Abstract
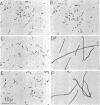
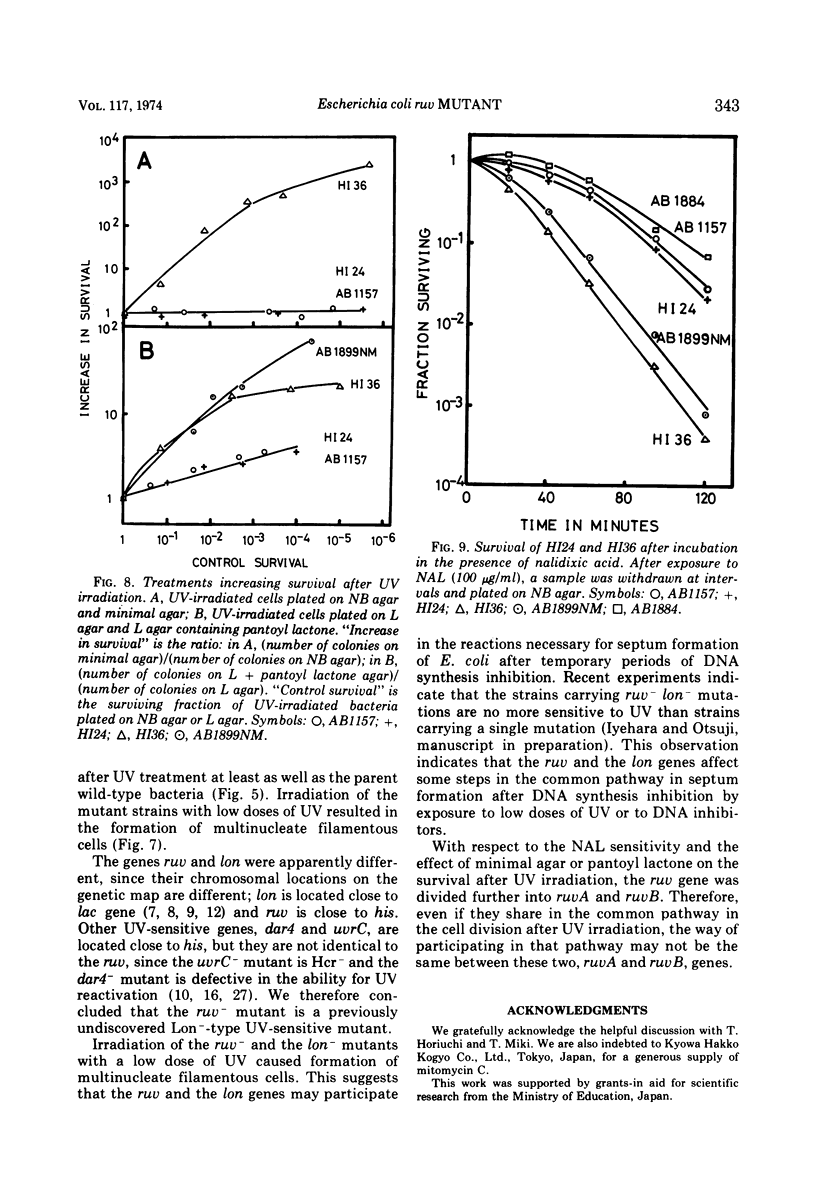
Two ultraviolet light (UV)-sensitive mutants have been isolated from Escherichia coli K-12. These mutants, designated RuvA− and RuvB−, were controlled by a gene located close to the his gene on the chromosome map. They were sensitive to UV (10- to 20-fold increase) and slightly sensitive to gamma rays (3-fold increase). Host cell reactivation, UV reactivation and genetic recombination were normal in these mutants. Irradiation of the mutants with UV resulted in the production of single-strand breaks in deoxyribonucleic acid, which was repaired upon incubation in a growth medium. After UV irradiation, these mutants resumed deoxyribonucleic acid synthesis at a normal rate, as did the parent wild-type bacteria, and formed nonseptate, multinucleate filaments. From these results we concluded that the mutants have some defect in cell division after low doses of UV irradiation, similar to the lon− or fil+ mutant of E. coli. The ruv locus was divided further into ruvA and ruvB with respect to nalidixic acid sensitivity and the effect of minimal agar or pantoyl lactone on survival of the UV-irradiated cell. The ruvB−mutant was more sensitive to nalidixic acid than were ruvA− and the parent strain. There was a great increase in the surviving fraction of the UV-irradiated ruvB− mutant when it was plated on minimal agar or L agar containing pantoyl lactone. No such increase in survival was observed in the ruvA− mutant.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER H. I., HARDIGREE A. A. POSTIRRADIATION GROWTH, DIVISION, AND RECOVERY IN BACTERIA. Radiat Res. 1965 May;25:92–102. [PubMed] [Google Scholar]
- ANDERSON E. H. Heat reactivation of ultraviolet-inactivated bacteria. J Bacteriol. 1951 Apr;61(4):389–394. doi: 10.1128/jb.61.4.389-394.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Hardigree A. A. Growth and Division of Filamentous Forms of Escherichia coli. J Bacteriol. 1965 Jul;90(1):223–226. doi: 10.1128/jb.90.1.223-226.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYCE R. P., HOWARD-FLANDERS P. GENETIC CONTROL OF DNA BREAKDOWN AND REPAIR IN E. COLI K-12 TREATED WITH MITOMYCIN C OR ULTRAVIOLET LIGHT. Z Vererbungsl. 1964 Dec 30;95:345–350. doi: 10.1007/BF01268667. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J., Green M. H., Greenberg J. Interaction of the exr and lon genes in Escherichia coli. J Bacteriol. 1968 Nov;96(5):1704–1710. doi: 10.1128/jb.96.5.1704-1710.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Loci of radiation sensitivity in Bs strains of Escherichia coli. Genet Res. 1968 Apr;11(2):183–191. doi: 10.1017/s0016672300011356. [DOI] [PubMed] [Google Scholar]
- Donch J., Greenberg J. Ultraviolet sensitivity gene of Escherichia coli B. J Bacteriol. 1968 May;95(5):1555–1559. doi: 10.1128/jb.95.5.1555-1559.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor G. J., Deering R. A. Effect of nalidixic acid and hydroxyurea on division ability of Escherichia coli fil+ and lon- strains. J Bacteriol. 1968 Feb;95(2):520–530. doi: 10.1128/jb.95.2.520-530.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor G. J., Deering R. A. Recovery of division ability in ultraviolet-irradiated Escherichia coli induced by photoreactivation, photoprotection, and liquid holding treatment. J Bacteriol. 1967 Dec;94(6):1946–1950. doi: 10.1128/jb.94.6.1946-1950.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE M. Effect of mitomycin C on interactions between temperate phages and bacteria. Virology. 1961 Apr;13:493–499. doi: 10.1016/0042-6822(61)90280-x. [DOI] [PubMed] [Google Scholar]
- Mattern I. E., van Winden M. P., Rörsch A. The range of action of genes controlling radiation sensitivity in Escherichia coli. Mutat Res. 1965 Apr;2(2):111–131. doi: 10.1016/0027-5107(65)90042-4. [DOI] [PubMed] [Google Scholar]
- OTSUJI N., SEKIGUCHI M., IIJIMA T., TAKAGI Y. Induction of phage formation in the lysogenic Escherichia coli K-12 by mitomycin C. Nature. 1959 Oct 3;184(Suppl 14):1079–1080. doi: 10.1038/1841079b0. [DOI] [PubMed] [Google Scholar]
- Otsuji N., Murayama I. Deoxyribonucleic acid damage by monofunctional mitomycins and its repair in Escherichia coli. J Bacteriol. 1972 Feb;109(2):475–483. doi: 10.1128/jb.109.2.475-483.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuji N. Properties of mitomycin C-sensitive mutants of Escherichia coli K-12. J Bacteriol. 1968 Feb;95(2):540–545. doi: 10.1128/jb.95.2.540-545.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. B., Aldous E. RECOVERY FROM ULTRAVIOLET IRRADIATION IN ESCHERICHIA COLI. J Bacteriol. 1949 Mar;57(3):363–375. doi: 10.1128/jb.57.3.363-375.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DE PUTTE P., WESTENBROEK C., ROERSCH A. THE RELATIONSHIP BETWEEN GENE-CONTROLLED RADIATION RESISTANCE AND FILAMENT FORMATION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Oct 15;76:247–256. doi: 10.1016/0006-3002(63)90037-4. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Conditional mutations involving septum formation in Escherichia coli. J Bacteriol. 1967 Jan;93(1):107–114. doi: 10.1128/jb.93.1.107-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Evidence for a relationship between deoxyribonucleic acid metabolism and septum formation in Escherichia coli. J Bacteriol. 1968 Jan;95(1):123–131. doi: 10.1128/jb.95.1.123-131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Putte P., van Sluis C. A., van Dillewijn J., Rörsch A. The location of genes controlling radiation sensitivity in Escherichia coli. Mutat Res. 1965 Apr;2(2):97–110. doi: 10.1016/0027-5107(65)90041-2. [DOI] [PubMed] [Google Scholar]