq is the formaldehyde concentration at n = 0 h−1, and K (m h−1) is the formaldehyde transfer coefficient. For particle board, K values lie between 0.40 m h−1 and 1.58 m h−1.(233) Similar equations have been derived by Berge et al.(234) and others.(235) The Berge equation (eq 21) assumes no air exchange and no concentration gradients in the chamber air. Formaldehyde emission is assumed to increase linearly with increasing relative humidity of the air. On the basis of previously reported results, an Arrhenius temperature dependence was selected.(236) Godish(237) pointed out that the Berge model is a relatively good predictor of formaldehyde concentration under standard conditions when measured under a variety of environmental conditions (Cx = standardized concentration, C = measured concentration, H = relative humidity under test conditions (%), H0 = relative humidity under standard conditions (%), T = temperature under test conditions (K), T0 = temperature under standard conditions (K), RB = coefficient of temperature (optimum value: 9799 K),(234)AB = coefficient of humidity (optimum value: 0.0175%−1)(234)). As an example, formaldehyde concentrations (in ppm) under steady-state conditions have been calculated with eq 21 as a function of humidity and temperature and are shown in Figure 10. The data have been standardized for 0.05 ppm, 296 K, and 50% relative humidity. The curves demonstrate the high influence of climatic parameters on formaldehyde concentrations.
Figure 10.
Variation of the formaldehyde concentration as a function of humidity and temperature, calculated with the Berge equation (eq 21). The initial conditions were T0 = 296 K, H0 = 50%, and Cx = 0.05 ppm.
Roffael(2) has expressed the criticism that eqs 19−21 do not take into account the influence of aging on the formaldehyde release capacity. Hawthorne and Matthews238,239 have stated that the release of formaldehyde from solid materials is limited by source-phase mass transfer from the interior of the source to the surface and can be described by eq 22.
Here, mp is the mass of formaldehyde in the material (source), mi is the mass of formaldehyde at the surface, and ks is the source-phase mass transfer coefficient. Equation 22 can be used for homogeneous materials but not for products which are multilayered or of complex construction. Zhang et al.(240) as well as Deng et al.(241) have proposed a more sophisticated model based on the diffusion coefficient, the partition coefficient, and the initial concentration in a dry building material in order to calculate the influence of temperature on formaldehyde emission. Xiong et al. describe an extraction method, where determination of the initial concentration in the material Cm,0 is based on Henry’s linear adsorption isotherm.(242) The material is placed in an airtight environmental chamber under static conditions, and the equilibrium concentration Ci is measured involving multiple flush/equilibrium cycles (see eq 23).
KH is the partition coefficient (dimensionless), V is the chamber volume, Vm is the volume of the material, and i is the number of flush/equilibrium cycles. Cm,o and KH are obtained from regression analysis.
If the emission rate is constant for a given temperature and humidity and not dependent on the air exchange rate, eq 15 can be used to calculate the concentration in the steady state. A long-term study of formaldehyde emission decay from particle board has been carried out by Zinn et al.(66) For products manufactured in 1986 and 1987, the overall three-quarter (75% of initial concentration) and half-lives were 38 and 216 days, respectively.
6.2. Emission from Wood-Based Materials
It is obvious from Table 6 that UF-bonded wood products constitute a group of comparatively strong formaldehyde emitters. The steady-state chamber value of Ceq = 0.7 ppm measured for chipboard by Andersen et al.(230) at T = 22 °C and r.h. = 35% can be converted to an area-specific emission rate of SERA = 255 μg m−2 h−1. The effect of temperature and humidity on UF-bonded particle board has been studied by Berge et al.,(234) who observed a strong increase in the steady-state concentration from 0.40 ppm to 1.70 ppm at n = 0.2 h−1 and L = 1.6 m2 m−3 when temperature and relative humidity were increased from 22 to 27 °C and from 30% to 60%, respectively. Kelly et al.(73) have compared different types of wood products. Uncoated UF products covered a range from 8.6 μg m−2 h−1 to 1560 μg m−2 h−1 with a median of 164 μg m−2 h−1. For coated UF wood products, the range was in the same order of magnitude but the median of 15 μg m−2 h−1 was distinctly lower. In contrast, the range of formaldehyde concentrations for uncoated PF wood products was between 9.5 μg m−2 h−1 and 13 μg m−2 h−1. Hodgson et al.(243) have studied cabinetry materials as sources of formaldehyde in a newly built house. The authors state that formaldehyde emission factors for five of the indoor sources were generally consistent with the results presented by Kelly et al.(73) Mølhave et al.(244) have measured formaldehyde emission from furniture and home equipment in a large-scale chamber. From their data they have estimated the long-term exposure of occupants.
Over the years, the formaldehyde emission potential of wood based products has continually decreased, a circumstance due to a varitey of regulations. In Germany, the so-called ETB guideline (ETB = Committee on Harmonized Prescriptions for Construction) was created in 1981 and classified particle board into three emission categories, E1−E3. The German Chemicals Act, which was revised in 1993, regulates the release of formaldehyde as follows: “coated and uncoated wood-based products (particle board, fiber board, plywood) must not be put onto the market if they exceed an equilibrium formaldehyde concentration of 0.1 ppm (0.12 mg m−3). The equilibrium concentration is to be determined in a large chamber using a test method in line with the scientific and technical states-of-the art”. Some voluntary quality certification systems such as the German “Blue Angel” label specify a steady-state concentration of 0.05 ppm.(245) In Germany, almost all wood-based materials produced meet the requirements of the Chemicals Act. This has recently been demonstrated for oriented-strand board (OSB). Ludewig et al.(246) determined the emission of formaldehyde from 31 samples. Steady-state concentrations ranged from 0.01 ppm to 0.09 ppm with a median of 0.04 ppm. Other counties have established similar test procedures, legislation, and labeling systems for regulating formaldehyde emissions from building products.
Wood-based composites such as laminate are also known to be low-emitting materials. Kim et al.(247) have measured emission rates of 0.03 mg m−2 h−1. Wiglusz et al.(248) were not able to detect any emissions of formaldehyde from laminate flooring. Emission rates for some other materials such as mineral wool,(249) PVC, parquet, ceilings, walls,(250) solid wood,(43) wall coverings,(70) carpeting,(251) fiberglass products,(73) etc. are also presented in Table 5.
6.3. Emission from Paints
In the case of wet products such as paint, it is necessary to take into account gas-phase mass transfer, which is based on molecular diffusion across a laminar boundary layer as described in eq 24.(252)
D is the diffusion coefficient, δ is the thickness of the boundary layer, CS is the concentration of formaldehyde at the source surface, Cair is the concentration of formaldehyde in the air, and kg is the gas-phase mass transfer coefficient. If the source phase mass transfer rate ks (see eq 22), which describes the diffusion from the interior of the source to the surface, is taken into account, three different scenarios must be considered:(173) (a) When kg ≫ ks, emission is controlled by the external diffusion process. This applies to most wet-applied or liquid products during drying or curing. (b) When kg ≪ ks, emission is controlled by the internal diffusion process and the influence of air flow conditions in the test facility should be negligible. This applies to most materials manufactured in the solid phase and also to wet-applied or liquid products after drying or curing. (c) A more difficult situation arises when kg ≈ ks or if the ratio ks/kg changes over time. For an aging product, ks/kg will normally increase and reach infinity for t → ∞. Chang et al.(75) have developed a semiempirical model assuming that the formaldehyde in the applied paint is distributed in a top layer (thin surface-coating section) and a bottom layer (paint embedded in the substrate). The analytical solution of the mass balance equations(75) is a triple exponential function (eq 25).
C(t) (mg m−3) is the formaldehyde concentration in the chamber, km (h−1) is the rate constant for emission from the top layer, kb (h−1) is the rate constant for emission from the bottom layer, and n is the air exchange rate. Figure 11 shows a modeled decay of formaldehyde from latex paint. The parameters were taken from Chang et al.(75) and are given in the figure captions. The curve shape is typical for the emission from freshly applied products. For km > kb, the first phase (<20 h) is controlled by evaporation of formaldehyde from the top layer, where the term exp(−kmt) dominates. With increasing t, the term exp(−kbt) more and more affects the progression of the curve.
Figure 11.
Emission of formaldehyde from latex paint, measured in a 0.053 m3 chamber with n = 0.5 h−1, T = 23 °C, r.h. = 50%, and loading L = 0.48 m2 m−3. The curve was obtained from a triple exponential fit of the experimental data (see eq 24). The parameters A1 = 1.926 mg m−3, n = 0.5 h−1, A2 = 0.513 mg m−3, km = 0.0745 h−1, A3 = 0.274 mg m−3, and kb = 0.0042 h−1 were taken from Chang et al.(75)
6.4. Other Products
In 2005, Bruinen de Bruin et al.(253) released a report on the characterization of indoor sources for formaldehyde and other pollutants. The authors examined wood and wood-based products, paper products, wall coverings, rubber, paint, glue, adhesives, lubricants, cosmetics, electronic equipment, and combustion. Emission factor tables are provided which derive from numerous sources. Kelly et al.(73) compared formaldehyde emissions from new and washed permanent-press shirts and found that normal laundering reduced emissions by about 60%.
6.5. Formaldehyde from Indoor VOC/Ozone Reactions
The formation of carbonyl compounds from the reactions of ozone with unsaturated VOCs has been known for decades, but the possible impact on indoor air quality was only recognized in the 1990s when Zhang et al.(254) conducted a field study of six residential houses during the summer of 1992. Based on these and other findings,(78) Zhang et al.(255) have investigated the indoor chemistry of ozone with styrene, limonene, and 4-vinylcyclohexene (VCH) in a 4.3 m3 Teflon chamber at temperatures of 21−24 °C and humidities of 17−56%, respectively. The styrene/ozone and limonene/ozone systems gave formaldehyde concentrations between 100 μg m−3 and 500 μg m−3. In the case of VCH, formaldehyde concentrations between 25 μg m−3 and 36 μg m−3 were measured. Although the authors state that the conditions and the chemicals employed in the reactions were similar to those for indoor environments, the concentrations of unsaturated hydrocarbons were higher than normally found in indoor air. A more realistic scenario was chosen by Morrison and Nazaroff,(85) who measured formaldehyde emission rates of 4−30 μg m−2 h−1 when carpet was exposed to 100 ppb ozone. The effect of ozone, infrared, sunlight, UV-A, and UV-B on the emission of formaldehyde from wood-based flooring materials was studied by Kagi et al.(256) Typical indoor/outdoor ratios of ozone concentrations range from 0.2 to 0.6, depending on the ventilation rate.79,257 Indoor concentrations of 10 ppb and higher are only possible during summer ozone episodes or in the case of strong indoor sources such as air- and water-cleaning devices. The study by Singer et al.(83) showed elevated formaldehyde indoor levels of the order of 10 ppb when household products were used with ca. 60 ppb ozone present. Nicolas et al.(71) exposed building products in a 17 L test chamber to 100−160 ppb of ozone at 23 °C and 30−50% r.h. and n = 12 h−1 for a period of 48 h. Increased formaldehyde emissions of the order of 5−40 μg m−2 h−1 associated with reduced emission rates of unsaturated compounds were observed as compared with the reference values (no ozone) of 0.5−25 μg m−2 h−1. Reiss et al.(88) reported an effect of heterogeneous ozone chemistry on latex paint. Waring et al.(258) have studied the efficiency of portable air cleaners. During an air cleaning period and in the presence of an air freshener, the formaldehyde concentration increased from 17−19 μg m−3 to 45−49 μg m−3. Fiedler et al.(259) have also shown that formaldehyde is formed from VOC/ozone mixtures. The authors exposed healthy women to a mixture of 23 volatile organic compounds (VOCs) with and without ozone. At a total VOC concentration of 26 mg m−3, the formaldehyde concentration increased from 13 μg m−3 (no ozone) to 40 μg m−3 in the presence of 40 ppb ozone.
6.6. Formaldehyde from Combustion
The formation of formaldehyde from combustion processes is well-known. Here the emission rate is often presented in the unit μg g−1 (mass emitted per mass of material burnt). Lee and Wang(260) measured formaldehyde concentrations up to 300 μg m−3 in a 18.26 m3 test chamber when burning incense sticks. The emission rate was 300−1700 μg per g stick. Wood combustion and cigarette-smoking have already been treated in section . Baek and Jenkins(98) measured an average formaldehyde concentration of 234 μg m−3 when six cigarettes were smoked in a 30 m3 chamber under almost static conditions. A study by Baker(261) with 13 experimental cigarettes (saccharides added) gave emission rates between 30 μg g−1 and 57 μg per cigarette smoked. In the study by Singer et al.,(262) distinctly higher formaldehyde emission rates of 950−1310 μg per cigarette smoked were measured. Maroni et al.(263) report 70−100 μg formaldehyde in the undiluted mainstream smoke of nonfilter cigarettes and 0.2 mg per cigarette in sidestream smoke. According to Baker et al.,261,264 formaldehyde is mainly generated from the pyrolysis of saccharides used as tobacco ingredients.
7. Formaldehyde Concentrations Indoors
7.1. Evaluation of Data
Residential and occupational indoor formaldehyde concentrations have been measured in numerous studies. However, it must be pointed out that different studies provide data of different quality and care has to be taken when comparing the studies presented in Table 7. Two strategies are commonly applied: (a) the collection of samples from a large number of randomly selected homes in order to estimate the exposure of the general population and (b) the monitoring of a limited number of homes to measure the time vs concentration behavior and to see the influence of aging. In general, concentrations of indoor pollutants are log−normally distributed.(265) This means that the geometric mean (GM) rather than the arithmetic mean (AM) should be used for the statistical evaluation. Moreover, a nonparametrical classification, e.g. 50-P (median) and 95-P, is convenient. In many cases, the range of measured concentrations (min−max) is also given. The critical evaluation of published data and comparison of different studies always require additional information, e.g. the criteria used in the selection of homes (at random or complaint cases), the type of sampling (active or passive), sampling time, and so on. Strategies for the measurement of formaldehyde and other indoor pollutants are available as ISO standards.184,266
Table 7. Comparison of Formaldehyde Levels in Indoor Air as Determined in Different International Studies (P = Percentile, 50-P = Median, GM = Geometric Mean, AM = Arithmetic Mean) on the Basis of Regiona.
| continent/country | location | Cindoor | comments | ref |
|---|---|---|---|---|
| Europe | ||||
| Germany (1991) | 327 residences | 55 μg m−3 | 50-P | (269) |
| 106 μg m−3 | 95-P | (269) | ||
| Germany (2008) | 586 residences | 23.5 μg m−3 | 50-P (survey for children) | (270) |
| 47.7 μg m−3 | 95-P (survey for children) | (270) | ||
| Germany (2003) | 14 office buildings, 1386 measurements | 6.0 μg m−3 | 50-P | (273) |
| Germany (2001) | 180 Berlin residences | 38 μg m−3 | 50-P | (271) |
| 98 μg m−3 | 95-P | (271) | ||
| Germany (1995) | 252 residences | 12−649 μg m−3 | range (complaint cases) | (64) |
| Germany (1993) | 190 residences | 62 ppb | 50-P | (461) |
| Austria (2002) | 160 homes | 8.8−115 μg m−3 | range | (462) |
| 25 μg m−3 | 50-P | (462) | ||
| Switzerland (1992) | private residences | 46 μg m−3 | 50-P (recently renovated) | (463) |
| 468 μg m−3 | 95-P (recently renovated) | (463) | ||
| Denmark (1987) | 14 Danish town halls | 40 μg m−3 | mean | (371) |
| 0−80 μg m−3 | range | (371) | ||
| Denmark (1991) | 2 new twin apartments | 63−384 μg m−3 | range (vacant) | (295) |
| 14−276 μg m−3 | range (occupied) | (295) | ||
| Denmark (1992) | 36 apartments | 37 μg m−3 | 50-P | (296) |
| Finland (2006) | 8 buildings | 19, 21, 26 μg m−3 | mean (0, 6, 12 months) | (313) |
| Finland (2009) | 23 office buildings | 11 μg m−3 | GM (suspected problems) | (464) |
| Sweden (2004) | 27 Uppsala dwellings | 8.3 μg m−3 | GM | (465) |
| Sweden (2005) | 64 bedrooms | 23 μg m−3 | 50-P (24 h) | (212) |
| 29 μg m−3 | 50-P (6 days) | (212) | ||
| Sweden (2001) | 181 classrooms | 3 μg m−3 | GM | (466) |
| <3−72 μg m−3 | range | (466) | ||
| France (2006) | Strasbourg libraries | 20 μg m−3 | 50-P | (108) |
| 95 μg m−3 | 95-P | (108) | ||
| France (2006) | Strasbourg locations | 5.3−73.8 μg m−3 | range (public spaces) | (267) |
| France (2008) | Strasbourg homes | 26.7 μg m−3 | 50-P (living rooms) | (467) |
| France (2003) | 61 Paris dwellings | 34.4 μg m−3 | GM (living room) | (468) |
| ≈ 78 μg m−3 | 95-P (living room) | (468) | ||
| France (2009) | 157−187 babies’ homes | 17.7−19.4 μg m−3 | GM | (277) |
| Italy (2009) | 20 homes | 12.9−9.3 μg m−3 | 50-P (January−June) | (469) |
| Poland (2005) | 5 office buildings | 2.3−32.3 μg m−3 | range | (470) |
| Turkey (2003) | 399 kitchens in Ankara | 0−2086 μg m−3 | range | (471) |
| 79.9 μg m−3 | AM | (471) | ||
| Turkey (2006) | 25 Ankara dwellings | 2.3−866.2 μg m−3 | range | (472) |
| 67.1 μg m−3 | GM | (472) | ||
| US/Canada | ||||
| USA (1995) | 14 residences | 11.1 ppb | mean (winter) | (473) |
| 26 residences | 16.1 ppb | mean (summer) | (473) | |
| USA (2000) | 4 manufactured houses | 21−47 ppb | range (new buildings) | (474) |
| 7 site-built houses | 14−58 ppb | range (new buildings) | (474) | |
| USA (2006) | different locations | 19.6 μg m−3 | GM (stores) | (475) |
| 14.3 μg m−3 | GM (dining) | (475) | ||
| USA (2007) | 234 homes | 20.1 μg m−3 | 50-P (RIOPA—indoor) | (275,282) |
| 32.5 μg m−3 | 95-P (RIOPA—indoor) | (275,282) | ||
| 20.5 μg m−3 | 50-P (RIOPA—personal) | (275,282) | ||
| 34 μg m−3 | 95-P (RIOPA—personal) | (275,282) | ||
| USA (1989) | 470 mobile homes | 70 ppb | AM | (302,475) |
| <30 to >300 ppb | range (built 1966−1984) | (302,475) | ||
| USA (2008) | 360 travel trailers | 81 ppb | GM (occupied) | (306) |
| 90 park models | 44 ppb | GM (occupied) | (306) | |
| 69 mobile homes | 57 ppb | GM (occupied) | (306) | |
| Canada (2003) | 151 homes (summary of 5 studies) | 29.8 μg m−3 | 50-P | (476) |
| Canada (2005) | 59 residences | 29.6 μg m−3 | 50-P | (417) |
| Canada (2008) | 96 Quebec homes | 9.6−90 μg m−3 | range | (310) |
| Latin America | ||||
| Brazil (2006) | academic institute | <1−82 μg m−3 | range (11 laboratories) | (358) |
| 7−8 μg m−3 | range (5 libraries) | (358) | ||
| 5−9 μg m−3 | range (3 classrooms) | (358) | ||
| Mexico (2003) | different locations | 4−122 μg m−3 | range | (321) |
| Asia | ||||
| Korea (2008) | 52 classrooms summer | 70 ppb | GM | (300) |
| 48 classrooms autumn | 40 ppb | GM | (300) | |
| 46 classrooms winter | 60 ppb | GM | (300) | |
| Korea (2008) | 6 apartments | 209−457 μg m−3 | range (new or renovated) | (298) |
| Korea (2009) | 50 school buildings | 150 ppb | GM (summer) | (477) |
| 45 school buildings | 100 ppb | GM (winter) | (477) | |
| Japan/Korea (2006) | 292 new homes | 134 μg m−3 | mean, first year | (299) |
| 60 new homes | 86 μg m−3 | mean, third year | (299) | |
| Japan (2006) | 25 Shimizu residences | 71.5 μg m−3 | 90-P (summer) | (268) |
| 21 Shimizu residences | 25.9 μg m−3 | 90-P (winter) | (268) | |
| Japan (2004) | 37 Nagoya residences | 17.6 μg m−3 | GM | (465) |
| Hong Kong (2002) | 6 residential homes | 11−24 μg m−3 | range | (478) |
| Hong Kong (2006) | 422 offices | 32 μg m−3 | GM (air-conditioned) | (315) |
| Hong Kong (2009) | 100 homes | 85.7 μg m−3 | 50-P | (301) |
| China (2004) | 28 hotel ballrooms | 29.7 μg m−3 | GM | (479) |
| China (2007) | public vehicles | 13−94 μg m−3 | range (taxi, bus, subway) | (285) |
| Bangladesh (2007) | 91 kitchens, impact on children | 26.2 μg m−3 | GM (biomass burning) | (293) |
| 36.9 μg m−3 | GM (fossil burning) | (293) | ||
| Africa | ||||
| Egypt (2000) | 294 Cairo residences | 96.6 μg m−3 | AM (winter and summer) | (480) |
| Australia/New Zealand | ||||
| Australia (2002) | 185 homes in Perth | 1−166 ppb | range (depends on room) | (278) |
| 20.4−23.8 ppb | GM (depends on room) | (278) | ||
| Australia (2006) | 4 schools | 3−38 μg m−3 | GM (depends on season) | (481) |
| Australia (2000) | 192 caravans | 29 ppb | GM (60 occupied) | (304) |
| 100 ppb | GM (132 unoccupied) | (304) | ||
| Other | ||||
| aircraft (simulated) | occupied cabin | 8−10 ppb | range (ozone initiated) | (89,90) |
| submarine (2006) | submerged operation | <10 μg m−3 | (1 week) | (482) |
The concentrations are shown in ppb or μg m−3 as they appear in the references.
7.2. Formaldehyde in Conventional Homes
Maroni et al.(263) have summarized a number of investigations carried out before 1990. A compilation of formaldehyde indoor concentrations in different European countries before 1990 may be found in an EU report.(207) References to more recent studies are available in other publications.17,267,268 The WHO(27) based an evaluation on 151 sets of figures from five Canadian studies conducted between 1989 and 1995. In indoor air, the 50-P and the 95-P were 29.8 μg m−3 and 84.6 μg m−3, respectively. In Germany, environmental surveys of indoor air have been performed by passive sampling since 1985. In the first survey, in 1985/86,(269) with 327 data, a 50-P and a 90-P of 55 μg m−3 and 106 μg m−3 were calculated. The recent survey, from 2003 to 2006,(270) (555 residences) showed reduced 50-P and 95-P values of 23.5 μg m−3 and 47.7 μg m−3. The results of both studies are directly compared in Figure 12. It should, however, be noted that the 1985/86 survey was carried out before German reunion and that the 2003−2006 survey focused on children. Schleibinger et al.(271) measured formaldehyde in 180 apartments in Berlin between 1988 and 1999 by passive sampling. It is interesting that their results of 38 μg m−3 (50-P) and 98 μg m−3 (95-P) fall between the data from the environmental survey but direct comparison is not possible since the sampling strategies differ. The Federal Institute for Risk Assessment (BfR)(272) has compared different German studies and comes to the conclusion that only the results of the German Environmental Survey can be regarded as representative for the German population. Salthammer et al.(64) measured formaldehyde concentrations in German dwellings between 1986 and 1993 at the request of persons complaining about irritation symptoms. The range was 12−649 μg m−3 with an arithmetic mean of 119 μg m−3. However, the annual specific arithmetic mean values decreased from 136 μg m−3 in 1986 to 91 μg m−3 in 1993. An interesting study in 14 office building with 1386 formaldehyde measurements was carried out by Bischof et al.(273) The overall median concentration was 6.0 μg m−3 while the highest median in one building was 26.5 μg m−3 (42 measurements). Moreover, there was a tendency for higher formaldehyde concentrations to be found in buildings with more than 600 employees and in open-plan offices.
Figure 12.
Comparison of two environmental surveys carried out by passive sampling in Germany in 1985/86 and 2003−2006. The data were taken from http://www.umweltbundesamt.de. According to the Federal Institute for Risk Assessment (BfR),(272) the results of the German Environmental Survey (GerES) can be regarded as representative for the German population.
For conventional homes, the WHO(274) cites average exposure concentrations to formaldehyde between 30 μg m−3 and 60 μg m−3. However, this statement is based on measurements carried out before the year 2000. In the 234 RIOPA homes (RIOPA = relationships of indoor, outdoor, and personal air), a median of 20.1 μg m−3 and a 95-P of 32.5 μg m−3 were measured.(275) Nevertheless, the authors assert that formaldehyde should still be regarded as belonging to the group of strongest indoor pollutant sources with an estimated median source strength of 3.9 mg h−1. In the BEAM study (BEAM = Boston Exposure Assessment in Microenvironments), geometric means of 19.6 μg m−3 and 14.3 μg m−3 were found in stores and restaurants, respectively.(276) A French study reports formaldehyde concentrations between 19 μg m−3 and 20 μg m−3 in Paris newborn babies’ homes.(277) When comparing these data with the results of the German Environmental Survey of 2003−2006 (the results from all German indoor surveys are available from http://www.umweltbundesamt.de) and other results from “normal” living spaces (see Table 7), average exposure concentrations between 20 μg m−3 and 40 μg m−3 seem to be more realistic. Although statistical parameters are valuable tools, a detailed risk assessment requires additional information such as the distribution of formaldehyde concentrations in histogram form and the number or percentage of data exceeding a defined value. Unfortunately, such information is only rarely found in the scientific literature. Dingle and Franklin(278) provide a distribution of mean formaldehyde concentrations in 185 homes in Perth, Western Australia. The geometric mean was 22.9 ppb, and for 86% of the homes, the level was <50 ppb. Seifert and Salthammer(279) have published the results of 595 formaldehyde measurements carried out by the Bremer Umweltinstitut before 1995. More than 60% of the indoor concentrations were below 0.05 ppm. On the other hand, about 10% of the concentrations exceeded 0.1 ppm. However, it is not certain that all of the dwellings included in this study were randomly selected.
Measuring personal exposure to formaldehyde and other airborne pollutants in the indoor environment is also of great interest.(280) This is usually done by placing passive samplers close to the breathing zone of test persons. A number of studies on this topic is known,212,281−283 and in most cases the personal exposure values were not significantly different from indoor concentrations.
7.3. Formaldehyde in New and Renovated Homes
Increased formaldehyde concentrations are often correlated with renovation work, new materials, or special conditions, e.g. private and public transport,284−286 medical laboratories,287−290 museums,291,292 combustion,(293) etc. Broder et al.(294) made comparisons of the health and house characteristics of the occupants of 231 control homes and 571 houses containing urea−formaldehyde foam insulation. Godish(237) compared measurements, carried out between 1978 and 1989, in different types of U.S. houses (houses insulated with urea−formaldehyde foam, prefabricated houses, and conventional houses). The WKI carried out 367 formaldehyde measurements in new prefabricated houses between 1996 and 2006. This type of housing is commonly made with wood-based materials such as particle board and OSB. The distribution of concentrations is shown in Figure 13 and is obviously log−normal (see solid curve in Figure 13). The median was 0.04 ppm, and the German guideline value of 0.1 ppm was exceeded by 14% of the data. The influence of modern building products, especially insulation materials made of mineral wool, on indoor formaldehyde levels was investigated in the EURIMA-WKI test house study.(154) The results of one experiment are shown in Figure 14 (for more details, see also the Supporting Information of ref (154)). Following installation of the test house (n = 0.3 h−1, no mineral wool, carpeting, adhesives, or furniture) in a 48 m3 stainless steel chamber (n = 2.0 h−1), the formaldehyde concentration inside the house increased to approximately 22 μg m−3. Although low-emitting and certificated materials were used, it was not possible to obtain formaldehyde levels below 20 μg m−3, since most common building products (e.g., wallpaper) release low amounts of formaldehyde. This indicates that low concentrations of formaldehyde can hardly be avoided in a new living space. It is interesting that the presence of mineral wool had no influence on the formaldehyde level in the house, but when the test house contained carpeting, carpet adhesive, and a sideboard made of lacquered particle board, an increase of the formaldehyde concentration up to 69 μg m−3 could be observed.
Figure 13.
Distribution of formaldehyde concentrations in new prefabricated houses between 1996 and 2006. The log−normal fit curve was obtained from nonlinear regression analysis. The 50-P value was 0.04 ppm, and 14% of the data exceeded the German guideline value of 0.1 ppm.
Figure 14.
Influence of building products on the formaldehyde concentration inside a test house (n = 0.3 h−1) and in the 48 m3 stainless steel chamber (n = 2.0 h−1). Measurements were performed between Oct 15 and Nov 29, 2007 (from Salthammer and Mentese(154)).
In the Danish twin apartment study,(295) formaldehyde was monitored in two apartments as a function of occupancy and season. After completion of the construction work, the formaldehyde concentration fell from 200−300 μg m−3 to 80 μg m−3. Subsequently, it rose to above 400 μg m−3 when the apartment was vacant during the spring and summer. In the occupied apartment, the formaldehyde concentration was 10 μg m−3 in the summer and 100 μg m−3 in the fall. These differences probably result from insufficient ventilation in the vacant apartment in the summer and from less ventilation of the occupied apartment in the fall. Harving(296) et al. calculated a median of 37 μg m−3 from measurements taken in 36 Danish apartments. They conclude that formaldehyde is of minor importance as an irritant in the indoor environment, but they also state that natural ventilation in a great number of Danish dwellings is too low from a health point of view. Brown(297) has studied indoor formaldehyde levels in Australian dwellings after construction. Within 246 days, the concentrations in living rooms decreased from 120 μg m−3 to 46 μg m−3. Surprisingly high formaldehyde concentrations between 209 μg m−3 and 457 μg m−3 were measured in new apartment buildings in Korea.(298) Park and Ikeda(299) measured formaldehyde levels over a period of three years in new and older homes. In the new homes, there was a falling trend from 134 μg m−3 (mean, first year) to 86 μg m−3 (mean, third year), while in the older homes the mean concentration of 88−90 μg m−3 became stable within three years. Sohn et al.(300) studied indoor and outdoor concentrations of formaldehyde as a function of the season. Geometric means ranged from 0.04 ppm (fall) to 0.09 ppm (winter). The indoor/outdoor ratios were about 5. Formaldehyde concentrations in Hong Kong homes (50-P = 85.7 μg m−3 for 100 samples) and the effect of aging were investigated by Guo et al.(301) A comparison with indoor formaldehyde concentrations in China, Taiwan, Korea, and Japan is presented in the same study.
7.4. Formaldehyde in Mobile Homes
Relatively high formaldehyde concentrations can be measured in mobile homes. This has already been stated by Sexton et al.,(302) who investigated 470 mobile homes manufactured between 1966 and 1984 and in 147 cases (31%) found concentrations > 0.1 ppm. Hanrahan et al.(303) have measured formaldehyde levels up to 2.8 ppm. The data by Dingle et al.(304) based on 192 caravans are consistent with other studies, but these authors have also found distinct differences between occupied and unoccupied caravans. Furthermore, Dingle et al. point out that the increased formaldehyde concentrations in mobile homes result from higher loading rates with wood-based materials of approximately 1.4 m2 m−3 and lower air exchange rates compared to conventional buildings. Main and Hogan(305) exposed 21 test persons to formaldehyde concentrations between 0.12 ppm and 1.6 ppm in two mobile trailers. Symptoms such as eye and throat irritation, headache, and fatigue were observed. In the United States, discussion about formaldehyde in mobile homes returned to public attention when survivors of hurricane Katrina, who live in trailers provided by the U.S. Department of Homeland Security (FEMA), complained about strange odors and adverse health effects. The U.S. Centers for Disease Control and Prevention (CDC) randomly selected 519 out of 120,000 trailers and mobile homes. The range of concentrations was between 3 and 590 ppb with a geometric mean of 77 ppb. In their study, CDC stated that the indoor temperature was a significant factor for formaldehyde levels irrespective of trailer make or model.(306) Maddalena et al.307,308 studied four unoccupied FEMA temporary housing units, each produced by a different manufacturer, to assess their indoor emissions. Steady-state indoor formaldehyde concentrations ranged from 378 μg m−3 (0.31 ppm) to 632 μg m−3 (0.52 ppm) in the morning and from 433 μg m−3 (0.35 ppm) to 926 μg m−3 (0.78 ppm) in the afternoon. Air exchange rates ranged from 0.15 h−1 to 0.39 h−1. Wolkoff and Kjaergaard(309) state that formaldehyde emission from wood-based materials is proportional to relative humidity at a given temperature. This might also play a role in the hot and humid climate of the southern U.S. states.
7.5. Influence of Climatic Parameters on Formaldehyde Levels
The effect of climatic parameters on indoor formaldehyde concentrations has been considered in several studies. Salthammer et al.(64) found a negative correlation between the formaldehyde concentration and the air exchange rate. A similar observation was made by Gilbert et al.(310) Ninety-six homes were studied in Quebec, and 80% of those homes had air exchange rates < 0.23 h−1. Based on the entire sample, the air exchange rate which ensured a formaldehyde concentration below Health Canada’s long-term exposure limit of 50 μg m−3 in 95% of the homes was 0.26 h−1. Gilbert et al. recommend that an air exchange rate meeting ASHRAE’s recommendation of 0.35 h−1 appears sufficient to ensure a concentration within the Canadian guideline in most homes. CDC has found slight associations of indoor temperature and relative humidity and logarithmic formaldehyde concentrations in occupied FEMA trailers.(306) A temperature effect was also reported by Jo and Sohn.(311) Tuomainen et al.(312) found that ventilation and occupancy had only a small effect on the formaldehyde concentration in one case and one control building. In this study, the formaldehyde concentrations were low (1−27 μg m−3) and the number of samples was 12 or less. Dingle and Franklin(278) investigated indoor formaldehyde concentrations as a function of the season and the age of the house. Higher levels were found in newer homes and in homes monitored in summer. Jaernstroem et al.(313) also report higher formaldehyde concentrations in the summer. Furthermore, these authors applied the method of principal component analysis (PCA)(314) to find out which variables affected indoor air quality most. These variables, which are not necessarily independent, were season, relative humidity, temperature, air exchange, floor coverings, ceilings, wall coverings, and occupancy. Wong et al.(315) attempted to relate indoor formaldehyde concentrations to other pollutants and climatic parameters but found only weak correlations. The data for prefabricated houses shown in Figure 15 were grouped by year. The results for 1996 and 2006 are shown in Figure 15A as Box−Whisker plots. At first sight, it seems surprising that both samples give the same median of 0.4 ppm. On closer inspection, however, the distribution is much smaller for the data from 2006 and, even more important, the air exchange rates (see Figure 15B) decreased from 1996 to 2006. This leads to the conclusion that formaldehyde emission rates in prefabricated houses will have fallen during the last 10 years, but this positive effect has been canceled out by decreasing air exchange rates.
Figure 15.
Box-and-whisker plots (min, max, mean, 25-P, 50-P, 75-P) of formaldehyde concentrations (A) and air exchange rates (B) for the years 1996, 2001, and 2006 (data from Figure 13).
8. Exposure and Risk Assessment
8.1. Calculation of the Daily Intake
Although outdoor air contributes adversely to indoor air quality,(268) it was believed that human exposure to formaldehyde mainly originates in the indoor environment rather than the ambient environment.(316) Two parameters are of major importance in the evaluation of indoor exposure to formaldehyde: (a) the concentration in air and (b) the time spent indoors. The latter depends on the age group and on the daily activities. Brasche and Bischof(317) have investigated the time spent indoors in German homes with regard to age, gender, characteristics, and location of the home. The overall mean time of 15.7 h per day is in line with results from the United States and Canada. Approximations made by the U.S. EPA(318) have been widely applied to calculate the daily intake of formaldehyde. The EPA indicated an inhalation rate of 0.63 m3 h−1 and 10 h per day for residential exposure. Stubenrauch et al.(319) estimate inhalation rates of 0.8 m3 h−1 for adults and 0.25 m3 h−1 for young children over 21 h per day. The total daily intake (DI) for an individual moving in n different environments can be computed from eq 26, where Cj, IRj, and tj represent the concentration, inhalation rate, and time spent in the environment j.
The WHO(27) has calculated probabilistic estimates of 24 h time-weighted average concentrations of formaldehyde in the air on the basis of five Canadian studies. The results indicate that one in two persons would be exposed to concentrations of 24−29 μg m−3, while 1 in 20 persons would be exposed to 80−94 μg m−3. Baez et al.(320) applied the EPA exposure scenario to Mexican homes and calculated a daily uptake of 173−600 μg day−1 on the 95-P level. It should, however, be pointed out that, for a respiratory tract irritant, the time-weighted average concentration and especially the peak exposure are more meaningful than the daily intake.
Acute, chronic (noncancer), and potential carcinogenic effects on humans have been reported for formaldehyde. Numerous case-control studies, cohort studies, and reviews have been published. The most relevant and frequently cited review papers, representing different views and perspectives, are summarized in Table 8. Results are also available for specific subgroups, such as children,321−327 special indoor environments, such as medical laboratories,288,328−331 workplaces,200,332,333 exposure studies,334−336 and others.(7) Animal studies are not included in this review. It should only be mentioned that Lu et al.(337) have recently reported an effect of inhaled formaldehyde on the learning and memory of mice due to oxidative stress.
Table 8. Reviews of Studies of the Acute and Chronic Adverse Health Effects of Formaldehyde.
| topic | ref |
|---|---|
| the INDEX report | Kotzias et al. (2005)(17) |
| World Health Organization—report on formaldehyde | WHO (1989, 2002)10,27 |
| classification of formaldehyde as human carcinogen | IARC (2006)(483) |
| sensory irritation in relation to carcinogenicity | Arts et al. (2006)(338) |
| evaluation of data on carcinogenicity | Appel et al. (2006)(348) |
| risk assessment for the population in Japan | Naya (2005)(357) |
| risk factors for respiratory and allergic effects in children | Mendell (2007)(229) |
| evaluation of epidemiological studies (1994−2006) | Duhayon et al. (2008)(200) |
| occupational exposure limit based on irritation | Paustenbach et al. (1997)(201) |
| hazard characterization and exposure−response relationship | Liteplo and Meek (2003)(476) |
| mode of action for carcinogenicity of formaldehyde | McGegor et al. (2006)(16) |
| evaluation of epidemiological studies | Bosetti et al. (2008)(351) |
| evaluation of literature related to effects with indoor air exposure | Arts et al. (2008)(349) |
| formaldehyde exposure and leukemia | Zhang et al. (2009)(202) |
8.2. Short-Term and Long-Term Exposure (Noncancer)
Acute (short-term) human exposure to formaldehyde causes discomfort; irritation of the eyes, nose, and throat; lachrymation; sneezing; coughing; nausea; and finally death.(27) Table 9 has been adopted from WHO(27) and provides an overview of acute human health effects at differrent levels of exposure. In the INDEX report,(17) studies of humans under controlled conditions are summarized as follows: “acute exposures to air concentrations ranging from 0.5 mg m−3 to 3.7 mg m−3 induce reversible eye, nose, and throat irritation, produce changes in nasal lavage fluid contents (indicative of irritation of the nasal epithelium), do not consistently or markedly affect pulmonary function variables in most individuals.” No-observed-(adverse)-effect levels (NO(A)EL) have been published on the basis of different criteria. The NOAEL of 0.03 mg m−3 mentioned in the INDEX report corresponds to the lowest odor threshold reported.(17) Studies of irritation in humans have been reviewed by Paustenbach et al.(201) and Arts et al.(338) These authors concluded that irritation starts at levels around 1 ppm. In a recent study designed on the basis of current standards, Lang et al.(24) examined the possible occurrence of sensory irritation and subjective symptoms in human volunteers exposed to formaldehyde concentrations relevant to the workplace. They concluded that sensory eye irritation is the most sensitive parameter and obtained a NOAEL of 0.5 ppm. This is in contrast to the WHO data in Table 9, which gives a lower threshold for throat and nose irritation of 0.1 mg m−3. However, in the case of the WHO publication cited, it is difficult to trace the original source, which makes a critical evaluation difficult. Wolkoff et al.(339) have also reviewed human odor and sensory irritation threshold values for formaldehyde and other compounds. They conclude that many sensory irritants are formed from alkene oxidation reactions, where formaldehyde is a major product.
Table 9. Effects of Formaldehyde in Humans after Short-Term Exposure(274).
| conc range or avg (mg m−3) | time range or avg | health effects in general population |
|---|---|---|
| 0.03 | repeated exposure | odor detection threshold (10-P)a |
| 0.18 | repeated exposure | odor detection threshold (50-P)a |
| 0.6 | repeated exposure | odor detection threshold (90-P)a |
| 0.1−3.1 | single and repeated exposure | throat and nose irritation threshold |
| 0.6−1.2 | single and repeated exposure | eye irritation threshold |
| 0.5−2.0 | 3−5 h | decreased nasal mucus flow rate |
| 2.4 | 40 min on 2 successive days with 10 min of moderate exercise on second day | postexposure (up to 24 h) headache |
| 2.5−3.7 | b | biting sensation in eyes and nose |
| 3.7 | single and repeated exposure | decreased pulmonary function only during heavy exercise |
| 5−6.2 | 30 min | tolerable for 30 min with lachrymation |
| 12−25 | b | strong lachrymation, lasting for 1 h |
| 37−60 | b | pulmonary edema, pneumonia, danger to life |
| 60−125 | b | death |
Frequency of effect in population.
Time range or average unspecified.
Formaldehyde is a well-known skin sensitizer, but its interrelationship with asthma has been debated for many years. Publications of different design and quality are available on this subject,323,324,335,340−346 but considering the widespread exposure to formaldehyde, reports on respiratory sensitization are few. In a recent review focusing on the effects of residential formaldehyde levels, Arts et al.(347) evaluated the data available, especially for children. They concluded that the question is still open whether there is a causal relationship between formaldehyde and allergic asthma or whether formaldehyde induces airway irritation resembling asthmatic reactions. Similarly, Appel et al.(348) were not able to adduce clear evidence for asthma induced by formaldehyde.
In occupational and residential environments, long-term exposure (chronic, noncancer) to increased levels of formaldehyde results in irritation of the upper and lower airways and eyes. Most studies related to chronic effects refer to the working environment, where formaldehyde is frequently used. The predominant effect of formaldehyde is sensory irritation at low concentrations, which will progress to cytotoxic irritation with cell destruction at higher concentrations. These effects are concentration- and not time-dependent. The threshold concentrations for sensory and cytotoxic irritation are therefore very similar for acute and chronic exposures. Concentrations not leading to sensory irritation after acute exposures are not expected to result in adverse effects after prolonged exposures.
8.3. Formaldehyde as a Human Carcinogen
The classification of formaldehyde as a known human carcinogen by IARC is based on cohort mortality studies of workers exposed to formaldehyde with an increased incidence of nasopharyngeal cancer.(332) However, the evaluation by IARC has been questioned by several authors. Marsh and Youk(349) pointed out that their reanalysis of the available data provided little evidence for a causal association between formaldehyde exposure and mortality and they recently provided evidence that the increased incidence of nasopharyngeal cancer might be related to exposures to several suspected risk factors for upper respiratory system cancer (e.g., sulfuric acid mists, mineral acid, metal dusts, and heat) in the metal industries of that area.(333) Moreover, Marsh et al.(350) criticized that the nasopharyngeal cancer risk models developed by Hauptmann et al.(332) and used by IARC to justify their classification of formaldehyde as a human carcinogen were mis-specified and nonrobust. Thus, the authors claimed that the decision of IARC should be reconsidered. Duhayon et al.(200) and Bosetti et al.(351) have reviewed epidemiological studies published after 1994. The authors state that the evidence for an association between formaldehyde exposure and nasopharyngeal cancer appears debatable and also suggest a need to reconsider the current carcinogenic classification by IARC. The German Federal Institute for Risk Assessment (BfR) and other institutions have stated that results from recent epidemiological studies support a possible causal relationship between inhalation exposure to formaldehyde and nasopharyngeal cancer.16,348 In their publication, the BfR points out that a slight sensory irritation response can be observed at concentrations of 0.2−0.3 ppm and that ocular and upper respiratory tract sensory irritation is not present below 0.1 ppm. A formaldehyde concentration of 0.1 ppm is therefore proposed as a safe level.
According to the evaluation by IARC, the epidemiologic evidence was strong but not sufficient to conclude that formaldehyde exposure causes leukemia in humans. Moreover, a plausible mechanism has not been identified how leukemia may be induced after formaldehyde inhalation. Two large cohort studies352,353 pointed at possible excesses while Coggon et al.(354) could detect no link between formaldehyde exposure and leukemia risk. Since the discussion is mainly driven by the National Cancer Institute (NCI) study,(352) the observation of Marsh and Youk(355) is relevant that the upward trend noted in the NCI study is produced by a deficit of leukemia cases among the unexposed and low exposed workers. Their reanalysis provided little evidence to support NCI’s suggestion of a causal association between formaldehyde exposure and mortality from leukemia. In the recent follow-up of the NCI study, Beane Freeman et al.(356) admitted that Hauptmann et al. had not included 1006 deaths in their previous analyses. The authors also showed that in some cases the original cause of death had to be changed. Bean Freeman et al.(356) summarize that the overall leukemia risk trends have decreased in comparison to their previous publication but still remain somewhat elevated and that further studies are needed to evaluate the risk of leukemia in other formaldehyde exposed poulations.
Some recent studies deal with the risk of cancer in relation to formaldehyde in air. Naya and Nakanishi(357) have evaluated case-control as well as cohort studies in humans and recommend a reference value of 0.01 ppm in outdoor air for the general population in Japan. The U.S. EPA provides an Integrated Risk Information system (IRIS) (http://www.epa.gov/iris) for calculating the cancer risk for formaldehyde and other chemicals.(358) Loh et al.(359) ranked the cancer risks of organic hazardous air pollutants in the United States and applied the unit risk model, where the chronic daily intake is multiplied by a cancer potency factor.(360) The Scientific Committee on Occupational Exposure Limits (SCOEL) recommends that regulations and health-based exposure limits for formaldehyde should be based on an established NOAEL.(361) Irigaray et al.(362) have identified formaldehyde as one of the compounds of major concern when evaluating lifestyle-related factors and environmental agents causing cancer.
For the risk assessment approach with regard to carcinogens, the mode of action is of pivotal importance. For genotoxic chemicals that lead to tumors by mutations of the DNA, it is generally accepted that a threshold cannot be defined and linearized mathematical models are used to define an exposure with acceptable risk as regards socioeconomic considerations. A different approach is applicable to nongenotoxic carcinogens which lead to tumors by a threshold mechanism, such as, for example, chronic irritation. Exposures below this threshold are not expected to have a carcinogenic effect. In 2000 the German MAK commission concluded that, at low exposure, concentrations without an increase of cell proliferation genotoxicity “play no or at most a minor part...so that no significant contribution to human cancer risk is expected”. This assessment has so far been annually confirmed by the commission.(363) This mode of action has recently also been supported by Liteplo and Meek(364) and has been refined and put into the context of the 2006 IPCS (International Programme on Chemical Safety) human framework for the analyses of cancer’s mode of action for humans.(16) Accordingly, concentrations not resulting in cytotoxic irritation with an increased cell proliferation would represent a threshold for carcinogenic action upon the upper respiratory tract. As cytotoxic irritation will only occur at concentrations clearly above those leading to sensory irritation, a carcinogenic action is not to be expected so long as sensory irritation is avoided. This sensory irritation is the decisive end point for all indoor air limits proposed in the last years by regulatory bodies, and these limits should therefore provide protection against tumor induction by formaldehyde.
8.4. Formaldehyde and SBS
The term “Sick Building Syndrome (SBS)” has been used to describe the mostly nonspecific complaints of occupants of buildings.(365) Brightman and Moss,(366) who have evaluated different studies, pointed out that SBS describes a constellation of symptoms which have no clear etiology and are attributable to exposure to a particular building environment. On the basis of a WHO report,(367) Mølhave(368) has suggested a definition for SBS. The most common symptoms are eye, nose, and throat irritation; dry and itching skin; nonspecific hypersensitivity; sensation of dry mucous membranes; headache, fatigue, and dizziness; airway infections and cough, wheezing and nausea. In general SBS does not correlate with any single factor, and only occasionally has formaldehyde been linked to SBS. Mendell(369) has summarized reported associations between work-related symptoms and a potential 37 factors and measurements from 33 studies conducted between 1984 and 1992. Formaldehyde was only mentioned by Skov et al.,(370) who did not find an association. Formaldehyde was therefore classed by Mendell as a compound with a “consistent lack of association with symptom reports”. Mainly consistent with symptom reports were air-conditioning, job dissatisfaction, and allergies/asthma. Similar results were obtained from the Danish town hall study371,372 and the German ProKlimA study.(273) An interesting aspect was raised by Sundell et al.,(373) who identified higher formaldehyde and lower TVOC (total volatile organic compound) levels as risk indicators for SBS. This was attributed to VOCs reacting with other air pollutants such as ozone to form irritant byproducts such as formaldehyde. Brightman and Moss(366) have called this theory “the missing VOCs”. A link between VOC exposures and SBS was demonstrated for the first time by TenBrinke et al.(374) SBS should not be confused with the so-called “Sick House Syndrome”. This term is mainly used in Japan and also takes into account the health problems of individuals in private dwellings.375,376
9. Reduction of Indoor Formaldehyde Pollution
Techniques for lowering the concentration of formaldehyde in the indoor environment have been discussed by several authors and can be classified into several groups:
• avoidance of sources and prevention of emissions right from the start
• removal of the source
• surface coating
• fumigation with ammonia
• increased ventilation
• catalytic reactions
• adsorption
The California Air Resources Board points out that “.. .the most effective way to reduce formaldehyde in indoor air is to remove or reduce sources of formaldehyde in the home and avoid adding new sources”.(219) However, the feasibility of source removal will sometimes depend on the circumstances. It may well be an easy matter in the case of furniture but be much more complicated in the case of a built structure. Surface treatment by use of reactive or diffusion resistant coatings and fumigation with ammonia were mainly applied in the case of very high formaldehyde levels in the 1980s and 1990s. The reaction with ammonia which yields hexamethylene tetramine (see eq 1) was in particular used in prefabricated houses. It is a drastic but very effective and long-lasting solution.2,377 Uchiyama et al.(378) proposed the application of natural compounds such as urea, catechin, and vanillin to suppress formaldehyde emission from plywood. Kim(379) suggested the use of volcanic pozzolan.
These days, formaldehyde source strengths are lower. This means that people are exposed to lower formaldehyde concentrations in the indoor environment, and it is more convenient to reduce pollutant levels by use of intelligent housing construction and ventilation.380,381 Here it is the responsibility of architects and engineers to ensure adequate air exchange with low heat loss. Sherman and Hodgson(382) suggested using measured formaldehyde emission and authoritative exposure standards to develop minimum ventilation rates for dwellings. Photocatalytic oxidation systems for the removal of formaldehyde have recently become popular.(383) One method works with high-flow photoreactors, where degradation of the pollutants is performed over a solid TiO2 catalyst and with UV light of high intensity.384−390 Hodgson et al.(391) as well as Mo et al.(392) have shown that such ultraviolet photocatalytic oxidation (UVPCO) systems produce formaldehyde due to incomplete mineralization of VOCs. The other technique uses wall paint equipped with modified TiO2(393) for the purpose of photocatalytically removing air pollutants under indoor conditions. Although such wall paints are frequently advertised, serious publications examining their efficiency have not yet become available. Moreover, Salthammer and Fuhrmann,(93) Gunschera et al.,(95) as well as Auvinen and Wirtanen(94) have shown that, under the influence of light, photocatalytic wall paints produce undesired secondary emissions such as formaldehyde. Quiller et al.(394) have shown that, in the presence of ozone, TiO2 surfaces promote the oxidation of styrene to form formaldehyde. From time to time, it is reported that plants act as air cleaners, but the literature on this topic is contradictory. The results of Godish and Guindon(395) do not support previous suggestions that botanical air purification using only plant leaves is an effective means of reducing residential formaldehyde levels. Giese et al.(396) demonstrated that formaldehyde is efficiently metabolized by the spider plant (Chlorophytum comosum). Schmitz et al.(397) conclude that the assimilation and metabolism of formaldehyde by leaves appears unlikely to be of value for indoor air purification. Wool fiber is also used for adsorption of formaldehyde under indoor conditions. Although there is no question that wool has the capability to adsorb formaldehyde, recent work refers to unreasonably high indoor concentrations of 1 ppm.398,399 Proof of the applicability of wool at lower formaldehyde concentrations is still absent. Matthews et al.(186) have demonstrated that gypsum board has some storage capacity for sorbed formaldehyde but appears to result in only a minor permanent loss mechanism.
A new aspect has recently been mentioned by Mui et al.(400) These authors evaluated the energy impact assessment for the reduction of carbon dioxide and formaldehyde exposure risk in air-conditioned offices in Hong Kong. They conclude that the energy impact should be an important factor in future ventilation strategies regarding indoor air quality.
10. Other Emissions Related to Formaldehyde
The appearance of other volatile organic compounds in conjunction with formaldehyde in indoor air can be classified by chemistry or by source. Two compounds are regarded as “chemistry-related” if one is formed by chemical reaction from the other. Two compounds are “source-related” if they are released from the same source but originate in different reactions. Formic acid, which is the oxidation product of formaldehyde (see eq 2), has been recognized as a hazard in the museum environment. Raychaudhuri and Brimblecombe(401) have found that formaldehyde can damage lead objects in display cases via conversion to formic acid. However, they also state that the question posed by Hatchfield and Carpenter,(402)“formaldehyde—how great is the danger to museums collections?”, remains unanswered. Tetrault(403) has pointed out that, with the exception of lead, very few studies support the proposition that formaldehyde is harmful to exhibits in a museum environment. In contrast, acetic acid seems to be a more aggressive indoor organic compound for a larger range of objects. Schieweck et al.(292) and Salthammer et al.(404) have measured formaldehyde, formic acid and acetic acid in different departments of a German museum. More references on museum-related sources, levels and associated damage to materials are available in ref (291).
Wood and wood-based materials are good examples for describing the occurrence of VOCs related to formaldehyde, as shown in Figure 16. The volatile ingredients of softwood mainly consist of monoterpenes, of which the most important are α-pinene, β-pinene, 3-carene, limonene, camphene, myrcene, and β-phellandrene.405,406 As outlined in section , terpenoids and other unsaturated hydrocarbons are precursors of formaldehyde in the presence of strong oxidants. The reaction of terpenes with ozone leads to the formation of terpene aldehydes(407) and secondary organic aerosols.408−410 Compounds such as methanol, acetaldehyde, and acetic acid are typically released from hardwood.(411) In the case of wood-based materials, aldehydes and other compounds are formed during the manufacturing process. Jiang et al.(412) as well as Makowski et al.413,414 have measured emissions of saturated and nonsaturated aldehydes (C3−C10) from the hot-pressing of mixed hardwood and Scots pine, respectively. Acetic acid and furfural result from the thermal degradation of hemicelluloses. These two compounds are most prominent in cork products.415,416 Acetic acid is formed from the degradation of acetyl groups, while furfural is produced from pentoses and hexoses under elimination of water. Furthermore, phenol−formaldehyde resins are frequently used in the manufacture of cork products. For this reason, formaldehyde and phenol are often measured together.(415)
Figure 16.
Possible VOC emissions related to formaldehyde for the case of a wood-based material.
In the case of other building materials such as carpet, latex paint, and so on, the occurrence of formaldehyde is related to the ozone-induced formation of other saturated and nonsaturated aldehydes (C2−C13, 2-octenal, 2-nonenal, 2,4-nonadienal, benzaldehyde, tolualdehyde).71,78,85,88 Test chamber experiments are often in good agreement with real-room measurements.243,254 Combustion processes can be identified by the simultaneous appearance of formaldehyde, acetaldehyde, and acrolein.260,261 Gilbert et al.(417) have pointed out that smoking is a source of acetaldehyde and acrolein in indoor air.
11. Summary and Outlook
Among the large variety of gaseous indoor pollutants, formaldehyde has always had an exceptional position, and there is no reason to believe that this view will change in the near future. Formaldehyde is a highly reactive aldehyde, it is an important chemical feedstock, it is a constituent of many industrial products, and it is ubiquitous outdoors and indoors due to natural and anthropogenic processes. As Pluschke(33) pointed out, formaldehyde was the hazardous substance “par excellence” in the 1980s. In 1970s Germany, the release of formaldehyde from wood-based materials and the high pollution levels in classrooms and daycare facilities for children triggered a public debate on indoor air pollutants. Every few years, the “formaldehyde discussion” is resuscitated—in 2004, for example, following classification of formaldehyde by IARC as a Group 1 carcinogen and recently, in the United States, when survivors of hurricane Katrina suffered adverse effects from the poor indoor air quality in their trailers.
Emission of formaldehyde from building products and consumer goods has been limited by authorities and by voluntary criteria. Reliable but sophisticated tools are available to measure formaldehyde concentrations even at low levels in rural environments. Indoor concentrations have been systematically monitored over the years. An evaluation of recent emission studies and indoor surveys has demonstrated that the situation has improved due to the progress made over recent decades regarding indoor products with reduced emissions. An examination of international studies carried out in 2005 or after (see Table 7) indicates that the average exposure of the population to formaldehyde seems to lie between 20 μg m−3 and 40 μg m−3 under normal living conditions. Although this trend toward decreasing concentrations is a positive one, it should be kept in mind that such average concentrations do not take into account the higher exposure which may result from new buildings or special indoor conditions, peak concentrations, and individual cases. It can be expected that advanced products and intelligent housing designs will bring about a further fall in formaldehyde concentrations indoors. However, the average indoor air concentrations of other compounds have also decreased and the RIOPA study has shown that among the carbonyls the indoor source strength of formaldehyde is still high in the USA. On the other hand, Eikmann et al.(418) have stated that in Germany formaldehyde exposure in the private environment can be regarded as manageable and controllable.
The previously mentioned classification of formaldehyde as a Group 1 carcinogen by IARC has started some controversial discussions. Different authorities in different countries and organizations as well as other epidemiologists have re-evaluated available data and come to different conclusions. Health Canada did not take IARC’s classification into consideration and in 2005 established a new formaldehyde indoor air guideline value of 50 μg m−3 (8 h). The German Federal Institute for Risk Assessment was aware of the IARC classification in 2006(419) but considered 0.1 ppm (124 μg m−3) to be a “safe level” and decided that a revision of the German guideline value was not required. Arts et al.(347) also concluded that 0.1 ppm can be considered a safe and appropriate level. A European working group stated in the INDEX report that formaldehyde should be regarded as a chemical of concern at indoor levels exceeding 1 μg m−3. The same group pointed out that from about 30 μg m−3 mild irritation of the eyes could be experienced by the general population and odor perceived. In other studies, odor thresholds and sensory irritation levels were found to be about one magnitude higher. Wolkoff(420) has stated that the guideline values for formaldehyde and other reactive VOCs appear to be overestimated. Taking into account average formaldehyde concentrations in indoor, rural, and urban air (see Figure 17) and the toxicological data available, interpreting values lower than 30 μg m−3 as guideline values or as recommendations seems to be somewhat unrealistic. Instead, established guideline values in the range of the WHO value could be used as a basis and should be complemented by application of the ALARA principle (ALARA = as low as reasonably achievable) and of recommendations regarding better ventilation and keeping temperatures moderate.
Figure 17.
Range of formaldehyde concentrations in different environments.
No one will reasonably doubt that formaldehyde is a relevant indoor pollutant. Regulations are urgently required, but the lower guideline value is not necessarily the better one. If indoor-related research is focused solely on formaldehyde and all efforts are applied to this compound, other pollutants will be easily neglected, something which can be counterproductive for human health. As an example, radon is also classified by IARC as a Group 1 human carcinogen, but formaldehyde has a considerably higher ranking in the public attention, probably as the result of a large number of previous incidents due to the acute health effects. Moreover, it seems questionable whether formaldehyde concentrations lower than 20 μg m−3 can be permanently achieved under normal living conditions in urban and rural environments. In addition to building materials and household products, we have to consider other formaldehyde sources, such as outdoor air, indoor chemical reactions, candles, cooking, gas heaters, etc. Although quantitative data are not available, we may conservatively estimate that these additional sources contribute 10−50% to formaldehyde indoor concentration levels. However, the uncertainty of this estimation indicates the need for further research on this topic.
Today, it is possible to produce low-emitting materials, and such products are already recommended by manufacturers of furniture and housing. On the other hand, the air exchange rates in houses have decreased in almost the same manner. Mechanical ventilation also consumes energy, and all the factors mentioned make for a vicious circle. The formaldehyde story will be continued.
13. Acknowledgments
The authors would like to thank the FormaCare sector group of CEFIC, Brussels, Belgium, for providing the financial support for this study. The statements made in this paper are those of the authors and are not necessarily in agreement with the views of CEFIC or related industries. We are also grateful to Dr. Tobias Schripp, WKI, for communicating his results (see Figure 5) prior to publication.
Biographies

Tunga Salthammer was born in Braunschweig, Germany, in 1960. He holds a diploma degree in chemistry (1986) and a Ph.D. in physical chemistry (1990) from the Technical University of Braunschweig, Germany. In 1989, he conducted research at the Physics Department of Strathclyde University in Glasgow, U.K. He joined the Fraunhofer Wilhelm-Klauditz-Institute (WKI) in 1990, where he currently serves as head of the Department of Material Analysis and Indoor Chemistry. From 2003 to 2009, he was a Professor of Indoor Hygiene at the University of Applied Sciences Braunschweig/Wolfenbüttel. Since 2007, he has been an Adjunct Professor at the Queensland University of Technology (QUT) in Brisbane, Australia. In June 2008, he received his state doctorate from the Technical University of Braunschweig. He was a Visiting Professor at the Technical University of Denmark in Lyngby (2006−2007) and at the Tsinghua University of Beijing, China (May 2007). He is a member of the Indoor Air Hygiene Commission of the German Federal Environment Agency. His research interests include analytical chemistry, VOC/SVOC emission studies on indoor materials using test chambers and cells, indoor chemistry, airborne particles, and settled dust.

Sibel Mentese was born in 1981 in Turkey. She received her B.S. degree in environmental engineering at Dokuz Eylül University in 2002, where she worked with Prof. Dr. Aysen Muezzinoglu. She obtained her M.S. degree in environmental engineering at Hacettepe University in 2004. She worked on the determination of residential formaldehyde levels for her M.S. thesis under the supervision of Prof. Dr. Gulen Gullu. In the same year, she commenced her Ph.D. degree in the same department, where she also started to work as a research assistant. She also received an M.A. degree in environmental economics at Ankara University in 2007. In the same year, she was awarded a scholarship by the Scientific and Technological Council of Turkey to conduct a project with Prof. Dr. Tunga Salthammer at the Fraunhofer WKI research center in Germany. In 2009, she received her Ph.D. in Environmental Engineering at the Hacettepe University on indoor air quality.

Rainer Marutzky was born in Halle, Germany, in 1947. From 1968 to 1972, he studied chemistry at the Technical University of Braunschweig. In 1975, he received his Ph.D. in biochemistry and enzyme kinetics. In 1976, he joined the Fraunhofer Wilhelm-Klauditz-Institute of Wood Research in Braunschweig as a research officer. In 1989 he became the director of the institute. Since 1996, he has been an Associate Professor of Wood Chemistry at the Technical University in Braunschweig. He has been engaged in formaldehyde research and testing for more than 30 years. Other research interests include wood combustion, wood-based panel recycling, and adhesive chemistry. He is the convener of the European standard committee for formaldehyde and other regulated dangerous substances.
12. Glossary
- ε
absorption coefficient
- δ
boundary layer
- τ
atmospheric lifetime
- ϕ
fluorescence yield
- A
surface of the passive sampler
- A1, A2, A3
prexponential factors (equation 25)
- AB
coefficient of humidity (Berge equation)
- acac
acetylacetone
- ACGIH
American Conference of Industrial Hygienists
- AFSSET
French Agency for Environmental and Occupational Health Safety
- AHMT
4-amino-3-hydrazino-5-mercapto-4H-1,2,4-triazole
- ALARA
as low as reasonably achievable
- AM
arithmetic mean
- ASHRAE
American Society of Heating, Refrigerating and Air Conditioning Engineers
- ASTM
American Society for Testing and Materials
- BfR
Bundesinstitut für Risikobewertung (German Federal Institute for Risk Assessment)
- CA
concentration on the surface of a passive sampler
- Cair
concentration in air
- Ceq
equilibrium concentration for n = 0 h−1 (Hoetjer equation)
- Ci
equilibrium concentration (Henry’s adsorption isotherm)
- Cj
concentration of a compound in the environment j
- Cm,o
initial concentration in the material (Henry’s adsorption isotherm)
- Cs
concentration at the source surface
- Css
steady-state chamber concentration (Andersen- and Hoetjer-equation)
- Cx
standardized concentration (Berge equation)
- C∞
steady-state concentration in a room for t → ∞
- C(t)
time-dependent concentration
- CARB
California Air Resources Board
- CDC
U.S. Centers for Disease Control and Prevention
- CEC
Commission of the European Communities
- CEN
European Committee for Standardization
- COMEAP
Committee on the Medical Effects of Air Pollutants
- D
diffusion coefficient
- Di
diffusion coefficient of a compound i (passive sampling)
- DIj
daily intake in the environment j
- DDL
3,5-diacetyl-1,4-dihydrolutidine
- DIN
Deutsche Industrie Norm
- DMC
dynamic microchamber
- DNPH
2,4-dinitrophenylhydrazine
- DOAS
differential optical absorption spectroscopy
- EN
European Norm (or European Standard)
- EPA
Environmental Protection Agency
- ETB
Eidgenössische Technische Baurichtlinien
- FEMA
U.S. Department of Homeland Security
- FiSIAQ
Finnish Society of Indoor Air Quality and Climate
- FLEC
field and laboratory emission cell
- FTIR
Fourier transform infrared
- GC/MS
gas chromatography/mass spectrometry
- GM
geometric mean
- H
relative humidity (Berge equation)
- H0
relative humidity under standard conditions (Berge equation)
- HPLC
high-performance liquid chromatography
- IARC
International Agency for Research on Cancer
- INDEX
Project Critical Appraisal of the Setting and Implementation of Indoor Exposure Limits in the EU
- IR
infrared
- IRj
inhalation rate in the environment j
- ISO
International Organization for Standardization
- JAS
Japanese Agricultural Standard
- JIS
Japanese Industrial Standard
- K
formaldehyde transfer coefficient (Hoetjer equation)
- KH
partition coefficient (Henry’s adsorption isotherm)
- kb
rate constant for emission from the bottom layer
- kg
gas-phase mass transfer coefficient
- km
rate constant for emission from the top layer
- kNO3
rate constant for the reaction with NO3
- kO3
rate constant for the reaction with O3
- kOH
rate constant for the reaction with OH
- KOW
octanol/water distribution coefficient
- ks
source-phase mass transfer coefficient
- l
diffusion length of a passive sampler
- L
surface-to-volume loading rate
- LEED
Leadership in Energy and Environmental Design
- LO(A)EL
lowest observed (adverse) effect level
- LOD
limit of detection
- MBTH
3-methyl-2-benzothiazolinone-hydrazone
- MDF
medium-density fiber board
- MF
melamine-formaldehyde
- MHW
Ministry of Health and Welfare
- mi
mass of a compound i on a passive sampler
- mp
mass in the product (source)
- ms
mass at the surface of a product
- MUF
melamine-urea-formaldehyde
- MUPF
melamine-urea-phenol-formaldehyde
- MWCNT
multiwall carbon nanotube
- n
air exchange rate
- NCI
National Cancer Institute
- NHD
Norwegian Health Directorate
- NHMRC
National Health and Medical Research Council of Australia
- NICNAS
Australia National Industrial Chemicals Notification and Assessment Scheme
- NIOSH
National Institute for Occupational Safety and Health
- NMR
nuclear magnetic resonance
- NO(A)EL
no observed (adverse) effect level
- OEHHA
Office of Environmental Health Hazard Assessment
- OSB
oriented-strand board
- OSHA
Occupational Safety Health Administration
- P
percentage (statistical, e.g. 50-P (= median), 90-P, 95-P, 98-P)
- PCH
4-phenylcyclohexene
- PF
phenol-formaldehyde
- PFBOA
o-(2,3,4,5,6-pentafluorobenzyl)hydroxylamine
- PMDI
polymeric methylene diphenyl diisocyanates
- PTR-MS
proton-transfer-reaction mass spectrometry
- PVC
polyvinyl chloride
- r.h.
relative humidity
- RB
coefficient of temperature (Berge equation)
- RD50
concentration that induces a 50% reduction in the respiratory rate
- REL
reference exposure limit
- RIOPA
relationships of indoor, outdoor, and personal air
- SBR
styrene-butadiene rubber
- SBS
sick building syndrome
- SCHER
Scientific Committee on Health and Environmental Risks
- SER
specific emission rate
- SERA
area-specific emission rate
- SIFT-MS
selected ion flow tube mass spectrometry
- STEL
short-term exposure level
- T
temperature
- T0
temperature under standard conditions (Berge equation)
- TDLS
tunable diode laser spectroscopy
- tj
time spent in environment j
- TLV
threshold limit value
- TWA
time-weighted average
- UF
urea-formaldehyde
- USGBC
US Green Building Council
- UV/vis
ultraviolet/visible
- V
room or chamber volume
- Vm
sample (material) volume
- VCH
4-vinylcyclohexene
- VDI
Verein Deutscher Ingenieure
- VOC
volatile organic compound
- WHO
World Health Organization
14. References
- Walker J. F.Formaldehyde; Reinhold Publishing Corporation: New York, 1964. [Google Scholar]
- Roffael E.Formaldehyde Release from Particleboard and Other Wood Based Panel; Forest Research Institute Malaysia: Kuala Lumpur, 1993. [Google Scholar]
- Kerns W. D.; Pavkov K. L.; Donofrio D. J.; Gralla E. J.; Swenberg J. A. Cancer Res. 1983, 43, 4382. [PubMed] [Google Scholar]
- Swenberg J. A.; Kerns W. D.; Mitchell R. I.; Gralla E. J.; Pavkov K. L. Cancer Res. 1980, 40, 3398. [PubMed] [Google Scholar]
- SCHER.Opinion on risk assessment on indoor air quality; European Commission: Brussels, 2007. [Google Scholar]
- Reuss G.; Disteldorf W.; Gamer A. O.; Hilt A.. Ullmann’s Encyclopedia of Industrial Chemistry; WILEY-VCH: Weinheim, 2002. [Google Scholar]
- Hauptmann M.; Straif K.; Pesch B. In Handbuch der Umweltmedizin; Wichmann H.-E., Schlipköter H.-W., Füllgraff G., Eds.; ECOMED-Verlag: Landsberg, 2006; Vol. 3, pp 1−28. [Google Scholar]
- Tang X.; Bai Y.; Duong A.; Smyth M. T.; Li L.; Zhang L. Environ. Int. 2009, 35, 1210. [DOI] [PubMed] [Google Scholar]
- Seinfeld J. H.; Pandis S. N.. Atmospheric Chemistry and Physics; John Wiley & Sons: New York, 2006. [Google Scholar]
- WHO.Formaldehyde; Environmental Health Criteria 89: Copenhagen, 1989. [Google Scholar]
- Atkinson R.; Arey J. Chem. Rev. 2003, 103, 4605. [DOI] [PubMed] [Google Scholar]
- Finlayson-Pitts B. J.; Pitts J. N. Jr.. Chemistry of the Upper and Lower Atmosphere; Academic Press: San Diego, 2000. [Google Scholar]
- Möller D.Luft; Walter de Gruyter: Berlin, 2003. [Google Scholar]
- Atkinson R. Atmos. Environ. 2000, 34, 2063. [Google Scholar]
- Braslavsky S.; Heicklen J. Int. J. Chem. Kinet. 1976, 8, 801. [Google Scholar]
- McGregor D.; Bolt H.; Cogliano V.; Richter-Reichhelm H.-B. Crit. Rev. Toxicol. 2006, 36, 821. [DOI] [PubMed] [Google Scholar]
- Kotzias D.; Koistinen K.; Kephalopulos S.; Schlitt C.; Carrer P.; Maroni M.; Jantunen M.; Cochet C.; Kirchner S.; Lindvall T.; McLaughlin J.; Molhave L.; de Oliveira Fernandez E.; Seifert B.. The INDEX project - Critical appaisal of the setting and implementation of indoor exposure limits in the EU; EUR-21590-EN; EC-JRC; Ispra, 2005. [Google Scholar]
- Nelson D. L.; Cox M. M.. Lehninger Principles of Biochemistry; Palgrave Macmillan: Houndmills, 2004. [Google Scholar]
- Skrzydlewska E. Toxicol. Mech. Methods 2003, 11, 277. [DOI] [PubMed] [Google Scholar]
- Spanel P.; Smith D.; Holland T. R.; Al Singary W.; Elder J. B. Rapid Commun. Mass Spectrom. 1999, 13, 1354. [DOI] [PubMed] [Google Scholar]
- Takeuchi A.; Takigawa T.; Abe M.; Kawai T.; Endo Y.; Yasugi T.; Endo G.; Ogino K. Bull. Environ. Contam. Toxicol. 2007, 79, 1. [DOI] [PubMed] [Google Scholar]
- Health CanadaProposed residential indoor air quality guidelines for formaldehyde; H128-1/05-432E; Health Canada: Ottawa, 2005. [Google Scholar]
- Kuwabara Y.; Alexeeff G. V.; Broadwin R.; Salmon A. G. Environ. Health Perspect. 2007, 115, 1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang I.; Bruckner T.; Triebig G. Regul. Toxicol. Pharmacol. 2008, 50, 23. [DOI] [PubMed] [Google Scholar]
- Devos M.; Patte F.; Rouault J.; Laffort P.; Van Gemert L. J.. Standardized human olfactory thresholds; Oxford University Press: Oxford, 1992. [Google Scholar]
- Koistinen K.; Kotzias D.; Kephalopoulos S.; Schlitt C.; Carrer P.; Jantunen M.; Kirchner S.; McLaughlin J.; Molhave L.; Fernandes E. O.; Seifert B. Allergy 2008, 63, 810. [DOI] [PubMed] [Google Scholar]
- WHO.Formaldehyde; Concise International Chemical Assessment Document 40: Geneva, 2002 [Google Scholar]; (http://www.inchem.org
- IARC.IARC classifies formaldehye as carcinogenic to humans; press release no.153; International Agency for Research on Cancer: Lyon, 2004. [Google Scholar]
- Pizzi A.; Mittal K. L.. Handbook of Adhesive Technology; Marcel Dekker Ltd.: New York, 2003. [Google Scholar]
- Zeppenfeld G.; Grunwald D.. Klebstoffe; DRW-Verlag: Leinfelden-Echterdingen, 2005. [Google Scholar]
- Marutzky R. In Wood Adhesives—Chemistry and Technology; Pizzi A., Ed.; Marcel Dekker Inc.: New York, 1989; Vol. 2, pp 307−387. [Google Scholar]
- Godish T. In Indoor Air Quality Handbook; Spengler J. D., Samet J. M., McCarthy J. F., Eds.; McGraw-Hill: New York, 2001. [Google Scholar]
- Pluschke P.Luftschadstoffe in Innenräumen; Springer-Verlag: Berlin, 1996. [Google Scholar]
- Koppmann R.; Wildt J. In Volatile Organic Compounds in the Atmosphere; Koppmann R., Ed.; Blackwell Publishing Ltd.: Oxford, 2007. [Google Scholar]
- Seco R.; Peñuelas J.; Filella I. Atmos. Environ. 2008, 42, 7907. [Google Scholar]
- Freudenberg K.; Harder M. Ber. Dtsch. Chem. Ges. (A and B Ser.) 1927, 60, 58. [Google Scholar]
- Müller K.; Haferkorn S.; Grabmer W.; Wisthaler A.; Hansel A.; Kreuzwieser J.; Cojocariu C.; Rennenberg H.; Herrmann H. Atmos. Environ. 2006, 40, 81. [Google Scholar]
- Trapp D.; Cooke K. M.; Fischer H.; Bonsang B.; Zitzelsberger R. U.; Seuwen R.; Schiller C.; Zenker T.; Parchatka U.; Nunes T. V.; Pio C. A.; Lewis A. C.; Seakins P. W.; Pilling M. J. Chemosphere 2001, 3, 295. [Google Scholar]
- Carter W. P. L.; Atkinson R. Int. J. Chem. Kinet. 1996, 28, 497. [Google Scholar]
- Kesselmeier J.; Kuhn U.; Wolf A.; Andreae M. O.; Ciccioli P.; Brancaleoni E.; Frattoni M.; Guenther A.; Greenberg J.; De Castro Vasconcellos P.; de Oliva T.; Tavares T.; Artaxo P. Atmos. Environ. 2000, 34, 4063. [Google Scholar]
- Smidt S.; Bauer H.; Pogodina O.; Puxbaum H. Atmos. Environ. 2005, 39, 4087. [Google Scholar]
- Solberg S.; Dye C.; Walker S.-E.; Simpson D. Atmos. Environ. 2001, 35, 195. [Google Scholar]
- Meyer B.; Boehme C. Forest Prod. J. 1996, 47, 45. [Google Scholar]
- Seco R.; Peñuelas J.; Filella I. Atmos. Environ. 2007, 41, 2477. [Google Scholar]
- Hanson A. D.; Roje S. Annu. Rev. Plant Phys. 2001, 52, 119. [DOI] [PubMed] [Google Scholar]
- Nuccio J.; Seaton P. J.; Kieber R. J. Limnol. Oceanogr. 1995, 40, 521. [Google Scholar]
- Atkinson R.; Arey J. Atmos. Environ. 2003, 37, 197. [Google Scholar]
- Atkinson R.; Tuazon E. C.; Aschmann S. M. Environ. Sci. Technol. 1995, 29, 1860–1866. [DOI] [PubMed] [Google Scholar]
- Grosjean E.; Grosjean D. Atmos. Environ. 1998, 32, 3393. [Google Scholar]
- Grosjean D.; Williams E. L.; Grosjean E.; Andino J. M.; Seinfeld J. H. Environ. Sci. Technol. 1993, 27, 2754. [Google Scholar]
- Nunes F. M. N.; Veloso M. C. C.; de P. Pereira P. A.; de Andrade J. B. Atmos. Environ. 2005, 39, 7715. [Google Scholar]
- Griesbaum K.; Miclaus V.; Jung I. C. Environ. Sci. Technol. 1998, 32, 647. [Google Scholar]
- Duan J.; Tan J.; Yang L.; Wu S.; Hao J. Atmos. Res. 2008, 88, 25. [Google Scholar]
- Marutzky R.Erkenntnisse zur Schadstoffbildung bei der Verbrennung von Holz und Spanplatten; Fraunhofer-WKI: Braunschweig, 1991. [Google Scholar]
- Hedberg E.; Kristensson A.; Ohlsson M.; Johansson C.; Johansson P.-Å.; Swietlicki E.; Vesely V.; Wideqvist U.; Westerholm R. Atmos. Environ. 2002, 36, 4823. [Google Scholar]
- Schauer J. J.; Kleeman M. J.; Cass G. R.; Simoneit B. R. T. Environ. Sci. Technol. 2001, 35, 1716. [DOI] [PubMed] [Google Scholar]
- Na K.; Cocker D. R. Environ. Res. 2008, 108, 7. [DOI] [PubMed] [Google Scholar]
- Reisen F.; Brown S. K. Environ. Int. 2009, 35, 342. [DOI] [PubMed] [Google Scholar]
- Roy M. M. Energy Convers. Manage. 2008, 49, 1111. [Google Scholar]
- Machado Corrêa S.; Arbilla G. Atmos. Environ. 2008, 42, 769. [Google Scholar]
- Guarieiro L. L. N.; Pereira P. A. d. P.; Torres E. A.; da Rocha G. O.; de Andrade J. B. Atmos. Environ. 2008, 42, 8211. [Google Scholar]
- Peng C.-Y.; Yang H.-H.; Lan C.-H.; Chien S.-M. Atmos. Environ. 2008, 42, 906. [Google Scholar]
- Nazaroff W. W.; Alvarez-Cohen L.. Environmental Engineering Science; John Wiley & Sons: New York, 2001. [Google Scholar]
- Salthammer T.; Fuhrmann F.; Kaufhold S.; Meyer B.; Schwarz A. Indoor Air 1995, 5, 120. [Google Scholar]
- Wittmann O. Holz Roh Werkst. 1962, 20, 221. [Google Scholar]
- Zinn T. W.; Cline D.; Lehmann W. F. Forest Prod. J. 1990, 40, 15. [Google Scholar]
- Kim S. Bioresour. Technol. 2009, 100, 744. [DOI] [PubMed] [Google Scholar]
- Pizzi A. In Wood Adhesives Chemistry and Technology; Pizzi A., Ed.; Marcel Dekker Inc.: New York, 1994. [Google Scholar]
- Roffael E.; Dix B.; Okum J. Holz Roh Werkst. 2000, 58, 301. [Google Scholar]
- Salthammer T.; Schriever E.; Marutzky R. Toxicol. Environ. Chem. 1993, 40, 121. [Google Scholar]
- Nicolas M.; Ramalho O.; Maupetit F. Atmos. Environ. 2007, 41, 3129. [Google Scholar]
- Wang H.; Morrison G. C. Environ. Sci. Technol. 2006, 40, 5263. [DOI] [PubMed] [Google Scholar]
- Kelly T. J.; Smith D. L.; Satola J. Environ. Sci. Technol. 1999, 33, 81. [Google Scholar]
- Chang J. C. S. Indoor Air 1999, 9, 253. [DOI] [PubMed] [Google Scholar]
- Chang J. C. S.; Guo Z.; Fortmann R.; Lao H. C. Indoor Air 2002, 12, 10. [DOI] [PubMed] [Google Scholar]
- Horn W.; Roβkamp E.; Ullrich D.. Biozidemissionen aus Dispersionsfarben; Umweltbundesamt, WaBoLu-Hefte 2/02: Berlin, 2002. [Google Scholar]
- Wolkoff P.; Clausen P. A.; Jensen B.; Nielsen G. D.; Wilkins C. K. Indoor Air 1997, 7, 92. [DOI] [PubMed] [Google Scholar]
- Weschler C. J.; Hodgson A. T.; Wooley J. D. Environ. Sci. Technol. 1992, 26, 2371. [Google Scholar]
- Weschler C. J. Indoor Air 2000, 10, 269. [DOI] [PubMed] [Google Scholar]
- Atkinson R.; Carter W. P. L. Chem. Rev. 1984, 84, 437. [Google Scholar]
- Atkinson R.; Hasegawa D.; Aschmann S. M. Int. J. Chem. Kinet. 1990, 22, 871. [Google Scholar]
- Destaillats H.; Lunden M. M.; Singer B. C.; Coleman B. K.; Hodgson A. T.; Weschler C. J.; Nazaroff W. W. Environ. Sci. Technol. 2006, 40, 4421. [DOI] [PubMed] [Google Scholar]
- Singer B. C.; Coleman B. K.; Destaillats H.; Hodgson A. T.; Lunden M. M.; Weschler C. J.; Nazaroff W. W. Atmos. Environ. 2006, 40, 6696. [DOI] [PubMed] [Google Scholar]
- Lamorena R. B.; Lee W. J. Hazard. Mater. 2008, 158, 471. [DOI] [PubMed] [Google Scholar]
- Morrison G. C.; Nazaroff W. W. Environ. Sci. Technol. 2002, 36, 2185. [DOI] [PubMed] [Google Scholar]
- Poppendieck D.; Hubbard H.; Ward M.; Weschler C.; Corsi R. L. Atmos. Environ. 2007, 41, 3166. [Google Scholar]
- Poppendieck D. G.; Hubbard H. F.; Weschler C. J.; Corsi R. L. Atmos. Environ. 2007, 41, 7614. [Google Scholar]
- Reiss R.; Ryan P. B.; Koutrakis P.; Tibbetts S. J. Environ. Sci. Technol. 1995, 29, 1906. [DOI] [PubMed] [Google Scholar]
- Weschler C. J.; Wisthaler A.; Cowlin S.; Tamas G.; Strom-Tejsen P.; Hodgson A. T.; Destaillats H.; Herrington J.; Zhang J.; Nazaroff W. W. Environ. Sci. Technol. 2007, 41, 6177. [DOI] [PubMed] [Google Scholar]
- Wisthaler A.; Tamas G.; Wyon D. P.; Strom-Tejsen P.; Space D.; Beauchamp J.; Hansel A.; Mark T. D.; Weschler C. J. Environ. Sci. Technol. 2005, 39, 4823. [DOI] [PubMed] [Google Scholar]
- Coleman B. K.; Destaillats H.; Hodgson A. T.; Nazaroff W. W. Atmos. Environ. 2008, 42, 642. [Google Scholar]
- Petrick L.; Dubowki Y. Indoor Air 2009, 19, 381. [DOI] [PubMed] [Google Scholar]
- Salthammer T.; Fuhrmann F. Environ. Sci. Technol. 2007, 41, 6573. [DOI] [PubMed] [Google Scholar]
- Auvinen J.; Wirtanen L. Atmos. Environ. 2008, 42, 4101. [Google Scholar]
- Gunschera J.; Andersen J. R.; Schulz N.; Salthammer T. Chemosphere 2009, 75, 476. [DOI] [PubMed] [Google Scholar]
- Balakrishnan K.; Ramaswamy P.; Sankar S. In Indoor Air Pollution; Pluschke P., Ed.; Springer: Berlin, 2003; Vol. 4F, pp 219−239. [Google Scholar]
- Fortman R.; Kariher P.; Clayton R.. Indoor Air Quality—Residential Cooking Exposures; ARB97−330CARB Research Division: Sacramento, 2001. [Google Scholar]
- Baek S. O.; Jenkins R. A. Atmos. Environ. 2004, 38, 6583. [Google Scholar]
- Li S.; Banyasz J. L.; Parrish M. E.; Lyons-Hart J.; Shafer K. H. J. Anal. Appl. Pyrolysis 2002, 65, 137. [Google Scholar]
- Martin P.; Heavner D. L.; Nelson P. R.; Maiolo K. C.; Risner C. H.; Simmons P. S.; Morgan W. T.; Ogden M. W. Environ. Int. 1997, 23, 75. [Google Scholar]
- Nazaroff W. W.; Singer B. C. J. Exposure Anal. Environ. Epidemiol. 2004, 14, S71. [DOI] [PubMed] [Google Scholar]
- Weschler C. J. Atmos. Environ. 2009, 43, 153. [Google Scholar]
- Lindinger W.; Hansel A.; Jordan A. Int. J. Mass Spectrom. Ion Processes 1998, 173, 191. [Google Scholar]
- Wehinger A.; Schmid A.; Mechtcheriakov S.; Ledochowski M.; Grabmer C.; Gastl G. A.; Amann A. Int. J. Mass Spectrom. 2007, 265, 49. [Google Scholar]
- Moser B.; Bodrogi F.; Eibl G.; Lechner M.; Rieder J.; Lirk P. Respir. Physiol. Neurobiol. 2005, 145, 295. [DOI] [PubMed] [Google Scholar]
- Kushch I.; Schwarz K.; Schwentner L.; Baumann B.; Dzien A.; Schmid A.; Unterkofler K.; Gastl G.; Spanel P.; Smith D.; Amann A. J. Breath Res. 2008, 2, 026002. [DOI] [PubMed] [Google Scholar]
- Vairavamurthy A.; Roberts J. M.; Newman L. Atmos. Environ. 1992, 26A, 1965. [Google Scholar]
- Hanoune B.; LeBris T.; Allou L.; Marchand C.; Le Calve S. Atmos. Environ. 2006, 40, 5768. [Google Scholar]
- Hanoune B.; Lemoine B.. Book of Abstracts; Progress in Biomedical Optics and Imaging; Massachusetts, October 1−4, 2006; SPIE: USA, 2006; p 90. [Google Scholar]
- Krüger U.; Kraenzmer M.; Strindehag O. Environ. Int. 1995, 21, 791. [Google Scholar]
- Wu P. C.; Li Y. Y.; Lee C. C.; Chiang C. M.; Su H. J. J. Indoor Air 2003, 13, 359. [DOI] [PubMed] [Google Scholar]
- Ferus M.; Cihelka J.; Civis S. Chem. Listy 2008, 102, 417. [Google Scholar]
- Cihelka J.; Matulková I.; Civis S. J. Mol. Spectrosc. 2009, 256, 68. [Google Scholar]
- de Gouw J.; Warneke C. Mass Spectrom. Rev. 2007, 26, 223. [DOI] [PubMed] [Google Scholar]
- Hansel A.; Singer W.; Wisthaler A.; Schwarzmann M.; Lindinger W. Int. J. Mass Spectrom. Ion Processes 1997, 167−168, 697. [Google Scholar]
- Thekedar B.; Szymczak W.; Hollriegl V.; Hoeschen C.; Oeh U. J. Breath Res. 2009, 3, 027007. [DOI] [PubMed] [Google Scholar]
- Hak C.; Pundt I.; Trick S.; Kern C.; Platt U.; Dommen J.; Ordonez C.; Prevot A. S. H.; Junkermann W.; Astorga-Llorens C.; Larsen B. R.; Mellqvist J.; Strandberg A.; Yu Y.; Galle B.; Kleffmann J.; Lörzer J. C.; Braathen G. O.; Volkamer R. Atmos. Chem. Phys. 2005, 5, 2881. [Google Scholar]
- van der Wal J. F.; Korf C.; Kuypers A. T. J. M.; Neele J. Environ. Int. 1989, 15, 517. [Google Scholar]
- Kleindienst T. E.; Shepson P. B.; Nero C. M.; Arnts R. R.; Tejada S. B.; Mackay G. I.; Mayne L. K.; Schiff H. I.; Lind J. A.; Kok G. L.; Lazrus A. L.; Dasgupta P. K.; Dong S. Atmos. Environ. 1988, 22, 1931. [DOI] [PubMed] [Google Scholar]
- Schulz M.; Salthammer T. In Organic Indoor Air Pollutants; Salthammer T., Ed.; WILEY-VCH: Weinheim, 1999; pp 15−30. [Google Scholar]
- Miksch R. R.; Anthon D. W.; Fanning L. Z.; Hollowell C. D.; Revzan K.; Glanville J. Anal. Chem. 1981, 53, 2118. [Google Scholar]
- Altshuller A. P.; Leng L. J. Anal. Chem. 1963, 35, 1541. [Google Scholar]
- Cohen I. R.; Altshuller A. P. Anal. Chem. 1966, 38, 1418. [Google Scholar]
- Hauser T. R.; Cummins R. L. Anal. Chem. 1964, 36, 679. [Google Scholar]
- Toda K.; Yoshioka K. I.; Mori K.; Hirata S. Anal. Chim. Acta 2005, 531, 41. [Google Scholar]
- Kawamura K.; Kerman K.; Fujihara M.; Nagatani N.; Hashiba T.; Tamiya E. Sens. Actuators, B 2005, 105, 495. [Google Scholar]
- Altshuller A. P.; Miller D. L.; Sleva S. F. Anal. Chem. 1961, 33, 621. [Google Scholar]
- NIOSH 3500: Formaldehyde by VIS; Manual of analytical methods, 4th ed.; NIOSH: Washington, DC, 1994. [Google Scholar]
- ASTM E 1333; American Society for Testing and Materials: West Conshohocken, 2002. [Google Scholar]
- ASTM D 6007; American Society for Testing and Materials: West Conshohocken, 2002. [Google Scholar]
- ASTM D 5582; American Society for Testing and Materials: West Conshohocken, 2006. [Google Scholar]
- Nash T. Biochem. J. 1953, 55, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risholm-Sundman M.; Larsen A.; Vestin E.; Weibull A. Atmos. Environ. 2007, 41, 3193. [Google Scholar]
- EN 717-1; European Committee for Standardization, Brussels, 2004. [Google Scholar]
- EN 717-2; European Committee for Standardization, Brussels, 1994 [Google Scholar]
- EN 717-3; European Committee for Standardization, Brussels, 1996 [Google Scholar]
- JIS A 1460; Japanese Standards Organization: Tokyo, 2001. [Google Scholar]
- Belman S. Anal. Chim. Acta 1963, 29, 120. [Google Scholar]
- Salthammer T. J. Photochem. Photobiol., A 1993, 74, 195. [Google Scholar]
- Pinheiro H. L. C.; De Andrade M. V.; Pereira P. A. D. P.; De Andrade J. B. Microchem. J. 2004, 78, 15. [Google Scholar]
- Junkermann W.; Burger J. M. J. Atmos. Ocean. Tech. 2006, 23, 38. [Google Scholar]
- Wisthaler A.; Apel E. C.; Bossmeyer J.; Hansel A.; Junkermann W.; Koppmann R.; Meier R.; Müller K.; Solomon S. J.; Steinbrecher R.; Tillmann R.; Brauers T. Atmos. Chem. Phys. 2008, 8, 2189. [Google Scholar]
- Sritharathikhun P.; Oshima M.; Motomizu S. Talanta 2005, 67, 1014. [DOI] [PubMed] [Google Scholar]
- Li Q.; Sritharathikhun P.; Motomizu S. Anal. Sci. 2007, 23, 413. [DOI] [PubMed] [Google Scholar]
- de Andrade J. B.; Tanner R. L. Atmos. Environ. 1992, 26A, 819. [Google Scholar]
- Sandner F.; Dott W.; Hollander J. Int. J. Hyg. Environ. Health 2001, 203, 275. [DOI] [PubMed] [Google Scholar]
- Andersson G.; Andersson K.; Nilsson C.-A.; Levin J.-O. Chemosphere 1979, 8, 823. [Google Scholar]
- Pires M.; Carvalho L. R. F. Anal. Chim. Acta 1998, 367, 223. [Google Scholar]
- U.S. EPA Method TO-11A; U.S. EPA: Cincinnati, 1999. [Google Scholar]
- ASTM D 5197; American Society for Testing and Materials: West Conshohocken, 2003. [Google Scholar]
- ISO 16000-3; ISO: Geneva, 2001. [Google Scholar]
- JIS A 1901; Japanese Standards Organization: Tokyo, 2003. [Google Scholar]
- JIS A 1911; Japanese Standards Organization: Tokyo, 2006. [Google Scholar]
- Salthammer T.; Mentese S. Chemosphere 2008, 73, 1351. [DOI] [PubMed] [Google Scholar]
- Trapp D.; De Serves C. Atmos. Environ. 1995, 29, 3239. [Google Scholar]
- Lv P.; Tang Z. A.; Yu J.; Zhang F. T.; Wei G. F.; Huang Z. X.; Hu Y. Sens. Actuators, B 2008, 132, 74. [Google Scholar]
- Zhou K.; Ji X.; Zhang N.; Zhang X. Sens. Actuators, B 2006, 119, 392. [Google Scholar]
- Wang J.; Liu L.; Cong S.-Y.; Qi J.-Q.; Xu B.-K. Sens. Actuators, B 2008, 134, 1010. [Google Scholar]
- Wang J.; Zhang P.; Qi J.-Q.; Yao P.-J. Sens. Actuators, B 2009, 136, 399. [Google Scholar]
- Achmann S.; Hermann M.; Hilbrig F.; Jérôme V.; Hämmerle M.; Freitag R.; Moos R. Talanta 2008, 75, 786. [DOI] [PubMed] [Google Scholar]
- Demkiv O.; Smutok O.; Paryzhak S.; Gayda G.; Sultanov Y.; Guschin D.; Shkil H.; Schuhmann W.; Gonchar M. Talanta 2008, 76, 837. [DOI] [PubMed] [Google Scholar]
- Vianello F.; Boscolo-Chio R.; Signorini S.; Rigo A. Biosens. Bioelectron. 2007, 22, 920. [DOI] [PubMed] [Google Scholar]
- Shinohara N.; Kajiwara T.; Ohnishi M.; Kodama K.; Yanagisawa Y. Environ. Sci. Technol. 2008, 42, 4472. [DOI] [PubMed] [Google Scholar]
- Seo J.; Kato S.; Tatsuma T.; Chino S.; Takada K.; Notsu H. Chemosphere 2008, 72, 1286. [DOI] [PubMed] [Google Scholar]
- Barro R.; Regueiro J.; Llompart M.; Garcia-Jares C. J. Chromatogr., A 2009, 1216, 540. [DOI] [PubMed] [Google Scholar]
- Salthammer T. Indoor Air 1997, 7, 189. [Google Scholar]
- Meyer U.; Möhle K.; Eyerer P.; Maresch L. Staub—Reinhalt. Luft 1994, 54, 137. [Google Scholar]
- Crump D. R.; Yu C. W. F.; Squire R. W.; Atkinson M. In Characterizing sources of indoor air pollution and related sink effects; Tichenor B. A., Ed.; American Society for Testing and Materials: West Conshohocken, 1996; Vol. STP 1287, pp 211−224. [Google Scholar]
- Anderson W. H.; Huber C. W.. U.S. Patent 5395494, 1995.
- CARB.Proposed Airborne Toxic Control Measure (ATCM) to Reduce Formaldehyde Emissions from Composite Wood Products; CARB: Sacramento, 2007. [Google Scholar]
- Groah W. J. Environ. Sci. Technol. 1991, 25, 117. [Google Scholar]
- Wolkoff P. Gefahrstoffe—Reinhalt. Luft 1996, 56, 151. [Google Scholar]
- Wolkoff P.; Salthammer T.; Woolfenden E. Gefahrstoffe—Reinhalt. Luft 2005, 65, 93. [Google Scholar]
- Uhde E.; Borgschulte A.; Salthammer T. Atmos. Environ. 1998, 32, 773. [Google Scholar]
- Zhang L. Z.; Niu J. L. Int. J. Heat Mass Transfer 2003, 46, 91. [Google Scholar]
- Risholm-Sundman M. Indoor Air 1999, 9, 268. [DOI] [PubMed] [Google Scholar]
- ISO 16000-10; ISO: Geneva, 2006. [Google Scholar]
- Colombo A.; Jann O.; Marutzky R. Staub—Reinhalt. Luft 1994, 54, 143. [Google Scholar]
- Marutzky R.; Margosian R. In Proceedings of Measuring and Controlling Volatile Organic Compound and Particulate Emissions from Wood Processing Operations and Wood-based Products; The Forest Prod Society: Madison, 1995; pp 62−73. [Google Scholar]
- Mehlhorn L. Adhesion 1986, 30, 27. [Google Scholar]
- Jann O. Deppe Holz Roh Werkst. 1990, 48, 365. [Google Scholar]
- Roffael E. Holz-Zentralblatt 1975, 101, 1403. [Google Scholar]
- Gavin M.; Crump D. R.; Brown V. M. Environ. Technol. 1995, 16, 579. [Google Scholar]
- ISO 16000-2; ISO: Geneva, 2004. [Google Scholar]
- Crump D. In Organic Indoor Air Pollutants, 2nd ed.; Salthammer T., Uhde E., Eds.; WILEY-VCH: Weinheim, 2009; pp 47−63. [Google Scholar]
- Matthews T. G.; Hawthorne A. R.; Thompson C. V. Environ. Sci. Technol. 1987, 21, 629. [DOI] [PubMed] [Google Scholar]
- Onishi M.; Sekine Y.; Sugihara K.; Kitasaka K.; Shimajiri H. J. Health Sci. 2007, 53, 413. [Google Scholar]
- Shinohara N.; Fujii M.; Yamasaki A.; Yanagisawa Y. Atmos. Environ. 2007, 41, 4018. [Google Scholar]
- Uchiyama S.; Aoyagi S.; Ando M. Atmos. Environ. 2004, 38, 6319. [Google Scholar]
- Yim B.; Jung E. S. Anal. Sci. 2006, 22, 993. [DOI] [PubMed] [Google Scholar]
- Fusaya M.; Ohura T.; Suzuki M.; Amagai T.; Matsushita H. Int. J. Environ. Anal. Chem. 2004, 84, 1035. [Google Scholar]
- Shinohara N.; Kumagai K.; Yamamoto N.; Yanagisawa Y.; Fujii M.; Yamasaki A. J. Air Waste Manage. Assoc. 2004, 54, 419. [DOI] [PubMed] [Google Scholar]
- Shinohara N.; Kai Y.; Mizukoshi A.; Fujii M.; Kumagai K.; Okuizumi Y.; Jona M.; Yanagisawa Y. Build. Environ. 2009, 44, 859. [Google Scholar]
- Gonzalez-Flesca N.; Cicolella A.; Bates M.; Bastin E. Environ. Sci. Pollut. Res. 1999, 6, 95. [DOI] [PubMed] [Google Scholar]
- Kotzias D. Exp. Toxicol. Pathol. 2005, 57, 5. [DOI] [PubMed] [Google Scholar]
- ISO 16000-4; ISO: Geneva, 2005. [Google Scholar]
- OSHA.Formaldehyde, 29CFR1910.1048, 7-1-06 ed.; U.S. Department of Labor: WA. [Google Scholar]
- ACGIH.TLVs and BEIs based on the documentation of the threshold limit values for chemical substances and physical agent & biological exposure indices; ACGIH: Cincinnati, 2006. [Google Scholar]
- NIOSH, NIOSH Pocket guide to chemical hazards; 2005-149;NIOSH: Cincinnati, 2005. [PubMed] [Google Scholar]
- Duhayon S.; Hoet P.; Van Maele-Fabry G.; Lison D. Int. Arch. Occup. Environ. Health 2008, 81, 695. [DOI] [PubMed] [Google Scholar]
- Paustenbach D.; Alarie Y.; Kulle T.; Schachter N.; Smith R.; Swenberg J.; Witschi H.; Horowitz S. B. J. Toxicol. Environ. Health, Part A 1997, 50, 217. [PubMed] [Google Scholar]
- Zhang L.; Steinmaus C.; Eastmond D. A.; Xin X. K.; Smith M. T. Mutat. Res., Rev. Mutat. Res. 2009, 681, 150. [DOI] [PubMed] [Google Scholar]
- OEHHA.Determination of acute reference exposure levels for airborne toxicants; Office of Environmental Health Hazard Assessment: California, U.S., 1999. [Google Scholar]
- USGBC.LEED for new construction & major renovations (LEED-NC) version 2.2 for Public use and display; USGBC: Washington, DC, 2005. [Google Scholar]
- ASHRAE 189.Standard for the High-Performance Green Buildings Except Low-Rise Residential Buildings; ASHRAE: Atlanta, 2007. [Google Scholar]
- CEC.Harmonisation of indoor material emissions labelling systems in the EU; EUR-21891-EN; CEC: Luxembourg, 2005. [Google Scholar]
- CEC.Indoor air pollution by formaldehyde in European countries; EUR-13216-EN; CEC: Luxembourg, 1990. [Google Scholar]
- COMEAP.Guidance on the Medical Effects of Air Pollutants; Department of Health, London, U.K., 2004. [Google Scholar]
- FiSIAQ.Classification of Indoor Climate 2000: Target values, Design Guidance and Product Requirements; Finnish Society of Indoor Air Quality and Climate: Espoo, Finland, 2001. [Google Scholar]
- NHD.Guidelines for indoor air quality; Report 6-90; Norwegian Health Directorate: Oslo, Norway, 1990. [Google Scholar]
- Stranger M.; Potgieter-Vermaak S. S.; Van Grieken R. Environ. Int. 2007, 33, 789. [DOI] [PubMed] [Google Scholar]
- Gustafson P.; Barregard L.; Lindahl R.; Sallsten G. J. Exposure Anal. Environ. Epidemiol. 2005, 15, 252. [DOI] [PubMed] [Google Scholar]
- AFSSET Working Group on Indoor Air Quality Guideline Values.Indoor Air Quality, Guideline Value Proposal—Formaldehyde; AFSSET: Maisons-Alfort Cedex, French, 2007. [Google Scholar]
- Mandin C.; Bonvallot N.; Kirchner S.; Keirsbulck M.; Alary R.; Cabanes P.-A.; Dor F.; Le Moullec Y.; Mullot J.-U.; Peel A.-E.; Rousselle C. CLEAN—Soil, Air, Water 2009, 37, 494. [Google Scholar]
- The Decree of Ministry of Health and Social Security of Poland.Maximum allowable concentrations of pollutants emitted by building materials and furnishings in inhabited closed areas; March 12, 1996.
- Ad hoc AG of Germany.Umweltmed. Forsch. Prax. 2006, 11, 362.
- CARB.Formaldehyde in the Home, Indoor Air Quality Guideline No. 1 and Supplement; CARB Research Division: Sacramento, CA, 1991. [Google Scholar]
- OEHHA.Chronic Toxicity Summary, Formaldehyde; California Office of Environmental Health Hazard Assessment: California, 2005. [Google Scholar]
- CARB.Indoor air Quality Guideline No. 1: Formaldehyde in the Home; CARB: Sacramento, CA, 2004. [Google Scholar]
- Health Canada.Exposure guidelines for residential indoor air quality: A Report of the Federal-Provincial Advisory Committee on Environmental and Occupational Health; Minister of Supply and Services Canada: Ottawa, Canada, 1995. [Google Scholar]
- Hong Kong Indoor Air Quality Management Group.Guidance Notes for the Management of Indoor Air Quality in Offices and Public Places; The Government of the Hong Kong Special Administrative Region: Hong Kong, 2003. [Google Scholar]
- Jeong J. Y.Recent issues on indoor air quality in Korea; Yongin University: http://www.kosha.net/shdb/common/getFile.jsp?contentId=319490&index=1. [Google Scholar]
- MHW of Japan.Committee on comfortable and healthy houses, final report; Ministry of Health and Welfare: Tokyo, Japan, 1997. [Google Scholar]
- Singapore Ministry of Environment.Guidelines for Indoor Air Quality in Office Premises; Institute of Environmental Epidemiology: Singapore, 1996. [Google Scholar]
- Indoor Air Quality Management Group of China.Indoor Air Quality Standard; GB/T 18883-2002; Ministry of Health: Beijing, China, 2002. [Google Scholar]
- NHMRC of Australia.Urea Formaldehyde Foam Insulation, Report of 93rd Session; NHMRC: Canberra, Australia, 1982. [Google Scholar]
- NICNAS.Priority Existing Chemical Assessment—Formaldehyde; 28; Department of Health and Ageing: Sydney, Australia, 2006. [Google Scholar]
- WHO.Air Quality Guidelines for Europe; WHO Regional Office for Europe: Copenhagen, 1987. [Google Scholar]
- Mendell M. J. Indoor Air 2007, 17, 259. [DOI] [PubMed] [Google Scholar]
- Andersen I.; Lundqvist G. R.; Molhave L. Atmos. Environ. (1967) 1975, 9, 1121. [Google Scholar]
- Mølhave L.; Bisgaard P.; Dueholm S. Atmos. Environ. (1967) 1983, 17, 2105. [Google Scholar]
- Myers G. E.; Nagaoka M. Forest Prod. J. 1981, 31, 39. [Google Scholar]
- Lehmann W. F. Forest Prod. J. 1987, 37, 31. [Google Scholar]
- Berge A.; Mellegaard B.; Hanetho P.; Ormstad E. Holz Roh—Werkst. 1980, 38, 251. [Google Scholar]
- Que Z.; Furuno T.; Katoh S.; Nishino Y. Build. Environ. 2007, 42, 2321. [Google Scholar]
- Berge A.; Mellegaard B. Forest Prod. J. 1979, 29, 21. [Google Scholar]
- Godish T.Indoor Environmental Quality; Lewis Publishers: Boca Raton, FL, 2001. [Google Scholar]
- Hawthorne A. R.; Matthews T. G. Atmos. Environ. 1987, 21, 419. [Google Scholar]
- Matthews T. G.; Fung K. W.; Tromberg B. J.; Hawthorne A. R. Environ. Int. 1986, 12, 301. [Google Scholar]
- Zhang Y.; Luo X.; Wang X.; Qian K.; Zhao R. Atmos. Environ. 2007, 41, 3203. [Google Scholar]
- Deng Q.; Yang X.; Zhang J. Atmos. Environ. 2009, 43, 2080. [Google Scholar]
- Xiong J.; Chen W.; Smith J. F.; Zhang Y.; Zhang J. Atmos. Environ. 2009, 43, 4102. [Google Scholar]
- Hodgson A. T.; Beal D.; McIlvaine J. E. R. Indoor Air 2002, 12, 235. [DOI] [PubMed] [Google Scholar]
- Mølhave L.; Dueholm S.; Jensen L. K. Indoor Air 1995, 5, 104. [Google Scholar]
- RAL UZ 38; RAL—Deutsches Institut für Gütesicherung und Kennzeichnung e.V.: St. Augustin, Germany, 2002.
- Ludewig K.; Meyer B.; Salthammer T.; Marutzky R. Holztechnologie 2008, 49, 36. [Google Scholar]
- Kim S.; Kim J.-A.; Kim H.-J.; Do Kim S. Polym. Test. 2006, 25, 605. [Google Scholar]
- Wiglusz R.; Sitko E.; Nikel G.; Jarnuszkiewicz I.; Igielska B. Build. Environ. 2002, 37, 41. [PubMed] [Google Scholar]
- Marutzky R.; Meyer B.; Schwarz A. Zentralbl. Arbeitsmed., Arbeitsschutz Ergon. 1993, 43, 334. [Google Scholar]
- Jaernstroem H.; Saarela K.; Kalliokoski P.; Pasanen A. L. Atmos. Environ. 2007, 41, 2290. [Google Scholar]
- Katsoyiannis A.; Leva P.; Kotzias D. J. Hazard. Mater. 2008, 152, 669. [DOI] [PubMed] [Google Scholar]
- Sparks L. E.; Tichenor B. A.; Chang J.; Guo Z. Indoor Air 1996, 6, 31. [DOI] [PubMed] [Google Scholar]
- Bruinen de Bruin Y.; Kotzias D.; Kephalopoulos S.. Characterization of indoor sources; European Commission; Joint Research Center; EU-21500-EN: Ispra, 2005. [Google Scholar]
- Zhang J.; He Q.; Lioy P. J. Environ. Sci. Technol. 1994, 28, 146. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Wilson W. E.; Lioy P. J. Environ. Sci. Technol. 1994, 28, 1975. [DOI] [PubMed] [Google Scholar]
- Kagi N.; Fujii S.; Tamura H.; Namiki N. Build. Environ. 2009, 44, 1199. [Google Scholar]
- Zhang J.; Lioy P. J. Indoor Air 1994, 4, 95. [Google Scholar]
- Waring M. S.; Siegel J. A.; Corsi R. L. Atmos. Environ. 2008, 42, 5003. [Google Scholar]
- Fiedler N.; Laumbach R.; Kelly-McNeil K.; Lioy P.; Fan Z.-H.; Zhang J.; Ottenweller J.; Ohman-Strickland P.; Kipen H. Environ. Health Perspect. 2007, 113, 1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C.; Wang B. Atmos. Environ. 2004, 38, 941. [Google Scholar]
- Baker R. R. Food Chem. Toxicol. 2006, 44, 1799. [DOI] [PubMed] [Google Scholar]
- Singer B. C.; Hodgson A. T.; Nazaroff W. W. Atmos. Environ. 2003, 37, 5551. [Google Scholar]
- Maroni M., Seifert B., Lindvall T., Eds.; Indoor Air Quality; Elsevier: Amsterdam, 1995. [Google Scholar]
- Baker R. R.; Coburn S.; Liu C. J. Anal. Appl. Pyrolysis 2006, 77, 12. [Google Scholar]
- Ott W. R.Environmental Statistics and Data Analysis; Lewis Publishers: Boca Raton, FL, 1995. [Google Scholar]
- ISO 16000-1; ISO: Geneva, 2004. [Google Scholar]
- Marchand C.; Bulliot B.; Le Calve S.; Mirabel P. Atmos. Environ. 2006, 40, 1336. [Google Scholar]
- Ohura T.; Amagai T.; Senga Y.; Fusaya M. Sci. Total Environ. 2006, 366, 485. [DOI] [PubMed] [Google Scholar]
- Krause C.; Chutsch M.; Henke M.; Huber M. K. C.; Leiske M.; Mailahn W.; Schulz C.; Schwarz E.; Seifert B.; Ullrich D.. Umwelt-Survey, Band IIIc: Wohn-Innenraum: Raumluft; 4/91; Federal Environment Agency: Berlin, Germany, 1991. [Google Scholar]
- Federal Environment Agency of Germany.Bundesgesundheitsblatt 2008, 51, 109.
- Schleibinger H.; Hott U.; Marchl D.; Braun P.; Plieninger P.; Rüden H. Gefahrstoffe—Reinhalt. Luft 2001, 61, 26. [Google Scholar]
- BfR.Inhalative Exposition des Verbrauchers gegenüber Formaldehyd; Federal Institute for Risk Assessment: Berlin, Germany, 2005 [Google Scholar]; (http://www.bfr.de)
- Bischof W.; Bullinger-Naber M.; Kruppa B.; Müller B. H.; Schwab R.. Exposition und gesundheitliche Beeinträchtigung in Bürogebäuden—Ergebnisse des ProKlimA-Projektes; Fraunhofer IRB Verlag: Stuttgart, 2003. [Google Scholar]
- WHO.Air Quality Guidelines for Europe; Regional Office for Europe: Copenhagen, Denmark, 2000. [Google Scholar]
- Liu W.; Zhang J.; Zhang L.; Turpin B. J.; Weisel C. P.; Morandi M. T.; Stock T. H.; Colome S.; Korn L. R. Atmos. Environ. 2006, 40, 2202. [Google Scholar]
- Loh M. M.; Houseman E. A.; Gray G. M.; Levy J. I.; Spengler J. D.; Bennett D. H. Environ. Sci. Technol. 2006, 40, 6903. [DOI] [PubMed] [Google Scholar]
- Dassonville C.; Demattei C.; Laurent A. M.; Le Moullec Y.; Seta N.; Momas I. Indoor Air 2009, 19, 314. [DOI] [PubMed] [Google Scholar]
- Dingle P.; Franklin P. Indoor Built Environ. 2002, 11, 111. [Google Scholar]
- Seifert B.; Salthammer T. In Handbuch der Umweltmedizin; Wichmann H. E., Schlipköter H.-W., Füllgraff G., Eds.; ECOMED Verlag: Landsberg, 2003; pp 1−32. [Google Scholar]
- Ashmore M. R.; Dimitroulopoulou C. Atmos. Environ. 2009, 43, 128. [Google Scholar]
- Jurvelin J. A.; Edwards R. D.; Vartiainen M.; Pasanen P.; Jantunen M. J. J. Air Waste Manage. Assoc. 2003, 53, 560. [DOI] [PubMed] [Google Scholar]
- Liu W.; Zhang J.; Korn L. R.; Zhang L.; Weisel C. P.; Turpin B.; Morandi M.; Stock T.; Colome S. Atmos. Environ. 2007, 41, 5280. [Google Scholar]
- Meininghaus R.; Bastin E.; Bourdet S.; Cicolella A.; Granier D.; Gonzalez-Flesca N. In Latini G., Brebbia C., Eds.; Air Pollution IX; Proceedings of the International Conference of Air Pollution; Ancona, 2001; pp 263−271. [Google Scholar]
- Zhang G.-S.; Li T.-T.; Luo M.; Liu J.-F.; Liu Z.-R.; Bai Y.-H. Build. Environ. 2008, 43, 315. [Google Scholar]
- Pang X.; Mu Y. Atmos. Environ. 2007, 41, 1819. [Google Scholar]
- Shinohara N.; Fernández-Bremauntz A. A.; Blanco Jiménez S.; Yanagisawa Y. Atmos. Environ. 2005, 39, 3481. [Google Scholar]
- Ohmichi K.; Komiyama M.; Matsuno Y.; Takanashi Y.; Miyamoto H.; Kadota T.; Maekawa M.; Toyama Y.; Tatsugi Y.; Kohno T.; Ohmichi M.; Mori C. Environ. Sci. Pollut. Res. 2006, 13, 120. [DOI] [PubMed] [Google Scholar]
- Wantke F.; Focke M.; Hemmer W.; Bracun R.; Wolf-Abdolvahab S.; Gotz M.; Jarisch R.; Tschabitscher M.; Gann M.; Tappler P. Allergy 2000, 55, 84. [DOI] [PubMed] [Google Scholar]
- Yamato H.; Nakashima T.; Kikuta A.; Kunugita N.; Arashidani K.; Nagafuchi Y.; Tanaka I. J. Occup. Health 2005, 47, 450. [DOI] [PubMed] [Google Scholar]
- Dascalaki E. G.; Lagoudi A.; Balaras C. A.; Gaglia A. G. Build. Environ. 2008, 43, 1945. [Google Scholar]
- Hatchfield P. B.Pollutants in the museum environment; Archetype Publications Ltd.: London, U.K., 2002. [Google Scholar]
- Schieweck A.; Lohrengel B.; Siwinski N.; Genning C.; Salthammer T. Atmos. Environ. 2005, 39, 6098. [Google Scholar]
- Khalequzzaman M.; Kamijima M.; Sakai K.; Chowdhury N. A.; Hamajima N.; Nakajima T. Indoor Air 2007, 17, 297. [DOI] [PubMed] [Google Scholar]
- Broder I.; Corey P.; Cole P.; Lipa M.; Mintz S.; Nethercott J. R. Environ. Res. 1988, 45, 141. [DOI] [PubMed] [Google Scholar]
- Wolkoff P.; Clausen P. A.; Nielsen P. A.; Molhave L. Indoor Air 1991, 1, 478. [Google Scholar]
- Harving H.; Dahl R.; Korsgaard J.; Linde S. A. Indoor Air 1992, 2, 121. [Google Scholar]
- Brown S. K. Indoor Air 2002, 12, 55. [DOI] [PubMed] [Google Scholar]
- Kim S.-S.; Kang D.-H.; Choi D.-H.; Yeo M.-S.; Kim K.-W. Build. Environ. 2008, 43, 320. [Google Scholar]
- Park J. S.; Ikeda K. Indoor Air 2006, 16, 129. [DOI] [PubMed] [Google Scholar]
- Sohn J.; Yang W.; Kim J.; Son B.; Park J. J. Environ. Manage. 2009, 90, 348. [DOI] [PubMed] [Google Scholar]
- Guo H.; Kwok N. H.; Cheng H. R.; Lee S. C.; Hung W. T.; Li Y. S. Indoor Air 2009, 19, 206. [DOI] [PubMed] [Google Scholar]
- Sexton K.; Petreas M. X.; Liu K. S. Environ. Sci. Technol. 1989, 23, 985. [Google Scholar]
- Hanrahan L.; Anderson H.; Dally K.; Eckmann A.; Kanarek M. JAPCA 1985, 35, 1164. [DOI] [PubMed] [Google Scholar]
- Dingle P.; Tan R.; Cheong C. Indoor Built Environ. 2000, 9, 233. [Google Scholar]
- Main D. M.; Hogan T. J. J. Occup. Med. 1983, 25, 896. [DOI] [PubMed] [Google Scholar]
- CDC.Final report on formaldehyde levels in FEMA-supplied travel trailers, park models and mobile homes; CDC: Washington, DC, 2008 [DOI] [PubMed] [Google Scholar]; (http://cdc.gov)
- Maddalena R.; Russell M.; Sullivan D. P.; Apte M. G.. Aldehyde and other volatile organic chemical emissions in four FEMA temporary housing units − final report; Lawrence Berkeley National Laboratory: Berkeley, 2008. [Google Scholar]
- Maddalena R.; Russell M.; Sullivan D. P.; Apte M. G. Environ. Sci. Technol. 2009, 43, 5626. [DOI] [PubMed] [Google Scholar]
- Wolkoff P.; Kjaergaard S. K. Environ. Int. 2007, 33, 850. [DOI] [PubMed] [Google Scholar]
- Gilbert N. L.; Guay M.; Gauvin D.; Dietz R. N.; Chan C. C.; Levesque B. Atmos. Environ. 2008, 42, 2424. [Google Scholar]
- Jo W.-J.; Sohn J.-Y. Build. Environ. 2009, 44, 1794. [Google Scholar]
- Tuomainen M.; Pasanen A. L.; Tuomainen A.; Liesivuori J.; Juvonen P. Atmos. Environ. 2001, 35, 305. [Google Scholar]
- Jaernstroem H.; Saarela K.; Kalliokoski P.; Pasanen A. L. Atmos. Environ. 2006, 40, 7178. [Google Scholar]
- Einax J. W.; Zwanzinger H. W.; Geiβ S.. Chemometrics in Environmental Analysis; WILEY-VCH: Weinheim, 1997. [Google Scholar]
- Wong L. T.; Mui K. W.; Hui P. S. Atmos. Environ. 2006, 40, 4246. [Google Scholar]
- Andreini B. P.; Baroni R.; Galimberti E.; Sesana G. Microchem. J. 2000, 67, 11. [Google Scholar]
- Brasche S.; Bischof W. Int. J. Hyg. Environ. Health 2005, 208, 247. [DOI] [PubMed] [Google Scholar]
- U.S. EPA.Exposure Factors Handbook; EPA 600/8-89/043; Office of Health and Environmental Assessment: Washington, DC, 1990. [Google Scholar]
- Stubenrauch S.; Hempfling R.; Doetsch P.; Grünhoff D. Umweltwiss. Schadst. Forsch. 1999, 11, 219. [Google Scholar]
- Baez A.; Padilla H.; Garcia R.; del Carmen Torres M.; Rosas I.; Belmont R. Sci. Total Environ. 2003, 302, 211. [DOI] [PubMed] [Google Scholar]
- Daisey J. M.; Angell W. J.; Apte M. G. Indoor Air 2003, 13, 53. [DOI] [PubMed] [Google Scholar]
- Franklin P.; Dingle P.; Stick S. Am. J. Respir. Crit. Care Med. 2000, 161, 1757. [DOI] [PubMed] [Google Scholar]
- Garrett M. H.; Hooper M. A.; Hooper B. M.; Rayment P. R.; Abramson M. J. Allergy 1999, 54, 330. [DOI] [PubMed] [Google Scholar]
- Krzyzanowski M.; Quackenboss J. J.; Lebowitz M. D. Environ. Res. 1990, 52, 117. [DOI] [PubMed] [Google Scholar]
- Wantke F.; Demmer C. M.; Tappler P.; Gotz M.; Jarisch R. Clin. Exp. Allergy 1996, 26, 276. [PubMed] [Google Scholar]
- Schwartz J. Pediatrics 2004, 113, 1037. [PubMed] [Google Scholar]
- Pohl H. R.; Abadin H. G. Toxicol. Appl. Pharmacol. 2008, 233, 116. [DOI] [PubMed] [Google Scholar]
- Kurose T.; Kodera H.; Aoyama H.; Kawamata S. Hiroshima J. Med. Sci. 2004, 53, 33. [PubMed] [Google Scholar]
- Suruda A.; Schulte P.; Boeniger M.; Hayes R. B.; Livingston G. K.; Steenland K.; Stewart P.; Herrick R.; Douthit D.; Fingerhut M. A. Cancer Epidemiol. Biomarkers Prev. 1993, 2, 453. [PubMed] [Google Scholar]
- Tanaka K.; Nishiyama K.; Yaginuma H.; Sasaki A.; Maeda T.; Kaneko S. Y.; Onami T.; Tanaka M. Kaibogaku Zasshi. J. Anatomy 2003, 78, 43. [PubMed] [Google Scholar]
- Dufresne A.; Infante-Rivard C.; Malo J. L.; Gautrin D. Am. Ind. Hyg. Assoc. J. 2002, 63, 647. [DOI] [PubMed] [Google Scholar]
- Hauptmann M.; Lubin J. H.; Stewart P. A.; Hayes R. B.; Blair A. Am. J. Epidemiol. 2004, 159, 1117. [DOI] [PubMed] [Google Scholar]
- Marsh G. M.; Youk A. O.; Buchanich J. M.; Erdal S.; Esmen N. A. Regul. Toxicol. Pharmacol. 2007, 48, 308. [DOI] [PubMed] [Google Scholar]
- Day J. H.; Lees R. E.; Clark R. H.; Pattee P. L. Can. Med. Assoc. J. 1984, 131, 1061. [PMC free article] [PubMed] [Google Scholar]
- Harving H.; Korsgaard J.; Pedersen O. F.; Molhave L.; Dahl R. Lung 1990, 168, 15. [DOI] [PubMed] [Google Scholar]
- Kulle T. J. Inhalation Toxicol. 1993, 5, 323. [Google Scholar]
- Lu Z.; Li C. M.; Qiao Y.; Yan Y.; Yang X. Indoor Air 2008, 18, 77. [DOI] [PubMed] [Google Scholar]
- Arts J. H. E.; Rennen M. A. J.; de Heer C. Regul. Toxicol. Pharmacol. 2006, 44, 144. [DOI] [PubMed] [Google Scholar]
- Wolkoff P.; Wilkins C. K.; Clausen P. A.; Nielsen G. D. Indoor Air 2006, 16, 7. [DOI] [PubMed] [Google Scholar]
- Ezratty V.; Bonay M.; Neukirch C.; Orset-Guillossou G.; Dehoux M.; Koscielny S.; Cabanes P.-A.; Lambrozo J.; Aubier M. Environ. Health Perspect. 2007, 115, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumchev K. B.; Spickett J. T.; Bulsara M. K.; Phillips M. R.; Stick S. M. Eur. Respir. J. 2002, 20, 403. [DOI] [PubMed] [Google Scholar]
- Sakamoto T.; Doi S.; Torii S. Allergol. Int. 1999, 48, 151. [Google Scholar]
- Sakula A. Lancet 1975, 2, 816. [DOI] [PubMed] [Google Scholar]
- Smedje G.; Norback D.; Edling C. Clin. Exp. Allergy 1997, 27, 1270. [PubMed] [Google Scholar]
- Vandenplas O.; Fievez P.; Delwiche J. P.; Boulanger J.; Thimpont J. Allergy: Europ. J. Allergy Clin. Immunol. 2004, 59, 115. [DOI] [PubMed] [Google Scholar]
- Baba K.; Yagi T.; Niwa S.; Sakakibara A.; Hattori T.; Koishikawa I.; Yoshida K.; Kobayashi T. Jpn J. Allergol. 2000, 49, 404. [PubMed] [Google Scholar]
- Arts J. H. E.; Muijser H.; Kuper F. C.; Woutersen R. A. Regul. Toxicol. Pharmacol. 2008, 52, 189. [DOI] [PubMed] [Google Scholar]
- Appel K.-E.; Bernauer U.; Herbst U.; Madle S.; Schulte A.; Richter-Reichhelm H.-B.; Gundert-Remy U. Umweltmed. Forsch. Prax. 2006, 11, 347. [Google Scholar]
- Marsh G. M.; Youk A. O. Regul. Toxicol. Pharmacol. 2005, 42, 275. [DOI] [PubMed] [Google Scholar]
- Marsh G. M.; Youk A. O.; Morfeld P. Regul. Toxicol. Pharmacol. 2007, 47, . [DOI] [PubMed] [Google Scholar]
- Bosetti C.; McLaughlin J. K.; Tarone R. E.; Pira E.; La Vecchia C. Ann. Oncol. 2008, 19, 29. [DOI] [PubMed] [Google Scholar]
- Hauptmann M.; Lubin J. H.; Stewart P. A.; Hayes R. B.; Blair A. J. Natl. Cancer Inst. 2003, 95, 1615. [DOI] [PubMed] [Google Scholar]
- Pinkerton L. E.; Hein M. J.; Stayner L. T. Occup. Environ. Med. 2004, 61, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggon D.; Harris E. C.; Poole J.; Palmer K. T. J. Natl. Cancer Inst. 2003, 95, 1608. [DOI] [PubMed] [Google Scholar]
- Marsh G. M.; Youk A. O. Regul. Toxicol. Pharmacol. 2004, 40, 113. [DOI] [PubMed] [Google Scholar]
- Beane Freeman L. E.; Blair A.; Lubin J. H.; Stewart P. A.; Hayes R. B.; Hoover R. N.; Hauptmann M. J. Natl. Cancer Inst. 2009, 101, 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya M.; Nakanishi J. Regul. Toxicol. Pharmacol. 2005, 43, 232. [DOI] [PubMed] [Google Scholar]
- Cavalcante R. M.; Campelo C. S.; Barbosa M. J.; Silveira E. R.; Carvalho T. V.; Nascimento R. F. Atmos. Environ. 2006, 40, 5701. [Google Scholar]
- Loh M. M.; Levy J. I.; Spengler J. D.; Houseman E. A.; Bennett D. H. Environ. Health Perspect. 2007, 115, 1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott W. R.; Steinmann A. C.; Wallace L. A.. Exposure Analysis; CRC Press: Boca Raton, FL, 2007. [Google Scholar]
- Bolt H.; Huici-Montagud A. Arch. Toxicol. 2008, 82, 61. [DOI] [PubMed] [Google Scholar]
- Irigaray P.; Newby J. A.; Clapp R.; Hardell L.; Howard V.; Montagnier L.; Epstein S.; Belpomme D. Biomed. Pharmacother. 2007, 61, 640. [DOI] [PubMed] [Google Scholar]
- Deutsche Forschungsgemeinschaft.MAK- und BAT-Werte-Liste 2008; WILEY-VCH: Bonn, 2008. [Google Scholar]
- Liteplo R. G.; Meek M. E. J. Toxicol. Environ. Health, Part B 2003, 6, 85. [DOI] [PubMed] [Google Scholar]
- Godish T.Sick Buildings: Definition, Diagnosis and Mitigation; Lewis Publishers: Boca Raton, FL, 1995. [Google Scholar]
- Brightman H. S.; Moss N. In Indoor Air Quality Handbook; Samet J. M., Spengler J. D., McCarthy J. F., Eds.; McGraw Hill: New York, 2001. [Google Scholar]
- WHO.Indoor Air Pollutants—Eposure and Health Effects; EURO Reports and Studies 78: Copenhagen, 1983. [Google Scholar]
- Molhave L. Environ. Int. 1989, 15, 65. [Google Scholar]
- Mendell M. J. Indoor Air 1993, 3, 227. [DOI] [PubMed] [Google Scholar]
- Skov P.; Valbjorn O.; Pedersen B. V. Scand. J. Work Environ. Health 1990, 16, 1. [DOI] [PubMed] [Google Scholar]
- Skov P.; Valbjorn O. Environ. Int. 1987, 13, 339. [Google Scholar]
- Skov P.; Valbjorn O.; Pedersen B. V. Scand. J. Work Environ. Health 1989, 15, 286. [DOI] [PubMed] [Google Scholar]
- Sundell J.; Anderson B.; Anderson K.; Lindvall T. Indoor Air 1993, 3, 82. [Google Scholar]
- TenBrinke J.; Selvin S.; Hodgson A. T.; Fisk W. J.; Mendell M. J.; Koshland C. P.; Daisey J. M. Indoor Air 1998, 8, 140. [Google Scholar]
- Sasagawa Y. Skin Res. 2005, 4, 3. [Google Scholar]
- Takigawa T.; Wang B.-L.; Sakano N.; Wang D.-H.; Ogino K.; Kishi R. Sci. Total Environ. 2009, 407, 5223. [DOI] [PubMed] [Google Scholar]
- Marutzky R. Holz Roh Werkst. 1989, 47, 207. [Google Scholar]
- Uchiyama S.; Matsushima E.; Kitao N.; Tokunaga H.; Ando M.; Otsubo Y. Atmos. Environ. 2007, 41, 8825. [Google Scholar]
- Kim S. Construct. Build. Mater. 2009, 23, 2319. [Google Scholar]
- Hayashi M.; Enai M.; Hirokawa Y. Build. Environ. 2001, 36, 721. [Google Scholar]
- Hayashi M.; Osawa H. Build. Environ. 2008, 43, 329. [Google Scholar]
- Sherman M. H.; Hodgson A. T. Indoor Air 2004, 14, 2. [DOI] [PubMed] [Google Scholar]
- Mo J.; Zhang Y.; Xu Q.; Lamson J. J.; Zhao R. Atmos. Environ. 2009, 43, 2229. [Google Scholar]
- Ao C. H.; Lee S. C.; Yu J. Z.; Xu J. H. Appl. Catal., B 2004, 54, 41. [Google Scholar]
- Ching W. H.; Leung M.; Leung D. Y. C. Sol. Energy 2004, 77, 129. [Google Scholar]
- Yang L.; Liu Z. Energy Convers. Manage. 2007, 48, 882. [Google Scholar]
- Yu H.; Zhang K.; Rossi C. Indoor Built Environ. 2007, 16, 529. [Google Scholar]
- Zhao J.; Yang X. Build. Environ. 2003, 38, 645. [Google Scholar]
- Shiraishi F.; Nomura T.; Yamaguchi S.; Ohbuchi Y. Chem. Eng. J. 2007, 127, 157. [Google Scholar]
- Shie J.-L.; Lee C.-H.; Chiou C.-S.; Chang C.-T.; Chang C.-C.; Chang C.-Y. J. Hazard Mater. 2008, 155, 164. [DOI] [PubMed] [Google Scholar]
- Hodgson A. T.; Destaillats H.; Sullivan D. P.; Fisk W. J. Indoor Air 2007, 17, 305. [DOI] [PubMed] [Google Scholar]
- Mo J.; Zhang Y.; Xu Q.; Zhu Y.; Lamson J. J.; Zhao R. Appl. Catal., B 2009, 89, 570. [Google Scholar]
- Sakthivel S.; Kisch H. Angew. Chem. 2003, 115, 5057. [DOI] [PubMed] [Google Scholar]
- Quiller R. G.; Benz L.; Haubrich J.; Colling M. E.; Friend C. M. J. Phys. Chem., C 2009, 113, 2063. [Google Scholar]
- Godish T.; Guindon C. Environ. Pollut. 1989, 62, 13. [DOI] [PubMed] [Google Scholar]
- Giese M.; Bauerdoranth U.; Langebartels C.; Sandermann H. Plant Physiol. 1994, 104, 1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz H.; Hilgers U.; Weidner M. New Phytol. 2000, 147, 307. [Google Scholar]
- Huang X.; Wang Y.-J.; Di Y.-H. Text. Res. J. 2007, 77, 946. [Google Scholar]
- Wang Y. J.; Huang X.; Di Y. H. Wool Textile J. 2007, 35. [Google Scholar]
- Mui K. W.; Wong L. T.; Chan W. Y. Energy Buildings 2008, 40, 1412. [Google Scholar]
- Raychaudhuri M. R.; Brimblecombe P. Stud. Conserv. 2000, 45, 226. [Google Scholar]
- Hatchfield P.; Carpenter J.. Formaldehyde: how great is the danger to museum collections?; Harvard University Art Museum: Cambridge, 1987. [Google Scholar]
- Tetrault J.Airborne pollutants in museums galleries and archives; Canadian Conservation Institute: Ottawa, 2003. [Google Scholar]
- Salthammer T.; Siwinski N.; Vogtenrath W.; Schieweck A. In Proceedings of Healthy Building; Fernandes E. O.,da Silva M. G.,Pinto J. R., Eds.; Lisboa, Portugal, 2006; Vol. 2, pp 283−286. [Google Scholar]
- Breitmaier E.Terpenes; WILEY-VCH: Weinheim, 2006. [Google Scholar]
- Fengel D.; Wegener G.. Wood; Walter De Gruyter: Berlin, 1989. [Google Scholar]
- Calogirou A.; Larsen B. R.; Kotzias D. Atmos. Environ. 1999, 33, 1423. [Google Scholar]
- Lamorena R. B.; Jung S.-G.; Bae G.-N.; Lee W. J. Hazard. Mater. 2007, 141, 245. [DOI] [PubMed] [Google Scholar]
- Sarwar G.; Corsi R. Atmos. Environ. 2007, 41, 959. [Google Scholar]
- Toftum J.; Freund S.; Salthammer T.; Weschler C. J. Atmos. Environ. 2008, 42, 7632. [Google Scholar]
- Risholm-Sundmann M.; Lundgren M.; Vestin E.; Herder P. Holz Roh Werkst. 1998, 56, 125. [Google Scholar]
- Jiang T.; Gardner D. J.; Baumann M. G. D. Forest Prod. J. 2002, 52, 66. [Google Scholar]
- Makowski M.; Ohlmeyer M. Holzforschung 2006, 60, 533. [Google Scholar]
- Makowski M.; Ohlmeyer M.; Meier D. Holzforschung 2005, 59, 519. [Google Scholar]
- Horn W.; Ullrich D.; Seifert B. Indoor Air 1998, 8, 39. [Google Scholar]
- Salthammer T.; Fuhrmann F. Indoor Air 2000, 10, 133. [DOI] [PubMed] [Google Scholar]
- Gilbert N. L.; Guay M.; Miller J. D.; Judek S.; Chan C. C.; Dales R. E. Environ. Res. 2005, 99, 11. [DOI] [PubMed] [Google Scholar]
- Eikmann T.; Knaust A.; Herr C. Umweltmed. Forsch. Prax. 2006, 11, 345. [Google Scholar]
- Edler L.Konzentrations-Wirkungsbeziehungen bei Formaldehyd—Bewertung neuer epidemiologischer Studien; Federal Institute für Risk Assessment (BfR): Berlin, Germany, 2005. [Google Scholar]
- Wolkoff P. Environ. Int. 2008, 34, 1204. [DOI] [PubMed] [Google Scholar]
- Criegee R. Angew. Chem., Int. Ed. 1975, 14, 745. [Google Scholar]
- Handbook of Chemistry and Physics, 82 ed.; Lide D. R., Ed.; CRC Press: Boca Raton, FL, 2001. [Google Scholar]
- Wang S. Y.; Yang T. H.; Lin L. T.; Lin C. J.; Tsai M. J. Build. Environ. 2007, 42, 2472. [Google Scholar]
- Wiglusz R.; Jarnuszkiewicz I.; Sitko E.; Wolska L. Bull. Inst. Maritime Tropical Med. Gdynia 1990, 41, 73. [PubMed] [Google Scholar]
- Wiglusz R.; Jarnuszkiewicz I.; Sitko E.; Wolska L. Bull. Inst. Maritime Tropical Med. Gdynia 1990, 41, 79. [PubMed] [Google Scholar]
- Kim S.; Kim H. J.; Moon S. J. Indoor Built Environ. 2006, 15, 511. [Google Scholar]
- Brown S. K. Indoor Air 1999, 9, 209. [DOI] [PubMed] [Google Scholar]
- Pickrell J. A.; Griffis L. C.; Mokler B. V.; Kanapilly G. M.; Hobbs C. H. Environ. Sci. Technol. 1984, 18, 682. [DOI] [PubMed] [Google Scholar]
- Pickrell J. A.; Mokler B. V.; Griffis L. C.; Hobbs C. H.; Bathija A. Environ. Sci. Technol. 1983, 17, 753. [DOI] [PubMed] [Google Scholar]
- Marutzky R.Formaldehydemissionen von Holzprodukten: von der Messung zur Bewertung. Holzindustrie und Holzprodukte im Wandel; Institut für Holzbiologie und Holztechnologie: Göttigen, 2005; pp 11−21. [Google Scholar]
- Wiglusz R.; Jarnuszkiewicz I.; Sitko E.; Nikel G. Build. Environ. 2000, 35, 53–57. [Google Scholar]
- Kim S.; Kim H. J. Indoor Air 2005, 15, 317. [DOI] [PubMed] [Google Scholar]
- Lévesque B.; Allaire S.; Gauvin D.; Koutrakis P.; Gingras S.; Rhainds M.; Prud’Homme H.; Duchesne J.-F. Sci. Total Environ. 2001, 281, 47. [DOI] [PubMed] [Google Scholar]
- Gustafson P.; Barregard L.; Strandberg B.; Sallsten G. J. Environ. Monitor. 2007, 9, 23. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Smith K. R. Environ. Sci. Technol. 1999, 33, 2311. [Google Scholar]
- Wang B.; Lee S. C.; Ho K. F.; Kang Y. M. Sci. Total Environ. 2007, 377, 52. [DOI] [PubMed] [Google Scholar]
- Lee S. C.; Wang B. Atmos. Environ. 2006, 40, 2128. [Google Scholar]
- Bakò-Birò Z.; Wargocki P.; Weschler C. J.; Fanger P. O. Indoor Air 2004, 14, 178. [DOI] [PubMed] [Google Scholar]
- Tuomi T.; Engstroem B.; Niemela R.; Svinhufvud J.; Reijula K. Appl. Occup. Environ. Hyg. 2000, 15, 629. [DOI] [PubMed] [Google Scholar]
- Leovic K.; Whitaker D.; Northeim C.; Sheldon L. J. Air Waste Manage. Assoc. 1998, 48, 915. [DOI] [PubMed] [Google Scholar]
- Leovic K. W.; Sheldon L. S.; Whitaker D. A.; Hetes R. G.; Calcagni J. A.; Baskir J. N. J. Air Waste Manage. Assoc. 1996, 46, 821. [DOI] [PubMed] [Google Scholar]
- Crump D. R.; Squire R. W.; Yu C. W. F. Indoor Built Environ. 1997, 6, 45. [Google Scholar]
- Wiglusz R.; Sitko E.; Jarnuszkiewicz I. Bull. Inst. Maritime Tropical Med. Gdynia 1991, 42, 51. [PubMed] [Google Scholar]
- Wiglusz R.; Sitko E.; Jarnuszkiewicz I. Bull. Inst. Maritime Tropical Med. Gdynia 1995, 46, 53. [PubMed] [Google Scholar]
- Nazaroff W. W.; Weschler C. J. Atmos. Environ. 2004, 38, 2841. [Google Scholar]
- Flyvholm M.-A.; Andersen P. Am. J. Ind. Med. 1993, 24, 533. [DOI] [PubMed] [Google Scholar]
- Wolkoff P.; Schneider T.; Kildeso J.; Degerth R.; Jaroszewski M.; Schunk H. Sci. Total Environ. 1998, 215, 135. [DOI] [PubMed] [Google Scholar]
- Singer B. C.; Destaillats H.; Hodgson A. T.; Nazaroff W. W. Indoor Air 2006, 16, 179. [DOI] [PubMed] [Google Scholar]
- Yu C.; Crump D. Build. Environ. 1998, 33, 357. [Google Scholar]
- Fan Z.; Lioy P.; Weschler C.; Fiedler N.; Kipen H.; Zhang J. Environ. Sci. Technol. 2003, 37, 1811. [DOI] [PubMed] [Google Scholar]
- Wolkoff P.; Clausen P. A.; Wilkins C. K.; Nielsen G. D. Indoor Air 2000, 10, 82. [DOI] [PubMed] [Google Scholar]
- Salthammer T. In Organic Indoor Air Pollutants; Salthammer T., Ed.; WILEY-VCH: Weinheim, 1999; pp 219−232. [Google Scholar]
- Platt U.; Perner D.; Pätz H. W. J. Geophys. Res. 1979, 84, 6329. [Google Scholar]
- Sawicki E.; Carnes A. E. Mikrochim. Acta 1968, 56, 148. [DOI] [PubMed] [Google Scholar]
- Sakaia T.; Tanaka S. I.; Teshima N.; Yasuda S.; Ura N. Talanta 2002, 58, 1271. [DOI] [PubMed] [Google Scholar]
- Sugaya N.; Sakurai K.; Nakagawa T.; Onda N.; Onodera S.; Morita M.; Masakatsu T. Anal. Sci. 2004, 20, 865. [DOI] [PubMed] [Google Scholar]
- Fan J.; Tang Y.; Feng S. Int. J. Environ. Anal. Chem. 2002, 82, 361. [Google Scholar]
- ASTM E 1333;American Society for Testing and Materials: West Conshohocken, 1996. [Google Scholar]
- JAS 233;Ministry of Agriculture and Forestry: Tokyo, 2003. [Google Scholar]
- EN 120;; European Committee for Standardization, Brussels, 1992. [Google Scholar]
- Scholz H. In Innenraumbelastungen; Diel F., Ed.; Bauverlag: Wiesbaden, 1993. [Google Scholar]
- Hutter H.-P.; Moshammer H.; Wallner P.; Damberger B.; Tappler P.; Kundi M. In Indoor Air 2002; Proceedings of the 9th International Conference on Indoor Air Quality and Climate, Monterey, 2002; Levin H., Ed.; Vol. 2, pp 239−243. [Google Scholar]
- Rothweiler H.; Wäger P. A.; Schlatter C. Atmos. Environ., Part A 1992, 26, 2219. [Google Scholar]
- Salonen H.; Pasanen A. L.; Lappalainen S.; Riuttala H.; Tuomi T.; Pasanen P.; Bäck B.; Reijula K. J. Occup. Environ. Hyg. 2009, 6, 239. [DOI] [PubMed] [Google Scholar]
- Sakai K.; Norbäck D.; Mi Y.; Shibata E.; Kamijima M.; Yamada T.; Takeuchi Y. Environ. Res. 2004, 94, 75. [DOI] [PubMed] [Google Scholar]
- Smedje G.; Norbäck D. Indoor Air 2001, 11, 127. [DOI] [PubMed] [Google Scholar]
- Marchand C.; Le Calve S.; Mirabel P.; Glasser N.; Casset A.; Schneider N.; de Blay F. Atmos. Environ. 2008, 42, 505. [Google Scholar]
- Clarisse B.; Laurent A. M.; Seta N.; Le Moullec Y.; El Hasnaoui A.; Momas I. Environ. Res. 2003, 92, 245. [DOI] [PubMed] [Google Scholar]
- Lovreglio P.; Carrus A.; Iavicoli S.; Drago I.; Persechino B.; Soleo L. J. Environ. Monitor. 2009, 11, 955. [DOI] [PubMed] [Google Scholar]
- Posniak M.; Makhniashvili I.; Koziel E. Indoor Built Environ. 2005, 14, 269. [Google Scholar]
- Vaizoglu S. A.; Aycan S.; Deveci M. A.; Acer T.; Bulut B.; Bayraktar U. D.; Akyollu B.; Celik M.; Arslan U.; Akpinar F.; Baris Z.; Arslan S.; Deniz A.; Evci E. D.; Güler C. Indoor Built Environ. 2003, 12, 329. [Google Scholar]
- Mentese S.; Gullu G. Indoor Built Environ. 2006, 15, 273. [Google Scholar]
- Reiss R.; Ryan B.; Tibbetts S. J.; Koutrakis P. J. Air Waste Manage. Assoc. 1995, 45, 811. [DOI] [PubMed] [Google Scholar]
- Hodgson A. T. Indoor Air 2000, 10, 178–192. [DOI] [PubMed] [Google Scholar]
- Liu K.-S.; Huang F.-Y.; Hayward S. B.; Wesolowski J.; Sexton K. Environ. Health Perspect. 1991, 94, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liteplo R. G.; Meek M. E. J. Toxicol. Environ. Health, Part B 2003, 6, 85. [DOI] [PubMed] [Google Scholar]
- Yang W.; Sohn J.; Kim J.; Son B.; Park J. J. Environ. Manage. 2009, 90, 348. [DOI] [PubMed] [Google Scholar]
- Lee S. C.; Li W. M.; Ao C. H. Atmos. Environ. 2002, 36, 225. [Google Scholar]
- Feng Y.; Wen S.; Wang X.; Sheng G.; He Q.; Tang J.; Fu J. Atmos. Environ. 2004, 38, 103. [Google Scholar]
- Khoder M. I.; Shakour A. A.; Farag S. A.; Abdel Hameed A. A. J. Environ. Monitor. 2000, 2, 123. [DOI] [PubMed] [Google Scholar]
- Zhang G.; Spickett J.; Rumchev K.; Lee A. H.; Stick S. Indoor Air 2006, 16, 74. [DOI] [PubMed] [Google Scholar]
- Persson O.; Oestberg C.; Pagels J.; Sebastian A. J. Environ. Monitor. 2006, 8, 1111. [DOI] [PubMed] [Google Scholar]
- IARC.IARC Monographs of the evaluation of Carcinogenic Risks to Humans, Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxy-2-propanol; Vol. 88; International Agency for Research on Cancer: Lyon, 2006. [PMC free article] [PubMed] [Google Scholar]
- Spanel P.; Smith D. J. Breath Res. 2008, 2, 046003. [DOI] [PubMed] [Google Scholar]
- Schripp T.; Fauck C.; Salthammer T.. Int. J. Mass Spectrom.2009, in press, doi: 10.1016/j.ijms.2009.11.001.. [Google Scholar]
- Blake R. S.; Monks P. S.; Ellis A. M. Chem. Rev. 2009, 109, 861. [DOI] [PubMed] [Google Scholar]