Abstract
Purpose
Insulin-like growth factor (IGF)-I and IGF-II stimulate neoplastic cell growth and inhibit apoptosis, whereas IGF-binding protein-3 (IGFBP-3) inhibits the bioavailability of IGF-I and has independent proapoptotic activity. We examined the influence of baseline plasma levels of IGF-I, IGF-II, IGFBP-3, and C-peptide on outcome among patients receiving first-line chemotherapy for metastatic colorectal cancer.
Experimental Design
The plasma levels of IGF-I, IGF-II, IGFBP-3, and C-peptide as well as data on prognostic factors and body size were measured at baseline among 527 patients participating in a randomized trial of first-line chemotherapy for metastatic colorectal cancer.
Results
Higher baseline plasma IGFBP-3 levels were associated with a significantly greater chemotherapy response rate (P = 0.03) after adjusting for other prognostic factors, whereas neither IGF-I nor IGF-II levels significantly predicted tumor response. Higher levels of IGF-I, IGF-II, and IGFBP-3 were all univariately associated with improved overall survival (P = 0.0001 for all). In a model that mutually adjusted for IGF-I and IGFBP-3, as well as other prognostic factors, increasing baseline-circulating IGFBP-3 was associated with a significantly longer time to tumor progression (P = 0.03), whereas circulating IGF-I was not associated with disease progression (P = 0.95). Levels of C-peptide were not associated with any measure of patient outcome.
Conclusion
Among colorectal cancer patients receiving first-line chemotherapy, increasing levels of IGFBP-3, an endogenous antagonist to IGF-I, are associated with an improved objective treatment response and a prolonged time to cancer progression. The IGF pathway may represent an important target for future treatment strategies.
The insulin-like growth factor (IGF) pathway is increasingly recognized for its roles in both normal growth and development as well as in tumorigenesis. IGF-I, IGF-II, and insulin have important mitogenic and antiapoptotic properties (1). IGF-I has characteristics of both a circulating hormone and a tissue growth factor; most IGF-I found in the circulation is produced by the liver. In laboratory models, inhibition of IGF-I receptor signaling seems to inhibit cell proliferation and increase the susceptibility of tumor cells to chemotherapeutic agents (2). Consequently, there has been considerable interest to develop effective targeted agents that inhibit the IGF-I receptor.
The bioavailability of IGFs is influenced by concentrations of specific IGF-binding proteins (IGFBP; ref. 1). At least six IGFBPs have been characterized, and their affinity for IGF-I and IGF-II is in the same order of magnitude as that of the IGF-I receptor. IGFBP-3 provides most of the IGF-binding capacity in serum, and IGFBP-3 is present in the circulation and in extravascular fluids. By binding to IGF-I, IGFBP-3 can attenuate IGF-I activity (3). Moreover, there is increasing evidence that the IGFBPs have growth inhibitory and proapoptotic actions that are independent of their capacity to bind IGFs (4-8). Further supporting the importance of IGFBP-3 in human colorectal carcinogenesis, a recent genome-wide survey of human colorectal cancers identified missense mutations in the IGFBP-3 gene (9).
In prospective studies of healthy subjects, elevated baseline levels of plasma IGF-I (10-16), IGF-II (16-18), and C-peptide (an indicator of insulin production; refs. 11, 14, 19-21) are associated with a greater subsequent risk of developing colorectal cancer, whereas increasing circulating IGFBP-3 (11-16) is associated with a significant reduction in the risk of developing colorectal cancer. Few studies have assessed the influence of these circulating biomarkers on the outcome of patients with established colorectal cancer. Nonetheless, based on the aforementioned findings, one might hypothesize that, among patients with newly established colorectal cancer, elevated baseline levels of plasma IGF-I would promote cancer progression, whereas increased circulating IGFBP-3 would delay or inhibit subsequent cancer progression.
Translational Relevance
The insulin-like growth factor (IGF) pathway is increasingly recognized for its roles in normal growth and development as well as in tumorigenesis. In laboratory models, inhibition of the IGF-I receptor seems to inhibit cell proliferation and increase susceptibility of tumor cells to chemotherapeutic agents. Consequently, there is a growing interest in the IGF-I receptor as a potential target for cancer therapy. Among 527 patients participating in a randomized trial of first-line chemotherapy for metastatic colorectal cancer, we measured baseline plasma levels of IGF-I, IGF-II, IGF-binding protein-3 (IGFBP-3), and C-peptide. We found that increasing levels of IGFBP-3, an endogenous antagonist to IGF-I, was associated with an improved objective response to chemotherapy and a prolonged time to tumor progression.The results of ongoing and proposed trials of IGF-I receptor antagonists in patients with advanced malignancy will hopefully provide further insight on the potential role of IGF pathway inhibition in colorectal cancer therapy.
We therefore examined the influence of pretreatment plasma levels of IGF-I, IGF-II, IGFBP-3, and C-peptide on cancer progression and survival among patients participating in a large randomized trial of first-line chemotherapy for metastatic colorectal cancer (N9741; ref. 22). By using patients enrolled in a prospective clinical trial with prescribed therapy and patient follow-up, we could minimize confounding by differences in the use of systemic chemotherapy, control for other clinical predictors of outcome, and directly examine the influence of these circulating biomarkers on patient outcome.
Materials and Methods
Patient population
Patients included in this study were drawn from a national, intergroup randomized trial of chemotherapy for metastatic colorectal cancer (22). Patients were randomized to receive (a) bolus irinotecan, 5-fluorouracil, and leucovorin; (b) infusional 5-fluorouracil, leucovorin, and oxaliplatin; or (c) irinotecan and oxaliplatin. Full details of the treatment trial and results have previously been published (22). Briefly, patients were required to have histologically proven unresectable colorectal adenocarcinoma, a baseline Eastern Cooperative Oncology Group performance status of ≤2, and adequate renal, liver, and bone marrow function. Exclusion criteria included prior therapy for advanced disease, baseline peripheral neuropathy or central nervous system disease, uncontrolled or severe comorbid illnesses, and a baseline of >3 loose stools per day. The protocol was reviewed and approved by the institutional review board of each participating institution. Patients signed informed consent for participation in the trial and were given the option of inclusion in a companion study of plasma for future research. In a previous report, we compared the baseline characteristics of the overall cohort of patients who enrolled in the treatment trial with the subset of patients participating in the biomarker studies (23). We did not detect any appreciable differences between these two groups. Further, patients experienced similar overall survival, with a median survival of 18.1 mo among all patients enrolled in the clinical trial and 18.2 mo among the patients who provided blood samples for plasma biomarkers.
Response and progression criteria
Study enrollment required at least one measurable lesion (≥2 cm in diameter) or disease that could be serially evaluated to establish whether the disease was getting better or worse (evaluable disease). Objective response to chemotherapy was calculated among patients with measurable disease (n = 474), whereas time to progression and overall survival were assessed among all study subjects (N = 527). Complete response required ≥50% reduction in the sum of the products of the longest perpendicular diameters of all measurable lesions. Regression required documented tumor reduction in evaluable patients who did not have disease that met the guidelines for measurable disease. Disease progression required ≥25% increase in measurable tumor or an increase in tumor size in patients whose lesions did not meet the criteria for measurable disease. After partial response, tumor measurements >50% of the maximal extent of a previously observed reduction constituted progression. Any new lesion constituted progression. Patients who did not meet the definitions of response or progression were classified as having stable disease.
Time to progression was calculated from study entry to disease progression, regardless of the patient’s treatment status. Deaths occurring within 30 d of treatment discontinuation were considered progressions. Survival was calculated from enrollment to death or last contact. Without contradictory data, patients who died or were lost to follow-up were assumed to have progressed at the time they were last documented to be progression-free.
Plasma biomarker measurement
Blood samples were collected upon study registration at the respective institutions and sent to the Mayo Central Laboratory for Clinical Trials in Rochester, Minnesota. Whole-blood samples were cooled and sent by overnight delivery to the laboratory. The stability of these biomarkers during the period of transport has been previously documented (24). Samples were centrifuged, divided, and frozen before use. IGF-I, IGF-II, IGFBP-3, and C-peptide levels were assayed in the laboratory of Dr. Michael Pollak, using enzyme-linked immunosorbent assays with reagents provided by Diagnostic Systems Laboratory. All assays were carried out by laboratory personnel who were blinded to patient outcome. Each sample was assayed in duplicate for each analyte, and correlations between replicates were >0.95. In previous studies, the mean intrabatch coefficients of variation calculated from the quality-control samples were 7%, 5%, 9%, and 10% for IGF-I, IGF-II, IGFBP-3 and C-peptide, respectively (11, 15, 25, 26).
Statistical analysis
In total, 1,379 patients were enrolled in N9741 after the incorporation of an amendment to collect blood samples for companion biomarker studies. Of this cohort, 527 patients provided blood samples for these analyses. Plasma biomarkers were each categorized according to quartiles. To assess the relation between the various plasma biomarkers and confirmed response rate to chemotherapy, linear tests for trend using multivariate logistic regression were conducted with each plasma biomarker modeled continuously in a model that included other potential predictors of patient outcome. The Kaplan-Meier method was used to describe the distribution of time to disease progression and overall survival time (27). Cox proportional hazards modeling was used to calculate hazard ratios and confidence intervals (28). In secondary analyses, we assessed the joint effects of plasma IGF-I and IGFBP-3 levels on patient outcome; to provide increased power for these cross-stratified subgroup analyses, plasma IGF-I and IGFBP-3 levels were categorized into tertiles only for these joint effect analyses. Tests for trend using two-sided P values were calculated by entering values for a specific biomarker as a continuous variable into the multivariate model. To satisfy the normality assumption, C-peptide was log-transformed when modeling. All statistical analyses used the SAS program package version 8.02 (SAS Institute). Two sided P values <0.05 were used to denote statistical significance.
Results
Patient characteristics
Among the 527 patients who provided baseline plasma samples for analysis, we examined baseline patient characteristics according to quartiles of plasma IGF-I and IGFBP-3 (Table 1). Participants on the highest level of IGF-I and IGFBP-3 were younger, possessed a higher body mass index (BMI), and were less likely to possess an Eastern Cooperative Oncology Group performance status of 2. Compared with the lowest categories, participants in the highest quartile of IGF-I were more often male, whereas those in the highest category of IGFBP-3 were more often female.
Table 1.
Baseline characteristics according to plasma IGF-I and IGFBP-3 (N = 527)
| IGF-I (quartiles) |
IGFBP-3 (quartiles) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Median age, y | 64 | 60 | 61 | 58 | 63 | 63 | 58 | 59 |
| Median BMI, kg/m2 | 25.3 | 25.4 | 26.5 | 27.1 | 25.5 | 26.5 | 25.9 | 27.1 |
| Male (%) | 39 | 50 | 70 | 76 | 63 | 56 | 66 | 49 |
| ECOG PS = 2 (%) | 11 | 2 | 3 | 2 | 7 | 7 | 2 | 2 |
| Treatment arm (%) | ||||||||
| IFL | 22 | 25 | 23 | 19 | 27 | 20 | 18 | 23 |
| FOLFOX4 | 52 | 56 | 60 | 63 | 52 | 62 | 61 | 57 |
| IROX | 26 | 19 | 17 | 18 | 21 | 18 | 21 | 20 |
| Race (%) | ||||||||
| White | 89 | 86 | 86 | 83 | 89 | 84 | 84 | 87 |
| Non-white | 11 | 14 | 14 | 17 | 11 | 16 | 16 | 13 |
Abbreviations: kg/m2, kilograms per meters squared; ECOG PS, Eastern Cooperative Oncology Group performance status; IFL, bolus irinotecan, 5-FU, and leucovorin; FOLFOX4, infusional 5-FU, leucovorin, and oxaliplatin; IROX, irinotecan and oxaliplatin.
Correlations between plasma markers, body mass index and performance status
We examined the relations among IGF axis biomarkers, C-peptide, BMI, and baseline performance status (Table 2). We defined correlation coefficients of >0.6 as strong, between 0.3 and 0.6 as moderate, and <0.3 as weak or nonexistent (29). Using Spearman correlations, we found strong correlations among IGF-I, IGF-II, and IGFBP-3 but weaker correlations for these proteins and either C-peptide, BMI, or performance status. Neither C-peptide nor BMI was associated with baseline performance status.
Table 2.
Correlation between plasma factors and BMI
| Level, mean (SD) | Spearman correlation between factors |
|||||
|---|---|---|---|---|---|---|
| IGF-I (r) | IGF-II | IGFBP-3(r) | C-peptide (r) | BMI (r) | ||
| IGF-I, ng/mL | 183.2 (83.9) | - | - | - | - | - |
| IGF-II, ng/mL | 842.4 (246.6) | 0.60* | - | - | - | - |
| IGFBP-3, ng/mL | 3,682 (1,030) | 0.68* | 0.87* | - | - | - |
| C-peptide, ng/mL | 4.01 (3.00) | 0.22* | 0.10* | 0.13* | - | - |
| BMI, kg/m2 | 27.0 (5.2) | 0.15* | 0.10* | 0.06 | 0.26* | - |
P < 0.05.
Plasma biomarkers levels and tumor response
We examined the influence of baseline plasma biomarker levels on the subsequent rates of objective response to systemic chemotherapy. Baseline levels of plasma IGF-I, IGF-II, and C-peptide were not significantly associated with objective tumor response (P = 0.59, 0.60, and 0.33, respectively). However, increasing baseline IGFBP-3 levels were associated with significantly higher rates of tumor response (multivariate P for trend = 0.03), after adjusting for age, gender, performance status, BMI, and treatment arm (Fig. 1). The response rate was 35% for individuals in the lowest quartile of IGFBP-3 compared with 53% in the highest quartile.
Fig. 1.
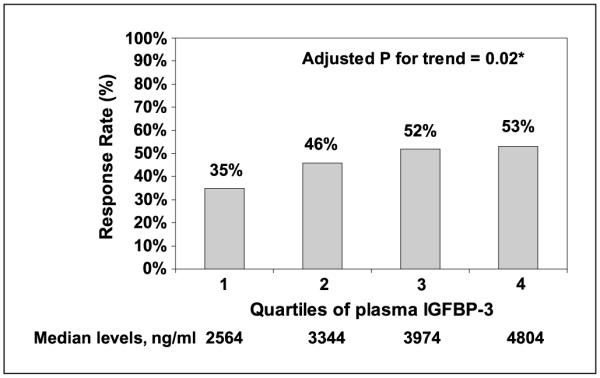
Chemotherapy response rate according to quartiles of plasma IGFBP-3. *, multivariate P value for trend adjusted for age, gender, performance status, BMI, and treatment arm.
Plasma biomarkers and patient outcome
We also examined the influence of baseline plasma biomarkers on time to progression and overall survival (Table 3). Higher baseline levels of IGF-I, IGF-II, and IGFBP-3 were each associated with significantly lower risks of disease progression and death, after adjusting for other patient characteristics. In contrast, levels of C-peptide were not predictive of either time to progression or mortality. Of note, the effect of IGF-I, IGF-II, and IGFBP-3 on patient outcome did not differ significantly according to treatment arm, age, gender, or BMI (data not shown).
Table 3.
Time to tumor progression and overall survival according to quartiles of plasma biomarkers
| Median time to tumor progression, d |
Hazard ratio for progression (95% CI) |
Median overall survival, d |
Hazard ratio for death (95% CI) |
|
|---|---|---|---|---|
| IGF-I, median (ng/mL) | ||||
| Q1, 89.4 | 201 | 1.0 | 463 | 1.0 |
| Q2, 149.2 | 253 | 0.83 (0.63-1.08) | 520 | 0.76 (0.58-0.99) |
| Q3, 203.2 | 286 | 0.62 (0.47-0.82) | 683 | 0.51 (0.39-0.68) |
| Q4, 280.9 | 256 | 0.76 (0.57-1.02) | 685 | 0.54 (0.41-0.72) |
| P, trend | <0.0 | 2 | <0.0001 | |
| IGF2, median (ng/mL) | ||||
| Q1, 575 | 201 | 1.0 | 392 | 1.0 |
| Q2, 747 | 237 | 0.80 (0.62-1.05) | 551 | 0.78 (0.60-1.02) |
| Q3, 917 | 266 | 0.67 (0.51-0.88) | 594 | 0.57 (0.43-0.75) |
| Q4, 1,128 | 302 | 0.66 (0.50-0.87) | 727 | 0.57 (0.44-0.75) |
| P, trend | 0.000 | 2 | <0.0001 | |
| IGFBP-3, median (ng/mL) | ||||
| Q1, 2564 | 204 | 1.0 | 466 | 1.0 |
| Q2, 3344 | 217 | 0.79 (0.60-1.04) | 530 | 0.81 (0.62-1.06) |
| Q3, 3974 | 257 | 0.65 (0.49-0.86) | 685 | 0.60 (0.46-0.80) |
| Q4, 4804 | 302 | 0.66 (0.50-0.87) | 666 | 0.59 (0.45-0.78) |
| P, trend | 0.002 | <0.0001 | ||
| C-peptide, median (ng/mL) | ||||
| Q1, 1.33 | 223 | 1.0 | 533 | 1.0 |
| Q2, 2.45 | 248 | 0.82 (0.62-1.08) | 613 | 0.94 (0.72-1.24) |
| Q3, 4.12 | 259 | 0.82 (0.62-1.08) | 605 | 0.84 (0.63-1.12) |
| Q4, 7.30 | 224 | 1.05 (0.79-1.39) | 540 | 0.98 (0.74-1.30) |
| P, trend | 0.66 | 0.98 | ||
Note: Multivariate hazard ratios, confidence intervals, and P values adjusted for age, gender, performance status, BMI, and treatment arm.
Abbreviations: Q, quartile; 95% CI, 95% confidence interval.
Because participants in the lowest categories of IGF-I, IGF-II, and IGFBP-3 tended to possess a lower BMI and worse performance status, we considered the possibility that the relation between these plasma biomarkers and patient outcome could simply reflect the influence of either an impaired patient nutritional or performance status. Therefore, beyond the aforementioned multivariate models, we repeated our analyses after excluding participants with a BMI <23 kg/m2 or an Eastern Cooperative Oncology Group performance status of 2. Among the 426 patients remaining after these exclusions, higher baseline levels of IGF-I, IGF-II, and IGFBP-3 each remained associated with significantly lower risks of disease progression (P for trend = 0.01, 0.0005, and 0.003, respectively) and death (P for trend < 0.0001 for all three biomarkers).
To assess the independent effects of circulating IGF-I and IGFBP-3, we mutually adjusted for plasma levels of both IGF-I and IGFBP-3 in our multivariate model (Table 4). Within the limitations of mutually adjusting for correlated biomarkers (30), higher circulating levels of IGF-I and IGFBP-3 were each associated with a declining mortality risk, although neither association with overall survival reached statistical significance (P for trend = 0.12 and 0.07 for IGF-I and IGFBP-3, respectively). In contrast, when we mutually adjusted for IGF-I and IGFBP-3, increasing circulating IGFBP-3 was associated with a significant improvement in time to progression (P for trend = 0.03), whereas levels of IGF-I were not associated with the risk of disease progression (P for trend = 0.95).
Table 4.
Patient outcome according to mutually adjusted levels of plasma IGF-I and IGFBP-3
| Quartile of plasma biomarker |
P for trend* | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Overall mortality | |||||
| IGF-I | |||||
| HR for death* (95% CI) | 1.0 (reference) | 0.83 (0.62-1.12) | 0.59 (0.42-0.84) | 0.67 (0.44-1.00) | 0.12 |
| IGFBP-3 | |||||
| HR for death* (95% CI) | 1.0 (reference) | 0.90 (0.68-1.20) | 0.72 (0.52-0.99) | 0.77 (0.52-1.13) | 0.07 |
| Tumor progression | |||||
| IGF-I | |||||
| HR for tumor progression* (95% CI) | 1.0 (reference) | 0.95 (0.70-1.29) | 0.77 (0.54-1.09) | 1.03 (0.69-1.55) | 0.95 |
| IGFBP-3 | |||||
| HR for tumor progression* (95% CI) | 1.0 (reference) | 0.80 (0.60-1.07) | 0.66 (0.48-0.91) | 0.60 (0.46-1.01) | 0.03 |
Abbreviation: HR, hazard ratio.
Multivariate hazard ratios, confidence intervals, and P values adjusted for age, gender, performance status, body mass index, treatment arm, and either plasma IGF-I or IGFBP-3 levels.
Finally, in exploratory analyses, we examined the joint effects of plasma IGF-I and IGFBP-3 levels on tumor response and the risk of tumor progression (Table 5). To provide increased power for these cross-stratified analyses, plasma IGF-I and IGFBP-3 levels were categorized into tertiles. As IGF-I and IGFBP-3 levels are strongly correlated (as shown in Table 2), the general distribution of subjects in Table 5 showed a relatively small number of subjects in the cells where IGF-I levels and IGFBP-3 levels were discordant. In contrast, most subjects appeared in the three cells where IGF-I and IGFBP-3 were in the same tertile. Interestingly, the relatively small number of “outlier” individuals found to simultaneously be in the highest tertile of IGF-I and the lowest tertile of IGFBP-3 seemed to experience the lowest response rate (17%) and the highest risk of tumor progression (hazard ratio, 2.65; 95% confidence interval, 1.07-6.56). In contrast, patients in the middle tertile of IGF-I and the highest tertile of IGFBP-3 seemed to experience the lowest risks of tumor progression (hazard ratio, 0.59; 95% confidence interval, 0.41-0.85).
Table 5.
Response rate and risk of tumor progression according to tertiles of plasma IGF-I and IGFBP-3
| IGF-I (tertiles) | IGFBP-3(tertiles) |
||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1 | |||
| No. patients* | 119 | 40 | 15 |
| Response rate | 40% | 48% | 40% |
| HR for progression† (95% CI) | 1.0 (reference) | 0.88 (0.58-1.33) | 1.32 (0.75-2.33) |
| 2 | |||
| No. patients* | 48 | 69 | 55 |
| Response rate | 42% | 52% | 51% |
| HR for progression† (95% CI) | 0.97 (0.66-1.41) | 0.68 (0.49-0.94) | 0.59 (0.41-0.85) |
| 3 | |||
| No. patients* | 6 | 65 | 57 |
| Response rate | 17% | 46% | 54% |
| HR for progression† (95% CI) | 2.65 (1.07-6.56) | 0.92 (0.65-1.31) | 0.72 (0.53-0.98) |
Number of patients reflects the number with protocol-defined measurable disease who were accessible for objective response (n = 474). Hazard ratios for disease progression were determined among all eligible subjects (N = 527).
Multivariate hazard ratios and P values adjusted for age, gender, performance status, body mass index, and treatment arm.
Discussion
In this cohort of patients with previously untreated metastatic colorectal cancer, higher baseline circulating levels of IGFBP-3 were associated with a significantly greater response rate to chemotherapy and a longer time to tumor progression and overall survival, even after adjusting for other potential predictors of patient outcome. Of note, higher baseline plasma levels of IGF-I and IGF-II also predicted longer times to tumor progression and overall survival, although neither factor was significantly associated with chemotherapy response rate. Moreover, when we mutually adjusted for IGF-I and IGFBP-3, only IGFBP-3 remained a significant predictor of tumor progression. Finally, baseline levels of C-peptide, a marker of circulating insulin, were not associated with any measure of treatment efficacy or patient survival.
The significant influence of higher baseline circulating levels of IGFBP-3 on chemotherapy response rate, time to progression, and survival bears interest. In the only other study of circulating IGFs in colorectal cancer patients, higher IGFBP-3 was similarly associated with a 48% reduction in colorectal cancer – specific mortality whereas IGF-I was not associated with patient outcome (31). IGFBP-3 induces apoptosis using both IGF-dependent and IGF-independent mechanisms (4-8, 32). In murine model systems, IGFBP-3 seems to act through IGF-I–dependent mechanisms early in cancer development; however, during the later stages of tumorigenesis, when angiogenesis, invasion, and inhibiting apoptosis are critical for the tumor, IGFBP-3 seems to act as a tumor suppressor entirely through an IGF-independent mechanism (33). Moreover, supporting the importance of IGFBP-3 in human colorectal carcinogenesis, a recent genome-wide survey of human colorectal cancers identified missense mutations in the IGFBP-3 gene (9).
In preclinical models, overexpression of IGFBP-3 conferred significant tumor reduction with only minimal effects on normal tissues (33). Moreover, in a previous analysis of this cohort, baseline circulating IGFBP-3 was not associated with baseline quality of life measures despite the significant benefit of IGFBP-3 levels on subsequent chemotherapy response and tumor progression (23). The possibility that IGFBP-3 inhibits tumor growth, with minimal apparent deleterious effects on normal host functions, represents an attractive feature in the development of IGFBP-3 as an anticancer agent (33).
We hypothesized that, among patients with newly established colorectal cancer, elevated baseline levels of plasma IGF-I would promote cancer progression, whereas increased circulating IGFBP-3 would delay or inhibit subsequent cancer progression. Although elevated baseline IGFBP-3 did predict a greater response rate to chemotherapy and a longer time to tumor progression and overall survival, the improved overall survival associated with higher circulating levels of IGF-I and IGF-II in this cohort seems counterintuitive, given the presumed tumor-promoting properties of these molecules. The levels of IGF-I, IGF-II, and C-peptide are influenced by malnutrition (1, 34, 35). As such, the inferior survival of patients with reduced levels of IGF-I and IGF-II could have reflected impaired nutritional status and/or impaired patient performance status secondary to a greater cancer burden. However, our findings remained significant after controlling BMI and performance status, and our results did not change after excluding leaner patients and those with an impaired performance status. Moreover, although C-peptide has also been associated with nutritional status (36, 37), levels of C-peptide were not predictive of patient survival in this cohort.
Alternatively, IGF-I and IGF-II may play an essential role in maintaining the functional capacity of the “host” (38, 39). In addition to their role in modulating the balance between cellular proliferation and apoptosis, the IGFs play key roles in regulating energy metabolism, body size, and various organspecific functions (1, 34, 35). Indirect support of this hypothesis is seen in patients with growth hormone deficiency treated with growth hormone replacement, in which IGF-I levels increase and the quality of life improves (40, 41). Administration of recombinant IGF-I in selected chronic diseases improved quality of life in some studies (42), but not others (43, 44). Notably, in a previous analysis of this cohort of colorectal cancer patients (23), higher baseline levels of IGF-I and IGF-II were both associated with a superior quality of life and diminished symptom distress. In contrast, C-peptide was not associated with quality of life measures (23), consistent with the failure of C-peptide to predict survival in the current analysis.
We acknowledge that this study has several limitations. Although baseline BMI was recorded in this trial, we did not collect information on weight loss that may have occurred immediately before study enrollment. Thus, residual confounding by cachexia secondary to advanced malignancy cannot be excluded. Nonetheless, in contrast to IGF-I, IGF-II, and C-peptide, levels of IGFBP-3 seem to be less influenced by nutritional factors (33). In addition, baseline protein levels may be influenced by morbidity from recent surgery or burden of cancer; however, our findings remained unchanged after adjusting for performance status and BMI and after exclusion of leaner patients with an impaired performance status. Moreover, as part of the clinical trial, all patients were required to have adequate biochemical parameters and performance status for enrollment, and >90% of study subjects had an Eastern Cooperative Oncology Group performance status of 0 or 1. In addition, because chemotherapy was defined by the clinical trial, residual confounding by choice of chemotherapy was minimized.
Although patients with poorly controlled diabetes mellitus or hyperglycemia were excluded from the trial, patients with adequately controlled diabetes mellitus were eligible. The clinical trial did not collect data on history of diabetes mellitus; nonetheless, previous studies have shown that circulating levels of IGF-I or IGFBP-3 are not materially influenced by abnormalities such as glucose intolerance or diabetes mellitus (45). Moreover, as shown in the analysis, plasma C-peptide, which is clearly influenced by glucose homeostasis, was not associated with chemotherapy response rate, time to progression, or overall survival among these 527 patients with metastatic colorectal cancer.
Plasma biomarker levels were only measured at the start of chemotherapy, and the effect of changes in the levels of these growth factors on patient outcome could not be assessed. However, one study of women receiving chemotherapy for advanced breast cancer showed that IGF-I did not change on therapy, whereas IGFBP-3 only modestly decreased (46). Additionally, our study sought to examine the influence of baseline plasma factors on patient prognosis and was not adequately powered to examine the predictive value of these markers with respect to specific chemotherapies. Nonetheless, we did not detect any significant interaction between IGF-I, IGF-II, or IGFBP-3 and chemotherapy treatment assignment in our analysis.
There is a growing interest in the IGF pathway as a potential target for cancer therapy (1, 2, 47). Among colorectal cancer patients receiving front-line chemotherapy, our data suggest that increasing levels of an endogenous antagonist to IGF-I, IGFBP-3, may substantially improve objective treatment response and delay cancer progression. However, our results also suggest that a minimum level of circulating IGF-I may be needed to maintain the functional capacity and/or survival of the host. The results of ongoing and proposed trials of IGF-I receptor antagonists in patients with advanced malignancy will hopefully provide further insight in the potential role of IGF pathway inhibition in cancer therapy.
Acknowledgments
Grant support: USPHS grants CA-118553, CA-25224, CA-32102, CA-38926, CA-21115, CA-37404, CA-35195, CA-35101, and P50 CA127003.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 2.Tao Y, Pinzi V, Bourhis J, Deutsch E. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway-therapeutic perspectives in cancer. Nat Clin Pract Oncol. 2007;4:591–602. doi: 10.1038/ncponc0934. [DOI] [PubMed] [Google Scholar]
- 3.Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–54. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 4.Rajah R, Valentinis B, Cohen P. Insulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-β1 on programmed cell death through a p53- and IGF-independent mechanism. J Biol Chem. 1997;272:12181–8. doi: 10.1074/jbc.272.18.12181. [DOI] [PubMed] [Google Scholar]
- 5.Williams AC, Smartt H, AM HZ, Macfarlane M, Paraskeva C, Collard TJ. Insulin-like growth factor binding protein 3 (IGFBP-3) potentiatesTRAIL-induced apoptosis of human colorectal carcinoma cells through inhibition of NF-κB. Cell Death Differ. 2007;14:137–45. doi: 10.1038/sj.cdd.4401919. [DOI] [PubMed] [Google Scholar]
- 6.AM HZ, Collard TJ, Malik K, Hicks DJ, Paraskeva C, Williams AC. Induction of apoptosis by the 16-kDa amino-terminal fragment of the insulin-like growth factor binding protein 3 in human colonic carcinoma cells. Int J Oncol. 2006;29:1279–86. [PubMed] [Google Scholar]
- 7.Kirman I, Poltoratskaia N, Sylla P, Whelan RL. Insulin-like growth factor-binding protein 3 inhibits growth of experimental colocarcinoma. Surgery. 2004;136:205–9. doi: 10.1016/j.surg.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins PJ, Khalaf S, Ogunkolade W, et al. Differential expression of IGF-binding protein-3 in normal and malignant colon and its influence on apoptosis. Endocr Relat Cancer. 2005;12:891–901. doi: 10.1677/erc.1.01080. [DOI] [PubMed] [Google Scholar]
- 9.Wood LD, Parsons DW, Jones S, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–13. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 10.Ma J, Pollak MN, Giovannucci E, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–5. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 11.Ma J, Giovannucci E, Pollak M, et al. Aprospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst. 2004;96:546–53. doi: 10.1093/jnci/djh082. [DOI] [PubMed] [Google Scholar]
- 12.Palmqvist R, Hallmans G, Rinaldi S, et al. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut. 2002;50:642–6. doi: 10.1136/gut.50.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Probst-Hensch NM, Yuan JM, Stanczyk FZ, Gao YT, Ross RK, Yu MC. IGF-1, IGF-2 and IGFBP-3 in prediagnostic serum: association with colorectal cancer in a cohort of Chinese men in Shanghai. Br J Cancer. 2001;85:1695–9. doi: 10.1054/bjoc.2001.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 15.Giovannucci E, Pollak MN, Platz EA, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev. 2000;9:345–9. [PubMed] [Google Scholar]
- 16.Manousos O, Souglakos J, Bosetti C, et al. IGF-I and IGF-II in relation to colorectal cancer. Int J Cancer. 1999;83:15–7. doi: 10.1002/(sici)1097-0215(19990924)83:1<15::aid-ijc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 17.Hunt KJ, Toniolo P, Akhmedkhanov A, et al. Insulinlike growth factor II and colorectal cancer risk in women. Cancer Epidemiol Biomarkers Prev. 2002;11:901–5. [PubMed] [Google Scholar]
- 18.Renehan AG, Jones J, Potten CS, Shalet SM, O’Dwyer ST. Elevated serum insulin-like growth factor (IGF)-II and IGF binding protein-2 in patients with colorectal cancer. Br J Cancer. 2000;83:1344–50. doi: 10.1054/bjoc.2000.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenab M, Riboli E, Cleveland RJ, et al. Serum C-peptide, IGFBP-1 and IGFBP-2 and risk of colon and rectal cancers in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2007;121:368–76. doi: 10.1002/ijc.22697. [DOI] [PubMed] [Google Scholar]
- 20.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S. Plasma C-peptide, insulin-like growth factor-I, insulinlike growth factor binding proteins and risk of colorectal cancer in a nested case-control study: the Japan public health center-based prospective study. Int J Cancer. 2007;120:2007–12. doi: 10.1002/ijc.22556. [DOI] [PubMed] [Google Scholar]
- 21.Wei EK, Ma J, Pollak MN, et al. A prospective study of C-peptide, insulin-like growth factor-I, insulin-like growth factor binding protein-1, and the risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2005;14:850–5. doi: 10.1158/1055-9965.EPI-04-0661. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg RM, Sargent DJ, Morton RF, et al. Arandomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 23.Meyerhardt JA, Sloan JA, Sargent DJ, et al. Associations between plasma insulin-like growth factor proteins and C-peptide and quality of life in patients with metastatic colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1402–10. doi: 10.1158/1055-9965.EPI-04-0862. [DOI] [PubMed] [Google Scholar]
- 24.Harris TG, Strickler HD, Yu H, et al. Specimen processing time and measurement of total insulin-like growth factor-I (IGF-I), free IGF-I, and IGF binding protein-3 (IGFBP-3) Growth Horm IGF Res. 2006;16:86–92. doi: 10.1016/j.ghir.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E, Pollak M, Liu Y, et al. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003;12:84–9. [PubMed] [Google Scholar]
- 26.Giovannucci E, Rimm EB, Liu Y, Willett WC. Height, predictors of C-peptide and cancer risk in men. Int J Epidemiol. 2004;33:217–25. doi: 10.1093/ije/dyh020. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 28.Cox DR. Regression models and life-tables [with discussion] J Royal Stat Soc B. 1972;34:187–220. [Google Scholar]
- 29.Cohen J. Statistical power analysis for behavioral sciences. Lawrence Erlbaum Associates; New Jersey: 1988. [Google Scholar]
- 30.Kleinbaum D, Kupper L, Nizam A, Muller K. Applied regression analysis and other multivariable methods. Duxbury Press; Pacific Grove (CA): 1997. [Google Scholar]
- 31.Haydon AM, Macinnis RJ, English DR, Morris H, Giles GG. Physical activity, insulin-like growth factor 1, insulin-like growth factor binding protein 3, and survival from colorectal cancer. Gut. 2006;55:689–94. doi: 10.1136/gut.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rechler MM. Editorial: Growth inhibition by insulinlike growth factor (IGF) binding protein-3-what’s igf got to do with it? Endocrinology. 1997;138:2645–7. doi: 10.1210/endo.138.7.5355. [DOI] [PubMed] [Google Scholar]
- 33.Cohen P. Insulin-like growth factor binding protein-3: insulin-like growth factor independence comes of age. Endocrinology. 2006;147:2109–11. doi: 10.1210/en.2006-0195. [DOI] [PubMed] [Google Scholar]
- 34.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21:215–44. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 35.Nakae J, Kido Y, Accili D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev. 2001;22:818–35. doi: 10.1210/edrv.22.6.0452. [DOI] [PubMed] [Google Scholar]
- 36.Dean DJ, Gazdag AC, Wetter TJ, Cartee GD. Comparison of the effects of 20 days and 15 months of calorie restriction on male Fischer 344 rats. Aging Milano. 1998;10:303–7. doi: 10.1007/BF03339792. [DOI] [PubMed] [Google Scholar]
- 37.Zuniga-Guajardo S, Garfinkel PE, Zinman B. Changes in insulin sensitivity and clearance in anorexia nervosa. Metabolism. 1986;35:1096–100. doi: 10.1016/0026-0495(86)90021-1. [DOI] [PubMed] [Google Scholar]
- 38.Juul A, Bang P, Hertel NT, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. 1994;78:744–52. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]
- 39.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low Serum Insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106:939–44. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 40.Carroll PV, Littlewood R, Weissberger AJ, et al. The effects of two doses of replacement growth hormone on the biochemical, body composition and psychological profiles of growth hormone-deficient adults. Eur J Endocrinol. 1997;137:146–53. doi: 10.1530/eje.0.1370146. [DOI] [PubMed] [Google Scholar]
- 41.Soares CN, Musolino NR, Cunha Neto M, et al. Impact of recombinant human growth hormone (RH-GH) treatment on psychiatric, neuropsychological and clinical profiles of GH deficient adults. A placebo-controlled trial. Arq Neuropsiquiatr. 1999;57:182–9. doi: 10.1590/s0004-282x1999000200003. [DOI] [PubMed] [Google Scholar]
- 42.Lai EC, Felice KJ, Festoff BW, et al. Effect of recombinant human insulin-like growth factor-I on progression of ALS: a placebo-controlled study. Neurology. 1997;49:1621–30. doi: 10.1212/wnl.49.6.1621. [DOI] [PubMed] [Google Scholar]
- 43.Lee PD, Pivarnik JM, Bukar JG, et al. Arandomized, placebo-controlled trial of combined insulin-like growth factor I and low dose growth hormone therapy for wasting associated with human immunodeficiency virus infection [published erratum appears in J Clin Endocrinol Metab 1996;81:3696] J Clin Endocrinol Metab. 1996;81:2968–75. doi: 10.1210/jcem.81.8.8768860. [DOI] [PubMed] [Google Scholar]
- 44.Waters D, Danska J, Hardy K, et al. Recombinant human growth hormone, insulin-like growth factor 1, and combination therapy in AIDS-associated wasting: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1996;125:865–72. doi: 10.7326/0003-4819-125-11-199612010-00001. [DOI] [PubMed] [Google Scholar]
- 45.Rajpathak SN, McGinn AP, Strickler HD, et al. Insulin-like growth factor-(IGF)-axis, inflammation, and glucose intolerance among older adults. Growth Horm IGF Res. 2008;18:166–73. doi: 10.1016/j.ghir.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holdaway IM, Mason BH, Lethaby AE, et al. Serum insulin-like growth factor-I and insulin-like growth factor binding protein-3 following chemotherapy for advanced breast cancer. ANZ J Surg. 2003;73:905. doi: 10.1046/j.1445-2197.2003.02817.x. [DOI] [PubMed] [Google Scholar]
- 47.Yee D. Targeting insulin-like growth factor pathways. Br J Cancer. 2006;94:465–8. doi: 10.1038/sj.bjc.6602963. [DOI] [PMC free article] [PubMed] [Google Scholar]


